Key Points
Question
How many patients are eligible for the 12 novel cardiovascular preventive therapies introduced in recent years and what are their preventive potential among secondary prevention patients?
Finding
This cohort study included 6292 patients with known ischemic heart disease and 2277 patients with prior myocardial infarction from the Copenhagen General Population Study. Most patients with known ischemic heart disease (8 of 10) or myocardial infarction (10 of 10) were eligible to receive novel preventive therapies.
Meaning
This study raises questions for the cardiovascular community about access to these potentially expensive therapies, including strategies for prioritizing their use.
Abstract
Importance
Recently, 12 randomized clinical trials (RCTs) have demonstrated the efficacy of novel therapies for mainly secondary prevention of atherosclerotic cardiovascular disease. However, given the potential overlapping eligibility of the RCTs, along with the cost of the new therapies, there are uncertainty and questions about implementing these RCT findings in real-world clinical practice.
Objective
To determine the eligibility and preventive potential for these new preventive therapies in a contemporary population.
Design, Setting, and Participants
This population-based contemporary cohort study included 6292 patients with known ischemic heart disease (IHD) and 2277 with a previous myocardial infarction (MI) enrolled between November 2003 and February 2015. Analyses were performed in the Copenhagen General Population Study with a mean (SD) of 7.7 (3.5) years of follow-up. The data were analyzed between January and October 2019.
Main Outcomes and Measures
We determined the drug eligibility and evidence-based potential for preventing major cardiovascular events of the 12 cardiovascular drugs tested in the following recent RCTs: IMPROVE-IT, PEGASUS, EMPA-REG, LEADER, SUSTAIN-6, FOURIER, CANVAS, REVEAL, CANTOS, COMPASS, ODYSSEY-OUTCOMES, and REDUCE-IT. The analyses were performed in patients with known IHD or with a previous MI at baseline.
Results
Of 6292 participants, 3861 (61%) were men and the mean (interquartile range) age was 69 (62-76) years. In patients with IHD or MI at baseline, eligibility for 1 or more new medications was 80% (n = 5036) and 99% (n = 2273), respectively, by meeting RCT enrollment criteria. Dividing the new therapies into 4 drug classes (lipid-modifying, antithrombotic, anti-inflammatory, and antidiabetic drugs), 2594 and 1834 patients with IHD or MI (41% and 81%, respectively) were eligible for 2 or more drug classes simultaneously. The 5-year estimated percentage of major cardiovascular events that could be prevented for each new therapy was 1% to 20% in patients with IHD or MI at baseline.
Conclusions and Relevance
Most patients with known IHD or previous MI are eligible for additional new secondary prevention therapies. This raises questions for the cardiovascular community and health care authorities about access to these potentially expensive therapies, including strategies for prioritizing their use.
This cohort study examines the drug eligibility of 12 cardiovascular drugs recently tested in several randomized clinical trials for Danish patients with known ischemic heart disease or prior myocardial infarction.
Introduction
The cornerstone of pharmacological therapy for secondary prevention of atherosclerotic cardiovascular disease (ASCVD) consists of lipid-lowering statin therapy, antihypertensive therapy, and an antithrombotic agent. However, despite the well-documented efficacy of these treatments, patients with existing ASCVD remain at high risk for recurrent events and mortality. Since 2015, 12 major randomized clinical trials (RCTs) of novel pharmacological treatments added to standard therapy have demonstrated further improvement in ASCVD outcomes in secondary prevention patients.1,2,3,4,5,6,7,8,9,10,11,12
The results from these RCTs are important to ASCVD prevention. However, to date, little is known about the potential implications of these studies on drug eligibility and ASCVD prevention. Thus, we assessed the evidence-based eligibility and preventive potential of new pharmacological therapies among patients with known ischemic heart disease (IHD) or prior myocardial infarction (MI) from a contemporary population-based study.
Methods
Study Population and Setting
The Copenhagen General Population Study (CGPS) is an ongoing prospective cohort study of the Danish general population.13 The studies were approved by Herlev and Gentoft Hospital and participants provided written informed consent. Additional details are described in the eMethods in the Supplement.
