This randomized clinical trial compares the efficacy and long-term outcomes of an online version of mindful-based cognitive therapy with usual depressive care for adults with residual depressive symptoms.
Key Points
Question
Can web-based treatment of residual depressive symptoms lead to incremental benefits for adults when added to usual depression care?
Finding
In this randomized clinical trial of 460 participants with residual depressive symptoms, those who received an online version of mindfulness-based cognitive therapy in addition to usual care had greater reductions in depressive and anxiety symptoms, higher rates of remission, and higher levels of quality of life compared with participants who received usual care only.
Meaning
The findings support the value of online mindfulness-based cognitive therapy as an adjunctive, scalable approach for the management of residual depressive symptoms.
Abstract
Importance
Patients with residual depressive symptoms face a gap in care because few resources, to date, are available to manage the lingering effects of their illness.
Objective
To evaluate the effectiveness for treating residual depressive symptoms with Mindful Mood Balance (MMB), a web-based application that delivers mindfulness-based cognitive therapy, plus usual depression care compared with usual depression care only.
Design, Setting, and Participants
This randomized clinical trial was conducted in primary care and behavioral health clinics at Kaiser Permanente Colorado, Denver. Adults identified with residual depressive symptoms were recruited between March 2, 2015, and November 30, 2018. Outcomes were assessed for a 15-month period, comprising a 3-month intervention interval and a 12-month follow-up period.
Interventions
Patients were randomized to receive usual depression care (UDC; n = 230) or MMB plus UDC (n = 230), which included 8 sessions delivered online for a 3-month interval plus minimal phone or email coaching support.
Main Outcomes and Measures
Primary outcomes were reduction in residual depressive symptom severity, assessed using the Patient Health Questionaire-9 (PHQ-9); rates of depressive relapse (PHQ-9 scores ≥15); and rates of remission (PHQ-9 scores <5). Secondary outcomes included depression-free days, anxiety symptoms (General Anxiety Disorder–7 Item Scale), and functional status (12-Item Short Form Survey).
Results
Among 460 randomized participants (mean [SD] age, 48.30 [14.89] years; 346 women [75.6%]), data were analyzed for the intent-to-treat sample, which included 362 participants (78.7%) at 3 months and 330 (71.7%) at 15 months. Participants who received MMB plus UDC had significantly greater reductions in residual depressive symptoms than did those receiving UDC only (mean [SE] PHQ-9 score, 0.95 [0.39], P < .02). A significantly greater proportion of patients achieved remission in the MMB plus UDC group compared with the UDC only group (PHQ-9 score, <5: β [SE], 0.38 [0.14], P = .008), and rates of depressive relapse were significantly lower in the MMB plus UDC group compared with the UDC only group (hazard ratio, 0.61; 95% CI, 0.39-0.95; P < .03). Compared with the UDC only group, the MMB plus UDC group had decreased depression-free days (mean [SD], 281.14 [164.99] days vs 247.54 [158.32] days; difference, −33.60 [154.14] days; t = −2.33; P = .02), decreased anxiety (mean [SE] General Anxiety Disorder–7 Item Scale score, 1.21 [0.42], P = .004), and improved mental functioning (mean [SE] 12-Item Short Form Survey score, −5.10 [1.37], P < .001), but there was no statistically significant difference in physical functioning.
Conclusions and Relevance
Use of MMB plus UDC resulted in significant improvement in depression and functional outcomes compared with UDC only. The MMB web-based treatment may offer a scalable approach for the management of residual depressive symptoms.
Trial Registration
ClinicalTrials.gov identifier: NCT02190968
Introduction
Depression is the second leading cause of disability worldwide, with the frequently chronic and recurrent nature of the disorder contributing significantly to the global burden of disease.1 Even low to moderate levels of residual depressive symptoms (RDS) are associated with significant impairment,2 greater social role strain,3 and risk of a negative prognosis.4 Despite the availability of antidepressant medication, most patients with depression who achieve a clinical response to antidepressant medications experience RDS.5 The public health risks of failing to address RDS are substantial, with the per capita costs of RDS ($2144) approaching the costs associated a major depressive episode ($3133).6 Patients with RDS often face a gap in care, whereby having achieved a marginal treatment response, they often are not provided resources for managing the lingering effects of the illness6,7 or achieving remission.
The RDS are important treatment targets and often require tailored management strategies that can be sequenced with acute-phase treatment8,9 and can be made widely accessible.10 The Mindful Mood Balance (MMB) treatment, which provides digital delivery of the skills of mindfulness-based cognitive therapy, is an important option for achieving these aims.
Mindfulness-based cognitive therapy was designed specifically to be used sequentially after response to acute-phase treatment and has a strong evidence base, including for depression relapse prevention and management of RDS.11,12 Studies of mindfulness-based cognitive therapy have reported moderate to large associations with reduction in RDS compared with antidepressant medications11 or usual care.13 The public health consequences of mindfulness-based cognitive therapy have been limited, however, because of dissemination challenges common to most in-person psychological interventions (eg, service costs, waiting lists, travel, and time training clinicians).14,15,16,17
Providing online therapies for patients who report RDS after routine care has been shown to be a promising approach to addressing these challenges and enhancing the dissemination of high-fidelity treatment of RDS.18,19 Dimidjian et al20 used a quasi-experimental design and tested MMB in patients who reported RDS after structured depression care at a large integrated health system (Kaiser Permanente Colorado) and reported that participants who received MMB showed a large effect size (Cohen d = 1.09) for the reduction of RDS that was maintained for 6 months.
In the context of these pilot data, the aim of the current study was to conduct a definitive trial for treating RDS with MMB compared with usual depression care (UDC). We hypothesized that adding MMB to UDC compared with UDC alone would lead to significant reductions in RDS severity, lower rates of depressive relapse, and higher rates of remission based on the Patient Health Questionnaire–9 (PHQ-9) results. We also believed that MMB plus UDC compared with UDC alone would lead to significantly more depression-free days, lower anxiety (General Anxiety Disorder–7 Item Scale [GAD-7]), and higher functional status (12-Item Short Form survey [SF-12]). Outcomes were assessed during a 15-month period, including a 3-month intervention interval and a 12-month follow-up period.
