This case-control cohort study analyzes patients with suspected coronary atherosclerosis and control patients to identify the factors associated with higher or lower risks for adverse cardiovascular events and acute coronary syndrome.
Key Points
Question
Is the density of coronary calcified plaque associated with future development of acute coronary syndrome?
Findings
In this case-control study of 189 patients who experienced vs 189 control individuals who did not experience an acute coronary syndrome after baseline coronary computed tomography angiography imaging, the volume of plaque with more than 1000 Hounsfield unit (termed 1K plaque) was associated with lower risk for acute coronary syndrome. The specific acute coronary syndrome precursor culprit lesion had less 1K plaque compared with the most stenotic lesion in control individuals.
Meaning
This study’s findings suggest that 1K plaque detected by coronary computed tomography angiography is associated with lower risk of future occurrence of acute coronary syndrome.
Abstract
Importance
Plaque morphologic measures on coronary computed tomography angiography (CCTA) have been associated with future acute coronary syndrome (ACS). However, the evolution of calcified coronary plaques by noninvasive imaging is not known.
Objective
To ascertain whether the increasing density in calcified coronary plaque is associated with risk for ACS.
Design, Setting, and Participants
This multicenter case-control cohort study included individuals enrolled in ICONIC (Incident Coronary Syndromes Identified by Computed Tomography), a nested case-control study of patients drawn from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry, which included 13 study sites in 8 countries. Patients who experienced core laboratory–verified ACS after baseline CCTA (n = 189) and control individuals who did not experience ACS after baseline CCTA (n = 189) were included. Patients and controls were matched 1:1 by propensity scores for age; male sex; presence of hypertension, hyperlipidemia, and diabetes; family history of premature coronary artery disease (CAD); current smoking status; and CAD severity. Data were analyzed from November 2018 to March 2019.
Exposures
Whole-heart atherosclerotic plaque volume was quantitated from all coronary vessels and their branches. For patients who underwent invasive angiography at the time of ACS, culprit lesions were coregistered to baseline CCTA lesions by a blinded independent reader. Low-density plaque was defined as having less than 130 Hounsfield units (HU); calcified plaque, as having more than 350 HU and subcategorized on a voxel-level basis into 3 strata: 351 to 700 HU, 701 to 1000 HU, and more than 1000 HU (termed 1K plaque).
Main Outcomes and Measures
Association between calcium density and future ACS risk.
Results
A total of 189 patients and 189 matched controls (mean [SD] age of 59.9 [9.8] years; 247 [65.3%] were male) were included in the analysis and were monitored during a mean (SD) follow-up period of 3.9 (2.5) years. The overall mean (SD) calcified plaque volume (>350 HU) was similar between patients and controls (76.4 [101.6] mm3 vs 99.0 [156.1] mm3; P = .32), but patients who experienced ACS exhibited less 1K plaque (>1000 HU) compared with controls (3.9 [8.3] mm3 vs 9.4 [23.2] mm3; P = .02). Individuals within the highest quartile of 1K plaque exhibited less low-density plaque, as a percentage of total plaque, when compared with patients within the lower 3 quartiles (12.6% [10.4%] vs 24.9% [20.6%]; P < .001). For 93 culprit precursor lesions detected by CCTA, the volume of 1K plaque was lower compared with the maximally stenotic lesion in controls (2.6 [7.2] mm3 vs 7.6 [20.3] mm3; P = .01). The per-patient and per-lesion results were similar between the 2 groups when restricted to myocardial infarction cases.
Conclusions and Relevance
Results of this study suggest that, on a per-patient and per-lesion basis, 1K plaque was associated with a lower risk for future ACS and that measurement of 1K plaque may improve risk stratification beyond plaque burden.
Introduction
Coronary computed tomography angiography (CCTA) allows for quantification of coronary atherosclerotic plaque. Overall plaque burden is a determinant of future major cardiovascular events. In addition, based on pathologic analysis and imaging results, lesions associated with acute coronary syndrome (ACS) or sudden coronary death exhibit necrotic lipid-laden cores, positive remodeling, spotty calcification, high cross-sectional plaque burden, high stenosis severity, and thin-cap fibroatheroma.1,2 Evolution of fibroatheromas, the development of sheets of calcification and reduction in necrotic core size, is considered plaque stabilizing.3
The evolution of calcified coronary plaques by noninvasive imaging is not known; however, it has been posited that increasing Hounsfield unit (HU) density (ie, brightness) on CCTA may reflect a stabilizing process. This hypothesis is consistent with the results from the Multi-Ethnic Study of Atherosclerosis (MESA), which used noncontrast computed tomographic (CT) imaging for coronary artery calcium scoring in which higher overall calcium density score was associated with lower risk for major cardiovascular events after adjusting for overall calcium volume.4
The present case-control cohort study analyzes the Incident Coronary Syndromes Identified by Computed Tomography (ICONIC), a study with, to our knowledge, the largest cohort of patients who experienced core laboratory–verified ACS after baseline CCTA. Individuals with ACS (referred to as cases or patients) were matched with those without ACS (referred to as control individuals) on the basis of cardiovascular risk factors and angiographic coronary artery disease (CAD) severity, with the total plaque volume not differing between the 2 groups. The aim of the present study was to ascertain the association between increasing density of calcified plaque and risk of future ACS.
