Abstract
The characteristics of wildland fire smoke exposures which initiate or exacerbate cardiopulmonary conditions are unclear. We previously reported that, on a mass basis, lung toxicity associated with particulate matter (PM) from flaming smoke aspirated into mouse lungs is greater than smoldering PM. In this study, we developed a computer-controlled inhalation system which can precisely control complex biomass smoke emissions from different combustion conditions. This system was used to examine the toxicity of inhaled biomass smoke from peat, eucalyptus and oak fuels generated under smoldering and flaming phases with emissions set to the same approximate concentration of carbon monoxide (CO) for each exposure (60–110 ppm), resulting in PM levels of ~4 mg/m3 for flaming and ~40 mg/m3 for smoldering conditions. Mice were exposed by inhalation 1 h/day for 2 days and assessed for lung toxicity at 4 and 24 h after the final exposure. Peat (flaming and smoldering) and eucalyptus (smoldering) smoke elicited significant inflammation (neutrophil influx) in mouse lungs at 4 h with the peat (flaming) smoke causing even greater lung inflammation at 24 h post-exposure. A significant alteration in ventilatory timing was also observed in mice exposed to the peat (flaming) and eucalyptus (flaming and smoldering) smoke immediately after each day of exposure. No responses were seen for exposures to similar concentrations of flaming or smoldering oak smoke. The lung toxicity potencies (neutrophil influx per PM mass) agreed well between the inhalation and previously reported aspiration studies, demonstrating that although flaming smoke contains much less PM mass than smoldering smoke, it is more toxic on a mass basis than smoldering smoke exposure, and that fuel type is also a controlling factor.
Keywords: Wildland fire smoke, Inhalation, Lung toxicity, Biomass smoke, Particulate matter
Introduction
Besides the direct threat of fire itself, wildland fire smoke poses significant threats to firefighter and public health, leading to an increased risk of serious symptoms (e.g., pneumonia, emphysema, and heart failure) (Liu et al. 2015; Reid et al. 2016; Youssouf et al. 2014). It is also well recognized that exposures to wildland fire smoke may worsen symptoms for people with pre-existing health conditions (e.g., asthma and cardiovascular disease) (Cascio 2018). Despite this evidence, relatively little has been done to evaluate and predict specific toxicity of wildland fire smoke including (1) how toxic is wildland fire smoke compared to other pollutants, (2) which components in wildland fire smoke are responsible for adverse health outcomes, and (3) whether the toxicity of wildland fire smoke is governed by fuel type and combustion conditions. Additionally, most mechanistic toxicity studies of wildland fire (or biomass) smoke have been conducted with in vitro cell or in vivo intratracheal instillation (or oropharyngeal aspiration) methods which are limited in the extent to which they mimic real-life exposure conditions (Driscoll et al. 2000; Osier and Oberdorster 1997). Thus, it is important to confirm findings with inhalation exposures in order to bridge the scientific knowledge gap between epidemiological and toxicological outcomes.
One challenge in conducting inhalation studies with biomass smoke from open (uncontrolled) combustions is that emissions vary widely through the course of combustion, leading to poor consistency and reproducibility of the resulting aerosol exposure (Sallsten et al. 2006; Tesfaigzi et al. 2002). Thus, it is difficult to accurately quantify the dose of pollutants during smoke inhalation, resulting in significant inter- or intra-laboratory variability in toxicological outcomes. To address this problem, we developed an automated smoke delivery system which can (1) control combustion phases (smoldering versus flaming), (2) precisely maintain biomass smoke concentrations at different levels during inhalation exposures, and (3) generate biomass smoke from different fuels with reproducible physico-chemical characteristics. To our knowledge, this represents the first fully-automated furnace system for biomass smoke inhalation exposures that can be used to provide accurate toxicological profiles from well-defined biomass smoke emissions.
We have reported previously that the relative toxicity of biomass smoke condensates is dependent on fuel type (oak, peat, pine needle, pine, and eucalyptus) and combustion phase (flaming and smoldering) following oropharyngeal aspiration in mice. Specifically we demonstrated that peat and eucalyptus smoke condensates are more potent at causing inflammation than oak smoke, and that the material from flaming smoke is, on an equal mass basis, more toxic than that from smoldering smoke (Kim et al. 2018). Here, we used the automated furnace system connected to a nose and mouth exposure apparatus to conduct acute inhalation exposures for three of these biomass fuels (peat, eucalyptus, and oak) under both flaming and smoldering combustion conditions. We assessed biological (lung injury and inflammation) and physiological responses (ventilatory function) in female Balb/cJ mice after the inhalation exposure, and estimated the deposited dose of biomass smoke PM in the mouse lungs. Finally, we compared the inflammatory responses with results of previously conducted aspiration tests after adjustment for the PM dosimetry, and determined whether the two routes of administration caused a similar toxicological profile between the different fuel and combustion conditions.
Methods
Fuel Types
We combusted three different biomass fuels: eucalyptus (Eucalyptus globulus), peat, and northern red oak (Quercus rubra). These fuels were selected based on our previous findings (Kim et al. 2018) that showed smoke from different biomass fuel types (eucalyptus, peat, red oak, pine, and pine needles) produced varying toxicity. The eucalyptus and peat fuels were purchased from commercial suppliers (Woodworkers Source, Phoenix, AZ and Glynn Bros, Boston, MA, respectively). Oak was obtained from the Air and Energy Management Division at the U.S. Environmental Protection Agency (EPA). The oak and eucalyptus wood samples were cut into approximately 2-cm long chips to help obtain uniform combustion. The peat sample was crumbled into loose agglomerates and then combusted without further processing. All biomass fuels were stored in a temperature- and humidity-controlled room (23°C and 39% relative humidity) prior to testing.
Automated Smoke Dosage Control System
We previously utilized a quartz-tube furnace system to control combustion phases (smoldering versus flaming) using various biomass fuels under constant conditions for up to 60 min (Kim et al. 2018). In this study, we modified the tube furnace system to precisely control both the combustion phases as well as the biomass smoke concentrations (i.e., PM and carbon monoxide). An automated smoke dosage control system incorporating a proportional-integral-derivative (PID) feedback loop coupled with a mass flow controller (Mass-Flo, MKS Instrument, Inc., Andover, MA) was designed and integrated into the quartz-tube furnace system to maintain constant smoke exposure. A schematic diagram of the automatic smoke dosage control system with an inhalation exposure chamber is shown in Figure 1. Biomass smoke (2 L/min) generated from the tube furnace system under smoldering or flaming conditions was diluted with air (nominally 2.5 and 40 L/min for 1st and 2nd dilution air, respectively) and then delivered to an inhalation chamber (64 port stainless steel chamber, Laboratory Products Inc., Seaford, DE). The smoke PM concentration was continuously monitored and used to adjust the PID feedback control loop linked to an exhaust flow control valve in the smoke inlet line. System flow adjustments were continuously made in response to changes in PM concentration in the exposure chamber, thus maintaining the PM concentrations within <10% of the target set value. The diluted biomass smoke through each port on the exposure chamber was maintained at a flow rate of approximately 1 L/min. The chamber temperature and relative humidity were maintained at 22°C and 40%, respectively. A pressure gauge (Magnehelic, Dwyer Instruments Inc., Michigan City, IN) was placed in the chamber to ensure constant pressure throughout the inhalation exposure. Carbon dioxide (CO2) and carbon monoxide (CO) levels were monitored using a non-dispersive infrared analyzer (Model: 602 CO/CO2; CAI Inc., Orange, CA) and nitrogen oxides (NO, NO2, and NOx) levels were measured with a chemiluminescent analyzer (Model: 42i NO/NO2/NOx; Thermo Scientific, Franklin, MA). PM was collected on a glass-fiber filter installed in the exhaust line of the inhalation chamber to determine mean PM concentrations gravimetrically by weighing the filter before and after inhalation exposure. Particle-size distributions (in the range of 10 nm to 10 μm) were monitored using a scanning mobility particle sizer (NanoScan SMPS, Model:3910; TSI Inc., Shoreview, MN) combined with an optical particle sizer (OPS, Model: 3330; TSI Inc.). Real-time measurements of biomass smoke properties and engineering parameters (e.g., temperatures, relative humidities, static pressures, and flow rates) were continuously monitored, recorded, and displayed using data acquisition software (Dasylab version 13.0, National Instruments, Austin, TX).
Figure 1.
