This cohort study provides a quantitative characterization and classification of central visual field loss among patients with end-stage glaucoma.
Key Points
Questions
What are the typical patterns of central visual field loss in end-stage glaucoma, and do deteriorations follow trajectories specific to their baseline patterns?
Findings
In this cohort study of 1103 patients with glaucoma, 14 central visual field patterns were determined by archetypal analysis, most of which preserve the inferotemporal regions entirely or partially. Initial encroachments on an intact central visual field at follow-up in end-stage glaucoma were more likely to be nasal loss.
Meaning
These results suggest that central visual field loss in end-stage glaucoma exhibits characteristic patterns that might be related to different subtypes, and initial central visual field loss is likely to be nasal loss.
Abstract
Importance
Although the central visual field (VF) in end-stage glaucoma may substantially vary among patients, structure-function studies and quality-of-life assessments are impeded by the lack of appropriate characterization of end-stage VF loss.
Objective
To provide a quantitative characterization and classification of central VF loss in end-stage glaucoma.
Design, Setting, and Participants
This retrospective cohort study collected data from 5 US glaucoma services from June 1, 1999, through October 1, 2014. A total of 2912 reliable 10-2 VFs of 1103 eyes from 1010 patients measured after end-stage 24-2 VFs with a mean deviation (MD) of −22 dB or less were included in the analysis. Data were analyzed from March 28, 2018, through May 23, 2019.
Main Outcomes and Measures
Central VF patterns were determined by an artificial intelligence algorithm termed archetypal analysis. Longitudinal analyses were performed to investigate whether the development of central VF defect mostly affects specific vulnerability zones.
Results
Among the 1103 patients with the most recent VFs, mean (SD) age was 70.4 (14.3) years; mean (SD) 10-2 MD, −21.5 (5.6) dB. Fourteen central VF patterns were determined, including the most common temporal sparing patterns (304 [27.5%]), followed by mostly nasal loss (280 [25.4%]), hemifield loss (169 [15.3%]), central island (120 [10.9%]), total loss (91 [8.3%]), nearly intact field (56 [5.1%]), inferonasal quadrant sparing (42 [3.8%]), and nearly total loss (41 [3.7%]). Location-specific median total deviation analyses partitioned the central VF into a more vulnerable superonasal zone and a less vulnerable inferotemporal zone. At 1-year and 2-year follow-up, new defects mostly occurred in the more vulnerable zone. Initial encroachments on an intact central VF at follow-up were more likely to be from nasal loss (11 [18.4%]; P < .001). One of the nasal loss patterns had a substantial chance at 2-year follow-up (8 [11.0%]; P = .004) to shift to total loss, whereas others did not.
Conclusions and Relevance
In this study, central VF loss in end-stage glaucoma was found to exhibit characteristic patterns that might be associated with different subtypes. Initial central VF loss is likely to be nasal loss, and 1 specific type of nasal loss is likely to develop into total loss.
Introduction
The preservation of the central visual field (VF) is particularly important for the quality of life of patients with glaucoma,1,2,3 especially in end-stage disease, when functionality largely depends on residual temporal vision. Therefore, enhanced understanding of patterns of central VF defects and their development over time is of great significance to improve management of end-stage glaucoma. Management of end-stage glaucoma is currently overlooked in clinical practice, even as recent advancements of retinal imaging show promise to monitor structural loss in advanced disease.4,5
To date, the most important work characterizing central VF loss has been conducted by Hood and coworkers.6,7 The investigators analyzed the association between macular structure and the 10-2 VF in eyes with normal 24-2 VF findings and identified 2 distinct zones associated with central vision. In particular, a predominantly inferior temporal zone less vulnerable to damage was described. The remaining region of 10-2 VF is described as more vulnerable to damage. The Hood model was validated for advanced and early glaucoma.6,7 In a subsequent study by Traynis and colleagues,8 68% of the abnormal 10-2 hemifields in mild glaucoma were arcuate like, 8% were widespread, and 25% were classified as other. However, what defects are present in end-stage glaucoma remains unclear.
Thus, the present study aims to quantitatively identify and systematically study central VF loss patterns and their shifts in end-stage glaucoma. First, we applied the unsupervised artificial intelligence method of archetypal analysis9,10 to characterize the spatial patterns of 10-2 VF loss in end-stage glaucoma. Then, we compared the 10-2 VF loss patterns with the more and less vulnerable zones proposed by the Hood model. Last, we quantified the development of 10-2 VF defects over time for the eyes with the patterns that preserve the entire less vulnerable zone at baseline.
