This randomized clinical trial examines the efficacy and safety of lumateperone for the short-term treatment of adults with schizophrenia.
Key Points
Question
Does 60 mg of lumateperone tosylate (42 mg of lumateperone) significantly reduce symptoms of schizophrenia compared with placebo without relevant motor, cardiometabolic, or endocrine adverse effects?
Findings
In this randomized clinical trial of 450 patients with acute exacerbation of schizophrenia, 42 mg of lumateperone demonstrated statistically significant differences in reducing symptoms of schizophrenia without treatment-emergent motor, cardiometabolic, or endocrine adverse effects compared with placebo.
Meaning
Lumateperone is a potential treatment for schizophrenia and has a favorable safety profile.
Abstract
Importance
Individuals living with schizophrenia are affected by cardiometabolic, endocrine, and motor adverse effects of current antipsychotic medications. Lumateperone is a serotonin, dopamine, and glutamate modulator with the potential to treat schizophrenia with few adverse effects.
Objective
To examine the efficacy and safety of lumateperone for the short-term treatment of schizophrenia.
Design, Setting, and Participants
This randomized, double-blind, placebo-controlled, phase 3 clinical trial was conducted from November 13, 2014, to July 20, 2015, with data analyses performed from August 13 to September 15, 2015. Patients with schizophrenia who were aged 18 to 60 years and were experiencing an acute exacerbation of psychosis were enrolled from 12 clinical sites in the United States.
Interventions
Patients were randomized 1:1:1 (150 patients in each arm) to receive lumateperone tosylate, 60 mg; lumateperone tosylate, 40 mg (equivalent to 42 or 28 mg, respectively, of the active moiety lumateperone); or placebo once daily for 4 weeks.
Main Outcomes and Measures
The prespecified primary efficacy end point was mean change from baseline to day 28 in the Positive and Negative Syndrome Scale (PANSS) total score vs placebo. The key secondary efficacy measure was the Clinical Global Impression–Severity of Illness (CGI-S) score. The PANSS subscale scores, social function, safety, and tolerability were also assessed.
Results
The study comprised 450 patients (mean [SD] age, 42.4 [10.2] years; 346 [77.1%] male; mean [SD] baseline PANSS score, 89.8 [10.3]; mean [SD] baseline CGI-S score, 4.8 [0.6]). In the prespecified modified intent-to-treat efficacy analysis (n = 435), 42 mg of lumateperone met the primary and key secondary efficacy objectives, demonstrating a statistically significant improvement vs placebo from baseline to day 28 on the PANSS total score (least-squares mean difference [LSMD], −4.2; 95% CI, −7.8 to −0.6; P = .02; effect size [ES], −0.3) and the CGI-S (LSMD, −0.3; 95% CI, −0.5 to −0.1; P = .003; ES, −0.4). For 28 mg of lumateperone, the LSMD from baseline to day 28 was −2.6 (95% CI, −6.2 to 1.1; P = .16; ES, −0.2) on the PANSS total score and −0.2 (95% CI, −0.5 to 0.0; P = .02; ES, −0.3) on the CGI-S. Both lumateperone doses were well tolerated without clinically significant treatment-emergent motor adverse effects or changes in cardiometabolic or endocrine factors vs placebo.
Conclusions and Relevance
Lumateperone demonstrated efficacy for improving the symptoms of schizophrenia and had a favorable safety profile.
Trial Registration
ClinicalTrials.gov identifier: NCT02282761
Introduction
Schizophrenia is a frequently chronic and debilitating disorder that affects approximately 1% of the general population.1 According to the recent Global Burden of Disease study,2 which used disability-adjusted life-years, schizophrenia had the highest functional burden among 235 physical and mental health states. Although current antipsychotic therapy is often effective for improving positive symptoms associated with schizophrenia (eg, hallucinations and delusions), efficacy is limited for negative symptoms, cognitive impairment, and social functioning.1 In addition, current treatments are associated with substantial adverse effects, including motor impairments, prolactin abnormalities, weight gain, metabolic disturbances, and cardiovascular risk factors3,4; these effects add to the already increased morbidity and mortality associated with schizophrenia.5,6,7,8
Lumateperone (lumateperone tosylate, ITI-007) is a mechanistically novel investigational agent for schizophrenia.9 The mechanism of action of lumateperone is unique because it simultaneously modulates serotonin, dopamine, and glutamate neurotransmission, the key neurotransmitters implicated in serious mental illness.9 Specifically, lumateperone acts as a potent serotonin 5-HT2A receptor antagonist, a dopamine D2 receptor presynaptic partial agonist and postsynaptic antagonist,10 a D1 receptor–dependent modulator of glutamate, and a serotonin reuptake inhibitor.9 In addition, lumateperone lacks interaction with off-target receptors that may contribute to the adverse effects of other antipsychotic drugs.9,11 Lumateperone is rapidly absorbed, with an effective half-life of 13 to 21 hours for lumateperone and metabolites, supporting once-daily administration.9 This randomized, double-blind, placebo-controlled, phase 3 clinical trial evaluated the efficacy and safety of lumateperone therapy after 4 weeks of treatment in patients experiencing an acute exacerbation of schizophrenia.
