Abstract
Background
Bulimia Nervosa (BN) represents an important public health problem and is related to serious morbidity and even mortality. This review attempted to systematically evaluate the use of antidepressant medications compared with placebo for the treatment of bulimia nervosa.
Objectives
The primary objective of this review was to determine whether using antidepressant medications was clinically effective for the treatment of bulimia nervosa.
The secondary objectives were: (i) to examine whether there was a differential effect for the various classes/types of antidepressants with regard to effectiveness and tolerability (ii) to test the hypothesis that the effect of antidepressants on bulimic symptoms was independent of its effect on depressive symptoms
Search methods
(1) electronic searches of MEDLINE (1966 to December 2002), EMBASE (1980‐December 2002) , PsycINFO (to December 2002), LILACS & SCISEARCH (to 2002) (2) the Cochrane Register of Controlled Trials and the Cochrane Depression, Anxiety and Neurosis Group Register ‐ ongoing (3) inspection of the references of all identified trials (4) contact with the pharmaceutical companies and the principal investigator of included trials (5) inspection of the International Journal of Eating Disorders ‐ ongoing
Selection criteria
Inclusion criteria: every randomised, placebo‐controlled trial in which antidepressant medications were compared to placebo to reduce the symptoms of bulimia nervosa in patients of any age or gender. Quality criteria: reports were considered adequate if they were classified as A or B according to the Cochrane Manual. The Jadad scale, with a cut off of 2 points, was applied to check the validity of the above referred criterion but was not used as an inclusion criterion.
Data collection and analysis
Data were extracted independently by two reviewers for each included trial. Dichotomous data were evaluated by the relative risk with 95% confidence intervals (CI) around this measure, based on the random effects model; continuous data were evaluated by the standardised mean difference with the 95% CI. NNT was calculated using the inverse of the absolute risk reduction.
Main results
Currently the review includes 19 trials comparing antidepressants with placebo: 6 trials with TCAs (imipramine, desipramine and amitriptyline), 5 with SSRIs (fluoxetine), 5 with MAOIs (phenelzine, isocarboxazid, moclobemide and brofaromine) and 3 with other classes of drugs (mianserin, trazodone and bupropion). Similar results were obtained in terms of efficacy for these different groups of drugs. The pooled RR for remission of binge episodes was 0.87 (95% CI 0.81‐0.93; p<0,001) favouring drugs. The NNT for a mean treatment duration of 8 weeks, taking the non‐remission rate in the placebo controls of 92% as a measure of the baseline risk was 9 (95% CI 6 ‐ 16). The RR for clinical improvement, defined as a reduction of 50% or more in binge episodes was 0.63 (95% CI 0.55‐0.74) and the NNT for a mean treatment duration of 9 weeks was 4 (95% CI 3 ‐ 6), with a non‐improvement rate of 67% in the placebo group. Patients treated with antidepressants were more likely to interrupt prematurely the treatment due to adverse events. Patients treated with TCAs dropped out due to any cause more frequently that patients treated with placebo. The opposite was found for those treated with fluoxetine, suggesting it may be a more acceptable treatment. Independence between antidepressant and anti‐bulimic effects could not be evaluated due to incomplete published data.
Authors' conclusions
The use of a single antidepressant agent was clinically effective for the treatment of bulimia nervosa when compared to placebo, with an overall greater remission rate but a higher rate of dropouts. No differential effect regarding efficacy and tolerability among the various classes of antidepressants could be demonstrated.
Plain language summary
Antidepressants compared with placebo for bulimia nervosa
Individual antidepressants are effective for the treatment of bulimia nervosa when compared to placebo treatment, with an overall greater remission rate but a higher rate of dropouts.
Background
Bulimia Nervosa is an eating disorder characterized by recurrent episodes binge eating (eating objectively large amounts of food over which the person has loss of control) followed by recurrent use of extreme compensatory behaviours in order to prevent weight gain, such as self‐induced vomiting; misuse of laxatives, diuretics, enemas or other medications; fasting; or excessive exercise. In addition, body shape and weight have an undue influence on sufferers self‐esteem and their self‐evaluation (APA 1994). The Diagnostic and Statistical Manual of the APA (APA 1994) specify frequency criteria for both binge eating and extreme compensatory behaviours, namely they occur a minimum of twice weekly over the preceding three months at the time of assessment.
Bulimia nervosa as a syndrome is not the same as "bulimia", meaning overeating, which can be found as a symptom in different psychiatric, neurologic or medical conditions. The syndrome as it is recognized by today's clinicians was first described by Russell in 1979 (Russell 1979) and was virtually unknown until the latter half of the 20th century (Russell 1997). A period of confusion followed the definition for the so‐called "bulimia" published in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (APA 1980). Bulimia was simply defined by recurrent episodes of binge eating, and the important criterion of morbid fear of fatness was omitted, and the criterion of attempted weight loss by self‐induced vomiting or other purging and non purging compensatory behaviours was optional. This confusion was addressed in the revision of the DSM in 1987 (APA 1987). During these seven years, and to a smaller extent thereafter, researchers included patients in trials who did not present the full syndrome as it is now understood.
Surveys indicate that the disorder affects 1.3% to 10.1% of North American women, depending on the diagnostic criteria used in the epidemiological studies (DSM‐III criteria being probably over inclusive). The prevalence among adolescent and young adult females is approximately 1% (APA 1994). Bulimia nervosa has been reported in all socio‐economic classes even if it is most commonly reported in developed countries and higher socio‐economic classes. At least 90% of individuals with bulimia nervosa are female. Bulimia nervosa may cause serious morbidity and even mortality (FBNCSG 1992) representing an important public health problem.
Two treatments have received the most support in controlled studies of bulimia nervosa: psychotherapeutic approaches, mainly cognitive behavior therapy, and antidepressant medication.
Binge eating is typically triggered by dysphoric mood stages and the episodes are often followed by depressed mood and self‐criticism. Moreover, there is an increased frequency of depressive symptoms or underlying mood disorders in individuals with bulimia nervosa. Thus, the connection between bulimia nervosa and depression has received particular attention. Although it is not clear whether depression antedates, coexists with or is a consequence of the eating disorders, this association led to the investigation of antidepressants in the treatment of bulimia nervosa (Advokat 1995).
All types of antidepressants seem to be beneficial in relieving bulimic symptoms (Wolfe 1995). There is no clear evidence of a differential effect among the various drugs used. It is not known if any particular class of antidepressants is more effective although selective serotonin re uptake inhibitors have been thought to produce benefits relative to placebo. It is thought that antidepressants have specific anti‐bulimic effects as they are both effective in bulimic patients who do and do not suffer depression.
Although antidepressants have been shown to be useful in the treatment of bulimia nervosa, results are far from optimal. Short‐term abstinence rates (on average 8 weeks) are about 30%, and overall reductions in bulimic behaviours are about 70% (Agras 1992, Leitenberg 1994). A significant relapse rate ( 30‐45%) is observed in patients followed for 4‐6 months ( Walsh 1991a). Pope 1983 also reported prompt relapse with discontinuation of antidepressant treatment, but good maintenance of effects when the drug was continued in a small 2‐year follow‐up of participants from a randomised controlled trial (RCT). In addition, two RCTs have specifically examined adverse effects of treatment. Walsh 1991a found significant increases in pulse rate, reclining systolic and diastolic blood pressures and orthostatic hypotension in young women with bulimia nervosa treated with desipramine. These effects appeared to be well‐tolerated. Carruba 2001 found no changes in blood pressure in participants randomised to moclobemide despite food diaries documenting a high consumption of tyramine‐containing foods. In addition, Wheadon 1992a in a meta‐analysis of two double‐blind RCTs of fluoxetine versus placebo found no significant difference in the incidence of suicidal acts or ideation. However, these were infrequent (suicide attempts 1.2%, none fatal; emergent suicidal ideation 3.1%). This review evaluated the use of antidepressants compared to placebo in the treatment of bulimia nervosa, both with regard to efficacy and acceptability.
Objectives
The primary objective of this review was to investigate whether the use of antidepressant medications is clinically effective for the treatment of bulimia nervosa when compared with placebo. Where possible, a meta‐analytic synthesis of the studies was performed.
The secondary objectives were: (i) to examine whether there was a differential effect for the various classes/types of antidepressants with regard to effectiveness and tolerability (ii) to test the hypothesis that the effect of antidepressants on bulimic symptoms was independent of their effect on depressive symptoms
Usefulness of antidepressants for more than 8 weeks was also investigated.
Methods
Criteria for considering studies for this review
Types of studies
We attempted to identify all relevant randomised placebo‐controlled trials.
Types of participants
People with bulimia nervosa defined by clinical state description or diagnosed by Russell's, DSM‐III, DSM‐III‐R, DSM‐IV or ICD‐10 criteria, irrespective of gender, age or treatment setting. Participants with both purging and non purging type bulimia nervosa, as defined in DSM‐IV (APA 1994), were included. As we intended to examine the influence of depression and obesity on clinical outcome, trials including patients with co morbid depression or obesity were also eligible.
Exclusion criteria: people with binge‐eating/purging type anorexia nervosa or binge‐eating disorder as defined in DSM‐IV (APA 1994).
Types of interventions
Trials were included if they compared antidepressant medications of any class/type to placebo for at least 4 weeks. The following antidepressants were included: a) tricyclic antidepressants (TCA):imipramine, amitriptyline, clomipramine, nortriptyline, desipramine; b) selective serotonin re uptake inhibitors (SSRI): fluoxetine, sertraline, paroxetine, citalopram, fluvoxamine; c) monoamine oxidase inhibitors (MAOI): phenelzine, isocarboxazid, moclobemide, brofaromine, tranylcypromine; d) other antidepressants: bupropion, trazodone, nefazodone, mianserin, mirtazapine, venlafaxine.
Trials with combined or augmentation drug therapies compared to placebo could also be included.
Types of outcome measures
Primary outcomes of interest were:
A. Changes in bulimic symptoms (i) the number of people per treatment group who did not show a remission in the bulimic symptoms, defined as 100% reduction in binge‐eating episodes from baseline at endpoint (ii) the number of people per treatment group who did not show a clinical improvement in the bulimic symptoms, defined as more than 50% reduction in binge‐eating episodes from baseline at endpoint (iii) the difference in the mean number of bulimic episodes at the end of the trial B. Comorbidity (i) difference in the severity of depressive symptoms at the end of the trial
C. Acceptability of the treatment (i) acceptability of the intervention to the participant group as measured by the number of people per treatment group dropping out during the trial for any cause (ii) tolerability of the intervention as measured by the number of people per treatment group dropping out during the trial due to adverse events
Search methods for identification of studies
See: Collaborative Review Group search strategy
A. Electronic searching Relevant randomised trials were identified by searching the following electronic databases by means of the Depression, Anxiety and Neurosis Group Strategy (see CCDAN module). A subsection of these trials was obtained by linking the CCDANCTR search with the following specific search for this review:
bulimia or bing* or overeat* or "compulsive eat*" or "compulsive vomit*" and pharmacotherapy*
(i) MEDLINE (January 1966 to December 2002).
(ii) EMBASE (January 1980 to December 2002)
(iii) LILACS (January 1982 to December 2002)
(iv) PsycINFO (January 1974 to December 2002)
This downloaded set of reports was searched for possible trials and re‐searched, within the bibliographic package, ProCite, with the phrase: [antidepressant* or tricyclic* or imipramine or amitriptyline or clomipramine or nortriptyline or desipramine or fluoxetine or sertraline or paroxetine or citalopram or fluvoxamine or bupropion or trazodone or nefazodone or phenelzine or isocarboxazid* or moclobemide or brofaromine or tranylcypromine or mianserin or mirtazapine]
(v) the Cochrane Depression, Anxiety and Neurosis Group Database of Trials
(vi) the Cochrane Central Register of Controlled Trials (CENTRAL)
(vii) SCISEARCH ‐ Science Citation Index Each of the included studies was sought as a citation on the SCISEARCH database. Reports of articles that had cited these studies were inspected in order to identify further trials.
Electronic search was performed by the DAN Group.
B. A second search was conducted with Clinical Evidence (Hay 2001) and comprised MEDLINE 1966‐December 2002, EMBASE 1980‐December 2002, PsycINFO 1989‐December 2002 and The Cochrane Library 2002 Issue 4. The following terms were used: (bulimia or bulimia nervosa or eating disorders or binge eating) and (therapy or treatments or trials or psychotherapy or cognitive‐behavioural therapy or pharmacotherapy or antidepressant or SSRI or MAOI)
C. Reference searching The reference lists of all papers selected were inspected for further relevant studies.
D. Pharmaceutical companies Companies carrying out comparative studies of their own products with placebo in the treatment of bulimia nervosa were contacted in order to obtain data on unpublished trials.
E. Personal contact The first authors of all included studies were contacted for further information or information regarding unpublished trials.
F. An inspection from the first issue of the International Journal of Eating Disorders is ongoing.
Data collection and analysis
Selection of trials The abstract of each reference identified by the search was evaluated by one reviewer (JB) in order to see if the study was likely to be relevant to this review. For this review all trials comparing drugs with placebo were eligible, regardless if other comparisons were made in the trial or not. Studies comparing two active drugs or drugs versus psychotherapy were not eligible for this review. For possible RCTs the full article was obtained and inspected to assess their relevance for this review based on the inclusion criteria.
Quality assessment Once the full relevant articles were obtained, two reviewers (JB and PH), independently, decided whether they met criteria to be included. In order to ensure that variation in results was not caused by systematic errors in the design of a study, the methodological quality of the trials was assessed by the two independent reviewers using the criteria described in the Cochrane Handbook (Mulrow 1996) and the Jadad Scale (Jadad 1996). The Cochrane Collaboration Handbook criteria are based on the evidence of a strong relationship between potential for bias in the results and allocation concealment (Schultz 1995) and are defined as below:
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment)
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
1. Was the study described as randomised? 2. Was the study described as double‐blind? 3. Was there a description of withdrawals and drop‐outs?
Each item received 1 point if the answer was positive. In addition, a point was deducted if either the randomisation or the blinding/masking procedures described were inadequate.
For the purpose of the analysis in this review, trials were included if they met the criteria A or B according to the Cochrane quality criteria (Sackett 1997). Additionally, a cut‐off of two points was used in the Jadad scale to check the assessment made by the Handbook criteria. However, the latter was not used to exclude trials in this review.
Reviewers were not blind to the names of the authors, institutions and journal of publication. Where disagreement could not be resolved by discussion and consensus, further information was sought contacting the authors for clarification. The articles were then added to the list of those awaiting assessment. The same methodology was used for the selection of trials identified by means of reference lists searched and for data on unpublished trials obtained through contacts with the pharmaceutical industry or with first authors. An inter‐rater reliability study between reviewers for the Cochrane Collaboration Handbook criteria (Sackett 1997) was performed by means of the kappa.
Data Management Data from the selected trials were extracted by the two reviewers. Again, any disagreement was discussed, decisions documented and, where necessary, the authors of the studies were contacted for clarification. Justification for excluding references from the review was documented. We anticipated that many trials would have inadequate reporting. Therefore, for studies that did not specify reasons for subjects who dropped out, we assumed that the subjects had no change in their bulimic symptoms. When insufficient data were provided to identify the original group size (prior to drop‐outs) the authors were contacted and the trials were allocated to the "awaiting assessment" list for a period of up to 1 year. If no information from authors was obtained, the trial was excluded.
We also expected that some trials would have used a crossover design. In order to exclude the potential additive effect in the second or later stages on these trials, only data from the first stage were analysed. If information from this first period was not obtained from authors, the trial was excluded from the meta‐analysis.
Analysis Dichotomous outcomes (remission, clinical improvement, drop‐outs) were analysed by the relative risk (RR) with 95% confidence intervals (CI) for each trial. The RR from the individual trials were combined using appropriate methods of meta‐analysis. Additionally, when overall results were significant, the number needed to harm (NNH) or the number needed to treat (NNT) to prevent (or to produce) one outcome was calculated on the inverse of the absolute risk reduction, by combining the RR with an estimate of the prevalence of the event in the control groups of the trials. The estimates of RR were based on the random effects model as it takes into account any difference between studies (even if there is no statistically significant heterogeneity) and gives the same result as the fixed effects model when there is no between study variance (DerSimonian 1986). For the analysis of continuous outcomes, the mean and standard deviation of bulimic symptoms and scores of depression scales for each trial were assessed, and the standardised mean difference with 95% CI calculated using the random effects method. Heterogeneity in the results of the trials ‐ i.e., whether differences were greater than would be expected by chance alone ‐ was assessed both by inspection of graphical presentations and by a test of heterogeneity using a Q ("combinability") statistic. A p value < 0.05 indicated statistical heterogeneity. The presence of publication bias from the tendency to publish only statistically significant results or results supporting the hypothesis was detected graphically in a "funnel plot" (Light 1984). When selective publication is present the distribution around the pooled RR of all studies is not homogeneous, indicating that small trials with non‐significant results are not being published, the "file‐drawer problem" (Rosenthal 1979). Sensitivity analyses were performed for: quality of trials; length of treatment (up to 8 weeks of treatment and 8 or more weeks of treatment); diagnostic criteria (DSM‐III bulimia versus DSM‐III‐R bulimia nervosa criteria); bulimic and purging symptoms; and class of drugs.
Results
Description of studies
Nineteen trials fulfilled inclusion criteria and had available data which could be used in this review (1436 patients in total). These trials were used for at least one of the main comparisons.
Further information for four trials could not be obtained from authors, therefore the following trials were excluded from this review: Alger 1991 (separate data for bulimic patients not obtained); Barlow 1988 and Blouin 1988 (data for the first crossover period not obtained); and Hughes 1986a (no evaluable data available). The first authors of these four papers have been contacted by courier. Three of them (Alger 1991, Barlow 1988, Blouin 1988) changed their address and did not receive the letters and no information could be obtained from the fourth (Hughes 1986a). Other twenty‐six studies were excluded. Most (n=15 ) were subsets from other studies, including follow‐up or interim analyses. Six studies compared antidepressants and psychotherapy without a placebo controlled group and were analysed in other specific reviews (Bacaltchuk 2003) (Fichter 1991; Agras 1992; Leitenberg 1994; Russell 1995b; Goldbloom 1996; and Walsh 1997). One study was excluded because patients did not fulfil diagnostic criteria for bulimia or bulimia nervosa (Box 1983; patients described as "overweight compulsive eaters"); one study compared antidepressants with no intervention (Scrimali 1994; no placebo controlled group); and one study did not provide separate data for obese binge eaters and bulimic patients (Marcus 1990; author replied to contact, data not available). When multiple publications of the same trial were found, all reports were checked and even if information of all trials was used only the main publication was referenced. In case of discrepancies among different publications of the same trial authors were contacted.
Design All studies were described as randomised. One study (FBNCSG 1992) compared two fixed doses of fluoxetine (20 and 60 mg daily) to placebo. As the 20 mg group showed no significant difference over placebo, we considered only the 60 mg group for comparisons; one study (Mitchell 1990) compared imipramine to psychotherapy and placebo. For the purpose of this review we considered only the imipramine and placebo groups; another study (Mitchell 1999c) compared fluoxetine to either placebo, self help manual or a combination of both active treatments. Only the fluoxetine and placebo groups were considered. Finally, one study (Rothschild 1994) compared three groups of patients with atypical depression presenting comorbid bulimia randomised to receive either imipramine, phenelzine or placebo. Outcomes were analysed retrospectively. We included the phenelzine and placebo groups in the comparisons as more patients were randomised to these groups than for imipramine. All other studies used parallel group design comparing one antidepressant drug to placebo. One further study (Walsh 1999) randomised patients to fluoxetine or placebo following a poor response to psychotherapy. Duration of trials ranged from 6 weeks to 16 weeks. A subgroup analysis was performed comparing trials with a duration of up to weeks versus those with a duration of more than 8 weeks.
Settings Two studies were multicenter, multinational (FBNCSG 1992; Wheadon 1992a), both comparing fluoxetine to placebo. Twelve trials were conducted in the United States of America (Agras 1987; Horne 1988; McCann 1990; Mitchell 1984, Mitchell 1990 and Mitchell 1999c; Pope 1983 and Pope 1989; Rothschild 1994; Walsh 1984a, Walsh 1991a and Walsh 1999), two in Canada (Kennedy 1988 and Kennedy 1993) one in the United Kingdom (Sabine 1983), one in Norway (Kanerva 1994) and one in Italy (Carruba 2001). Trials recruited outpatients either seeking treatment "spontaneously" from eating disorders clinics/programs (6 trials) or included also "volunteers" for research recruited through advertisement in local newspapers and media (13 trials).
Participants Eight trials included in the main comparisons used DSM‐III criteria for bulimia, 9 trials used DSM‐III‐R criteria for bulimia nervosa and one trial used DSM IV criteria. Sabine 1983 did not use a formal diagnostic criterion but described patients clinically as bulimics. As the DSM‐III diagnostic criteria for "bulimia" omitted the important criterion of a morbid fear of fatness and even the criterion of attempted weight loss by self‐induced vomiting was optional, we can not state that patients diagnosed as bulimics according to the DSM‐III criteria are equivalent to those presenting the DSM‐III‐R criteria for bulimia nervosa. We then performed a sensitivity analysis comparing trials using bulimic patients diagnosed according to DSM‐III with those diagnosed according to DSM‐III‐R or Russell's criteria. The study population included in the trials had comparable demographic and behavioral features. All studies except one (McCann 1990) included purging type bulimics, according to the DSM‐III‐R definition ("the person regularly engages in self‐induced vomiting or misuse laxatives, diuretics or enemas"). Patients were mostly adult and young adult females, few adolescents were included. The median mean number of binges at baseline was 10.1, ranging from 3.8 to 12.0. A constant inclusion criterion was that patients should be between 80 and 120% of the expected ideal weight or body mass index (BMI). Most patients were not depressed, and mean baseline scores in depression scales ranged from 12.2 to 16.9 for the studies assessing depression by means of the Hamilton Depression Rating Scale ‐ 21 items and from 12.4 to 18.8 for those using the Beck Depression Inventory. Mean duration of symptoms ranged from 5.0 to 14.0 years. The number of participants randomised in the trials ranged from 18 to 398. No concomitant psychotherapy was performed.
Interventions All comparisons were of a single antidepressant versus placebo. No trial compared two or more combined drugs or augmentation therapies with placebo. Six trials compared TCAs with placebo: imipramine (Agras 1987; Mitchell 1990; Pope 1983) desipramine (McCann 1990; Walsh 1991a) and amitriptyline (Mitchell 1984); five compared MAOIs with placebo: phenelzine (Rothschild 1994; Walsh 1984a) isocarboxazid (Kennedy 1988), brofaromine (Kennedy 1993) and moclobemide (Carruba 2001); five studies compared fluoxetine with placebo (FBNCSG 1992; Wheadon 1992a; Kanerva 1994; Mitchell 1999c; Walsh 1999); and three compared other antidepressants with placebo: mianserin (Sabine 1983) trazodone (Pope 1989) and bupropion (Horne 1988).
Outcomes Four dichotomous outcomes were used in this review. Two concerned changes in bulimic symptoms, and were considered primary efficacy outcomes: remission and clinical improvement. Remission was described in 10 trials and clinical improvement in 8 studies. Remission in bulimic symptoms was considered the more stringent criteria for improvement and was defined as 100% reduction in binge‐eating episodes from baseline to endpoint. Clinical improvement was defined as a reduction of 50% or more in binge‐eating episodes. As we could not assume beforehand that all patients included in the studies would be purging type bulimic individuals, binge‐eating episodes were considered the main bulimic symptom. So, where binge eating and purging episodes were reported, only binge‐eating episodes were considered. Where only purging episodes were reported, they were considered as binge‐eating episodes. A sensitivity analysis was performed to verify if purging and bingeing could be conflated. The other two dichotomous outcomes used concerned acceptability and tolerability of treatment. The first, 'number of drop‐outs due to adverse events' was recorded where adverse experiences were so severe that patients stopped treatment prematurely. This was extracted from 13 trials. The second, 'drop‐outs due to any cause', (causes included adverse events, lack of improvement, protocol violations, patient choice) could be extracted from 15 trials. Continuous outcomes analysed were the mean difference in bulimic symptoms (mean number of binges per treatment group) and mean difference in depressive symptoms (mean scores in depression scales per treatment group) at endpoint. Three trials reported only median scores with range for both bulimic and depressive symptoms (FBNCSG 1992; Wheadon 1992a and Walsh 1999) and data were not used in the pooled analysis. In some other cases data on standard deviations were lacking, so these trials could not be included in the analysis. For analysis of improvement in depressive symptoms, studies were pooled together, as far as all of the trials were comparable even if measures of depression were from different scales (according to Cucherat 1997, pp. 250).
Risk of bias in included studies
Methodological quality was assessed independently by two reviewers (JB and PH). Inter‐rater agreement for the Cochrane Collaboration Handbook criteria between reviewers employed the kappa statistic as a measure of agreement. Kappa for this analysis was calculated by means of the ARCUS Quickstat software for windows (Buchan 1998) and the result (0.81) indicated a very good agreement beyond chance. Letters requesting additional information on allocation concealment for all trials were sent to authors. Only two trials (Wheadon 1992a and Sabine 1983) were graded as "A" according to the first quality assessment criteria (Mulrow 1996), thanks to further information provided by the authors to our inquiry. All other 14 trials were classified as "B" because no information on allocation concealment was given in the report.
In general, the quality of reporting was poor. All trials reported the randomisation procedure without adequate information on allocation concealment. Blinding procedures were also inadequately described in all trials. Some did not reported the number of drop‐outs or did not specified reasons for drop‐out. Several trials excluded patients after randomisation from analysis and did not perform an intention‐to‐treat analysis. Some trials also omitted the standard deviations of continuos outcomes. The most frequently reported outcome was mean percentage change in bulimic episodes, the omission of standard deviations being common again for this measure.
Effects of interventions
Publication bias The dysymetric funnel plot histogram for the dichotomous outcomes "remission" and "clinical improvement" reflects the absence of smaller studies favouring placebo or showing lower RR in favour of antidepressants, consistent with possible publication bias. This bias was confirmed by the identification of at least two unpublished reports of a negative multicenter, multinational study comparing fluvoxamine with placebo (Corcos 1996), showing a lack of greater efficacy of fluvoxamine compared to placebo. Authors were contacted (Russell GFM in England and Jeammet PH in France) but we could not obtain their unpublished results.
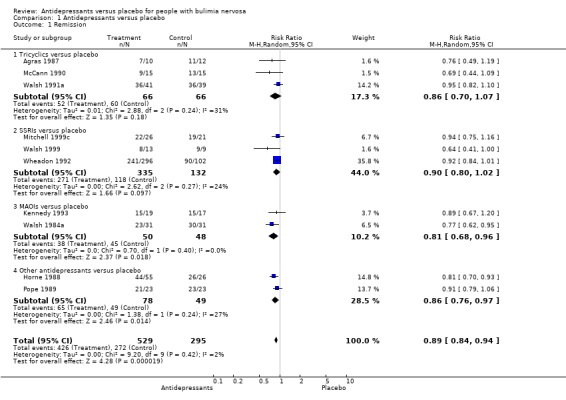
Remission This outcome was reported in ten trials (Agras 1987, McCann 1990, Walsh 1991a[TCAs]; Wheadon 1992a[SSRI]; Kennedy 1993, Walsh 1984a [MAOIs]; Horne 1988 [bupropion]; Pope 1989 [trazodone] and Walsh 1999 and Mitchell 1999c [SSRI]). In general, short‐term remission of binge episodes was more likely on antidepressants than placebo. TCAs (three studies, 66 patients per treatment group) showed a RR of 0.86 (95% CI 0.70‐1.07). Concerning MAOIs (two trials, 50 patients in the antidepressant group and 48 patients in the placebo group), the RR was 0.81 (95% CI 0.68‐0.96) and the NNT for a mean treatment duration of 8 weeks was 6 (95% CI 3 ‐ 27), considering a non‐remission rate of 94% in the placebo group. SSRIs (three trials, 335 treated with fluoxetine and 132 with placebo) showed a RR of 0.89 (95% CI 0.76‐1.03). Two trials evaluating other drugs (trazodone and bupropion) reported remission rates. As no evidence of heterogeneity in terms of non‐remission was found considering all studies together by using a chi‐square statistic (X2=10.63; df=9; p=0.3) it was possible to perform a further analysis: "any antidepressant" versus placebo. The pooled RR considering the eight studies reporting this outcome was 0.88 (95% CI 0.82‐0.93; p<0.001) favouring drugs. The NNT for a mean treatment duration of 8 weeks (min‐max=6‐16), taking the non‐remission rate in the placebo controls of 92% as a measure of the baseline risk, was 9 (95% CI 6 ‐ 16).
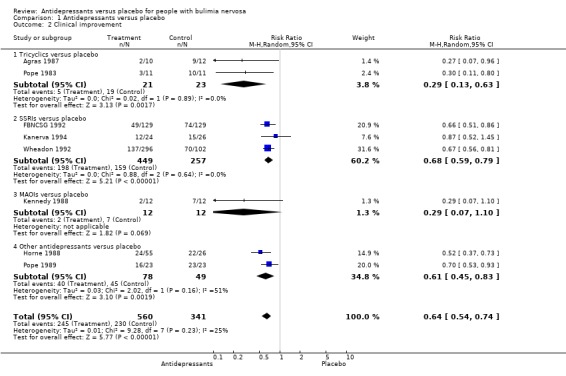
Clinical improvement This outcome was reported in eight trials (Agras 1987, Pope 1983 [TCAs]; FBNCSG 1992, Wheadon 1992a, Kanerva 1994 [SSRI]; Kennedy 1988 [MAOI]; Horne 1988 [bupropion] and Pope 1989 [trazodone]). Effects were much stronger than those observed for remission, specially for TCAs, but the two studies were small, including only 44 patients (21 for active drug and 23 for placebo) in total. The RR for these two trials was 0,29 (95% CI 0,13‐0,63) and the NNT for a mean treatment duration of 11 weeks was 2 (95% CI 1 ‐ 3), for a non‐improvement rate in the placebo group of 83%. For fluoxetine, the only SSRI studied, the RR was 0,68 (95% CI 0.59‐0.79). The three trials considered in the pooled analysis included 706 patients (449 and 257 for fluoxetine and placebo respectively), representing 78% of the total patients included in the analysis of this outcome. The NNT for a mean treatment duration of 10 weeks with fluoxetine was 6 (95% CI 4 ‐ 10), for a non‐improvement rate of 62% in the placebo group. Considering the effect of any drug, the RR was 0.63 (95% CI 0.55‐0.74). This estimate was based on the random effects model and possible differences between studies (chi‐square heterogeneity test: 9.22 (df=7; p=0.237) were taken into account. The NNT for a mean treatment duration of 9 weeks (min‐max=6‐16) with any drug for clinical improvement was 4 (95% CI 3 ‐ 6), with a non‐improvement rate of 67% in the placebo group.
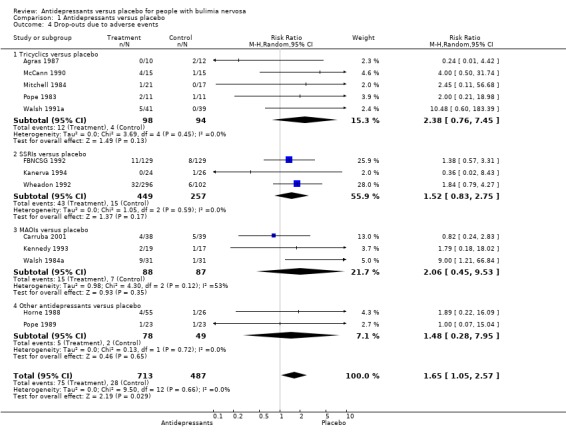
Tolerability No significant results among and within classes of drugs were found in the number of people per treatment group dropping out during the trial due to adverse events, the measure of tolerability used in this review, as reported in thirteen trials (Agras 1987, McCann 1990, Mitchell 1984, Pope 1983, Walsh 1991a[TCAs]; FBNCSG 1992, Wheadon 1992a, Kanerva 1994 [SSRI]; Kennedy 1993, Walsh 1984a and Carruba 2001[MAOIs]; Horne 1988[bupropion] and Pope 1989 [trazodone]). The RR for TCAs (five trials and 192 patients analysed) was 2.38 (95% CI 0.76‐7.45; chi‐square heterogeneity test: 3.69 (df=4; p= 0.45)). For fluoxetine (SSRI) the RR was 1.52 (95% CI 0.83‐2.75 ; three trials and 706 patients analysed; chi‐square heterogeneity test: 1.05 (df=2; p=0.59)) The pooled RR for MAOIs (three trials and 175 patients) was 2.06 (95% CI 0.45‐9.53; X2 heterogeneity test: 4.30 (df=2; p=0.12)). Other drugs (bupropion: one trial, RR= 1.89 [95% CI 0,22‐16,09] and trazodone: one trial, RR= 1.00 [95% CI 0.07‐15.04]) reported similar results for drop‐outs. MAOIs presented the higher rates of drop‐outs due to adverse events of all classes of antidepressants analysed (22% versus 4% for placebo). As no heterogeneity was found among classes of drugs, the comparison "any drug" versus placebo was performed. The pooled analysis of all trials reporting drop‐outs due to adverse events showed a statistically significant result in favour of placebo: RR= 1.65 (95% CI 1.05‐2.57); chi‐square statistic for heterogeneity=9.50 (df=12); p=0.64. The NNH for a mean treatment duration of 9 weeks (min‐max=6‐16) was 19 (95% CI 12 ‐ 46), given a prevalence of drop‐outs of 5.1% in the placebo group.
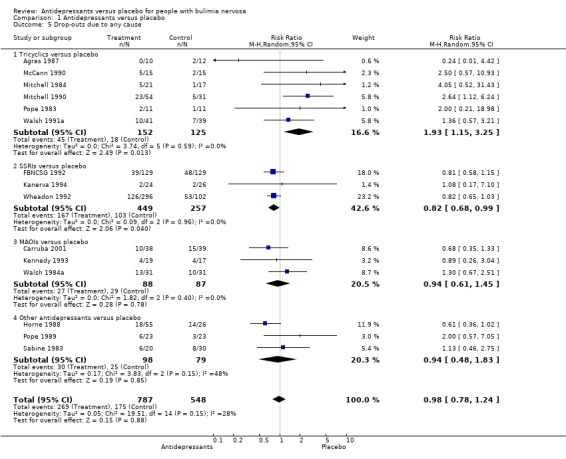
Acceptability of treatment TCAs and SSRIs showed statistically significant results for drop‐outs due to any cause, but in opposite directions. The RR for TCAs (six trials and 277 patients [Agras 1987, McCann 1990, Mitchell 1984 and Mitchell 1990, Pope 1983 and Walsh 1991a]) was 1.93 (95% CI 1.15‐3.25), drop‐outs being more likely in the TCA group. No heterogeneity was found among TCA studies (X2=3,74[df=5]; p=0.577) and the NNH for a mean treatment duration of 10 weeks (min‐max=6‐16) was 7 (95% CI 4 ‐ 18), given a prevalence of drop‐outs due to adverse events of 14.4% in the placebo group. For fluoxetine (SSRI; three trials and 706 patients [FBNCSG 1992, Wheadon 1992a, Kanerva 1994]) the RR was 0.82 (95% CI 0.68‐0.99) but the drop‐outs were more likely in the placebo group. As no heterogeneity was found among these three trials the NNH was calculated (35 [95% CI 10 ‐ infinity]). No statistically significant increase in drop‐outs due to any cause was found for MAOIs (three trials and 175 patients [Kennedy 1993; Walsh 1984a and Carruba 2001]), bupropion (Horne 1988), trazodone (Pope 1989) or mianserin (Sabine 1983). As the test of heterogeneity for the comparison "any drug" versus placebo was not significant (chi‐square heterogeneity test: 19.51 (df=14; p=0.15) this comparison was performed. No statistically significant difference in acceptability of treatment between antidepressants and placebo has been shown (RR= 0.98,95% CI 0.78‐1.24).
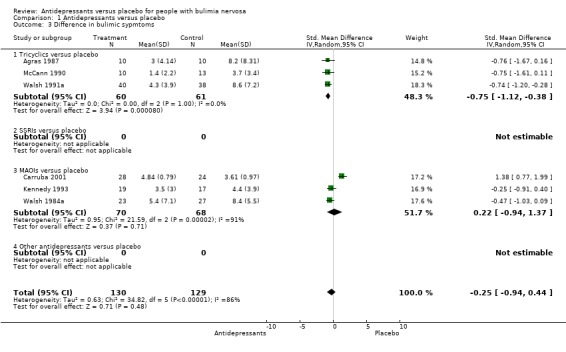
Difference in bulimic symptoms This analysis was based on the number of patients who reported the number of bulimic episodes at the end of the trial, and it was not an intention to treat analysis because of missing data. No difference was found among treatment groups in baseline mean rates of bulimic episodes, and all trials reporting this outcome were included in the analysis (Agras 1987, McCann 1990, Walsh 1991a [TCAs]; Kennedy 1993, Walsh 1984a and Carruba 2001[MAOIs]). As heterogeneity was found among these 6 trials, they were not pooled. When the outlier (Carruba 2001) identified through the graphic analysis of SMD results was excluded from the pooled studies no heterogeneity was found among the remaining 5 trials (X2= 4.67, df=4; p=0,323)). A possible reason for the heterogeneity was that this was the only study significantly favouring placebo. Pooled estimate of standardised effect size for the comparison "any antidepressant" versus placebo was ‐0.59 (approximate 95% CI = ‐0,87 to ‐0,31; p < 0,0001) in favour of active antidepressant drugs.
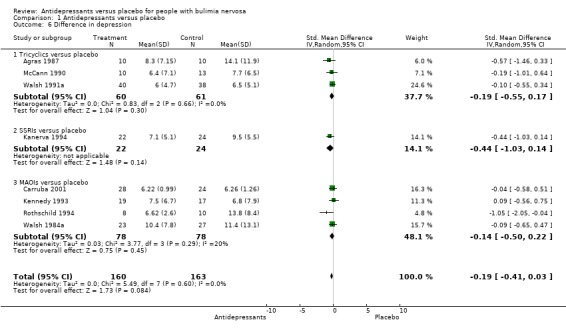
Difference in depressive symptoms This analysis was based on the number of patients who reported depression scores at the end of the trial, and it was not an intention to treat analysis due to missing data. Eight trials reported mean and standard deviations for depressive symptoms at endpoint (Agras 1987, Carruba 2001, Kanerva 1994, Kennedy 1993, McCann 1990, Rothschild 1994, Walsh 1984a, Walsh 1999). No statistically significant difference was observed between classes of drugs. Baseline mean depression scores were low, except for one trial (Rothschild 1994) that analysed retrospectively atypical depressive patients with comorbid bulimia. Other trials usually did not include depressive patients. No heterogeneity in the results of these eight trials has been found (X2= 5.49 (df=7; p= 0.6).
Secondary methodological assessment Trials were rated according to the Jadad Scale (see methods section for a description of the items). Scores for each trial are summarized in the table below: ====================================================== STUDY JADAD SCORE STUDY JADAD SCORE ====================================================== AGRAS 1987 1 FBNCSG 1992 1 CARRUBA 2001 1 GOLDSTEIN 1995 5 HORNE 1988 2 McCANN 1990 1 KANERVA 1994 2 KENNEDY 1988 0 KENNEDY 1993 2 MITCHELL 1984 1 MITCHELL 1990 2 MITCHELL 2001 1 POPE 1983 2 POPE 1989 2 ROTHSCHILD 1994 0 SABINE 1983 2 WALSH 1988 2 WALSH 1991 1 WALSH 2000 0 ======================================================
Sensitivity analyses These analyses were defined a priori and subsidiary to the main review question, and were based on non‐randomised comparisons. The comparison "any drug" versus placebo was used in order to evaluate the main efficacy outcomes (remission and clinical improvement) according to:
(i) Quality of the trial As only two trials were classified as "A" according to the quality assessment criteria (Wheadon 1992a and Sabine 1983) this comparison was not performed.
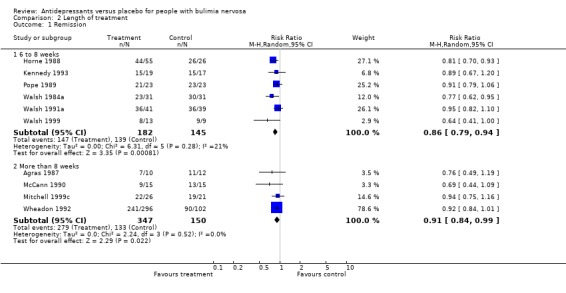
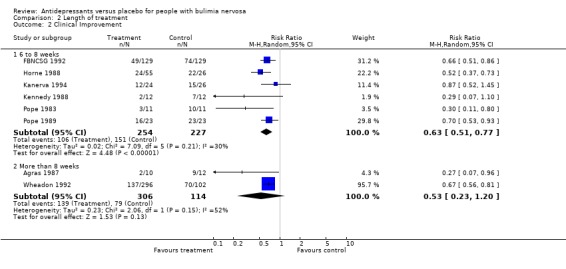
(ii) Length of treatment Remission: Six trials (Horne 1988, Kennedy 1993, Pope 1989, Walsh 1984a, Walsh 1991a and Walsh 1999) reporting this outcome had a duration of up to 8 weeks and four trials had a duration longer than 8 weeks (Agras 1987, Wheadon 1992a, McCann 1990 and ). No statistical difference was found between RR and 95% confidence intervals for these two groups of trials (RR= 0.85 [0.78‐0.94] versus 0,91 [0.84‐0.99]). Clinical improvement: six trials (Horne 1988, FBNCSG 1992, Kanerva 1994, Kennedy 1988, Pope 1983 and Pope 1989) reporting this outcome had a duration of 6 to 8 weeks and two trials had a duration longer than 8 weeks (Agras 1987 and Wheadon 1992a). No statistical difference was found between RR and 95% confidence intervals for these two groups of trials (RR= 0.6257 [0.5096‐0.768] versus 0.5264 [0.2313‐1.198]).
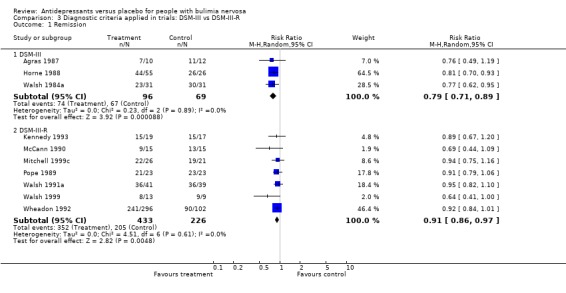
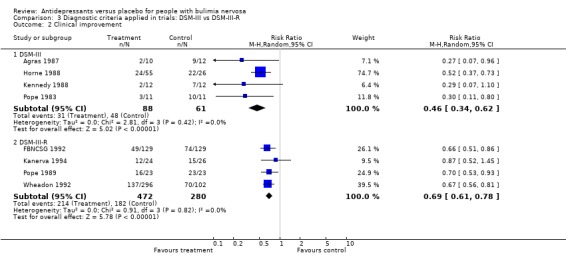
(iii) Diagnostic criteria (DSM‐III bulimia versus DSM‐III‐R bulimia nervosa criteria) Remission: three trials (Agras 1987, Horne 1988 and Walsh 1984a) reporting this outcome used DSM‐III criteria and seven trials used DSM‐III‐R more strict criteria (Wheadon 1992a, Kennedy 1993, McCann 1990, Mitchell 1999c, Pope 1989 , Walsh 1991a and Walsh 1999). No statistical difference was found between RR and 95% confidence intervals for these two groups of trials (RR= 0.79 [0.71‐0.88] versus 0.91 [0.86‐0.97]). A non significant difference favouring antidepressants in trials using the less restrictive criteria of DSM‐III was observed. Clinical improvement: four trials (Agras 1987, Horne 1988, Kennedy 1988 and Pope 1983) reporting this outcome used DSM‐III criteria and four trials used DSM‐III‐R criteria (FBNCSG 1992, Wheadon 1992a,Kanerva 1994 and Pope 1989). No statistical difference was found between RR and 95% confidence intervals for these two groups of trials (RR= 0.4575 [0.3372‐0.6208] versus 0.6876 [0.6056‐0.7808]). A slightly more pronounced difference favouring antidepressants in trials using the less restrictive criteria of DSM‐III was observed.
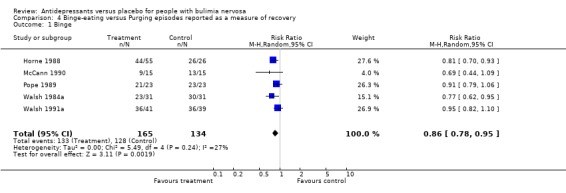
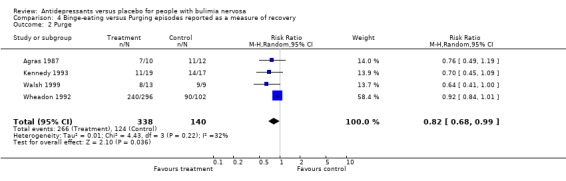
(iv) Bulimic versus purging episodes Five studies reported remission of binge episodes (Horne 1988, McCann 1990, Pope 1989, Walsh 1984a, Walsh 1991a), one of purge episodes (Agras 1987) and three of both purging and bulimic episodes (Wheadon 1992a, Kennedy 1993 and Walsh 1999). The subgroup analysis confirmed that antidepressants remained superior to placebo when either bingeing or purging was considered the main bulimic symptom (RR =0.86; 95% CI= 0.79‐0.94 for bingeing and RR= 0.80; 95% CI= 0.65‐0.98 for purging).
(v) Class of drugs When different classes of antidepressants were compared, approximate 95% confidence intervals for estimates of DL‐RR for remission and clinical improvement of bulimic episodes overlapped (TCA= 0.86; 0.7‐1.07; SSRI= 0.89; 0.76‐1.03; MAOI= 0.81; 0.68‐0.96 for remission; and TCA= 0.29; 0.13‐0.63; SSRI= 0.68; 0.59‐0.79; MAOI= 0.29; 0.07‐1.10 for clinical improvement).Therefore, there is no conclusive evidence to support statistically significant differences in efficacy among these different classes of antidepressants.
Discussion
BN is considered a complex and multi factorial clinical condition that may be difficult to treat, with a chronic course. Investigations of drug therapy with antidepressants for bulimia nervosa have been limited by several conceptual and methodological problems, including definition of cases, control of relevant variables and methods of assessment and strategies for treatment outcome trials (Shaw 1990). Many of the papers reviewed lacked important methodological information, such as details about adequacy of randomisation, blinding procedures and reasons for non‐completion. Some trials were published more than once, reporting preliminary results and sub‐samples with post‐hoc analysis. Cross‐over studies did not provide information for the first period and carry‐over or withdrawn effects were not taken into account. The most frequently reported outcome for efficacy was the mean percent reduction in bulimic episodes, some trials lacking data on standard deviations of this measure. We did not consider this outcome helpful for clinical decision making as it does not translate benefits of treatment. A 70% reduction in binge episodes in the experimental group compared to a 50% reduction in the placebo control group does not help clinicians advise their patients regarding the probability for them of being free of bulimic symptoms or presenting a relevant clinical improvement with that specific drug. Remission and clinical improvement were considered more appropriate outcomes for efficacy. Nevertheless, data could not be obtained for a considerable number of trials. These methodological inconsistencies, added to a possible publication bias, may have altered the differences in efficacy between antidepressants and placebo and should be taken into account in the appraisal of results of the present review.
Concerning generalisation of main findings from this review, bulimia nervosa patients included in the trials seem similar to those seen in clinical settings in terms of age, duration of illness, settings and severity of symptoms. Most studies included strictly defined bulimic patients, according to the diagnostic criteria used at the time of study implementation. Most patients did not present severe depression or other concurrent DSM‐IV axis I major disorder. Rates of patients with personality disorders or other conditions (e.g.. multi‐impulsive patients, substance abuse) that may be more difficult to treat were not systematically reported. Few adolescents were included in the trials, due to restrictive inclusion criteria regarding age (in general, patients were over 18 years old). However, although the onset of symptoms in bulimia nervosa is during late adolescence, patients usually seek treatment in average five years later (Johnson 1987). Therefore, results of this review should be directed to young adult bulimic patients without severe co‐morbidity.
Efficacy This meta‐analysis provides statistical evidence that, in general, a single antidepressant agent is clinically effective for the treatment of BN when compared to placebo, but the effect is modest. Although the differences in terms of definition of illness according to different diagnostic criteria, length of treatment, quality of trials, and classes of drugs, a homogeneous therapeutic effect was found. No statistically significant differential effect regarding efficacy among TCAs, SSRIs, MAOIs and other classes of antidepressants could be demonstrated. Remission rates were low and a considerable fraction of patients did not show a reduction of at least 50% in bulimic symptoms in a short‐term. Clinical improvement was consistent for all classes of antidepressants, though most of the patients would still fulfil criteria for BN at the end of the trials.
Acceptability and tolerability Medication studies without concurrent psychological approaches included in this review confirmed the high dropout rates observed in most trials evaluating single pharmacological treatments, in part because of side effects and in part because of patients' negative attitudes towards medication use. The better acceptability of SSRIs may be related to its short‐term effects on appetite and weight (Advokat 1995).
Depression Even if most patients included in the studies presented low scores in depression rating scales, it is possible that antidepressants have both a direct effect over bulimic symptoms and an effect over depressive symptoms, reducing indirectly the disturbed eating behaviours. However, as individual patient data for concurrent depression was not systematically provided, it was not possible to conclude how depression affected short‐term efficacy of antidepressants on bulimic symptoms.
Dose The doses used in TCAs and MAOIs trials have generally been similar to those employed in the treatment of depression. The only dose‐response study conducted was the first multicenter fluoxetine study, which compared a high dose (60 mg) and a low dose (20 mg) of fluoxetine with placebo. The high dose regimen was clearly superior to both other treatments in this trial (FBNCSG 1992). The other SSRI trials used doses of 60 mg of fluoxetine, which are higher than the usual 20 mg dose used in the treatment of depression.
Relapse and follow‐up Few trials followed patients after this short‐term period of double‐blind treatment. Two trials reported a significant relapse rate (30‐45%) in improved patients followed for 4‐6 months (Mitchell 1987a) following patients reported by Mitchell and colleagues (Mitchell 1990; Walsh 1991a). Only one published trial was designed to specifically evaluate prevention of relapse (Fichter 1996b). In this randomised, double‐blind, placebo controlled trial, 72 inpatients successfully treated with psychotherapy were randomised to fluvoxamine (SSRI) or placebo for the following 15 weeks (12 as outpatients). Drop‐out rates were high (33% or 27 patients, 19 out of 33 randomised to fluvoxamine). Relapse rates were significantly higher for placebo. It was not possible to include data from this maintenance study as it used a different randomisation scheme.
Authors' conclusions
Implications for practice.
Antidepressants are part of the therapeutic armamentarium for the treatment of bulimia nervosa patients. However, their use as sole therapy does not seem sufficient to effectively treat these patients. Fluoxetine is the most systematically studied antidepressant agent. Even if it is not superior to other drugs in terms of efficacy, its' better acceptability may justify its' use as a first line antidepressant in bulimia nervosa. A daily dose of 60 mg is more effective than antidepressant doses of 20 mg. Eight weeks seems to be an appropriate period to obtain a relevant clinical improvement. If no or only partial response is noted, an alternative therapeutic approach is indicated.
Implications for research.
It is of note that the number of studies is declining with time, with little new research since the inception of this review in 1996. There are likely to be several reasons for this, and include the possible greater acceptance of psychotherapy by clinicians and patients and the costs of further trials for the use of medication in areas other than that of their first indication. The effect of other SSRIs than fluoxetine, as well as newer antidepressants (e.g. venlafaxine, mirtazepine and reboxetine) still need to be studied. Future research should systematically include bulimic patients with co‐morbid major depression, personality disorders, substance abuse and other relevant clinical conditions. Other relevant dimensions of response, for example the modulation of the cognitive aspects of bulimia nervosa, namely extreme weight and shape concern, and less specific outcomes such as social functioning and quality of life, should be assessed in future trials. The impact of depression and other co‐morbid disorders on prognosis should be evaluated, improving generalization of results. The option of trying a second antidepressant agent to reduce side effects or increase efficacy should also be evaluated. Given the chronic, frequently relapsing course of treated patients, short‐term results are of limited clinical value and long‐term studies as well as more studies evaluating maintenance of change are needed. The relative low efficacy of antidepressants in both research and clinical setting has prompted investigators to assess some new and provocative pharmacological approaches, including the 5‐HT3 inhibitor ondansetron and the antiepileptic drug topiramate.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 11 August 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Professors Jair J Mari (Federal University of Sao Paulo) and Irismar R de Oliveira (Federal University of Bahia), and Roberta P Trefiglio, who provided invaluable advice and support; Hugh McGuire and the Cochrane Collaboration Depression, Anxiety and Neuroses Group who provided the access to the CCDAN database and advice and support and the search updates. All the authors who provided trial information, some many years after the studies were competed.
Data and analyses
Comparison 1. Antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission | 10 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.84, 0.94] |
| 1.1 Tricyclics versus placebo | 3 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 1.2 SSRIs versus placebo | 3 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 1.3 MAOIs versus placebo | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 1.4 Other antidepressants versus placebo | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.76, 0.97] |
| 2 Clinical improvement | 8 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.54, 0.74] |
| 2.1 Tricyclics versus placebo | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.13, 0.63] |
| 2.2 SSRIs versus placebo | 3 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.79] |
| 2.3 MAOIs versus placebo | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.10] |
| 2.4 Other antidepressants versus placebo | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 3 Difference in bulimic sypmtoms | 6 | 259 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.94, 0.44] |
| 3.1 Tricyclics versus placebo | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.12, ‐0.38] |
| 3.2 SSRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 MAOIs versus placebo | 3 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.94, 1.37] |
| 3.4 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Drop‐outs due to adverse events | 13 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.05, 2.57] |
| 4.1 Tricyclics versus placebo | 5 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.76, 7.45] |
| 4.2 SSRIs versus placebo | 3 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.83, 2.75] |
| 4.3 MAOIs versus placebo | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.45, 9.53] |
| 4.4 Other antidepressants versus placebo | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.28, 7.95] |
| 5 Drop‐outs due to any cause | 15 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.24] |
| 5.1 Tricyclics versus placebo | 6 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [1.15, 3.25] |
| 5.2 SSRIs versus placebo | 3 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 0.99] |
| 5.3 MAOIs versus placebo | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.45] |
| 5.4 Other antidepressants versus placebo | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.48, 1.83] |
| 6 Difference in depression | 8 | 323 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.41, 0.03] |
| 6.1 Tricyclics versus placebo | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.55, 0.17] |
| 6.2 SSRIs versus placebo | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.03, 0.14] |
| 6.3 MAOIs versus placebo | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.22] |
1.1. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 1 Remission.
1.2. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 2 Clinical improvement.
1.3. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 3 Difference in bulimic sypmtoms.
1.4. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 4 Drop‐outs due to adverse events.
1.5. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 5 Drop‐outs due to any cause.
1.6. Analysis.
Comparison 1 Antidepressants versus placebo, Outcome 6 Difference in depression.
Comparison 2. Length of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 6 to 8 weeks | 6 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.94] |
| 1.2 More than 8 weeks | 4 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 2 Clinical Improvement | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 6 to 8 weeks | 6 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.51, 0.77] |
| 2.2 More than 8 weeks | 2 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.23, 1.20] |
2.1. Analysis.
Comparison 2 Length of treatment, Outcome 1 Remission.
2.2. Analysis.
Comparison 2 Length of treatment, Outcome 2 Clinical Improvement.
Comparison 3. Diagnostic criteria applied in trials: DSM‐III vs DSM‐III‐R.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 DSM‐III | 3 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 1.2 DSM‐III‐R | 7 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 2 Clinical improvement | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 DSM‐III | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.34, 0.62] |
| 2.2 DSM‐III‐R | 4 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.61, 0.78] |
3.1. Analysis.
Comparison 3 Diagnostic criteria applied in trials: DSM‐III vs DSM‐III‐R, Outcome 1 Remission.
3.2. Analysis.
Comparison 3 Diagnostic criteria applied in trials: DSM‐III vs DSM‐III‐R, Outcome 2 Clinical improvement.
Comparison 4. Binge‐eating versus Purging episodes reported as a measure of recovery.
4.1. Analysis.
Comparison 4 Binge‐eating versus Purging episodes reported as a measure of recovery, Outcome 1 Binge.
4.2. Analysis.
Comparison 4 Binge‐eating versus Purging episodes reported as a measure of recovery, Outcome 2 Purge.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agras 1987.
| Methods | RCT, 16 weeks, drop‐outs replaced, standard interviews (15 min), physicians different from measurement team, participants self‐monitoring | |
| Participants | DSM‐III bulimia, > 18 years old. >= 2 bulimic episodes (binge + purge) 1 week prior the trial. Age: 30,9. Duration of BN: 8,7 years. BDI: 17,8. Purges /w: 11,8 | |
| Interventions | Imipramine: 50 mg/3days; 100 mg/4days; 150 mg for 1 week; 200 mg/1 week; 250 mg/1week; 300 mg for the rest of period. Mean: 167 mg versus placebo | |
| Outcomes | Remission
Clinical Improvement Change in bulimic symptoms Drop‐outs for any cause and side effects Depression: BDI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Carruba 2001.
| Methods | RCT, 6 weeks, Multicenter in Italy | |
| Participants | 77 women aged 18‐40 years (mean around 25) diagnosed according DSM IV criteria for Bulimia Nervosa, mean BMI around 20 | |
| Interventions | Moclobemide 600 mg/day versus placebo | |
| Outcomes | Binge and vomiting episodes per week Depression: HAM‐D Drop‐outs for AE and any cause | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
FBNCSG 1992.
| Methods | Multicenter, multicountry, RCT, 8 weeks, single‐blind placebo run in, weekly interviews, | |
| Participants | BN DSM‐III‐R patients, 3 episodes/week/6 mo. > 18 years old, 85‐130% of ideal body weight. Mean age: 27.7(pl);26.4(fl). Binges/w: 11. Purges/w: 11.Weight(kg): 60‐61. BMI: 22.5; HDRS: 11.9 | |
| Interventions | Fluoxetine 20 and 60 mg (only 60 mg included) versus placebo | |
| Outcomes | Clinical improvement Drop‐out due to adverse effects and any cause Depr:HDRS | |
| Notes | No psychotherapy but self‐monitoring of eating behavior | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Horne 1988.
| Methods | RCT, D‐B, 8 weeks, ITT possible for dichotomic outcomes | |
| Participants | 81 Bulimia DSM‐III, 3 episodes/week/6 months, BN > 1year, not depressed, 18‐55 years old, mean 26(b) and 27(PL), weight=80‐130% desirable body weight, mean 57 (b), 60 (pl), duration of BN=6.5 years, binges/week= 12(b)/8.5(pl), HDRS baseline 10.6, patients seeking treatment and advertisement | |
| Interventions | Bupropion 450mg versus placebo, no other concomitant ttt (no psychotherapy) | |
| Outcomes | Remission Clinical improvement Drop‐outs dus to adverse events and any cause | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kanerva 1994.
| Methods | RCT, D‐B, 8 weeks, 1 week run‐in placebo, self assessments (diaries) | |
| Participants | BN DSM‐III‐R, > 15 years old, mean 25.2 years, BMI > 16, wight baseline 63 kg, 8 non‐purging, duration of illness: 5.7 years, age of onset: 19.6 years old, binges/week baseline=10HDRS baseline: 12 | |
| Interventions | Fluoxetine 60 mg versus placebo | |
| Outcomes | Clinical improvement Drop‐outs due to adverse events and any cause Depression: HDRS 21 items | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kennedy 1988.
| Methods | RCT, D‐B, 6 weeks, crossover | |
| Participants | Bulimia DSM‐III, >= 3 binges/week, BN > 1 year, 18‐40 years old, mean 26.4, referred to hospital for ED ttt, 75‐125% of ideal body weight, mean 95%, age at onset: 19 | |
| Interventions | Isocarboxazida 60 mg (increases of 10 mg, 60 mg by the end of 4 weeks) versus placebo | |
| Outcomes | Clinical improvement | |
| Notes | Lack data for 1st period of crossover. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kennedy 1993.
| Methods | RCT, D‐B, 8 weeks, self‐report (diary) and observer reted questionaires, 1 week placebo run‐in. | |
| Participants | BN DSM‐III‐R, women, 85‐125% ideal body weight, mean 70.2kg(b)/62.8kg(pl), BMI 26.2(b)/24.2(pl), 18‐40 years old, mean 27.6(b)/25.9(pl), referred consultations at the hospital, duration of illness 14 years, binges/week baseline=9, purges/week baseline 10.2(b)/7.5(pl) | |
| Interventions | Brofaromine 175 mg versus placebo | |
| Outcomes | Remission Change in bulimic symptoms Drop‐outs due to adverse events and any cause Depression:HDRS 17 item | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McCann 1990.
| Methods | RCT, D‐B, 12 weeks, follow‐up visit week 16. Food diaries. Not ITT | |
| Participants | Non‐purging BN DSM‐III‐R, 2 episode/week/1 years plus overconcern with body shape & weight, women, adverstisement in newspapers and TV. Binges/week baseline= 3.8(d)/2.5(pl), BDI baseline= 12.4; BMI= 31. Weight:90 kg. | |
| Interventions | Desipramine 188mg versus placebo. 25 mg/day 2 days, increasind os 25 mg every 2 days during first week and 5o mg every 2 days second week till maximum tolerated dose up to 300 mg. | |
| Outcomes | Remission Change in bulimic symptoms Drp‐outs due to adverse events and any cause Depression: BDI ‐ not ITT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mitchell 1984.
| Methods | RCT, D‐B, 8 weeks, routine patients in eating disorder clinic | |
| Participants | 32 Bulimia, DSM III, > 6 months, age 18‐45, median 26(a)/24.5(pl), duration of illness 5 years | |
| Interventions | Amitriptyline versus Placebo. 50 mg 3 days, 100 mg 4 days and then 150 mg. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mitchell 1990.
| Methods | RCT, 10 weeks, physicians responsible for medication management were blind to randomization. | |
| Participants | Bulimia DSM‐III, 3 episodes/week/6 months, patients being evaluated in ED clinic and advertisements. Age 18‐40, mean 24, female, 80‐120% ideal body weight, mean 107%, duration of illness 6.5 years,binges/week baseline=7,3(i)/ 8,0(pl)/ 9,2(pt)/ 8.4(comb).HDRS baseline=11 | |
| Interventions | Imipramine initial dose 50 mg, increments to 200 mg for 2 weeks, maintained for 2 weeks, if necessary 300 mg.CBT= 3 phases. 1= 2 hour sessions for each week 2 weeks: mael planning and CBT techniques. 2=arttempt to interrupt bulimic behaviors begin eating regular meals, use of CBT techniques,5 nights a week, 3 hour sessions, then 2 sessions/week (lecture+diner+psychotherapy). 3= last month: 1 and half hour sessions, exposure and relapse prevention | |
| Outcomes | Relapse Drop‐outs due to any cause | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mitchell 1999c.
| Methods | RCT, 16 weeks, D‐B, 4 comparison groups | |
| Participants | DSM‐III‐R, 91 adult women, mean age: 26.6 years old | |
| Interventions | Fluoxetine 60 mg, placebo | |
| Outcomes | Remission | |
| Notes | Data provide by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pope 1983.
| Methods | RCT, D‐B, 6 weeks | |
| Participants | 22 Bulimia DSM‐III, 16‐55 years old, mean 27.7. Inclusion criteria: 2 binges/week followed by purging, BN > 1 year. Recruitment from advertisements and referrals, weight between 80‐115% normal weight. | |
| Interventions | Imipramine 200mg versus placebo | |
| Outcomes | Clinical improvement Deterioration Change in bulimic symptoms Drop‐outs due to adverse events and any cause Depression: HDRS (difference or change from baseline to endpoint) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pope 1989.
| Methods | RCT, D‐B, 6 weeks, 2 weeks wash out placebo run‐in, | |
| Participants | BN DSM‐III‐R + 3 episodes/week/6 months, age 18‐55, mean 26, weight 80‐140% of ideal weight, mean 98.3%, purging type, from advertisements, baseline=12 binges/week, duration of BN=7.4 years | |
| Interventions | Trazodone 355 mg (maximum 400 mg) versus placebo, increasing dose of 50 mg every 2 days | |
| Outcomes | Remission Clinical improvement Drop‐outs due to adverse events and any cause Depression (differene baseline‐endpoint): HDRS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rothschild 1994.
| Methods | RCT, D‐B, 6 weeks after 10 day placebo period, raters blind to ttt | |
| Participants | Patients with atypic depression and BN diagnosed post‐hoc according to DSM‐III criteria. Age: 32.8. Binges/week baseline=5.2. HDRS baseline= 15.2 | |
| Interventions | Phenelzine 45mg versus imipramine 150mg versus placebo | |
| Outcomes | Clinical improvement (not ITT) Depression: HDRS (21 items) | |
| Notes | Retrospective data!! | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sabine 1983.
| Methods | RCT, D‐B, 8 weeks | |
| Participants | 50 bulimic female patients referred to a hospital, 1‐2 episodes/week+ preoccupation with food, fear of fatness, weight... Russell's criteria!! Age=16‐65, mean 22.4(m) / 25(pl), weight in kg= 63.3(m) / 61 (pl) | |
| Interventions | Mianserin 30‐60 mg versus placebo | |
| Outcomes | Drop‐outs due to any cause | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Walsh 1984a.
| Methods | RCT, D‐B, 8 weeks, single‐blind plecebo phase of 2 weeks, diary, seen weekly | |
| Participants | Bulimia DSM III + 3 episodes/week, 18‐45 years old, mean 27, women, 80‐120% ideal body weight, referral from therapists and advertisements. Duration of illness= 9.0‐9.8 years, mean binges baseline= 11.9(ph) / 9.2 (pl), HDRS= 10.8 (ph) / 7.5 (pl) :significant difference | |
| Interventions | Phenelzine 60‐90 mg versus placebo. 30 mg first weeks, 60 mg 2nd week, 90 mg by week 6. | |
| Outcomes | Remission Change in bulimic symptoms Drop‐outs due to adverse events and any cause Depression: HDRS 17 items | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Walsh 1991a.
| Methods | RCT, D‐B, 6 weeks, 2 week single blind wash out placebo, 30 min weekly sessions, diary | |
| Participants | 80 BN DSM‐III‐R for 1 year, 18‐45 years old, mean 25, 85‐120% ideal weight, mean 134 lb, BMI 22, trough advertisements, duration of illness=6.6 years, binges/week baseline=8, HDRS baseline 7.3(pl) / 8.3 (d), BDI 15(pl) / 10.4 (d): signifficant difference | |
| Interventions | Desipramine 200‐300 mg versus placebo. 200 mg till week 4, 300 if not better. | |
| Outcomes | Remission Change in bulimic symptoms Drop‐outs due to any cause and adverse events Depression: BDI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Walsh 1999.
| Methods | RCT, two sites in US, D‐B 8 weeks, following poor response to psychotherapy | |
| Participants | 22 patients diagnosed according DSM‐III‐R criteria, purging type self‐inducing vomiting at once a week over 1 month | |
| Interventions | Fluoxetine 60 mg/day versus placebo | |
| Outcomes | Remission, depression (BDI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wheadon 1992.
| Methods | RCT, D‐B, 16 weeks, ITT, multicenter, run‐in one week period, sample size calculation | |
| Participants | BN DSM‐III‐R, >= 3 episodes of vomiting/week, BN for 6 months, > 18 years old, 96.2% women, 96.7% white, age: 27, weight: 58kg, vomiting/week baseline: 9, binges/week baseline: 9, median days /week binges:6 | |
| Interventions | Fluoxetine 60 mg versus placebo | |
| Outcomes | Remission Clinical improvement Drop‐outs due to adverse events and any cause | |
| Notes | Median number of binges/purges and median scores of HDRS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agras 1992 | No placebo control group. |
| Agras 1994 | No placebo control group |
| Alger 1991 | Separate data for bulimic and BED patients not obtained |
| Barlow 1988 | Data for first phase of crossover not obtained |
| Blouin 1988 | Data for first phase of crossover not obtained |
| Box 1983 | Not BN patients. |
| Fichter 1991 | Antidepressants plus psychotherapy versus psychotherapy plus placebo. Absence of "drug versus placebo alone" comparisons. |
| Fichter 1996 | Additional report of Fichter 1991. |
| Goldbloom 1996 | No placebo control group. Ads versus psychotherapy. |
| Hahn 1989 | Letter discussin Horne 1988 trial. |
| Hughes 1986a | No data for any outcome obtained |
| Kennedy 1986 | Letter with subset data of Kennedy 1988. |
| Koran 1995 | Not BN patients |
| Leitenberg 1994 | No placebo control group. Control=CBT |
| Marcus 1990 | Paper did not provide separate data for obese bulimic and non bulimic patients. Author contacted, did not provide data. |
| Margittai 1987 | Subset of Barlow 1988. Evaluation of drop‐outs of crossover study not providing data for first period. |
| Mitchell 1987a | Subset of Mitchell 1990. 6‐month follow‐up maintenance data. |
| Price 1987 | Letter to the editor. Not described as RCT. Phenelzine study. No address from authors to contact. |
| Russell 1995b | No placebo control group. Ads versus psychotherapy. |
| Scrimali 1994 | No placebo control group. Control=no drug, only diet |
| Walsh 1984 | Subset of Walsh 1988. Partial data on phenelzine trial. |
| Walsh 1992 | Subset of Walsh 1991. Pulse and blood pressure analysis from desipramine trial. |
| Walsh 1997 | No placebo group. Controls= CBT & SPT |
Declarations of interest
None
JB has received fees from Janssen‐Cilag Farmaceutica, Brazil. PH has received support to attend conferences and meetings from Pfizer PTY Ltd, Solvay Pharmaceuticals and Bristol‐Myers Squibb Pharmaceuticals.
Edited (no change to conclusions)
References
References to studies included in this review
Agras 1987 {published data only}
- Agras WS, Dorian B, Kirkley BG, Arnow B, Bachman J. Imipramine in the treatment of bulimia: a double‐blind controlled study. International Journal of Eating Disorders 1987;6(1):29‐38. [Google Scholar]
Carruba 2001 {published data only}
- Carruba MO, Cuzzolaro M, Riva L, Bosello O, Liberti S, Castra R, et al. Efficacy and tolerability of moclobemide in bulimia nervosa: a placebo‐controlled trial. International Journal of Psychopharmacology 2001;16(1):27‐32. [DOI] [PubMed] [Google Scholar]
FBNCSG 1992 {published data only}
- Fluoxetine Bulimia Nervosa Collaborative Study Group. Fluoxetine in the treatment of bulimia nervosa. A multicenter, placebo‐controlled, double‐blind trial. Archives of General Psychiatry 1992;49(2):139‐47. [PubMed] [Google Scholar]
- Goldbloom DS, Olmsted MP. Pharmacotherapy of bulimia nervosa with fluoxetine: assessment of clinically significant attitudinal change. American Journal of Psychiatry 1993;150(5):770‐4. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wilson MG, Ascroft RC, Al‐Banna M. Effectiveness of fluoxetine therapy in bulimia nervosa regardless of comorbid depression. International Journal of Eating Disorders 1999;25(1):19‐27. [DOI] [PubMed] [Google Scholar]
- McCarthy MK, Goff DC, Baer L, Cioffi J, Herzog DB. Dissociation, childhood trauma, and the response to fluoxetine in bulimic patients. International Journal of Eating Disorders 1994;15(3):219‐26. [DOI] [PubMed] [Google Scholar]
- Wheadon DE, Rampey AH Jr, Thompson VL, Potvin JH, Masica DN, Beasley CM Jr. Lack of association between fluoxetine and suicidality in bulimia nervosa. Journal of Clinical Psychiatry 1992;53(7):235‐41. [PubMed] [Google Scholar]
- Wood A. Pharmacotherapy of bulimia nervosa‐ experience with fluoxetine. International Clinical Psychopharmacology 1993;8(4):295‐9. [DOI] [PubMed] [Google Scholar]
Horne 1988 {published data only}
- Horne RL, Ferguson JM, Pope HG Jr, Hudson JI, Lineberry CG, Ascher J, et al. Treatment of bulimia with bupropion: a multicenter controlled trial. Journal of Clinical Psychiatry 1988;49(7):262‐6. [PubMed] [Google Scholar]
Kanerva 1994 {published data only}
- Kanerva R, Rissanen A, Sarna S. Fluoxetine in the treatment of anxiety, depressive symptoms, and eating‐related symptoms in bulimia nervosa. Nordic Journal of Psychiatry 1994;49(7):237‐42. [Google Scholar]
Kennedy 1988 {published data only}
- Kennedy SH, Piran N, Warsh JJ, Prendergast P, Mainprize E, Whynot C, et al. A trial of isocarboxazid in the treatment of bulimia nervosa. Journal of Clinical Psychopharmacology 1988;8(6):391‐6. [PubMed] [Google Scholar]
Kennedy 1993 {published data only}
- Davis BA, Kennedy SH, Durden DA, D'Souza J, Goldbloom DS, Boulton AA. The effect of the MAO‐A selective inhibitor brofaromine on the plasma and urine concentrations of some biogenic amines and their acidic metabolites in bulimia nervosa. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1993;17(5):747‐63. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Davis BA, Brown GM, Ford CG, D'Souza J. Effects of chronic brofaromine administration on biogenic amines including sulphatoxymelatonin and acid metabolites in patients with bulimia nervosa. Neurochemical Research 1993;18(12):1281‐5. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Goldbloom DS, Ralevski E, Davis C, D'Souza JD, Lofchy J. Is there a role for selective monoamine oxidase inhibitor therapy in bulimia nervosa? A placebo‐controlled trial of braforamine. Journal of Clinical Psychopharmacology 1993;13(6):415‐22. [PubMed] [Google Scholar]
McCann 1990 {published data only}
- McCann UD, Agras WS. Successful treatment of nonpurging bulimia nervosa with desipramine: a double‐blind, placebo‐controlled study. American Journal of Psychiatry 1990;147(11):1509‐13. [DOI] [PubMed] [Google Scholar]
Mitchell 1984 {published data only}
- Mitchell JE, Groat R. A placebo‐controlled double‐blind trial of amitriptyline in bulimia. Journal of Clinical Psychopharmacology 1984;4(4):186‐93. [PubMed] [Google Scholar]
Mitchell 1990 {published data only}
- Keel PK, Mitchell JE, Davis TL, & Crow SJ. Long‐term impact of treatment in women diagnosed with bulimia nervosa. International Journal of Eating Disorders 2002;31(2):151‐8. [DOI] [PubMed] [Google Scholar]
- Keel PK, Mitchell JE, Miller KB, Davis TL, Crow SJ. Long‐term outcome of bulimia nervosa. Archives of General Psychiatry 1999;56(1):63‐9. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Pyle RL, Eckert ED, Hatsukami D, Pomeroy C, Zimmerman R. A comparison study of antidepressants and structured intensive group psychoterapy in the treatment of bulimia nervosa. Archives of General Psychiatry 1990;47(2):149‐57. [DOI] [PubMed] [Google Scholar]
Mitchell 1999c {published data only}
- Mitchell J, Crosby R. Manual‐based self‐help and fluoxetine in treating bulimia nervosa. Current Opinion in Psychiatry 1999;12(Suppl 1):128. [Google Scholar]
- Mitchell JE, Fletcher L, Hanson K, Mussell MP, Seim H, Crosby R, et al. The relative efficacy of fluoxetine and manual‐based self‐help in the treatment of outpatients with bulimia nervosa. Journal of Clinical Psychopharmacology 2001;21(3):298‐304. [DOI] [PubMed] [Google Scholar]
Pope 1983 {published data only}
- Pope HG Jr, Hudson JI. Antidepressant drug therapy for bulimia: current status. Journal of Clinical Psychiatry 1986;47(7):339‐45. [PubMed] [Google Scholar]
- Pope HG Jr, Hudson JI, Jonas JM, Yurgelum‐Todd D. Bulimia treated with imipramine: a placebo‐controlled, double‐blind study. American Journal of Psychiatry 1983;140(5):554‐8. [DOI] [PubMed] [Google Scholar]
- Pope HG, Hudson JI, Jonas JM, Yurgelun‐Todd, D. Antidepressant treatment of bulimia: A two‐year follow‐up study. Journal of Clinical Psychopharmacology 1985;5(6):320‐7. [PubMed] [Google Scholar]
Pope 1989 {published data only}
- Hudson JI, Pope HG Jr, Keck PE Jr, McElroy SL. Treatment of bulimia nervosa with trazodone: short‐term response and long‐term follow‐up. Clinical Neuropharmacology 1989;12(Suppl 1):38‐46. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, Keck PE, McElroy S, Hudson JI. A placebo‐controlled study of trazodone in bulimia nervosa. Journal of Clinical Psychopharmacology 1989;9(4):254‐9. [PubMed] [Google Scholar]
Rothschild 1994 {published data only}
- Rothschild R, Quitkin HM, Quiktin FM, Stewart JW, Ocepek‐Welikson K, McGrath PJ, et al. A double‐blind placebo‐controlled comparison of phenelzine and imipramine in the treatment of bulimia in atypical depressives. International Journal of Eating Disorders 1994;15(1):1‐9. [DOI] [PubMed] [Google Scholar]
Sabine 1983 {published data only}
- Collings S, King M. Ten‐year follow‐up of 50 patients with bulimia nervosa. British Journal of Psychiatry 1994;164:80‐7. [DOI] [PubMed] [Google Scholar]
- Sabine EJ, Yonace A, Farrington AJ, Barrant KH, Wakeling A. Bulimia nervosa: a placebo‐controlled double‐blind therapeutic trial of mianserin. British Journal of Clinical Pharmacology 1983;15 Suppl 2:195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1984a {published data only}
- Walsh BT, Gladis M, Roose SP, Stewart JW, Glassman AH. A controlled trial of phenelzine in bulimia. Psychopharmacology Bulletin 1987;23(1):49‐51. [PubMed] [Google Scholar]
- Walsh BT, Gladis M, Roose SP, Stewart JW, Glassman AH. Phenelzine vs placebo in 50 patients with bulimia. Archives of General Psychiatry 1988;45(5):471‐5. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Stewart JW, Roose SP, Gladis M, Glassman AH. A double‐blind trial of phenelzine in bulimia. Journal of Psychiatric Research 1985;19(2‐3):485‐9. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Stewart JW, Roose SP, Gladis M, Glassman AH. Treatment of bulimia with phenelzine. A double‐blind, placebo‐controlled study. Archives of General Psychiatry 1984;41(11):1105‐9. [DOI] [PubMed] [Google Scholar]
Walsh 1991a {published data only}
- Ames‐Frankel J, Devlin MJ, Walsh BT, Strasser TJ, Sadik C, Oldham JM, et al. Personality disorder diagnoses in patients with bulimia nervosa. Clinical correlates and changes with treatment. Journal of Clinical Psychiatry 1992;53(3):90‐6. [PubMed] [Google Scholar]
- Strasser TJ, Pike KM, & Walsh BT. The impact of prior substance abuse on treatment outcome for bulimia nervosa. Addictive Behaviors 1992;17(4):387‐95. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Hadigan CM, Devlin MJ, Gladis M, Roose SP. Long‐term outcome of antidepressant treatment for bulimia nervosa. American Journal of Psychiatry 1991;148(9):1206‐12. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Hadigan CM, Wong LM. Increased pulse and blood pressure associated with desipramine treatment of bulimia nervosa. Journal of Clinical Psychopharmacology 1992;12(3):163‐8. [PubMed] [Google Scholar]
Walsh 1999 {published data only}
- Walsh BT, Agras WS, Devlin MJ, Fairburn CG, Wilson GT, Kahn CM, et al. Fluoxetine for bulimia nervosa following poor response to psychotherapy. American Journal of Psychiatry 2000;157(8):1332‐4. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Agras WS, Devlin MJ, Kahn C, Chally K. Fluoxetine in bulimia following failure of psychotherapy. 152nd Annual Meeting of the American Psychiatric Association. Washington DC, USA. 15 20th May. 1999.
- Wilson GT, Fairburn CC, Agras WS, Walsh BT, Kraemer H. Cognitive‐behavioral therapy for bulimia nervosa: Time course and mechanisms of change. Journal of Consulting & Clinical Psychology 2002;70(2):267‐74. [PubMed] [Google Scholar]
Wheadon 1992 {published and unpublished data}
- Goldstein DJ, Wilson M, Ascroft R. Effectiveness of fluoxetine therapy in bulimia nervosa regardless of baseline depression. 150th Annual Meeting of the American Psychiatric Association. San Diego, California, USA. 17 22 May. 1997.
- Goldstein DJ, Wilson MG, Ascroft RC, Al‐Banna M. Effectiveness of fluoxetine therapy in bulimia nervosa regardless of comorbid depression. International Journal of Eating Disorders 1999;25(1):19‐27. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wilson MG, Thomson VL, Potvin JH, Rampey AH Jr. Long‐term fluoxetine treatment of bulimia nervosa. British Journal of Psychiatry 1995;166:660‐6. [DOI] [PubMed] [Google Scholar]
- Wheadon DE, Rampey AH Jr, Thompson VL, Potvin JH, Masica DN, Beasley CM Jr. Lack of association between fluoxetine and suicidality in bulimia nervosa. Journal of Clinical Psychiatry 1992;53(7):235‐41. [PubMed] [Google Scholar]
References to studies excluded from this review
Agras 1992 {published data only}
- Agras WS, Rossiter EM, Arnow B, Schneider JA, Telch CF, Raeburn SD, et al. Pharmacologic and cognitive‐behavioral treatment for bulimia nervosa: a controlled comparison. American Journal of Psychiatry 1992;149(1):82‐7. [DOI] [PubMed] [Google Scholar]
- Agras WS, Rossiter EM, Arnow B, Telch CF, Raeburn SD, Bruce B, et al. One‐year follow‐up of psychosocial and pharmacologic treatments for bulimia nervosa. