Key Points
Question
What is the objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab?
Findings
This phase 2 interventional trial of 20 patients showed that the best objective response rate (determined by Response Evaluation Criteria In Solid Tumors, version 1.1) at 24 weeks was 30% (95% CI, 12%-54%; n = 6) and that the best objective response rate overall was 35% (95% CI, 15%-59%; n = 7).
Meaning
Talimogene laherparepvec and pembrolizumab was associated with antitumor activity in advanced sarcoma, across a range of sarcoma histologic subtypes, with a manageable safety profile.
Abstract
Importance
Patients with advanced sarcoma have limited treatment options. Talimogene laherparepvec (T-VEC) has been shown to increase tumor-specific immune activation via augmenting antigen presentation and T-cell priming.
Objective
To examine whether T-VEC in combination with pembrolizumab is associated with increased tumor-infiltrating lymphocyte infiltration and programmed death-ligand 1 expression and thus with increased antitumor activity in patients with locally advanced or metastatic sarcoma.
Design, Setting, and Participants
This open-label, single-institution phase 2 interventional trial of T-VEC plus pembrolizumab enrolled 20 patients with locally advanced or metastatic sarcoma between March 16 and December 4, 2017, for whom at least 1 standard systemic therapy had failed. The median duration of therapy was 16 weeks (range, 7-67 weeks). Reported analyses include data through December 14, 2018.
Intervention
Patients received pembrolizumab (200-mg flat dose) intravenously and T-VEC (first dose, ≤4 mL × 106 plaque-forming units [PFU]/mL; second and subsequent doses, ≤4 mL × 108 PFU/mL) injected into palpable tumor site(s) on day 1 of each 21-day cycle.
Main Outcomes and Measures
The primary end point was objective response rate (ORR; complete response and partial response) at 24 weeks determined by Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1, criteria. Secondary end points included best ORR by immune-related RECIST criteria, progression-free survival rate at 24 weeks, overall survival, and safety.
Results
All 20 patients (12 women [60%]; median age, 63.5 years [range, 24-90 years]) were evaluable for response. The study met its primary end point of evaluating the best ORR at 24 weeks determined by RECIST, version 1.1, criteria; the best ORR was 30% (95% CI, 12%-54%; n = 6). The ORR overall was 35% (95% CI, 15%-59%; n = 7). The incidence of grade 3 treatment-related adverse events was low (4 patients [20%]). There were no grade 4 treatment-related adverse events or treatment-related deaths.
Conclusions and Relevance
In this phase 2 clinical trial, treatment with T-VEC plus pembrolizumab was associated with antitumor activity in advanced sarcoma across a range of sarcoma histologic subtypes, with a manageable safety profile. This combination therapy met its predefined primary study end point; further evaluation of T-VEC in combination with pembrolizumab for patients with select sarcoma subtypes is planned.
Trial Registration
ClinicalTrials.gov identifier: NCT03069378
This phase 2 clinical trial examines whether talimogene laherparepvec in combination with pembrolizumab is associated with increased tumor-infiltrating lymphocyte infiltration and programmed death-ligand 1 expression, and thus with increased antitumor activity in patients with locally advanced or metastatic sarcoma.
Introduction
Sarcomas are rare cancers of mesenchymal origin.1,2,3,4 Chemotherapy remains the cornerstone of management for advanced sarcoma. In the first-line setting, doxorubicin or gemcitabine and docetaxel yield objective response rates (ORRs) of approximately 20%.5 Second-line options such as pazopanib and trabectedin yield lower ORRs (4%-10%).6,7 There is a need for more effective treatment options for sarcomas.
Immunotherapy has emerged as a revolutionary oncologic therapy during the last decade.8,9,10,11,12,13 A significant focus of research has centered on T-cell immune checkpoint inhibitors (ICIs),14 which are now approved for treatment of several cancers.9,10,11,12,13
The SARC028 study evaluated treatment with pembrolizumab for patients with sarcoma.15 In the initial group with soft tissue sarcoma (n = 40), the ORR was 18%. Partial responses (PRs) were primarily observed in patients with undifferentiated pleomorphic sarcoma (UPS) and in patients with dedifferentiated and pleomorphic liposarcoma (LPS). A follow-up expansion cohort demonstrated ORRs of 23% (9 of 40) among patients with UPS and 10% (4 of 39) among patients with LPS.16 The Alliance (A091401) noncomparative, phase 2 study randomized patients with advanced sarcoma to receive nivolumab or nivolumab and ipilimumab.17 The ORR in the combination therapy group was 16%, and the ORR in the monotherapy group was 5%. Activity was seen in patients with UPS, leiomyosarcoma, myxofibrosarcoma (MFS), angiosarcoma, and alveolar soft-part sarcoma, yet most patients did not respond, and there was a lack of prognostic biomarkers.
The expression of immune biomarkers differs by sarcoma subtype and may be associated with response to immunotherapy.2 Immune cell infiltration has commonly been detected in genomically complex sarcomas such as dedifferentiated LPS, UPS, and MFS.2 In the SARC028 study, most (75%) of the patients with UPS who responded had programmed death-ligand 1 (PD-L1)–positive tumors.16 Responses were also seen in patients with PD-L1–negative tumors (2 of 8 UPS tumors and 3 of 3 LPS tumors).
Talimogene laherparepvec (T-VEC) is an oncolytic immunotherapy derived from a modified human herpes simplex virus type 1 designed to self-replicate within and lyse tumor cells, thereby releasing tumor antigens and promoting regional and systemic antitumor immunity.18 Talimogene laherparepvec is approved by the US Food and Drug Administration (FDA) for the treatment of melanoma.18,19 Pembrolizumab is an anti–programmed cell death protein 1 (anti–PD-1) monoclonal antibody that negatively regulates effector T-cell activity.14
We hypothesized that T-VEC in combination with pembrolizumab would increase tumor-infiltrating lymphocyte (TIL) infiltration and PD-L1 expression, thereby augmenting antitumor activity. We conducted a phase 2 study of T-VEC and pembrolizumab for patients with sarcoma.
Methods
Study Design and Participants
This was a single-institution, phase 2 study of T-VEC plus pembrolizumab (eFigure 1 in Supplement 1). Key inclusion criteria were being 18 years of age or older; having histologically confirmed locally advanced or metastatic sarcoma; having 1 or more injectable subcutaneous, cutaneous, or nodal lesions (≥10 mm); having a disease measurable by use of Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1,20 criteria; having 1 or more lines of prior standard systemic therapy when available; having an Eastern Cooperative Oncology Group performance status of 0 or 1; and having adequate organ function. Key exclusion criteria included having known active central nervous system metastases, symptomatic autoimmune disease, clinically significant immunosuppression, and/or active herpetic skin lesions; long-term use of systemic antiherpetic agents (eg, acyclovir); having known active tuberculosis, HIV, or acute or chronic hepatitis B or C virus; and having prior therapy with T-VEC. The study protocol was approved by the FDA and the Memorial Sloan Kettering Cancer Center Institutional Review Board (trial protocol in Supplement 2). The study was conducted in accordance with the Declaration of Helsinki21 and the Guidelines for Good Clinical Practice. Each participant provided protocol-specific, written informed consent in accordance with institutional guidelines.
Procedures
Participants received both study drugs administered on day 1 of each 21-day cycle. Talimogene laherparepvec was administered by intratumoral injection (first dose, ≤4 mL × 106 plaque-forming units [PFU]/mL; second and subsequent doses, ≤4 mL × 108 PFU/mL). Talimogene laherparepvec dosing guidelines are shown in eTable 1 in Supplement 1. Pembrolizumab, 200 mg/dose, was administered intravenously. Treatment was repeated until the patient developed a complete response (CR), progressive disease, or unacceptable toxic effects or had received the maximum duration of therapy (12 months). Patients experiencing disease progression were permitted to continue treatment beyond progression if they were deriving clinical benefit. The protocol was amended (amendment 10; approved by the institutional review board on August 30, 2018) to allow patients who stopped treatment after completing 12 months to resume treatment in the setting of disease progression while not receiving therapy. Treatment could be delayed and/or withheld because of toxic effects; delays longer than 9 weeks because of treatment-related adverse events (TRAEs) resulted in permanent discontinuation of study therapy. If T-VEC was delayed and/or discontinued, treatment with pembrolizumab alone could continue, and vice versa. The study was sequentially monitored for TRAEs based on a predefined early stopping rule (eFigure 1 in Supplement 1).
Radiographic tumor assessments were performed at baseline, every 8 weeks for the first 56 weeks, and every 12 weeks thereafter until disease progression. Primary response assessments were measured per RECIST, version 1.1.20 Secondary response assessments were measured per immune-related RECIST criteria.22 All adverse events were categorized using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.23
Mandatory tumor biopsies (from distant noninjected tumor sites) were performed at baseline and week 8, when feasible. Blood samples for correlative analysis were obtained during screening and at selected time points in the study.
Outcomes
The primary end point was ORR (CR and PR) at 24 weeks determined by RECIST, version 1.1, criteria. Secondary end points included best ORR determined by immune-related RECIST criteria, progression-free survival rate at 24 weeks, overall survival, and safety.
Statistical Analysis
The study used a 1-stage design based on the exact binomial test. A 5% ORR was considered not promising; a 30% ORR was considered promising. The planned population was 20 patients. The design had a type I error rate of 0.08 and a type II error rate of 0.04. Efficacy analyses included patients who received study therapy and underwent evaluation for response. Safety analyses included patients who received at least 1 dose of treatment. Statistical tests were 2-sided. Categorical data analyses and summary statistics were used to report adverse events. The Kaplan-Meier method was used to estimate the distributions of time-to-event end points.
Correlative Analyses
Paraffin-embedded tumor material taken before and after treatment was examined for TIL immune biomarker expression, including PD-L1, PD-1, CD3, CD4, FOXP3, CD8, CD68, CD163, and MHC1 by Qualtek Laboratory. Programmed death-ligand 1 tumor membranous expression was determined using the Merck 22C3 antibody. The threshold for positive PD-L1 expression was greater than 1%. The immunohistochemistry for all other immune biomarkers was performed on automated strainers. Slides were imaged using a Mirax slide scanner (Zeiss Inc). Images were generated using Caseviewer software (3DHistech).
The TIL score was determined by examining a hematoxylin-eosin stain for relative abundance based on a morphologic assessment (mean, 3-5 cells per high-power field [40 × objective]). The TIL score was assigned as follows: 3 (>20 cells per high-power field), 2 (11-20 cells per high-power fields), 1 (1-10 cells per high-power field), and 0 (<1 or 0 cells per high-power field).
Results
Between March 16 and December 4, 2017, 20 patients were enrolled in the trial. Reported analyses include data through December 14, 2018. The baseline demographic characteristics of the patients are listed in Table 1. A total of 12 patients (60%) had received 3 or more prior lines of therapy; 5 patients (25%) had previously received ICI. Among responsive patients, the mean number of prior lines of therapy was 1 (range, 0-3). One patient had microsatellite instability–high sarcoma. The median duration of study therapy was 16 weeks (range, 7-67 weeks). Two patients discontinued T-VEC owing to resolution of palpable disease. Two patients stopped pembrolizumab because of toxic effects. The median follow-up time for survival analysis was 56.2 weeks.
Table 1. Baseline Demographic and Disease Characteristics of Patients.
| Characteristic | Patients, No. (%) (N = 20) |
|---|---|
| Age, median (range), y | 63.5 (24-90) |
| Sex | |
| Female | 12 (60) |
| Male | 8 (40) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 9 (45) |
| 1 | 11 (55) |
| Clinical disease status | |
| Recurrent locally advanced with or without local regional lymph node involvement | 8 (40) |
| Distant metastases | 12 (60) |
| HSV serostatus at baseline | |
| Positive | 18 (90) |
| Negative | 2 (10) |
| Injected lesion at baseline, median (range) | |
| Maximum dimension, cm | 5 (2-16.8) |
| No. of lesions injected | 1 (1-4) |
| Sarcoma histologic subtype | |
| Leiomyosarcoma | 5 (25) |
| Angiosarcoma | 3 (15) |
| Head and neck, No. | 2 |
| Radiotherapy associated, No. | 1 |
| Undifferentiated pleomorphic sarcoma | 2 (10) |
| Undifferentiated or unclassified sarcomaa | 3 (15) |
| Otherb | 7 (35) |
| Prior lines of therapy | |
| Median (range) | 3 (0-9) |
| Nonec | 1 (5) |
| 1-2 | 7 (35) |
| ≥3 | 12 (60) |
| Doxorubicin- or liposomal doxorubicin–based treatment | 13 (65) |
| Pazopanib | 9 (45) |
| Prior immunotherapy | 5 (25) |
| Nivolumab and ipilimumab | 3 (15) |
| Nivolumab alone | 1 (5) |
| Clinical trial (atezolizumab and TIGIT antibody) | 1 (5) |
Abbreviations: HSV, herpes simplex virus; TIGIT, T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine based inhibitory motif domain.