Methods
Patients
This randomized clinical trial was conducted from November 13, 2014, to July 20, 2015. Data analysis was performed from August 13 to September 15, 2015. Patients were recruited at 12 US clinical sites and admitted to an inpatient research unit for a screening period of 2 to 7 days before randomization (trial protocol Supplement 1). The trial was conducted in compliance with the principles of the Good Clinical Practice guideline and was approved by the Copernicus Group institutional review board. All participating patients provided written informed consent, and data were deidentified. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Eligible participants were aged 18 to 60 years and had a clinical diagnosis of schizophrenia according to the DSM-5,12 confirmed by the Structured Clinical Interview for DSM-IV-TR Axis I disorders, clinical trials version13 (modified for use in this study).14 Patients were included if they were experiencing an acute exacerbation of psychosis, defined as a total score on the Brief Psychiatric Rating Scale15 of 40 or higher, with a score of 4 or higher on 2 or more positive symptoms (with a 7-point Likert scale with 1 indicating not present and 7 indicating extremely severe), and onset of the acute episode within 4 weeks of screening. Patients were required to have a score of 4 or higher, indicating moderate to severe disease severity, on the Clinical Global Impression–Severity of Illness (CGI-S)16 at screening and baseline. Severity of illness was confirmed at baseline by a Positive and Negative Syndrome Scale (PANSS)17 total score of 70 or higher, indicating moderate to extreme symptoms of schizophrenia. Patients had to have a previous response to antipsychotic therapy. Additional inclusion and exclusion criteria are given in the eMethods in Supplement 2.
Design, Intervention, and Randomization
In this randomized, 4-week, double-blind, placebo-controlled, inpatient trial, patients were randomized centrally across sites in a 1:1:1 ratio to 1 of 3 treatments administered orally once daily in the morning: 42 mg of lumateperone (equivalent to 60 mg of lumateperone tosylate), 28 mg of lumateperone (equivalent to 40 mg of lumateperone tosylate), or placebo. Lumateperone is the active free base form of the drug administered orally as a tosylate salt (lumateperone tosylate, also known as ITI-007) in capsule form. Central randomization occurred via an automated telephone or web-based system, with a unique randomization number assigned to each patient for use in electronic case report forms. Immediately after the 4-week study treatment period or at early discontinuation, patients were stabilized with standard-of-care treatment for 5 days in preparation for discharge. A follow-up safety assessment was performed approximately 2 weeks after the last dose of study medication.
Measures and Procedures
Primary and key secondary efficacy measures were assessed weekly by remote structured clinical interviews conducted via secure videoconferencing (MedAvante Inc). Highly trained centralized raters, independent of the study sites and blinded to treatment and study visit, were used to minimize baseline score inflation.18,19 Additional secondary efficacy end points and safety measures were assessed at each site by trained raters. The primary efficacy end point was the mean change from baseline to day 28 on the PANSS total score vs placebo. The key secondary efficacy end point was the CGI-S score. Other secondary efficacy measures included the PANSS positive, negative, and general psychopathology subscales, the Personal and Social Performance (PSP) scale,20 the PANSS-derived prosocial factor (P3, P6, N2, N4, N7, and G16),21 and the Calgary Depression Scale for Schizophrenia.22
Safety was assessed by treatment-emergent adverse events (TEAEs), modified physical examinations, 12-lead electrocardiograms (ECGs), vital signs, and clinical laboratory tests (eMethods in Supplement 2). Motor tolerability and safety were assessed by the Simpson-Angus Scale,23 Barnes Akathisia Rating Scale,24 and Abnormal Involuntary Movement Scale.16 Suicidality was evaluated by the Columbia Suicide Severity Rating Scale.25
Statistical Analysis
In each treatment arm, 132 patients were expected to have evaluable data. The study was designed to have 90% power to demonstrate an effect size of 0.4, corresponding to a 6-point difference in change from baseline to day 28 in PANSS total score between active treatment and placebo, at a 2-sided significance level of .05.
The treatment effect on the primary efficacy end point was evaluated using a mixed-effects model for repeated measures (MMRM). A mixture-based gatekeeping procedure26,27,28 was implemented to preserve the type I error rate at the 2-sided .05 level for the multiple-dose group comparisons of the primary and key secondary efficacy end points. All prespecified efficacy analyses were performed for a prespecified modified intent-to-treat analysis set, which includes all randomized patients who received at least 1 dose of study medication and had a valid baseline and at least 1 valid postdose assessment. See additional statistical analysis information in the eMethods in Supplement 2.
Safety results were summarized descriptively by treatment group and visit (when applicable). The incidences of clinical laboratory tests, vital signs, and ECG results that met predefined markedly abnormal criteria were summarized. Selected safety end points (fasting total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, glucose, insulin, triglycerides, and prolactin levels) for treatment groups were compared with placebo using MMRM whenever applicable and analysis of covariance for variables that were measured only twice during the study (once at screening or baseline and once after baseline). SAS statistical software, version 9.4 or higher (SAS Institute Inc) was used for the statistical analyses.