Methods
The VFs used in this cohort study were measured from June 1, 1999, through October 1, 2014, and were obtained by the Glaucoma Research Network, a consortium consisting of the following institutions: Massachusetts Eye and Ear, Boston; Wilmer Eye Institute, Baltimore, Maryland; New York Eye and Ear Infirmary of Mount Sinai, New York, New York; Bascom Palmer Eye Institute, Miami, Florida; and Wills Eye Hospital, Philadelphia, Pennsylvania. The institutional review boards of each institution approved this retrospective study and waived the need for informed consent for the use of these deidentified patient data. This study adheres to the tenets of the Declaration of Helsinki11 as well as all federal and state laws. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants and Data
From the entire Glaucoma Research Network data set of Swedish interactive thresholding algorithm standard 24-2 and 10-2 VFs with stimulus size III measured with the Humphrey Field Analyzer (HFA; Carl Zeiss Meditec), all reliable VFs were selected. The reliability criteria for VF selection were fixation loss rate of 33% or less, false-negative rate of 20% or less, and false-positive rate of 20% or less.12,13,14,15,16,17 Although using strict reliability criteria can potentially exclude VFs that represent true defects in end-stage glaucoma, because poor VF reliability is known to be particularly prevalent in this disease phase,14,18,19 we intentionally retained criteria used in pre–end-stage glaucoma to ensure that the VFs included are sufficiently reliable.
Based on the definition from the US Social Security Administration, eyes with VF mean deviation (MD) of −22 dB or less on 30-2 VF testing are classified as legally blind.20 Because in clinical practice, glaucomatous visual loss is often assessed by the 24-2 pattern instead of the 30-2 pattern, the definition of legal blindness has frequently been extended to the 24-2 test as well.21,22 Therefore, we chose a 24-2 VF MD of −22 dB as the cutoff point for end-stage glaucoma. The 10-2 VFs with test dates on or after any 24-2 VFs with an MD of −22 dB or less were selected for our data analyses. The total deviation (TD) values were used as primary inputs for assessing VF loss pattern by archetypal analysis. All VFs were converted to right eye format and were accordingly analyzed and plotted in right eye format.
Statistical Analyses
Archetypal Analysis
An unsupervised machine learning method, archetypal analysis, was used to identify representative VF patterns in end-stage glaucoma. Compared with unsupervised artificial intelligence methods of axis learning (eg, principal component analysis,23 independent component analysis24) and center learning (eg, k-means,25 Gaussian mixture modeling26) that have been applied to analyze VF loss patterns, archetypal analysis determines representative VF patterns lying at the corners of the data space. The VF patterns identified by archetypal analysis closely resemble VF patterns identified by physicians10,27 and therefore are readily interpretable.28 Because VF loss occurs in stereotypical patterns that adhere to the anatomy of the visual pathways, this corner learning approach is particularly suited to detect glaucomatous and nonglaucomatous visual loss as well as artifactitious VF loss patterns. The archetypal VF patterns have been used to improve glaucoma diagnosis and progression detection.29,30
Cross-Sectional Analyses
The most recent reliable VF of each eye was used for identifying the representative patterns of VFs. Specifically, we applied the archetypal analysis to 10-2 VFs with test dates on or after any 24-2 VFs with an MD of −22 dB or less to determine the central VF loss patterns in end-stage glaucoma. Each 10-2 VF was decomposed as a weighted sum of the identified archetypes, and the archetype with the largest decomposition coefficient was assigned as the primary archetype for that VF. The optimal number k of representative patterns was determined by 10-fold cross-validation.31 The combined 24-2 and 10-2 VFs with the same test dates were also analyzed by archetypal analysis to obtain the correspondence between 24-2 and 10-2 patterns. The 10-2 VF defect patterns were then manually compared with the more and less vulnerable zones described by the Hood model,7 as well as the more and less vulnerable zones constructed by segmenting the mean TD plot over all 10-2 VFs by median value of the mean TDs at all test locations.
Longitudinal Analyses
For eyes with primary 10-2 VF patterns that preserve the entire less vulnerable zone at baseline, the development of 10-2 VF defects at 1- and 2-year follow-ups were quantified, which longitudinally validates whether the development of central VF defects mostly affects the more vulnerable zone compared with the less vulnerable zone. For the primary 10-2 archetype at baseline, we determined whether the archetype remained the same at follow-up or shifted into other primary archetypes with the χ2 test. In addition, when a primary archetype at baseline shifted into other primary archetypes at follow-up, we determined whether any of the other primary archetypes at follow-up had statistically higher or lower probability than random chance with the χ2 test. Two-sided P < .05 was considered statistically significant. Data were analyzed from March 28, 2018, through May 23, 2019.
Results
Cross-Sectional Analysis Results
The prevalence of eyes with a 24-2 VF MD of −22 dB or less in the Glaucoma Research Network data set was 4.2%. The mean (SD) age for patients with an MD of −22 dB or less at the most recent reliable 24-2 VF test was 68.0 (16.0) years. A total of 1103 eyes from 1010 patients (mean [SD] age, 70.4 [14.3] years; mean [SD] 10-2 MD, −21.5 [5.6] dB) had 10-2 VF on or after the date of the 24-2 test, which was 1103 (14.4%) of 7680 total eyes with a 24-2 VF MD of −22 dB or less.
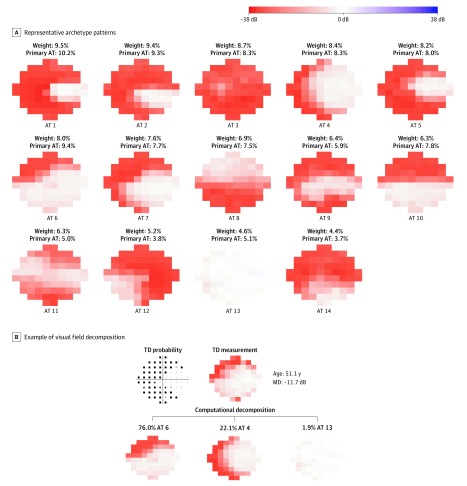
Figure 1A shows the 14 central VF patterns in end-stage glaucoma. The fourteen 10-2 VF archetypes are ordered by their respective decomposition weights. The fourteen 10-2 archetypes include nearly intact central vision (archetype 13), temporal-sparing loss (archetypes 1, 2, and 5), hemifield loss (archetypes 8 and 10), inferonasal quadrant sparing (archetype 12), central island loss (archetype 9 and 11), nasal loss (archetypes 4, 6, and 7), nearly total loss (archetype 14), and total loss (archetype 3). Figure 1B illustrates an example of 10-2 VF decomposition to those central VF patterns. The decomposition coefficient ranging from 0 to 100% represents the content percentage of a particular central VF pattern making up a central VF. The decomposition coefficients of all 14 central VF patterns (Figure 1A) add up to 100%. In other words, a central VF can be approximated by adding the multiplication of the 14 decomposition coefficients and their respective central VF patterns. In Figure 1B, a central VF was approximated as a linear combination of 76.0% of superonasal loss (archetype 6), 22.1% of bihemifield nasal loss (archetype 4), and 1.9% intact field (archetype 13).
Figure 1. The 14 Representative Archetype (AT) Patterns of 10-2 Visual Fields (VFs).
A, Visual fields were identified from the 1103 most recent VFs of 1103 eyes. B, An example of a VF decomposition to the 10-2 VF AT patterns. The 14 ATs are ordered by their mean contribution to all VFs. For each VF, the AT with the largest decomposition coefficient is assigned as the primary archetype for that VF. The percentage of each AT as the primary AT over all VFs is also reported. All VFs are plotted in right eye format. MD indicates mean deviation; TD, total deviation.
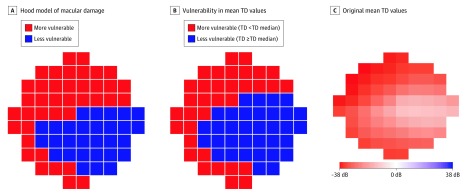
Figure 2A shows the more vulnerable (red) and less vulnerable (blue) zones of the 10-2 central VF according to the Hood model of macular damage in glaucoma.6,7 Figure 2B shows the more vulnerable (red; TD values are less than the TD median) and less vulnerable (blue; TD values are at least as great as the TD median) zones of the 10-2 VF by segmenting the mean TD values over 2912 10-2 VFs in end-stage glaucoma with the median value of the mean TD at all test locations. Figure 2C displays the original mean TD values over all 10-2 VFs. The less and more vulnerable zone derived by thresholding the mean TD values was highly consistent with the less vulnerable zone demonstrated in the Hood model.6,7
Figure 2. More Vulnerable and Less Vulnerable Zones of 10-2 Central Visual Field (VF).
A, The model of macular damage in glaucoma is described by Hood et al.7 B, The more vulnerable (total deviation [TD] values are less than TD median value) and less vulnerable (TD values are at least the TD median value) zones of 10-2 central VF according to the mean TD values over 2912 10-2 VFs in end-stage glaucoma. C, The original mean TD values over 2912 10-2 VFs in end-stage glaucoma are shown. All VFs are plotted in right eye format.
Four of the 14 central VF patterns (archetypes 4, 6, 7, and 13) preserved the main less vulnerable zone proposed by the Hood model, whereas only archetype 13 preserved the main more vulnerable zone. Seven central VF patterns (archetypes 1, 2, 3, 7, 8, 9, and 14) lost their main more vulnerable zone, whereas only 2 central VF patterns (archetypes 3 and 8) lost their less vulnerable zone. Furthermore, the 3 most frequent 10-2 archetypes by weights of 2 temporal sparing patterns (archetypes 1 and 2) and total loss (archetype 3) all lost their more vulnerable zone entirely, whereas archetypes 1 and 2 spared part of their less vulnerable zone. Compared with the mean more and less vulnerable zones proposed in the Hood model, the quantified 10-2 VF defects show that individual more and less vulnerable zones may have various spatial patterns. We also quantified the 24-2 VF patterns and the combined 24-2 and 10-2 VF patterns in end-stage glaucoma, which were detailed in eMethods and eFigures 1 and 2 in the Supplement.
Longitudinal Analysis Results
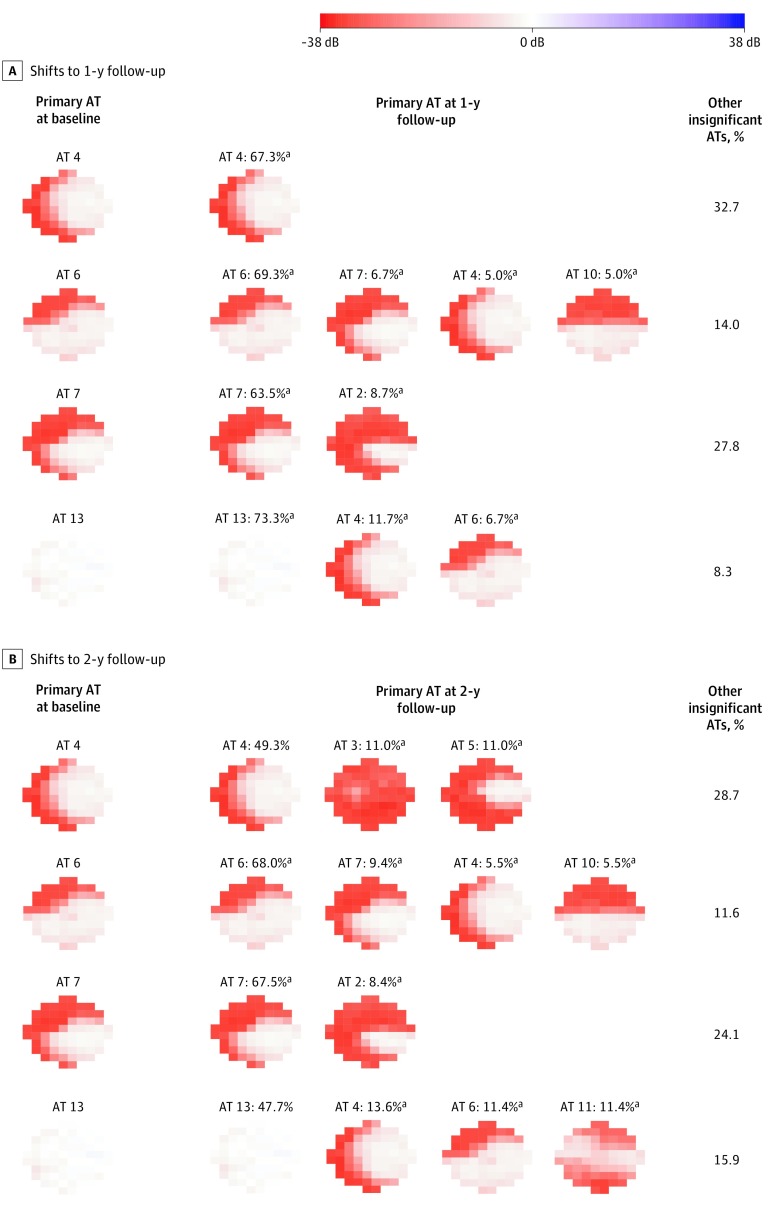
The primary 10-2 VF pattern shift from baseline VF to 1-year (1290 VFs) and 2-year (894 VFs) follow-up VFs were assessed for eyes with primary 10-2 VF patterns at baseline preserving the main less vulnerable zone by the χ2 test, namely the patterns of nasal loss (archetypes 4, 6, and 7) and the nearly intact vision of archetype 13. At the 1-year follow-up, shown in Figure 3A, eyes with archetypes 4 (67.3%), 6 (69.3%), 7 (63.5%), and 13 (73.3%) as the primary pattern were more likely (P < .001 for all) to remain the same than to shift to a different VF pattern. Among eyes with archetype 13 as the primary pattern, 7 (11.7%; P < .001) shifted to the nasal loss pattern consisting of archetype 4 and 4 (6.7%; P = .03), archetype 6. Among eyes with archetype 6 as the primary pattern, 12 (6.7%; P < .001) shifted to archetype 7, 9 (5.0%; P = .03) shifted to archetype 4, and 9 (5.0%; P = .03) shifted to archetype 10. Among eyes with archetype 7 as the primary pattern, 10 (8.7%; P < .001) shifted to archetype 2.
Figure 3. The 10-2 Primary Visual Field (VF) Pattern Shifts From Baseline to 1- and 2-Year Follow-up.
For any primary archetypes (ATs) at baseline, we determined whether the primary AT was more or less likely to stay the same at follow-up. In addition, when a primary baseline AT shifted into another archetype at follow-up, we determined whether this shift was more or less likely than chance alone. All VFs are plotted in right eye format.
aDenotes significant presence of follow-up patterns for each baseline pattern (P < .05).
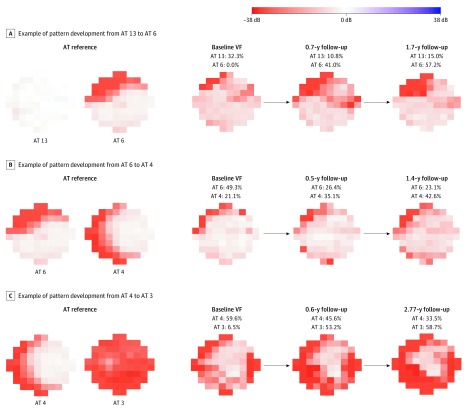
At the 2-year follow-up, shown in Figure 3B, eyes with archetypes 6 (87 [68.0%]) and 7 (56 [67.5%]) as the primary pattern were more likely (P < .001 for all) to remain the same as opposed to shifting to a different VF pattern, whereas eyes with archetypes 4 (36 [49.3%]) and 13 (21 [47.7%]) as the primary patterns were not. Among eyes with archetype 13 as the primary pattern, 6 (13.6%; P = .004) shifted to a nasal loss pattern consisting of archetype 4; 5 (11.4%; P = .03), archetype 6; and 5 (11.4%; P = .03), archetype 11. Among eyes with archetype 6 as the primary pattern, 12 (9.4%; P < .001) shifted to archetype 7, 7 (5.5%; P = .049) shifted to archetype 4, and 7 (5.5%; P = .049) shifted to archetype 10. Among eyes with archetype 7 as the primary pattern, 7 (8.4%; P = .001) shifted to archetype 2. Remarkably, among eyes with archetype 4 as the primary pattern, 8 (11.0%; P = .004) shifted to archetype 3 (total loss), and 8 (11.0%; P = .004) shifted to archetype 5 (temporal sparing). Figure 4 shows 3 examples of the pattern shift characterized by 10-2 primary archetypes shifting from nearly intact vision (archetype 13) to initial superior nasal loss (archetype 6), from initial superior nasal loss (archetype 6) to moderate nasal loss (archetype 4), and from moderate nasal loss (archetype 4) to total loss (archetype 3).
Figure 4. Three Examples of Primary Pattern Shift.
All visual fields (VFs) are plotted in right eye format. AT indicates archetype.
Discussion
In this cohort study, we have quantitatively determined the representative central VF patterns in end-stage glaucoma, including patterns of nearly intact VF, nearly total loss, total loss, temporal sparing, quadrant sparing, nasal loss, and central islands. Those central VF patterns were compared with the more and less vulnerable zones proposed by the Hood model.7 The central VF patterns show how individual more and less vulnerable zones present and vary in end-stage glaucoma. Furthermore, for eyes with the 4 primary VF patterns preserving the main less vulnerable zone, the development trajectories from baseline to 1- and 2-year follow-up were quantified. Statistical tests were applied to ensure that those pattern shifts were not simply due to random measurement noise or test-retest variability.
Hood and colleagues7 previously introduced a model of glaucomatous central VF loss that associates retinal structure with visual function. Based on visual inspection of patients with glaucoma with central visual loss, they reported a specific area of the 10-2 VF to be specifically vulnerable and another area to be less vulnerable to visual loss (Figure 2A). We partitioned the 10-2 locations of our end-stage VFs by median TDs into more and less affected areas and obtained 2 coherent VF areas that are similar to the visual inspection–based Hood model (Figure 2B). Although this quantitative confirmation of the Hood model is interesting, the major contribution of this work was to determine representative VF loss patterns by our unsupervised artificial intelligence model, which turned out to be consistent with the Hood model. More specifically, the 14 central VF patterns in end-stage glaucoma showed that VF damages in more vulnerable zones can take different forms. For example, although archetypes 6 and 7 have VF loss confined in the more vulnerable zone, archetype 6 is superonasal loss, whereas archetype 7 is bihemifield nasal loss. Among the 14 representative patterns we discovered, 7 patterns lost specific areas of the more vulnerable zone, whereas only 2 patterns were subject to visual loss in the less vulnerable zone. Moreover, for the 4 patterns for which the main areas of the less vulnerable zone were preserved, the subsequent development of central VF defects affected the more vulnerable zone more than the less vulnerable zone.
A particularly interesting finding was that the initial encroachments on an intact central VF (archetype 13) at follow-up in end-stage glaucoma were more likely to take the form of nasal loss patterns (archetypes 4 and 6) that are analogous to the known nasal step patterns in early glaucoma development measured by 24-2 VF.27 Arcuate-like defects have been reported to be the most frequent central VF defect in mild glaucoma.8 Our results quantitatively showed this to be similar for the onset of central VF loss in end-stage glaucoma. Deeper knowledge about this nasal loss in central VF might help us to better understand the pathophysiological mechanism of the onset of central VF loss.
Another important finding of potentially high relevance to patients with end-stage glaucoma is that 11.0% of eyes with archetype 4 as the primary pattern at baseline shifted to total loss (archetype 3). However, this was not the case for eyes with archetype 7 at baseline, although archetype 7 is highly similar to archetype 4 and seems to have even more VF loss. Those results suggest that archetypes 4 and 7 might be 2 different subtypes of early central VF loss.
Limitations
Our study has several limitations. First, we only had 2-year follow-up for most eyes. The short follow-up period limited our ability to confirm whether the primary pattern shift for an individual eye can be repeated in subsequent tests. However, we used a statistical test to confirm that the primary pattern shift is significant for a group of eyes, which ensures the pattern shift results are not due to random fluctuations. Second, sex and race information were not available in our deidentified data set. Third, information about visual acuity was not available in our data set; thus, we were unable to study how central VF loss patterns are associated with visual acuity. Fourth, some VF patterns, such as the hemifield loss pattern (archetype 8), could be associated with nonglaucomatous causes of visual loss, such as anterior ischemic optic neuropathy. More structural data and detailed medical records will be needed to elucidate the exact pathological causes of those VF patterns. Fifth, many patients with end-stage glaucoma are unable to perform the 24-2 or 10-2 VF test with stimulus size III. It has been reported that stimulus size V is preferable for more advanced glaucoma.32 Unfortunately, there were no 10-2 VFs tested with stimulus size V in the Glaucoma Research Network data set used in this study. Because our data set contained the entire patient population of 5 large glaucoma services, this observation indicates that size V stimuli are hardly used in clinical practice to assess glaucomatous vision loss. In future studies, it might be beneficial to repeat our data analyses on specifically collected sets of size V VFs. Finally, the present work does not document any residual low spatial resolution islands of vision outside the angular radius of the central 24°.33,34
Conclusions
We applied an unsupervised artificial intelligence method to a large data set to quantitatively determine central VF loss patterns in end-stage glaucoma. Those central VF patterns confirmed the mean more and less vulnerable zones proposed in the Hood model and quantified the individual variation of those more and less vulnerable zones. The early onset of central VF loss is nasal loss, which is analogous to the nasal step in 24-2 VF. Furthermore, for the 2 highly similar patterns of archetypes 4 and 7, eyes with the former archetype as the primary pattern had a significant chance of developing into total loss, whereas the latter archetype did not. Such a finding suggests that archetypes 4 and 7 might be 2 different subtypes of early central VF loss.