Methods
Trial Design
This 2-group, single-blind randomized clinical trial was approved by the institutional review boards of the University of Toronto, Kaiser Permanente Colorado, and the University of Colorado Boulder. All patients provided written informed consent before beginning study procedures. We compared the effectiveness of MMB plus UDC vs UDC alone (trial protocol in Supplement 1). Participants were randomized with an allocation ratio of 1:1 using the Research Electronic Data Capture randomization module with a file created by a random number generator in SAS, version 9.4 (SAS Institute Inc).21 Study staff were blinded to the contents of the randomization file. Figure 1 gives the study recruitment, randomization, and patient flow. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Figure 1. CONSORT Diagram.
MDE indicates major depressive disorder; MMB, mindful mood balance; and PHQ-9, Patient Health Questionnaire -9.
Sample Recruitment
Study activities were completed online between March 2, 2015, and November 30, 2018, and consistent with a pragmatic trial, inclusion criteria were minimal. All participants were Kaiser Permanente Colorado members and were identified through either electronic medical records that indexed real-time PHQ-9 scores and depression diagnoses, electronic clinician referral, or medical office flyers. Participants were aged 18 years or older with at least 1 prior episode of major depressive disorder confirmed via telephone interview and had a current PHQ-9 score between 5 and 9.22 Exclusion criteria included presence of schizophrenia, bipolar disorder, current psychosis, organic mental disorder, or pervasive developmental delay.
On the basis of effect sizes reported in Dimidjian et al,20 we estimated 80% power for detecting an effect size of 0.36 or greater with α = .05 based on a 2-tailed test. A total of 1045 patients were screened, 785 completed telephone interviews, and 460 were randomized.
Interventions
The MMB treatment was developed to provide the core components of the in-person mindfulness-based cognitive therapy program23 in an online, 8-session, self-administered platform. The MMB treatment teaches participants how to disengage from habitual, automatic, dysfunctional cognitive patterns (ie, depression-related ruminative thought patterns), to reduce RDS and vulnerability to relapse. Each MMB session incorporates experiential practice, video-based vicarious learning, and didactic information.24,25 During the intervention phase, participants were supported by a coach who provided motivational and technical support. Participants received a mean of 2.34 hours of coaching (sum of all hours spent coaching, divided by the number of coaches) during 12 weeks; this included a 45-minute orientation telephone call, 10-minute telephone check-ins for the first 2 weeks that tapered to weekly motivational emails, or telephone calls (eMethods 1 and 3 in Supplement 2).
Usual depression care followed the Kaiser Permanente Adult Depression National Guideline, an adaptation of STAR*D (Sequenced Treatment Alternatives to Relieve Depression),26 for antidepressant management. Patients also had access to individual or group psychotherapy through Kaiser Permanente’s behavioral health clinics. Care pathways were determined by severity level and included treatment with antidepressants, psychotherapy, or both (eMethods 2 in Supplement 2).
Outcomes
Primary outcomes were reduction in RDS severity, rates of remission, and rates of depressive relapse. These were assessed via the PHQ-9, a 9-item self-report measure with a range of scores from 0 to 27 and higher scores indicating greater depression severity. Remission was defined as scores less than 5, and relapse was defined as scores of 15 or higher, a severity threshold consistent with a determination of clinical relapse.27
Secondary outcomes were reductions in anxiety symptoms, indexed by the Generalized Anxiety Disorder–7 (GAD-7),28 a 7-item self-report measure with a range of scores from 0 to 21, with higher scores indicating greater anxiety severity. Functional status was assessed using the physical and mental functioning subscales of the Short Form 12 (SF-12) survey.29 This measure comprises 12 questions and is scored on a range from 0 to 100, with 0 indicating lower levels of health. Depression-free days were calculated to characterize depression-related morbidity based on converting consecutive scores on the PHQ-9 into a scale from 0 to 1, with 1 corresponding to depression-free days (PHQ-9 score, <5) and 0 corresponding to continuing symptomatic status (PHQ-9 score, ≥15). Intermediate scores were assigned a linear prorated value between 0 and 1.30
Statistical Analysis
All analyses were conducted with the intent-to-treat sample. We compared groups on baseline demographic and clinical characteristics using t tests for continuous variables and χ2 tests for categorical variables. To test our primary hypotheses that participants receiving MMB would report a reduction in RDS during the 3-month intervention phase and that this reduction would be sustained across the 12-month follow-up period, we implemented hierarchical linear modeling.31,32,33 To assess the best mathematical trajectory of change over time, every outcome was inspected visually through spaghetti plots and intervention-based mean profiles as well as quantitatively by comparison of the –2 (log-likelihood) Akaike information criterion and the Bayesian information criterion of various models, in which we compared linear change, log-linear change, polynomial change, and piecewise change with breakpoint at the 3-month time point corresponding to the end of the intervention phase (eTable 3 in Supplement 2). Consistently across outcomes, the piecewise linear change model with 2 phases provided the best fit. We also included a covariate-of-assessment time point to account for a systematic spike in PHQ-9 scores at assessment points at which participants completed an assessment battery comprised of all questionnaires compared with a briefer screening battery administered at other time points. Hierarchical linear models were replaced with hierarchical generalized linear models to accommodate binary outcomes, such as remission or nonremission, based on a PHQ-9 score of less than 5,31 and analyses focusing on time-to (ie, first relapse) were compared using Cox proportional hazard regression models.34 When fitting hierarchical linear models or hierarchical generalized linear models, an intent-to-treat analysis was used with the intake score as the first outcome, instead of a covariate. Effect sizes for the respective within-intervention and between-intervention change measures for hierarchical linear models were derived as Cohen d per Feingold.35 Percent differences were reported for hierarchical generalized linear models, and hazard ratios were reported for the time-to models.