Secondary Prevention Trials
Listed in chronological order by publication date, the following studies are included in our analyses (full names are listed in eMethods in the Supplement): IMPROVE-IT,1 PEGASUS,2 EMPA-REG,3 LEADER,4 SUSTAIN-6,5 FOURIER,6 CANVAS,7 REVEAL,8 CANTOS,9 COMPASS,10 ODYSSEY-OUTCOMES,11 and REDUCE-IT.12 Enrollment criteria are shown in eTable 1 in the Supplement. To assess maximal eligibility over time by implementing RCT evidence, we did not require strict adherence to time criteria for recent/prior ASCVD event used in trials. However, in sensitivity analyses, we followed such time criteria.
It is unlikely that patients will be assigned 2 of the novel drugs that target the same biological pathway (ie, 2 novel antithrombotics). Thus, to determine if patients could be eligible to take 2 or more drugs simultaneously, we divided the drugs into 4 drug classes: lipid-modifying, antithrombotic, anti-inflammatory, and antidiabetic.
Outcomes and Statistical Analysis
Myocardial infarction, stroke, cardiovascular deaths, and all-cause mortality were identified by linkage to the nationwide Danish registries. We determined the proportion of patients with IHD or MI who were eligible for novel preventive therapies by meeting enrollment criteria (inclusion/exclusion criteria) in RCTs. Further, we assessed the proportion who met the criteria in several trials and by several drug classes simultaneously. Finally, we estimated the evidence-based potential for reducing major cardiovascular events over 5 years by each novel therapy according to trial criteria, assuming similar efficacy as in the RCTs. Stata, version 15.1 (StataCorp) was used and statistical significance was set at P < .05.
Results
The study consisted of 6292 patients with known IHD enrolled from November 2003 to February 2015, of whom 2277 had a previous MI. Baseline characteristics are shown in the Table. During a mean of 7.7 years of follow-up, 474 MIs, 456 strokes, 646 cardiovascular deaths, and 1410 all-cause deaths occurred.
Table. Baseline Characteristics of Patients With Known Ischemic Heart Disease or Prior Myocardial Infarction From the Copenhagen General Population Study and of Those Eligible for New Medication to Prevent Atherosclerotic Cardiovascular Disease According to Inclusion and Exclusion Criteria Used in 12 Different Trials.
| Characteristicsa | All | Eligibility According to Trial Criteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMPROVE-IT | PEGASUS | EMPA-REG | LEADER | SUSTAIN-6 | FOURIER | CANVAS | REVEAL | CANTOS | COMPASS | ODYSSEY | REDUCE-IT | ||
| Ischemic heart disease | |||||||||||||
| Individuals, No. | 6292 | 2004 | 1660 | 811 | 810 | 810 | 1727 | 818 | 845 | 924 | 4176 | 1664 | 977 |
| Sex, male, No. (%) | 3861 (61) | 1423 (71) | 1205 (73) | 559 (69) | 552 (68) | 552 (68) | 1291 (75) | 562 (69) | 689 (82) | 637 (69) | 2682 (63) | 1156 (69) | 743 (76) |
| Age, y | 69 (62-76) | 71 (64-78) | 74 (69-79) | 71 (65-77) | 71 (65-77) | 71 (65-77) | 70 (64-77) | 71 (65-77) | 72 (66-78) | 72 (65-79) | 74 (69-79) | 69 (62-77) | 70 (64-76) |
| Blood pressure, mm Hg | |||||||||||||
| Systolic | 144 (130-158) | 144 (130-158) | 144 (130-160) | 144 (130-158) | 144 (130-158) | 144 (130-158) | 142 (129-156) | 144 (130-158) | 140 (126-154) | 144 (130-159) | 145 (132-160) | 144 (130-159) | 142 (130-157) |
| Diastolic | 82 (75-90) | 81 (74-89) | 80 (72-88) | 80 (71-86) | 80 (71-86) | 80 (71-86) | 80 (73-88) | 80 (71-86) | 79 (71-85) | 80 (72-89) | 80 (73-89) | 82 (75-90) | 80 (73-88) |
| Cholesterol, mg/dL | |||||||||||||
| Total cholesterol | 190 (163-224) | 178 (159-209) | 174 (151-205) | 166 (143-197) | 166 (143-197) | 166 (143-197) | 166 (147-189) | 166 (143-197) | 139 (128-147) | 178 (151-213) | 186 (159-217) | 190 (170-217) | 166 (151-182) |
| HDL cholesterol | 55 (44-69) | 53 (43-66) | 53 (43-66) | 48 (39-61) | 48 (39-61) | 47 (39-61) | 51 (41-64) | 48 (39-61) | 46 (38-56) | 49 (40-63) | 56 (44-70) | 53 (43-66) | 45 (37-56) |
| LDL cholesterol | 101 (76-130) | 93 (74-116) | 86 (67-112) | 77 (61-104) | 77 (60-104) | 77 (60-104) | 81 (66-101) | 77 (61-104) | 62 (50-73) | 93 (70-120) | 93 (72-124) | 101 (85-124) | 74 (62-85) |
| Triglycerides | 137 (96-202) | 139 (96-203) | 139 (97-205) | 168 (112-245) | 169 (112-251) | 169 (112-251) | 139 (97-207) | 168 (112-251) | 124 (89-180) | 153 (112-224) | 136 (96-198) | 142 (100-205) | 210 (175-274) |
| C-reactive protein, mg/L | 1.6 (1.0-2.9) | 1.6 (1.0-3.1) | 1.7 (1.1-3.4) | 1.8 (1.1-3.5) | 1.8 (1.1-3.7) | 1.8 (1.1-3.7) | 1.5 (1.0-2.8) | 1.8 (1.1-3.6) | 1.5 (1.0-3.1) | 3.8 (2.6-5.9) | 1.7 (1.1-3.2) | 1.6 (1.0-3.0) | 1.6 (1.1-2.9) |
| Current smokers, No. (%) | 984 (16) | 364 (18) | 269 (16) | 115 (14) | 113 (14) | 114 (14) | 289 (17) | 118 (14) | 126 (15) | 206 (22) | 593 (14) | 322 (19) | 134 (14) |
| Diabetes, No. (%) | 830 (13) | 274 (14) | 334 (21) | 811 (100) | 810 (100) | 810 (100) | 309 (18) | 818 (100) | 316 (38) | 168 (18) | 747 (17) | 195 (12) | 275 (28) |
| Statin use, No. (%) | 3535 (56) | 1455 (73) | 1245 (75) | 642 (79) | 646 (80) | 646 (80) | 1727 (100) | 648 (79) | 785 (93) | 629 (68) | 2633 (62) | 1097 (66) | 977 (100) |
| Myocardial Infarction | |||||||||||||
| Individuals, No. | 2277 | 2004 | 1660 | 332 | 334 | 334 | 1624 | 334 | 629 | 924 | 1608 | 1664 | 474 |
