This genome-wide association study examines the extent of overlap between the genetic architectures of major psychiatric disorders and body mass index and identifies common genetic loci.
Key Points
Question
Are there genome-wide genetic factors underlying both body mass index and major psychiatric disorders?
Findings
In this study of combined genome-wide association data from 1 380 284 individuals, genetic overlap between body mass index and major psychiatric disorders (ie, schizophrenia, bipolar disorder, and major depression) was found.
Meaning
These findings identify common genetic loci body mass index and major psychiatric disorders and indicate mixed association directions with mainly opposite associations in schizophrenia and body mass index.
Abstract
Importance
People with major psychiatric disorders (MPDs) have a 10- to 20-year shorter life span than the rest of the population, and this difference is mainly due to comorbid cardiovascular diseases. Genome-wide association studies have identified common variants involved in schizophrenia (SCZ), bipolar disorder (BIP), and major depression (MD) and body mass index (BMI), a key cardiometabolic risk factor. However, genetic variants jointly influencing MPD and BMI remain largely unknown.
Objective
To assess the extent of the overlap between the genetic architectures of MPDs and BMI and identify genetic loci shared between them.
Design, Setting, and Participants
Using a conditional false discovery rate statistical framework, independent genome-wide association study data on individuals with SCZ (n = 82 315), BIP (n = 51 710), MD (n = 480 359), and BMI (n = 795 640) were analyzed. The UK Biobank cohort (n = 29 740) was excluded from the MD data set to avoid sample overlap. Data were collected from August 2017 to May 2018, and analysis began July 2018.
Main Outcomes and Measures
The primary outcomes were a list of genetic loci shared between BMI and MPDs and their functional pathways.
Results
Genome-wide association study data from 1 380 284 participants were analyzed, and the genetic correlation between BMI and MPDs varied (SCZ: r for genetic = −0.11, P = 2.1 × 10−10; BIP: r for genetic = −0.06, P = .0103; MD: r for genetic = 0.12, P = 6.7 × 10−10). Overall, 63, 17, and 32 loci shared between BMI and SCZ, BIP, and MD, respectively, were analyzed at conjunctional false discovery rate less than 0.01. Of the shared loci, 34% (73 of 213) in SCZ, 52% (36 of 69) in BIP, and 57% (56 of 99) in MD had risk alleles associated with higher BMI (conjunctional false discovery rate <0.05), while the rest had opposite directions of associations. Functional analyses indicated that the overlapping loci are involved in several pathways including neurodevelopment, neurotransmitter signaling, and intracellular processes, and the loci with concordant and opposite association directions pointed mostly to different pathways.
Conclusions and Relevance
In this genome-wide association study, extensive polygenic overlap between BMI and SCZ, BIP, and MD were found, and 111 shared genetic loci were identified, implicating novel functional mechanisms. There was mixture of association directions in SCZ and BMI, albeit with a preponderance of discordant ones.
Introduction
Cardiometabolic comorbidity in major psychiatric disorders (MPDs) is a major public health concern. The high rates of cardiometabolic risk factors, particularly obesity,1,2 contribute greatly to cardiovascular disease among individuals with MPDs, which is a main cause of the 10- to 20-year shorter life expectancy.3,4 Cardiometabolic comorbidity and the associated mortality have remained high during the last decades,5,6,7 illustrating that most patients with MPDs such as schizophrenia (SCZ), bipolar disorder (BIP), and major depression (MD) have not benefited from the recent improvements in medicine. However, comorbidity may be a source of new pathophysiologic knowledge as indicated in recent genetic studies.8,9,10
Cardiometabolic risk factors and overt cardiovascular disease in MPD are closely linked to lifestyle, including diet, physical activity, and smoking habits. Further, several psychopharmacologic agents, in particular antipsychotics, are obesogenic and have metabolic adverse events.2 At the same time, the polygenic nature of MPDs11,12 and cardiometabolic risk factors13,14,15 is becoming increasingly clear. Both body mass index (BMI) obesity and MPDs have substantial heritability, estimated to be 24% to 90% for BMI,16 40% to 70% for obesity,17 31% to 42% for MD,18 79% to 93% for BIP,19 and 79% for SCZ.20 Various genetic studies have established a relationship between BMI and MPD,21,22,23,24 and increased weight has been associated with treatment response across MPDs.25,26 Owing to the strong association between obesity and MPD, neurobiologic hypotheses associated with potential underlying mechanisms have been proposed.27 However, the relationship is complex, as both weight gain and loss are associated with depressive episodes, and there seems to be a link between low BMI and SCZ,28,29 recently supported by genetic studies.30,31 A bidirectional relationship is also suggested from studies of obesity and depression.32
New powerful statistical methods specifically designed to analyze the polygenic architectures of complex traits have enabled improved gene discovery and replication rates.8,33,34 Specifically, we have shown how genetic pleiotropy enrichment increases the statistical power for identifying shared genetic variants.8,34 By assessing the contribution of all single-nucleotide polymorphisms (SNPs) from 2 independent genome-wide association studies (GWAS), we can determine their common associations, both in the presence and in the absence of overall genetic correlation.34,35,36,37
In this study, we analyzed GWAS summary statistics of BMI, SCZ, BIP, and MD using the pleiotropy-based conditional and conjunctional false discovery rate (FDR) statistics8,34 to investigate the shared genetic architectures of BMI and SCZ, BIP, and MD. We hypothesized that the conditional and conjunctional FDR statistics would enhance the discovery of genetic loci for BMI and MPDs, thereby disclosing more of their shared polygenic architecture independent of their overall association directions.
Methods
Genome-wide Association Study Samples
We used GWAS summary statistics results for BMI,38 SCZ,11 BIP,39 and MD.40 The BMI data were obtained from GIANT and UK BioBank (n = 795 640). The MD data were generated by meta-analyzing the Psychiatric Genomics Consortium GWAS and the 23andMe GWAS, which also included cases with self-reported depression. The meta-analysis was performed using the inverse-weighted fixed-effects model implemented in the software METAL (http://csg.sph.umich.edu//abecasis/Metal/).41 GWAS summary statistics for SCZ11 and BIP39 were provided by the Psychiatric Genomics Consortium. All GWASs performed and investigated in the present study were approved by the local ethics committees, and written informed consent was obtained from all participants. Additional details are provided in the original GWAS articles.11,38,39,40 Furthermore, the Norwegian Institutional Review Board for the southeast Norway region evaluated the current protocol and found that no additional institutional review board approval was needed because no individual data were used. Data were collected from August 2017 to May 2018.
Statistical Analyses
Analysis began July 2018. To visually assess the presence of enrichment, we generated conditional quantile-quantile (Q-Q) plots,8 conditioning BMI on SCZ, BIP, and MD and vice versa. We also estimated the genetic correlation between BMI and SCZ, BIP, and MD using linkage disequilibrium (LD) score regression.42,43 Details about these methods can be found in the eMethods in Supplement 1.
To improve the discovery of genetic variants associated with BMI and SCZ, BIP, and MD, we computed conditional FDR statistics.8 The conditional FDR method builds on an empirical Bayesian statistical framework and uses GWAS summary statistics from a trait of interest (eg, BMI) together with those of a conditional trait (eg, MD) to estimate the posterior probability that a SNP has no association with the primary trait, given that the P values for that SNP in both the primary and conditional traits are as small as or smaller than the observed P value. Thus, the method improves the detection of genetic variants associated with the primary trait via reranking the test statistics of the primary phenotype based on the strength of the association with the secondary phenotype.
To determine any loci likely to be shared by 2 phenotypes, we computed conjunctional FDR statistics.8,34,35 The conjunctional FDR is an extension of the conditional FDR and is defined as the maximum of the 2 conditional FDR statistics for a specific SNP and estimates the posterior probability that a SNP is null for either trait or both, given that the P values for both phenotypes are as small as or smaller than the P values for each trait individually. For more details, see the original8 and subsequent publications.35,44,45 In the present study, we used a conservative FDR level of 0.01 per pairwise comparison for conditional FDR and conjunctional FDR, but for subsequent analyses of association directions, we also included shared SNPs with conjunctional FDR less than 0.05. Manhattan plots were constructed based on the conjunctional FDR to show the genomic location of the shared genetic loci.8
All analyses were performed after excluding SNPs in the extended major histocompatibility complex (hg19 location chr 6: 25119106-33854733) and the 8p23.1 (hg19 location chr 8: 7242715-12483982) regions to avoid potential biases owing to intricate LD patterns.
Genomic Loci Definition and Functional Annotation
To define the independent genomic loci, we used FUMA.46 Single-nucleotide polymorphisms with FDR less than 0.01 and at LD r2 < 0.6 with each other were considered as independent significant SNPs, and a fraction of the independent significant SNPs in approximate LD with each other at r2 < 0.1 were considered lead SNPs. We outlined the distinct genomic loci and their borders based on FUMA’s default parameters.46
FUMA was also deployed to annotate the significantly associated SNPs with functional categories, Combined Annotation Dependent Depletion scores,47 RegulomeDB scores,48 and chromatin states.49,50 A Combined Annotation Dependent Depletion score above 12.37 shows an association of deleterious protein with outcomes.47 The RegulomeDB score indicates the regulatory functionality of SNPs based on expression quantitative trait loci and chromatin marks.48 The chromatin state indicates the accessibility of genomic regions using 15 categories, as predicted by ChromHMM based on 5 chromatin marks for 127 epigenomes.49,50 To place them in potential biologic context, we matched the candidate loci to the brain tissue expression quantitative trait loci (expression quantitative trait loci) databases from GTEx (http://gtexportal.org) and Braineac (http://www.braineac.org).51,52 Further details are provided in the eMethods in Supplement 1.
To identify overrepresented pathways for the genes nearest the identified shared loci for each MPD, we used ConsensusPathDB.53 All analyses were corrected for multiple comparisons. Significance was set at a 2-sided P value less than .05.
Results
The MD sample originally consisted of 135 458 individuals with MD and 344 901 controls.40 To avoid sample overlaps, we excluded the UK Biobank cohort (n = 29 740). The SCZ sample included 34 241 individuals with SCZ and 45 604 controls.11 The BIP sample consisted of 20 352 individuals with BIP and 31 358 controls.39 A total of 1 380 284 individuals were included in this analysis.
Genetic Overlap and Correlation Between BMI and MPDs
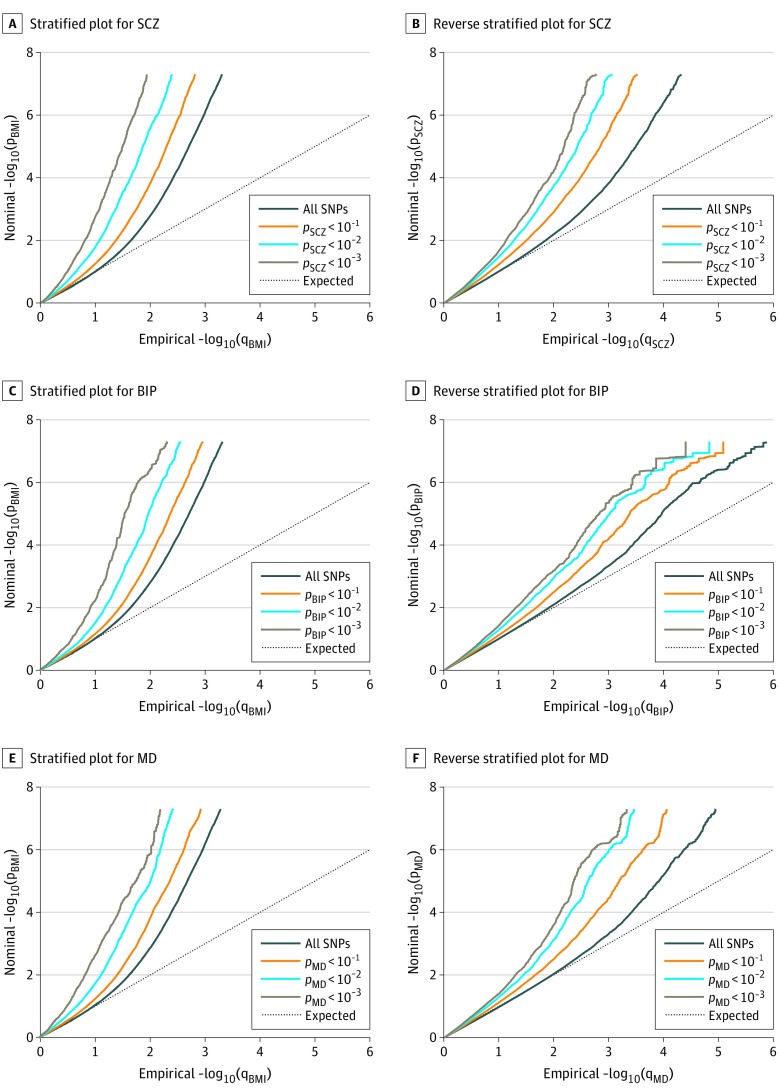
The stratified Q-Q plots indicate successive increments of SNP enrichment for BMI conditioned on association P values for SCZ, BIP, and MD (Figure 1A, C, and E). These suggest polygenic overlap between the phenotypes. The reverse stratified Q-Q plots also display enrichment of associations for SCZ, BIP, and MD given BMI (Figure 1B, D, and F).
Figure 1. Conditional Quantile-Quantile Plots.
A, C, and E, Conditional quantile-quantile plots of nominal vs empirical body mass index (BMI) −log10 P values (corrected for inflation) below the standard genome-wide association study threshold of P < 5 × 10−8 as a function of the significance of the association with schizophrenia (SCZ), bipolar disorder (BIP), and major depression (MD) at the level of P ≤ .10, P ≤ .01, and P ≤ .001, respectively. B, D, and F, Conditional quantile-quantile plots of nominal vs empirical SCZ, BIP, and MD −log10 P values (corrected for inflation) below the standard genome-wide association study threshold of P < 5 × 10−8 as a function of the significance of the association with BMI, at the level of P ≤ .10, P ≤ .01, and P ≤ .001, respectively. The dashed line indicates the null hypothesis. SNP indicates single-nucleotide polymorphism.
Successive leftward shifts from the dashed line of no association for decreasing nominal BMI P values indicate that the proportion of non-null SNPs in BMI increase with higher levels of association with the MPDs (SCZ, BIP, or MD) (Figure 1).
We used partitioned LD score regression43 to assess the statistical significance of the enrichment for the Q-Q plot strata and the analyses returned significant enrichment for BMI given SCZ, BIP, and MD as well as for SCZ, BIP, and MD given BMI for all strata (eTables 1, 10, and 17 in Supplement 1).
Genome-wide LD score regression analyses showed significant negative genetic correlation between BMI and SCZ (r for genetic = −0.11; P = 2.4909 × 10−10), nonsignificant negative genetic correlation between BMI and BIP (r for genetic = −0.06; P = .0103), and significant positive genetic correlation between BMI and MD (r for genetic = 0.12; P = 6.7040 × 10−10).
BMI-Associated Loci Identified With Conditional FDR
To enhance the discovery of genetic variants associated with BMI and MPDs, we applied the conditional FDR statistical analysis on the association of BMI with MPDs, and vice versa. At conditional FDR less than 0.01, we identified 723, 679, and 710 loci associated with BMI conditionally on SCZ, BIP, and MD, respectively (Supplement 2, Supplement 4, and Supplement 6). The reversed conditional FDR analysis showed 170, 52, and 70 loci associated with SCZ, BIP, and MD, respectively, conditionally on BMI (eTables 2, 11, and 18 in Supplement 1).
Loci Shared Between BMI and MPDs
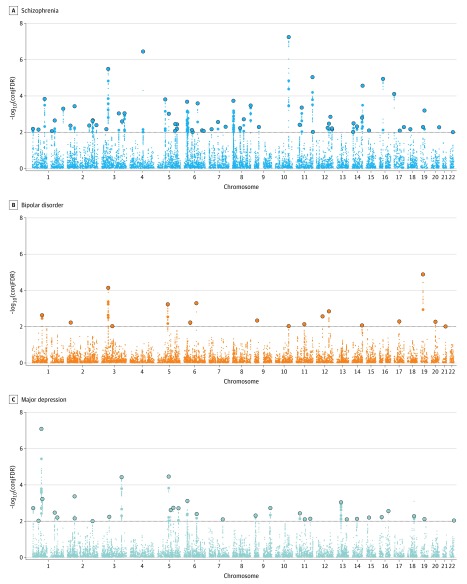
To identify loci shared between BMI and MPDs, we performed conjunctional FDR analyses. A total of 63 distinct loci were shared between BMI and SCZ at conjunctional FDR less than 0.01; of those, 12 were not identified in the original BMI GWAS,38 26 were not identified in the original SCZ GWAS,11 and 7 were novel for both phenotypes (Figure 2 and eTable 3 in Supplement 1). Seventeen loci emerged as shared between BMI and BIP at conjunctional FDR less than 0.01 (Figure 2 and eTable 12 in Supplement 1). One of these was not significantly associated in the original BMI GWAS, while 9 were not identified in the original BIP GWAS39 (eTable 12 in Supplement 1). Finally, 32 distinct genomic loci were associated with both BMI and MD at conjunctional FDR less than 0.01 (Figure 2 and eTable 19 in Supplement 1); 6 of these were not identified in the original BMI GWAS, 14 were novel for MD,40 and 4 were novel for both traits. All identified shared SNPs at conjunctional FDR less than 0.05 are reported in eTables 4, 13, and 20 in Supplement 1.
Figure 2. Common Genetic Variants Jointly Associated With Body Mass Index, Schizophrenia, Bipolar Disorder, and Major Depression at Conjunctional False Discovery Rate (conjFDR) Less Than 0.01.
Manhattan plot showing the –log10 transformed conjFDR values for each single-nucleotide polymorphism on the y-axis and chromosomal positions along the x-axis. The dotted horizontal line represents the threshold chosen for reporting shared associations (conjFDR < 0.01). Independent lead single-nucleotide polymorphisms are encircled in outlined circle. The significant shared signal in the major histocompatibility complex region (chr6:25119106–33854733) is represented by 1 independent lead single-nucleotide polymorphism. Further details are provided in eTables 3, 12, and 19 in Supplement 1.
In addition to those shared with BMI, the MPDs had also several genetic loci shared among them. Of the SNPs in the loci shared by SCZ and BMI, 2 were observed in the original BIP39 and 3 in the MD GWASs.40 Seventeen of the shared loci were below conjunctional FDR 0.05 for BMI and BIP (eTable 3 in Supplement 1). Further, 12 of the loci shared between BMI and SCZ at conjunctional FDR less than 0.01 were below conjunctional FDR 0.05 for BMI and MD, and 6 of these were below conjunctional FDR 0.05 for BMI and BIP (eTable 3 in Supplement 1). Of the 9 new loci BIP shared with BMI, 2 were also identified in the conjunctional FDR analyses for BMI and SCZ and also for BMI and MD (eTable 12 in Supplement 1). Of the loci shared by MD and BMI, 9 were identified in the conjunctional FDR analysis for BMI and SCZ and 2 of these were novel for MD (eTable 19 in Supplement 1). Of the 4 novel loci, 1 was also identified at conjunctional FDR 0.05 for BMI and SCZ. However, 3 and 1 of the 32 shared loci were identified in the original SCZ and BIP GWASs, respectively (eTable 19 in Supplement 1).
By comparing the association directions of the top lead SNPs at conjunctional FDR less than 0.05, a clear pattern of mixed association directions appears, with SNPs with concordant association direction in 73 of 213 loci (34%) in BMI and SCZ, 56 of 99 loci (57%) in BMI and MD, and 36 of 69 loci (52%) in BMI and BIP (eTables 4, 13, and 20 in Supplement 1). This can explain the observed negative, negligible, and positive genetic correlations between BMI and SCZ, BMI and BIP, and BMI and MD, respectively.
Annotation of Loci Shared Between BMI and the MPDs
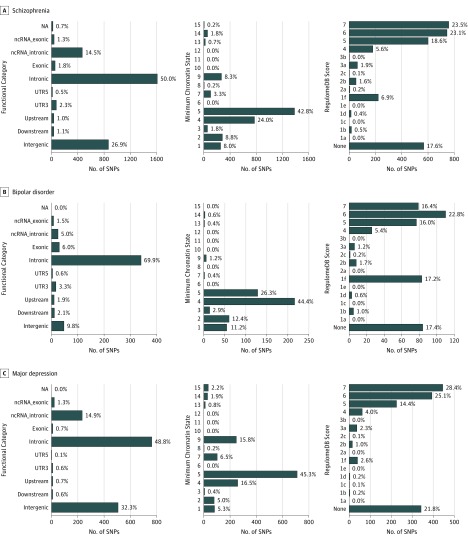
The functional annotation of all SNPs at conjunctional FDR less than 0.10 for BMI vs SCZ, BIP, and MD are shown in Figure 3, Supplement 3, Supplement 5, and Supplement 7. The shared loci revealed variants associated with brain tissue gene expression and several biologic and molecular processes including central nervous system development, hormone, GABAergic and glutamatergic signaling, and intracellular processes. Interestingly, the analyses of genes with concordant and opposite association directions for BMI and the MPDs indicated only minor overlap in the overrepresented pathways. More genetic analyses are provided in the eResults and eTables 5, 6, 8, 9, 14, 15, 21, and 22 in Supplement 1.
Figure 3. Distribution of the Annotation for All SNPs Jointly Associated With Body Mass Index and Schizophrenia, Bipolar Disorder, and Major Depression at Conjunctional False Discovery Rate Less Than 0.10 Including Functional Consequences of SNPs.
A low RegulomeDB score indicates a higher likelihood of having a regulatory function. A lower minimum chromatin state across 127 tissue and cell types indicates higher accessibility, and states 1 to 7 refer to open chromatin states. NA indicates not applicable; ncRNA, noncoding RNA; SNP, single-nucleotide polymorphism; UTR, untranslated region.
Pathway Analysis of Loci Shared Between BMI and the MPDs
To determine the overrepresented pathways among the genes nearest the identified loci shared between BMI and MPDs at conjunctional FDR less than 0.01, we carried out pathway overrepresentation analyses. There were 20 pathways significantly overrepresented among the genes nearest the identified loci shared between BMI and SCZ with the proton-pump inhibitor pathway and AKT phosphorylates targets in the nucleus pathway as the most significant (eTable 7 in Supplement 1). We found the neural cell adhesion molecule signaling for neurite outgrowth pathway and L1 cell adhesion molecule interactions pathway to be significantly overrepresented among the genes nearest the 17 loci shared between BMI and BIP (P = .001, Q = 0.007 and P = .003, Q = 0.011, respectively) (eTable 16 in Supplement 1). Finally, the RAB guanine nucleotide exchange factors exchange GTP for GDP on RABs pathway and RAB regulation of trafficking pathway were significantly overrepresented among the genes nearest the loci shared between BMI and MD (P = 9.29 × 10−5, Q = 0.000557 and P = 0.000244, Q = 0.000731, respectively) (eTable 23 in Supplement 1). For analysis of loci with concordant and opposite association directions respectively, see eResults in Supplement 1.
Discussion
In the current study, we demonstrated polygenic overlap between BMI and MPDs. We identified 63 genetic loci shared between BMI and SCZ, 17 loci shared between BMI and BIP, and 32 loci shared between BMI and MD (conjunctional FDR < 0.01). There was a striking pattern of bidirectional associations for the shared loci. In total, 57% of the shared MD SNPs had positive associations with BMI, against 52% of the shared BIP SNPs and as few as 34% of the shared SCZ SNPs (conjunctional FDR < 0.05), suggesting different genetic liability to weight gain across these disorders.
The current findings of polygenic overlap between BMI and MPDs are in line with epidemiologic evidence of associations between BMI and psychotic and affective disorders,1,22,54 which has been suggested as an important contributor to increased cardiovascular morbidity and mortality in MPDs. However, the current findings of a mixture of association directions for the shared loci underscores the complexity of this genetic relationship and suggest that factors other than disease-specific genetics play a significant role in weight gain in MPDs, particularly in SCZ. Most (66%) of the SNPs overlapping between BMI and SCZ (conjunctional FDR < 0.05) have opposite association directions, in line with the estimated negative genetic correlation (r for genetic = −0.11). The finding has clinical implications suggesting that factors such as antipsychotic treatment, diet, or lifestyle may be the main drivers of weight gain in treated patient groups with long-term disease. Further, low BMI has been indicated as a risk factor for SCZ,55,56,57 and there seems to be an increased frequency of underweight in patients with SCZ.29,58 Body mass index forms the basis of nutritional status definitions, and genetic loci associated with variation in BMI could be associated with clinical subphenotypes ranging from underweight to obesity. Weight gain associated with negative symptoms in SCZ59 may constitute such a subgroup. The current findings could be of relevance for the variation in proneness to weight gain during antipsychotic medication, which seems to be partly genetically mediated.60
Our findings of overlapping genetic architecture between BMI and MD and BIP are in line with clinical and epidemiologic evidence.1,22,54 Most BIP and MD loci shared with BMI (52% and 57%, respectively) have positive associations with BMI, while only MD showed significant positive genetic correlation with BMI (r for genetic = 0.12). The mixed association directions can explain the clinical features of both loss and gain of weight during a depressive episode.61 A recent mendelian randomization study indicated elevated BMI as a causal factor in the development of depression.62 Moreover, our results are supported by findings of preponderance of genetic risk variants for BMI in MD with the atypical features weight gain and/or increased appetite,63 suggesting genetically determined subphenotypes. It is also of interest that several drugs used in the treatment of affective disorders are prone to weight gain adverse events, including antidepressants and mood stabilizers.
Our results go beyond standard genetic correlations as the conditional FDR tool can assess the direction of association of each overlapping genetic variant, independently of the overall genetic correlation. For example, despite the lack of genetic correlation in BIP and BMI (r for genetic = −0.06), we identified 17 loci shared by the 2. The discovery of shared loci may form the basis for developing risk prediction tools of comorbid obesity, enabling targeted interventions of importance for cardiometabolic health. Of interest, we observed that several of the discovered risk loci are shared across the MPDs, implying common mechanisms of comorbid obesity across several mental disorders.64 The notion of common genetic mechanisms across mental illness is supported by recent gene expression studies65 as well as the recent finding of overlapping SNPs from the large Psychiatric Genomics Consortium cross-disorder group66 showing moderate to high genetic correlations between BIP, MD, and SCZ. However, a major part of the genetic loci are still to be discovered.11 A large Swedish registry-based study recently confirmed the shared genetic liability between BIP and MD/SCZ, respectively.67
The functional annotations of the shared loci revealed variants associated with brain tissue gene expression and several biologic and molecular processes including central nervous system development, hormone, GABAergic, and glutamatergic signaling, and intracellular processes. The identification of glucocorticoid receptor binding with variants conveying similar association directions in BIP and BMI may be of special clinical interest68,69 given the established role of the hypothalamic-pituitary-adrenal axis in MPD68,70,71 and the hypothalamic-pituitary-adrenal axis’s suggested contribution to obesity in MPD.27 Further, a large proportion of the shared genetic loci are brain-related, suggesting that BMI regulation is for a large part involving brain-related mechanisms.
Here, we used summary statistics from GWAS of large cohorts, which we screened for overlapping samples. However, some cryptic overlap may persist across BMI and mental disorders samples owing to the comorbidity of these phenotypes. In fact, weight disturbances are often included in mental illness syndromes (eg, diagnostic criteria in MD). Owing to their more severely impaired functioning, individuals with SCZ are less likely to participate in scientific studies.72 Therefore, lower cryptic overlap is to be expected for SCZ than for MD, with BIP somewhere in between. As the level of genetic overlap will increase with increasing degree of sample overlap, the number of shared genetic loci may be overestimated, especially for MD whose samples include individuals with less severe conditions. Further, brain function determines behavior, which is a key factor in lifestyle choices such as diet and exercise, which in turn affect BMI. Thus, it is possible that similar brain mechanisms are involved in behavior related to mental illness and BMI. Although we were able to identify overlapping genetic loci and the direction of their association, the complexity of the mechanisms underlying the respective conditions and their comorbidity are apparent, as is the need for detailed characterized samples to provide additional insight. As the pathophysiologic mechanisms of the studied phenotypes are not fully known, we are cautious about claiming a specific type of pleiotropy73 based on findings of shared genetic loci.
Limitations
As with all GWAS findings, any SNP represents through LD a genomic region including potentially many causal SNPs. Hence, further studies are required to determine the true causal variants underlying the shared associations detected here and whether the same causal variants are involved in BMI, SCZ, BIP, and MD. Furthermore, it is challenging to evaluate small effect sizes and to speculate about molecular mechanisms behind the effective variants when examining such potentially overlapping phenotypes.
Conclusions
Here, we demonstrate extensive genetic overlap between BMI and psychotic and affective disorders with a striking pattern of bidirectional associations, suggesting a complex interplay of metabolism-related gene pathways in the pathophysiology of SCZ, BIP, and MD. While two-thirds of the genetic overlap had opposite associations directions in SCZ and BMI, suggesting environmental causes of observed weight gain rather than disease-specific genetics, most had concordant association directions in BMI and BIP and MD, pointing to genetic susceptibility as a potential cause of weight gain. The findings have implications for the discovery of drugs with fewer adverse events and potential future individualized treatment to reduce weight gain.
eMethods
eResults.
eReferences.
