Key Points
Question
What are the temporal trends in race-specific 30-day outcomes after acute myocardial infarction in the contemporary era?
Findings
In this cohort study of patients admitted with acute myocardial infarction to hospitals participating in the Chest Pain–MI registry, stable declines in 30-day readmission rates were observed between 2008 and 2016 for both black and nonblack patients; 30-day mortality rates also declined over time in nonblack patients, with stable temporal trends in black patients. The association between race and 30-day outcomes did not vary before and after the Hospital Readmissions Reduction Program began.
Meaning
In this analysis, racial differences in 30-day outcomes were not modified by Hospital Readmissions Reduction Program implementation across high-performing and low-performing hospitals.
This cohort study assesses temporal trends in 30-day readmission and mortality rates among black and nonblack patients discharged after hospitalization for acute myocardial infarction at hospitals defined as low-performing and high-performing per their Hospital Readmissions Reduction Program penalty status.
Abstract
Importance
The association of the Hospital Readmission Reduction Program (HRRP) with reductions in racial disparities in 30-day outcomes for myocardial infarction (MI), is unknown, including whether this varies by HRRP hospital penalty status.
Objective
To assess temporal trends in 30-day readmission and mortality rates among black and nonblack patients discharged after hospitalization for acute MI at low-performing and high-performing hospitals, as defined by readmission penalty status after HRRP implementation.
Design, Setting, and Participants
This observational cohort analysis used data from the multicenter National Cardiovascular Data Registry Chest Pain–MI Registry centers that were subject to the first cycle of HRRP, between January 1, 2008, and November 30, 2016. All patients hospitalized with MI who were included in National Cardiovascular Data Registry Chest Pain–MI Registry were included in the analysis. Data were analyzed from April 2018 to September 2019.
Exposures
Hospital performance category and race (black compared with nonblack patients). Centers were classified as high performing or low performing based on the excess readmission ratio (predicted to expected 30-day risk adjusted readmission rate) for MI during the first HRRP cycle (in October 2012).
Main Outcomes and Measures
Thirty-day all-cause readmission and mortality rates.
Results
Among 753 hospitals that treated 155 397 patients with acute MI (of whom 11 280 [7.3%] were black), 399 hospitals (53.0%) were high performing. Thirty-day readmission rates declined over time in both black and nonblack patients (annualized 30-day readmission rate: 17.9% vs 20.8%). Black (compared with nonblack) race was associated with higher unadjusted odds of 30-day readmission in both low-performing and high-performing centers (odds ratios: before HRRP: low-performing hospitals, 1.14 [95% CI, 1.03-1.26]; P = .01; high-performing hospitals, 1.17 [95% CI, 1.04-1.32]; P = .01; after HRRP: low-performing hospitals, 1.23 [95% CI, 1.13-1.34]; P < .001; high-performing hospitals, 1.25 [95% CI, 1.12-1.39]; P < .001). However, these racial differences were not significant after adjustment for patient characteristics. The 30-day mortality rates declined significantly over time in nonblack patients, with stable (nonsignificant) temporal trends among black patients. Adjusted associations between race and 30-day mortality showed that 30-day mortality rates were significantly lower among black (compared with nonblack) patients in the low-performing hospitals (odds ratios: pre-HRRP, 0.79 [95% CI, 0.63-0.97]; P = .03; post-HRRP, 0.80 [95% CI, 0.68-0.95]; P = .01) but not in high-performing hospitals. Finally, the association between race and 30-day outcomes did not vary after the HRRP period began in either high-performing or low-performing hospitals.
Conclusions and Relevance
In this analysis, 30-day readmission rates among patients with MI declined over time for both black and nonblack patients. Differences in race-specific 30-day readmission rates persisted but appeared to be attributable to patient-level factors. The 30-day mortality rates have declined for nonblack patients and remained stable among black patients. Implementation of the HRRP was not associated with improvement or worsening of racial disparities in readmission and mortality rates.
Introduction
The Hospital Readmissions Reduction Program (HRRP) was designed to reduce readmissions for heart failure, acute myocardial infarction (AMI), and pneumonia through financial penalties levied by the US Centers for Medicare & Medicaid Services (CMS) on hospitals with higher-than-expected 30-day readmission rates.1,2,3 Since HRRP implementation, a decline has been noted in 30-day readmission rates for targeted conditions, with a greater decline in hospitals that were subjected to readmission penalties.4,5,6 However, concerns have been raised that the HRRP had resulted in disproportionately higher penalties among hospitals caring for patients who are socioeconomically disadvantaged and/or members of racial/ethnic minorities, which could lead to further exacerbation of health care disparities.7,8,9 This is particularly relevant considering the higher risk of readmission among patients who are socioeconomically disadvantaged and the lack of adjustment for socioeconomic status (SES) and race in CMS 30-day readmission models.7,10,11,12,13,14,15,16,17
Recent studies have evaluated the effect of the HRRP on racial disparities in readmission rates for targeted conditions. However, these studies were limited by the use of CMS claims data, which lacks granularity in patient-level factors, such as disease severity. These studies also did not report the association of HRRP with racial disparities in penalized vs nonpenalized hospitals. This represents an important knowledge gap, given the concern that readmission penalties may add to the financial resource deprivation of lower-performing hospitals, leading to a pernicious cycle that further widens disparities in care and outcomes.8,9 Furthermore, the association of HRRP implementation with race-specific trends in 30-day mortality have not been evaluated previously. Accordingly, we evaluated the temporal trends in 30-day readmission and mortality outcomes among black and nonblack patients who were admitted with AMI across hospitals stratified by their penalty status, using data from the National Cardiovascular Data Registry (NCDR) Chest Pain–MI Registry.
Methods
Data Source
The NCDR Chest Pain–MI Registry (formerly the ACTION Registry–GWTG) is a voluntary national registry for outcomes-based quality improvement. Inclusion criteria, exclusion criteria, and techniques of data collection have been described previously.18 Briefly, hospital personnel are trained in standardized data collection techniques, and they record patient demographics, clinical presentation, hospital course, and patient outcomes. The registry consists of consecutive patients with AMI who are treated at participating centers across the United States.
