Key Points
Question
Is neighborhood socioeconomic disadvantage associated with hippocampal and total brain volume in a cognitively unimpaired population?
Findings
In this cross-sectional study of 951 cognitively unimpaired individuals, living in the most socioeconomically disadvantaged neighborhoods was associated with significantly lower hippocampal and total brain tissue volume, adjusted for premorbid brain volume. Increased cardiovascular risk mediated the association with total brain tissue but not hippocampal volume.
Meaning
Neighborhood disadvantage may be associated with brain tissue volume during the aging process in the absence of clinical cognitive impairment.
Abstract
Importance
Identifying risk factors for brain atrophy during the aging process can help direct new preventive approaches for dementia and cognitive decline. The association of neighborhood socioeconomic disadvantage with brain volume in this context is not well known.
Objective
To test whether neighborhood-level socioeconomic disadvantage is associated with decreased brain volume in a cognitively unimpaired population enriched for Alzheimer disease risk.
Design, Setting, and Participants
This study, conducted from January 6, 2010, to January 17, 2019, at an academic research neuroimaging center, used cross-sectional data on 951 participants from 2 large, ongoing cohort studies of Alzheimer disease (Wisconsin Registry for Alzheimer’s Prevention and Wisconsin Alzheimer’s Disease Research Center clinical cohort). Participants were cognitively unimpaired based on National Institute on Aging–Alzheimer’s Association workgroup diagnostic criteria for mild cognitive impairment and Alzheimer disease, confirmed through a consensus diagnosis panel. The cohort was enriched for Alzheimer disease risk based on family history of dementia. Statistical analysis was performed from April 3 to September 27, 2019.
Main Outcomes and Measures
The Area Deprivation Index, a geospatially determined index of neighborhood-level disadvantage, and cardiovascular disease risk indices were calculated for each participant. Linear regression models were fitted to test associations between relative neighborhood-level disadvantage (highest 20% based on state of residence) and hippocampal and total brain tissue volume, as assessed by magnetic resonance imaging.
Results
In the primary analysis of 951 participants (637 women [67.0%]; mean [SD] age, 63.9 [8.1] years), living in the 20% most disadvantaged neighborhoods was associated with 4.1% lower hippocampal volume (β = −317.44; 95% CI, −543.32 to −91.56; P = .006) and 2.0% lower total brain tissue volume (β = −20 959.67; 95% CI, −37 611.92 to −4307.43; P = .01), after controlling for intracranial volume, individual-level educational attainment, age, and sex. Robust propensity score–matched analyses determined that this association was not due to racial/ethnic or demographic characteristics. Cardiovascular risk score, examined in a subsample of 893 participants, mediated this association for total brain tissue but not for hippocampal volume.
Conclusions and Relevance
For cognitively unimpaired individuals, living in the most disadvantaged neighborhoods was associated with significantly lower cerebral volumes, after controlling for maximal premorbid (total intracranial) volume. This finding suggests an association of community socioeconomic context, distinct from individual-level socioeconomic status, with brain volume during aging. Cardiovascular risk mediated this association for total brain tissue volume but not for hippocampal volume, suggesting that neighborhood-level disadvantage may be associated with these 2 outcomes via distinct biological pathways.
This cross-sectional study examines whether neighborhood-level socioeconomic disadvantage is associated with decreased brain volume in a cognitively unimpaired population enriched for Alzheimer disease risk.
Introduction
Alzheimer disease and vascular dementia are the most common causes of aging-related cognitive impairment in the United States and are major factors associated with morbidity, mortality, and health care–related expense. In both diseases, hippocampal and cortical volume loss precedes clinically identifiable cognitive deficits.1,2 Given the close association between brain tissue loss and cognitive decline, it is critical to identify the factors associated with this process. Previously identified individual-level risk factors include age, male sex, cardiovascular risk factors, and cardiovascular disease.3,4,5,6,7,8,9,10,11
Another important class of risk factors are social determinants of health—the social, physical, and economic environment in which people are born, live, work, and age—which are a cornerstone of mechanistic theories of health disparities.12,13 Social factors are associated with neurobiology, and the role they play in neurodegeneration and dementia are active areas of study.6,7,8,9,10,11,14,15,16 More proximal individual-level socioeconomic factors (referred to hereafter as individual socioeconomic status [SES]), such as higher educational level, income, and occupational complexity, are associated with decreased incident mild cognitive impairment and Alzheimer disease.15,16 Results from previous studies on individual SES and preclinical neurodegeneration have been mixed, with studies showing negative,6,7,8,9 positive,10,11 and no significant14 associations.
Studying factors that are more distal than individual SES, such as the socioeconomic context of an individual’s area of residence (ie, his or her neighborhood), may provide complementary and clarifying evidence on the association between the social determinants of health and neurodegenerative processes. The association of an individual’s neighborhood context with health and disease is distinct from—and sometimes greater than—the association of individual SES with health and disease.17,18,19,20,21 Neighborhood-level disadvantage, comprising poverty, educational level, income level, employment, and infrastructure within a geographic region, is associated with numerous negative health outcomes, including mortality,22 hospital readmission,23 ischemic stroke,24 and cardiometabolic risk factors.22 The translational utility of neighborhood-level disadvantage metrics is substantial; metrics are publicly available, can be harmonized across all US states, and are proving highly advantageous for policy-, clinical-, and therapeutic-based interventions.18
From a neurobiological perspective, certain brain regions, such as the hippocampus, may be uniquely vulnerable to neighborhood-level disadvantage. Neighborhood-level disadvantage is associated with altered diurnal patterns of cortisol and response to stressors,25,26 and experimental animal studies27,28 and observational human studies29,30 have shown chronic stress to be associated with altered hippocampal structure and function. In addition, neighborhood-level disadvantage has been observationally31 and experimentally17 associated with cardiometabolic risk factors, which in turn are associated with hippocampal and global brain atrophy.2,32,33,34,35
In the present study, we examined the association of neighborhood disadvantage with cerebral volumes in 2 large studies comprising cognitively unimpaired individuals, hypothesizing that living in highly disadvantaged neighborhoods would be associated with lower total brain and hippocampal volume (HV). Based on previous work finding age- and sex-associated differential hippocampal vulnerability,32,36,37 we also tested for interactions between age, sex, and neighborhood-level disadvantage. Finally, we tested mediation by cardiovascular risk, given its association with both neighborhood-level disadvantage17,22 and neurodegeneration.32,33,34,35
Methods
Study Participants
Neuroimaging data were acquired for participants enrolled in 1 of 2 longitudinal cohort studies of community-dwelling adults (the Wisconsin Registry for Alzheimer’s Prevention38 and the Wisconsin Alzheimer’s Disease Research Center cohorts). The present cross-sectional analysis, conducted from January 6, 2010, to January 17, 2019, included 951 cognitively unimpaired individuals who had undergone T1-weighted structural magnetic resonance imaging (MRI). Complete inclusion and exclusion criteria are shown in eFigure 1 in the Supplement. Participants underwent a comprehensive neuropsychological battery to determine their cognitive status in accordance with the National Institute on Aging–Alzheimer’s Association workgroup diagnostic criteria.39 For the cardiovascular risk mediation analysis, a subsample of the study data was used (n = 893) based on additional exclusion criteria (eFigure 1 and eTable 3 in the Supplement). Demographic characteristics, including age, sex, race/ethnicity, parental dementia history, and educational level, were obtained by participant self-report. The University of Wisconsin Institutional Review Board approved all study procedures and all participants provided written informed consent.