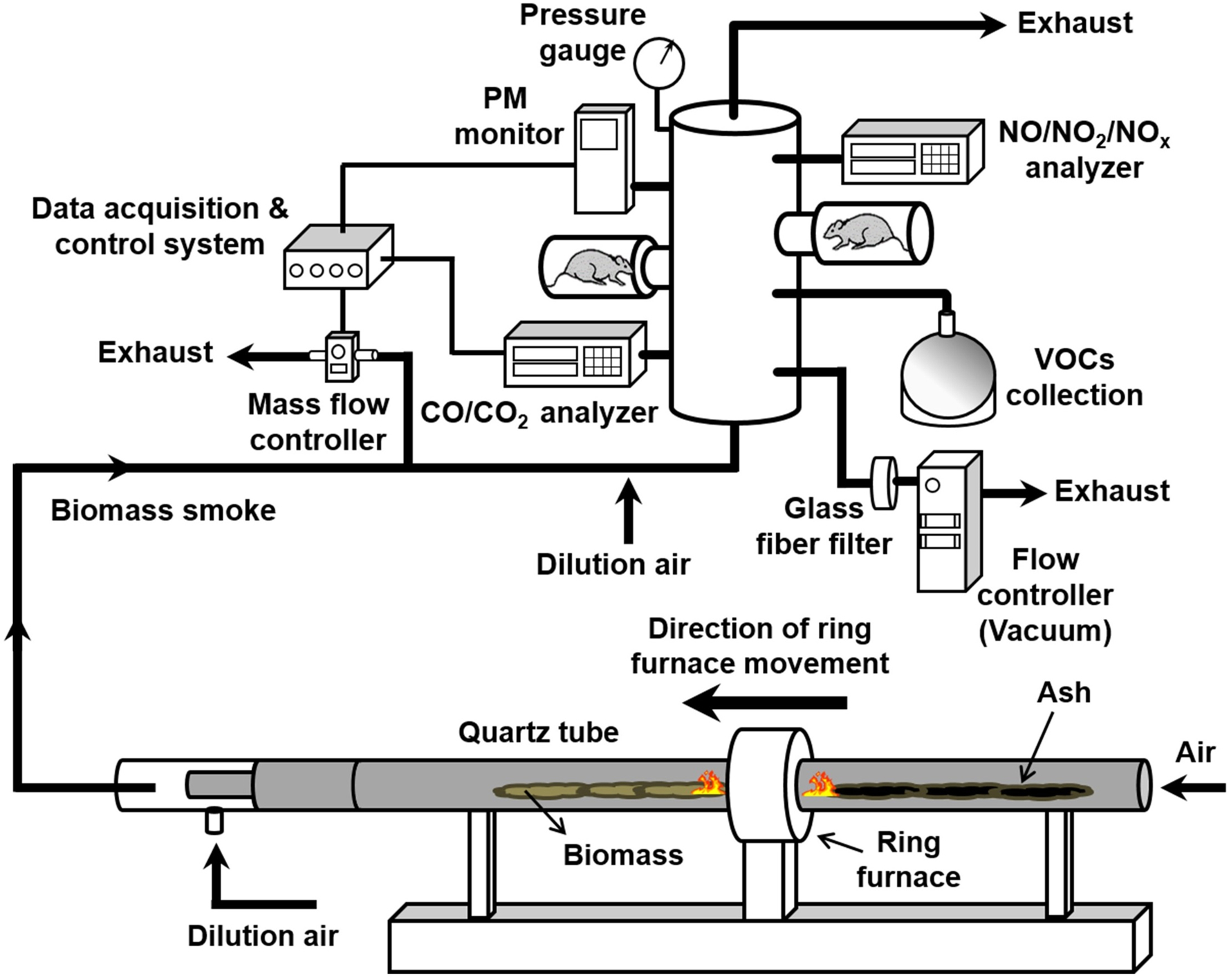
Diagram of the automated smoke dosage control system for inhalation exposure to biomass smoke.
Characterization of Biomass Smoke
CO2, CO, and PM concentrations during the exposures to biomass smoke emissions were continuously measured in the inhalation chamber under both flaming and smoldering conditions. Combustion phases were defined using target levels within the modified combustion efficiency (MCE) framework, which is defined as MCE (%) = (ΔCO2 / (ΔCO2 + ΔCO)) × 100, where ΔCO2 and ΔCO are the excess concentrations of CO2 and CO (Ward and Hardy 1991). Urbanski (2014) has suggested that combustion be considered flaming when the MCE is > 95% and smoldering when the MCE is 65–85%.
Sampling Procedures
Volatile organic compounds (VOCs) and PM samples were obtained from ports on the inhalation chamber for further speciation and analysis. VOCs in the biomass smoke and filtered air (control) were sampled using SUMMA canisters and carbonyls were sampled with 2,4-dinitrophenylhydrazine (DNPH)-coated silica cartridges (PN 505323, Sigma-Aldrich Co., St. Louis, MO). The sampling flow rates through the evacuated canister (filled to approximately 0.7 atm) were controlled using a critical orifice at a flow rate of approximately 70 mL/min. Cartridge sampling flow rates were controlled with a SKC Aircheck Sampling Pump (Model: 224-PCXR8, SKC Inc., Eighty Four, PA) with flow rates in the range of 0.5 – 0.7 L/min. PM in the biomass smoke and filtered air (control) was collected on a pre-baked quartz filter for analysis of carbon species or Teflon filters for elemental and ionic analyses. The PM sampling flow rate (approximately 1 L/min) was controlled with a vacuum controller (Model: XC-40; Apex Instruments Inc., Fuquay-Varina, NC).
Sample Analysis
VOCs in the canisters were analyzed by gas chromatography-mass spectrometry (GC-MS) in accordance with U.S. EPA TO-15 Method. DNPH cartridge samples were extracted with 6 mL of carbonyl-free acetonitrile (Burdick & Jackson, VWR International, Radnor, PA). Carbonyl hydrazones were analyzed in the extracts by high performance liquid chromatography (HPLC) according to U.S. EPA Method TO-11A. Detailed descriptions of the TO-15 and TO-11A analytical procedures were reported previously (George et al. 2014). PM was extracted from Teflon filters for inorganic elemental and ion analysis. PM was digested in aqua regia and then assayed for inorganic elements by high-resolution magnetic-sector-field inductively coupled plasma mass spectrometry (HR-ICPMS; Model: Element 2; Thermo Scientific, Franklin, MA). Additional samples of PM were diluted in ultrapure water and then analyzed for ionic species using a dual-ion chromatography system (Model: ICS-2000; Dionex, Sunnyvale, CA). For carbon analysis, we analyzed quartz filters with a thermal-optical analyzer (Model: 107A; Sunset Laboratory Inc., Tigard, OR). Additional details of the PM analysis are provided in the Supporting Information.
Experimental Animals
Adult pathogen-free female Balb/cJ mice (approximately 20 g body weight) were purchased from the Jackson Laboratory (Bar Harbor, ME), housed in groups of four in polycarbonate cages with hardwood chip bedding at the U.S. Environmental Protection Agency (EPA) Animal Care Facility (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care) and were maintained on a 12-h light to dark cycle at 22.3 ± 1.1°C temperature and 50 ± 10% relative humidity. Mice were given access to rodent chow and water ad libitum and were acclimated for at least 10 days before the study began. The studies were conducted after approval by the EPA Institutional Animal Care and Use Committee.
Inhalation Exposure
The inhalation exposures were conducted with mice being individually housed in larger rat restraint tubes that were inserted into ports of a “nose and mouth only” exposure chamber, allowing free movement (Wong 2007). Mice were exposed to the biomass smoke (smoldering and flaming) for 1 h per day for 2 consecutive days. Because CO in biomass smoke can elicit its own health impacts in terms of altering respiratory rate, vascular function and other endpoints (Canova et al. 2010; Otterbein et al. 2000; Rose et al. 2017), we elected to hold the exposures to similar concentrations of CO to compare how different PM levels might affect the pulmonary responses. Preliminary testing during combustion runs showed that the highest PM concentrations achievable from flaming combustion with no dilution approximated 4 mg/m3, with CO levels between 50 and 100 ppm. When smoldering conditions were used, the similar range of CO obtained through a PID feedback dilution mechanism yielded PM levels of approximately 40 mg/m3. Thus, biomass smoke concentrations in the inhalation chamber were set at 40 and 4 mg/m3 of PM for smoldering and flaming combustions, respectively. In addition, the selection of the smoldering smoke PM level was also based on the following information. We previously conducted lung toxicity tests of biomass smoke PM in mice after oropharyngeal aspiration of 100 μg of PM (Kim et al. 2018) and the PM dose to the lung (deposited PM in the respiratory tract) was calculated to be 81 μg assuming the respiratory deposition fraction by the aspiration method is 0.81 (Foster et al. 2001). With inhalation exposure in the current study, we calculated that 83 μg of PM could be deposited in the respiratory tract of mice following inhalation of 40 mg/m3 of the smoldering smoke PM if the minute ventilation and deposition fraction are assumed to be 54 mL/min (de Hennezel et al. 2001) and 0.32 (Foster et al. 2001), respectively. Since the deposited PM dose (83 μg) from the inhalation exposure was similar to the PM (81 μg) from the aspiration exposure, the PM target set value for the smoldering smoke was chosen to be 40 mg/m3 with levels of CO (50 – 100 ppm) set to the same range as the flaming exposure. As a control, another group of mice was exposed in an identical chamber to filtered air under the same experimental conditions.
Ventilatory Function Assessment
Ventilatory function was monitored in unanesthetized/unrestrained mice using whole body plethysmography (Buxco Electronics, Wilmington, NC). Ventilatory function data included: minute ventilation (MV), tidal volume (TV), breathing frequency (F), relaxation time (RT), inspiratory (Ti) and expiratory (Te) time, and peak inspiratory (PIF) and peak expiratory (PEF) flow. Additionally, time and flow rate parameters were used to evaluate an index of ventilator timing (enhanced pause; Penh). To reduce the possibility of homeostatic changes related to time of day, respiratory function data were collected for 27 min at 24 h before exposure (Baseline), immediately after exposure (Day 1 and Day 2), and 24 h after the end of the second day of exposure (Post), following a 5-min acclimation period for each mouse in its individual plethysmograph. All data were averaged for each 9-min portion of the measurement period.
Blood and Lung Assay
Carboxyhemoglobin (COHb) levels were determined in blood of mice collected by facial vein venipuncture immediately before the scheduled end of the second exposure. COHb was analyzed using a CO-Oximeter (Model: 682, Instrumentation Laboratory, Lexington, MA) immediately after the collection. At 4 or 24 h after the end of the second day of exposure, mice were euthanized by intraperitoneal injection of Euthasol (Virbac AH Inc., Fort Worth, TX). Blood was collected by cardiac puncture, and hematology values were measured using a Coulter AcT 10 Hematology Analyzer (Beckman Coulter Inc., Miami, FL). Bronchoalveolar lavage fluid (BALF) was collected from the right lung lobes and used to determine the total cell count and differential analysis of macrophage and neutrophil numbers. We also analyzed the recovered BALF supernatant to determine cellular injury as indicated by protein, albumin, lactate dehydrogenase (LDH), γ-glutamyl transferase (GGT), and N-acetyl-β-D-glucoaminidase (NAG) levels. Pro-inflammatory cytokine responses were assessed by measuring tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and macrophage inhibitory protein-2 (MIP-2). Additional details of the biochemical/cytokine analyses are provided in the Supporting Information.
Dosimetry of Inhaled Biomass Smoke PM
We used the publicly available multiple-path particle dosimetry (MPPD, v3.04, Applied Research Associates Inc., Arlington, VA) model to estimate deposition fractions of PM in different compartments (extrathoracic, tracheobronchial, and pulmonary regions) of the mouse respiratory tract during inhalation exposure to the biomass smoke. A detailed description of the MPPD model is given elsewhere (Asgharian et al. 2014). Briefly, input parameters to the MPPD model for the PM characteristic (e.g., PM concentration, size, and geometric standard deviation (GSD)) were obtained from the PM monitoring data. Input parameters for the mouse lung physiology (i.e., tidal volume and breathing frequency) were obtained from the whole-body plethysmography data. A set of default values for other input parameters in the MPPD model was used if the parameters were not specified. Because the lung toxicity data (e.g., neutrophil numbers in BALF) after inhalation were associated with different PM masses in the respiratory tract, the calculated PM deposition fraction values were used to normalize the lung toxicity response data (# neutrophil/μg PM) so that the toxicity results were comparable at different biomass smoke exposure conditions. In order to further evaluate the toxicity responses of the inhaled biomass smoke PM, we obtained normalized lung toxicity data of the same biomass smoke PM from our previous aspiration study (Kim et al. 2018) and compared them with the inhalation toxicity data presented here.
Statistical Analysis
Data were expressed as mean ± the standard error of the mean (SEM). For the analysis of lung toxicity data on pro-inflammatory cytokine/cell numbers, protein, albumin, NAG, LDH and GGT values in BALF, hematology value, and COHb levels, we used one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison adjustment to compare the biological responses between biomass smoke-exposed groups and filtered air controls. For the analysis of lung function data, we used two-way analysis of variance (ANOVA) followed by the Fisher’s Least Significant Difference test to compare the time course of the respiratory parameters between smoke-exposed groups and the filtered air control group. We also modeled the lung toxicity potencies (# neutrophils/μg PM) of the biomass smoke PM with linear regression analysis to characterize their association with different exposure methods (e.g., inhalation and aspiration methods). Statistical analyses were performed using GraphPad Prism software (version 6.07, GraphPad Software Inc., San Diego, CA). We assigned the statistical significance level at a probability value of p < 0.05.
Results
Properties of Smoldering and Flaming Smoke in the Inhalation Chamber
The smoke generated from three biomass fuels (peat, eucalyptus, and oak) under smoldering and flaming combustion phases, and their concentration profiles during the exposure are shown in Figure 2. Specific properties of each biomass smoke in the inhalation chamber are also summarized in Table 1. Average PM concentrations for the biomass smoke exposures were 40.5 mg/m3 during the smoldering (85% of MCE) and 3.7 mg/m3 during the flaming phase (98% of MCE), and the PM levels stayed within 1 – 5% of the smoldering and 5 – 15% of the flaming phase target set value over the entire exposure period. Under the two distinct levels of PM in the inhalation chamber, CO levels ranged from approximately 60 to 110 ppm for all the biomass smoke emissions. NOx levels in the flaming smoke were approximately 30 – 70 times higher than those of the smoldering smoke while selected VOC levels were up to approximately 3 times higher in the smoldering smoke. Mass median aerodynamic diameters of PM in the biomass smoke ranged from 0.1 – 0.3 μm with low GSD values (approximately 1.43) for all tested fuel combustion emissions.
Figure 2.
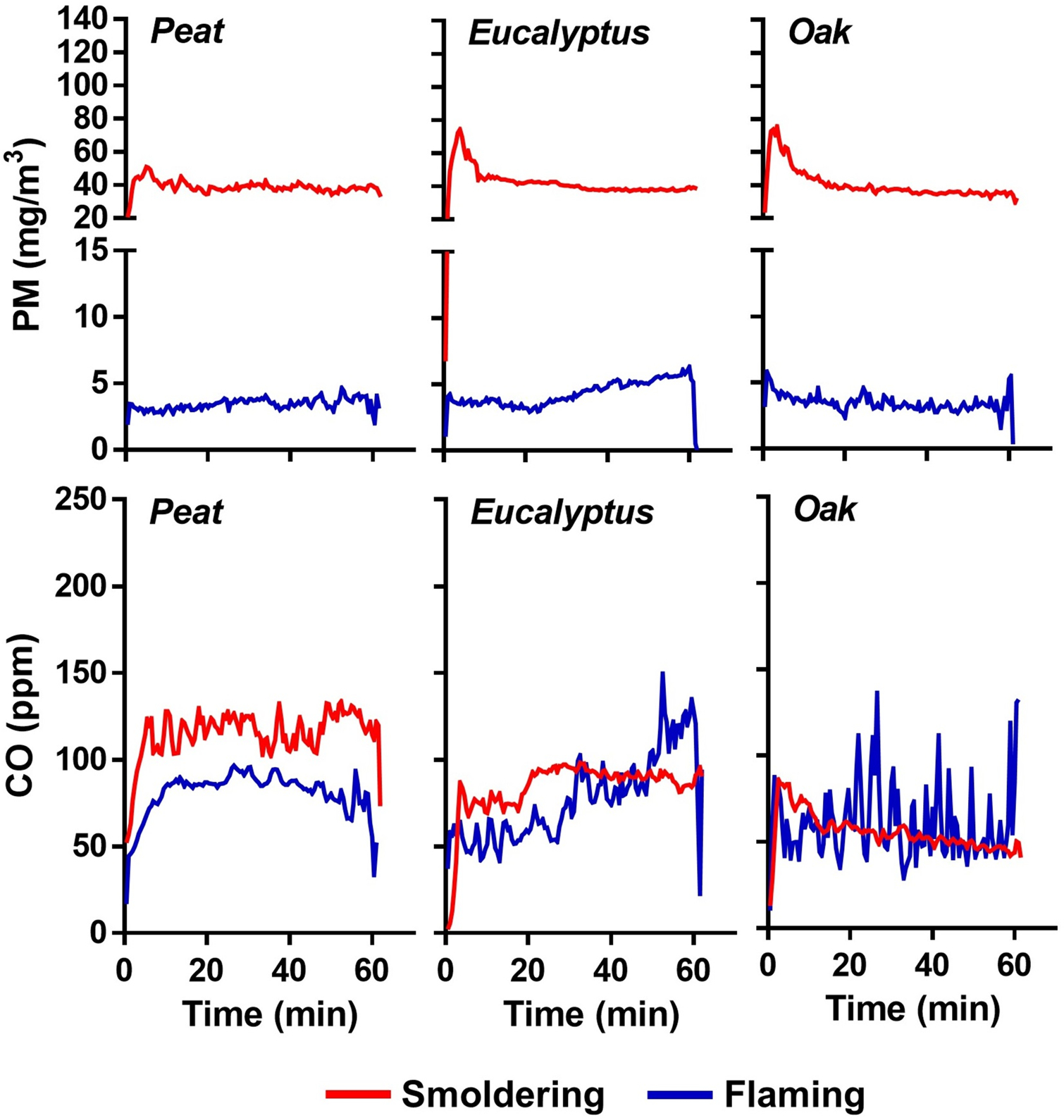
Average concentrations of PM and CO of the biomass smoke in the inhalation chamber during exposures.
Table 1.
Characteristics of the biomass smoke in the inhalation chamber.
| Characteristic (unit) | Peat | Eucalyptus | Oak | |||
|---|---|---|---|---|---|---|
| Smoldering | Flaming | Smoldering | Flaming | Smoldering | Flaming | |
| MCE (%)1) | 86.0±0.1 | 97.0±0.1 | 83.7±0.3 | 98.4±0.1 | 83.6±0.3 | 98.0±0.1 |
| PM (mg/m3) | 38.7±0.4 | 3.4±0.1 | 42.2±0.6 | 4.2±0.1 | 40.5±0.8 | 3.5±0.1 |
| CO (ppm) | 115±1 | 81±1 | 84±2 | 76±2 | 56±1 | 59±2 |
| CO2 (ppm) | 721±5 | 2,794±40 | 427±8 | 4,790±78 | 297±5 | 2,942±65 |
| NO (ppb) | 227±54 | 5,109±892 | 30±1 | 1,449±61 | 38.9±0.1 | 1,812±233 |
| NO2 (ppb) | 0±0 | 1,292±187 | 0±0 | 666±19 | 0±0 | 502±154 |
| NOx (ppb) | 227±54 | 6,380±1,079 | 30±1 | 2,106±77 | 39±0 | 2,303±384 |
| VOCs (ppb) | 2,677 | 1,694 | 1,911 | 760 | 4,072 | 1,682 |
| MMAD (nm)2) | 151[1.46] | 133[1.29] | 283[1.46] | 165[1.40] | 180[1.52] | 158[1.32] |
Note: Error ranges represent standard error of the mean (SEM)
Modified combustion efficiency (MCE) = ΔCO2 / (ΔCO2 + ΔCO) × 100
Mass median aerodynamic diameter (MMAD) of PM; values in brackets represent the geometric standard deviation (GSD)
Major chemical characteristics of particulate- and gas-phase compounds in the biomass smoke are shown in Figures 3 – 4 and summarized in Tables S1 – S3. Total carbon accounted for 40 – 70% of PM mass from all three fuels from either the smoldering or the flaming phase. Ionic species and inorganic elements each constituted approximately 1% of the PM mass from smoldering combustion and constituted approximately 5% of the PM mass from the flaming phase. With respect to the gas phase components, the highest levels of VOCs were measured in the oak smoke (smoldering or flaming), whereas the eucalyptus smoke contained the lowest VOCs levels (Figures 3B and 4). Major VOC components were acetaldehyde (up to ~25% of VOCs in all smoldering exposures), acrolein (~15% of VOCs in flaming peat smoke), formaldehyde (~43% of VOCs in flaming eucalyptus smoke), and benzene (~30% of VOCs in flaming oak smoke).
Figure 3. Major chemical components of the biomass smoke in the inhalation chamber during exposures.
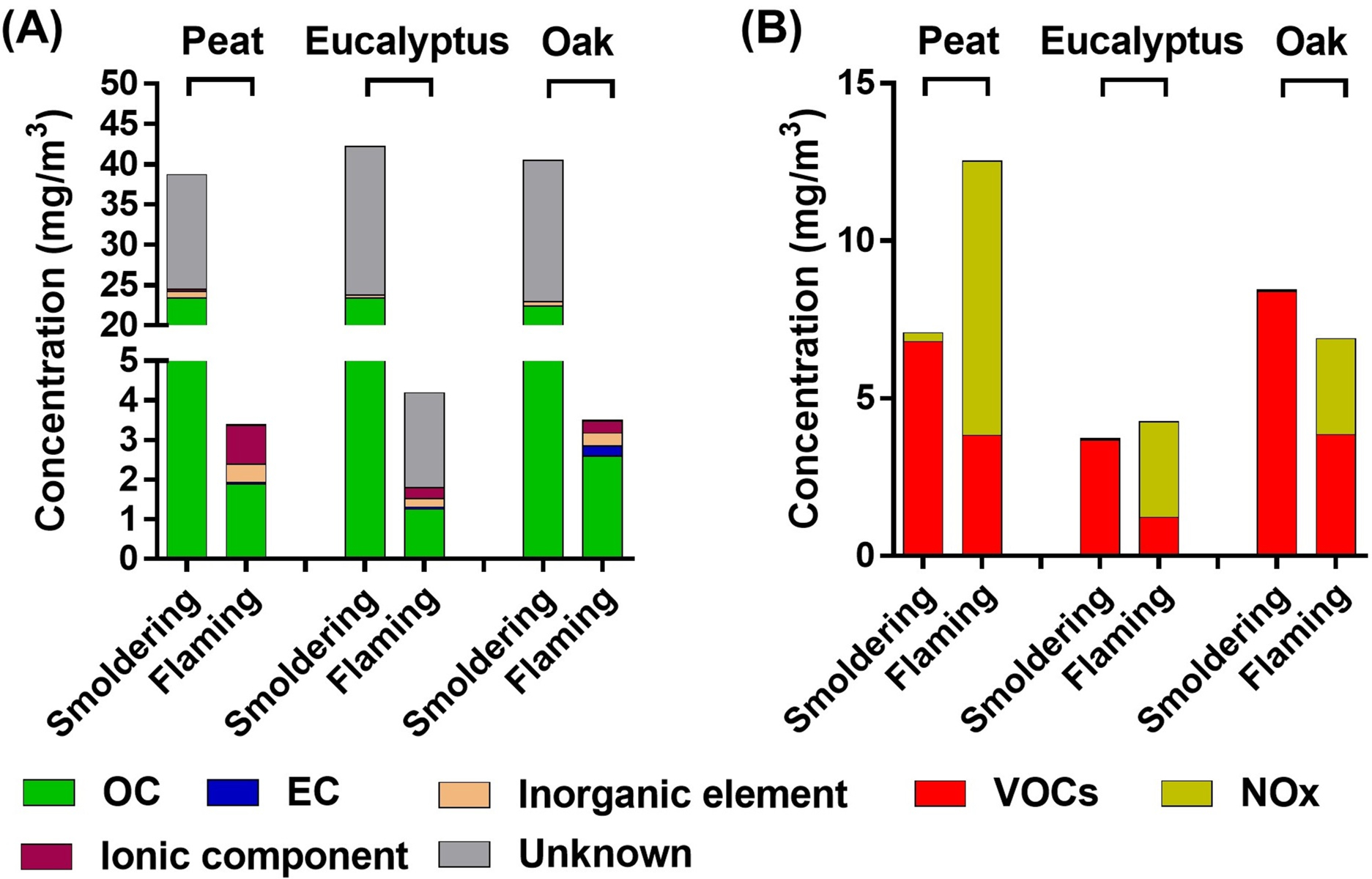
(A) particulate-phase chemical components. (B) gas-phase chemical components.
Figure 4. Major constituents of VOCs in the biomass smoke during exposures.
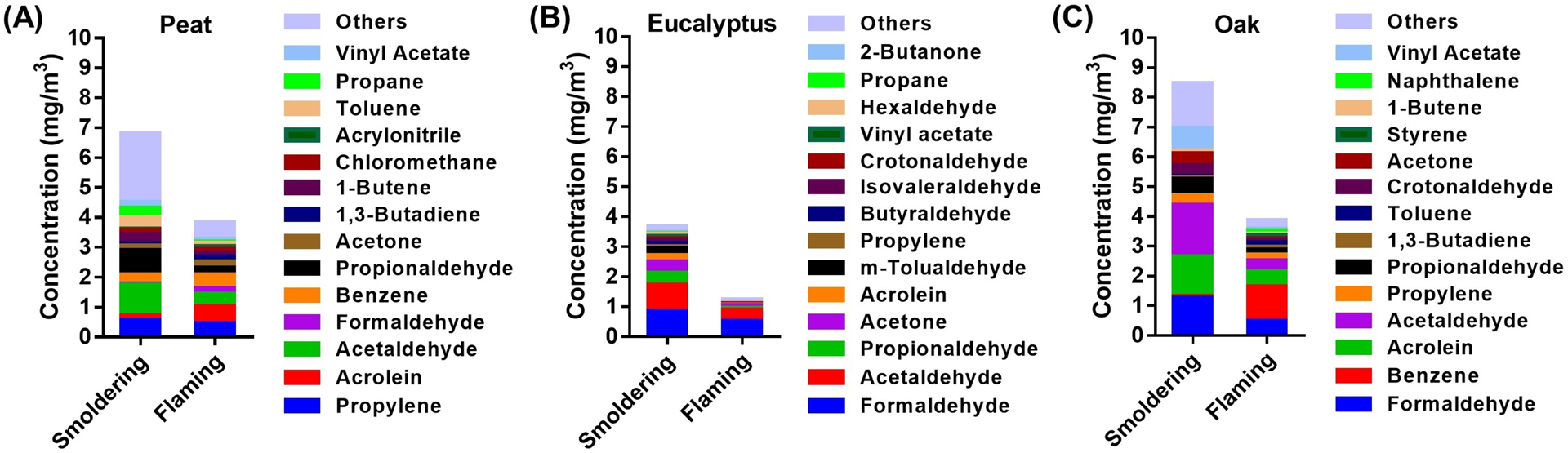
(A) peat smoke (B) eucalyptus smoke (C) oak smoke.
Lung Toxicity of Biomass Smoke Inhalation
COHb levels in blood of mice exposed to the biomass smoke for 1 h ranged from approximately 7 to 13% and corresponded reasonably well to the exposure concentration pattern (approximately 60 to 110 ppm CO) (Figure 5A). At 4 and 24 h after the end of the second day of exposure, BALF of the mice was analyzed for various biomarkers of lung cell injury and inflammation. A small but significant increase in neutrophil influx into the lung was observed in mice exposed to the smoldering peat and eucalyptus and flaming peat smoke at 4 h post-exposure, with the neutrophil response further increasing (up to ~6% of total BALF cells) at 24 h after the flaming peat and eucalyptus smoke exposures (Figure 5B). Neutrophil numbers were not significantly increased at 4 and 24 h after exposure to the oak smoke from either smoldering or flaming combustion. Total numbers of macrophages in BALF were largely unchanged for any biomass smoke at 4 and 24 h post-exposure (Figure S1). While the number of neutrophils rose under inhalation exposures to certain types of the biomass smoke, levels of pro-inflammatory cytokines in BALF were not significantly elevated at any of the biomass smoke exposure conditions (Figure S2). Small (but significant) increases in lung injury biomarkers (protein, albumin, LDH, and GGT) were observed for the peat (smoldering and flaming) and eucalyptus (flaming) smoke but not the oak smoke exposure (Figure S3). None of the smoke exposures significantly altered any of the hematological parameters studied (data not shown).
Figure 5. Biological responses in mice exposed to the biomass smoke.
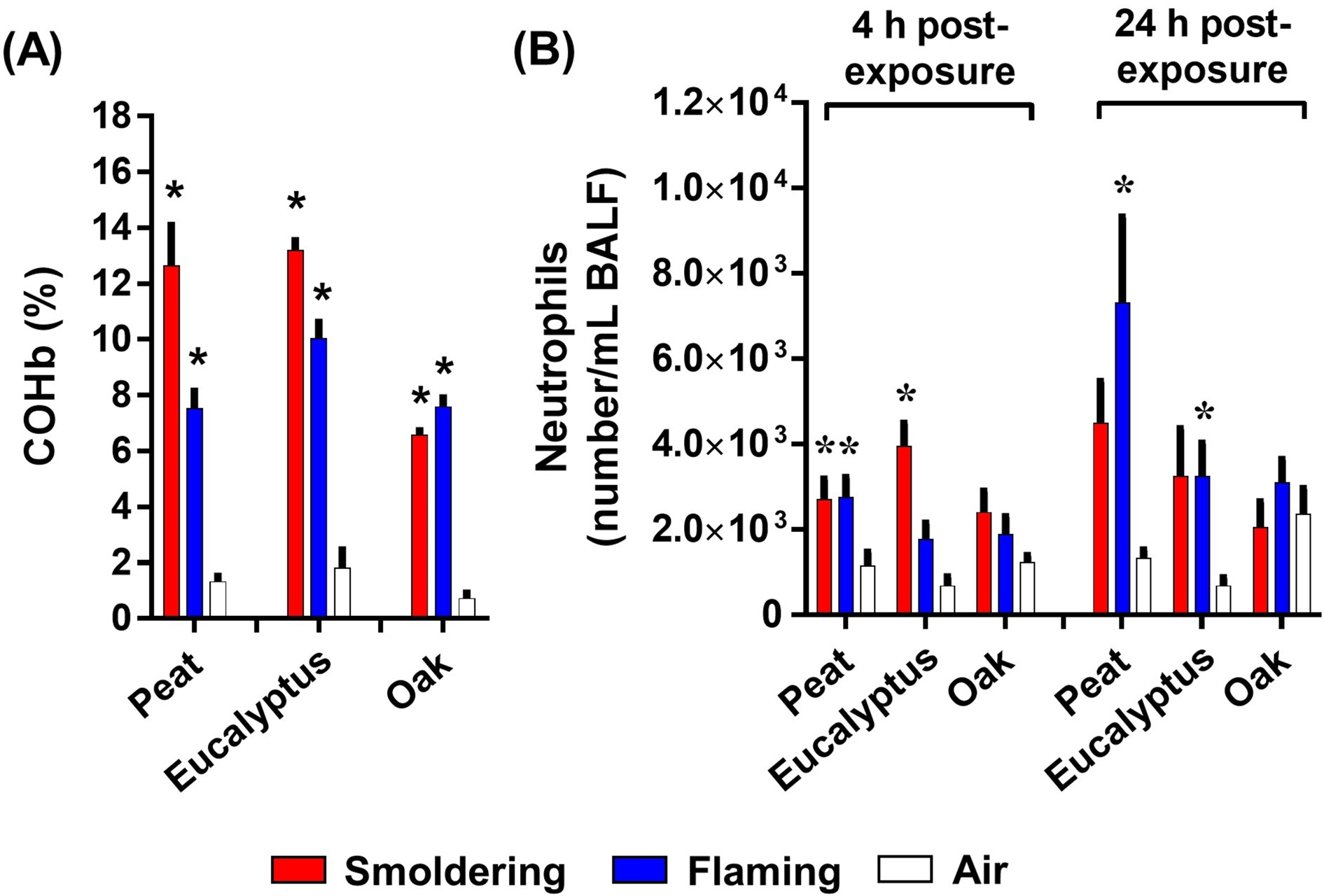
(A) COHb levels in blood of mice exposed to the biomass smoke. (B) neutrophil numbers in BALF of mouse lungs exposed to the biomass smoke. Data are mean ± SEM and obtained from 8 mice for each group. *p < 0.05 compared with the air-exposed group. The statistical tests were performed using one-way ANOVA followed by the Dunnett’s multiple comparison.
Ventilation Parameters following Biomass Smoke Inhalation
Changes in respiratory (ventilation) parameters (Te, Ti, PIF, PEF, RT, MV, TV, and F) in mice were monitored prior to the biomass smoke exposure (baseline), immediately after each day of exposure (Day 1 and Day 2), and at 24 h after the second day of exposure (Post). All respiratory parameters are expressed as a percentage (%) after normalization to the average pre-exposure values (baseline values) as shown in Figures S4 – S5. Under the flaming peat smoke exposure condition, Te, Ti, and RT were significantly elevated on both Day 1 and Day 2, while other parameters (PEF, MV, TV, and F) only increased on Day 2. The smoldering peat smoke did not significantly increase any respiratory parameters but decreased Te and RT on Day 1. Like the flaming peat smoke, the flaming eucalyptus smoke significantly increased all respiratory parameters on both Day 1 and Day 2 and this increase continued at 24 h post-exposure (Post). The smoldering eucalyptus smoke further elevated all the parameters throughout the study period (Day 1, Day 2, and Post). Slower breathing reflected by increases in measures of respiratory time (Te, Ti and RT) paired with increases in ventilation volume (MV and TV) is consistent with airway constriction and increased airflow resistance associated with exposure to the peat and eucalyptus smoke. This alteration of breathing patterns also indicates an increased effort to maintain adequate air exchange during exposures. Unlike the peat and eucalyptus smoke, most respiratory parameters (Ti, PIF, MV, TV, and F) were not significantly increased in mice exposed to the oak smoke with the exception that smoldering oak smoke increased Te and RT on Day 2 only. Based on the recorded measurements for PEF, PIF, Te, and RT, changes in ventilatory timing (as measured by Penh) associated with the biomass smoke exposure were calculated as follows Penh = PEF/PIF × (Te – RT)/RT and shown in Figure 6. Overall, a significant increase in ventilatory timing, potentially indicating airflow obstruction, was observed in mice exposed to the flaming peat smoke (Day 2) and both flaming and smoldering eucalyptus smoke (Day 1, Day 2, and Post), while the flaming and smoldering oak smoke at approximately the same CO and PM concentrations did not significantly alter ventilatory timing after each day of exposure (Day 1 and Day 2). Furthermore, the Penh values indicate that the alteration in the breathing pattern response to the peat and eucalyptus smoke was mostly driven by expiratory ventilation parameters (i.e., Te, PEF and RT) associated with possible airway narrowing and inflammation.
Figure 6. Ventilatory function of mice exposed to the biomass smoke.
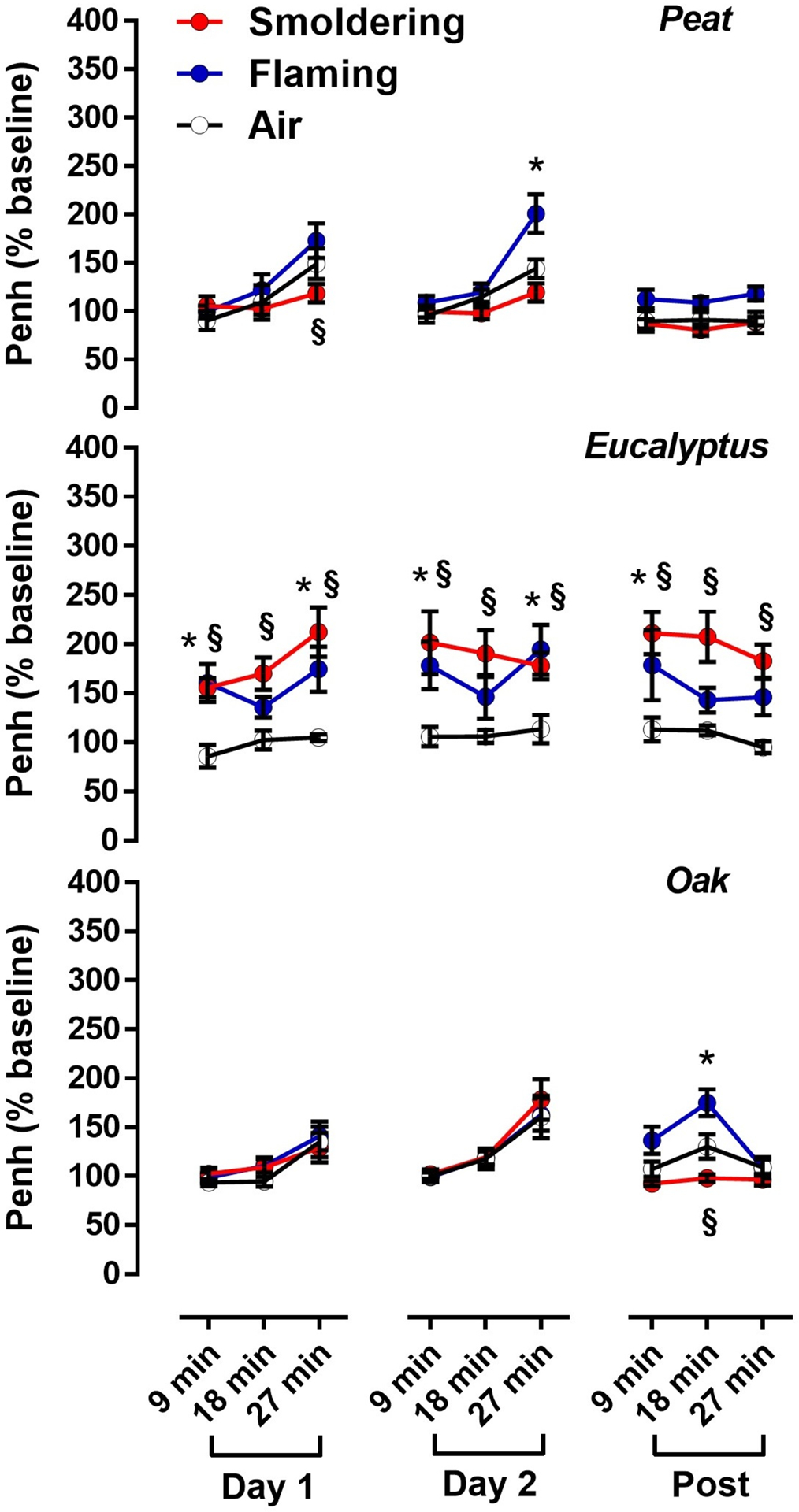
Penh values (as an index of ventilatory timing) are expressed as the Penh % after normalized by the average Penh values for pre-exposure conditions (baseline values). Data are mean ± SEM and obtained from 8 mice for each group. *p < 0.05 for the flaming smoke exposed group compared with the air-exposed group from the same time point. §p < 0.05 for the smoldering smoke exposed group compared with the air-exposed group from the same time point. The statistical tests were performed using two-way ANOVA followed by the Fisher’s Least Significant Difference test.
Evaluation of Dose Dependent Responses to Biomass Smoke
The fraction of PM deposited in the respiratory tract following inhalation exposure was estimated with the MPPD model using empirical input parameters (Tables 1 and S4) and calculated to be 0.32 – 0.39 for the various exposure conditions (Figure 7A). Based on this modeled value, lung toxicity potency (i.e., neutrophil numbers normalized by the deposited PM mass in the respiratory tract) of the biomass smoke PM after the inhalation exposure was calculated (Table 2). In order to evaluate lung toxicity potency, neutrophil numbers induced by aspiration exposure to the same biomass smoke PM were used from our previous study (Kim et al. 2018) to calculate the lung toxicity potency (# neutrophil/μg PM) based on the assumption that 81% of PM was aspirated into lungs (Foster et al. 2001). Like the results from the aspiration exposure study, the flaming biomass smoke (per unit mass of PM) from peat and eucalyptus fuels was associated with significantly higher neutrophil recruitment than the smoldering smoke. This finding was further supported by linear regression analysis of the lung toxicity potencies between two exposure methods (Figure 7B). A good correlation of the lung toxicity potencies between the inhalation and aspiration methods was observed in mice exposed to the peat (p < 0.001) and eucalyptus (p = 0.0058) smoke PM. Specifically, strong correlations were seen with ions (p = 0.0009), carbon (p = 0.0069), and inorganic elements (p = 0.0290) of PM in the eucalyptus smoke, and carbon (p < 0.0001) and inorganic elements (p = 0.0003) of PM in the peat smoke (Figure S6). This indicates that a PM composition-dependent correlation analysis provides more specific information on which chemical component might predict potential lung toxicity following different exposure routes. Since the lung toxicity associated with the oak smoke exposures was not significant, the inhalation and aspiration exposure outcomes did not correlate with each other (p = 0.3250).
Figure 7. Dosimetric analysis of lung toxicity of inhaled biomass smoke PM in mice.
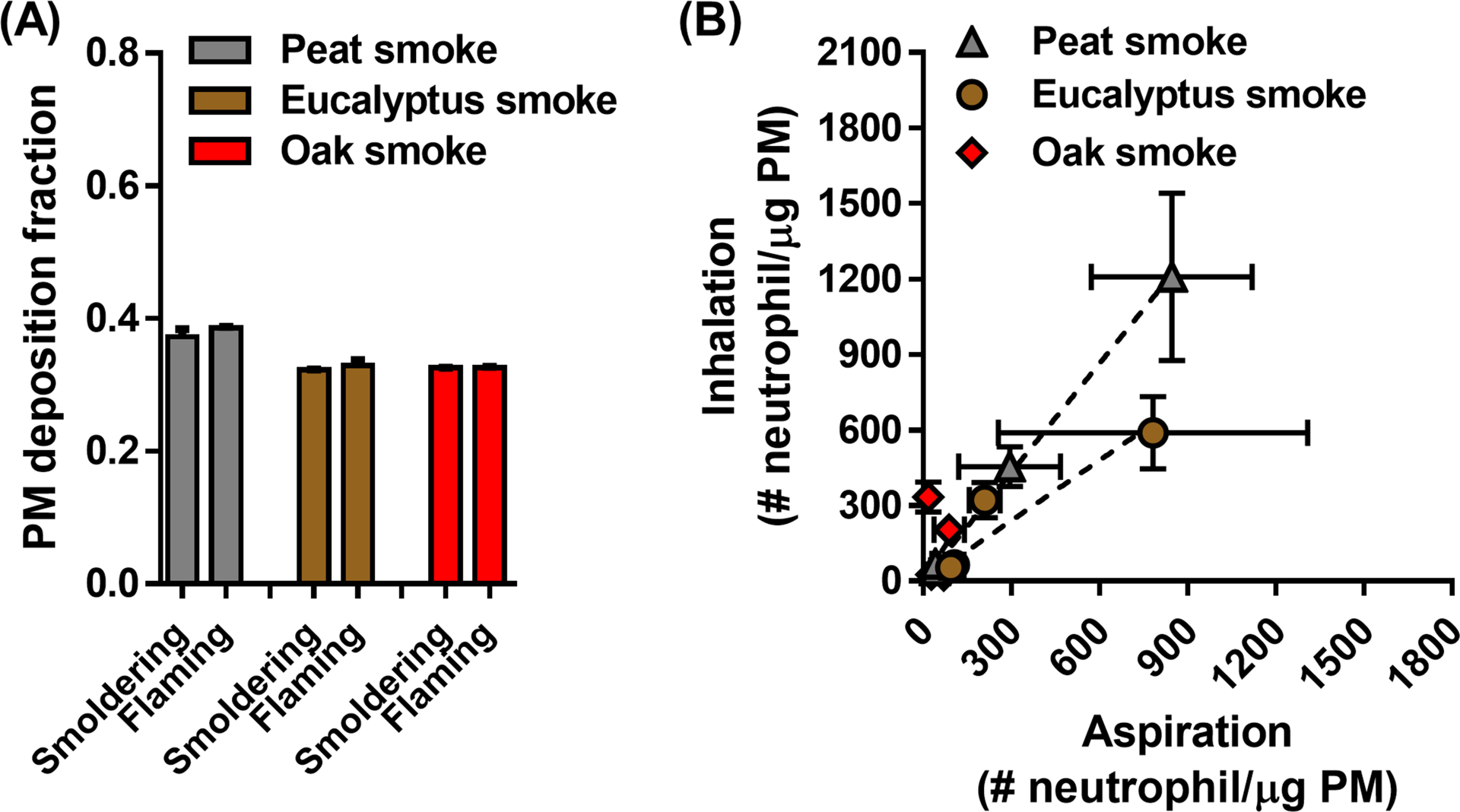
(A) calculated deposition fraction for inhaled biomass smoke PM in the entire respiratory tract using the MPPD model. Input parameters (i.e., particle characteristics and breathing parameters) for the MPPD model were obtained from experimental data (Tables 1 and S4). (B) comparison of lung toxicity potencies (# neutrophil/μg PM) of the biomass smoke PM from inhalation and aspiration methods after adjustment for PM dosimetry. Neutrophil numbers determined from each delivery method were normalized by the deposited PM mass in the entire respiratory tract of mice at different exposure conditions. Concordance of lung toxicity potencies between the inhalation and aspiration methods was observed in mice exposed to the peat (p < 0.0001) and eucalyptus (p = 0.0058) smoke but not the oak (p = 0.3250) smoke. All data are presented as mean ± SEM.
Table 2.
Comparison of lung deposited PM mass (μg PM) and lung toxicity potencies (# neutrophil/μg PM) after inhalation and aspiration of biomass smoke PM.
| Exposure method | Peat | Eucalyptus | Oak | ||||
|---|---|---|---|---|---|---|---|
| Smoldering | Flaming | Smoldering | Flaming | Smoldering | Flaming | ||
| Deposited PM mass | |||||||
| Aspiration1) | 81 | 81 | 81 | 81 | 81 | 81 | |
| Inhalation2) | 65.8±1.8 | 6.1±0.2 | 60.9±2.0 | 5.5±0.3 | 97.1±9.7 | 9.2±0.7 | |
| Lung toxicity potency | |||||||
| Aspiration3) | 4 h | 39±14* | 294±173* | 105±33* | 209±52* | 27±12 | 88±52* |
| 24 h | 41±12 | 846±274* | 94±35* | 782±527* | 70±52 | 17±7 | |
| Inhalation4) | 4h | 41±7* | 454±78* | 64±9* | 321±70 | 25±5 | 203±44 |
| 24h | 68±15 | 1,208±332* | 53±18 | 589±144* | 21±6 | 333±59 | |
Note: Error ranges represent standard error of the mean (SEM)
Deposited PM mass = PM deposition fraction in the respiratory tract (Foster et al. 2001) × PM dose (Kim et al. 2018)
Deposited PM mass = PM deposition fraction in the respiratory tract (Figure 7A) × PM dose (Table 1) × Total respiratory volume (Table S4)
Lung toxicity potency = Neutrophil counts (Kim et al. 2018) / Deposited PM mass
Lung toxicity potency = Neutrophil counts (Figure 5B) / Deposited PM mass
These values were statistically significant from the respective negative control groups.
Discussion
Well-Controlled Biomass Smoke Emitted from the Automated Combustion System
We demonstrated that the mass median aerodynamic diameter of PM emitted from the smoldering and flaming combustions of peat, eucalyptus and oak ranged from 0.1 to 0.3 μm, consistent with previous reports (Kleeman et al. 1999). Reid and Hobbs (1998) reported that fresh smoke particles emitted from forest fires with different vegetation and changing combustion phases had a size range from 0.2 to 0.3 μm, and further suggested that particle size in the biomass smoke appear to be mainly governed by condensation and coagulation processes rather than combustion intensity. Although the different combustion conditions (e.g., fuel types and burning temperatures) had some effects on particle size, they profoundly altered the chemical properties of the biomass smoke. Specifically, flaming combustion was associated with higher emissions of ions and inorganic elements because the increased temperature promotes vaporization of ionic and inorganic species (Frey et al. 2009; Rau 1989). Likewise, flaming combustion also led to higher NOx emissions (Jaffe and Wigder 2012) although this is also influenced by increased nitrogen content in the fuels, such as with peat, which produced higher NOx emissions than the other two biomass fuels (Lacaux et al. 1996). In contrast VOC emissions showed the opposite trend with higher emissions from the smoldering than the flaming combustion. Greenberg et al. (2006) reported that the reaction of oxygen with organic content in biomass fuel at low combustion temperatures (approximately 300°C) produced high VOC emissions, while oxidation at high temperatures (>500°C) generated low VOC with higher CO2 emissions. George et al. (2016) reported VOC emission factors (EFs) from wildland peat fire smoke: propylene (0.5 – 3.5 g/kg), acetaldehyde (0.7 – 3.3 g/kg), benzene (0.8 – 3.2 g/kg), acetone (0.3 – 1.3 g/kg), and toluene (0.1 – 1.6 g/kg), and we found relatively similar VOC EFs in the smoldering peat smoke reported here (propylene, acetaldehyde, benzene, acetone, and toluene at 1.8, 2.1, 2.0, 0.5, and 2.8 g/kg, respectively), calculated as EF t = (fuel carbon fraction × mass of carbon emitted as t × molecular weight t × 1,000) / (molecular weight carbon × total mass of carbon). While it could be argued that the biomass smoke generated from our system is less complex than natural wildland fire smoke, the precise control offers better reproducibility of biomass smoke production from other laboratory- or field-based open burning combustions.
Inhalation Exposure to Well-Defined Concentrations of Biomass Smoke
In experimental settings, biomass smoke is difficult to maintain at a stable concentration because the emission characteristics are strongly influenced by the dynamic and relatively unpredictable nature of combustion processes. Thus, biological responses to smoke inhalation studies can vary widely according to the dose of particles and gases delivered. The automated active combustion feedback system described here monitors biomass smoke concentrations in the inhalation chamber and adjusts the dilution air in real time to deliver stable PM and gas concentrations during the entire exposure period with an overall average within 7% of the target concentration, and a standard error of 2% (at 40 and 4 mg/m3 from smoldering and flaming combustion, respectively).
Since this study was mainly focused on the potential toxicity of smoke particles it was important to avoid potential interfering effects of inhaled CO during exposure. It is well accepted that relatively low concentrations (<100ppm) of CO exposure can induce cardiovascular effects such as myocardial ischemia, arrythmias and angina (ATSDR 2012; WHO 2000) in susceptible individuals. Short term exposure to levels such as these however, is not known to cause lung injury or inflammation, and in fact several studies have reported that CO exposure actually reduces lung inflammation induced by exogenous agents as bacterial endotoxin or immune mediated lung disease (Dolinay et al. 2004; Goebel et al. 2008; Otterbein et al. 2000; Wilson et al. 2010). Furthermore, blood COHb levels (7 – 13%) in the smoke exposed mice were lower than the proposed biological threshold limit value (15 – 20% in human and 15 – 30% in rodents) (Foresti et al. 2008; Wilson et al. 2010), supporting the notion that CO-mediated lung toxicity was not likely to occur over the course of the exposure. Since NOx levels (6.4 ppm) in the flaming peat smoke were three-fold higher than in other flaming smokes however, potential effects caused by NOx exposure cannot be ruled out (Dowell et al. 1971; NRC 1998; Thomas et al. 1967).
Previously, we demonstrated that oropharyngeal aspiration of the peat and eucalyptus smoke condensates had higher lung toxicity (based on neutrophil counts) than the oak smoke, and this was associated with organic carbon and heavy metals (Kim et al. 2018). Since no gas phase products were involved in this response, only the chemical components of the particles could have caused the lung inflammation. Given that the smoke from oak had approximately the same amounts of VOCs and gases than the other fuels, it is likely that the increased responses from inhalation of eucalyptus and peat smoke were caused by differences in particle chemistry. Interestingly oropharyngeal aspiration of smoke condensates further increased pro-inflammatory cytokines in the lungs (Kim et al. 2018), which was not evident after the inhalation exposure. It is possible that cytokines were upregulated after the first exposure and then waned by the second exposure, or that the bolus instillation increased pro-inflammatory signaling more so than the cumulative dosing by inhalation (Driscoll et al. 2000; Lam et al. 2004). It should also be noted that despite the ten-fold lower PM concentration of the flaming smoke, the mice had significantly increased lung neutrophils at 24 h post-exposure, further confirming that the lung toxicity of biomass smoke emissions is, on a PM mass basis, greater for the flaming than the smoldering phase (Kim et al. 2018).
In addition to the lung inflammatory responses, alterations in respiratory pattern (or function) after exposure to the biomass smoke were determined with unrestrained whole-body plethysmography (WBP). Although evaluating true lung function or respiratory mechanics using noninvasive unrestrained WBP has been criticized (Bates and Irvin 2003; Hoymann 2012; Vanoirbeek et al. 2010), respiratory parameters obtained from WBP devices have been shown to be a useful probe for respiratory health of experimental animals (Lim et al. 2014; Vaickus et al. 2010). In this study the enhanced pause (Penh), which has been correlated with airflow obstruction in some mouse strains (e.g., Balb/cJ) (Adler et al. 2004), showed significant increases only in mice exposed to flaming peat and flaming or smoldering eucalyptus smoke. As noted above, since there were no striking differences in gas phase components (i.e., VOCs, CO and CO2) among the three fuels under the same combustion phase, the significant ventilatory alterations following exposure to peat and eucalyptus smoke were likely associated with the PM, The lack of ventilatory alterations of the oak smoke exposure is consistent with our previous report (Gibbs-Flournoy et al. 2018) suggesting that smoke from this fuel is less toxic than from the other fuels tested. Since this study only assessed lung toxicity after acute inhalation exposures, further research is needed to determine if the differential toxicity is still valid for long-term exposures, or for that matter, other endpoints.
The physiological measurements across the various fuels and combustion conditions were in good agreement with the lung toxicity endpoints and there was also correlation between the lung toxicity potencies (# neutrophil/μg PM) between inhalation and aspiration exposures. Although there are differences in the exposure concentration and distribution of material administered by aspiration and inhalation (Driscoll et al. 2000), these variables can be minimized by precisely controlling and delivering accurate exposure concentrations of inhaled material. Thus, it was possible to calculate a more accurate delivered (or deposited) dose of inhaled PM in the biomass smoke in animals and compare dose-related toxicity outcomes between inhalation and aspiration exposures. The calculated dose of inhaled PM in this study averaged 75 μg for the smoldering smoke PM (deposited dose = deposited fraction of the inhaled PM in the respiratory tract (0.34) × PM concentration (40.3 mg/m3) × duration of exposure (2 h) × minute ventilation of the mice (46 L/min)) and was in the same range as the deposited dose of aspirated PM (81 μg; assuming a respiratory tract deposition fraction of 81% (Foster et al. 2001)) in our previous study (Kim et al. 2018). In contrast, the calculated dose for the flaming smoke ranged from 5 to 9 μg which was ten to fifteen times less than the dose delivered during the smoldering exposures. Since the inflammatory responses between the two combustion conditions were quite similar this would confirm our previous conclusion that flaming smoke particles are, on a mass basis, more toxic than smoldering particles.
Conclusions
Our data suggest that biomass smoke inhalation causes lung inflammation that is influenced by the type of biomass smoke (peat, eucalyptus and oak) and combustion conditions (flaming and smoldering), and since few appreciable contrasts were seen with the gas phase components, PM composition appears to be the key factor responsible for the adverse lung toxicity and physiologic outcomes. In agreement with our previous studies, the flaming smoke appeared on a mass basis to cause more lung inflammation than PM from fuels combusted under smoldering conditions. Despite the acute exposure period, some biomass smoke conditions affected respiratory parameters at least 24 h after the last exposure. Using this novel technical approach, reproducible and reliable biomass smoke inhalation toxicity data was generated that has the potential for broad general application in future studies to improve our collective understanding of the health effects of smoke and could contribute to the development of a quantitative risk assessment model for smoke generated from a variety of different fuels and conditions. Importantly, the application of this exposure system with validated higher throughput in vitro cell based models will speed this process and reduce the need for further animal use.
Supplementary Material
Acknowledgements
The authors would like to thank Wanda Williams, Rachel Grindstaff, and Judy Richards for technical assistance in toxicological analyses; Dr. Kasey Kovalcik for technical assistance in PM chemical analyses; and Drs. Aimen Farraj and Barbara Buckley for their careful review of this manuscript.
This study was supported by the Joint Fire Science Program Project (14-1-04-16) and was performed while Dr. Yong Ho Kim held a National Research Council Senior Research Associateship Award at the U.S. Environmental Protection Agency. Additional support was provided by the intramural research program of the Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC.
The research described in this manuscript has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use. The authors declare that they have no competing financial interests.
References
- Adler A, Cieslewicz G, Irvin CG (2004) Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice J Appl Physiol 97:286–292 doi: 10.1152/japplphysiol.00821.2003 [DOI] [PubMed] [Google Scholar]
- Asgharian B et al. (2014) Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract Inhal Toxicol 26:829–842 doi: 10.3109/08958378.2014.935535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (2012) Toxicological Profile for Carbon Monoxide. U.S. Department of Health and Human Servies, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia: [PubMed] [Google Scholar]
- Bates JH, Irvin CG (2003) Measuring lung function in mice: the phenotyping uncertainty principle J Appl Physiol 94:1297–1306 doi: 10.1152/japplphysiol.00706.2002 [DOI] [PubMed] [Google Scholar]
- Canova C et al. (2010) Carbon monoxide pollution is associated with decreased lung function in asthmatic adults Eur Respir J 35:266–272 doi: 10.1183/09031936.00043709 [DOI] [PubMed] [Google Scholar]
- Cascio WE (2018) Wildland fire smoke and human health Sci Total Environ 624:586–595 doi: 10.1016/j.scitotenv.2017.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hennezel L, Debarre S, Ramisse F, Delamanche S, Harf A, Alonso JM, Calvet JH (2001) Plethysmography for the assessment of pneumococcal pneumonia and passive immunotherapy in a mouse model Eur Respir J 17:94–99 [DOI] [PubMed] [Google Scholar]
- Dolinay T, Szilasi M, Liu M, Choi AM (2004) Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury Am J Respir Crit Care Med 170:613–620 doi: 10.1164/rccm.200401-023OC [DOI] [PubMed] [Google Scholar]
- Dowell AR, Kilburn KH, Pratt PC (1971) Short-term exposure to nitrogen dioxide. Effects on pulmonary ultrastructure, compliance, and the surfactant system Arch Intern Med 128:74–80 [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB (2000) Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations Toxicological sciences : an official journal of the Society of Toxicology 55:24–35 [DOI] [PubMed] [Google Scholar]
- Foresti R, Bani-Hani MG, Motterlini R (2008) Use of carbon monoxide as a therapeutic agent: promises and challenges Intensive Care Med 34:649–658 doi: 10.1007/s00134-008-1011-1 [DOI] [PubMed] [Google Scholar]
- Foster WM, Walters DM, Longphre M, Macri K, Miller LM (2001) Methodology for the measurement of mucociliary function in the mouse by scintigraphy J Appl Physiol 90:1111–1117 doi: 10.1152/jappl.2001.90.3.1111 [DOI] [PubMed] [Google Scholar]
- Frey AK et al. (2009) Chemical composition and mass size distribution of fine particulate matter emitted by a small masonry heater Boreal Env Res 14:255–271 [Google Scholar]
- George IJ, Black RR, Geron CD, Aurell J, Hays MD, Preston WT, Gullett BK (2016) Volatile and semivolatile organic compounds in laboratory peat fire emissions Atmos Environ 132:163–170 doi: 10.1016/j.atmosenv.2016.02.025 [DOI] [Google Scholar]
- George IJ, Hays MD, Snow R, Faircloth J, George BJ, Long T, Baldauf RW (2014) Cold temperature and biodiesel fuel effects on speciated emissions of volatile organic compounds from diesel trucks Environ Sci Technol 48:14782–14789 doi: 10.1021/es502949a [DOI] [PubMed] [Google Scholar]
- Gibbs-Flournoy EA et al. (2018) Differential exposure and acute health impacts of inhaled solid-fuel emissions from rudimentary and advanced cookstoves in female CD-1 mice Environ Res 161:35–48 doi: 10.1016/j.envres.2017.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel U et al. (2008) Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs Anesthesiology 108:1025–1036 doi: 10.1097/ALN.0b013e3181733115 [DOI] [PubMed] [Google Scholar]
- Greenberg JP, Friedli H, Guenther AB, Hanson D, Harley P, Karl T (2006) Volatile organic emissions from the distillation and pyrolysis of vegetation Atmos Chem Phys 6:81–91 doi: 10.5194/acp-6-81-2006 [DOI] [Google Scholar]
- Hoymann HG (2012) Lung function measurements in rodents in safety pharmacology studies Front Pharmacol 3:156 doi: 10.3389/fphar.2012.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe DA, Wigder NL (2012) Ozone production from wildfires: A critical review Atmos Environ 51:1–10 doi: 10.1016/j.atmosenv.2011.11.063 [DOI] [Google Scholar]
- Kim YH et al. (2018) Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires Environ Health Perspect 126:017011 doi: 10.1289/ehp2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman MJ, Schauer JJ, Cass GR (1999) Size and composition distribution of fine particulate matter emitted from wood burning, meat charbroiling, and cigarettes Environ Sci Technol 33:3516–3523 [Google Scholar]
- Lacaux JP, Delmas R, Jambert C, Kuhlbusch TAJ (1996) NOx emissions from African savanna fires J Geophys Res 101:23585–23595 doi:doi: 10.1029/96JD01624 [DOI] [Google Scholar]
- Lam CW, James JT, McCluskey R, Hunter RL (2004) Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation Toxicol Sci 77:126–134 doi: 10.1093/toxsci/kfg243 [DOI] [PubMed] [Google Scholar]
- Lim R et al. (2014) Measuring respiratory function in mice using unrestrained whole-body plethysmography J Vis Exp: e51755 doi: 10.3791/51755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML (2015) A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke Environ Res 136:120–132 doi: 10.1016/j.envres.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (1998) Acute toxicity of nitrogen dioxide In: Assessment of Exposure-Response Functions for Rocket-Emission Toxicants. National Academy Press, Washington, D.C., [PubMed] [Google Scholar]
- Osier M, Oberdorster G (1997) Intratracheal inhalation vs intratracheal instillation: differences in particle effects Fundam Appl Toxicol 40:220–227 [DOI] [PubMed] [Google Scholar]
- Otterbein LE et al. (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway Nat Med 6:422–428 doi: 10.1038/74680 [DOI] [PubMed] [Google Scholar]
- Rau JA (1989) Composition and size distribution of residential wood smoke particles Aerosol Sci Technol 10:181–192 doi: 10.1080/02786828908959233 [DOI] [Google Scholar]
- Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT (2016) Critical review of health impacts of wildfire smoke exposure Environ Health Perspect 124:1334–1343 doi: 10.1289/ehp.1409277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JS, Hobbs PV (1998) Physical and optical properties of young smoke from individual biomass fires in Brazil J Geophys Res Atmos 103:32013–32030 doi:doi: 10.1029/98JD00159 [DOI] [Google Scholar]
- Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT (2017) Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy Am J Respir Crit Care Med 195:596–606 doi: 10.1164/rccm.201606-1275CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallsten G et al. (2006) Experimental wood smoke exposure in humans Inhal Toxicol 18:855–864 doi: 10.1080/08958370600822391 [DOI] [PubMed] [Google Scholar]
- Tesfaigzi Y et al. (2002) Health effects of subchronic exposure to low levels of wood smoke in rats Toxicol Sci 65:115–125 [DOI] [PubMed] [Google Scholar]
- Thomas HV, Mueller PK, Wright R (1967) Response of rat lung mast cells to nitrogen dioxide inhalation J Air Pollut Control Assoc 17:33–35 doi: 10.1080/00022470.1967.10468939 [DOI] [PubMed] [Google Scholar]
- Urbanski S (2014) Wildland fire emissions, carbon, and climate: Emission factors Forest Ecol Manage 317:51–60 doi: 10.1016/j.foreco.2013.05.045 [DOI] [Google Scholar]
- Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG (2010) Assessing pulmonary pathology by detailed examination of respiratory function Am J Pathol 177:1861–1869 doi: 10.2353/ajpath.2010.100053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoirbeek JA et al. (2010) Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases Am J Respir Cell Mol Biol 42:96–104 doi: 10.1165/rcmb.2008-0487OC [DOI] [PubMed] [Google Scholar]
- Ward DE, Hardy CC (1991) Smoke emissions from wildland fires Environ Int 17:117–134 doi: 10.1016/0160-4120(91)90095-8 [DOI] [Google Scholar]
- WHO (2000) Carbon monoxide vol European Series, No.91, 2nd edn WHO Regional Publications, [Google Scholar]
- Wilson MR, O’Dea KP, Dorr AD, Yamamoto H, Goddard ME, Takata M (2010) Efficacy and safety of inhaled carbon monoxide during pulmonary inflammation in mice PloS one 5:e11565 doi: 10.1371/journal.pone.0011565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BA (2007) Inhalation exposure systems: design, methods and operation Toxicol Pathol 35:3–14 doi: 10.1080/01926230601060017 [DOI] [PubMed] [Google Scholar]
- Youssouf H et al. (2014) Non-accidental health impacts of wildfire smoke Int J Environ Res Public Health 11:11772–11804 doi: 10.3390/ijerph111111772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