The registry is approved by individual institutional review boards of each participating hospital or considered quality improvement data and therefore not subject to institutional review board approval. The Chest Pain–MI Registry was granted a waiver of informed consent in accordance with 45 CFR 46.116 (d) (more details are presented in eMethods in the Supplement). Data on postdischarge outcomes were obtained in participants in the Chest Pain–MI Registry who were 65 years or older and had fee-for-service Medicare coverage by linking inpatient administrative claims and the Medicare file using strict Social Security number matching, along with indirect identifiers, such as patient sex and date of birth.
Patient Population
The present analysis included the centers from the Chest Pain–MI Registry that participated in the first cycle of HRRP and treated patients with MI between January 1, 2008, and November 30, 2016 (eTable 1 in the Supplement). Exclusion criteria included patients transferred out of the presenting hospital, patients who left against medical advice, patients who were not eligible for Medicare fee-for-service benefits at discharge, patients who died during the index hospitalization, and patients with missing linkage data. Patients admitted to hospitals that had fewer than 25 AMI cases from July 2008 to June 2011 were also excluded.
The cohort was stratified at the implementation of the HRRP into 2 temporal groups. A pre-HRRP group consisted of patients hospitalized in the time prior to the implementation of HRRP (January 2008 to September 2012). The post-HRRP group consisted of patients hospitalized in the postimplementation period (October 2012 to November 2016).
Safety-net hospitals in the study cohort were identified based on the Disproportionate Share Hospital patient percentage for 2016, as determined by CMS.19 Specifically, Disproportionate Share Hospital patient percentage is determined by Medicare Supplemental Security Income eligibility and Medicaid enrollment, by the formula (Medicare Supplemental Security Income Days / Total Medicare Days) + (Medicaid, Non-Medicare Days / Total Patient Days). Consistent with prior literature, hospitals in the top quintile of DSH patient percentage were considered safety-net hospitals in this cohort.20,21,22
Exposure Variables
Primary exposure variables were race and hospital performance status. Race was self-reported on the data collection form. Hospital performance status (high performing vs low performing) was determined based on the HRRP penalty status during the first cycle (July 1, 2008, and June 30, 2011): hospitals with an excess readmission ratio (ERR) for MI greater than 1 (MI-ERR >1) that were thus liable for a readmission penalty for MI were defined as low-performing hospitals.23 Hospitals are penalized if the ratio of predicted to expected 30-day readmission rates is greater than 1 (as determined by the CMS via a complex series of risk adjustments and the following calculation: ERR = Risk-Adjusted Predicted Readmissions / Risk-Adjusted Expected Readmissions), which indicates a higher-than-expected readmission rate. Readmission rates are risk adjusted using a previously standardized protocol.14,17
Study Outcomes
The primary outcomes of interest were 30-day all-cause readmission and 30-day all-cause mortality from the day of discharge for the study participants. These were determined based on the linked inpatient CMS claims and the denominator file, respectively.3
Statistical Analysis
The study cohort was stratified into 4 groups based on race (black compared with nonblack patients) and hospital performance status (high-performing vs low-performing). Clinical and demographic characteristics, in-hospital management, and discharge medications were reported across the 4 groups as percentages for categorical variables and medians with interquartile ranges for continuous variables. Differences in patient characteristics across groups were assessed using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables.
The observed rates of 30-day readmission and mortality over time were compared across race and hospital performance groups using the Cochran-Armitage trend test. Unadjusted and multivariable logistic regression models with a random intercept for hospital type were used to assess the association between race (black compared with nonblack patient groups) and 30-day outcomes (all-cause readmission and all-cause mortality rates) in the pre-HRRP and post-HRRP periods using a difference-in-differences approach. Separate models were constructed for high-performing and low-performing hospitals, and the associations were reported as odds ratios (ORs) with 95% CIs. The multivariable models adjusted the following covariates: demographic characteristics (age, sex, weight, and socioeconomic status [SES] score), index admission clinical signs and symptoms (systolic blood pressure, heart rate, ST-segment MI or non-ST segment MI, ST depression or transient ST elevation, presence of heart failure, and heart failure with shock on admission), laboratory test results (initial hemoglobin, initial serum creatinine, and peak troponin levels), comorbidities (peripheral arterial disease, hypertension, diabetes mellitus, tobacco use history, dyslipidemia, prior MI, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior heart failure, and prior stroke), and a time trend. The SES score was created through linking a patient’s zip code of residence to American Community Survey data. The score is based on 5 variables: median household income; median home value; the percentage of households receiving interested, dividend, or net rental income; the percentage of adults 25 years or older who had completed high school; and the percentage of adults 25 years or older who had completed college.24 The SES score is the sum of the z scores for the 5 variables, and an increasing SES score indicates increasing neighborhood socioeconomic advantages. Since missingness in the data was small (<3.1%), missing continuous variables were imputed to sex, and ST-segment MI specific median values and missing categorical variables were imputed to the mode. The continuous adjustment variables were truncated at the first and 99th percentiles. Flexible splines of continuous adjustment variables were used to fit models. The time trend was assessed for linearity and linear splines of calendar month were used to account for nonlinearity. A spline knot was chosen that balanced model fit by maximizing model likelihood and the knot point for time trend was chosen at month 49 (October 2012, the month of HRRP implementation). Sensitivity analysis was also performed to evaluate the adjusted association between race and 30-day readmission and mortality rates in the pre-HRRP and post-HRRP period among patients hospitalized in safety-net hospitals. All analyses were performed using SAS version 9.4 (SAS Inc). All significance testing was 2-sided, with P values less than .05 indicating significance.
Results
The original cohort included 1012 centers that treated 343 212 consecutive patients with MI between January 1, 2008, and November 30, 2016. The final population included 155 397 participants from 753 clinical sites. The pre-HRRP grouping included 62 751 patients at 483 sites, and the post-HRRP grouping included 92 646 patients at 678 sites. A total of 17 755 participants were treated at 128 hospitals classified as safety-net hospitals.
Of 753 hospitals that treated 155 397 patients (11 280 black patients [7.3%]) with AMI, 354 sites (treating 63 318 patients) were low performing (MI-ERR >1), and 399 sites (53.0%; treating 92 079 patients) were high performing (MI-ERR ≤1), based on 30-day readmissions for AMI during the first HRRP period. Low-performing and high-performing hospitals treated 6657 black patients (10.5%) and 4623 black patients (5.0%), respectively.