As a sensitivity analysis for missing measures (eTable 4 in Supplement 2), we implemented a Markov Chain Monte Carlo imputation method through PROC MI of SAS, version 9.4 (SAS Institute).21,36 Markov Chain Monte Carlo constructs a Markov chain long enough for the distribution of the elements to stabilize to a common, stationary distribution. Data augmentation is applied to bayesian inference with missing data by repeating a series of imputation and posterior steps. These 2 steps are iterated long enough for the results to be reliable for the imputed data set.37,38
Results
Baseline demographic and clinical history variables for each group are presented in Table 1. Among 460 total participants (230 in each group), the mean (SD) age was 48.3 (14.9) years and 346 were women (75.6%). Of 456 total participants categorized by race, 419 (91.9%) were white and 8 (1.8%) were black; of 446 participants categorized by ethnicity, 39 (8.7%) were Hispanic, 8 (1.8%) were black, and 7 (1.5%) were Asian. Participants reported a mean (SD) of 7.5 (3.1) previous episodes of depression and at study intake; 78% of participants (355 of 455) reported receiving antidepressant medications, and 50% (219 of 435) reported receiving current psychotherapy.
Table 1. Participant Demographics and Clinical Characteristics.
| Characteristic | MMB Plus UDC Group (n = 230) | UDC Only Group (n = 230) | Total (N = 460) |
|---|---|---|---|
| Scores at intake, mean (SD) | |||
| PHQ-9 | 7.20 (1.41) | 7.29 (1.53) | 7.24 (1.47) |
| GAD-7 | 6.51 (3.15) | 6.20 (3.28) | 6.35 (3.22) |
| SF-12 PCS | 51.03 (9.88) | 51.77 (9.61) | 51.40 (9.74) |
| SF-12 MCS | 34.27 (7.92) | 34.22 (8.63) | 34.25 (8.28) |
| Age, mean (SD), y | 48.3 (15.1) | 48.2 (14.7) | 48.3 (14.9) |
| Sex, No. (%) | |||
| Male | 58 (25.3) | 54 (23.6) | 112 (24.4) |
| Female | 171 (74.7) | 175 (76.4) | 346 (75.6) |
| Marital status, No. (%) | |||
| Never married | 55 (24.0) | 48 (21.1) | 103 (22.5) |
| Married, civil union, or common-law marriage | 105 (45.8) | 112 (49.1) | 217 (47.5) |
| Divorced or separated | 60 (26.2) | 60 (26.3) | 120 (26.3) |
| Widowed | 9 (3.9) | 8 (3.5) | 17 (3.7) |
| Race/ethnicity, No. (%)a | |||
| American Indian or Alaskan Native | 1 (0.4) | 2 (0.9) | 3 (0.7) |
| Asian | 3 (1.3) | 4 (1.8) | 7 (1.5) |
| Black or African American | 4 (1.8) | 4 (1.8) | 8 (1.8) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.4) | 1 (0.2) |
| White | 212 (3.0) | 207 (90.8) | 419 (91.9) |
| Other | 8 (3.5) | 10 (4.4) | 18 (4.0) |
| Hispanic or Latino, No. (%) | 21 (9.4) | 18 (8.1) | 39 (8.7) |
| Educational level, No. (%)b | |||
| Did not complete high school | 3 (1.3) | 3 (1.3) | 6 (1.3) |
| Completed high school | 33 (14.5) | 24 (10.5) | 57 (12.5) |
| Completed college or university (includes undergraduate, graduate, or professional degree) | 191 (84.1) | 202 (88.2) | 393 (86.2) |
| Employment, No. (%) | |||
| Full-time | 131 (57.0) | 124 (54.2) | 255 (55.6) |
| Part-time | 25 (10.9) | 30 (13.1) | 55 (12.0) |
| Student | 5 (2.2) | 7 (3.1) | 12 (2.6) |
| Other | 69 (30.0) | 68 (29.7) | 137 (29.8) |
| Income, US $, No. (%) | |||
| 0-29 999 | 25 (11.0) | 24 (10.5) | 49 (10.8) |
| 30 000-69 999 | 91 (40.1) | 93 (40.8) | 184 (40.4) |
| 70 000-99 999 | 52 (22.9) | 57 (25.0) | 109 (24.0) |
| ≥100 000 | 59 (26.0) | 54 (23.7) | 113 (24.8) |
| Age at onset of first episode of depression, mean (SD) | 23.13 (13.29) | 23.57 (13.28) | 23.35 (13.27) |
| Weeks since last episode, mean (SD) | 64.00 (150.86) | 58.82 (114.16) | 61.46 (134.00) |
| No. of previous episodes of depression, mean (SD) | 7.44 (3.15) | 7.48 (3.14) | 7.46 (3.14) |
| Previous hospitalization for depression, No. (%) | 36 (15.79) | 36 (15.79) | 72 (15.79) |
| Previous suicide attempt, No. (%) | 39 (17.18) | 43 (18.94) | 82 (18.06) |
| Antidepressant at intake, No. (%) | 178 (77.39) | 177 (77.63) | 355 (77.51) |
| Current psychotherapy, No. (%) | 110 (50.69) | 109 (50.00) | 219 (50.34) |
| Current psychotherapy and antidepressant, No. (%) | 84 (38.71) | 85 (39.17) | 169 (38.94) |
Abbreviations: GADS-7 indicates Generalized Anxiety Disorder–7; MCS, mental component summary; MMB, Mindful Mood Balance; PCS, physical component summary; PHQ-9, Patient Health Questionnaire–9; SF-12, 12-item Short Form Survey; UDC, usual depression care.
Race/ethnicity comparison is white vs nonwhite.
Fisher exact test was used for educational level, part-time student, and unemployed comparison.