Results
Patients
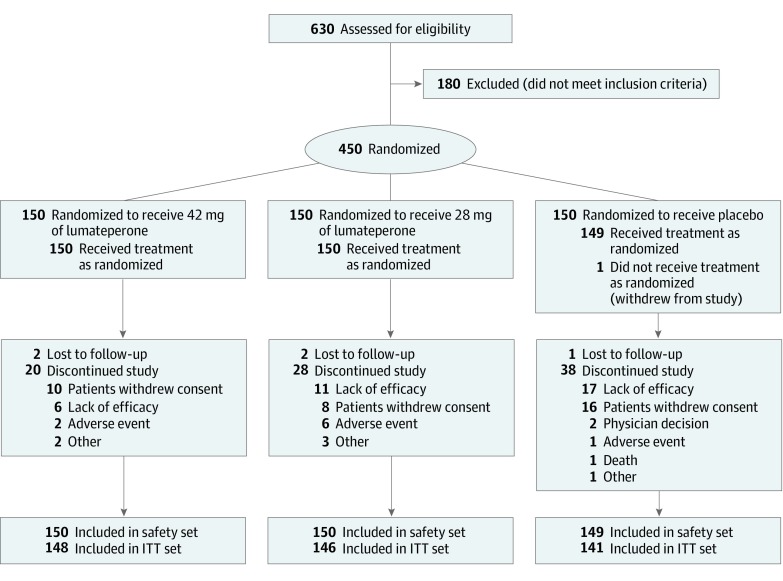
Of 630 patients screened, 450 (71.4%) were randomized to treatment (mean [SD] age, 42.4 [10.2] years; 346 [77.1%] male; mean [SD] baseline PANSS score, 89.8 [10.3]; mean [SD] baseline CGI-S score, 4.8 [0.6]); 150 patients were randomized to each treatment arm. A total of 449 were included in the safety population, and 435 were included in the efficacy intent-to-treat population (Figure 1 and eTable 1 in Supplement 2). Altogether, 366 patients (81.3%) completed the 4-week treatment, and 359 (79.8%) completed the study (Figure 1). Study completion rates were 85.3% in the 42 mg of lumateperone group, 80.0% in the 28 mg of lumateperone group, and 74.0% in the placebo group. Overall, withdrawal of consent and lack of efficacy were the most common reasons for study discontinuation, which was higher in the placebo group (16 withdrew consent and 17 discontinued because of lack of efficacy) than in the 42 mg of lumateperone group (10 withdrew consent and 6 discontinued because of lack of efficacy) and 28 mg of lumateperone group (8 withdrew consent and 11 discontinued because of lack of efficacy). The median time to discontinuation for any reason was 15 days (range, 1-23 days) for the 42 mg of lumateperone group, 13 days (range, 2-26 days) for the 28 mg of lumateperone group, and 13 days (range, 2-24 days) for placebo, with the difference between the 42 mg of lumateperone and placebo groups being statistically significant (P = .006).
Figure 1. Study Flow and Patient Disposition.
ITT indicates intent-to-treat.
Baseline demographic and clinical characteristics were similar across groups (Table 1). The median time since diagnosis of schizophrenia was 15.0 years (range, <1 to 46 years). There were no meaningful between-group differences in prior medication use at study entry (eTable 2 in Supplement 2).
Table 1. Demographic and Baseline Characteristicsa.
| Characteristic | Lumateperone, 42 mg (n = 150) | Lumateperone, 28 mg (n = 150) | Placebo (n = 149) | Total (N = 449) |
|---|---|---|---|---|
| Demographic characteristics of safety population | ||||
| Male | 110 (73.3) | 113 (75.3) | 123 (82.6) | 346 (77.1) |
| Age, mean (SD), y | 42.4 (10.3) | 43.5 (10.1) | 41.4 (10.3) | 42.4 (10.2) |
| Age ≤40 y | 62 (41.3) | 56 (37.3) | 71 (47.7) | 189 (42.1) |
| Race/ethnicity | ||||
| Black | 108 (72.0) | 94 (62.7) | 96 (64.4) | 298 (66.4) |
| White | 33 (22.0) | 42 (28.0) | 42 (28.2) | 117 (26.1) |
| Other | 9 (6.0) | 14 (9.3) | 11 (7.4) | 34 (7.6) |
| Non-Hispanic/Latino | 137 (91.3) | 133 (88.7) | 135 (90.6) | 405 (90.2) |
| Weight, mean (SD), kg | 86.3 (17.0) | 85.8 (16.7) | 85.9 (17.0) | 86.0 (16.9) |
| BMI, mean (SD) | 28.7 (5.4) | 28.4 (5.1) | 28.2 (5.3) | 28.4 (5.3) |
| Baseline efficacy characteristics in the ITT population | 148 | 146 | 141 | 435 |
| Time since initial schizophrenia diagnosis, mean (SD), y | 16.5 (10,4) | 17.0 (10.6) | 17.4 (10.6) | 17.0 (10.5) |
| PANSS total score, mean (SD) | 90.1 (9.5) | 89.3 (10.2) | 90.1 (11.1) | 89.8 (10.3) |
| PANSS positive symptom subscale score, mean (SD) | 26.0 (3.5) | 25.8 (3.9) | 25.8 (3.9) | 25.9 (3.8) |
| PANSS negative symptom subscale score, mean (SD) | 20.6 (3.8) | 20.4 (4.2) | 21.0 (4.4) | 20.7 (4.1) |
| PANSS general psychopathology subscale score, mean (SD) | 43.5 (6.1) | 43.1 (6.0) | 43.2 (6.3) | 43.3 (6.1) |
| PANSS prosocial subscale score, mean (SD) | 25.0 (3.4) | 24.5 (3.5) | 24.3 (3.3) | 24.6 (3.4) |
| Central CGI-S score, mean (SD) | 4.8 (0.5) | 4.7 (0.6) | 4.8 (0.6) | 4.8 (0.6) |
| Site CGI-S score, mean (SD) | 4.8 (0.6) | 4.8 (0.6) | 4.8 (0.6) | 4.8 (0.6) |
| BPRS score, mean (SD) | 53.7 (7.4) | 53.3 (7.2) | 54.2 (7.7) | 53.7 (7.4) |
| PSP scale score, mean (SD) | 47.8 (11.9) | 48.2 (12.2) | 47.7 (12.4) | 47.9 (12.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); BPRS, Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impression–Severity of illness; ITT, intent-to-treat; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance.
Data are presented as number (percentage) of study participants unless otherwise indicated.
Efficacy
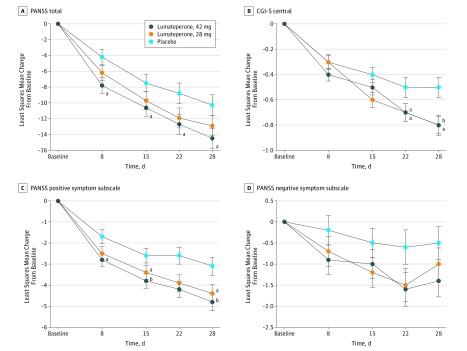
Treatment with 42 mg of lumateperone demonstrated a statistically significant improvement in change from baseline to day 28 in PANSS total score vs placebo (least-squares mean difference [LSMD], −4.2; 95% CI, −7.8 to −0.6; effect size, −0.3; unadjusted P = .02; multiplicity-adjusted P = .04) (Table 2 and Figure 2A). The LSMD observed with 28 mg of lumateperone vs placebo was −2.6 (95% CI, −6.2 to 1.1; effect size, −0.2; nominal P = .16; multiplicity-adjusted P = .18). Sensitivity analyses using a pattern-mixture model and an analysis of covariance last observation carried forward confirmed the robustness of the result from the MMRM analysis for the change from baseline to day 28 in PANSS total score in the 42 mg of lumateperone group vs the placebo group (LSMD, −3.9 95% CI, −7.4 to −0.4; effect size, −0.3; nominal P = .03; and LSMD, −5.0; 95% CI, −8.5 to −1.5; effect size, −0.3; nominal P = .006, respectively) (eTable 3 in Supplement 2). Statistically significant differences from placebo in the PANSS total score were observed at the day 8 assessment and continued through the day 28 assessment with 42 mg of lumateperone (Figure 2A). Interaction analyses suggested consistent treatment effects for demographic subgroups of race (black vs not black), ethnicity (Hispanic/Latino vs not Hispanic/Latino), age (≤40 vs >40 years), and sex for the comparison of 42 mg of lumateperone vs placebo in PANSS total score. A responder analysis indicated that 54 patients (36.5%) treated with 42 mg of lumateperone, 53 (36.3%) treated with 28 mg of lumateperone, and 36 placebo-treated patients (25.5%) had 30% or greater improvement in PANSS total score (eTable 4 in Supplement 2).
Table 2. Efficacy End Point Measures (Intent-to-Treat Population).
| Measure | Lumateperone, 42 mg | Lumateperone, 28 mg | Placebo |
|---|---|---|---|
| PANSS total score, MMRM, No. | 148 | 146 | 141 |
| Change from baseline to day 28, mean (SE) | −15.6 (1.21) | −13.7 (1.22) | −12.4 (1.15) |
| Change from baseline to day 28, LS mean (SE) | −14.5 (1.3) | −12.9 (1.3) | −10.3 (1.3) |
| Difference from placebo, LS mean (95% CI) | −4.2 (−7.8 to −0.6) | −2.6 (−6.2 to 1.1) | NA |
| Effect size | −0.30 | −0.18 | NA |
| Unadjusted P valuea | .02 | .16 | NA |
| Multiplicity-adjusted P value | .04 | .18 | NA |
| CGI-S central score, MMRM, No. | 130 | 123 | 109 |
| Change from baseline to day 28, mean (SE) | −0.9 (0.08) | −0.8 (0.08) | −0.6 (0.08) |
| Change from baseline to day 28, LS mean (SE) | −0.8 (0.07) | −0.8 (0.08) | −0.5 (0.08) |
| Difference from placebo, LS mean (95% CI) | −0.3 (−0.5 to −0.1) | −0.2 (−0.5 to 0.0) | NA |
| Effect size | −0.39 | −0.30 | NA |
| Unadjusted P valuea | .003 | .03 | NA |
| Multiplicity adjusted P value | .04 | NA | NA |
| PANSS positive symptom subscale score, ANCOVA-LOCF, No. | 146 | 145 | 141 |
| Change from baseline to day 28, mean (SE) | −4.8 (0.45) | −4.4 (0.43) | −3.1 (0.43) |
| Change from baseline to day 28, LS mean (SE) | −4.8 (0.42) | −4.4 (0.42) | −3.1 (0.43) |
| Difference from placebo, LS mean (95% CI) | −1.7 (−2.9 to −0.5) | −1.2 (−2.4 to −0.1) | NA |
| Effect size | −0.33 | −0.24 | NA |
| P valuea | .006 | .04 | NA |
| PANSS negative symptom subscale score, ANCOVA-LOCF, No. | 146 | 145 | 141 |
| Change from baseline to day 28, mean (SE) | −1.4 (0.41) | −0.9 (0.39) | −0.7 (0.45) |
| Change from baseline to day 28, LS mean (SE) | −1.4 (0.38) | −1.0 (0.38) | −0.5 (0.39) |
| Difference from placebo, LS mean (95% CI) | −0.9 (−2.0 to 0.2) | −0.5 (−1.6 to 0.6) | NA |
| Effect size | −0.20 | −0.11 | NA |
| P valuea | .09 | .36 | NA |
| PANSS general psychopathology subscale score, ANCOVA-LOCF, No. | 146 | 145 | 141 |
| Change from baseline to day 28, mean(SE) | −7.7 (0.73) | −6.3 (0.77) | −5.2 (0.70) |
| Change from baseline to day 28, LS mean (SE) | −7.6 (0.69) | −6.4 (0.69) | −5.2 (0.70) |
| Difference from placebo, LS mean (95% CI) | −2.4 (−4.3 to −0.5) | −1.2 (−3.1 to 0.7) | NA |
| Effect size | −0.29 | −0.14 | NA |
| P valuea | .01 | .22 | NA |
| PANSS-derived prosocial factor score,ANCOVA-LOCF, No.b | 146 | 145 | 141 |
| Change from baseline to day 28, mean (SE) | −4.9 (0.43) | −4.5 (0.42) | −3.4 (0.42) |
| Change from baseline to day 28, LS mean (SE) | −4.7 (0.39) | −4.5 (0.39) | −3.6 (0.40) |
| Difference from placebo, LS mean (95% CI) | −1.1 (−2.2 to 0) | −1.0 (−2.1 to 0.2) | NA |
| Effect size | −0.24 | −0.20 | NA |
| P valuea | .04 | .09 | NA |
| PSP, ANCOVA, No. | 128 | 121 | 109 |
| Change from baseline to day 28, mean (SE) | 10.9 (1.48) | 10.5 (1.15) | 7.8 (1.38) |
| Change from baseline to day 28, LS mean (SE) | 11.0 (1.13) | 10.5 (1.16) | 7.7 (1.22) |
| Difference from placebo, LS mean (95% CI) | 3.3 (0.1 to 6.6) | 2.9 (−0.4 to 6.2) | NA |
| Effect size | 0.26 | 0.23 | NA |
| P valuea | .05 | .09 | NA |
Abbreviations: ANCOVA, analysis of covariance; CGI-S, Clinical Global Impression–Severity of illness; LOCF, last observation carried forward; LS, least squares; MMRM, mixed-effects model for repeated measures; NA, not applicable; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance.
P values are nominal and unadjusted for multiplicity unless noted.
The PANSS-derived prosocial factor is composed of PANSS items P3 (hallucinatory behavior), P6 (suspiciousness), N2 (emotional withdrawal), N4 (passive-apathetic social withdrawal), N7 (stereotyped thinking), and G16 (active social avoidance).
Figure 2. Least-Squares Mean Change in Positive and Negative Syndrome Scale (PANSS) Total Score and Clinical Global Impression–Severity of Illness (CGI-S) and Mean Change in PANSS Positive and Negative Symptom Subscales.
Analysis of covariance last observation carried forward was used. P values are nominal and unadjusted for multiplicity. Whiskers represent SEs.
aP < .05 vs placebo.
bP < .01 vs placebo.
Individuals in the 42 mg of lumateperone group also met the key secondary end point with a statistically significant change in CGI-S score from baseline to day 28 vs placebo (LSMD, −0.3; 95% CI, −0.5 to −0.1; effect size, −0.4; unadjusted P = .003; multiplicity-adjusted P = .04); although there was no significant difference between the 28 mg of lumateperone group and the placebo group for the primary end point, the groups significantly differed on CGI-S (LSMD, −0.2; 95% CI, −0.5 to 0; effect size; −0.3; nominal P = .02) (Table 2 and Figure 2B). Treatment with 42 and 28 mg of lumateperone significantly improved the PANSS positive subscale score from baseline to day 28 compared with placebo (42 mg: LSMD, −1.7; 95% CI, −2.9 to −0.5; effect size, −0.3; nominal P = .006; and 28 mg: LSMD, −1.2; 95% CI, −2.4 to −0.1; effect size, −0.2; nominal P = .04) (Figure 2C); the changes in the PANSS negative subscale score from baseline to day 28 compared with placebo were not significant (Figure 2D). Statistically significant improvements vs placebo were observed with 42 mg of lumateperone in the general psychopathology subscale score and in psychosocial function (measured by the PANSS-derived prosocial factor and PSP scale) (general psychopathology subscale: LSMD, −2.4; 95% CI, −4.3 to −0.5; effect size, −0.3; nominal P = .01; PANSS-derived prosocial factor: LSMD, −1.1; 95% CI, −2.2 to 0.0; effect size, −0.2.; nominal P = .04; and PSP scale: LSMD, 3.3; 95% CI, 0.1 to 6.6; effect size, −0.3; nominal P = .05) (Table 2). Change in Calgary Depression Scale for Schizophrenia score from baseline to day 28 was not significantly different from that in the placebo group after treatment with 42 mg of lumateperone (LSMD, 0.4; 95% CI, −0.24 to 0.96; nominal P = .24) or 28 mg of lumateperone (LSMD, 0.2; 95% CI, −0.43 to 0.79; nominal P = .57).
Safety
Treatment-emergent adverse events occurred in 97 patients (64.7%) in the 42 mg of lumateperone group, 85 (56.7%) in the 28 mg of lumateperone group, and 75 (50.3%) in the placebo group (eTable 5 in Supplement 2). The TEAEs occurring in either lumateperone group in 5% or more of patients and more than 2 times the rate in the placebo group were somnolence (42 mg of lumateperone group, 26 [17.3%]; 28 mg of lumateperone group, 17 [11.3%]; and placebo, 6 [4.0%]), sedation (19 [12.7%] in the 42 mg of lumateperone group, 14 [9.3%] in the 28 mg of lumateperone group, and 8 [5.4%] in the placebo group), fatigue (8 [5.3%] in the 42 mg of lumateperone group, 7 [4.7%] in the 28 mg of lumateperone group, and 2 [1.3%] in the placebo group), and constipation (10 [6.7%] in the 42 mg of lumateperone group, 6 [4.0%] in the 28 mg of lumateperone group, and 4 [2.7%] in the placebo group) (eTable 5 in Supplement 2). Two patients experienced severe-intensity TEAEs and discontinued treatment: orthostatic hypotension (1 patient [0.7%] in the 42 mg of lumateperone group) and convulsions (1 patient [0.7%] in the 28 mg of lumateperone group; this patient had preexisting risk factors and relevant medical history regarding seizures; this TEAE was deemed serious). All other TEAEs were mild or moderate in intensity. One patient in the placebo group experienced a serious TEAE of asthma. Additional TEAEs leading to discontinuation by treatment arm were as follows: headache (2 [1.3%]) in the 42 mg of lumateperone group and schizophrenia (1 [0.7%]) in the placebo group. One placebo-treated patient (0.7%) died of an unknown cause 13 days after discontinuing the study.
No serious and unexpected drug reactions were reported during the study. There was no increase in suicidal ideation or behavior with lumateperone at either dose as measured by TEAEs or the Columbia Suicide Severity Rating Scale (eTable 6 in Supplement 2). No extrapyramidal symptoms (EPS)–related TEAEs occurred in 5% or more of patients in any treatment arm (eTable 5 in Supplement 2); EPS-related TEAEs were rare (eTable 7 in Supplement 2). Treatment with 42 or 28 mg of lumateperone was not associated with increased EPS as measured by the Simpson-Angus Scale, Barnes Akathisia Rating Scale, or Abnormal Involuntary Movement Scale (eTable 8 in Supplement 2). Three patients (2.0%) treated with 42 mg of lumateperone, 1 patient (0.7%) treated with 28 mg of lumateperone, and 4 patients (2.7%) in the placebo group used benztropine as a rescue medication for EPS during the 28-day treatment period (eTable 9 in Supplement 2).
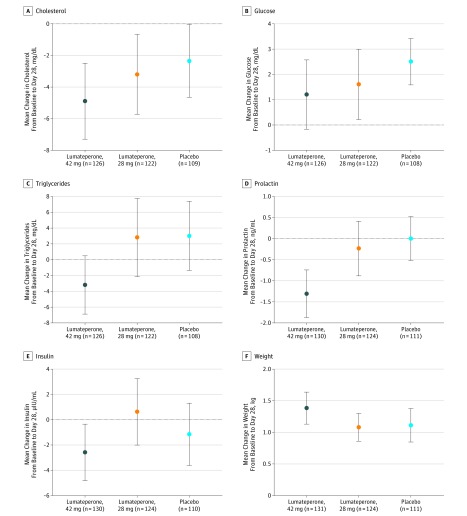
Mean change in weight from baseline to day 28 was similar in all treatment arms (Figure 3). Median change in weight from baseline to day 28 was 0.9 kg (range, −36 to 11 kg) for 42 mg of lumateperone, 0.6 kg (range, −12 to 13 kg) for 28 mg of lumateperone, and 0.7 kg (range, −12 to 16) for placebo. Weight changes of 7% or greater and shifts in body mass index from overweight to obese were infrequent and similar among groups (eFigure in Supplement 2). There were no significant mean changes in metabolic parameters from baseline to day 28 compared with placebo (Figure 3).
Figure 3. Change in Cholesterol, Glucose, Triglycerides, Prolactin, and Insulin Levels and Weight From Baseline.
Error bars indicate SE. SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; triglycerides to millimoles per liter, multiply by 0.0113; prolactin to nanograms per liter, multiply by 0.0435; and insulin to picomoles per liter, multiply by 6.945.
No clinically meaningful changes from baseline or differences observed in physical examination results, vital signs, or ECG findings were found between the lumateperone and placebo groups. One patient in the placebo group (before treatment) and 1 patient in the 28 mg of lumateperone group (before and after treatment) had a corrected QT interval by Fredericia greater than 450 milliseconds. No patients had a corrected QT interval by Fredericia greater than 500 milliseconds or a change greater than 60 milliseconds from baseline.
Discussion
Treatment with 42 mg of lumateperone compared with placebo significantly improved symptoms in patients with acute exacerbation of schizophrenia without causing many of the adverse effects commonly observed with currently available antipsychotics.3,4 These findings support the results of a previous 4-week study that found that treatment with 42 mg of lumateperone (60 mg of lumateperone tosylate) was effective in treating schizophrenia symptoms compared with placebo and had an effect size (0.4) for reduction in PANSS total symptoms similar to that of 4 mg of risperidone.29 The effect sizes for PANSS total (0.30) and CGI-S (0.39) reduction in this study are broadly comparable with standard of care and, especially, newly approved antipsychotic effect sizes for overall reduction in symptoms (PANSS total), including brexpiprazole (0.26), cariprazine (0.34), and lurasidone (0.36).4,30,31
Treatment with 42 mg of lumateperone significantly improved symptoms, with significant reductions beginning at the first week and maintained throughout treatment. A responder analysis indicated that 54 patients (36.5%) who received 42 mg of lumateperone improved on the PANSS total score by 30% or more compared with 36 patients (25.5%) who received placebo (number needed to treat of 9.1). Furthermore, patients treated with 42 mg of lumateperone had significant improvement across a broad range of PANSS subscales and on the CGI-S, consistent with clinical meaningfulness of the improvement.
A higher dose (84 mg) of lumateperone (equivalent to 120 mg of lumateperone tosylate) was not effective in a previous study29; therefore, a lower dose of lumateperone (28 mg) was explored in this study. Although patients treated with 28 mg of lumateperone did not have a statistically significant decrease in the PANSS total score, decreases on the PANSS positive symptom subscale and on the CGI-S outperformed placebo, establishing a dose-response curve for the efficacy of lumateperone.29
The improvement in psychosocial function is a highly desired yet often unrealized outcome in patients with schizophrenia.1 Improvements in psychosocial function were suggested by significant improvements assessed by the site-rated PSP and the centrally rated PANSS-derived prosocial factor, which was previously identified via factor analysis in patients with schizophrenia and mood disorders21 and has been used to assess other antipsychotics.32,33 The 6-item PANSS-derived prosocial factor includes PANSS items of active social avoidance, passive social withdrawal, and emotional withdrawal, 3 symptoms reported to be highly indicative of interpersonal functioning.34
Prior research35 indicates that improvement in schizophrenia symptoms after treatment with lumateperone is associated with approximately 40% striatal D2 receptor occupancy (D2RO) at peak plasma concentrations, which is a substantially lower occupancy at an efficacious dose than most currently available antipsychotics that exhibit 60% to 80% D2RO. The ability to treat schizophrenia at low postsynaptic D2RO and with low EPS and low hyperprolactinemia liability may be attributed to the following characteristics of lumateperone: (1) substantially greater affinity for 5-HT2A–receptor than D2-receptor modulation, greater than that of clozapine9 (60-fold greater affinity for 5-HT2A receptors in vitro and confirmed high cortical 5-HT2A–receptor occupancy and relatively lower striatal D2RO)10; (2) unique interaction with D2 receptors as a presynaptic partial agonist and postsynaptic antagonist with functional mesolimbic and mesocortical selectivity10; (3) inhibition of serotonin reuptake10; and (4) indirect enhancement of glutamate neurotransmission downstream from activation of D1-receptors9 (with 3 and 4 predicting antidepressant effects in addition to antipsychotic effects).10 The observed safety profile may also be associated with lumateperone’s minimal binding at histaminergic or muscarinic receptors,10 which have been traditionally associated with cardiometabolic effects and other tolerability issues of existing antipsychotics.11
