This cohort study compares decompressing stoma vs self-expandable metal stent as a bridge to surgery for nonlocally advanced left-sided obstructive colon cancer using propensity score matching.
Key Points
Question
Is a decompressing stoma better than self-expandable metal stent as a bridge to surgery for nonlocally advanced left-sided obstructive colon cancer?
Findings
In this cohort study of 443 patients with left-sided obstructive colon cancer, after propensity score matching, patients treated with a decompressing stoma had a longer hospital stay during the bridging interval, more primary anastomoses, more stomas after resection, fewer major resection–related complications, and more subsequent interventions. No significant differences in locoregional recurrence, disease-free survival, and overall survival were found.
Meaning
The findings suggest that equipoise still exists in the management of left-sided obstructive colon cancer.
Abstract
Importance
Bridge to elective surgery using self-expandable metal stent (SEMS) placement is a debated alternative to emergency resection for patients with left-sided obstructive colon cancer because of oncologic concerns. A decompressing stoma (DS) might be a valid alternative, but relevant studies are scarce.
Objective
To compare DS with SEMS as a bridge to surgery for nonlocally advanced left-sided obstructive colon cancer using propensity score matching.
Design, Setting, and Participants
This national, population-based cohort study was performed at 75 of 77 hospitals in the Netherlands. A total of 4216 patients with left-sided obstructive colon cancer treated from January 1, 2009, to December 31, 2016, were identified from the Dutch Colorectal Audit and 3153 patients were studied. Additional procedural and intermediate-term outcome data were retrospectively collected from individual patient files, resulting in a median follow-up of 32 months (interquartile range, 15-57 months). Data were analyzed from April 7 to October 28, 2019.
Exposures
Decompressing stoma vs SEMS as a bridge to surgery.
Main Outcomes and Measures
Primary anastomosis rate, postresection presence of a stoma, complications, additional interventions, permanent stoma, locoregional recurrence, disease-free survival, and overall survival. Propensity score matching was performed according to age, sex, body mass index, American Society of Anesthesiologists score, prior abdominal surgery, tumor location, pN stage, cM stage, length of stenosis, and year of resection.
Results
A total of 3153 of the eligible 4216 patients were included in the study (mean [SD] age, 69.7 [11.8] years; 1741 [55.2%] male); after exclusions, 443 patients underwent bridge to surgery (240 undergoing DS and 203 undergoing SEMS). Propensity score matching led to 2 groups of 121 patients each. Patients undergoing DS had more primary anastomoses (104 of 121 [86.0%] vs 90 of 120 [75.0%], P = .02), more postresection stomas (81 of 121 [66.9%] vs 34 of 117 [29.1%], P < .001), fewer major complications (7 of 121 [5.8%] vs 18 of 118 [15.3%], P = .02), and more subsequent interventions, including stoma reversal (65 of 113 [57.5%] vs 33 of 117 [28.2%], P < .001). After DS and SEMS, the 3-year locoregional recurrence rates were 11.7% for DS and 18.8% for SEMS (hazard ratio [HR], 0.62; 95% CI, 0.30-1.28; P = .20), the 3-year disease-free survival rates were 64.0% for DS and 56.9% for SEMS (HR, 0.90; 95% CI, 0.61-1.33; P = .60), and the 3-year overall survival rates were 78.0% for DS and 71.8% for SEMS (HR, 0.77; 95% CI, 0.48-1.22; P = .26).
Conclusions and Relevance
The findings suggest that DS as bridge to resection of left-sided obstructive colon cancer is associated with advantages and disadvantages compared with SEMS, with similar intermediate-term oncologic outcomes. The existing equipoise indicates the need for a randomized clinical trial that compares the 2 bridging techniques.
Introduction
A substantial number of patients with colon cancer present with a colonic obstruction. These patients have an increased risk of postoperative morbidity and mortality if resection is performed in the emergency care setting.1 Patients with left-sided obstructive colon cancer (LSOCC) are often older and frail, with a deteriorated clinical condition because of inadequate oral intake. A distended bowel impedes laparoscopic surgery in this context.2 These observations have led to (inter)national guidelines recommending a bridge to elective surgery (BTS) approach in older and frail patients with LSOCC.3,4 A BTS can be accomplished by self-expandable metal stent (SEMS) placement or decompressing stoma (DS) construction.
Implementation of SEMS as a BTS in routine practice is still hampered by oncologic concerns, at least in the Netherlands.5 These concerns include a high risk of tumor cell dissemination attributable to tumor manipulation and locoregional recurrence after stent- or guidewire-related perforations.6,7 However, a recent meta-analysis8 did not confirm an association of SEMS with decreased survival compared with emergency surgery.
The DS technique revealed fewer permanent stomas and more primary anastomoses than emergency resection.9 Compared with SEMS, the tumor is left untouched without potential oncologic risks.10 However, studies on DS are limited, with a significant number of studies published before 1995.11,12,13,14 This lack of data might reflect infrequent use of DS in daily practice probably because of the need for an additional procedure for stoma reversal (3-stage) and the risk of stoma prolapse and other stoma-related complications.
In contrast to numerous studies15,16,17 comparing SEMS with emergency resection, there is almost no literature comparing the 2 BTS techniques. To provide more evidence regarding which BTS option is preferable, the aim of the current study was to compare DS with SEMS as a BTS for LSOCC in a large national cohort using propensity score matching.
Methods
Study Design and Population
For this cohort study, a collaborative research project was performed in the Netherlands under the umbrella of the Dutch Snapshot Research Group according to a predefined protocol.18 Short-term outcomes of 3879 patients who had a resection of LSOCC from January 1, 2009, to December 31, 2016, were retrieved from the Dutch Colorectal Audit (DCRA). From August 1, 2017, to December 31, 2017, surgical residents extended this database with additional diagnostic, procedural, and intermediate-term outcome data gathered from original patient files under the supervision of 1 or 2 consultant surgeons. Subsequently, the supplied data were checked for discrepancies and missing values, which were communicated to the collaborators to recheck and complete the data.19 Data were analyzed from April 7 to October 28, 2019. This study was approved by the institutional review board of the Academic Medical Center in Amsterdam, the Netherlands. Because of the retrospective design with anonymized data, an exemption status for individual informed consent was provided. The study design and preparation of the original manuscript were performed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.20
Patients were selected from the DCRA in case of (1) a symptomatic colonic obstruction (abdominal distention, nausea, and/or vomiting) by a (2) tumor in the distal colon (sigmoid, descending colon, or splenic flexure), (3) proven malignant tumor by histologic analysis, and (4) confirmation of the obstruction on radiography or computed tomography (CT), defined as dilated colon proximal to the primary tumor with or without distension of small bowel. For the present analysis, the following exclusion criteria were used: (1) signs of bowel perforation at initial presentation, (2) palliative treatment intent, (3) emergency resection, and (4) locally advanced tumor (cT4 stage, neoadjuvant therapy, and/or multivisceral resection). Treatment intent was retrieved from the original patient files and based on judgment of the local multidisciplinary team. Centers that performed SEMS were categorized for the purpose of subgroup analysis, thereby defining high-volume centers as those with more than 70% of the patients with LSOCC treated with SEMS and a treating endoscopist who has performed at least 20 procedures.19
End Points
Short-term outcomes were primary anastomoses, 90-day mortality, complications, additional interventions, hospital stay, postresection presence of a stoma, and SEMS-related perforations. Long-term outcomes included 3-year locoregional recurrence, 3-year disease-free survival (DFS), 3-year overall survival (OS), complications, additional interventions, total hospital stay, and permanent stoma.