Neighborhood Disadvantage
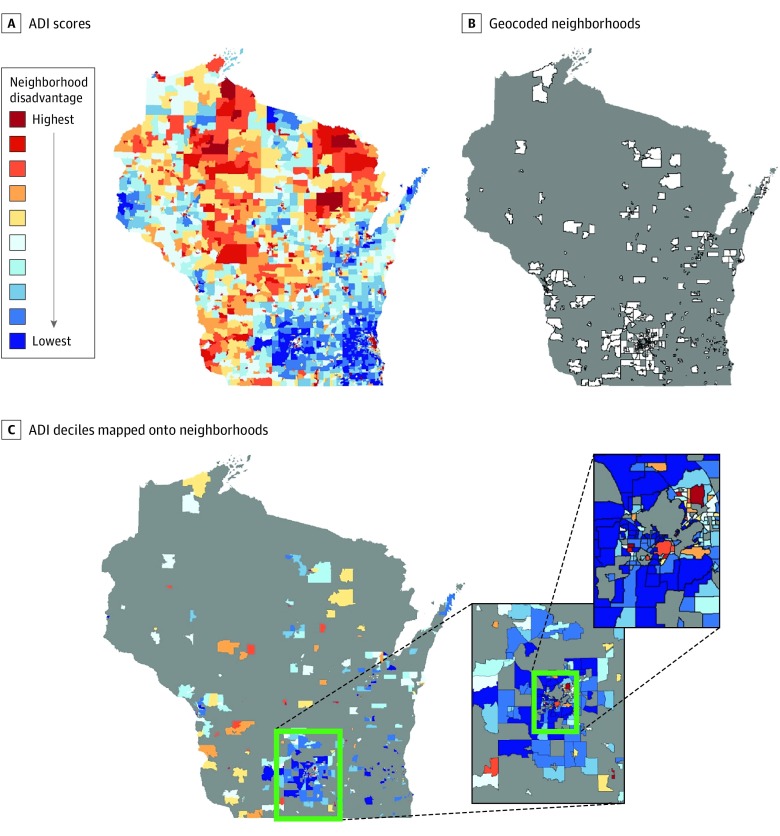
The Area Deprivation Index (ADI), used to indicate neighborhood-level disadvantage, is constructed using 17 area-level US Census indicators of poverty, education, employment, and physical environment from 2013 American Community Survey data.18,23 Complete methods for ADI score determination and the 17 indicators have been previously described,23 and these ADI metrics are made available through the Neighborhood Atlas.18 Census block group ADI scores were ranked into relative deciles based on statewide distributions. Most recent participant addresses were used to assign geocodes based on zip code +4, which were then linked to associated ADI deciles (Figure 1).
Figure 1. Mapping Neighborhood Socioeconomic Disadvantage Onto the Study Population.
A, Area disadvantage (ADI) scores are calculated for every census block group in each state and converted to relative deciles based on statewide norms. The state of Wisconsin is shown as an illustrative example. B, Study population residential zip codes +4 (neighborhoods) are geocoded and mapped to nearest block group. C, ADI deciles from matching block groups are mapped onto participant neighborhood. Pop-out highlights the increased density of block groups characteristic of urban areas. Gray areas indicate no study participants residing in corresponding block group.
MRI Acquisition and Processing
High-resolution T1-weighted structural MRI was performed using equipment and acquisition parameters described in eTables 1 and 2 in the Supplement. All MRI scans were read by an experienced clinical neuroradiologist and excluded from analysis if major structural abnormalities or other pathologic findings were described. Scans flagged for minor abnormalities were further visually inspected and excluded if image processing or volumetric measurements were noticeably affected. For preprocessing, T1-weighted volumes underwent segmentation into tissue classes (gray matter, white matter, and cerebrospinal fluid) using Statistical Parametric Mapping, version 12 (https://www.fil.ion.ucl.ac.uk/spm). For HV, automated subcortical tissue segmentation was performed using FSL First.40 All segmentations were visually quality checked for accuracy, and segmentation failures were corrected if possible or excluded from analysis. Total intracranial volume was determined in Statistical Parametric Mapping, version 12 using the reverse brain mask method.41
Cardiovascular Risk Factors
Cardiovascular risk factors were assessed at the most recent previous study visit (mean [SD] number of years before MRI scan, 1.1 [1.2]). Systolic blood pressure measurements were obtained by sphygmomanometry with the participant seated. Blood samples were obtained after a 12-hour fast by venipuncture, and total and high-density lipoprotein cholesterol levels were measured from serum. Data on smoking history, diabetes status, and antihypertensive medication use were obtained from participant questionnaires. Ten-year atherosclerotic cardiovascular disease (ASCVD) risk indices were calculated using the American College of Cardiology–American Heart Association 2013 calculator.42 The validated ranges of ASCVD index components and the number of participants excluded from analysis for results that were outside these ranges are listed in eTable 3 in the Supplement.
Study Variables
For all analyses, relative statewide ADI distributions were used to create a dichotomous variable using the highest quintile (most disadvantaged neighborhoods) vs the lowest 4 quintiles (least disadvantaged neighborhoods), consistent with previous literature demonstrating negative health outcomes using cutoffs between the highest 25% and 15% levels of neighborhood disadvantage.23,24 Hippocampal volume (sum of left and right) and total brain tissue volume (TBV; sum of total white and gray matter) were selected as outcomes based on their previously reported associations with Alzheimer disease and related dementias. Volumetric measures were normalized to total intracranial volume by the residual correction method, which has increased sensitivity to HV loss.43 Adjusting volumetric measures for total intracranial volume increases sensitivity to detect relative tissue volume loss from a premorbid state. Sex was treated as a dichotomous variable, while morphometric measures, age, ASCVD index, and educational level, were treated as continuous variables for all analyses. The MRI scanner and head coil were included as categorical nuisance variables.