Given the high burden of schizophrenia,2 an unmet medical need still exists to treat the broad range of symptoms without increasing adverse effects of antipsychotics, including risk of EPS, parkinsonism and akathisia, hyperprolactinemia, weight gain, dyslipidemia, diabetes, and cardiovascular disease.3,36,37,38,39,40,41 Furthermore, tolerability and safety issues associated with many antipsychotics lead to nonadherence.3,36,42 In this study, treatment with lumateperone demonstrated a safety profile similar to placebo, confirming results observed in a previous clinical trial.29 The high completion rates and low discontinuation rates attributable to adverse events support the favorable safety profile of lumateperone. There was 1 event of convulsion that was severe and deemed serious; this patient had preexisting risk factors and relevant medical history regarding seizures but was inappropriately randomized because of incomplete medical history and inaccurate self-reporting at screening. Antipsychotics are, in general, associated with risk of seizure43,44; however, the specific risks associated with lumateperone could not be determined from this study because patients with a history of seizure disorder were excluded. The adverse events in the lumateperone groups that occurred at a clinically meaningful rate were sedation, somnolence, fatigue, and constipation, which all were predominantly mild. Of note, lumateperone was administered in the morning to capture important safety measures during the day around the time of peak plasma levels. In clinical practice, lumateperone will likely be administered in the evening with maintenance of sleep as a potential benefit of the mild sedative effects in some patients. Additional safety studies are being conducted to evaluate lumateperone administered in the evening.
Limitations
The following limitations should be noted. First, patients had to meet specific inclusion criteria, which may limit generalizability of these data to broader populations. Second, the 4-week treatment duration may not elucidate the safety profile associated with longer-term treatment or address maintenance of effectiveness; however, an open-label trial of long-term treatment with lumateperone in patients with schizophrenia is currently in progress,45 which may extend the generalizability of the current results. Third, the prosocial factor, which improved with lumateperone, includes negative symptoms and positive symptoms. The improvements in the prosocial factor may have been secondary to improvements in positive symptoms in this acutely ill population. Additional studies are warranted to examine the benefit of lumateperone treatment for social function and negative symptoms.
Conclusions
The unique pharmacologic mechanisms of lumateperone10 seem to confer antipsychotic efficacy with favorable safety and tolerability. The efficacy and safety profiles of lumateperone may differ in important ways from existing treatments for patients with schizophrenia.3
Trial Protocol
eMethods. Supplemental Methods
eTable 1. Patient Randomization by Study Sitea
eTable 2. Most Frequently Reported Prior Medicationsa (≥5% in the safety set)
eTable 3. Sensitivity Analysis for Efficacy Endpoint Measures for Lumateperone Vs Placebo (ITT)
eTable 4. Responder Analysis for Lumateperone in Patients With Schizophreniaa (ITT)
eTable 5. Incidence of Treatment-Emergent Adverse Events Occurring in ≥5% of Patients in Any Treatment Group (Safety Set)
eTable 6. Lumateperone Was Not Associated With Suicidal Ideation or Behavior as Measured by the Columbia-Suicide Severity Rating Scale (Safety Set)
eTable 7. Incidence of Treatment-Emergent Adverse Events Related to Extrapyramidal Symptoms (Safety Set)
eTable 8. Extrapyramidal Symptoms as Measured by Objective Clinician-Administered Scales (Mean Change From Baseline to Day 28 on the Barnes Akathisia Rating Scale, Abnormal Involuntary Movement Scale, and Simpson-Angus Scale; Safety Set)
eTable 9. Concomitant Psychotropic Medicationa Use for the Management of Extrapyramidal Symptoms or Agitation (Safety Set)
eFigure. Weight Change ≥7% and BMI Shift From Overweight to Obese (Safety Set)
eReferences
Data Sharing Statement
References
- 1.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67 [DOI] [PubMed] [Google Scholar]
- 2.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712-e723. doi: 10.1016/S2214-109X(15)00069-8 [DOI] [PubMed] [Google Scholar]
- 3.Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757-777. doi: 10.2147/TCRM.S117321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 5.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119-136. doi: 10.1002/wps.20204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181. doi: 10.1001/jamapsychiatry.2015.1737 [DOI] [PubMed] [Google Scholar]
- 7.Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163-180. doi: 10.1002/wps.20420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4(4):295-301. doi: 10.1016/S2215-0366(17)30078-0 [DOI] [PubMed] [Google Scholar]
- 9.Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614. doi: 10.1080/14737175.2016.1174577 [DOI] [PubMed] [Google Scholar]
- 10.Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621. doi: 10.1007/s00213-014-3704-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(suppl 2):S12-S21. doi: 10.1016/S0924-9338(10)71701-6 [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 13.First MB, Williams JBW, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV-TR Axis I disorders, clinical trials version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- 14.First MB, Williams JB, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 Disorders—Clinical Trials Version (SCID-5-CT, Modified for ITI-007-301). Arlington, VA: American Psychiatric Association; 2014. [Google Scholar]