Baseline hospital and patient characteristics of the study population are shown in Table 1. Compared with nonblack patients, black patients in both high-performing and low-performing hospitals had a younger mean (SD) age (low-performing hospitals: black patients, 73.0 [68.0-80.0] years; nonblack patients, 75.0 [69.0-82.0] years; high-performing hospitals: black patients, 73.0 [68.0-80.0] years; nonblack patients, 75.0 [69.0-82.0] years; P < .001) and were more commonly women (low-performing hospitals: black patients, 3487 of 6657 [52.4%]; nonblack patients, 24 000 of 56 661 [42.4%]; high-performing hospitals: black patients, 2395 of 4623 [51.8%]; nonblack patients, 35 564 of 87 456 [40.7%]; P < .001). Black patients also had a higher burden of cardiovascular risk factors, such as hypertension (low-performing hospitals: black patients, 6141 [92.2%]; nonblack patients, 46 955 [82.9%]; high-performing hospitals: black patients, 4243 [91.8%]; nonblack patients, 70 562 [80.7%]; P < .001), diabetes mellitus (low-performing hospitals: black patients, 3322 [49.9%]; nonblack patients, 20 271 [35.8%]; high-performing hospitals: black patients, 2313 [50.0%]; nonblack patients, 30 155 [34.5%]; P < .001), and smoking (low-performing hospitals: black patients, 1422 [21.4%]; nonblack patients, 9531 [16.8%]; high-performing hospitals: black patients, 954 [20.6%]; nonblack patients, 13 764 [15.7%]; P < .001), and established cardiovascular disease, such as peripheral arterial disease (low-performing hospitals: black patients, 1013 [15.2%]; nonblack patients, 7482 [13.2%]; high-performing hospitals: black patients, 677 [14.7%]; nonblack patients, 10 990 [12.6%]; P < .001), congestive heart failure (low-performing hospitals: black patients, 1599 [24.1%]; nonblack patients, 9878 [17.5%]; high-performing hospitals: black patients, 1106 [23.9%]; nonblack patients, 13 383 [15.3%]; P < .001), and stroke (low-performing hospitals: black patients, 1032 [15.5%]; nonblack patients, 6108 [10.8%]; high-performing hospitals: black patients, 765 [16.6%]; nonblack patients, 8646 [9.9%]; P < .001) across both penalized and nonpenalized hospital groups. Furthermore, black patients were less likely to present with ST-segment elevation MI (low-performing hospitals: black patients, 1684 [25.3%]; nonblack patients, 17 505 [30.9%]; high-performing hospitals: black patients, 1142 [24.7%]; nonblack patients, 27 193 [31.1%]; P < .001) and had more severe clinical presentations, with higher heart rates (low-performing hospitals: black patients, 84 [70-100] beats/minute; nonblack patients, 82 [69-98] beats/minute; high-performing hospitals: black patients, 84 [71-99] beats/minute; nonblack patients, 80 [68-96] beats/minute; P < .001), higher prevalence of clinical signs of heart failure (low-performing hospitals: black patients, 1588 [23.9%]; nonblack patients, 10 716 [18.9%]; high-performing hospitals: black patients, 947 [20.5%]; nonblack patients, 14 326 [16.4%]; P < .001), and less likelihood of receiving defect-free care (low-performing hospitals: black patients, 4203 [65.2%]; nonblack patients, 37 395 [68.4%]; high-performing hospitals: black patients, 2909 [65.0%]; nonblack patients, 60 411 [71.6%]; P < .001) compared with nonblack patients. These differences in clinical severity of AMI among black compared with nonblack patients were consistent across both penalized and nonpenalized hospital groups.
Table 1. Baseline Characteristics of the Study Participants Stratified by Hospital Performance Status and Race.
| Characteristic | Patients, No. (%) | P Valuea | |||
|---|---|---|---|---|---|
| Low-Performing Hospitals (n = 354) | High-Performing Hospitals (n = 399) | ||||
| Black Patients (n = 6657) | Nonblack Patients (n = 56 661) | Black Patients (n = 4623) | Nonblack Patients (n = 87 456) | ||
| Age, median (IQR), y | 73.0 (68.0-80.0) | 75.0 (69.0-82.0) | 73.0 (68.0-80.0) | 75.0 (69.0-82.0) | <.001 |
| Female | 3487 (52.4) | 24 000 (42.4) | 2395 (51.8) | 35 564 (40.7) | <.001 |
| BMI, median (IQR) | 27.8 (24.2-32.3) | 27.4 (24.1-31.2) | 27.7 (24.0-32.1) | 27.4 (24.2-31.3) | <.001 |
| Socioeconomic status score, median (IQR) | −0.8 (−2.9 to 2.6) | −0.2 (−3.4 to 3.0) | −0.5 (−3.0 to 2.0) | −0.5 (−3.1 to 1.9) | <.001 |
| Hypertension | 6141 (92.2) | 46 955 (82.9) | 4243 (91.8) | 70 562 (80.7) | <.001 |
| Dyslipidemia | 4482 (67.4) | 38 657 (68.3) | 3046 (65.9) | 59 027 (67.5) | .004 |
| Diabetes mellitus | 3322 (49.9) | 20 271 (35.8) | 2313 (50.0) | 30 155 (34.5) | <.001 |
| Current/recent smoker (<1 y) | 1422 (21.4) | 9531 (16.8) | 954 (20.6) | 13 764 (15.7) | <.001 |
| Peripheral arterial disease | 1013 (15.2) | 7482 (13.2) | 677 (14.7) | 10 990 (12.6) | <.001 |
| Medical history | |||||
| Myocardial infarction | 1697 (25.5) | 14 831 (26.2) | 1247 (27.0) | 22 420 (25.7) | <.001 |
| PCI | 1523 (22.9) | 15 122 (26.7) | 1166 (25.2) | 23 425 (26.8) | <.001 |
| CABG | 916 (13.8) | 11 320 (20.0) | 686 (14.8) | 16 681 (19.1) | <.001 |
| Congestive heart failure | 1599 (24.1) | 9878 (17.5) | 1106 (23.9) | 13 383 (15.3) | <.001 |