One patient with undifferentiated sarcoma had microsatellite instability–high disease.
Other included chondrosarcoma, extraskeletal myxoid chondrosarcoma, malignant peripheral nerve sheath tumor, epithelioid sarcoma, alveolar soft part sarcoma, myxofibrosarcoma, and synovial sarcoma.
No standard systemic therapy option was available.
All patients were evaluable for response. The ORR determined by RECIST, version 1.1, criteria at 24 weeks was 30% (95% CI, 12%-54%) (Table 2). One patient with cutaneous angiosarcoma experienced a delayed response at 32 weeks, making the overall ORR 35% (95% CI, 15%-59%). The median time to response was 14.4 weeks (range, 6.6-31.9 weeks). The median duration of response was 56.1 weeks (range, 49.4-87.0 weeks). At the time of analysis, 5 patients had completed the maximal duration of study therapy (12 months), and 4 patients remained in the study (2 were progression-free while not receiving treatment and 2 resumed study therapy in the setting of progression while not receiving therapy, and disease control was recaptured). The disease control rate (CR, PR, and stable disease) determined by RECIST, version 1.1, criteria was 70%.
Table 2. Objective Response Rate per RECIST, Version 1.1, and Immune-Related RECIST Criteria.
| Variable | RECIST v1.1 (n = 20) | Immune-Related RECIST (n = 20) |
|---|---|---|
| Objective response, No. (%) | ||
| Best overall response | 7 (35) | 7 (35) |
| Complete response | 0 | 0 |
| Partial response | 7 (35) | 7 (35) |
| Stable disease | 7 (35) | 5 (25) |
| Progressive disease | 6 (30) | 8 (40) |
| Best objective response rate, No. (%) | 7 (35) | 7 (35) |
| At 24 wk | 6 (30) | 6 (30) |
| Disease control rate, No. (%) | 14 (70) | 12 (60) |
| Duration of response | ||
| Patients with response, No. (%) | 7 (35) | 7 (35) |
| Duration of response, median (range), wk | 56.1 (49.4-87) | 56.1 (49.4-87) |
Abbreviation: RECIST, Response Evaluation Criteria In Solid Tumors.
Partial response was observed in 5 histologic subtypes: cutaneous angiosarcoma of head and neck (n = 2), UPS (n = 2), MFS (n = 1), epithelioid sarcoma (n = 1), and sarcoma unclassified (n = 1). The demographic characteristics of the patients who achieved a PR are shown in eTable 2 in Supplement 1. One patient with UPS achieved a PR at week 8, and another patient with MFS achieved a PR at week 24; both patients completed 52 weeks of treatment with pembrolizumab and maintained PR while not receiving therapy. Another patient with UPS achieved a PR at week 16, which was maintained until the patient left the study at week 49 because of noncompliance. Two patients with cutaneous angiosarcoma experienced a PR (1 at week 16 and another at week 32). Both of these patients completed 52 weeks of study therapy; one experienced progression after 1 month of not receiving therapy, and the other experienced progression after 4 months of not receiving therapy. Study treatment was reinitiated, and disease control was recaptured in both patients. Another PR was seen in a patient with epithelioid sarcoma at 8 weeks. This patient left the study at week 40 owing to recurrent immune-mediated uveitis. A patient with unclassified sarcoma achieved a PR at week 8 and left the study owing to progression of disease at week 52. Six of the 7 patients who responded to treatment had locally advanced, multifocal, unresectable disease or operable disease necessitating morbid palliative surgery (n = 2). Three patients who responded to treatment had a measurable distant disease beyond the injectable tumor site(s); a response was observed in all 3 patients in both injected and noninjected tumor(s), including at visceral sites.