Intervention Exposure and Costs
Participants assigned to MMB plus UDC completed a mean (SD) of 4.8 (2.8) sessions of MMB of 8 total sessions, with 210 of 230 (91.3%) completing at least 1 treatment session, 144 (62.6%) completing at least 4 sessions, and 63 (27.4%) completing all 8 sessions. With respect to the use of therapy skills in the MMB sessions, participants practiced formal or informal mindfulness meditation for a mean (SD) of 46.1 (44.1) times during the 3-month intervention phase.
Pharmacy dispensing and psychotherapy data were available for 100% of the sample across the 15-month study period and indicated that 166 participants (72.2%) assigned to MMB plus UDC and 170 (73.9%) assigned to UDC only were dispensed psychotropic medication. With respect to psychotherapy, 111 (48.3%) assigned to MMB plus UDC and 114 (49.6%) assigned to UDC only had 2 or more psychotherapy visits. Differences between the groups were not significant for either utilization category.
The MMB treatment was designed as a stand-alone online intervention with minimal support. The cost of coaching support for 12 weeks, based on the average salary reported by the US Bureau of Labor Statistics ($28.68 per hour, plus 31% benefit rate and 10% overhead rate [rent, information technology, and infrastructure] for a health educator in the United States),39 was $96.67 for a mean of 2.34 hours per participant (sum of all hours spent coaching, divided by the number of coaches) and included orientation and follow-up telephone calls, emails, website tracking, and supervision.
Serious Adverse Events
The number of serious adverse events reported was small and consistent with findings from previous trials12,20; 1 serious adverse event (overdose) was reported by a participant assigned to the MMB plus UDC group, with none reported by participants in the UDC group. With respect to clinical deterioration, we examined referrals to behavioral health for PHQ-9 scores of 13 or higher and crisis calls for PHQ-9, item 9 (suicide ideation endorsement). Our data indicated that clinical deterioration was more prevalent in the UDC group (eTable 1 in Supplement 2). Although the proportions did not differ between the groups (χ2 = 0.49), on an absolute basis, the UDC group had more than twice as many alerts (127) as the MMB group (51).
Intervention Effects on Primary Outcomes
Consistent with our hypothesis, patients assigned to MMB plus UDC had significantly greater reduction in RDS during the entire study period compared with patients assigned to UDC only (mean [SE] difference, −2.55 [0.29] vs −1.64 [0.27]); the mean (SE) between-group difference in improvement in PHQ-9 score was 0.91 (0.39; t = 2.34, P = .02). During the intervention phase, the mean (SE) estimated improvements in PHQ-9 score were −2.70 (0.23) for the MMB plus UDC group and −0.80 (0.20) for the UDC only group, with a significantly greater reduction in RDS for participants in the MMB plus UDC group compared with the UDC only group (mean [SE] between-group difference in reduction in PHQ-9 score, 1.89 [0.33]; t = 5.85; P < .001). During the 12-month follow-up period, patients in the MMB plus UDC group maintained their initial gains on the PHQ-9, with a mean (SE) increase of 0.15 (0.26), which was not statistically significant. Participants in the UDC group had continued improvement (−0.84 [0.24]) that was significantly greater than that in participants in the MMB plus UDC group (0.98 [0.35]; t = 2.81; P = .003).
Because a systematic increase in PHQ-9 scores occurred when data were acquired at assessment points when participants completed a comprehensive assessment battery compared with time points when participants completed only a brief screening battery and the PHQ-9 score increase at these time points was statistically significant (F1,430 = 132.68, P < .001), we ran these analyses again controlling for this effect. Patients assigned to MMB plus UDC had a significantly greater mean (SE) reduction in RDS on the PHQ-9 during the entire study period (−2.65 [0.29]) compared with patients assigned to UDC only (−1.70 [0.27]) (Figure 2), and the mean (SE) between-group difference in improvement in PHQ-9 score was 0.95 (0.39; t = 2.43, P < .02). During the intervention phase, the mean (SE) estimated reductions in PHQ-9 score were −2.83 (0.24) for the MMB plus UDC group and −0.94 (0.22) for the UDC only group, with a significantly greater mean (SE) reduction in RDS for participants in the MMB plus UDC group compared with those in the UDC only group (1.89 [0.32]; t = 5.84; P < .001). During the 12-month follow-up period, patients in the MMB plus UDC group maintained their initial gains, an increase of 0.19 (0.26) that was not statistically significant. Participants in the UDC only group showed continued improvement, (−0.76 [0.24]) that was significantly greater than that among participants in the MMB plus UDC group (0.95 [0.35] mean reduction in PHQ-9 scores in the follow-up for the UDC, analogous to reductions in RDS; t = 2.71; P = .007).
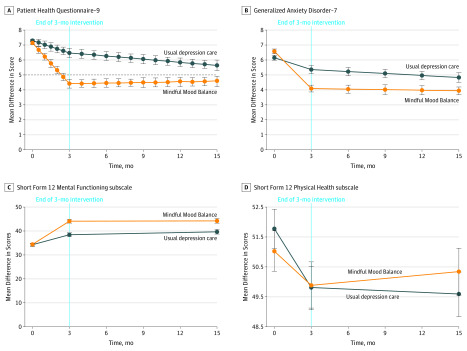
Figure 2. Differences Between the Mindful Mood Balance (MMB) Plus Usual Depression Care (UDC) Group and the UDC Only Group on Primary and Secondary Measures.
The vertical dotted line represents the end of the treatment interval; horizontal black dashed line, Patient Health Questionnaire–9 threshold of 5.
Consistent with our hypothesis that MMB would increase rates of remission (PHQ-9, <5) among participants, we found that a significantly greater mean (SE) proportion of participants during the entire study period in the MMB plus UDC group (59.4% [2.7%]) compared with the UDC alone group (47.0% [2.6%]) achieved remission. Mean (SE) between-group differences on the log-odds scale were 0.50 (0.15; t = 3.25; P < .001). During the intervention phase, both groups showed significant mean (SE) increases in the numbers of participants below the remission threshold (MMB plus UDC group: 57.2% [2.6%]; UDC only group: 35.7% [2.4%]), with a significantly greater number of participants achieving remission in the MMB plus UDC group compared with the UDC only group (mean [SE] between-group difference on log-odds scale, 0.88 [0.15]; t = −5.89; P < .001). Similar to the 12-month outcomes reported above, gains were maintained in the MMB plus UDC group during the 12-month follow-up period, corresponding to a mean (SE) increase in the rate of remission of 2.2% (2.6%) that was not statistically significant. The UDC only group showed significantly greater mean (SE) rates of remission compared with the MMB plus UDC group (11.3% [2.4%]; between-group differences in log-odds, 0.38 [0.14]; t = 2.65, P = .008) (Table 2 and Table 3).
Table 2. Mean Group Differences and Effect Sizes for Primary and Secondary Outcomes.
| Measure | Within-Group Difference, Mean (SE) | Between-Group Effect Size, Cohen d (95% CI) | ||
|---|---|---|---|---|
| MMB Plus UDC Group | UDC Group | Difference | ||
| PHQ-9 score | ||||
| Total | −2.65 (0.29)a | −1.70 (0.27)a | 0.95 (0.39)b | 0.23 (0.04 to 0.41) |
| Intervention phase | −2.83 (0.24)a | −0.94 (0.22)a | 1.89 (0.32)a | 0.55 (0.36 to 0.73) |
| Follow-up phase | 0.19 (0.26) | −0.76 (0.24)c | −0.95 (0.35)c | −0.25 (−0.44 to –0.07) |
| PHQ-9 remission (PHQ-9, ≤5) | ||||
| Total | 1.23 (0.11)a | 0.86 (0.10)a | −0.37 (0.12)c | −0.28 (−0.46 to –0.09) |
| Intervention phase | 1.16 (0.12)a | 0.37 (0.12)c | −0.79 (0.14)a | −0.55 (−0.73 to –0.36) |
| Follow-up phase | 0.07 (0.10) | 0.49 (0.10)a | 0.42 (0.14)c | 0.28 (0.10 to 0.47) |
| GAD-7 score | ||||
| Total | −2.48 (0.31)a | −1.27 (0.29)a | 1.21 (0.42)c | 0.27 (0.09 to 0.45) |
| Intervention phase | −2.34 (0.28)a | −0.75 (0.26)c | 1.60 (0.37)a | 0.40 (0.22 to 0.59) |
| Follow-up phase | −0.14 (0.30) | −0.53 (0.27) | −0.39 (0.40) | −0.09 (−0.27 to 0.09) |
| SF-12 score (PCS) | ||||
| Total | −1.86 (0.68)c | −2.39 (0.63)c | −0.53 (0.93) | −0.12 (−0.30 to 0.06) |
| Intervention phase | −1.64 (0.57)c | −2.38 (0.53)a | −0.74 (0.78) | −0.06 (−0.24 to 0.12) |
| Follow-up phase | −0.22 (0.69) | −0.003 (0.64) | 0.22 (0.94) | −0.05 (−0.23 to 0.13) |
| SF-12 score (MCS) | ||||
| Total | 10.27 (1.01)a | 5.17 (0.93)a | −5.10 (1.37)c | 0.45 (0.26 to 0.63) |
| Intervention phase | 9.78 (0.81)a | 4.11 (0.76)a | −5.67 (1.11)a | 0.54 (0.35 to 0.73) |
| Follow-up phase | 0.49 (0.98) | 1.06 (0.90) | 0.57 (1.33) | 0.05 (−0.13 to 0.23) |
| Total depression-free daysd | 281.43 (164.99) | 247.46 (158.32) | −33.97 (149.66)c | 0.22 (0.04 to 0.40) |
Abbreviations: GADS-7 indicates Generalized Anxiety Disorder–7; MCS, mental component summary; MMB, Mindful Mood Balance; PCS, physical component summary; PHQ-9, Patient Health Questionnaire–9; SF-12, 12-Item Short Form Survey; UDC, usual depression care.
P < .001.
P < .05.
P < .01.
Data are mean (SD) days.
Table 3. Descriptive Data for Primary and Secondary Outcomes Over Assessment Points.
| Month, Group | Score, Mean (SD) | |||
|---|---|---|---|---|
| PHQ-9 | GAD-7 | SF-12 MCS | SF-12 PCS | |
| Intervention Period | ||||
| 0 | ||||
| UDC only (n = 230) | 7.29 (1.53) | 6.20 (3.28) | 34.22 (8.63) | 51.77 (9.61) |
| MMB plus UDC (n = 230) | 7.20 (1.41) | 6.51 (3.15) | 34.27 (7.92) | 51.03 (9.88) |
| 0.5 | ||||
| UDC only (n = 204) | 7.45 (3.28) | NA | NA | NA |
| MMB plus UDC (n = 205) | 6.44 (3.15) | NA | NA | NA |
| 1.0 | ||||
| UDC only (n = 202) | 6.77 (3.58) | NA | NA | NA |
| MMB plus UDC (n = 190) | 5.49 (2.99) | NA | NA | NA |
| 1.5 | ||||
| UDC only (n = 211) | 7.68 (4.08) | NA | NA | NA |
| MMB plus UDC (n = 181) | 5.90 (3.57) | NA | NA | NA |
| 2.0 | ||||
| UDC only (n = 194) | 6.60 (3.59) | NA | NA | NA |
| MMB plus UDC (n = 172) | 4.98 (3.37) | NA | NA | NA |
| 2.5 | ||||
| UDC only (n = 194) | 6.09 (3.53) | NA | NA | NA |
| MMB plus UDC (n = 172) | 4.48 (3.19) | NA | NA | NA |
| 3.0 | ||||
| UDC only (n = 198) | 7.40 (4.27) | 5.41 (3.75) | 38.46 (10.34) | 49.81 (9.55) |
| MMB plus UDC (n = 164) | 5.19 (3.69) | 3.71 (2.98) | 44.10 (9.87) | 49.87 (9.93) |
| End of Active Treatment | ||||
| 4 | ||||
| UDC only (n = 193) | 6.48 (4.09) | NA | NA | NA |
| MMB plus UDC (n = 157) | 4.39 (3.07) | 4.43 (3.94)a | NA | NA |
| 5 | ||||
| UDC only (n = 191) | 6.22 (3.84) | NA | NA | NA |
| MMB plus UDC (n = 160) | 4.62 (3.59) | NA | NA | NA |
| 6 | ||||
| UDC only (n = 191) | 6.16 (3.96) | 5.25 (3.90) | NA | NA |
| MMB plus UDC (n = 158) | 4.70 (3.55) | 4.22 (3.48) | NA | NA |
| 7 | ||||
| UDC only (n = 189) | 6.24 (4.13) | NA | NA | NA |
| MMB plus UDC (n = 158) | 4.36 (3.42) | NA | NA | NA |
| 8 | ||||
| UDC only (n = 191) | 5.89 (3.86) | NA | NA | NA |
| MMB plus UDC (n = 161) | 3.90 (3.17) | NA | NA | NA |
| 9 | ||||
| UDC only (n = 191) | 6.30 (4.23) | 4.96 (3.85) | NA | NA |
| MMB plus UDC (n = 156) | 4.65 (4.11) | 3.89 (3.99) | NA | NA |
| 10 | ||||
| UDC only (n = 181) | 5.95 (4.12) | NA | NA | NA |
| MMB plus UDC (n = 155) | 4.15 (3.66) | NA | NA | NA |
| 11 | ||||
| UDC only (n = 182) | 5.44 (3.56) | NA | NA | NA |
| MMB plus UDC (n = 154) | 4.20 (3.71) | NA | NA | NA |
| 12 | ||||
| UDC only (n = 184) | 5.57 (3.79) | 4.74 (3.77) | NA | NA |
| MMB plus UDC (n = 151) | 4.83 (3.74) | 4.03 (3.80) | NA | NA |
| 13 | ||||
| UDC only (n = 184) | 5.48 (3.82) | NA | NA | NA |
| MMB plus UDC (n = 157) | 4.30 (3.47) | NA | NA | NA |
| 14 | ||||
| UDC only (n = 181) | 5.16 (3.57) | NA | NA | NA |
| MMB plus UDC (n = 155) | 4.01 (3.52) | NA | NA | NA |
| 15 | ||||
| UDC only (n = 176) | 7.06 (4.76) | 4.92 (4.23) | 39.64 (11.93) | 49.58 (9.82) |
| MMB plus UDC (n = 154) | 5.10 (4.19) | 3.49 (3.21) | 44.37 (10.51) | 50.34 (9.29) |
Abbreviations: GADS-7 indicates Generalized Anxiety Disorder–7; MCS, mental component summary; MMB, Mindful Mood Balance; NA, not applicable; PCS, physical component summary; PHQ-9, Patient Health Questionnaire–9; SF-12, 12-Item Short Form Survey; UDC, usual depression care.
Patients assigned MMB plus UDC (n = 58) were assessed using the GAD-7 at month 4.
Treatment with MMB plus UDC also was associated with lower rates of relapse during the 12-month follow-up period, with 31 of 230 participants (13.5%) in this group crossing the relapse threshold (PHQ-9, ≥15) compared with 53 (23.0%) in the UDC only group (hazard ratio, 0.61; 95% CI, 0.39-0.95; χ2 = 4.83; P < .03). We additionally fit the time to first response, which yielded a significant intervention effect (χ2 = 11.89, P < .001) with a hazard ratio of 1.46 (95% CI, 1.18-1.80), indicating that the rate of response for MMB plus UDC was 45.6% greater than UDC alone.
Intervention Effects on Anxiety and Quality of Life Outcomes
Consistent with our hypothesis, MMB plus UDC was significantly associated with greater improvement in anxiety symptom severity compared with UDC only, with participants in the MMB plus UDC group showing a mean (SE) decrease in GAD-7 score of 2.48 (0.31) points compared with 1.27 (0.29) points in the UDC group (mean [SE] between-group difference, 1.21 [0.42]; t = 2.90, P = .004).28 With respect to quality of life outcomes, controlling for intake, MMB plus UDC was associated with more depression-free days during the entire study period compared with UDC alone (mean [SD], 281.14 [164.99] days vs 247.54 [158.32] days; difference: −33.60 [154.14] days; t = −2.33; P = .02).
Participation in MMB plus UDC was also associated with an increase in SF-12 mental functioning subscale score (mean [SD] increase, 10.27 [1.01] points vs 5.17 [0.93] points for UDC; mean [SD] difference, −5.10 [1.37]; t = −3.72; P = .001). There was no statistically significant difference between groups on the physical health subscale of the SF-12 (mean [SE], −1.50 [1.44]; t = 1.04; P = .30).
Discussion
Treatment with MMB plus UDC for adults with partially remitted depression resulted in significant reductions in RDS compared with UDC only delivered by a large integrated health system. A greater percentage of MMB plus UDC participants achieved remission (PHQ-9 score, <5) and did not experience relapse (PHQ-9 score, ≥15). Benefits of MMB were evident within the 3-month intervention period and were maintained across the 12-month follow-up period.
Our findings align with prior evidence for the effects of mindfulness-based cognitive therapy on RDS11,12,13 and showed that teaching affect-regulation skills to individuals with RDS can be extended through web-based delivery with MMB. In settings that use routine monitoring of depressive symptoms, MMB can be integrated as an augmentation strategy or second care step for patients who achieve only partial remission after acute phase treatment.9,10 Providing the right treatment at the right time can optimize depression outcomes by reducing undertreatment40 and lowering patients’ future risk profiles.4 However, as Mohr and others41 have cautioned, health system implementation must be engineered, rather than assumed. Our experience suggests that batch messaging through the Kaiser Permanente Colorado patient portal and clinician endorsement or recommendation were drivers of patient uptake and engagement.
Unlike most web-based interventions that address acute phase disorders, MMB targets psychological vulnerability after initial treatment and teaches skills to reverse symptom perpetuation and return.42,43 This may be apparent in the differential effects reported on the SF-12, in which participants receiving MMB plus UDC showed improved mental functioning but no change in physical health.
The benefits of MMB plus UDC compared with UDC alone were evident on some of the secondary outcomes, including lower anxiety severity, more depression-free days, and improved functional outcomes. A focus on the overall illness burden in this population is vital because it can be rate limiting for the resumption of work and social roles.44,45 For example, comorbid anxiety is associated with a diminished response to first-line treatments as well as poor long-term outcomes in patients with RDS,46,47 and the presence of RDS has been associated with increased absenteeism and reduced productivity at work.48 In a sample of 771 workers, Beck et al49 estimated that every 1-point increase in PHQ-9 score led to a mean productivity loss of 1.65%. This represents a significant labor cost to employers. Given its potentially lower costs, confidentiality, and increased accessibility offered by its online format, integrating MMB into occupational health or employee assistance programs may be a solution for addressing even minor depression in the workplace.
Limitations
There are a number of limitations that deserve mention, including a lack of diversity in the sample that we studied. Consistent with a pragmatic trial, our assessment of patients with RDS relied on self-reported symptoms in the absence of a confirmatory diagnostic or clinical interview. This allowed us to detect RDS but not to fully characterize the prior duration of RDS, a variable that has been linked to illness course. Although the PHQ-9 has been subjected to considerable validation,27 depressive relapse is often assessed via structured interview, and as such, the group difference in survival times favoring MMB plus UDC may reflect different rates of clinical deterioration rather than a discrete episode of major depressive disorder or full remission. Also, the spikes in PHQ-9 scores across both groups at the assessment points when a more comprehensive assessment battery was administered compared with scores at other time points with the brief symptom screener are another possible limitation. We interpreted this pattern as a measurement artifact because of the order of the PHQ-9 (being placed last in the full assessment battery and first in the brief symptom screener) and controlled for these elevations in our statistical models; however, it is possible that the spikes reflect other, as yet unidentified processes. In addition, although MMB completion rates of 27% were lower than rates in recent reviews (eg, 63%),50 the percentage of participants who received a minimum therapeutic exposure of 4 or more sessions was 62.6% (eTable 2 in Supplement 2). Future studies should explore methods for enhancing engagement, such as providing support tied to each individual MMB session or offering adherence-focused guidance.51
Conclusions
Use of MMB plus UDC resulted in significant improvement in depression and functional outcomes compared with UDC only. The addition of MMB to UDC delivered by an integrated health system provides a pragmatic and accessible strategy to address the suboptimal treatment of patients with RDS in primary care and employment settings.52,53 Further research on and design of effective implementation models is required to optimize the public health outcomes of MMB.41
Trial Protocol
eMethods 1. Information on Coaches for Mindful Mood Balance
eMethods 2. Description of Usual Depression Care at KPCO
eMethods 3. Description of Mindful Mood Balance
eTable 1. SOAR Clinical Deterioration Descriptive Data
eTable 2. Demographic and Clinical History Variables for Participants Completing <4 vs ≥4 Sessions of MMB
eTable 3. Model Fit Parameters for HLM
eTable 4. Sensitivity Analyses for Missing Data
Data Sharing Statement
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41(6):1165-1174. doi: 10.1017/S0033291710001911 [DOI] [PubMed] [Google Scholar]
- 3.Cuijpers P, Beekman ATF, Reynolds CF III. Preventing depression: a global priority. JAMA. 2012;307(10):1033-1034. doi: 10.1001/jama.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patten SB, Williams JVA, Lavorato DH, Bulloch AGM, MacQueen G. Depressive episode characteristics and subsequent recurrence risk. J Affect Disord. 2012;140(3):277-284. doi: 10.1016/j.jad.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Zajecka J, Kornstein SG, Blier P. Residual symptoms in major depressive disorder: prevalence, effects, and management. J Clin Psychiatry. 2013;74(4):407-414. doi: 10.4088/JCP.12059ah1 [DOI] [PubMed] [Google Scholar]
- 6.Bet PM, Hugtenburg JG, Penninx BWJH, van Balkom A, Nolen WA, Hoogendijk WJG. Treatment inadequacy in primary and specialized care patients with depressive and/or anxiety disorders. Psychiatry Res. 2013;210(2):594-600. doi: 10.1016/j.psychres.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 7.Thornicroft G, Chatterji S, Evans-Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119-124. doi: 10.1192/bjp.bp.116.188078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50. doi: 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop BW, LoParo D, Kinkead B, et al. Benefits of sequentially adding cognitive-behavioral therapy or antidepressant medication for adults with nonremitting depression. Am J Psychiatry. 2019;176(4):275-286. doi: 10.1176/appi.ajp.2018.18091075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016;173(2):128-137. doi: 10.1176/appi.ajp.2015.15040476 [DOI] [PubMed] [Google Scholar]
- 11.Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76(6):966-978. doi: 10.1037/a0013786 [DOI] [PubMed] [Google Scholar]
- 12.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256-1264. doi: 10.1001/archgenpsychiatry.2010.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geschwind N, Peeters F, Huibers M, van Os J, Wichers M. Efficacy of mindfulness-based cognitive therapy in relation to prior history of depression: randomised controlled trial. Br J Psychiatry. 2012;201(4):320-325. doi: 10.1192/bjp.bp.111.104851 [DOI] [PubMed] [Google Scholar]