Methods
Study Design
ICONIC was a nested case-control study that comprised patients with no known CAD drawn from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry, which included 13 sites in 8 countries (United States, Canada, Italy, Germany, South Korea, Austria, Portugal, and the Netherlands).2 Each participating site obtained local institutional review board or ethics board approval. Written or oral patient informed consent or a waiver of consent was obtained according to site-specific regulations.
The specific methods for inclusion and exclusion of patients and the construction of the ICONIC cohort have been previously described.2 Briefly, the CONFIRM registry prospectively collected baseline demographic and clinical data of patients who underwent clinically indicated CCTA for suspected CAD and monitored them for the occurrence of major adverse cardiovascular events.5 Patients who experienced site-adjudicated ACS were matched 1:1 to within-site control individuals who did not experience ACS during the mean (SD) follow-up period of 3.9 (2.5) years. Hence, the results of the present study were prospective but observational. Sites submitted clinical, electrocardiographic, imaging, and laboratory data that supported ACS to the Clinical and Data Coordinating Center (Dalio Institute of Cardiovascular Imaging, New York, New York) for uniform adjudication of ACS while masked to CCTA evaluation findings. In addition, DICOM (Digital Imaging and Communications in Medicine) files were sent to the CCTA core laboratory (Severance Cardiovascular Hospital, Seoul, South Korea), which performed quantitative, whole-heart, 18-segment coronary tree analysis and was blinded to case status.
After exclusion of site-reported ACS cases with insufficient adjudication data, ACS cases in revascularized coronary segments, and CCTA scans with insufficient image quality for quantitative analysis, the final ICONIC cohort consisted of 234 cases of ACS and 234 propensity score–matched control individuals. The propensity score was based on age; male sex; the presence of hypertension, hyperlipidemia, and diabetes; family history of premature CAD; current smoking status; and CAD severity by CCTA (defined as nonobstructive CAD or as 1-vessel, 2-vessel, or 3-vessel or left main obstructive CAD [≥50% stenosis]).2
Patients or control individuals older than 75 years were excluded (along with their counterparts) from the present study because current guidelines do not recommend routine risk assessment above this age threshold and to avoid excessive calcification.6 Data for patient or matched control individuals older than 75 years are presented in eTable 2 in the Supplement.
ACS Event Adjudication
A detailed description of event adjudication has been published previously.2 The Clinical and Data Coordinating Center reviewed symptoms of ischemia, cardiac laboratory biomarker data, electrocardiogram results, and invasive coronary angiograms for site-reported ACS cases and adjudicated cases according to the World Health Organization definition and the third universal definition of myocardial infarction7,8 or unstable angina. Myocardial infarction cases consisted of ST-segment elevation myocardial infarction (STEMI), non–STEMI, or unclassified myocardial infarction (abnormal cardiac enzyme level [>99% of the upper limit of normal], presence of other supporting information, and ambiguous electrocardiogram unsuitable for STEMI or non–STEMI classification). Six physicians (F.Y.L., L.B., I.C., D.H., J.-H.L., and A.R.) who were blinded to CCTA results adjudicated ACS cases, and 2 additional physicians (F.Y.L., D.H.) adjudicated ambiguous cases. Acute coronary syndrome cases related to previous percutaneous coronary intervention, stent thrombosis, or coronary artery bypass graft were excluded because these events could not be related to baseline CCTA plaques.
In addition, 1 culprit lesion per patient was adjudicated for patients who underwent invasive angiography at the time of ACS.2 In patients with 1 single significant stenosis, this lesion was considered the culprit lesion. In cases with 2 or more lesions, the culprit lesion was defined on the basis of luminal narrowing, the complexity of the lesion’s morphologic structure, and the electrocardiogram for the distribution of ischemia. In cases with multiple candidate culprits, consensus reading with a second cardiologist was performed. Subsequently, the invasive angiography–defined culprit lesion was coregistered to baseline CCTA lesions by coronary segment coding, distance from ostia, and vessel branch points as landmarks. Unblinded comparison of invasive coronary angiography with CCTA was allowed for coregistration of ambiguous cases.