eMethods. Identification of Representative Patterns of 24-2 VF in End-Stage Glaucoma
eFigure 1. The 18 Representative Archetype Patterns of 24-2 VFs and an Example of the VF Decomposition
eFigure 2. The Archetype VF Patterns Obtained From 197 Combined 24-2 and 10-2 VFs Tested on the Same Dates
References
- 1.Murata H, Hirasawa H, Aoyama Y, et al. Identifying areas of the visual field important for quality of life in patients with glaucoma. PLoS One. 2013;8(3):e58695. doi: 10.1371/journal.pone.0058695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CPB, Baig S, Medeiros FA. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology. 2016;123(3):552-557. doi: 10.1016/j.ophtha.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R; Los Angeles Latino Eye Study Group . Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941-948.e1. doi: 10.1016/j.ophtha.2007.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Joiner DB, Tsamis E, et al. Optical coherence tomography circle scans can be used to study many eyes with advanced glaucoma. Ophthalmol Glaucoma. 2019;2(3):130-135. doi: 10.1016/j.ogla.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim G-N, Lee EJ, Kim H, Kim T-W. Dynamic range of the peripapillary retinal vessel density for detecting glaucomatous visual field damage. Ophthalmol Glaucoma. 2019;2(2):103-110. doi: 10.1016/j.ogla.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Hood DC, Raza AS, de Moraes CGV, et al. Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(2):940-946. doi: 10.1167/iovs.10-5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood DC, Raza AS, de Moraes CGV, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1-21. doi: 10.1016/j.preteyeres.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10° of the visual field. JAMA Ophthalmol. 2014;132(3):291-297. doi: 10.1001/jamaophthalmol.2013.7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler A, Breiman L. Archetypal analysis. Technometrics. 1994;36(4):338-347. doi: 10.1080/00401706.1994.10485840 [DOI] [Google Scholar]
- 10.Elze T, Pasquale LR, Shen LQ, Chen TC, Wiggs JL, Bex PJ. Patterns of functional vision loss in glaucoma determined with archetypal analysis. J R Soc Interface. 2015;12(103):20141118. doi: 10.1098/rsif.2014.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Leung CKS, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118(8):1551-1557. doi: 10.1016/j.ophtha.2010.12.035 [DOI] [PubMed] [Google Scholar]
- 13.Leung CK-S, Yu M, Weinreb RN, Lai G, Xu G, Lam DS-C. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119(9):1858-1866. doi: 10.1016/j.ophtha.2012.03.044 [DOI] [PubMed] [Google Scholar]
- 14.Birt CM, Shin DH, Samudrala V, Hughes BA, Kim C, Lee D. Analysis of reliability indices from Humphrey visual field tests in an urban glaucoma population. Ophthalmology. 1997;104(7):1126-1130. doi: 10.1016/S0161-6420(97)30173-0 [DOI] [PubMed] [Google Scholar]
- 15.Newkirk MR, Gardiner SK, Demirel S, Johnson CA. Assessment of false positives with the Humphrey Field Analyzer II perimeter with the SITA algorithm. Invest Ophthalmol Vis Sci. 2006;47(10):4632-4637. doi: 10.1167/iovs.05-1598 [DOI] [PubMed] [Google Scholar]
- 16.Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113(7):1081-1086. doi: 10.1016/j.ophtha.2006.01.066 [DOI] [PubMed] [Google Scholar]
- 17.Pasquale LR, Willett WC, Rosner BA, Kang JH. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology. 2010;117(8):1521-1529. doi: 10.1016/j.ophtha.2009.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz J, Sommer A, Witt K. Reliability of visual field results over repeated testing. Ophthalmology. 1991;98(1):70-75. doi: 10.1016/S0161-6420(91)32339-X [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000;41(8):2201-2204. doi: 10.1016/S0002-9394(00)00758-3 [DOI] [PubMed] [Google Scholar]
- 20.Social Security Administration, Office of Disability. Disability evaluation under Social Security: 2.00 special senses and speech–adult Vol 2018. https://www.ssa.gov/disability/professionals/bluebook/2.00-SpecialSensesandSpeech-Adult.htm#203. Accessed June 13, 2018.