Statistical Analysis
Statistical analysis was performed from April 3 to September 27, 2019, using R, version 3.5.2 (R Foundation for Statistical Computing). Fixed-effect ordinary least squares linear regression models were fit to test associations between neighborhood-level disadvantage and volumetric outcomes, with age, sex, educational level, and MRI scanner variables included as additional covariates. Interaction terms were added to test for differential associations of neighborhood-level disadvantage by age or sex. To test whether cardiovascular risk statistically mediated the association between neighborhood-level disadvantage and brain morphometry, we performed model-based path analysis and estimated the indirect (mediation) and direct effects using a quasi-bayesian Monte Carlo simulation with 10 000 iterations (R package mediation, version 4.4.6).44 A critical α of 0.05 defined statistical significance and all statistical tests were 2 sided unless otherwise noted. Regression diagnostics were assessed for all statistical models.
Results
Demographic Characteristics
The demographic characteristics of the participants are described in Table 1. Participants living in the most disadvantaged neighborhoods reported significantly fewer years of education (mean [SD], 15.0 [2.7] vs 16.4 [2.6] years), were less likely to identify as white (27 of 41 [65.9%] vs 818 of 910 [89.9%]), and were more likely to identify as black or African American (14 of 41 [34.1%] vs 64 of 910 [7.0%]) than participants in less disadvantaged neighborhoods, but they did not differ from participants in less disadvantaged neighborhoods by age, sex, parental dementia history, or APOE-ε4 allele status.
Table 1. Demographic Characteristics of Study Participants.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| Total Morphometry Sample (N = 951) | Least Disadvantaged Neighborhoods (n = 910) | Most Disadvantaged Neighborhoods (n = 41) | |
| Demographic variablesa | |||
| Participants | |||
| WRAP | 439 (46.2) | 423 (46.5) | 16 (39.0)b |
| ADRC | 512 (53.8) | 487 (53.5) | 25 (61.0)b |
| Age, mean (SD) [range], y | 63.9 (8.1) [44.4-90.4] | 63.9 (8.0) [44.4-89] | 63.7 (9.5) [46.8-90.4]b |
| Female sex | 637 (67.0) | 607 (66.7) | 30 (73.2)b |
| Education, mean (SD) [range], y | 16.4 (2.6) [8-24] | 16.4 (2.6) [8-24] | 15.0 (2.7) [10-21]c |
| APOE-ε4 positive, No./total No. (%) | 315/910 (34.6)d | 303/870 (34.8) | 12/40 (30.0)b |
| Parental dementia history | 614 (64.6) | 589 (64.7) | 25 (61.0)b |
| Primary race/ethnicity | |||
| White | 845 (88.9) | 818 (89.9) | 27 (65.9)e |
| Black or African American | 78 (8.2) | 64 (7.0) | 14 (34.1)e |
| American Indian or Alaska Native | 23 (2.4) | 23 (2.5) | 0b |
| Other | 5 (0.5) | 5 (0.5) | 0b |
| Hispanic ethnicity | 8 (0.8) | 8 (0.9) | 0b |
| MRI outcomes, mean (SD) [range], mm3 | |||
| Total hippocampal volume | 7747 (776) [5208-10 088] | 7761 (776) [5208-10 088] | 7423 (720) [5288-8654] |
| Total brain tissue volume | 1 072 985 (70 389) [766 605-1 237 097] | 1 074 018 (70 460) [766 605-1 237 097] | 1 050 074 (65 504) [910 662-1 184 179] |
| Total Cardiovascular Risk Sample (N = 893) | Least Disadvantaged Neighborhoods (n = 857) | Most Disadvantaged Neighborhoods (n = 36) | |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mean (SD) [range], mm Hg | 125 (16) [90-193] | 125 (16) [90-193] | 131 (16.3) [98-162]f |
| Cholesterol, mean (SD) [range], mg/dL | |||
| Total | 202 (35) [131-316] | 203 (35) [131-316] | 197 (38) [140-301]b |
| HDL | 61 (16) [22-100] | 61 (16) [22-100] | 58 (15) [30-98]b |
| Current smoker | 33 (3.7) | 29 (3.4) | 4 (11.1)f |
| Diabetes diagnosis | 76 (8.5) | 67 (7.8) | 9 (25.0)e |
| Taking antihypertensive medication | 295 (33.0) | 277 (32.3) | 18 (50.0)f |
| ASCVD 10-y risk score, mean (SD) [range] | 9.5 (9.0) [0.22-60.4] | 9.4 (8.9) [0.22-60.4] | 12.7 (10.8) [0.68-40.5] |
Abbreviations: ADRC, Wisconsin Alzheimer’s Disease Research Center; ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein, MRI, magnetic resonance imaging; WRAP, Wisconsin Registry for Alzheimer’s Prevention.
SI conversion factor: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259.
The χ2 or 2-tailed t test was used to test differences in sample demographics.
P > .05 (most vs least neighborhood disadvantage).
P < .01.
Forty-one participants missing APOE genetic data.
P < .001.
P < .05.
Neighborhood-Level Disadvantage and Brain Morphometry
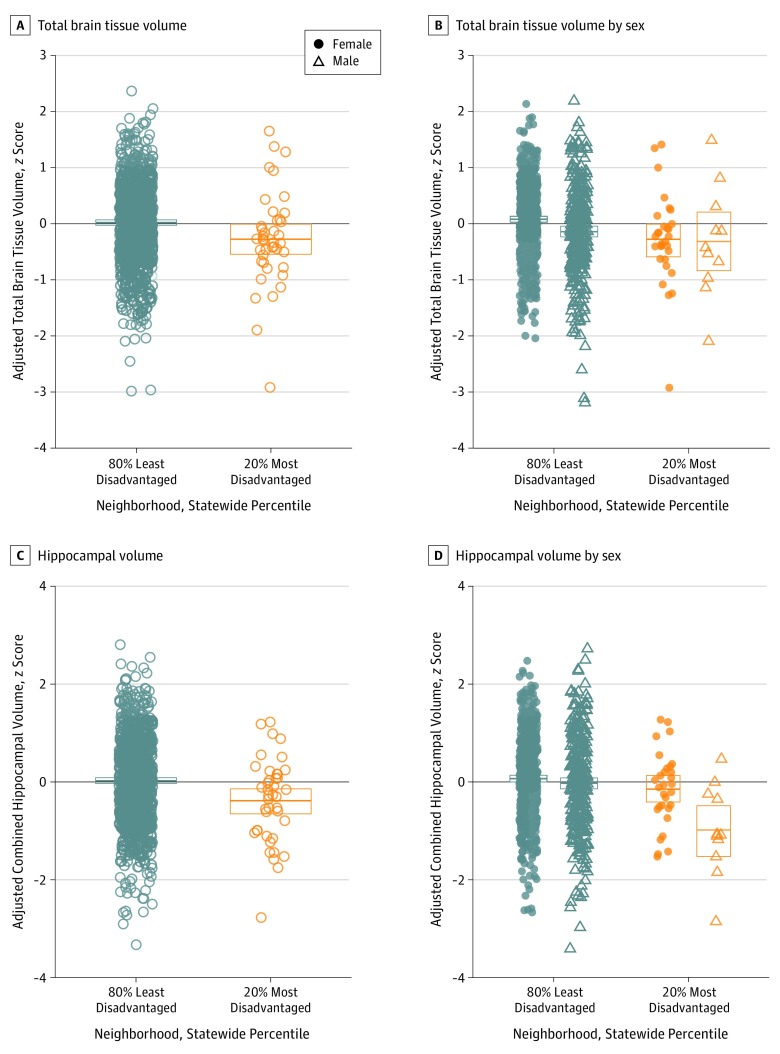