Two of the patients who responded to treatment (1 with angiosarcoma and 1 with epithelioid sarcoma) had disease progression while receiving immunotherapy regimens incorporating ICI immediately before entering this study (concurrent radiotherapy was also administered in 1 case), suggesting synergism between ICI and T-VEC in this study. The patient with microsatellite instability–high sarcoma achieved stable disease as the best response before experiencing disease progression at week 16.
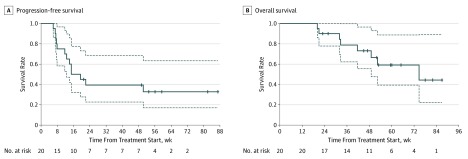
The median progression-free survival was 17.1 weeks (95% CI, 12.6 weeks to not estimable; Figure, A). The 12-week progression-free survival rate was 70% (2-sided 95% CI, 52.5%-93.3%), and the 24-week progression-free survival rate was 39.4% (2-sided 95% CI, 22.7%-68.3%). At the data cutoff, 8 patients had died. The median disease-specific survival was 74.7 weeks (3-sided 95% CI, 49.0 weeks to not estimable; Figure, B).
Figure. Kaplan-Meier Analysis of Progression-Free Survival and Overall Survival.
Dashed lines indicate the 95% CIs.
Talimogene laherparepvec and pembrolizumab were well tolerated in combination. The most frequent TRAEs occurring at any grade in 20% or more of patients included fatigue (16 [80%]), fever (9 [45%]), chills (9 [45%]), nausea (6 [30%]), anemia (5 [25%]), vomiting (4 [20%]), hypothyroidism (4 [20%]), pruritus (4 [20%]), and an increase in aspartate aminotransferase level (4 [20%]) (eTable 3 in Supplement 1). Grade 3 TRAEs were reported for 4 patients and included pneumonitis (1 [5%]), anemia (1 [5%]), fever (1 [5%]), and hypophosphatemia (1 [5%]). There were no grade 4 TRAEs and no treatment-related deaths.
Immune-mediated TRAEs (any grade) included pneumonitis (2 [10%]), uveitis (1 [5%]), hypothyroidism (4 [20%]), and thrombocytopenia (1 [5%]) (eTable 3 in Supplement 1). One patient experienced grade 3 pneumonitis. Another patient experienced recurrent grade 2 uveitis and discontinued study therapy.
Pretreatment tumor tissue was adequate for correlative analysis (PD-L1 and TIL characterization) for 16 patients, and posttreatment tumor tissue was adequate for correlative analysis for 14 patients. Eleven patients had adequate paired tumor specimens suitable for analysis.
PD-L1 Tumor Membrane Expression
One patient with epithelioid sarcoma had PD-L1–positive sarcoma at baseline. This patient obtained a PR after previously experiencing disease progression while receiving nivolumab and ipilimumab concurrent with radiotherapy. Among the 11 patients with paired evaluable tumor samples, 6 (55%) converted from PD-L1 negative at baseline to PD-L1 positive after treatment. eTable 4 in Supplement 1 includes the efficacy outcomes of these patients stratified by PD-L1 tumor expression and histologic subtype. Tumor material suitable for analysis was available from 6 of the 7 patients who experienced a response and 13 patients with refractory disease. In the responsive cohort, 1 patient had PD-L1–positive tumor expression at baseline and 4 patients had PD-L1–positive tumor expression after treatment. In the refractory group, no patients had PD-L1–positive disease at baseline and 5 patients had PD-L1–positive disease after treatment.
TIL Score Assessment
The mean TIL score was higher in the responsive group compared with the refractory group. The mean TIL score detected among responsive patients was 3, compared with a mean score of 2 among patients in the refractory group (eTable 4 in Supplement 1).