eTable 1. Test for enrichment of strata in the QQ plots for BMI and SCZ
eTable 2. Distinct genomic loci associated with SCZ condFDR<0.01 given association with BMI
eTable 3. Distinct genomic loci associated with both BMI and SCZ at conjFDR<0.01
eTable 4. Distinct genomic loci associated with both BMI and SCZ at conjFDR<0.05
eTable 5. Significant eQTL functionality of SNPs in conj_BMI_SCZ in the GTEx database
eTable 6. cisEQTL- SNPs in conj_BMI_SCZ in the Braineac database
eTable 7. Gene Ontology gene-sets significantly associate with genes nearest loci in conj_BMI_SCZ at conjFDR<0.01
eTable 8. Pathway analysis for gene-set with concordant effect direction in conj_BMI vs MPDs at conjFDR<0.05
eTable 9. Pathway analysis for gene-set with opposite effect direction in conj_BMI vs MPDs at conjFDR<0.05
eTable 10. Test for enrichment of strata in the QQ plots for BMI and BIP
eTable 11. Distinct genomic loci associated with BIP condFDR<0.01 given association with BMI
eTable 12. Distinct genomic loci associated with both BMI and BIP at conjFDR<0.01
eTable 13. Distinct genomic loci associated with both BMI and BIP at conjFDR<0.05
eTable 14. Significant eQTL functionality of SNPs in conj_BMI_BIP in the GTEx database
eTable 15. cisEQTL- SNPs in conj_BMI_BIP in the Braineac database
eTable 16. Gene Ontology gene-sets significantly associated with genes nearest loci in conj_BMI_BIP at conjFDR<0.01
eTable 17. Test for enrichment of strata in the QQ plots for BMI and MD
eTable 18. Distinct genomic loci associated with major depression(MD) condFDR<0.01 given association with BMI
eTable 19. Distinct genomic loci associated with both BMI and major depression (MD) at conjFDR<0.01
eTable 20. Distinct genomic loci associated with both BMI and major depression (MD) at conjFDR<0.05
eTable 21. Significant eQTL functionality of SNPs in conj_BMI_major depression (MD) in the GTEx database
eTable 22. cisEQTL- SNPs in conj_BMI_major depression (MD) in the Braineac database
eTable 23. Gene Ontology gene-sets significantly associated with genes nearest loci in conj_BMI_major depression (MD) at conjFDR<0.01; pathway analysis for gene-sets significantly associated with genes nearest loci in conj_BMI_MDD at conjFDR<0.01
eTable
eTable
eTable
eTable
eTable
eTable
References
- 1.McElroy SL, Keck PE Jr. Obesity in bipolar disorder: an overview. Curr Psychiatry Rep. 2012;14(6):650-658. doi: 10.1007/s11920-012-0313-8 [DOI] [PubMed] [Google Scholar]
- 2.Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease: a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. 2014;5:137. doi: 10.3389/fpsyt.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry. 2011;199(6):453-458. doi: 10.1192/bjp.bp.110.085100 [DOI] [PubMed] [Google Scholar]
- 4.Laursen TM, Plana-Ripoll O, Andersen PK, et al. Cause-specific life years lost among persons diagnosed with schizophrenia: is it getting better or worse? Schizophr Res. 2019;206:284-290. doi: 10.1016/j.schres.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One. 2011;6(9):e24597. doi: 10.1371/journal.pone.0024597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rødevand L, Steen NE, Elvsåshagen T, et al. Cardiovascular risk remains high in schizophrenia with modest improvements in bipolar disorder during past decade. Acta Psychiatr Scand. 2019;139(4):348-360. doi: 10.1111/acps.13008 [DOI] [PubMed] [Google Scholar]
- 7.Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350-1363. doi: 10.1001/jamapsychiatry.2014.1314 [DOI] [PubMed] [Google Scholar]
- 8.Andreassen OA, Djurovic S, Thompson WK, et al. ; International Consortium for Blood Pressure GWAS; Diabetes Genetics Replication and Meta-analysis Consortium; Psychiatric Genomics Consortium Schizophrenia Working Group . Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92(2):197-209. doi: 10.1016/j.ajhg.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreassen OA, Harbo HF, Wang Y, et al. ; Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; International Multiple Sclerosis Genetics Consortium (IMSGC) . Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20(2):207-214. doi: 10.1038/mp.2013.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709-717. doi: 10.1038/ng.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JR, Cai Y, Zhang Q, Zhang W, Nogales-Cadenas R, Zhang ZD. Integrated post-GWAS analysis sheds new light on the disease mechanisms of schizophrenia. Genetics. 2016;204(4):1587-1600. doi: 10.1534/genetics.116.187195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreassen OA, McEvoy LK, Thompson WK, et al. ; International Consortium for Blood Pressure Genome-Wide Association Studies, Genetic Factors for Osteoporosis Consortium . Identifying common genetic variants in blood pressure due to polygenic pleiotropy with associated phenotypes. Hypertension. 2014;63(4):819-826. doi: 10.1161/HYPERTENSIONAHA.113.02077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidel E, Scholl UI. Genetic mechanisms of human hypertension and their implications for blood pressure physiology. Physiol Genomics. 2017;49(11):630-652. doi: 10.1152/physiolgenomics.00032.2017 [DOI] [PubMed] [Google Scholar]
- 15.Paththinige CS, Sirisena ND, Dissanayake V. Genetic determinants of inherited susceptibility to hypercholesterolemia: a comprehensive literature review. Lipids Health Dis. 2017;16(1):103. doi: 10.1186/s12944-017-0488-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elks CE, den Hoed M, Zhao JH, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne). 2012;3:29. doi: 10.3389/fendo.2012.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comuzzie AG, Allison DB. The search for human obesity genes. Science. 1998;280(5368):1374-1377. doi: 10.1126/science.280.5368.1374 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552-1562. doi: 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 19.Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164(1):331-343. doi: 10.1016/j.neuroscience.2009.03.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoge EA, Pollack MH, Kaufman RE, Zak PJ, Simon NM. Oxytocin levels in social anxiety disorder. CNS Neurosci Ther. 2008;14(3):165-170. doi: 10.1111/j.1755-5949.2008.00051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera M, Locke AE, Corre T, et al. Interaction between the FTO gene, body mass index and depression: meta-analysis of 13701 individuals. Br J Psychiatry. 2017;211(2):70-76. doi: 10.1192/bjp.bp.116.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afari N, Noonan C, Goldberg J, et al. Depression and obesity: do shared genes explain the relationship? Depress Anxiety. 2010;27(9):799-806. doi: 10.1002/da.20704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuellar-Barboza AB, Winham SJ, McElroy SL, et al. Accumulating evidence for a role of TCF7L2 variants in bipolar disorder with elevated body mass index. Bipolar Disord. 2016;18(2):124-135. doi: 10.1111/bdi.12368 [DOI] [PubMed] [Google Scholar]
- 24.Williams MJ, Klockars A, Eriksson A, et al. The drosophila ETV5 homologue Ets96B: molecular link between obesity and bipolar disorder. PLoS Genet. 2016;12(6):e1006104. doi: 10.1371/journal.pgen.1006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong C, Park C, Rosenblat JD, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health. 2018;15(4):E771. doi: 10.3390/ijerph15040771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raben AT, Marshe VS, Chintoh A, Gorbovskaya I, Müller DJ, Hahn MK. The complex relationship between antipsychotic-induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front Neurosci. 2018;11:741. doi: 10.3389/fnins.2017.00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18-33. doi: 10.1038/s41380-018-0017-5 [DOI] [PubMed] [Google Scholar]
- 28.Padmavati R, McCreadie RG, Tirupati S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr Res. 2010;121(1-3):199-202. doi: 10.1016/j.schres.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 29.Inamura Y, Sagae T, Nakamachi K, Murayama N. Body mass index of inpatients with schizophrenia in Japan. Int J Psychiatry Med. 2012;44(2):171-181. doi: 10.2190/PM.44.2.h [DOI] [PubMed] [Google Scholar]
- 30.Duncan LE, Shen H, Ballon JS, Hardy KV, Noordsy DL, Levinson DF. Genetic correlation profile of schizophrenia mirrors epidemiological results and suggests link between polygenic and rare variant (22q11.2) cases of schizophrenia. Schizophr Bull. 2018;44(6):1350-1361. doi: 10.1093/schbul/sbx174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solmi F, Mascarell MC, Zammit S, Kirkbride JB, Lewis G. Polygenic risk for schizophrenia, disordered eating behaviours and body mass index in adolescents. Br J Psychiatry. 2019;215(1):428-433. doi: 10.1192/bjp.2019.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajan TM, Menon V. Psychiatric disorders and obesity: a review of association studies. J Postgrad Med. 2017;63(3):182-190. doi: 10.4103/jpgm.JPGM_712_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JZ, Hov JR, Folseraas T, et al. ; UK-PSCSC Consortium; International PSC Study Group; International IBD Genetics Consortium . Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670-675. doi: 10.1038/ng.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreassen OA, Thompson WK, Schork AJ, et al. ; Psychiatric Genomics Consortium (PGC); Bipolar Disorder and Schizophrenia Working Groups . Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. doi: 10.1371/journal.pgen.1003455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smeland OB, Bahrami S, Frei O, et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. [published online January 4, 2019]. Mol Psychiatry. 2019. doi: 10.1038/s41380-018-0332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schork AJ, Wang Y, Thompson WK, Dale AM, Andreassen OA. New statistical approaches exploit the polygenic architecture of schizophrenia: implications for the underlying neurobiology. Curr Opin Neurobiol. 2016;36:89-98. doi: 10.1016/j.conb.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin RL, Schijven D, van Rheenen W, et al. ; Project MinE GWAS Consortium; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Genetic correlation between amyotrophic lateral sclerosis and schizophrenia. Nat Commun. 2017;8:14774. doi: 10.1038/ncomms14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turcot V, Lu Y, Highland HM, et al. ; CHD Exome+ Consortium; EPIC-CVD Consortium; ExomeBP Consortium; Global Lipids Genetic Consortium; GoT2D Genes Consortium; EPIC InterAct Consortium; INTERVAL Study; ReproGen Consortium; T2D-Genes Consortium; MAGIC Investigators; Understanding Society Scientific Group . Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet. 2018;50(1):26-41. doi: 10.1038/s41588-017-0011-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl EA, Breen G, Forstner AJ, et al. Genomewide association study identifies 30 loci associated with bipolar disorder. Bio Rxiv. https://www.biorxiv.org/content/10.1101/173062v4. Published January 24, 2018. Accessed November 12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulik-Sullivan B, Finucane HK, Anttila V, et al. ; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3 . An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236-1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smeland OB, Frei O, Kauppi K, et al. ; NeuroCHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) Cognitive Working Group . Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74(10):1065-1075. doi: 10.1001/jamapsychiatry.2017.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shadrin AA, Smeland OB, Zayats T, et al. Novel loci associated with attention-deficit/hyperactivity disorder are revealed by leveraging polygenic overlap with educational attainment. J Am Acad Child Adolesc Psychiatry. 2018;57(2):86-95. doi: 10.1016/j.jaac.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310-315. doi: 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle AP, Hong EL, Hariharan M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790-1797. doi: 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kundaje A, Meuleman W, Ernst J, et al. ; Roadmap Epigenomics Consortium . Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317-330. doi: 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Zhang F, Hu H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481-487. doi: 10.1038/ng.3538 [DOI] [PubMed] [Google Scholar]
- 51.Battle A, Brown CD, Engelhardt BE, Montgomery SB; GTEx Consortium; Laboratory, Data Analysis & Coordinating Center (LDACC)—Analysis Working Group; Statistical Methods groups—Analysis Working Group; Enhancing GTEx (eGTEx) groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA; Biospecimen Collection Source Site—NDRI; Biospecimen Collection Source Site—RPCI; Biospecimen Core Resource—VARI; Brain Bank Repository—University of Miami Brain Endowment Bank; Leidos Biomedical—Project Management; ELSI Study; Genome Browser Data Integration & Visualization—EBI; Genome Browser Data Integration & Visualization—UCSC Genomics Institute, University of California Santa Cruz; Lead analysts; Laboratory, Data Analysis & Coordinating Center (LDACC); NIH program management; Biospecimen collection; Pathology; eQTL manuscript working group . Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204-213. doi: 10.1038/nature24277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramasamy A, Trabzuni D, Guelfi S, et al. ; UK Brain Expression Consortium; North American Brain Expression Consortium . Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17(10):1418-1428. doi: 10.1038/nn.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamburov A, Stelzl U, Lehrach H, Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013;41(database issue):D793-D800. doi: 10.1093/nar/gks1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population-based studies. Obes Rev. 2011;12(5):e438-e453. doi: 10.1111/j.1467-789X.2010.00843.x [DOI] [PubMed] [Google Scholar]