- 21.Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. 2014;55(1):102-109. doi: 10.1167/iovs.13-13006 [DOI] [PubMed] [Google Scholar]
- 22.Foulsham WS, Fu L, Tatham AJ. Prior rates of visual field loss and lifetime risk of blindness in glaucomatous patients undergoing trabeculectomy. Eye (Lond). 2015;29(10):1353-1359. doi: 10.1038/eye.2015.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wold S, Esbensen K, Geladi P. Principal component analysis. Chemom Intell Lab Syst. 1987;2(1-3):37-52. doi: 10.1016/0169-7439(87)80084-9 [DOI] [Google Scholar]
- 24.Comon P. Independent component analysis, a new concept? Signal Processing. 1994;36(3):287-314. doi: 10.1016/0165-1684(94)90029-9 [DOI] [Google Scholar]
- 25.Hartigan JA, Wong MA. Algorithm as 136: a k-means clustering algorithm. J R Stat Soc Ser C. 1979;28(1):100-108. doi: 10.2307/2346830 [DOI] [Google Scholar]
- 26.Yousefi S, Balasubramanian M, Goldbaum MH, et al. Unsupervised Gaussian mixture-model with expectation maximization for detecting glaucomatous progression in standard automated perimetry visual fields. Transl Vis Sci Technol. 2016;5(3):2. doi: 10.1167/tvst.5.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keltner JL, Johnson CA, Cello KE, et al. ; Ocular Hypertension Treatment Study Group . Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2003;121(5):643-650. doi: 10.1001/archopht.121.5.643 [DOI] [PubMed] [Google Scholar]
- 28.Cai S, Elze T, Bex PJ, Wiggs JL, Pasquale LR, Shen LQ. Clinical correlates of computationally derived visual field defect archetypes in patients from a glaucoma clinic. Curr Eye Res. 2017;42(4):568-574. doi: 10.1080/02713683.2016.1205630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Pasquale LR, Shen LQ, et al. Reversal of glaucoma hemifield test results and visual field features in glaucoma. Ophthalmology. 2018;125(3):352-360. doi: 10.1016/j.ophtha.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Shen LQ, Pasquale LR, et al. An artificial intelligence approach to detect visual field progression in glaucoma based on spatial pattern analysis. Invest Ophthalmol Vis Sci. 2019;60(1):365-375. doi: 10.1167/iovs.18-25568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH. Estimating classification error rate: repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal. 2009;53(11):3735-3745. doi: 10.1016/j.csda.2009.04.009 [DOI] [Google Scholar]
- 32.Wall M, Doyle CK, Eden T, Zamba KD, Johnson CA. Size threshold perimetry performs as well as conventional automated perimetry with stimulus sizes III, V, and VI for glaucomatous loss. Invest Ophthalmol Vis Sci. 2013;54(6):3975-3983. doi: 10.1167/iovs.12-11300 [DOI] [PubMed] [Google Scholar]
- 33.Trope GE, Britton R. A comparison of Goldmann and Humphrey automated perimetry in patients with glaucoma. Br J Ophthalmol. 1987;71(7):489-493. doi: 10.1136/bjo.71.7.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez JA, Brown RH, Lynch MG, Caplan MB. Risk of postoperative visual loss in advanced glaucoma. Am J Ophthalmol. 1993;115(3):332-337. doi: 10.1016/S0002-9394(14)73584-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Identification of Representative Patterns of 24-2 VF in End-Stage Glaucoma
eFigure 1. The 18 Representative Archetype Patterns of 24-2 VFs and an Example of the VF Decomposition
eFigure 2. The Archetype VF Patterns Obtained From 197 Combined 24-2 and 10-2 VFs Tested on the Same Dates