Immunohistochemical Analysis
Fourteen patients, including 7 responsive and 7 nonresponsive patients, had pretreatment and posttreatment biopsy samples that underwent immunohistochemical analysis. On review of hematoxylin-eosin stains, 1 case from each group was excluded because there was limited tissue present for examination, leaving each group with 6 informative sample pairs. Within the responsive group, the results of the morphologic examination showed no residual viable tumor in 3 posttherapy samples (including 2 UPS and 1 angiosarcoma cases). The remaining 3 samples had a residual viable tumor. A consistent pattern seen in all the pretreatment samples of responsive patients was the presence of clusters and nodular aggregates of CD3+/CD8+ TILs at the infiltrating edge of the tumor (eFigure 2A in Supplement 1). A smaller number of TILs were present admixed within the tumor. The posttherapy samples showed an increased number of CD3+/CD8+ TILs overall compared with the pretherapy samples. In contrast, the samples from nonresponsive patients, either before or after therapy, had minimal CD3+/CD8+ TIL infiltrates within the tumor, and, more important, they all lacked clusters or aggregates of TILs at the infiltrative edge of the tumor (eFigure 2B in Supplement 1).
Discussion
This study met its primary end point and demonstrated that T-VEC plus pembrolizumab was associated with an ORR at 24 weeks of 30%. A delayed response was observed in 1 patient at 32 weeks, which increased the ORR to 35%. This finding, together with the median time to response of 14.4 weeks, highlights that the maximal response to therapy may take a prolonged period to achieve. The median duration of response was 56.1 weeks, suggesting durable disease control. To our knowledge, this is one of the highest ORRs reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy.
The safety of this combination therapy for sarcoma is consistent with a previously reported experience of patients with melanoma.24 The incidence of grade 3 TRAEs was low (20%). The incidence of grade 3 TRAEs observed with standard chemotherapy is at least comparable but generally higher.5,6,7,25,26 There were no grade 4 TRAEs or treatment-related deaths.
Responses were observed among 5 histologic subtypes, including UPS, MFS, epithelioid sarcoma, cutaneous angiosarcoma, and unclassified sarcoma. Most (60%) of the study population had received 3 or more prior lines of therapy. Among responsive patients, the mean number of prior lines of therapy was 1 (range, 0-3). This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course. It has been observed in other cancer types, such as Merkel cell carcinoma, that higher response rates to immunotherapy are achieved among chemotherapy-naive patients compared with chemotherapy-refractory patients.11,27
Given the nature of T-VEC administration, the trial was enriched for patients with locally advanced disease. Patients in this group had either unresectable or operable disease that would necessitate morbid palliative surgery. Eight patients with recurrent, locally advanced disease enrolled, of whom 6 (75%) achieved a PR. Neoadjuvant chemotherapy is considered for patients presenting with localized, large, high-grade soft tissue sarcomas. The standard neoadjuvant regimen is anthracycline-ifosfamide–based chemotherapy. An ORR of 16% to 28% has been reported with this neoadjuvant regimen.28,29,30,31 The high response rate observed among patients with locally advanced sarcoma in this study supports the investigation of T-VEC and pembrolizumab as neoadjuvant strategy for disease subtypes in which activity was demonstrated. Response in distant, noninjected sites was observed among the patients with stage IV and recurrent locally advanced disease (eFigure 2B in the Supplement). This finding underscores the merit of continuing to explore this combination therapy for metastatic sarcoma as well.
There is a need to develop prognostic biomarkers. The patients in this study underwent pretreatment and posttreatment tumor biopsies. Only 1 patient demonstrated PD-L1–positive disease at baseline; this patient achieved a PR. Most (9 [64%]) of the posttreatment tumor samples were PD-L1 positive; 4 patients experienced PR, while the remainder experienced stable or progressive disease. Responsive patients’ tumors had a higher TIL score. In all the responsive patients, across various subtypes, pretreatment samples had aggregates of CD3+/CD8+ TILs at the infiltrating tumor edge. This finding is consistent with previous reports of patients with melanoma treated with pembrolizumab, in which the responsive patients had increased TILs at the tumor edge.32
Limitations
Although this trial had positive results, as a phase 2 study, it has certain limitations. Adequate tumor material was not available from all patients, which limits the interpretation of the correlative analyses. There was no control group, which leads to difficulty in discerning the benefit of the combination therapy compared with either drug alone. Response to pembrolizumab monotherapy in patients with advanced UPS and cutaneous angiosarcoma has previously been reported in the literature.15,33 However, 2 responsive patients in our study were refractory to combination ICI prior to study entry, which suggests that T-VEC may augment the efficacy of pembrolizumab and offer clinical benefit over ICI alone. Further investigation of this combination therapy in a randomized clinical trial of patients with select sarcoma subtypes represents the ideal next step.
Conclusions
This phase 2 study of T-VEC and pembrolizumab for patients with advanced sarcoma met its primary end point. Expansion of this study is in progress for angiosarcoma, UPS and MFS, and epithelioid sarcoma. In addition, a neoadjuvant cohort of patients with localized, operable UPS and MFS is being explored. Continued exploration of immune-related biomarkers to inform the future selection of patients with sarcomas most likely to benefit from this treatment remains a priority for the expansion cohort.
eFigure 1. Study Schema
eFigure 2A. Morphologic Appearance and Immunohistochemical Results of Responders
eFigure 2B. Morphologic Appearance and Immunohistochemical Results of Nonresponders
eTable 1. Talimogene Laherparepvec Injection Volume Guideline Based on Tumor Size
eTable 2. Demographic and Disease Characteristics of Responders (n = 7)
eTable 3. Incidence of Treatment-Related Adverse Events
eTable 4. Tumor Membranous PD-L1 Expression and Characterization of Tumor-Infiltrating Lymphocytes
Trial Protocol
References
- 1.D’Angelo SP, Tap WD, Schwartz GK, Carvajal RD. Sarcoma immunotherapy: past approaches and future directions. Sarcoma. 2014;2014:391967. doi: 10.1155/2014/391967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4):950-965. doi: 10.1016/j.cell.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 4.Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75-85. doi: 10.1016/S1470-2045(00)00016-4 [DOI] [PubMed] [Google Scholar]
- 5.Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397-1410. doi: 10.1016/S1470-2045(17)30622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Graaf WT, Blay JY, Chawla SP, et al. ; EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group . Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879-1886. doi: 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793. doi: 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti–CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384. doi: 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133. doi: 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9):e180077. doi: 10.1001/jamaoncol.2018.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493-1501. doi: 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess MA, Bolejack V, Schuetze S, et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): final results of SARC028 expansion cohorts. J Clin Oncol. 2019;37(15_suppl):11015. doi: 10.1200/JCO.2019.37.15_suppl.11015 [DOI] [Google Scholar]
- 17.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416-426. doi: 10.1016/S1470-2045(18)30006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780-2788. doi: 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 19.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763-5771. doi: 10.1200/JCO.2009.24.3675 [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Published May 28, 2009. Accessed December 9, 2019.
- 24.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec and pembrolizumab for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(15_suppl):9568. doi: 10.1200/JCO.2016.34.15_suppl.9568 [DOI] [Google Scholar]
- 25.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637. doi: 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 26.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497. doi: 10.1016/S0140-6736(16)30587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812-822. doi: 10.1016/S1470-2045(17)30334-0 [DOI] [PubMed] [Google Scholar]
- 29.Gortzak E, Azzarelli A, Buesa J, et al. ; E.O.R.T.C. Soft Tissue Bone Sarcoma Group and the National Cancer Institute of Canada Clinical Trials Group/Canadian Sarcoma Group . A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096-1103. doi: 10.1016/S0959-8049(01)00083-1 [DOI] [PubMed] [Google Scholar]
- 30.Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol. 2016;27(12):2283-2288. doi: 10.1093/annonc/mdw430 [DOI] [PubMed] [Google Scholar]
- 31.Pasquali S, Gronchi A. Neoadjuvant chemotherapy in soft tissue sarcomas: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(6):415-429. doi: 10.1177/1758834017705588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7(1):213. doi: 10.1186/s40425-019-0689-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Schema
eFigure 2A. Morphologic Appearance and Immunohistochemical Results of Responders
eFigure 2B. Morphologic Appearance and Immunohistochemical Results of Nonresponders
eTable 1. Talimogene Laherparepvec Injection Volume Guideline Based on Tumor Size
eTable 2. Demographic and Disease Characteristics of Responders (n = 7)
eTable 3. Incidence of Treatment-Related Adverse Events
eTable 4. Tumor Membranous PD-L1 Expression and Characterization of Tumor-Infiltrating Lymphocytes
Trial Protocol