We first tested the association between neighborhood-level disadvantage and brain volumetry. Living in the most disadvantaged neighborhoods was associated with significantly lower HV and TBV after controlling for all covariates (Figure 2A and C). Living in the most disadvantaged neighborhoods was associated with a 4.1% lower HV (β = −317.44; 95% CI, −543.32 to −91.56; P = .006) (3.0% after adjusting for TBV) and a 2.0% lower TBV (β = −20 959.67; 95% CI, −37 611.92 to −4307.43; P = .01) (Table 2). Exploratory region-of-interest and voxelwise analyses (eMethods in the Supplement) revealed significantly lower gray matter volume in cortical and subcortical regions throughout the brain, with robust associations observed within temporal lobe areas (eFigure 2 and eTables 4 and 5 in the Supplement). Sensitivity analyses testing neighborhood-level disadvantage across deciles, quintiles, dichotomously split by median, eighth or tenth decile, and continuously by US percentile rank showed consistent associations with lower HV and/or TBV (eFigures 3-8 and eTables 6-11 in the Supplement). Older age and male sex were associated with lower HV and TBV, while educational level was associated with higher TBV only (Table 2). To address possible hidden measured or unmeasured risk factors represented in the demographic differences in educational level and racial/ethnic identity observed in the study cohort (Table 1), we conducted a follow-up propensity score–matched cohort analysis (eMethods and eFigure 9 in the Supplement). Our matched cohort showed no significant differences in age, sex, race/ethnicity, educational level, APOE-ε4 allele status, or parental dementia history (eTable 12 in the Supplement). In regression analyses using our matched cohort, living in the most disadvantaged neighborhoods was still associated with significantly lower HV and TBV (eTable 13 in the Supplement). Nearly identical results were observed when matching order was reversed or randomized (eTable 14 in the Supplement).
Figure 2. Association of Neighborhood-Level Disadvantage With Lower Total Brain Tissue Volume and Hippocampal Volume.
A, Total brain tissue volume. B, Total brain tissue volume by sex. C, Hippocampal volume. D, Hippocampal volume by sex. Total brain tissue and hippocampal volume were significantly lower in participants living in the most disadvantaged neighborhoods. Male participants living in the most disadvantaged neighborhoods had significantly lower mean hippocampal volume than female participants living in the most disadvantaged neighborhoods. There was no significant difference in total brain tissue volume between male and female participants living in the most disadvantaged neighborhoods. All volumetric values are adjusted for intracranial volume, age, sex, educational level, and magnetic resonance imaging scanner variables and are standardized (z score) for direct comparison between brain volume outcomes. Box plots show means with 95% CIs estimated by bootstrapping with 1000 iterations.
Table 2. Results of Regression Models of Association Between Neighborhood-Level Disadvantage and Morphometric Brain Volumesa.
| Characteristic | Unstandardized β Coefficient (95% CI) | ||||
|---|---|---|---|---|---|
| Total Hippocampus | Total Brain Tissue | ||||
| I | II | III | IV | V | |
| 20% Most disadvantaged neighborhoods | –317.44 (–543.32 to –91.56)b | –235.39 (–452.47 to –18.30)c | –163.74 (–426.54 to 99.05) | –20 959.67 (–37 611.92 to –4307.43)c | –24 638.84 (–44 057.77 to –5219.91)c |
| Total brain tissue | NA | 0.004 (0.003 to 0.005)d | NA | NA | NA |
| Age | −37.47 (−43.27 to −31.67)d | −14.82 (−22.16 to −7.47)d | −37.85 (−43.66 to −32.05)d | −5786.09 (−6214.00 to −5358.17)d | −5776.90 (−6205.65 to −5348.16)d |
| Male sex | −97.95 (−196.02 to 0.12)e | −36.91 (−131.74 to 57.92) | −74.82 (−174.77 to 25.14) | –15 592.66 (–22 822.52 to –8362.80)d | –16 146.51 (–23 532.70 to –8760.32)d |
| Years of education completed | 10.16 (−7.56 to 27.89) | 4.79 (−12.23 to 21.81) | 9.04 (−8.67 to 26.76) | 1372.84 (66.09 to 2679.60)c | 1399.63 (90.53 to 2708.73)c |
| Neighborhood disadvantage: male sex | NA | NA | −573.74 (−1078.04 to −69.44)c | NA | 13 733.99 (−23 531.01 to 50 998.99) |
| Model intercept | 9993.62 (9526.49 to 10 460.74)d | 4419.66 (3156.87 to 5682.44)d | 10 025.95 (9558.94 to 10 492.96)d | 1 423 783.00 (1 389 346.00 to 1 458 220.00)d | 1 423 009.00 (1 388 500.00 to 1 457 519.00)d |
| R2 | 0.16 | 0.23 | 0.16 | 0.44 | 0.44 |
| Adjusted R2 | 0.15 | 0.22 | 0.16 | 0.44 | 0.44 |
Abbreviations: ADI, Area Deprivation Index, MRI, magnetic resonance imaging; NA, not applicable.
Presented as unstandardized β coefficient estimate (95% CI) for each model variable for comparison of absolute effect sizes of variables on morphometry outcomes. Neighborhood-level disadvantage is based on statewide ADI distributions. All volumes are in cubic millimeters. All models included 951 cognitively unimpaired participants. Models also include MRI scanner nuisance variables.
P < .01.
P < .05.
P < .001.
P < .10.
Association of Neighborhood-Level Disadvantage, Sex, and Age With Brain Morphometry
Next, we assessed differential associations between neighborhood-level disadvantage and volumetric outcomes based on age and sex. We found a significant sex × neighborhood-level disadvantage interaction on HV (Table 2). For participants living in the most disadvantaged neighborhoods, male participants had a significantly lower HV than did female participants (Figure 2D). There was an interaction of age × neighborhood-level disadvantage on TBV, although when a single young participant with a particularly low TBV was removed from the analysis, the interaction did not remain significant (eTable 15 and eFigure 10 in the Supplement).
Cardiovascular Risk Mediation of Neighborhood-Level Disadvantage and Brain Morphometry
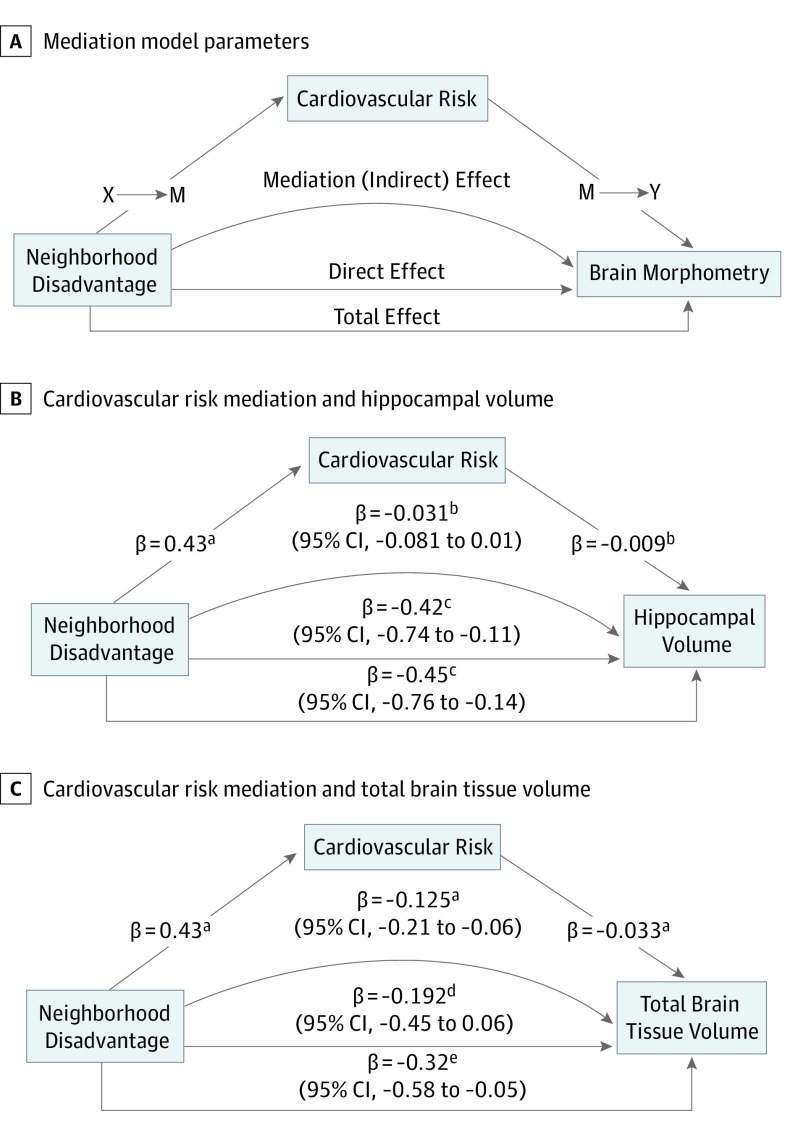
To test whether cardiovascular risk mediated the association between neighborhood-level disadvantage and brain morphometry, we conducted a stepwise path analysis and a mediation analysis to test indirect effects (Figure 3A). We first confirmed the association between neighborhood-level disadvantage and brain volumes in our cardiovascular risk subsample (eTable 16 in the Supplement). We then examined 10-year ASCVD score (Figure 3A; X → M), which was significantly higher, by 3.9% on average, for participants living in the most disadvantaged neighborhoods (eTable 16 in the Supplement). Next, we tested the association between cardiovascular risk and brain morphometry (Figure 3A; M → Y), which revealed significant negative associations between ASCVD score and TBV (Figure 3C) but not HV (Figure 3B). Individual ASCVD index components (diabetes diagnosis, systolic blood pressure, positive smoking status, and antihypertensive medication use) were higher for participants from the most disadvantaged neighborhoods (Table 1), and all except smoking status were significantly associated with TBV (eTable 17 in the Supplement). Finally, we found a significant mediation effect of cardiovascular risk on the association between neighborhood-level disadvantage and TBV with no additional direct effect (Figure 3C). We did not observe similar mediation for HV, although, notably, HV mediation models showed a significant direct effect (Figure 3B).
Figure 3. Cardiovascular Risk Mediation of Association Between Neighborhood-Level Disadvantage and Global Brain Volume.
A, Conceptual model for path analysis and mediation model parameters. B, Results of cardiovascular risk mediation of neighborhood-level disadvantage on total hippocampal volume. C, Results of cardiovascular risk mediation of neighborhood-level disadvantage on total brain tissue volume. z-Score–scaled β coefficients (Cohen d statistic) for relevant variables are shown for stepwise multiple regression models in path analysis (X → M and M → Y), Cohen d and 95% CIs are shown for estimation of indirect, direct and total effects using quasi-bayesian Monte Carlo simulation mediation modeling. Effect sizes can be directly compared between the 2 outcomes. Cardiovascular risk significantly mediated the association between neighborhood disadvantage and total brain tissue volume, but not for hippocampal volume. All models control for intracranial volume, age, sex, educational level, and magnetic resonance imaging scanner variables.
aP < .001.
bP < .10.
cP < .01.
dP > .10.
eP < .05.
Discussion
Association of Neighborhood-Level Disadvantage With Brain Morphometry
In this cross-sectional study of 951 cognitively unimpaired participants in middle to late adulthood, a high level of neighborhood disadvantage was associated with significantly lower HV and TBV. Applying general population–derived nomograms45 to our average study participant (a 64-year-old woman in the 50th percentile for HV), living in the most disadvantaged neighborhoods was equivalent to a mean of 7 years (95% CI, 2.5-12 years) of age-associated hippocampal atrophy. The association between neighborhood-level disadvantage and HV remained significant after controlling for TBV, indicating distinct global and hippocampal-specific associations. Differences in individual educational level—previously used as a proxy for individual SES20,21,46—did not account for the association between neighborhood-level disadvantage and brain morphometry. Distinct from neighborhood-level disadvantage, educational level was associated with total brain tissue but not with HV (Table 2). Together, these findings suggest that individual-level and neighborhood-level disadvantage may have unique associations with brain morphometry, echoing findings in other fields.20,21 Along with recent work showing associations between neighborhood-level disadvantage, cerebrospinal fluid biomarkers of neuronal injury and gliosis, and memory-related cognitive performance (M. Zuelsdorff, PhD, unpublished data, November 2019), this study provides additional in vivo neuroimaging evidence for a unique association of neighborhood disadvantage with brain volume during aging.
To our knowledge, our study is the first to show a robust association between neighborhood-level disadvantage and HV. A previous study of younger adults (mean age, 42.6 years) found a similar association between neighborhood-level disadvantage and HV that did not remain statistically significant when controlling for sex, age, intracranial volume, and individual SES.47 Our findings might be explained by an expanded sample size, but it is possible that our study cohort, which is enriched for Alzheimer disease risk owing to older age and parental dementia history, might be particularly vulnerable to the deleterious effects of neighborhood-level disadvantage on the hippocampus.
Sex, Neighborhood-Level Disadvantage, and Brain Morphometry
We found that male participants living in the most disadvantaged neighborhoods had significantly lower HV than did female participants (Figure 2D). This finding is consistent with previous findings of increased male vulnerability of the hippocampus to other insults, such as obstructive sleep apnea36 and type 1 diabetes,32 and of greater age-associated cerebral atrophy in cognitively unimpaired older men.3,48 Explanations for the observed sex differences could be further explored, including assessing the protective effects of social connectivity49 or the effect of age-related sex hormones.
Cardiovascular Risk Mediation of the Association Between Neighborhood-Level Disadvantage and TBV
We found that cardiovascular risk significantly mediated the association between neighborhood-level disadvantage and TBV, with no resulting direct effect (Figure 3C). Differences associated with diabetes and hypertension (systolic blood pressure and antihypertensive medication use) (Table 1; eTable 17 in the Supplement) appeared to be particularly relevant. This finding is consistent with a biologically plausible theoretical causal pathway between neighborhood-level disadvantage, cardiovascular risk, and brain volume loss during aging (Figure 3A). Neighborhoods with greater socioeconomic disadvantage have disproportionately less access to health-promoting resources such as healthy food options50 and high-quality recreational areas51 and concurrently higher rates of obesity31 and diabetes.17 Cardiometabolic dysfunction and disease, including hypertension, obesity, and diabetes, are established risk factors for neurodegeneration.2,4,5 Cardiovascular risk appears to have no mediation effect between neighborhood disadvantage and HV in these data (Figure 3B). This finding suggests that distinct biological pathways may link neighborhood-level disadvantage and HV compared with TBV. Testing other plausible biological pathways, such as exposure to chronic stress and the subsequent loss of hippocampal dendritic architecture and impaired neurogenesis,52 will be important areas for future research.
Limitations
Some limitations should be considered in interpreting the results of this study. Given the cross-sectional and observational study design, caution should be exercised in inferring causality. For example, children growing up in poverty have altered cortical and hippocampal development53 and greater later-life white matter lesion burden indicative of cerebrovascular disease,54 suggesting that socioeconomic disadvantage may have differential neurobiological effects over the course of lifetime. We found that neighborhood-level disadvantage was associated with decreased HV and decreased TBV, controlling for maximum premorbid brain volume (total intracranial volume). This finding is suggestive of brain tissue loss, although neurodevelopmental associations with our observed findings cannot be ruled out. It also remains to be determined whether neighborhood-level disadvantage is associated with accelerated age-related cerebral atrophy, pathologic neurodegenerative processes, or both. Endeavors to formally test the association of exposure to neighborhood-level disadvantage at various life stages with brain volume in later life are currently under way.
The socioeconomic and demographic features of our study cohorts provide important context to the present findings. Among the study participants from the most disadvantaged neighborhoods, educational levels were higher and the reported rates of medical comorbidities (such as diabetes) were lower compared with the mean education level and the mean rates of medical comorbidities reported in the United States, which possibly led to an underestimation of the effect of neighborhood-level disadvantage. Furthermore, our characterization of individual-level SES was limited to educational level. Incorporation of individual-level income, occupation, and life course factors (including parental occupation and education level) would better differentiate between neighborhood-level and individual-level SES associations with neurodegeneration. Similarly, socioeconomic inequality—the unequal distribution of resources between individuals within a community or society—is a unique determinant of health and disease that was not considered in the present study. The synergistic association between neighborhood-level disadvantage and high level of socioeconomic inequality remains an open and worthwhile research question. The educational level and racial/ethnic composition of participants were unequally distributed between levels of neighborhood disadvantage. Racial/ethnic identity alone is not an established risk factor for structural neurodegeneration, although differences in racial/ethnic composition and/or educational level between levels of neighborhood disadvantage in our study cohort may be proxies for other measured or unmeasured risk factors (eg, cardiovascular risk, genetic susceptibility, or environmental exposures).
In a robust set of matched cohort analyses, neighborhood-level disadvantage was not associated with “hidden” risk factors represented in these demographic differences. However, a dissection of differential associations between neighborhood-level disadvantage and neurodegeneration based on individual SES or race/ethnicity would be a valuable future endeavor. Finally, the number of study participants from more disadvantaged neighborhoods was proportionately smaller than those from less disadvantaged neighborhoods. Participants living in the 20% most disadvantaged neighborhoods comprised 4.3% of our total study population. This underrepresentation may well be a common characteristic of Alzheimer disease study cohorts nationally, with implications for interpretation and generalizability of study findings more broadly. The geoanalytical tools used to characterize this disparity are now available in a publicly accessible form—the Neighborhood Atlas18—which could guide outreach and recruitment efforts to underrepresented participants from more disadvantaged neighborhoods.
Clinical Implications
The present study provides correlational evidence connecting neighborhood-level disadvantage and brain volume in an older population. Separate from determining causality, understanding neighborhood-level disadvantage as a risk indicator for brain volume loss during the aging process is an important endeavor in its own right. Indices of neighborhood-level disadvantage can be quickly determined using easy-to-use and publicly available tools.18 Relative disadvantage scores can be ascertained using a patient’s residential address, without the clinician gathering additional social history or without the patient taking a questionnaire. In the future, neighborhood-level disadvantage could be factored into clinical decision-making or could guide public health initiatives promoting healthy brain aging in at-risk areas.18 Previous policy interventions such as the Moving to Opportunity study have demonstrated that intervening to modify neighborhood quality can be a viable health promotion strategy with measurable cardiovascular benefits.17
Conclusions
In this study, we found evidence that neighborhood-level disadvantage is associated with brain volumes in cognitively unimpaired older middle-aged adults, independent of individual-level SES. The association with total brain tissue was mediated by increased cardiovascular disease risk. The reported findings fit into a growing body of evidence on the unique negative health effects of neighborhood-level disadvantage21,23,24 and suggest that residential location may be a risk marker for brain volume loss during aging. Although a major effort is made to promote healthy behaviors, our findings provide additional evidence that larger-scale efforts to reduce community-level disadvantage and disparities may be important to promote healthy aging and dementia prevention.
eFigure 1. Inclusion/Exclusion Criteria for Constructing Study Sample
eFigure 2. Results of Exploratory Voxel-Wise and Region of Interest Analyses
eFigure 3. Association Between Brain Morphometry and Neighborhood Disadvantage by State-Wide Decile
eFigure 4. Association Between Brain Morphometry and Neighborhood Disadvantage by State-Wide Quintile
eFigure 5. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide Median (50%) Split
eFigure 6. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide 8th Decile (70%) Split
eFigure 7. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide 10th Decile (90%) Split
eFigure 8. Association Between Brain Morphometry and Neighborhood Disadvantage by US Percentile
eFigure 9. Distribution of Propensity Scores for Matched Cohort Analysis
eFigure 10. Age x Neighborhood Disadvantage Interaction on Brain Morphometry
eTable 1. MRI Scanner and Head Coil Used for Structural Scans
eTable 2. Structural MRI Acquisition Parameters
eTable 3. Previously Validated Ranges for 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Index and Number of Study Participants Outside These Ranges
eTable 4. Results of Exploratory Voxel-Based Morphometry Analysis Comparing Gray Matter Volume in Participants From Highest Disadvantage Neighborhoods to All Others
eTable 5. Results of Regression Models of Exploratory Analysis of Associations Between Neighborhood Disadvantage and Gray Matter Volume in Cortical and Subcortical Regions of Interest
eTable 6. Results of Regression Models of Association Between Neighborhood Disadvantage Deciles and Morphometric Brain Volumes
eTable 7. Results of Regression Models of Association Between Neighborhood Disadvantage Quintiles and Morphometric Brain Volumes
eTable 8. Results of Regression Models of Association Between 50% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 9. Results of Regression Models of Association Between 30% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 10. Results of Regression Models of Association Between 10% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 11. Results of Regression Models of Association Between US Percentile ADI Score and Morphometric Brain Volumes
eTable 12. Propensity Score-Matched Cohort Demographics
eTable 13. Results of Regression Models of Association Between Neighborhood Disadvantage and AD-Related Morphometric Brain Volumes Using Propensity Score-Matched Cohort
eTable 14. Results of Regression Models of Association Between Neighborhood Disadvantage and AD-Related Morphometric Brain Volumes in Additional Propensity Score-Matched Cohorts
eTable 15. Results of Regression Models of Neighborhood Disadvantage x Age Interaction on Morphometric Brain Volumes With Outlier Included and Excluded
eTable 16. Results of Stepwise Regression Analysis of the Mediation of Neighborhood Disadvantage on AD-Related Morphometric Brain Volumes by Cardiovascular Risk
eTable 17. Results of Regression Analyses Including Cardiovascular Risk Components of the ASCVD Risk and Neighborhood Disadvantage on Morphometric Brain Volumes
eMethods
eReferences
References
- 1.Villemagne VL, Burnham S, Bourgeat P, et al. ; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group . Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367. doi: 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 2.Lambert C, Zeestraten E, Williams O, et al. . Identifying preclinical vascular dementia in symptomatic small vessel disease using MRI. Neuroimage Clin. 2018;19:925-938. doi: 10.1016/j.nicl.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Wiste HJ, Weigand SD, et al. . Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol. 2017;16(6):435-444. doi: 10.1016/S1474-4422(17)30077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vemuri P, Knopman DS, Lesnick TG, et al. . Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. 2017;74(6):718-726. doi: 10.1001/jamaneurol.2017.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enzinger C, Fazekas F, Matthews PM, et al. . Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64(10):1704-1711. doi: 10.1212/01.WNL.0000161871.83614.BB [DOI] [PubMed] [Google Scholar]
- 6.Noble KG, Grieve SM, Korgaonkar MS, et al. . Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Julkunen V, Paajanen T, et al. ; AddNeuroMed Consortium . Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology. 2012;54(9):929-938. doi: 10.1007/s00234-012-1005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solé-Padullés C, Bartrés-Faz D, Junqué C, et al. . Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30(7):1114-1124. doi: 10.1016/j.neurobiolaging.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Foubert-Samier A, Catheline G, Amieva H, et al. . Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging. 2012;33(2):423.e15-423.e25. doi: 10.1016/j.neurobiolaging.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 10.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65(1):113-120. doi: 10.1001/archneurol.2007.27 [DOI] [PubMed] [Google Scholar]
- 11.Boots EA, Schultz SA, Almeida RP, et al. . Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin Neuropsychol. 2015;30(7):634-642. doi: 10.1093/arclin/acv041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services Healthy People 2020: an opportunity to address societal determinants of health in the United States. https://www.healthypeople.gov/2010/hp2020/advisory/SocietalDeterminantsHealth.htm. Published July 26, 2010. Accessed May 13, 2019.
- 13.Hill CV, Pérez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging health disparities research framework. Ethn Dis. 2015;25(3):245-254. doi: 10.18865/ed.25.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B. Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging. 2018;68:142-150. doi: 10.1016/j.neurobiolaging.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattler C, Toro P, Schönknecht P, Schröder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196(1):90-95. doi: 10.1016/j.psychres.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 16.Karp A, Kåreholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159(2):175-183. doi: 10.1093/aje/kwh018 [DOI] [PubMed] [Google Scholar]
- 17.Ludwig J, Sanbonmatsu L, Gennetian L, et al. . Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011;365(16):1509-1519. doi: 10.1056/NEJMsa1103216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the Neighborhood Atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Kind AJH, Nerenz D. Area Deprivation Index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493-501. doi: 10.1177/1062860617753063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honjo K, Iso H, Nakaya T, et al. ; Japan Public Health Center-based Prospective Study Group . Impact of neighborhood socioeconomic conditions on the risk of stroke in Japan. J Epidemiol. 2015;25(3):254-260. doi: 10.2188/jea.JE20140117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsay SE, Morris RW, Whincup PH, et al. . The influence of neighbourhood-level socioeconomic deprivation on cardiovascular disease mortality in older age: longitudinal multilevel analyses from a cohort of older British men. J Epidemiol Community Health. 2015;69(12):1224-1231. doi: 10.1136/jech-2015-205542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health. 1998;52(6):399-405. doi: 10.1136/jech.52.6.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind AJH, Jencks S, Brock J, et al. . Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AF, Liang L-J, Vassar SD, et al. . Neighborhood disadvantage and ischemic stroke: the Cardiovascular Health Study (CHS). Stroke. 2011;42(12):3363-3368. doi: 10.1161/STROKEAHA.111.622134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Soc Sci Med. 2012;75(6):1038-1047. doi: 10.1016/j.socscimed.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. doi: 10.3389/fnhum.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee T, Jarome T, Li S-J, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20(17):1554-1558. doi: 10.1097/WNR.0b013e328332bb09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O’Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6(1):29127. doi: 10.1038/srep29127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccolo LR, Noble KG; Pediatric Imaging, Neurocognition, and Genetics Study . Perceived stress is associated with smaller hippocampal volume in adolescence. Psychophysiology. 2018;55(5):e13025. doi: 10.1111/psyp.13025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villarreal G, Hamilton DA, Petropoulos H, et al. . Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52(2):119-125. doi: 10.1016/S0006-3223(02)01359-8 [DOI] [PubMed] [Google Scholar]