Definitions
Short-term complications included BTS-related or resection-related adverse events within 90 days after resection. Major resection–related complications were defined as Clavien-Dindo grade III or higher.21 Short-term reinterventions included surgical, endoscopic, or radiologic reinterventions within 90 days after resection attributable to complications, including those related to BTS. The SEMS-related perforations included clinically overt perforations, silent perforations observed during the resection, and microperforations. Disease-free survival included the interval between first presentation with colonic obstruction until locoregional recurrence, distant metastasis, death, or last follow-up. Overall survival included the interval between first presentation until death or last follow-up. Total complication rate included BTS-related, resection-related, and stoma-related complications after resection. Total number of interventions included BTS, tumor resection, additional interventions, and stoma reversal. Total hospital stay included any initial or subsequent admissions during the BTS interval and after tumor resection. Permanent stomas were stomas in situ at the end of follow-up.
Statistical Analysis
Normally distributed continuous variables were reported as means (SDs) and nonnormally distributed variables as medians (interquartile ranges [IQRs]). Before matching, variables were compared with the t test or the Mann-Whitney test. Categorical variables were presented as percentages and compared using the χ2 test or the Fisher exact test.
Propensity score matching was performed rather than inverse probability weighting or addition of the propensity score to a regression model because we wanted to restrict our analysis to the most optimally balanced patient groups. Because some patients might be preferably treated with DS, regression analysis using the whole cohort of patients was considered to be inappropriate. Patients were propensity score matched according to the following variables that might influence treatment choice: age, sex, body mass index, American Society of Anesthesiologists score, prior abdominal surgery, tumor location, pN stage, cM stage, length of stenosis on CT, and year of resection. Before matching, missing data were imputed with multiple imputation by chained equations (MICE package in R software). Then 1-to-1 nearest neighbor matching was performed without replacement with a caliper of 0.25 logit of the SD of the propensity score (optimal matching).22 Covariate balance within the selected and unselected cohorts was assessed with mean standardized differences (MSDs). An MSD of less than 10% represented a negligible difference in outcomes.23 In addition, covariates were compared between selected and unselected patients within the DS and the SEMS groups using the χ2 test or Fisher exact test. Outcomes in the matched cohort were analyzed with conditional logistic regression to account for the paired nature of the data. Conditional odds ratios (cORs) with 95% CIs were calculated. For outcomes with fewer than 5 observations, the McNemar test was performed. Kaplan-Meier curves were plotted, and survival probabilities were compared using Cox proportional hazards regression with shared frailty, with SEMS as a reference. These analyses were performed for the following end points: DFS, OS, stoma-free survival, and BTS-related complications. The proportional hazards assumption was evaluated using a log − log plot. For analyzing DFS, OS, and stoma-free survival, patients were censored when they were lost to follow-up during the study period. Regarding BTS-related complications, patients were censored in case of death or loss to follow-up. Subgroup analyses between SEMS and different types of DS (double loop or blowhole) were performed using conditional logistic regression as well. A 2-sided P < .05 was considered to be statistically significant. Analyses were performed on an intention-to-treat basis, meaning that DS after unsuccessful SEMS was still analyzed within the SEMS group. Analyses were performed with IBM SPSS statistics, version 25.0 (IBM Corp) and R software version R3.3.2 (Matching and Frailtypack packages, R Foundation for Statistical Computing).
Results
Baseline Characteristics
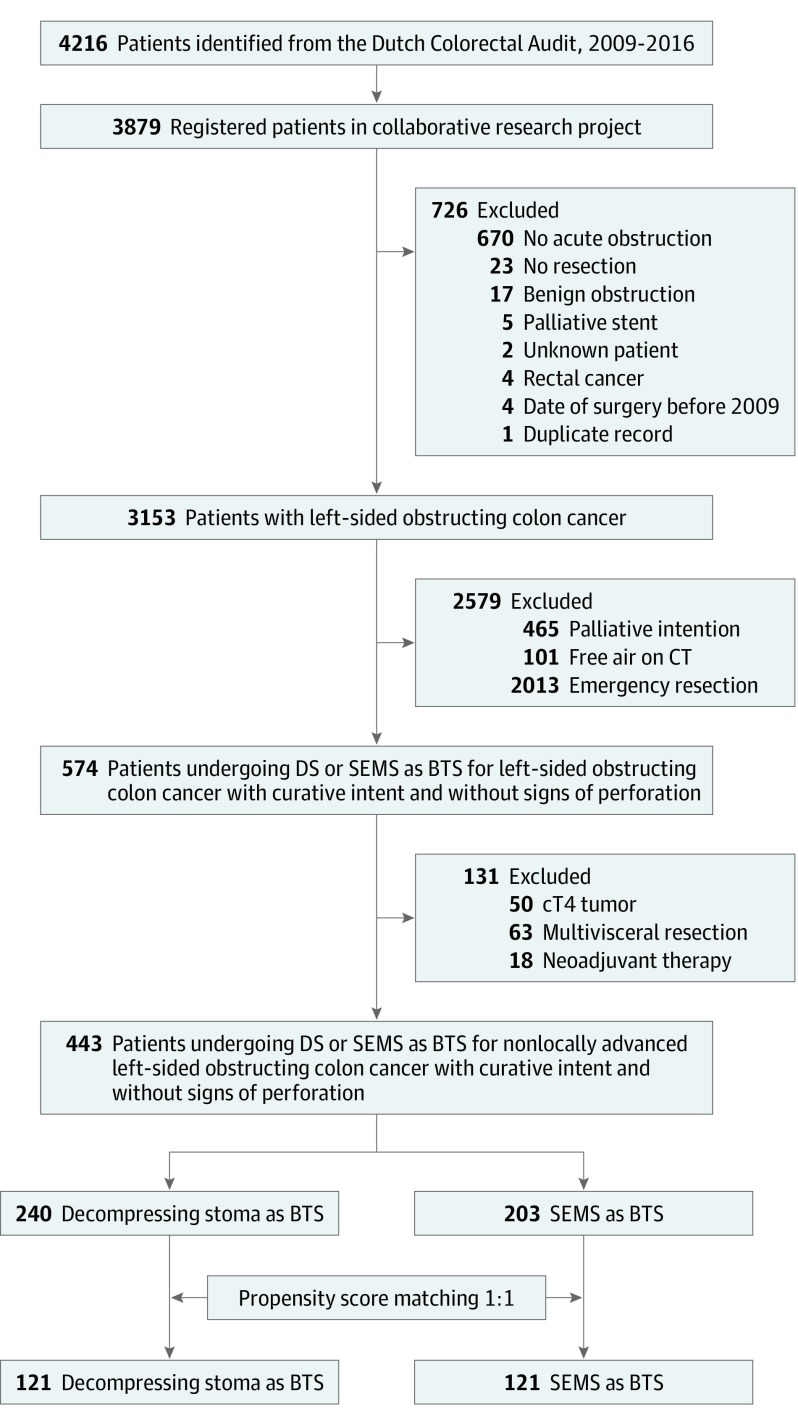