| Sex, male, No. (%) | 1637 (72) | 1423 (71) | 1205 (73) | 256 (77) | 255 (76) | 255 (76) | 1221 (75) | 257 (77) | 528 (84) | 637 (69) | 1189 (73) | 1156 (69) | 387 (82) |
| Age, y | 70 (63-77) | 71 (64-78) | 74 (69-79) | 71 (66-76) | 71 (66-77) | 71 (66-77) | 70 (63-77) | 71 (66-76) | 72 (66-78) | 72 (65-79) | 74 (69-79) | 69 (62-77) | 70 (63-76) |
| Blood pressure, mm Hg | |||||||||||||
| Systolic | 142 (130-157) | 144 (130-158) | 144 (130-160) | 144 (130-156) | 144 (130-156) | 144 (130-156) | 142 (129-156) | 144 (130-156) | 140 (125-152) | 144 (130-159) | 144 (130-160) | 144 (130-159) | 141 (130-156) |
| Diastolic | 80 (73-89) | 81 (74-89) | 80 (72-88) | 79 (70-86) | 79 (70-86) | 79 (70-86) | 80 (73-88) | 79 (70-86) | 79 (70-85) | 80 (72-89) | 80 (72-88) | 82 (75-90) | 80 (72-88) |
| Cholesterol, mg/dL | |||||||||||||
| Total cholesterol | 174 (151-209) | 178 (159-209) | 174 (151-205) | 163 (143-190) | 163 (139-190) | 163 (139-190) | 166 (147-190) | 163 (143-190) | 139 (128-147) | 178 (151-213) | 174 (151-205) | 190 (170-217) | 163 (147-178) |
| HDL cholesterol | 52 (42-65) | 53 (43-66) | 53 (43-66) | 47 (38-59) | 46 (38-59) | 46 (38-59) | 51 (41-63) | 46 (38-59) | 46 (38-59) | 49 (40-63) | 53 (42-66) | 53 (43-66) | 44 (36-54) |
| LDL cholesterol | 89 (70-116) | 93 (74-116) | 86 (67-112) | 76 (58-102) | 75 (58-101) | 76 (58-101) | 81 (66-101) | 76 (58-102) | 63 (51-73) | 93 (70-120) | 85 (67-112) | 101 (85-124) | 74 (62-85) |
| Triglycerides | 140 (97-206) | 139 (96-203) | 139 (97-205) | 174 (118-245) | 174 (118-248) | 174 (118-248) | 140 (97-207) | 174 (118-249) | 121 (89-174) | 153 (112-224) | 139 (97-204) | 142 (100-205) | 214 (177-277) |
| C-reactive protein, mg/L | 1.6 (1.0-3.0) | 1.6 (1.0-3.1) | 1.7 (1.1-3.4) | 1.9 (1.1-3.9) | 1.9 (1.1-3.9) | 1.9 (1.1-3.9) | 1.5 (1.0-2.7) | 1.9 (1.1-3.9) | 1.5 (1.0-3.1) | 3.8 (2.6-5.9) | 1.7 (1.1-3.3) | 1.6 (1.0-3.0) | 1.7 (1.1-3.0) |
| Current smokers, No. (%) | 412 (18) | 364 (18) | 269 (16) | 63 (19) | 62 (19) | 62 (19) | 278 (17) | 64 (19) | 107 (17) | 206 (22) | 276 (17) | 322 (19) | 80 (17) |
| Diabetes, No. (%) | 338 (15) | 274 (14) | 334 (21) | 330 (100) | 334 (100) | 334 (100) | 284 (17) | 334 (100) | 133 (21) | 168 (18) | 308 (21) | 195 (12) | 119 (25) |
| Statin use, No. (%) | 1671 (74) | 1455 (73) | 1245 (75) | 284 (86) | 287 (86) | 287 (86) | 1624 (100) | 286 (86) | 591 (94) | 629 (68) | 1228 (75) | 1097 (66) | 474 (100) |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert HDL and LDL cholesterol to mmol/L, multiply by 0.0259; C-reactive protein to nmol/L, multiply by 9.524; triglycerides to mmol/L, multiply by 0.0113.
Baseline characteristics are presented as proportions for categorical variables and as medians (interquartile range) for continuous variables.
Trial Eligibility
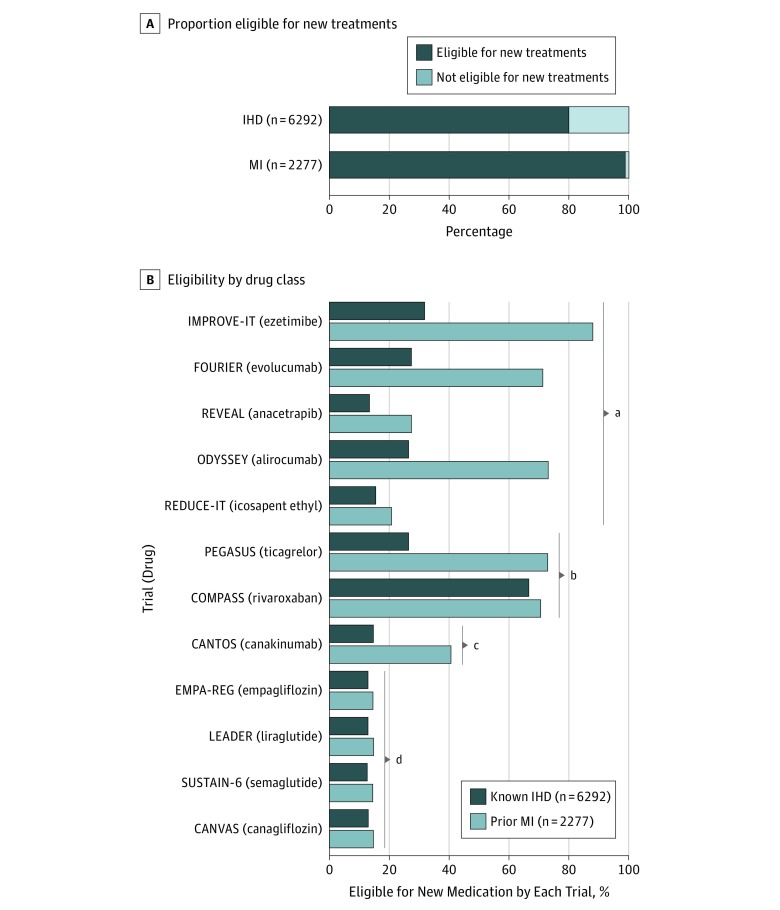
In patients with known IHD or previous MI, 5036 of 6292 (80%) and 2273 of 2277 (99%), respectively, were eligible to receive at least 1 of the new preventive therapies by meeting enrollment criteria in 1 or more RCTs (Figure 1). Eligibility was highest with the COMPASS criteria and lowest with the studies of antidiabetic drugs (Figure 1).
Figure 1. Eligibility for Novel Therapies.
Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death in patients with known ischemic heart disease (IHD) or prior myocardial infarction (MI) from a contemporary general population. A, Proportion eligible to receive at least 1 new secondary prevention medication. B, Eligibility by each trial individually ordered by drug classes. Data are based on individuals in the Copenhagen General Population Study.
aLipid-modifying.
bAntithrombotic.
cAnti-inflammatory.
dAntidiabetic.
There was a large overlap in trial eligibility. Thus, 2304 of 6292 patients with IHD (37%) and 1830 of 2277 patients with MI (80%) were eligible to receive 4 or more of the new therapies simultaneously (eFigure 1 in the Supplement). Assessing eligibility according to the 4 drug classes, 2594 of 6292 patients with IHD (41%) and 1834 of 2277 patients with prior MI (81%) qualified to receive therapies from 2 or more drug classes simultaneously (eFigure 1 in the Supplement).
Outcomes in Trial-Eligible Patients
As shown in eTable 2 in the Supplement, trial-eligible patients experienced slightly higher rates of major events than the overall IHD or MI groups. The rates of major cardiovascular events in trial-eligible CGPS patients compared with actual trial patients were similar for most of the RCTs (eFigure 2 in the Supplement).
Potential Event Prevention
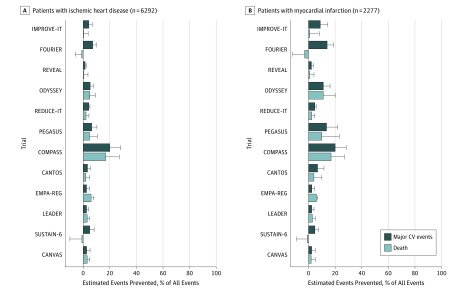
The 5-year estimated percentage of major cardiovascular events or deaths that could be prevented by each new therapy varied from −3% to 20% (Figure 2 and eTables 3 and 4 in the Supplement). For example, the maximum potential reduction of events was 20% for major cardiovascular events with COMPASS evidence among patients with previous MI.
Figure 2. Potential Evidence-Based Prevention of Major Cardiovascular Events and All-Cause Deaths by Each Trial.
A, Potential prevention of events in patients with ischemic heart disease at baseline. B, Potential prevention of events in patients with prior myocardial infarction. Based on patients from the Copenhagen General Population Study. Major cardiovascular (CV) events is the composite end point of myocardial infarction, stroke, and cardiovascular death.
Sensitivity Analysis
In sensitivity analyses, we followed the time criteria used in the RCTs. As shown in eTables 5 through 8 and eFigures 3 through 5 in the Supplement, the results were similar to the main analyses.
Excluding patients with diabetes also yielded similar results; 4206 of 5462 patients with IHD (77%) and 1935 of 1939 patients with MI (99%) were eligible to receive novel therapies (eFigures 6 and 7 in the Supplement). Allowing eligibility for multiple lipid-modifying drugs in combination with only 1 drug from each of the other 3 drug classes, 2896 of 6292 patients with IHD (46%) and 2210 of 2277 patients with MI (97%) MI qualified to receive 2 or more drugs simultaneously (eFigure 8 in the Supplement).
Discussion
We found that for most patients with known IHD or MI there is RCT-based evidence that adding novel preventive therapies may reduce rates of recurrent ASCVD events or death. Notably, many patients with IHD or MI met the criteria for several different drug classes simultaneously, indicating that they could be candidates for receiving several new drugs.
To our knowledge, before 2015, practically no new pharmacological therapies beyond blood pressure–lowering medications were successful in reducing residual ASCVD risk when added to standard care. The results from the 12 RCTs therefore represent major steps forward for secondary prevention by showing that residual risk can be reduced by targeting distinct biological pathways in lipid metabolism, coagulation cascade, inflammation, or glucose homeostasis.1,2,3,4,5,6,7,8,9,10,11,12
Event rates of major cardiovascular events, the primary end point in most RCTs, were comparable between trial-eligible CGPS participants and actual trial patients. These observations are important, as a common critique of trials is that the results are not generalizable because of highly selected trial participants.14 Our results align with a report from the REACH Registry showing a high rate of eligibility for COMPASS among patients with known ASCVD.15
The improved outcomes observed with these different treatment paradigms will have future implications for treating secondary prevention patients. However, the overlap in drug eligibility presents a challenge for their appropriate implementation. The challenge will be to personalize treatment on a case-by-case basis, choosing the intervention that provides the best trade-off between efficacy, safety, and cost for the individual patient. However, given the eligibility for multiple drugs in many patients, payers (ie, insurance companies) are likely to manage access to these medications closely.
Limitations
The limitations of our study should be considered. White individuals were studied, and extrapolation to other races/ethnicities should be done cautiously. We included patients with IHD or MI; however, some of the therapies were also tested in primary prevention patients while others (ie, ezetimibe) are routinely used in this setting. Finally, our estimations for event reductions are based on modeling and extrapolating results from RCTs. While reasonable, it should be emphasized that the preventive potential estimates depend on how many patients are eligible for treatment and the treatment effect. A strength of the study is that the results originate from a contemporary population with no losses to follow-up. This is essential for a valid assessment of the potential association of therapies with ASCVD prevention.
Conclusions
For most patients with IHD and nearly all with prior MI, there is evidence from recent RCTs that treatment with novel preventive therapies added to standard care will reduce the risk of recurrent ASCVD events. Our results indicate opportunities for improved evidence-based secondary prevention. However, there is a clear need for prioritizing the use of the new preventive therapies that considers the overlap of RCT evidence, preventive effectiveness, and costs.
eMethods. Search strategy for Medline (using PubMed)
eTable 1. Main inclusion criteria used in the 12 randomized controlled trials for prevention of atherosclerotic cardiovascular disease.
eTable 2. Event rates for major cardiovascular events, myocardial infarction, stroke and all-cause mortality per 1000 person-years among trial-eligible individuals and in the total Copenhagen General Population Study cohort.
eTable 3. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with ischemic heart disease by each trial.
eTable 4. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with prior myocardial infarction by each trial.
eTable 5. Baseline characteristics of patients with ischemic heart disease or prior myocardial infarction from the Copenhagen General Population Study and of those eligible for new medication to prevent atherosclerotic cardiovascular disease according to inclusion and exclusion criteria used in ten different trials
eTable 6. Event rates for myocardial infarction, stroke and all-cause mortality per 1000 person-years among trial-eligible individuals and in the total Copenhagen General Population Study cohort
eTable 7. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with ischemic heart disease by each trial
eTable 8. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with prior myocardial infarction by each trial
eFigure 1. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death by number of trials or drug classes simultaneously
eFigure 2. Comparison of event rate per 1000 person-years of major cardiovascular events in trial-eligible Copenhagen General Population Study participant and actual patients from the randomized controlled trials.
eFigure 3. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death in patients with known ischemic heart disease or prior myocardial infarction from a contemporary general population
eFigure 4. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death by number of trials or drug classes simultaneously
eFigure 5. Potential evidence-based prevention of major cardiovascular events, myocardial infarctions, strokes and all-cause mortality by each trial
eFigure 6. Eligibility by each trial individually in individuals without diabetes ordered by drug classes.
eFigure 7. Eligibility by number of trials or drug classes simultaneously in individuals without diabetes.
eFigure 8. Eligibility for number of drug classes simultaneously while allowing for multiple lipid-modifying drugs
eFigure 9. Eligibility by number of drug classes simultaneously while allowing for multiple lipid-modifying drugs but only drug from each of the other classes.
References
- 1.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 2.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators . Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. doi: 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 8.Bowman L, Hopewell JC, Chen F, et al. ; HPS3/TIMI55–REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217-1227. doi: 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2018;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 13.Mortensen MB, Nordestgaard BG. Comparison of five major guidelines for statin use in primary prevention in a contemporary general population. Ann Intern Med. 2018;168(2):85-92. doi: 10.7326/M17-0681 [DOI] [PubMed] [Google Scholar]
- 14.Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233-1240. doi: 10.1001/jama.297.11.1233 [DOI] [PubMed] [Google Scholar]
- 15.Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39(9):750-757a. doi: 10.1093/eurheartj/ehx658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search strategy for Medline (using PubMed)
eTable 1. Main inclusion criteria used in the 12 randomized controlled trials for prevention of atherosclerotic cardiovascular disease.
eTable 2. Event rates for major cardiovascular events, myocardial infarction, stroke and all-cause mortality per 1000 person-years among trial-eligible individuals and in the total Copenhagen General Population Study cohort.
eTable 3. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with ischemic heart disease by each trial.
eTable 4. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with prior myocardial infarction by each trial.
eTable 5. Baseline characteristics of patients with ischemic heart disease or prior myocardial infarction from the Copenhagen General Population Study and of those eligible for new medication to prevent atherosclerotic cardiovascular disease according to inclusion and exclusion criteria used in ten different trials
eTable 6. Event rates for myocardial infarction, stroke and all-cause mortality per 1000 person-years among trial-eligible individuals and in the total Copenhagen General Population Study cohort
eTable 7. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with ischemic heart disease by each trial
eTable 8. Estimated major cardiovascular events, myocardial infarctions, strokes and deaths prevented in 5 years among individuals with prior myocardial infarction by each trial
eFigure 1. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death by number of trials or drug classes simultaneously
eFigure 2. Comparison of event rate per 1000 person-years of major cardiovascular events in trial-eligible Copenhagen General Population Study participant and actual patients from the randomized controlled trials.
eFigure 3. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death in patients with known ischemic heart disease or prior myocardial infarction from a contemporary general population
eFigure 4. Eligibility for novel therapies to prevent atherosclerotic cardiovascular disease and death by number of trials or drug classes simultaneously
eFigure 5. Potential evidence-based prevention of major cardiovascular events, myocardial infarctions, strokes and all-cause mortality by each trial
eFigure 6. Eligibility by each trial individually in individuals without diabetes ordered by drug classes.
eFigure 7. Eligibility by number of trials or drug classes simultaneously in individuals without diabetes.
eFigure 8. Eligibility for number of drug classes simultaneously while allowing for multiple lipid-modifying drugs
eFigure 9. Eligibility by number of drug classes simultaneously while allowing for multiple lipid-modifying drugs but only drug from each of the other classes.