| Stroke, overall | 1032 (15.5) | 6108 (10.8) | 765 (16.6) | 8646 (9.9) | <.001 |
| Presentation with STEMI | 1684 (25.3) | 17 505 (30.9) | 1142 (24.7) | 27 193 (31.1) | <.001 |
| Systolic blood pressure, median (IQR), mm Hg | 150.0 (128.0-174.0) | 147.0 (126.0-169.0) | 149.0 (126.0-173.0) | 146.0 (125.0-168.0) | <.001 |
| Heart rate on admission, median (IQR), beats per minute | 84.0 (70.0-100.0) | 82.0 (69.0-98.0) | 84.0 (71.0-99.0) | 80.0 (68.0-96.0) | <.001 |
| Cardiogenic shock at presentation | 160 (2.4) | 1437 (2.5) | 116 (2.5) | 2281 (2.6) | .73 |
| Signs of heart failure | 1588 (23.9) | 10 716 (18.9) | 947 (20.5) | 14 326 (16.4) | <.001 |
| Initial creatinine value, median (IQR), mg/dL | 1.2 (1.0-1.6) | 1.1 (0.9-1.4) | 1.2 (1.0-1.6) | 1.1 (0.9-1.4) | <.001 |
| Initial hemoglobin value, median (IQR), g/dL | 12.4 (11.0-13.6) | 13.4 (12.0-14.6) | 12.5 (11.1-13.8) | 13.5 (12.1-14.8) | <.001 |
| Left ventricular ejection fraction assessed, % | 6215 (93.4) | 53 075 (93.7) | 4313 (93.3) | 81 908 (93.7) | .03 |
| Interventions during index admission | |||||
| Primary PCI for STEMI | 1242 (93.9) | 13 961 (94.5) | 902 (96.5) | 21 113 (92.5) | <.001 |
| PCI (non-STEMI) | 1906 (38.4) | 17 236 (44.0) | 1334 (38.4) | 28 586 (47.5) | <.001 |
| CABG | 526 (7.9) | 5518 (9.8) | 302 (6.5) | 7540 (8.6) | <.001 |
| Discharge medication status | |||||
| Aspirin | 5839 (97.2) | 50 167 (97.6) | 4062 (97.1) | 77 909 (98.0) | <.001 |
| P2Y12 receptor inhibitors | 4466 (72.7) | 39 832 (75.9) | 3109 (72.8) | 62 197 (76.5) | <.001 |
| Statin | 5663 (93.3) | 47 717 (93.2) | 3912 (91.9) | 73 775 (93.3) | .002 |
| β-Blockers | 5703 (96.3) | 48 285 (96.5) | 3953 (96.4) | 74 801 (96.8) | .66 |
| ACE inhibitor or ARB | 4027 (72.2) | 34 225 (70.5) | 2777 (70.7) | 53 670 (70.9) | <.001 |
| Ideal patients for ACE inhibitor or ARB | 1047 (86.7) | 8618 (86.8) | 659 (85.0) | 11 968 (87.8) | .22 |
| Defect-free care | 4203 (65.2) | 37 395 (68.4) | 2909 (65.0) | 60 411 (71.6) | <.001 |
| In-hospital mortality risk score, median (IQR) | 34.0 (28.0-41.0) | 34.0 (28.0-40.0) | 34.0 (28.0-41.0) | 34.0 (28.0-40.0) | <.001 |
| In-hospital major bleeding risk score, median (IQR) | 32.0 (27.0-38.0) | 30.0 (25.0-35.0) | 32.0 (27.0-37.0) | 29.0 (24.0-34.0) | <.001 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; IQR, interquartile range; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; hemoglobin to g/L, multiply by 10.0; and troponin to μg/L, multiply by 1.0.
P values are based on Pearson χ2 tests for all categorical row variables. P values are based on χ2 rank based group mean score statistics for all continuous or ordinal row variables. These are equivalent to Kruskal-Wallis tests.
Race-Associated Differences in 30-Day Readmission Rates for AMI Across Hospital Performance Groups
At the start of the study period (2009), nonblack patients had lower 30-day readmission rates compared with black patients in the overall cohort (annualized 30-day readmission rate: 17.94% vs 20.84%). In unadjusted analysis, black race was associated with higher odds of 30-day readmission (Table 2); this risk was consistent across both high-performing and low-performing hospitals in both the pre-HRRP period (ORs: low-performing hospitals, 1.14 [95% CI, 1.03-1.26]; P = .01; high-performing hospitals, 1.17 [95% CI, 1.04-1.32]; P = .01) and the post-HRRP period (ORs: low-performing hospitals, 1.23 [95% CI, 1.13-1.34]; P < .001; high-performing hospitals, 1.25 [95% CI, 1.12-1.39]; P < .001). However, the association between race and 30-day readmission was no longer significant after adjustment for patient-level variables. Additional adjustment for SES did not modify the association between race and 30-day readmission rates. Furthermore, the racial difference in 30-day readmission rates for black compared with nonblack patients did not vary from the pre-HRRP period to the post-HRRP period in either high-performing or low-performing hospitals. In a sensitivity analysis limited to patients hospitalized in safety net hospitals (n = 17 755 patients from 128 centers), race was not associated with 30-day readmission rates in adjusted analysis in the pre-HRRP or post-HRRP periods (eTable 2 in the Supplement).
Table 2. Association Between Race and 30-Day Readmission Rates by Hospital Performance Statusa.
| Variable | Unadjusted | Adjusted Without Socioeconomic Status Score | Adjusted With Socioeconomic Status Score | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95%CI) | P Value | Odds Ratio (95%CI) | P Value | Odds Ratio (95%CI) | P Value | |
| Low-performing hospitalsb | ||||||
| Pre-HRRP periodc | 1.14 (1.03-1.26) | .01 | 0.96 (0.86-1.07) | .48 | 0.96 (0.86-1.07) | .48 |
| Post-HRRP periodd | 1.23 (1.13-1.34) | <.001 | 1.01 (0.92-1.10) | .90 | 1.01 (0.92-1.10) | .91 |
| High-performing hospitalse | ||||||
| Pre-HRRP periodc | 1.17 (1.04-1.32) | .01 | 0.99 (0.87-1.11) | .81 | 0.98 (0.87-1.11) | .80 |
| Post-HRRP periodd | 1.25 (1.12-1.39) | <.001 | 1.01 (0.90-1.12) | .89 | 1.01 (0.90-1.12) | .89 |
Abbreviation: HRRP, Hospital Readmission Reduction Program.