- 14.Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 15.Kessler RC, Berglund P, Demler O, et al. ; National Comorbidity Survey Replication . The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105. doi: 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- 16.Andrews G, Issakidis C, Sanderson K, Corry J, Lapsley H. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. Br J Psychiatry. 2004;184(6):526-533. doi: 10.1192/bjp.184.6.526 [DOI] [PubMed] [Google Scholar]
- 17.Weissman MM, Verdeli H, Gameroff MJ, et al. National survey of psychotherapy training in psychiatry, psychology, and social work. Arch Gen Psychiatry. 2006;63(8):925-934. doi: 10.1001/archpsyc.63.8.925 [DOI] [PubMed] [Google Scholar]
- 18.Buntrock C, Ebert DD, Lehr D, et al. Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA. 2016;315(17):1854-1863. doi: 10.1001/jama.2016.4326 [DOI] [PubMed] [Google Scholar]
- 19.Compen F, Bisseling E, Schellekens M, et al. Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36(23):2413-2421. doi: 10.1200/JCO.2017.76.5669 [DOI] [PubMed] [Google Scholar]
- 20.Dimidjian S, Beck A, Felder JN, Boggs JM, Gallop R, Segal ZV. Web-based mindfulness-based cognitive therapy for reducing residual depressive symptoms: an open trial and quasi-experimental comparison to propensity score matched controls. Behav Res Ther. 2014;63:83-89. doi: 10.1016/j.brat.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute Inc SAS® 9.4 Language Reference: Concepts. 5th ed Cary, NC: SAS Institute Inc; 2016. [Google Scholar]
- 22.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191-E196. doi: 10.1503/cmaj.110829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal ZV, Williams JM, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. 2nd ed New York, NY: Guilford Press; 2013. [Google Scholar]
- 24.Dirkx JM. The meaning and role of emotions in adult learning. New Dir Adult Contin Educ. 2008;120:7-18. doi: 10.1002/ace.311 [DOI] [Google Scholar]
- 25.Eastmond DV. Adult learners and internet-based distance education. New Dir Adult Contin Educ. 1998;78:33-41. [Google Scholar]
- 26.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. [DOI] [PubMed] [Google Scholar]
- 27.Levis B, Benedetti A, Thombs BD; DEPRESsion Screening Data (DEPRESSD) Collaboration . Accuracy of Patient Health Questionnaire–9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 29.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 30.Vannoy SD, Arean P, Unützer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv. 2010;61(2):160-163. doi: 10.1176/ps.2010.61.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed London, UK: Sage Publications; 2002. [Google Scholar]
- 32.Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50(4):933-944. doi: 10.2307/2533433 [DOI] [PubMed] [Google Scholar]
- 33.Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462-1470. [DOI] [PubMed] [Google Scholar]
- 34.Collett D. Modelling Survival Data in Medical Research. London, England: Taylor & Francis; 2014. [Google Scholar]
- 35.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14(1):43-53. doi: 10.1037/a0014699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y. Multiple imputation using SAS software. J Stat Softw. 2011;45(6):1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 38.Rubin DB. Multiple imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 39.US Bureau of Labor Statistics Occupational Employment and Wages, May 2018. https://www.bls.gov/oes/current/oes211091.htm. Updated March 29, 2019. Accessed July 5, 2019.
- 40.Köhler-Forsberg O, Cusin C, Nierenberg AA. Evolving issues in the treatment of depression. JAMA. 2019;321(24):2401-2402. doi: 10.1001/jama.2019.4990 [DOI] [PubMed] [Google Scholar]
- 41.Mohr DC, Lyon AR, Lattie EG, Reddy M, Schueller SM. Accelerating digital mental health research from early design and creation to successful implementation and sustainment. J Med Internet Res. 2017;19(5):e153. doi: 10.2196/jmir.7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275-287. doi: 10.1037/0022-006X.70.2.275 [DOI] [PubMed] [Google Scholar]
- 43.Kruijt AW, Antypa N, Booij L, et al. Cognitive reactivity, implicit associations, and the incidence of depression: a two-year prospective study. PLoS One. 2013;8(7):e70245. doi: 10.1371/journal.pone.0070245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan DV, Nakagome K, Asami Y, Pappadopulos EA, Boucher M. Restoring function in major depressive disorder: systematic review. J Affect Disord. 2017;215:299-313. doi: 10.1016/j.jad.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 45.Lin CH, Chou LS, Chen MC, Chen CC. The relationship between symptom relief and functional improvement during acute fluoxetine treatment for patients with major depressive disorder. J Affect Disord. 2015;182:115-120. doi: 10.1016/j.jad.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 46.Kennedy JC, Dunlop BW, Craighead LW, Nemeroff CB, Mayberg HS, Craighead WE. Follow-up of monotherapy remitters in the PReDICT study: maintenance treatment outcomes and clinical predictors of recurrence. J Consult Clin Psychol. 2018;86(2):189-199. doi: 10.1037/ccp0000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farabaugh A, Alpert J, Wisniewski SR, et al. Cognitive therapy for anxious depression in STAR(*) D: what have we learned? J Affect Disord. 2012;142(1-3):213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135-3144. doi: 10.1001/jama.289.23.3135 [DOI] [PubMed] [Google Scholar]
- 49.Beck A, Crain AL, Solberg LI, et al. Severity of depression and magnitude of productivity loss. Ann Fam Med. 2011;9(4):305-311. doi: 10.1370/afm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):329-342. doi: 10.1016/j.cpr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 51.Ebert DD, Buntrock C, Lehr D, et al. Effectiveness of web- and mobile-based treatment of subthreshold depression with adherence-focused guidance: a single-blind randomized controlled trial. Behav Ther. 2018;49(1):71-83. doi: 10.1016/j.beth.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 52.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314-2321. doi: 10.1001/archinte.166.21.2314 [DOI] [PubMed] [Google Scholar]
- 53.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53(10):924-932. doi: 10.1001/archpsyc.1996.01830100072009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Information on Coaches for Mindful Mood Balance
eMethods 2. Description of Usual Depression Care at KPCO
eMethods 3. Description of Mindful Mood Balance
eTable 1. SOAR Clinical Deterioration Descriptive Data
eTable 2. Demographic and Clinical History Variables for Participants Completing <4 vs ≥4 Sessions of MMB
eTable 3. Model Fit Parameters for HLM
eTable 4. Sensitivity Analyses for Missing Data
Data Sharing Statement