- 31.Rachele JN, Kavanagh A, Brown WJ, Healy AM, Schmid CJ, Turrell G. Neighborhood socioeconomic disadvantage and body mass index among residentially stable mid-older aged adults: findings from the HABITAT multilevel longitudinal study. Prev Med. 2017;105:271-274. doi: 10.1016/j.ypmed.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 32.Foland-Ross LC, Reiss AL, Mazaika PK, et al. ; Diabetes Research in Children Network (DirecNet) . Longitudinal assessment of hippocampus structure in children with type 1 diabetes. Pediatr Diabetes. 2018;19(6):1116-1123. doi: 10.1111/pedi.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirabayashi N, Hata J, Ohara T, et al. . Association between diabetes and hippocampal atrophy in elderly Japanese: the Hisayama Study. Diabetes Care. 2016;39(9):1543-1549. doi: 10.2337/dc15-2800 [DOI] [PubMed] [Google Scholar]
- 34.Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44(1):29-34. doi: 10.1161/01.HYP.0000132475.32317.bb [DOI] [PubMed] [Google Scholar]
- 35.Tchistiakova E, Anderson ND, Greenwood CE, MacIntosh BJ. Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. Neuroimage Clin. 2014;5:36-41. doi: 10.1016/j.nicl.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macey PM, Prasad JP, Ogren JA, et al. . Sex-specific hippocampus volume changes in obstructive sleep apnea. Neuroimage Clin. 2018;20:305-317. doi: 10.1016/j.nicl.2018.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653-660. doi: 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- 38.Johnson SC, Koscik RL, Jonaitis EM, et al. . The Wisconsin Registry for Alzheimer’s Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2017;10:130-142. doi: 10.1016/j.dadm.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907-922. doi: 10.1016/j.neuroimage.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keihaninejad S, Heckemann RA, Fagiolo G, Symms MR, Hajnal JV, Hammers A; Alzheimer’s Disease Neuroimaging Initiative . A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T). Neuroimage. 2010;50(4):1427-1437. doi: 10.1016/j.neuroimage.2010.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. [published correction appears in Circulation. 2014;129(25)(suppl 2):S74-S75]. Circulation. 2014;129(25)(suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 43.Hansen TI, Brezova V, Eikenes L, Håberg A, Vangberg TR. How does the accuracy of intracranial volume measurements affect normalized brain volumes? sample size estimates based on 966 subjects from the HUNT MRI cohort. AJNR Am J Neuroradiol. 2015;36(8):1450-1456. doi: 10.3174/ajnr.A4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1-38. doi: 10.18637/jss.v059.i05 26917999 [DOI] [Google Scholar]
- 45.Nobis L, Manohar SG, Smith SM, et al. . Hippocampal volume across age: nomograms derived from over 19,700 people in UK Biobank. Neuroimage Clin. 2019;23:101904. doi: 10.1016/j.nicl.2019.101904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans DA, Hebert LE, Beckett LA, et al. . Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54(11):1399-1405. doi: 10.1001/archneur.1997.00550230066019 [DOI] [PubMed] [Google Scholar]
- 47.Gianaros PJ, Kuan DC, Marsland AL, et al. . Community socioeconomic disadvantage in midlife relates to cortical morphology via neuroendocrine and cardiometabolic pathways. Cereb Cortex. 2017;27(1):460-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176-193. doi: 10.1016/j.neuroimage.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14(2):163-164. doi: 10.1038/nn.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilmers A, Hilmers DC, Dave J. Neighborhood disparities in access to healthy foods and their effects on environmental justice. Am J Public Health. 2012;102(9):1644-1654. doi: 10.2105/AJPH.2012.300865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughey SM, Walsemann KM, Child S, Powers A, Reed JA, Kaczynski AT. Using an environmental justice approach to examine the relationships between park availability and quality indicators, neighborhood disadvantage, and racial/ethnic composition. Landsc Urban Plan. 2016;148:159-169. doi: 10.1016/j.landurbplan.2015.12.016 [DOI] [Google Scholar]
- 52.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3-23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noble KG, Houston SM, Brito NH, et al. . Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray AD, McNeil CJ, Salarirad S, Whalley LJ, Staff RT. Early life socioeconomic circumstance and late life brain hyperintensities–a population based cohort study. PLoS One. 2014;9(2):e88969. doi: 10.1371/journal.pone.0088969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Inclusion/Exclusion Criteria for Constructing Study Sample
eFigure 2. Results of Exploratory Voxel-Wise and Region of Interest Analyses
eFigure 3. Association Between Brain Morphometry and Neighborhood Disadvantage by State-Wide Decile
eFigure 4. Association Between Brain Morphometry and Neighborhood Disadvantage by State-Wide Quintile
eFigure 5. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide Median (50%) Split
eFigure 6. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide 8th Decile (70%) Split
eFigure 7. Association Between Brain Morphometry and Neighborhood Disadvantage by Statewide 10th Decile (90%) Split
eFigure 8. Association Between Brain Morphometry and Neighborhood Disadvantage by US Percentile
eFigure 9. Distribution of Propensity Scores for Matched Cohort Analysis
eFigure 10. Age x Neighborhood Disadvantage Interaction on Brain Morphometry
eTable 1. MRI Scanner and Head Coil Used for Structural Scans
eTable 2. Structural MRI Acquisition Parameters
eTable 3. Previously Validated Ranges for 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Index and Number of Study Participants Outside These Ranges
eTable 4. Results of Exploratory Voxel-Based Morphometry Analysis Comparing Gray Matter Volume in Participants From Highest Disadvantage Neighborhoods to All Others
eTable 5. Results of Regression Models of Exploratory Analysis of Associations Between Neighborhood Disadvantage and Gray Matter Volume in Cortical and Subcortical Regions of Interest
eTable 6. Results of Regression Models of Association Between Neighborhood Disadvantage Deciles and Morphometric Brain Volumes
eTable 7. Results of Regression Models of Association Between Neighborhood Disadvantage Quintiles and Morphometric Brain Volumes
eTable 8. Results of Regression Models of Association Between 50% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 9. Results of Regression Models of Association Between 30% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 10. Results of Regression Models of Association Between 10% Highest Neighborhood Disadvantage and Morphometric Brain Volumes
eTable 11. Results of Regression Models of Association Between US Percentile ADI Score and Morphometric Brain Volumes
eTable 12. Propensity Score-Matched Cohort Demographics
eTable 13. Results of Regression Models of Association Between Neighborhood Disadvantage and AD-Related Morphometric Brain Volumes Using Propensity Score-Matched Cohort
eTable 14. Results of Regression Models of Association Between Neighborhood Disadvantage and AD-Related Morphometric Brain Volumes in Additional Propensity Score-Matched Cohorts
eTable 15. Results of Regression Models of Neighborhood Disadvantage x Age Interaction on Morphometric Brain Volumes With Outlier Included and Excluded
eTable 16. Results of Stepwise Regression Analysis of the Mediation of Neighborhood Disadvantage on AD-Related Morphometric Brain Volumes by Cardiovascular Risk
eTable 17. Results of Regression Analyses Including Cardiovascular Risk Components of the ASCVD Risk and Neighborhood Disadvantage on Morphometric Brain Volumes
eMethods
eReferences