The multivariable models adjusted the following covariates: demographic characteristics (age, sex, weight, and socioeconomic status score), index admission clinical signs and symptoms (systolic blood pressure, heart rate, ST-segment myocardial infarction or non–ST-segment myocardial infarction, ST depression or transient ST elevation, presence of heart failure, heart failure with shock on admission), laboratory test results (initial hemoglobin, initial serum creatinine, and peak troponin levels), comorbidities (peripheral arterial disease, hypertension, diabetes mellitus, tobacco use history, dyslipidemia, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior heart failure, and prior stroke), and time trend.
Low-performing hospitals: hospitals that were penalized for higher than expected readmission rate for acute myocardial infarction during the first HRRP cycle (excess readmission ratio for myocardial infraction >1).
Pre-HRRP: before implementation of the HRRP.
Post-HRRP: after implementation of the HRRP.
High-performing hospitals: hospitals that were not penalized for higher than expected readmission rate for acute myocardial infarction during the first HRRP cycle (excess readmission ratio for myocardial infraction ≤1).
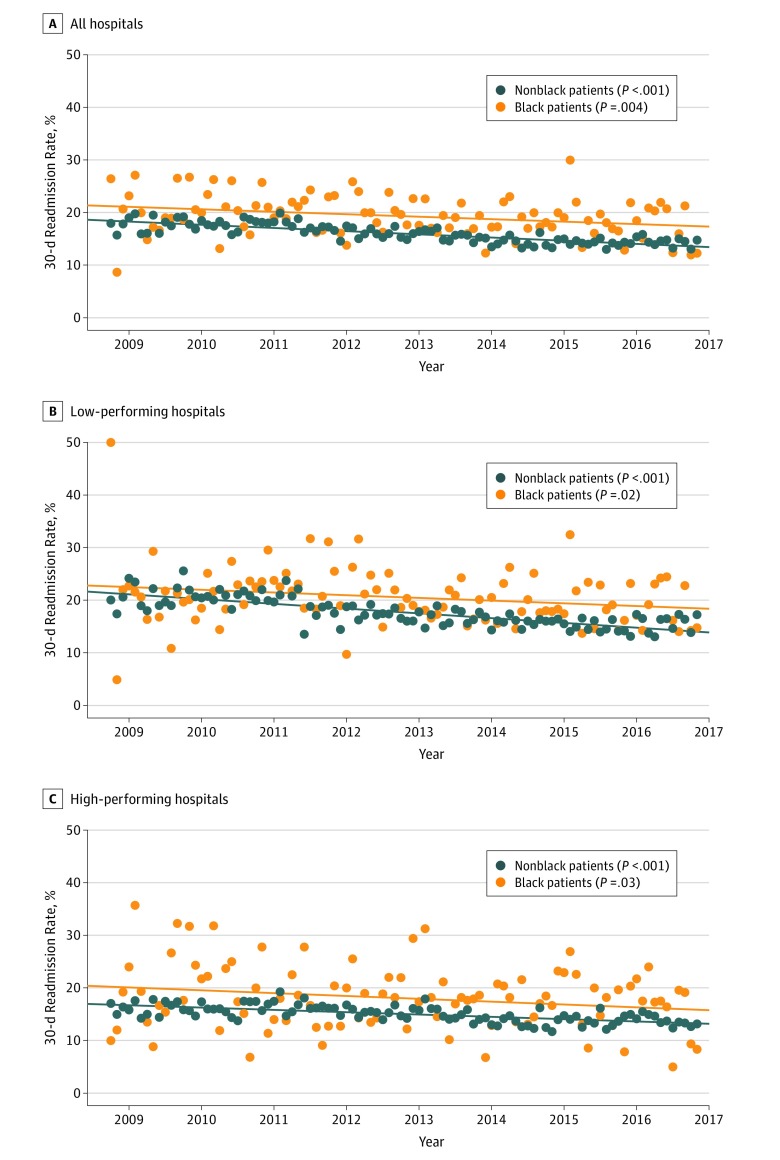
Temporal trends in the race-specific 30-day readmission rates are shown in Figure 1. The observed 30-day readmission rates were higher among black compared with nonblack patients throughout the study period. There was a significant temporal decline in the observed 30-day readmission rates for both black and nonblack patients in the overall cohort (annualized 30-day readmission rates in 2009 vs 2016: black patients, 20.8% vs 17.4%; P = .004 for trend; nonblack patients, 17.9% vs 14.5%; P < .001 for trend) as well as among the low-performing hospital subgroup (annualized 30-day readmission rates in 2009 vs 2016: black patients, 19.6% vs 18.2%; P = .02 for trend; nonblack patients, 21.1% vs 15.6%; P < .001 for trend) and high-performing hospital subgroup (annualized 30-day readmission rates in 2009 vs 2016: black patients, 22.1% vs 15.9%; P = .03; nonblack patients, 16.0% vs 13.8%; P < .001 for trend; eTable 3 in the Supplement).
Figure 1. Trends in 30-Day Observed Readmission Rates for Black and Nonblack Patients.
Orange circles indicate black patients, and dark blue circles indicate nonblack patients.
Racial Differences in 30-Day Mortality Rates for AMI Across Hospital Performance Groups
In adjusted analysis accounting for patient-level characteristics, black race was associated with a lower 30-day mortality rate in low-performing hospitals (ORs: pre-HRRP, 0.79 [95% CI, 0.63-0.97]; P = .03; post-HRRP, 0.80 [95% CI, 0.68-0.95]; P = .01). This association remained significant with further adjustment for SES and was consistent for both the pre-HRRP period (OR, 0.78 [95% CI, 0.63-0.97]; P = .02) and the post-HRRP period (OR, 0.80 [95% CI, 0.68-0.95]; P = .01; P = .85 for the interaction of race and time; Table 3). In high-performing hospitals, there was no significant association between race and 30-day mortality rates after adjustment for patient-level variables across the pre-HRRP and post-HRRP periods, with no significant change in the association over time. In sensitivity analysis limited to patients hospitalized in safety-net hospitals, race was not associated with 30-day mortality rates across both the pre-HRRP and the post-HRRP period (eTable 2 in the Supplement).
Table 3. Association Between Race and 30-Day Mortality Rates by Hospital Performance Tiera.
| Variable | Unadjusted | Adjusted Without Socioeconomic Status Score | Adjusted With Socioeconomic Status Score | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Low-performing hospitalsb | ||||||
| Pre-HRRP periodc | 0.89 (0.72-1.09) | .25 | 0.79 (0.63-0.97) | .03 | 0.78 (0.63-0.97) | .02 |
| Post-HRRP periodd | 0.98 (0.83-1.15) | .79 | 0.80 (0.68-0.95) | .01 | 0.80 (0.68-0.95) | .01 |
| High-performing hospitalse | ||||||
| Pre-HRRP periodc | 0.97 (0.78-1.20) | .77 | 0.85 (0.68-1.06) | .15 | 0.84 (0.67-1.06) | .14 |
| Post-HRRP periodd | 1.24 (1.03-1.49) | .02 | 0.99 (0.81-1.20) | .89 | 0.99 (0.81-1.20) | .89 |
Abbreviation: HRRP, Hospital Readmission Reduction Program.
The multivariable models adjusted the following covariates: demographic characteristics (age, sex, weight, and socioeconomic status score), index admission clinical signs and symptoms (systolic blood pressure, heart rate, ST-segment myocardial infarction or non–ST-segment myocardial infarction, ST-depression or transient ST elevation, presence of heart failure, and heart failure with shock on admission), laboratory test results (initial hemoglobin, initial serum creatinine, and peak troponin), comorbidities (peripheral arterial disease, hypertension, diabetes mellitus, tobacco use history, dyslipidemia, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior heart failure, and prior stroke), and time trend.
Pre-HRRP: before implementation of the HRRP.
Post-HRRP: after implementation of the HRRP.
Low-performing hospitals: hospitals that were penalized for higher than expected readmission rate for acute myocardial infarction during the first HRRP cycle (excess readmission ratio for myocardial infarction >1).
High-performing hospitals: hospitals that were not penalized for higher than expected readmission rate for acute myocardial infarction during the first HRRP cycle (excess readmission ratio for myocardial infarction ≤1).
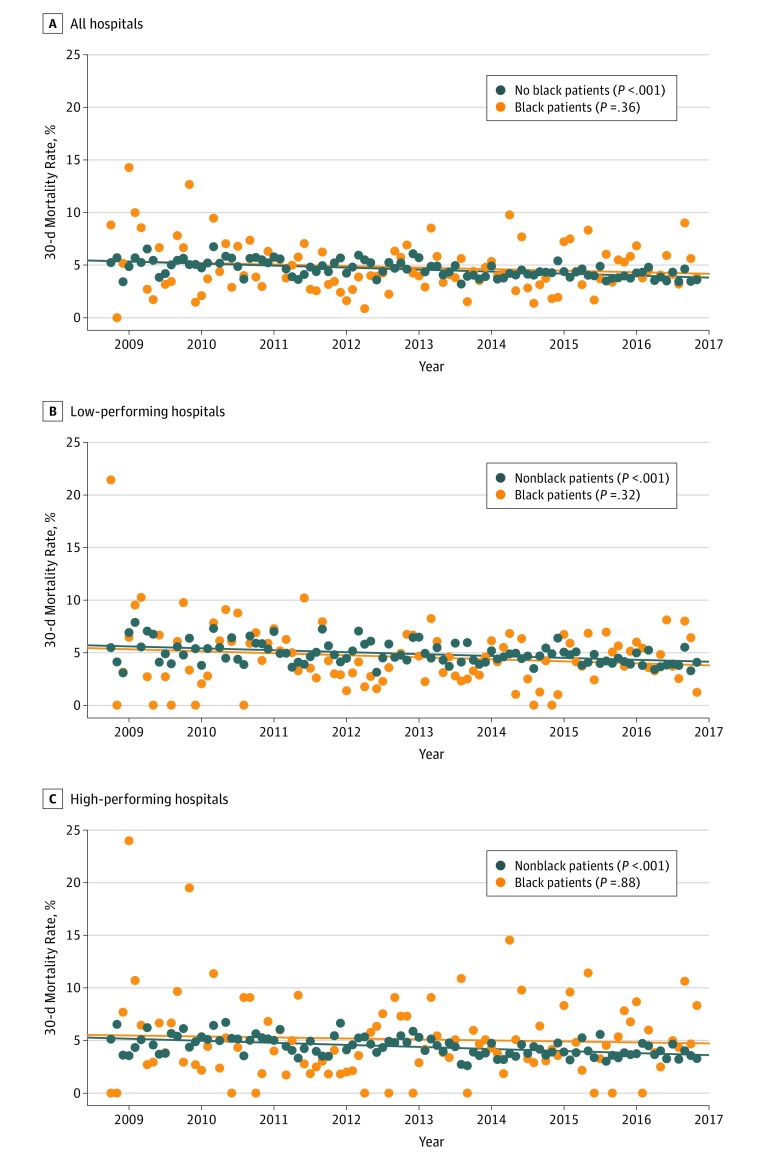
Temporal trends in race-specific 30-day mortality rates are shown in Figure 2. Consistent with 30-day readmission rates, a significant decline in the 30-day mortality rates was also observed among nonblack patients in both low-performing hospitals (annualized 30-day mortality rates in 2009 vs 2016, 5.8% vs 4.1%) and high-performing hospitals (annualized 30-day mortality rates in 2009 vs 2016, 4.8% vs 3.9%) (P < .001 for both trends). In contrast, the 30-day mortality rates did not change significantly over time among black patients across both low-performing hospitals and high-performing hospitals (Figure 2; eTable 4 in the Supplement).
Figure 2. Trends in 30-Day Observed Mortality Rates for Black and Nonblack Patients.
Orange circles indicate black patients, and dark blue circles indicate nonblack patients.
Discussion
We observed several important findings in this study. First, unadjusted 30-day readmission rates were significantly higher in black compared with nonblack patients discharged after a hospitalization for AMI at centers participating in the Chest Pain–MI registry. However, the racial differences in 30-day readmission rates were largely driven by differences in patient-level factors, such as clinical characteristics and disease severity. Second, we observed a significant decline in 30-day readmission rates for both black and nonblack patients hospitalized with MI throughout the study period, which did not appear to be modified by either hospital performance status or implementation of HRRP. Third, the association between race and 30-day mortality rates differed based on hospital performance strata. Thirty-day mortality rates were significantly lower among black (compared with nonblack) patients in the low-performing hospitals but not in high-performing hospitals, with no significant changes in these associations by implementation of HRRP. Finally, a significant decline in 30-day mortality rates was observed throughout the study period for nonblack patients and stable temporal trends among black patients. Taken together, the study findings suggest that the implementation of HRRP has not been associated with either widening or narrowing of racial disparities in 30-day outcomes among patients discharged with AMI.
Consistent with prior literature, we observed a steady decline in the 30-day readmission rates across all race-based and hospital performance–based groups throughout the study period.6,25 Despite the temporal decline in 30-day readmission rates, we observed a consistently higher burden of readmission within 30 days after discharge among black compared with nonblack patients with AMI, throughout the study period. However, this race-based difference in 30-day readmission rates was largely driven by higher burden of cardiovascular risk factors and higher severity of disease in black patients. Along these lines, prior studies have also identified traditional cardiovascular risk factors as important determinants of readmission within 30 days.2,7,11,12,26,27,28,29,30 Future studies are needed to determine if a more patient-focused approach aimed at optimizing guideline-directed medical therapies along with targeting readmission diversion programs to patients with a higher disease severity burden may lower racial differences in 30-day readmission rates.
Recent studies have evaluated the association of HRRP with racial differences in 30-day readmission rate trends for target conditions in safety-net and non–safety-net hospitals.31,32 One study28 demonstrated a widening in disparities in safety-net hospitals after HRRP implementation, while another29 reported a narrowed but persistent gap in readmission rates among black and nonblack patients. Findings from this study suggest that there remain persistent racial differences in 30-day readmission; however, the higher readmission rates seen in black patients are largely associated with higher disease severity. This study is unique in that it uses high-quality clinical data rather than administrative claims for risk adjustment, which allows for a more complete risk adjustment. Consistent with this notion, we observed that adding SES adjustment to the robust clinical risk-adjusted model did not appreciably affect the observed associations between race and 30-day readmission rates, suggesting that the information contained by SES may be captured by clinical risk factors that may not be ascertainable in administrative data sets that are less clinically robust. Our study findings also add to the existing literature by evaluating race-specific readmission trends across hospital performance groups and suggest that the racial differences in the 30-day readmission rates have remained unchanged since the implementation of HRRP and are not different among high-performing vs low-performing hospitals.
Another significant contribution of this study to the existing literature is the assessment of temporal trends in race-specific 30-day mortality rates among patients hospitalized with MI. We observed that black race was significantly associated with a lower risk of 30-day mortality in low-performing hospitals but not high-performing hospitals. Previous studies have demonstrated a similar lower risk of mortality among black patients with coronary artery disease or MI.33,34,35 It is plausible that the lack of association between black race and a lower risk of mortality in the high-performing hospitals may be associated with the smaller number of black patients in these centers (<5%). Future studies are needed to determine if differences in care patterns may account for the differing patterns of association between race and 30-day mortality rates at high-performing vs low-performing hospitals. We also observed a significant temporal decline in 30-day mortality rates among nonblack patients and stable trends in 30-day mortality among black patients, with consistent patterns of race-specific associations in the pre-HRRP and post-HRRP period. Taken together, these findings potentially allay concerns that penalizing low-performing hospitals with reduced CMS payments under HRRP has led to worsening racial disparities in clinical outcomes for AMI.
Limitations
This study has several limitations. First, we only included hospitals that voluntarily joined the NCDR Chest Pain–MI registry. While participating hospitals are located across the United States and contained a mix of urban, rural, teaching, and nonteaching institutions, these study findings may not be generalizable to all hospitals across the country. Hospitals in the NCDR Chest Pain–MI registry may have better resources than nonparticipating centers, and nonparticipating centers may be more vulnerable to financial penalties levied by the HRRP. However, our findings were consistent in the sensitivity analysis limited to the patients hospitalized in safety-net hospitals who were included in the study cohort. Second, owing to the observational nature of this study, we cannot establish a cause-and-effect association with regards to HRRP implementation and changes in readmission and mortality rates. Third, unmeasured sociodemographic factors that differ across racial groups may have influenced the observed associations between race and clinical outcomes. Finally, we do not have information of racial differences in access to outpatient, emergency, and observation-based care, which may have influenced the observed patterns of 30-day readmission and mortality in black compared with nonblack patients.
Conclusions
In conclusion, among patients receiving Medicare fee-for-service benefits who were admitted to hospitals in the NCDR Chest Pain–MI registry, racial differences in 30-day readmission rates persist that are largely associated with patient-levels factors, such as risk factor burden and disease severity. Implementation of HRRP has not been associated with worsening or improvement in these racial differences in 30-day outcomes, and there has been a consistent decline in 30-day readmission after an AMI hospitalization for both black and nonblack patients. The 30-day mortality rates have also declined for nonblack patients and remained stable among black patients.
eMethods.
eTable 1. Cohort Derivation for the study
eTable 2. Association between race and 30-day readmission and mortality rates in hospitals identified as safety-net in the study cohort based on the Disproportionate Share hospital patient percent
eTable 3. Observed 30-day readmission rates across different patient groups at the start and end of the study period.
eTable 4. Observed annualized 30-day mortality rates across different patient groups at the start and end of the study period.
References
- 1.Hospital Readmissions Reduction Program. Patient Protection and Affordable Care Act. 2124 Stat. 119 § 3025.2010.
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 3.US Centers for Medicare and Medicaid Services Hospital Readmissions Reduction Program (HRRP): acute inpatient PPS 2018; https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Published 2018. Accessed December 24, 2018.
- 4.Zuckerman RB, Joynt Maddox KE, Sheingold SH, Chen LM, Epstein AM. Effect of a hospital-wide measure on the Readmissions Reduction Program. N Engl J Med. 2017;377(16):1551-1558. doi: 10.1056/NEJMsa1701791 [DOI] [PubMed] [Google Scholar]
- 5.Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the Hospital Readmissions Reduction Program: a pre-post analysis. Ann Intern Med. 2017;166(5):324-331. doi: 10.7326/M16-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the Hospital Readmission Reduction Program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342-343. doi: 10.1001/jama.2012.94856 [DOI] [PubMed] [Google Scholar]
- 8.Bhalla R, Kalkut G. Could Medicare readmission policy exacerbate health care system inequity? Ann Intern Med. 2010;152(2):114-117. doi: 10.7326/0003-4819-152-2-201001190-00185 [DOI] [PubMed] [Google Scholar]
- 9.Gu Q, Koenig L, Faerberg J, Steinberg CR, Vaz C, Wheatley MP. The Medicare Hospital Readmissions Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49(3):818-837. doi: 10.1111/1475-6773.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa JF, Joynt KE, Zhou X, Orav EJ, Jha AK. Safety-net hospitals face more barriers yet use fewer strategies to reduce readmissions. Med Care. 2017;55(3):229-235. doi: 10.1097/MLR.0000000000000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495-504. doi: 10.1093/geront/48.4.495 [DOI] [PubMed] [Google Scholar]
- 12.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A, Golwala H, Hall HM, et al. Association of US Centers for Medicare and Medicaid Services hospital 30-day risk-standardized readmission metric with care quality and outcomes after acute myocardial infarction: findings from the National Cardiovascular Data Registry/Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines. JAMA Cardiol. 2017;2(7):723-731. doi: 10.1001/jamacardio.2017.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Centers for Medicare and Medicaid Services QualityNet Readmission measures methodology: 2018 readmission measures updates 2018. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1219069855841. Published 2018. Accessed December 12, 2018.
- 15.US Centers for Medicare and Medicaid Services Outcome measures: hospital quality initiative 2017. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Published 2017. Accessed November 14, 2019.
- 16.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29-37. doi: 10.1161/CIRCOUTCOMES.108.802686 [DOI] [PubMed] [Google Scholar]
- 17.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. doi: 10.1161/CIRCOUTCOMES.110.957498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson ED, Roe MT, Rumsfeld JS, et al. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2(5):491-499. doi: 10.1161/CIRCOUTCOMES.108.847145 [DOI] [PubMed] [Google Scholar]
- 19.US Centers for Medicare and Medicaid Services Disproportionate share hospital (DSH). https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/dsh.html. Updated November 13, 2019. Accessed November 27, 2019.
- 20.Samson LW, Chen LM, Epstein AM, Maddox KEJ. Dually enrolled beneficiaries have higher episode costs on the Medicare spending per beneficiary measure. Health Aff (Millwood). 2018;37(1):86-94. doi: 10.1377/hlthaff.2017.0914 [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158 [DOI] [PubMed] [Google Scholar]
- 22.Jha AK, DesRoches CM, Shields AE, et al. Evidence of an emerging digital divide among hospitals that care for the poor. Health Aff (Millwood). 2009;28(6):w1160-w1170. doi: 10.1377/hlthaff.28.6.w1160 [DOI] [PubMed] [Google Scholar]
- 23.Horwitz LI, Grady JN, Cohen DB, et al. Development and validation of an algorithm to identify planned readmissions from claims data. J Hosp Med. 2015;10(10):670-677. doi: 10.1002/jhm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99-106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 25.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood). 2014;33(5):778-785. doi: 10.1377/hlthaff.2013.0816 [DOI] [PubMed] [Google Scholar]
- 27.Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48(11):981-988. doi: 10.1097/MLR.0b013e3181ef60d9 [DOI] [PubMed] [Google Scholar]
- 28.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675-681. doi: 10.1001/jama.2011.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenauer PK, Lagu T, Rothberg MB, et al. Income inequality and 30 day outcomes after acute myocardial infarction, heart failure, and pneumonia: retrospective cohort study. BMJ. 2013;346:f521. doi: 10.1136/bmj.f521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vest JR, Gamm LD, Oxford BA, Gonzalez MI, Slawson KM. Determinants of preventable readmissions in the United States: a systematic review. Implement Sci. 2010;5:88. doi: 10.1186/1748-5908-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueroa JF, Zheng J, Orav EJ, Epstein AM, Jha AK. Medicare program associated with narrowing hospital readmission disparities between black and white patients. Health Aff (Millwood). 2018;37(4):654-661. doi: 10.1377/hlthaff.2017.1034 [DOI] [PubMed] [Google Scholar]
- 32.Chaiyachati KH, Qi M, Werner RM. Changes to racial disparities in readmission rates after Medicare’s Hospital Readmissions Reduction Program within safety-net and non-safety-net hospitals. JAMA Netw Open. 2018;1(7):e184154. doi: 10.1001/jamanetworkopen.2018.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downing NS, Wang C, Gupta A, et al. Association of racial and socioeconomic disparities with outcomes among patients hospitalized with acute myocardial infarction, heart failure, and pneumonia: an analysis of within- and between-hospital variation. JAMA Netw Open. 2018;1(5):e182044. doi: 10.1001/jamanetworkopen.2018.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polsky D, Lave J, Klusaritz H, et al. Is lower 30-day mortality posthospital admission among blacks unique to the Veterans Affairs health care system? Med Care. 2007;45(11):1083-1089. doi: 10.1097/MLR.0b013e3180ca960e [DOI] [PubMed] [Google Scholar]
- 35.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43(4):308-319. doi: 10.1097/01.mlr.0000156848.62086.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Cohort Derivation for the study
eTable 2. Association between race and 30-day readmission and mortality rates in hospitals identified as safety-net in the study cohort based on the Disproportionate Share hospital patient percent
eTable 3. Observed 30-day readmission rates across different patient groups at the start and end of the study period.
eTable 4. Observed annualized 30-day mortality rates across different patient groups at the start and end of the study period.