Each hospital in the Netherlands (N = 77) was invited to participate, of which 75 hospitals participated, resulting in the registration of 3879 of all 4216 eligible patients (92.0%) (Figure 1). A total of 3153 of the eligible 4216 patients were included in the study (mean [SD] age, 69.7 [11.8] years; 1741 [55.2%] male). Exclusion for factors such as absence of obstruction (n = 670), palliative intent (n = 465), and locally advanced tumors (n = 131) led to 443 BTS patients: 240 undergoing DS and 203 undergoing SEMS (Table 1). In the unmatched data set, there were no missing data for age, sex, tumor location, and year of resection. Data for the other variables were missing for less than 2%, except for stenosis length (225 [50.8%]) and body mass index (45 [10.2%]). Multiple imputation resulted in completion of these variables. The 1-to-1 propensity score matching resulted in 121 patients undergoing DS and 121 undergoing SEMS. Selected patients undergoing DS had less often undergone prior abdominal surgery than unselected DS patients (36 [29.8%] vs 52 [43.7%], P = .03) (eTable 1 in the Supplement). Selected patients undergoing SEMS had more often undergone prior abdominal surgery (32 [26.4%] vs 11 [13.4%], P = .03) and had more pN2 tumors than unselected patients undergoing SEMS (25 [20.7%] vs 7 [8.5%], P = .04). After matching, a comparison of the selected patients undergoing DS and SEMS resulted in an MSD less than 10% for all baseline characteristics except tumor location (MSD, 12.5%).
Figure 1. Flowchart of Patient Selection.
BTS indicates bridge to surgery; CT, computed tomography; DS, decompressing stoma; SEMS, self-expandable metal stent.
Table 1. Baseline Characteristicsa.
| Characteristic | Before Propensity Score Matching | After Propensity Score Matching | Unselected Groups After Matching | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DS as BTS (n = 240) | SEMS as BTS (n = 203) | SMD, % | DS as BTS (n = 121) | SEMS as BTS (n = 121) | SMD, % | DS as BTS (n = 119) | SEMS as BTS (n = 82) | SMD, % | |
| Male | 149 (62.1) | 115 (56.7) | 16.9 | 72 (59.5) | 73 (60.3) | 1.7 | 77 (64.7) | 42 (51.2) | 27.6 |
| Age, mean (SD), y | 68.5 (11.4) | 70.5 (11.7) | 11.1 | 69.8 (11.0) | 70.1 (12.1) | 2.1 | 67.2 (11.7) | 71.1 (11.2) | 33.9 |
| BMI, mean (SD) | 25.0 (4.0) | 25.5 (5.0) | 12.7 | 25.3 (4.3) | 25.2 (3.9) | 2.5 | 24.6 (3.6) | 26.3 (6.5) | 33.6 |
| ASA score 3-4 | 53 (22.1) | 48 (23.6) | 3.7 | 26 (21.5) | 26 (21.5) | <0.1 | 27 (22.7) | 22 (26.8) | 9.6 |
| Prior abdominal surgery | 88 (36.7) | 43 (21.2) | 34.7 | 36 (29.8) | 32 (26.4) | 7.4 | 52 (43.7) | 11 (13.4) | 71.2 |
| Tumor location | |||||||||
| Splenic flexure | 42 (17.5) | 10 (4.9) | 41.1 | 6 (5.0) | 8 (6.6) | 12.5 | 36 (30.3) | 2 (2.4) | 81.2 |
| Descending colon | 44 (18.3) | 38 (18.7) | 23 (19.0) | 18 (14.9) | 21 (17.6) | 20 (24.4) | |||
| Sigmoid | 154 (64.2) | 155 (76.4) | 92 (76.0) | 95 (78.5) | 62 (52.1) | 60 (73.2) | |||
| pN stageb | |||||||||
| pN0 | 95 (39.6) | 101 (49.8) | 22.8 | 51 (42.1) | 54 (44.6) | 8.6 | 44 (37.0) | 49 (59.8) | 59.6 |
| pN1 | 89 (37.1) | 69 (34.0) | 47 (38.8) | 42 (34.7) | 41 (34.5) | 26 (31.7) | |||
| pN2 | 56 (23.3) | 33 (16.3) | 23 (19.0) | 25 (20.7) | 34 (28.6) | 7 (8.5) | |||
| cM1 stage | 16 (6.7) | 16 (7.9) | 10 (8.3) | 9 (7.4) | 6 (5.0) | 8 (9.8) | |||
| Lung | 1 (0.4) | 1 (0.5) | 4.7 | 0 | 1 (0.8) | 3.1 | 1 (0.8) | 0 | 18.1 |
| Liver | 14 (5.8) | 11 (5.4) | 10 (8.3) | 5 (4.1) | 4 (3.4) | 6 (7.3) | |||
| Peritoneal | 3 (1.3) | 3 (1.5) | 0 | 2 (1.7) | 3 (2.5) | 1 (1.2) | |||
| Other | 1 (0.4) | 2 (1.0) | 0 | 1 (0.8) | 1 (0.8) | 1 (1.2) | |||
| Length of stenosis, mean (SD), cm | 4.4 (2.0) | 4.0 (1.5) | 27.1 | 4.1 (1.7) | 3.9 (1.5) | 7.6 | 4.6 (2.0) | 3.8 (1.6) | 43.9 |
| Year of resectionc | |||||||||
| 2009 | 5 (9.8) | 46 (90.2) | 93.1 | 5 (31.3) | 11 (68.8) | 0.4 | 0 | 35 (100) | 358.4 |
| 2010 | 17 (27.4) | 45 (72.6) | 17 (47.2) | 19 (52.8) | 0 | 26 (100) | |||
| 2011 | 24 (53.3) | 21 (46.7) | 20 (69.0) | 9 (31.0) | 4 (25.0) | 12 (75.0) | |||
| 2012 | 21 (43.8) | 27 (56.3) | 13 (39.4) | 20 (60.6) | 8 (53.3) | 7 (46.7) | |||
| 2013 | 39 (65.0) | 21 (35.0) | 25 (55.6) | 20 (44.4) | 14 (93.3) | 1 (6.7) | |||
| 2014 | 32 (88.9) | 4 (11.1) | 12 (75.0) | 4 (25.0) | 20 (100) | 0 | |||
| 2015 | 52 (72.2) | 20 (27.8) | 16 (45.7) | 19 (54.3) | 36 (97.3) | 1 (2.7) | |||
| 2016 | 50 (72.5) | 19 (27.5) | 13 (40.6) | 19 (59.4) | 37 (100) | 0 | |||
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by square of height in meters); BTS, bridge to surgery; DS, decompressing stoma; SEMS, self-expandable metal stent; SMD, standardized mean difference.
Data are presented as number (percentage) of patients unless otherwise indicated.
pT stage was not included in propensity score matching and therefore not shown in Table 1. After propensity score matching, pT stage did not significantly differ between the groups (P = .22).
Percentage of row instead of column total.
Procedural Characteristics and Short-term Outcomes
The median interval between DS and resection was 30 days (IQR, 19-47 days), which was 18 days (IQR, 8-31 days) after SEMS (cOR, 0.95; 95% CI, 0.93-0.97; P < .001) (Table 2). The median hospital stay during the BTS interval was 7 days (IQR, 5-12 days) for DS and 4 days (IQR, 2-6 days) for SEMS (cOR, 0.86; 95% CI, 0.79-0.92; P < .001). No differences were observed in BTS-related complications (cOR, 0.78; 95% CI, 0.29-2.09; P = .62; hazard ratio [HR], 0.80; 95% CI, 0.32-2.04; P = .64). More primary anastomoses were constructed in the DS group (104 of 121 [86.0%] vs 90 of 120 [75.0%]; cOR, 0.41; 95% CI, 0.19-0.89; P = .02). Patients undergoing SEMS had fewer postresection stomas (34 of 117 [29.1%] vs 81 of 121 [66.9%]; cOR, 0.15; 95% CI, 0.07-0.32; P < .001). The 90-day complication rate did not significantly differ between the groups, although patients undergoing SEMS had more major resection–related complications (18 of 118 [15.3%] vs 7 of 121 [5.8%]; cOR, 3.20; 95% CI, 1.17-8.74; P = .02). The 90-day mortality rates were 1.7% for DS and 5.0% for SEMS (P = .29). eTable 2 and eTable 3 in the Supplement provide an overview of DS- and SEMS-specific complications. The overall SEMS-related perforation rate was 8.0%. Three patients had clinically overt perforations, all of whom underwent subsequent emergency resection. Two of these patients died at 11- and 18-month follow-up. eTable 4 in the Supplement gives a comparison of hospital stay between patients undergoing SEMS and different types of DS. Within the DS group, patients undergoing double-loop DS had a shorter median postresection hospital stay than those undergoing blowhole DS (6 vs 8 days, P = .04). High- vs low-volume SEMS centers did not have any significant differences in technical success and SEMS-related complications (eTable 5 in the Supplement).
Table 2. Procedural Characteristics and Short-term Treatment Outcomes (Within 90 Days After Resection) of Propensity Score–Matched Samplesa.
| Characteristic or Outcome | DS as BTS (n = 121) | SEMS as BTS (n = 121) | cOR (95% CI) | P Value |
|---|---|---|---|---|
| Time from first presentation to DS or SEMS, median (IQR), d | 1.0 (0.0-2.0) | 1.0 (0.0-1.0) | 0.82 (0.67-1.00) | .05 |
| Time from DS or SEMS to resection in days, median (IQR), d | 30.0 (19.0-47.0) | 18.0 (8.0-30.8) | 0.95 (0.93-0.97) | <.001 |
| Laparoscopic approach for primary tumor resection | 60/121 (49.6) | 62/120 (51.7) | 1.07 (0.65-1.74) | .80 |
| Surgical procedure | .44 | |||
| Sigmoid resection | 81/121 (66.9) | 84/121 (69.4) | 1.13 (0.65-1.98) | .67 |
| Left hemicolectomy | 31/121 (25.6) | 33/121 (27.3) | 1.10 (0.60-2.02) | .76 |
| Subtotal colectomy | 6/121 (5.0) | 3/121 (2.5) | NAb | .51b |
| Extended left hemicolectomy | 3/121 (2.5) | 0/121 (0.0) | NAc | NAc |
| Transverse resection | 0/121 | 1/121 (0.8) | NAc | NAc |
| Completeness of resection | .53 | |||
| R0 | 116/121 (95.9) | 112/114 (98.2) | 2.50 (0.49-12.89) | .27 |
| R1 | 5/121 (4.1) | 1/114 (0.9) | NAb | .22b |
| R2 | 0/121 | 1/114 (0.9) | NAc | NAc |
| No. of lymph nodes harvested, median (IQR) | 15.0 (12.0-21.0) | 19.0 (14.0-24.0) | 1.04 (1.01-1.07) | .01 |
| No. of positive lymph nodes harvested, median (IQR) | 1.0 (0.0-3.0) | 1.0 (0.0-3.0) | 1.02 (0.95-1.10) | .57 |
| Angioinvasion | 31/106 (29.2) | 34/113 (30.1) | 1.05 (0.56-1.97) | .87 |
| Extramural venous invasion | 23/106 (21.7) | 31/113 (27.4) | 1.39 (0.68-2.83) | .37 |
| Lymphatic invasion | 6/106 (5.7) | 2/111 (1.8) | NAb | .45b |
| Intramural venous invasion | 2/106 (1.9) | 1/113 (0.9) | NAb | >.99b |
| Stoma in situ directly after tumor resection | 81/121 (66.9) | 34/117 (29.1) | 0.15 (0.07-0.32) | <.001 |
| (New) stoma constructed during resection | 13/81 (16.0) | 34/34 (100) | NA | NA |
| Type of stoma in situ directly after tumor resection | .01 | |||
| Diverting ileostomy | 9/77 (11.7)d | 4/34 (11.8) | 0.50 (0.09-2.73) | .42 |
| End ileostomy | 4/77 (5.2) | 2/34 (5.9) | NAb | >.99b |
| Diverting colostomy | 57/77 (74.0) | 0/34 | NAc | NAc |
| End colostomy | 7/77 (9.1) | 28/34 (82.4) | 65.29 (1.63-2611.50) | .03 |
| Primary anastomosis | 104/121 (86.0) | 90/120 (75.0) | 0.41 (0.19-0.89) | .02 |
| Hospital stay during BTS interval, median (IQR), d | ||||
| Directly after DS or SEMS without readmissions | 7.0 (5.0-12.0) | 4.0 (2.0-6.0) | 0.85 (0.79-0.92) | <.001 |
| Readmission during BTS interval | 2/111 (1.8) | 1/113 (0.9) | NAb | >.99b |
| Including readmissions during BTS interval | 7.0 (5.0-12.0) | 4.0 (2.0-6.0) | 0.86 (0.79-0.92) | <.001 |
| Hospital stay directly after resection, median (IQR), de | 7.0 (5.0-9.0) | 7.0 (5.0-14.0) | 1.03 (1.00-1.06) | .09 |
| BTS-related complications during bridging interval | 13/116 (11.2) | 8/115 (7.0) | 0.78 (0.29-2.09) | .62 |
| SEMS-related perforation | NA | 9/112 (8.0) | NA | NA |
| Resection-related complications within 90 d | 24/121 (19.8) | 34/118 (28.8) | 1.56 (0.86-2.81) | .14 |
| Major resection–related complications | 7/121 (5.8) | 18/118 (15.3) | 3.20 (1.17-8.74) | .02 |
| Anastomotic leakage | 3/104 (2.9) | 5/90 (5.6) | NAb | >.99b |
| Abscess (intra-abdominal) | 2/121 (1.7) | 7/121 (5.8) | NAb | .18b |
| Fascia dehiscence | 3/119 (2.5) | 5/118 (4.2) | NAb | .73b |
| Wound infection | 13/120 (10.8) | 6/117 (5.1) | 0.42 (0.15-1.18) | .10 |
| Postoperative ileus | 4/120 (3.3) | 9/117 (7.7) | NAb | .27b |
| Gastroparesis | 0/120 | 4/117 (3.4) | NAc | NAc |
| Bleeding | 0/120 | 2/117 (1.7) | NAd | NAc |
| 90-d Complication ratef | 34/118 (28.8) | 39/114 (34.2) | 1.27 (0.73-2.23) | .40 |
| 90-d Subsequent intervention rateg | 8/112 (7.1) | 16/115 (13.9) | 2.33 (0.90-6.07) | .08 |
| 90-d Mortality | 2/121 (1.7) | 6/121 (5.0) | NAb | .29b |
| Adjuvant chemotherapy | 47/120 (39.2) | 54/121 (44.6) | 1.26 (0.76-2.09) | .37 |
| Time from resection until start of adjuvant chemotherapy, median (IQR), wk | 5.0 (4.0-7.0) | 7.0 (4.0-9.5) | 1.13 (0.91-1.39) | .28 |
Abbreviations: BTS, bridge to surgery; cOR, conditional odds ratio; DS, decompressing stoma; IQR, interquartile range; NA, not applicable; SEMS, self-expandable metal stent.
Data are presented as number/total number (percentage) of patients unless otherwise indicated.
McNemar test instead of conditional logistic regression because of fewer than 5 observations; therefore, no cOR and 95% CI were reported.
Because of 0 observations in 1 or both group(s), no P value could be calculated with the McNemar test.
Type of stoma in situ directly after tumor resection missing for 4 patients undergoing DS.
Without readmissions.
Combination of complications after BTS and less than 90 days after resection.
Additional interventions within the BTS interval or less than 90 days after resection solely because of complications, excluding complications related to the stoma in situ after resection and excluding stoma reversals.
Long-term Outcomes
The median follow-up was 36 months (IQR, 15-59 months) for the DS group and 31 months (IQR, 15-56 months) for the SEMS group (cOR for follow-up time, 1.00; 95% CI, 0.99-1.01; P = .59) (Table 3). Three-year locoregional recurrence was 11.7% for the DS group and 18.8% for the SEMS group (HR, 0.62; 95% CI, 0.30-1.28; P = .20), 3-year DFS was 64.0% for the DS group and 56.9% for the SEMS group (HR, 0.90; 95% CI, 0.61-1.33; P = .60), and 3-year OS was 78.0% for the DS group and 71.8% for the SEMS group (HR, 0.77; 95% CI, 0.48-1.22; P = .26) (Figure 2 and eFigure 1 in the Supplement). Permanent stoma rate did not significantly differ between the DS and SEMS groups (28.9% vs 19.2%; cOR 0.63, 95% CI, 0.36-1.13; P = .12) (Table 3 and eFigure 2 and eFigure 3 in the Supplement). The total median hospital stay was 15 days (IQR, 11-23 days) for the DS group and 13 days (IQR, 9-20 days) for the SEMS group (cOR, 0.99; 95% CI, 0.97-1.01; P = .22). Patients undergoing double-loop DS had a shorter total hospital stay than those undergoing blowhole DS (15 vs 23 days; P = .003). More subsequent interventions were performed after DS than after SEMS (57.5% vs 28.2%; cOR, 0.28; 95% CI, 0.15-0.53; P < .001), which did not remain significant after exclusion of stoma reversals. Patients undergoing DS had a higher number of interventions, with a median of 3.0 (IQR, 2.0-3.0) vs 2.0 (IQR, 2.0-3.0) in the SEMS group (cOR, 0.42; 95% CI, 0.25-0.72; P = .002). High- vs low-volume SEMS centers did not reveal any significant differences in locoregional recurrence, DFS, and OS (eTable 5 and eFigure 4 in the Supplement).
Table 3. Long-term Treatment Outcomes in Propensity Score–Matched Samplesa.
| Outcome | DS as BTS (n = 121) | SEMS as BTS (n = 121) | cOR (95% CI) | P Value |
|---|---|---|---|---|
| Follow-up, median (IQR), mo | 35.5 (15.0-58.5) | 31.0 (15.0-56.0) | 1.00 (0.99-1.01) | .59 |
| Permanent stoma at time of last follow-up | 35/121 (28.9) | 23/120 (19.2) | 0.63 (0.36-1.13) | .12 |
| In patients with a minimum follow-up of 12 mo | 24/101 (23.8) | 15/93 (16.1) | 0.65 (0.30-1.38) | .26 |
| Total hospital stay, median (IQR), db | 15.0 (11.0-23.0) | 13.0 (9.0-20.0) | 0.99 (0.97-1.01) | .22 |
| After resection, including readmissions | 7.0 (5.0-12.3) | 7.5 (5.0-15.0) | 1.02 (0.99-1.04) | .28 |
| Total complication rate during entire follow-upc | 56/116 (48.3) | 44/109 (40.4) | 0.78 (0.46-1.32) | .36 |
| Related to stoma after resection | 28/77 (36.4) | 8/26 (30.8) | 0.86 (0.29-2.55) | .78 |
| Parastomal hernia | 5/77 (6.5) | 3/30 (10.0) | NA | NA |
| Incisional hernia | 8/74 (10.8) | 0/29 | NA | |
| Stoma prolapse | 9/77 (11.7) | 0/30 | NA | |
| Stoma necrosis | 0/76 | 3/29 (10.3) | NA | |
| Ileus caused by stoma | 1/76 (1.3) | 0/30 | NA | |
| Dehydration | 0/76 | 1/28 (3.6) | NA | |
| High output | 2/76 (2.6) | 3/30 (10.0) | NA | |
| Additional interventions (entire follow-up) | ||||
| Including stoma reversald | 65/113 (57.5) | 33/117 (28.2) | 0.28 (0.15-0.53) | <.001 |
| Excluding stoma reversale | 21/113 (18.6) | 22/116 (19.0) | 1.07 (0.53-2.16) | .86 |
| No. of interventions, median (IQR)f | 3.0 (2.0-3.0) | 2.0 (2.0-3.0) | 0.42 (0.25-0.72) | .002 |
| Locoregional recurrence | 12/118 (10.2) | 18/116 (15.5) | 1.40 (0.62-3.15) | .42 |
| Anastomotic | 3/12 (25.0) | 3/18 (16.7) | NA | NA |
| Locoregional lymph node(s) | 0/12 | 1/18 (5.6) | NA | |
| Peritoneal metastasis | 6/12 (50.0) | 11/18 (61.1) | NA | |
| Unknown | 3/12 (25.0) | 3/18 (16.7) | NA | |
| Distant metastases | 26/119 (21.8) | 27/117 (23.1) | 1.17 (0.62-2.19) | .63 |
| Liver | 15/26 (57.7) | 12/27 (44.4) | NA | NA |
| Lung | 1/26 (3.8) | 4/27 (14.8) | NA | |
| Distant lymph node | 0/26 | 1/27 (3.7) | NA | |
| Combination | 9/26 (34.6) | 10/27 (37.0) | NA | |
| Other | 1/26 (3.8) | 0/27 | NA | |
| 3-y Locoregional recurrence, % | 11.7 | 18.8 | NA | .20 |
| No. of events after 36 mo of follow-up | 11 | 16 | NA | NA |
| No. of patients at risk after 36 mo of follow-up | 57 | 51 | NA | |
| 3-y Disease-free survival, % | 64.0 | 56.9 | NA | |
| No. of events after 36 mo of follow-up | 37 | 42 | NA | .60 |
| No. of patients at risk after 36 mo of follow-up | 49 | 46 | NA | |
| 3-y Overall survival, % | 78.0 | 71.8 | NA | |
| No. of events after 36 mo of follow-up | 21 | 27 | NA | .26 |
| No. of patients at risk after 36 mo of follow-up | 60 | 56 | NA |
Abbreviations: BTS, bridge to surgery; cOR, conditional odds ratio; DS, decompressing stoma; IQR, interquartile range; NA, not applicable; SEMS, self-expandable metal stent.
Data are presented as number/total number (percentage) of patients unless otherwise indicated.
Combination of hospital stay during BTS interval, including readmissions, and hospital stay after resection, including readmissions.
Combination of complications after BTS and resection, including stoma-related complications that occurred after resection during entire follow-up.
Additional interventions within the BTS interval or after resection because of complications and/or stoma reversal during entire follow-up.
Additional interventions within the BTS interval or after resection solely because of complications during entire follow-up, excluding stoma reversal.
Combination of initial BTS intervention, subsequent tumor resection, additional interventions related to complications, and stoma reversal during entire follow-up.
Figure 2. Survival Curves for Decompressing Stoma (DS) vs Self-expandable Metal Stent (SEMS) as a Bridge to Elective Surgery (Propensity Score–Matched Samples).
Discussion
This nationwide, propensity score–matched study compared short-term and intermediate-term outcomes in patients who underwent DS or SEMS as BTS for nonlocally advanced LSOCC. Patients undergoing DS had more postresection stomas, required more repeated interventions, had a higher number of additional interventions, and had a longer hospital stay during the BTS interval. However, patients undergoing DS had more primary anastomoses and fewer major postresection complications. Permanent stoma rate and oncologic outcomes did not significantly differ between the DS and SEMS groups.
Minimally invasive relief of colonic obstruction in LSOCC can be accomplished by DS construction or SEMS placement. The construction of a DS is a relatively fast and controlled procedure with an almost 100% success rate and can be performed in almost every patient. Placement of an SEMS is a technically demanding procedure that requires specific skills, needs a certain patient selection depending on tumor characteristics (length and location of the stenosis), and has a risk of perforation and unsuccessful decompression. However, SEMS seems to be the least invasive intervention considering the shortest BTS-related hospital admission, similar to observations in the palliative setting.24 This finding might be related to the need for general anesthesia and to more postoperative pain after DS construction and the in-hospital organization of domiciliary stoma care. Available guidelines recommend SEMS, provided that the lesion is amenable to stenting and the endoscopist has sufficient experience with SEMS placement. However, these recommendations are based on low-quality evidence.3,4 In fact, at the time of publication of these guidelines, no studies were available that directly compared DS and SEMS.
The first published study on DS vs SEMS as BTS for LSOCC was limited by a small sample size and potential selection bias, and no matching was performed. In accordance with our results, Amelung et al25 observed fewer postresection stomas for SEMS than DS (18% vs 62%) and no significant differences in hospital stay and permanent stoma rate.
Recently, a French population-based study26 on DS (n = 327) vs SEMS (n = 191) was published. In contrast to our results, DS was associated with more complications and a longer hospital stay than was SEMS. Median DFS was 54 months in both groups, but OS was significantly longer in the DS group (median, 124 vs 59 months). The authors concluded that SEMS might negatively influence survival, but whether they sufficiently corrected for confounding baseline differences is unclear. Furthermore, outcomes of the French study26 might have been influenced by the slightly higher age and American Society of Anesthesiologists score of the included patients compared with the present Dutch population study.
More major postresection complications were observed in the SEMS group, which is in line with the study by Amelung et al.25 This observation might be explained by more anastomoses that were defunctioned in our DS group because most of the decompressing stomas were reversed later. In contrast, Amelung et al25 reported fewer long-term complications (30% vs 10%) than observed in our study (48% vs 40%) for DS vs SEMS.25 Stoma-related complications that occurred after resection were not included in that study. In fact, only incisional hernias, enterocutaneous fistulas, abscesses of the abdominal wall, and anastomotic stenoses were mentioned as long-term complications. The current study specifically focused on complete and detailed data collection regarding intermediate-term surgical complications. Nevertheless, there might still be underreporting of outcomes as suggested by the relatively few incisional hernias of 11% for DS and 0% for SEMS (Table 3). Studies27,28 reported incisional hernia rates of up to 30%, of which approximately 50% required a surgical reintervention.
A blowhole DS was performed in only a few patients. Although success of decompression is comparable, a blowhole DS is easier to construct with shorter operative time compared with a double-loop DS.29 A double-loop DS is less likely to retract and does not need revision for diverting a primary anastomosis at the time of resection, but it is a bulky stoma with a high risk of prolapse. In contrast to the study by Kasten et al,29 we found a significantly shorter postresection and total hospital stay for double-loop DS. Current evidence does not allow for clear recommendations on type of DS.
The premature closure of the Dutch Stent-in II trial owing to relatively high perforation rates has contributed to an ongoing discussion on the oncologic safety of SEMS.5,30 Concerns include a high risk of tumor cell dissemination because of manipulation during SEMS placement and expanding pressure as well as tumor recurrence after a perforation.6,7 These concerns were fueled by reports31,32 of a higher rate of perineural and lymph node invasion after SEMS than after emergency resection. However, meta-analyses33,34 did not reveal an association with decreased survival. Current SEMS-related perforation rates are lower than those of the Dutch Stent-in II trial (8.0% vs 19.1%), and technical success has improved as well (86.0% vs 70.2%),5 which might be associated with an increase in stenting experience and proper patient selection in routine daily practice. A recent study19 reported on the oncologic outcomes after SEMS-related perforations in this population-based data set, which revealed a 3-year locoregional recurrence of 17.9% vs 11.0% (P = .43), a 3-year DFS rate of 48.5% vs 59.6% (P = .72), and a 3-year OS rate of 61.1% vs 75.1% (P = .53). However, with only 17 SEMS-related perforations, no firm conclusions could be drawn. The survival curves of the present analysis (Figure 2) seem to separate in favor of DS, but substantial overlap between the CIs does not allow for any conclusion.
Limitations
The current study has several limitations. Despite collecting detailed data, some information was still lacking because of the retrospective study design, such as the physician’s reasons for DS or SEMS, quality of life, and treatment costs. Moreover, multiple imputation had to be performed for length of stenosis in more than half of the patients, which might have caused bias. Furthermore, the variables on which propensity scores were computed were chosen based on a potential influence on treatment choice, and residual confounding by unknown factors might still be present, which might have resulted in groups that were still not fully comparable. Exclusion of cases that did not meet the matching criteria might have reduced statistical power and caused bias. For example, the increased proportion of pN2 tumors in unselected vs selected patients undergoing SEMS might have negatively influenced the results on SEMS. However, because the SMDs were less than 10%, differences between the 2 selected BTS groups were neglible.23 Furthermore, median follow-up was relatively short to reliably estimate long-term oncologic outcomes, and patient numbers were particularly low after 2 years. Therefore, data regarding DFS and OS should be interpreted with caution. In addition, patients without any tumor resection are not included in the DCRA and therefore were not included in this study. Such patients might have died shortly after the bridging intervention or have been treated with palliative systemic therapy or best supportive care, which might have introduced selection bias if overrepresented in 1 of the 2 groups. Also, data on endoscopist experience were lacking. Stents are also placed in the palliative care setting without subsequent resection; therefore, centers’ experience can be underestimated based on the DCRA data set. Furthermore, experience may also depend on other advanced endoscopic techniques, including other gastrointestinal stenting procedures. Therefore, we could not reliably assess stenting experience and did not include this potential confounder in our analyses.
Conclusions
This nationwide, propensity score–matched study comparing DS and SEMS for nonlocally advanced LSOCC revealed advantages and disadvantages of the 2 bridging techniques. Oncologic outcome was slightly in favor of DS, but statistical significance was not reached and follow-up was relatively short. Randomized clinical trials are indicated to determine the best BTS strategy considering the equipoise.
eTable 1. Comparison of Baseline Characteristics Between Selected Patients Versus Unselected Patients After Propensity Score Matching, for DS and SEMS Separately
eTable 2. DS-Related Complications
eTable 3. SEMS-Specific Outcomes
eTable 4. Sub Analysis of Either Blowhole or Double Loop DS Versus SEMS Patients
eTable 5. Comparison of SEMS Patients in High-Volume Versus Low-Volume SEMS Centers
eFigure 1. Disease Free Survival (2A) and Overall Survival (2B) for DS Versus SEMS as BTS, Truncated at a Third of Patients at Risk (Propensity-Score Matched Samples)
eFigure 2. Stoma-Free Survival After Resection for DS Versus SEMS as BTS (Propensity-Score Matched)
eFigure 3. Flowchart of Patients With a Stoma In Situ Directly After Resection
eFigure 4. Disease Free Survival (3A) and Overall Survival (3B) Following SEMS Placement for High-Volume Versus Low-Volume SEMS Centers
References
- 1.Bakker IS, Snijders HS, Grossmann I, Karsten TM, Havenga K, Wiggers T. High mortality rates after nonelective colon cancer resection: results of a national audit. Colorectal Dis. 2016;18(6):612-621. doi: 10.1111/codi.13262 [DOI] [PubMed] [Google Scholar]
- 2.Tanis PJ, Paulino Pereira NR, van Hooft JE, Consten EC, Bemelman WA; Dutch Surgical Colorectal Audit . Resection of obstructive left-sided colon cancer at a national level: a prospective analysis of short-term outcomes in 1,816 patients. Dig Surg. 2015;32(5):317-324. doi: 10.1159/000433561 [DOI] [PubMed] [Google Scholar]
- 3.Greenaway K. National Bowel Cancer Audit Annual Report. London, UK: Healthcare Quality Improvement Partnership, National Bowel Cancer Audit; 2015. [Google Scholar]
- 4.van Hooft JE, van Halsema EE, Vanbiervliet G, et al. ; European Society of Gastrointestinal Endoscopy . Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46(11):990-1053. doi: 10.1055/s-0034-1390700 [DOI] [PubMed] [Google Scholar]
- 5.van Hooft JE, Bemelman WA, Oldenburg B, et al. ; collaborative Dutch Stent-In study group . Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction. Lancet Oncol. 2011;12(4):344-352. doi: 10.1016/S1470-2045(11)70035-3 [DOI] [PubMed] [Google Scholar]
- 6.Sloothaak DA, van den Berg MW, Dijkgraaf MG, et al. ; collaborative Dutch Stent-In study group . Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg. 2014;101(13):1751-1757. doi: 10.1002/bjs.9645 [DOI] [PubMed] [Google Scholar]
- 7.Yamashita S, Tanemura M, Sawada G, et al. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol Lett. 2018;15(1):400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amelung FJ, Burghgraef TA, Tanis PJ, et al. Critical appraisal of oncological safety of stent as bridge to surgery in left-sided obstructing colon cancer. Crit Rev Oncol Hematol. 2018;131:66-75. doi: 10.1016/j.critrevonc.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Amelung FJ, Mulder CL, Verheijen PM, Draaisma WA, Siersema PD, Consten EC. Acute resection versus bridge to surgery with diverting colostomy for patients with acute malignant left sided colonic obstruction. Surg Oncol. 2015;24(4):313-321. doi: 10.1016/j.suronc.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Jiang JK, Lan YT, Lin TC, et al. Primary vs. delayed resection for obstructive left-sided colorectal cancer: impact of surgery on patient outcome. Dis Colon Rectum. 2008;51(3):306-311. doi: 10.1007/s10350-007-9173-4 [DOI] [PubMed] [Google Scholar]
- 11.Carson SN, Poticha SM, Shields TW. Carcinoma obstructing the left side of the colon. Arch Surg. 1977;112(4):523-526. doi: 10.1001/archsurg.1977.01370040175027 [DOI] [PubMed] [Google Scholar]
- 12.Fielding LP, Stewart-Brown S, Blesovsky L. Large-bowel obstruction caused by cancer. BMJ. 1979;2(6189):515-517. doi: 10.1136/bmj.2.6189.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandrup P, Lund L, Balslev I. Surgical treatment of acute malignant large bowel obstruction. Eur J Surg. 1992;158(8):427-430. [PubMed] [Google Scholar]
- 14.Huddy SP, Shorthouse AJ, Marks CG. The surgical treatment of intestinal obstruction due to left sided carcinoma of the colon. Ann R Coll Surg Engl. 1988;70(1):40-43. [PMC free article] [PubMed] [Google Scholar]
- 15.Foo CC, Poon SHT, Chiu RHY, Lam WY, Cheung LC, Law WL. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction. Surg Endosc. 2019;33(1):293-302. doi: 10.1007/s00464-018-6487-3 [DOI] [PubMed] [Google Scholar]
- 16.Amelung FJ, Borstlap WAA, Consten ECJ, et al. ; Dutch Snapshot Research Group . Propensity score-matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg. 2019;106(8):1075-1086. doi: 10.1002/bjs.11172 [DOI] [PubMed] [Google Scholar]
- 17.Yang P, Lin XF, Lin K, Li W. The role of stents as bridge to surgery for acute left-sided obstructive colorectal cancer. Rev Invest Clin. 2018;70(6):269-278. doi: 10.24875/RIC.18002516 [DOI] [PubMed] [Google Scholar]
- 18.Dutch Snapshot Research Group . Benchmarking recent national practice in rectal cancer treatment with landmark randomized controlled trials. Colorectal Dis. 2017;19(6):O219-O231. doi: 10.1111/codi.13644 [DOI] [PubMed] [Google Scholar]
- 19.Amelung FJ, Borstlap WAA, Consten ECJ, et al. ; Dutch Snapshot Research Group . Population based comparison of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg. 2019;106(8):1075-1086. doi: 10.1002/bjs.11172 [DOI] [PubMed]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014;179(2):226-235. doi: 10.1093/aje/kwt212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abelson JS, Yeo HL, Mao J, Milsom JW, Sedrakyan A. Long-term postprocedural outcomes of palliative emergency stenting vs stoma in malignant large-bowel obstruction. JAMA Surg. 2017;152(5):429-435. doi: 10.1001/jamasurg.2016.5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amelung FJ, Ter Borg F, Consten EC, Siersema PD, Draaisma WA. Deviating colostomy construction versus stent placement as bridge to surgery for malignant left-sided colonic obstruction. Surg Endosc. 2016;30(12):5345-5355. doi: 10.1007/s00464-016-4887-9 [DOI] [PubMed] [Google Scholar]
- 26.Mege D, Sabbagh C, Manceau G, et al. ; AFC (French Surgical Association) Working Group . What is the best option between primary diverting stoma or endoscopic stent as a bridge to surgery with a curative intent for obstructed left colon cancer? results from a propensity score analysis of the French Surgical Association multicenter cohort of 518 patients. Ann Surg Oncol. 2019;26(3):756-764. doi: 10.1245/s10434-018-07139-0 [DOI] [PubMed] [Google Scholar]
- 27.Bhangu A, Nepogodiev D, Futaba K; West Midlands Research Collaborative . Systematic review and meta-analysis of the incidence of incisional hernia at the site of stoma closure. World J Surg. 2012;36(5):973-983. doi: 10.1007/s00268-012-1474-7 [DOI] [PubMed] [Google Scholar]
- 28.Amelung FJ, de Guerre LEVM, Consten ECJ, et al. Incidence of and risk factors for stoma-site incisional herniation after reversal. BJS Open. 2018;2(3):128-134. doi: 10.1002/bjs5.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasten KR, Midura EF, Davis BR, Rafferty JF, Paquette IM. Blowhole colostomy for the urgent management of distal large bowel obstruction. J Surg Res. 2014;188(1):53-57. doi: 10.1016/j.jss.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 30.van Hooft JE, Fockens P, Marinelli AW, et al. ; Dutch Colorectal Stent Group . Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40(3):184-191. doi: 10.1055/s-2007-995426 [DOI] [PubMed] [Google Scholar]
- 31.Sabbagh C, Browet F, Diouf M, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? Ann Surg. 2013;258(1):107-115. doi: 10.1097/SLA.0b013e31827e30ce [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28(3):407-414. doi: 10.1007/s00384-012-1556-x [DOI] [PubMed] [Google Scholar]
- 33.Matsuda A, Miyashita M, Matsumoto S, et al. Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction. Ann Surg Oncol. 2015;22(2):497-504. doi: 10.1245/s10434-014-3997-7 [DOI] [PubMed] [Google Scholar]
- 34.Ceresoli M, Allievi N, Coccolini F, et al. Long-term oncologic outcomes of stent as a bridge to surgery versus emergency surgery in malignant left side colonic obstructions. J Gastrointest Oncol. 2017;8(5):867-876. doi: 10.21037/jgo.2017.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of Baseline Characteristics Between Selected Patients Versus Unselected Patients After Propensity Score Matching, for DS and SEMS Separately
eTable 2. DS-Related Complications
eTable 3. SEMS-Specific Outcomes
eTable 4. Sub Analysis of Either Blowhole or Double Loop DS Versus SEMS Patients
eTable 5. Comparison of SEMS Patients in High-Volume Versus Low-Volume SEMS Centers
eFigure 1. Disease Free Survival (2A) and Overall Survival (2B) for DS Versus SEMS as BTS, Truncated at a Third of Patients at Risk (Propensity-Score Matched Samples)
eFigure 2. Stoma-Free Survival After Resection for DS Versus SEMS as BTS (Propensity-Score Matched)
eFigure 3. Flowchart of Patients With a Stoma In Situ Directly After Resection
eFigure 4. Disease Free Survival (3A) and Overall Survival (3B) Following SEMS Placement for High-Volume Versus Low-Volume SEMS Centers