- 55.Sørensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Height, weight and body mass index in early adulthood and risk of schizophrenia. Acta Psychiatr Scand. 2006;114(1):49-54. doi: 10.1111/j.1600-0447.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 56.Zammit S, Rasmussen F, Farahmand B, et al. Height and body mass index in young adulthood and risk of schizophrenia: a longitudinal study of 1 347 520 Swedish men. Acta Psychiatr Scand. 2007;116(5):378-385. doi: 10.1111/j.1600-0447.2007.01063.x [DOI] [PubMed] [Google Scholar]
- 57.Verma SK, Subramaniam M, Liew A, Poon LY. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry. 2009;70(7):997-1000. doi: 10.4088/JCP.08m04508 [DOI] [PubMed] [Google Scholar]
- 58.Sugawara N, Maruo K, Sugai T, et al. Prevalence of underweight in patients with schizophrenia: A meta-analysis. Schizophr Res. 2018;195:67-73. doi: 10.1016/j.schres.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 59.Jakobsen AS, Speyer H, Nørgaard HCB, et al. Dietary patterns and physical activity in people with schizophrenia and increased waist circumference. Schizophr Res. 2018;199:109-115. doi: 10.1016/j.schres.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 60.Zhang JP, Lencz T, Zhang RX, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42(6):1418-1437. doi: 10.1093/schbul/sbw058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9:14. doi: 10.1186/1471-2458-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyrrell J, Mulugeta A, Wood AR, et al. Using genetics to understand the causal influence of higher BMI on depression. Int J Epidemiol. 2019;48(3):834-848. doi: 10.1093/ije/dyy223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milaneschi Y, Lamers F, Peyrot WJ, et al. ; CHARGE Inflammation Working Group and the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry. 2017;74(12):1214-1225. doi: 10.1001/jamapsychiatry.2017.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curran JE, McKay DR, Winkler AM, et al. Identification of pleiotropic genetic effects on obesity and brain anatomy. Hum Hered. 2013;75(2-4):136-143. doi: 10.1159/000353953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandal MJ, Haney JR, Parikshak NN, et al. ; CommonMind Consortium; PsychENCODE Consortium; iPSYCH-BROAD Working Group . Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359(6376):693-697. doi: 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landén M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;17(2):184-193. doi: 10.1111/bdi.12242 [DOI] [PubMed] [Google Scholar]
- 68.Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43(3):449-469. doi: 10.1017/S0033291712000955 [DOI] [PubMed] [Google Scholar]
- 69.Soria V, González-Rodríguez A, Huerta-Ramos E, et al. ; PNECAT Group . Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: a systematic review and narrative synthesis. Psychoneuroendocrinology. 2018;93:8-19. doi: 10.1016/j.psyneuen.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 70.Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191-218. doi: 10.1016/j.neubiorev.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 71.Belvederi Murri M, Prestia D, Mondelli V, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327-342. doi: 10.1016/j.psyneuen.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 72.Candilis PJ, Geppert CMA, Fletcher KE, Lidz CW, Appelbaum PS. Willingness of subjects with thought disorder to participate in research. Schizophr Bull. 2006;32(1):159-165. doi: 10.1093/schbul/sbj016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14(7):483-495. doi: 10.1038/nrg3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eResults.
eReferences.
eTable 1. Test for enrichment of strata in the QQ plots for BMI and SCZ
eTable 2. Distinct genomic loci associated with SCZ condFDR<0.01 given association with BMI
eTable 3. Distinct genomic loci associated with both BMI and SCZ at conjFDR<0.01
eTable 4. Distinct genomic loci associated with both BMI and SCZ at conjFDR<0.05
eTable 5. Significant eQTL functionality of SNPs in conj_BMI_SCZ in the GTEx database
eTable 6. cisEQTL- SNPs in conj_BMI_SCZ in the Braineac database
eTable 7. Gene Ontology gene-sets significantly associate with genes nearest loci in conj_BMI_SCZ at conjFDR<0.01
eTable 8. Pathway analysis for gene-set with concordant effect direction in conj_BMI vs MPDs at conjFDR<0.05
eTable 9. Pathway analysis for gene-set with opposite effect direction in conj_BMI vs MPDs at conjFDR<0.05
eTable 10. Test for enrichment of strata in the QQ plots for BMI and BIP
eTable 11. Distinct genomic loci associated with BIP condFDR<0.01 given association with BMI
eTable 12. Distinct genomic loci associated with both BMI and BIP at conjFDR<0.01
eTable 13. Distinct genomic loci associated with both BMI and BIP at conjFDR<0.05
eTable 14. Significant eQTL functionality of SNPs in conj_BMI_BIP in the GTEx database
eTable 15. cisEQTL- SNPs in conj_BMI_BIP in the Braineac database
eTable 16. Gene Ontology gene-sets significantly associated with genes nearest loci in conj_BMI_BIP at conjFDR<0.01
eTable 17. Test for enrichment of strata in the QQ plots for BMI and MD
eTable 18. Distinct genomic loci associated with major depression(MD) condFDR<0.01 given association with BMI
eTable 19. Distinct genomic loci associated with both BMI and major depression (MD) at conjFDR<0.01
eTable 20. Distinct genomic loci associated with both BMI and major depression (MD) at conjFDR<0.05
eTable 21. Significant eQTL functionality of SNPs in conj_BMI_major depression (MD) in the GTEx database
eTable 22. cisEQTL- SNPs in conj_BMI_major depression (MD) in the Braineac database
eTable 23. Gene Ontology gene-sets significantly associated with genes nearest loci in conj_BMI_major depression (MD) at conjFDR<0.01; pathway analysis for gene-sets significantly associated with genes nearest loci in conj_BMI_MDD at conjFDR<0.01
eTable
eTable
eTable
eTable
eTable
eTable