CCTA Evaluation
Coronary computed tomography angiography scans were acquired with at least 64-slice scanners in accordance with the Society of Cardiovascular Computed Tomography guidelines, and sites visually interpreted images for coronary plaque presence, stenosis severity, location, and composition from an 18-segment coronary tree model.9,10 The DICOM files were sent to the CCTA core laboratory, where independent level III–experienced readers performed quantitative plaque analysis using semiautomated software (QAngioCT Research Edition, version 2.1.9.1; Medis Medical Imaging Systems).
The methods, including routine interreproducibility and intrareproducibility checks, are described elsewhere.2 Briefly, lumen, vessel, and plaque volumes were measured in each coronary segment from the coronary tree 2 mm or more in diameter on every 1-mm cross-section; data were summed to per-segment and per-patient levels. Plaque composition was defined as necrotic core, fibro-fatty, fibrous, and calcified, using the following HU strata: 30 or less, 31 to 130, 131 to 350, and more than 350.11,12 The present study focused on calcified plaque, and additional calcification subgroups were created by calculating plaque strata starting from 351 HU: 351 to 700, 701 to 1000, and more than 1000 HU which, was referred to as 1K plaque and resembled very dense calcification. These thresholds differed from categories of HU densities for calculation of Agatston score because all measurements were performed on contrast-enhanced CCTA, which is known to increase the attenuation (ie, HU) of all vascular structures. Besides segmental analysis, measurements of length, volume, composition, stenosis, and maximal cross-sectional plaque burden were performed for each coronary lesion.13 The same plaque composition HU strata as in a per-segment level were calculated on a per-lesion level analysis. Plaque data were provided by absolute volume. Primary analysis was performed between ACS cases and control individuals, and secondary analysis included comparisons between cases of myocardial infarction and control individuals.
Statistical Analysis
Continuous data were described as mean (SD) regardless of distribution, for uniformity of presentation. The 2-tailed, unpaired t test or Mann-Whitney test was used for comparisons of unpaired continuous data; the paired t test or Wilcoxon rank sum test was used for paired data. Categorical data were compared with the McNemar test. First, at the per-patient level, the calcified plaque strata were compared between patients and control individuals. Second, patients and control individuals in the top quartile of 1K plaque were compared with the remaining 3 quartiles of individuals for relative amounts of calcified, fibrous, fibro-fatty, and necrotic core plaques (calculated by dividing by the total plaque volume and multiplying by 100%). Third, calcified plaque strata of culprit precursor lesions were compared with the lesion with the highest diameter stenosis in the matched control individual.
Two-sided P < .05 was considered statistically significant. All analyses were performed with SAS, version 9.4 (SAS Institute Inc), and R, version 3.3.0 (R Foundation for Statistical Computing). Data analyses were performed from November 2018 to March 2019.
Results
Patients
The study population included 189 patients experiencing core laboratory–verified ACS and 189 propensity score–matched control individuals. The mean (SD) age of the population was 59.9 (9.8) years, and 247 (65.3%) were male. Patients and control individuals were well matched on their mean (SD) propensity scores (0.07 [0.04] vs 0.07 [0.04]; P = .76). Of the individual score components, the presence of diabetes was lower in patients with ACS compared with control individuals (37 [19.6%] vs 57 [30.2%]; P = .006), whereas CAD severity tended to be more severe in the control individuals compared with cases (Table 1). Patients more frequently underwent interval coronary revascularization (92 patients [48.7%] vs 42 [22.2%]; P < .001) of nonculprit lesions. Patients, compared with control individuals, displayed higher mean (SD) maximal stenosis severity (42.5% [25.9%] vs 31.9% [21.1%]; P < .001). The total mean (SD) plaque volume was similar in both groups (258.9 [271.0] mm3 vs 252.9 [286.9] mm3; P = .38) (Table 2).
Table 1. Propensity Score and Clinical Characteristics.
| Variable | Frequency, No. (%) | P Value | |
|---|---|---|---|
| ACS Group (n = 189) | Control Group (n = 189) | ||
| Included in propensity score | |||
| Age, mean (SD), y | 59.6 (10.3) | 60.3 (9.3) | .33 |
| Male sex | 126 (66.7) | 121 (64.0) | .33 |
| Diabetes | 37 (19.6) | 57 (30.2) | .006 |
| Hypertension | 116 (62.0) | 106 (56.1) | .21 |
| Hyperlipidemia | 105 (55.6) | 102 (54.0) | .82 |
| Familial history of CAD | 80 (43.5) | 78 (41.3) | .44 |
| Current smoker | 56 (29.6) | 43 (22.8) | .09 |
| CAD severity | |||
| None | 13 (7.3) | 25 (13.2) | .06 |
| Nonobstructive | 69 (38.8) | 75 (39.7) | |
| 1-Vessel obstructive | 26 (14.6) | 38 (20.1) | |
| 2-Vessel obstructive | 31 (17.4) | 21 (11.1) | |
| 3-Vessel/left main obstructive | 39 (21.9) | 30 (15.9) | |
| Angina type | |||
| Asymptomatic | 25 (14.0) | 64 (35.0) | .001 |
| Noncardiac | 20 (11.2) | 23 (12.6) | |
| Atypical | 8 (47.8) | 69 (37.7) | |
| Typical | 48 (27.0) | 27 (14.8) | |
| Race/ethnicity | |||
| White | 85 (62.0) | 89 (62.2) | .59 |
| East Asian | 43 (31.4) | 42 (29.4) | |
| Other | 9 (6.6) | 12 (8.4) | |
| Lipid profile, mean (SD), mg/dL | |||
| Cholesterol | |||
| Total | 190.0 (48.2) | 186.7 (40.7) | .67 |
| Low-density lipoprotein | 116.6 (42.6) | 119.2 (32.1) | .78 |
| High-density lipoprotein | 43.5 (10.9) | 47.8 (13.2) | .09 |
| Medications | |||
| Statins | 75 (58.1) | 64 (49.2) | .24 |
| Aspirin | 73 (52.5) | 66 (47.5) | .78 |
| ACEI/ARB | 51 (37.0) | 52 (37.4) | >.99 |
| β-Blocker | 49 (35.5) | 49 (35.3) | .79 |
| Interval coronary revascularization | 92 (48.7) | 42 (22.2) | <.001 |
| ACS type | |||
| Myocardial infarction | |||
| ST-segment elevation | 33 (17.5) | NA | NA |
| Non–ST-segment elevation | 90 (47.6) | NA | NA |
| Unclassified | 3 (1.6) | NA | NA |
| Unstable angina | 63 (33.0) | NA | NA |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; CAD, coronary artery disease; NA, not applicable.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
Table 2. Per-Patient Atherosclerotic Characteristics.
| Variable | Mean (SD) | P Value | |
|---|---|---|---|
| ACS Group (n = 189) | Control Group (n = 189) | ||
| Atherosclerotic feature, % | |||
| Maximal | |||
| Diameter stenosis | 42.5 (25.9) | 31.9 (21.1) | <.001 |
| Area stenosis | 60.2 (27.1) | 49.1 (27.9) | <.001 |
| Cross-sectional plaque burden | 64.8 (25.7) | 54.4 (29.1) | <.001 |
| Mean plaque burden | 10.4 (9.5) | 10.5 (10.5) | .45 |
| Total plaque volume, mm3 | 258.9 (271.0) | 252.9 (286.9) | .38 |
| Calcified (>350 HU) | 76.4 (101.6) | 99.0 (156.1) | .32 |
| Fibrous (131-350 HU) | 117.4 (121.6) | 109.3 (121.8) | .30 |
| Fibro-fatty (31-130 HU) | 58.3 (82.9) | 40.7 (62.5) | .007 |
| Necrotic core (<30 HU) | 6.8 (14.9) | 4.1 (8.7) | .01 |
| Fibro-fatty + necrotic core (<30-130 HU) | 65.1 (93.1) | 44.7 (68.9) | .006 |
| Calcium density, mm3 | |||
| Calcification (351-700 HU) | 57.1 (73.6) | 66.8 (99.12) | .61 |
| Calcification (701-1000 HU) | 14.8 (23.8) | 23.0 (44.5) | .11 |
| 1K plaque (>1000 HU) | 3.9 (8.3) | 9.4 (23.2) | .02 |
Abbreviations: ACS, acute coronary syndrome; HU, Hounsfield units.
Patient-Level Calcified Plaque in Patients vs Controls
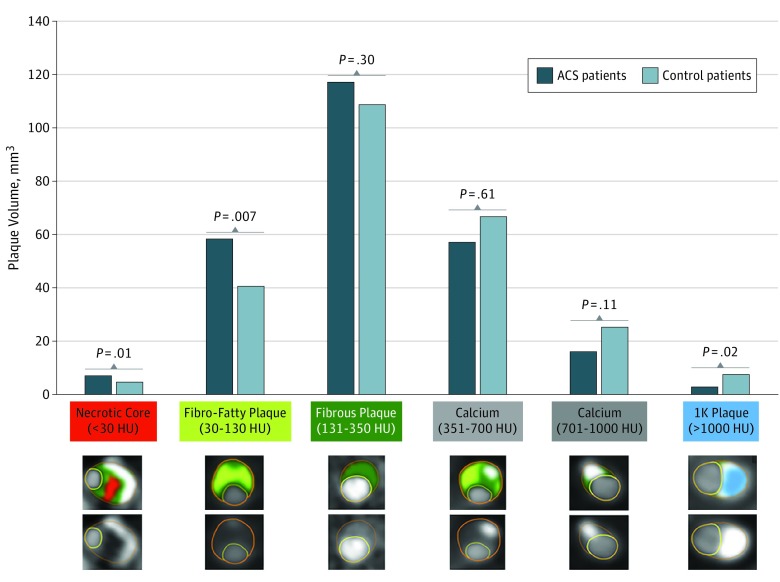
Total mean (SD) calcified plaque volume (>350 HU) was not different between patients and control individuals (76.4 [101.6] mm3 vs 99.0 [156.1] mm3; P = .32) (Table 2). With each increasing calcium density stratum, calcification volume was lower in patients than in control individuals with increasing levels of significance. The mean (SD) volume of 1K plaque (>1000 HU) was 3.9 (8.3) mm3 in patients and 9.4 (23.2) mm3 in control individuals (P = .02) (Figure 1). An example of a coronary artery with 1K plaque is shown in Figure 2. When restricting to patients who experienced myocardial infarction (n = 126), the results were similar (eTable 1 in the Supplement). The mean (SD) volume of 1K plaque in patients who experienced myocardial infarction was 3.6 (7.2) mm3 compared with that in control individuals of 10.0 (23.6) mm3 (P = .01) (eTable 1 in the Supplement). The 1K plaque did not differ between patients and control individuals older than 75 (eTable 2 in the Supplement).
Figure 1. Whole-Heart Plaque Volume by Composition for Patients With Acute Coronary Syndrome (ACS) and Control Individuals .
Per-patient volumes of necrotic core (<30 Hounsfield units [HU]), fibro-fatty (30-130 HU), fibrous (131-350 HU), calcified (351-700 HU), calcified (701-1000 HU), and 1K (>1000 HU) plaques are reported. Patients with ACS reported significantly more necrotic core and fibro-fatty plaques and less 1K plaque compared with control individuals. Cross-sectional examples of the different plaque composition types are displayed by different colors. 1K plaque is shown in blue.
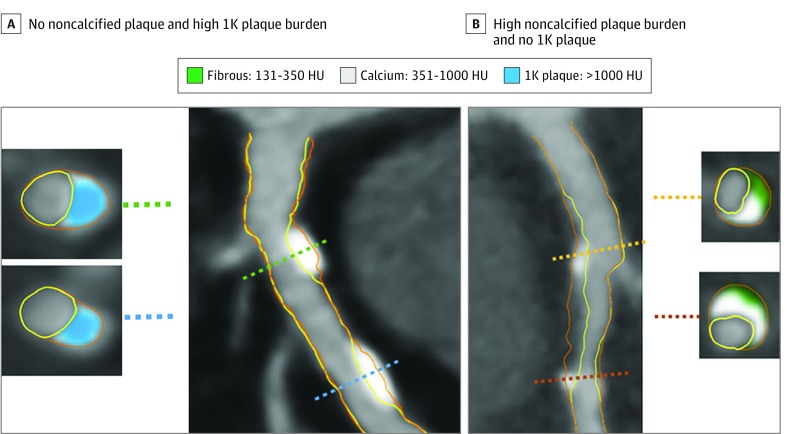
Figure 2. Example of a Vessel With 1K Plaque.
The artery segment in the left panel (A) shows 2 lesions composed of 1K plaque (ie, plaque with a volume >1000 Hounsfield units [HU]), without noncalcified plaque. Cross-sectional examples are shown with 1K plaque (blue). The artery segment in the right panel (B) shows calcifications between 351 and 1000 HU (gray) intermingled in noncalcified plaque. Two cross-sections show 351- to 1000-HU calcium plaque together with 130- to 350-HU fibrous plaque tissue (green).
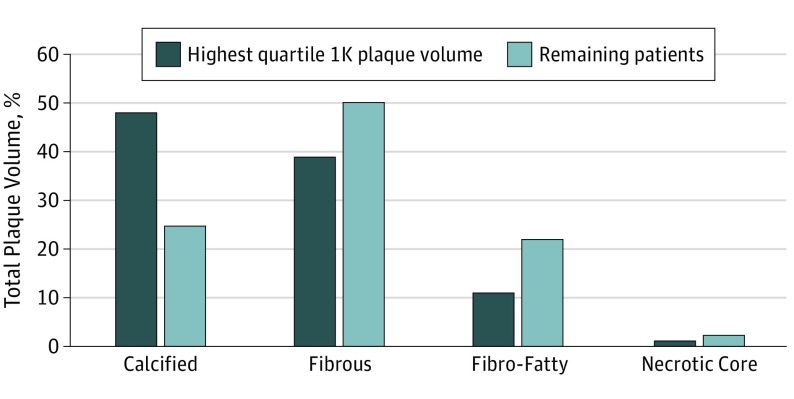
Patients or control individuals in the top quartile of 1K plaque by volume were characterized by a higher overall mean (SD) plaque volume compared with the patients in the other 3 quartiles (539.6 [339.7] mm3 vs 206.9 [196.6] mm3; P < .001) (Figure 3). Individuals in the highest quartile of 1K plaque had relatively more calcified plaque (48.3% [17.3%] vs 24.9% [21.0%]; P < .001) and relatively less necrotic core plus fibro-fatty plaque (12.6% [10.4%] vs 24.9% [20.6%]; P < .001) compared with the other 3 quartiles of the population (Figure 3).
Figure 3. Plaque Composition in Individuals With a Large 1K Plaque Volume.
Individuals with the highest quartile of 1K plaque (ie, plaque with a volume >1000 Hounsfield units [HU]) had relatively more calcified plaque but less fibrous, fibro-fatty, and necrotic core plaques compared with the other 3 quartiles of patients. Absolute volumes and comparisons are provided in eTable 5 in the Supplement.
Lesion-Level Calcified Plaque in Culprit Precursors vs Controls
In 93 patients (49.2%), a CCTA precursor lesion for future ACS culprit lesion could be identified at the time of invasive angiography. Compared with the lesion associated with the greatest stenosis in control individuals, the culprit precursor lesion had a lower mean (SD) volume of 1K plaque (2.6 [7.2] mm3 vs 7.6 [20.3] mm3; P = .01) (eTable 3 in the Supplement). When restricted to myocardial infarction cases, the results were similar; the mean (SD) volume of 1K plaque in myocardial infarction culprit precursors was 2.0 [4.7] mm3 compared with that in control lesions of 9.2 [20.7] mm3 (P = .02) (eTable 4 in the Supplement).
Discussion
ICONIC was a study of patients who experienced ACS after baseline CCTA and control individuals who did not have the same experience, and these 2 groups were propensity matched on overall plaque burden and cardiovascular risk factors. We observed that the per-patient volume of 1K plaque was lower in patients than in control individuals (3.9 [8.3] mm3 vs 9.4 [23.2] mm3; P = .02). Furthermore, the precursor lesion at baseline CCTA associated with development of future ACS demonstrated a smaller volume of 1K plaque compared with the most severely stenotic lesion in control individuals (2.6 [7.2] mm3 vs 7.6 [20.3] mm3; P = .01). We believe these results support the identification of varied atherosclerotic plaque features associated with low risk to high risk prognostic results. For 1K plaque, these results support the hypothesis that higher-density plaque signifies stability and a reduced occurrence of ACS.
Calcified Component of Plaque and Risk for ACS
Studies have demonstrated that patients who underwent imaging at the time of ACS compared with patients who presented with stable CAD were more likely to have a larger proportion of noncalcified plaque.14,15,16,17,18 Leber et al16 observed that, among 21 patients who presented with acute myocardial infarction and 19 patients with stable angina who underwent CCTA, the proportion of plaque being noncalcified was higher in patients with myocardial infarction.
These observations are well supported by histopathologic findings that ruptured coronary plaques are characterized by fibroatheromas with large necrotic cores and thin, inflamed fibrous caps.19 Fibroatheromas (which consist mainly of lipid-rich plaque and necrotic core) may evolve into fibrocalcific plaques or healed ruptures, which are associated with an increase in calcification and a decrease of necrotic core. This process is considered a factor in the stabilization of coronary plaque and is supported by data from intravascular ultrasonography and CCTA, which show that statins are associated with increased calcification burden on follow-up imaging and reduced necrotic core volume.20,21
However, the detection of calcification on imaging is not, per se, a reassuring sign. In CT, the calcified plaque represents histopathologic sheets of calcium that only develop later in the atherosclerotic disease process and therefore represent more advanced plaque formation. On a noncontrast-enhanced CT examination, a high coronary artery calcium score has been associated with a large coronary plaque burden and elevated cardiovascular event rates, whereas the absence of calcium excluded advanced plaque and portended excellent cardiovascular outcomes.22,23 An increasing burden of calcification is equal to an increasing amount of coronary atherosclerosis, thus explaining the strong prognostic value of coronary calcium scoring.
Hence, calcified plaque represents advanced atherosclerosis but has a more stable histopathological appearance, either owing to the stabilizing property of calcification sheets or the lower burden of necrotic core. These observations suggest that the relative burden of calcium is important and may be associated with the risk for future cardiovascular events. The answer to this question requires a baseline plaque evaluation with follow-up and is therefore not possible with histopathologic analysis.
The MESA study investigated whether an increase in calcium density on noncontrast calcium scans was prognostically important among 3398 individuals who experienced 175 coronary heart disease events during the 7½ years of follow-up.4 In a noncontrast CT study, coronary artery calcium density is scored from 1 to 4, representing HU densities from more than 130 to more than 400. In the MESA report, the mean per-patient calcium density score was derived by dividing the Agatston score by the calcium volume and adjusted for slice thickness. In Cox proportional hazards regression models that adjusted for the overall volume of coronary artery calcium, the increasing density (specifically, the highest density category) was associated with lower risk for myocardial infarction, resuscitated cardiac arrest, or coronary heart disease death.4 This finding from the MESA study was supportive of the present findings that higher plaque density was associated with a reduced event risk.
The strongest association was observed for 1K plaque, whereas calcium between 351 and 1000 HU was not statistically significantly lower in patients compared with control individuals. This higher attenuation threshold corresponds with previous reports that histopathologically defined calcification has CT attenuation of 715 HU24 or 966 HU.25 Absence of the prognostic effect for calcification with lower than 1000 HU may be associated with the partial volume effect, whereby small calcifications are associated with blooming artifacts compared with more lipid-rich plaque, which results in CCTA calcification with Hounsfield units between 351 and 1000 (Figure 2). In addition to the per-patient analysis, we analyzed 1K plaque in the specific precursor lesion associated with future occurrence of ACS. Similarly, culprit precursor lesions had less 1K plaque than the most severe lesion in control individuals, supporting the concept of plaque stability on per-patient and per-lesion levels. Furthermore, we observed that patients with a large amount of 1K plaque showed relatively less noncalcified plaque, which was associated with reduced risk for ACS.2 This finding may suggest that higher-density atherosclerosis in the coronary tree presents a plaque phenotype with lower adverse event risk. However, the findings of this study are merely suggestive; prospective studies should confirm our observations. Clinically, the findings of lower risk for a per-lesion and per-patient volume of 1K plaque can help refine risk stratification beyond the coronary plaque burden. In addition, the burden of 1K plaque may serve as a surrogate marker of risk for clinical outcomes in serial CCTA studies.
Limitations
This study has some limitations. Patients and control individuals were drawn from a large observational registry, which had inherent limitations such as selection bias and unmeasured confounders. For adjudication of ACS, patients who died without enough evidence of ACS were excluded. In the small subset of patients older than 75 years, the 1K plaque density was not lower in patients with ACS. The higher prevalence of total plaque and calcified plaque with age may be explanatory for the diminished relationship of 1K plaque in patients with ACS. Coronary lesions with 100% stenosis could not be evaluated with quantitative CCTA. Although the propensity scores were similar between the 2 groups, some imbalance between the individual propensity score components may be observed. No information was available on post-CCTA medication use patterns. The HU of different plaque composition types is dependent on the luminal contrast attenuation and provided kilovolt during acquisition, which was not standardized for all included patients.
Conclusions
Identifying the type of atherosclerotic plaque that serves as a determinant of both higher and lower risk status is an ever-unfolding story. Previous research has indicated that lower plaque density in calcified plaque was associated with a higher risk of major coronary events.4 In the present analysis of the ICONIC study, higher-density calcified plaque, referred to as 1K plaque, was associated with a reduced risk for future ACS on per-patient and per-lesion bases. We believe these results support the plaque stabilization hypothesis with coronary calcium and help in understanding the varying risk signatures that can be detected in atherosclerotic plaque.
eTable 1. Per-Patient Calcification Density Data Restricted to Myocardial Infarction Cases
eTable 2. Per-Patient Calcification Density Data for Age >75
eTable 3. Lesion Specific Calcification Volumes for Culprit Precursors Versus Control Lesions
eTable 4. Lesion Specific Calcification Volumes for Culprit Precursors of Myocardial Infarction Versus Control Lesions
eTable 5. Plaque Composition in Individuals With a Large 1K Plaque Volume
References
- 1.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337-346. doi: 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 2.Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511-2522. doi: 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi: 10.1016/j.jcmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311(3):271-278. doi: 10.1001/jama.2013.282535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5(2):84-92. doi: 10.1016/j.jcct.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935-2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) . Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 8.Mendis S, Thygesen K, Kuulasmaa K, et al. ; Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction . World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40(1):139-146. doi: 10.1093/ije/dyq165 [DOI] [PubMed] [Google Scholar]
- 9.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-1170. doi: 10.1016/j.jacc.2007.03.067 [DOI] [PubMed] [Google Scholar]
- 10.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10(6):435-449. doi: 10.1016/j.jcct.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Park HB, Lee BK, Shin S, et al. Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol. 2015;25(10):3073-3083. doi: 10.1007/s00330-015-3698-z [DOI] [PubMed] [Google Scholar]
- 12.Brodoefel H, Reimann A, Heuschmid M, et al. Characterization of coronary atherosclerosis by dual-source computed tomography and HU-based color mapping: a pilot study. Eur Radiol. 2008;18(11):2466-2474. doi: 10.1007/s00330-008-1019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cury RC, Abbara S, Achenbach S, et al. Coronary Artery Disease - Reporting and Data System (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging. 2016;9(9):1099-1113. doi: 10.1016/j.jcmg.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101(6):598-603. doi: 10.1161/01.CIR.101.6.598 [DOI] [PubMed] [Google Scholar]
- 15.van Velzen JE, de Graaf FR, Jukema JW, et al. Comparison of the relation between the calcium score and plaque characteristics in patients with acute coronary syndrome versus patients with stable coronary artery disease, assessed by computed tomography angiography and virtual histology intravascular ultrasound. Am J Cardiol. 2011;108(5):658-664. doi: 10.1016/j.amjcard.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Leber AW, Knez A, White CW, et al. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol. 2003;91(6):714-718. doi: 10.1016/S0002-9149(02)03411-2 [DOI] [PubMed] [Google Scholar]
- 17.Shemesh J, Apter S, Itzchak Y, Motro M. Coronary calcification compared in patients with acute versus in those with chronic coronary events by using dual-sector spiral CT. Radiology. 2003;226(2):483-488. doi: 10.1148/radiol.2262011903 [DOI] [PubMed] [Google Scholar]
- 18.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424-3429. doi: 10.1161/01.CIR.0000148131.41425.E9 [DOI] [PubMed] [Google Scholar]
- 19.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262-1275. doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Chang HJ, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11(10):1475-1484. doi: 10.1016/j.jcmg.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi: 10.1016/j.jacc.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Young R, Burke G, et al. Ten-year Association of Coronary Artery Calcium With Atherosclerotic Cardiovascular Disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157-2162. doi: 10.1161/01.CIR.92.8.2157 [DOI] [PubMed] [Google Scholar]
- 24.Schroeder S, Kuettner A, Leitritz M, et al. Reliability of differentiating human coronary plaque morphology using contrast-enhanced multislice spiral computed tomography: a comparison with histology. J Comput Assist Tomogr. 2004;28(4):449-454. doi: 10.1097/00004728-200407000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Chopard R, Boussel L, Motreff P, et al. How reliable are 40 MHz IVUS and 64-slice MDCT in characterizing coronary plaque composition? An ex vivo study with histopathological comparison. Int J Cardiovasc Imaging. 2010;26(4):373-383. doi: 10.1007/s10554-009-9562-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Per-Patient Calcification Density Data Restricted to Myocardial Infarction Cases
eTable 2. Per-Patient Calcification Density Data for Age >75
eTable 3. Lesion Specific Calcification Volumes for Culprit Precursors Versus Control Lesions
eTable 4. Lesion Specific Calcification Volumes for Culprit Precursors of Myocardial Infarction Versus Control Lesions
eTable 5. Plaque Composition in Individuals With a Large 1K Plaque Volume