- 15.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99. [PubMed] [Google Scholar]
- 16.US Department of Health Education, and Welfare. ; ECDEU Assessment Manual for Psychopharmacology. MD: US Dept of Health Education, and Welfare; 1976. [Google Scholar]
- 17.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Kobak KA, Zhao Y, Alexander MM, Kane JM. Use of remote centralized raters via live 2-way video in a multicenter clinical trial for schizophrenia. J Clin Psychopharmacol. 2008;28(6):691-693. doi: 10.1097/JCP.0b013e31818c9ba3 [DOI] [PubMed] [Google Scholar]
- 19.Kobak KA, Leuchter A, DeBrota D, et al. Site versus centralized raters in a clinical depression trial: impact on patient selection and placebo response. J Clin Psychopharmacol. 2010;30(2):193-197. doi: 10.1097/JCP.0b013e3181d20912 [DOI] [PubMed] [Google Scholar]
- 20.Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25(2):325-338. doi: 10.1185/03007990802611919 [DOI] [PubMed] [Google Scholar]
- 21.Purnine DM, Carey KB, Maisto SA, Carey MP. Assessing positive and negative symptoms in outpatients with schizophrenia and mood disorders. J Nerv Ment Dis. 2000;188(10):653-661. doi: 10.1097/00005053-200010000-00003 [DOI] [PubMed] [Google Scholar]
- 22.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251. doi: 10.1016/0920-9964(90)90005-R [DOI] [PubMed] [Google Scholar]
- 23.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19. doi: 10.1111/j.1600-0447.1970.tb02066.x [DOI] [PubMed] [Google Scholar]
- 24.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. doi: 10.1192/bjp.154.5.672 [DOI] [PubMed] [Google Scholar]
- 25.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J. 2008;50(5):667-677. doi: 10.1002/bimj.200710464 [DOI] [PubMed] [Google Scholar]
- 27.Dmitrienko A, Tamhane AC. Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med. 2011;30(13):1473-1488. doi: 10.1002/sim.4008 [DOI] [PubMed] [Google Scholar]
- 28.Dmitrienko A, Kordzakhia G, Brechenmacher T. Mixture-based gatekeeping procedures for multiplicity problems with multiple sequences of hypotheses. J Biopharm Stat. 2016;26(4):758-780. doi: 10.1080/10543406.2015.1074917 [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961. doi: 10.1016/j.biopsych.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 30.Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927-942. doi: 10.1176/appi.ajp.2017.16121358 [DOI] [PubMed] [Google Scholar]
- 31.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951. doi: 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleischhacker W, Galderisi S, Laszlovszky I, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry. 2019;58:1-9. doi: 10.1016/j.eurpsy.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 33.Correll CU, Stanford AD, Claxton A, Du Y, Weiden PJ. Social and functional outcomes with two doses of aripiprazole lauroxil vs placebo in patients with schizophrenia: a post-hoc analysis of a 12-week phase 3 efficacy study. Psychiatry Res. 2019;274:176-181. doi: 10.1016/j.psychres.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 34.Harvey PD, Khan A, Keefe RSE. Using the positive and negative syndrome scale (PANSS) to define different domains of negative symptoms: prediction of everyday functioning by impairments in emotional expression and emotional experience. Innov Clin Neurosci. 2017;14(11-12):18-22. [PMC free article] [PubMed] [Google Scholar]
- 35.Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605. doi: 10.1038/s41386-018-0251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs A, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes. 2008;6(1):105. doi: 10.1186/1477-7525-6-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correll CU. What are we looking for in new antipsychotics? J Clin Psychiatry. 2011;72(suppl 1):9-13. doi: 10.4088/JCP.10075su1.02 [DOI] [PubMed] [Google Scholar]
- 38.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. doi: 10.1038/nrendo.2011.156 [DOI] [PubMed] [Google Scholar]
- 39.Vancampfort D, Wampers M, Mitchell AJ, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12(3):240-250. doi: 10.1002/wps.20069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339-347. doi: 10.1002/wps.20252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166-174. doi: 10.1002/wps.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. doi: 10.1002/wps.20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018:1-10. doi: 10.1080/15622975.2018.1500030 [DOI] [PubMed] [Google Scholar]
- 44.Wu C-S, Wang S-C, Yeh I-J, Liu S-K. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579. doi: 10.4088/JCP.15m09898 [DOI] [PubMed] [Google Scholar]
- 45.Vanover K, Dmitrienko A, Glass S, et al. Lumateperone (ITI-007) for the treatment of schizophrenia: placebo-controlled clinical trials and an open-label safety switching study. Schizophr Bull. 2018;44(suppl 1):S341-S341. doi: 10.1093/schbul/sby018.831 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Supplemental Methods
eTable 1. Patient Randomization by Study Sitea
eTable 2. Most Frequently Reported Prior Medicationsa (≥5% in the safety set)
eTable 3. Sensitivity Analysis for Efficacy Endpoint Measures for Lumateperone Vs Placebo (ITT)
eTable 4. Responder Analysis for Lumateperone in Patients With Schizophreniaa (ITT)
eTable 5. Incidence of Treatment-Emergent Adverse Events Occurring in ≥5% of Patients in Any Treatment Group (Safety Set)
eTable 6. Lumateperone Was Not Associated With Suicidal Ideation or Behavior as Measured by the Columbia-Suicide Severity Rating Scale (Safety Set)
eTable 7. Incidence of Treatment-Emergent Adverse Events Related to Extrapyramidal Symptoms (Safety Set)
eTable 8. Extrapyramidal Symptoms as Measured by Objective Clinician-Administered Scales (Mean Change From Baseline to Day 28 on the Barnes Akathisia Rating Scale, Abnormal Involuntary Movement Scale, and Simpson-Angus Scale; Safety Set)
eTable 9. Concomitant Psychotropic Medicationa Use for the Management of Extrapyramidal Symptoms or Agitation (Safety Set)
eFigure. Weight Change ≥7% and BMI Shift From Overweight to Obese (Safety Set)
eReferences
Data Sharing Statement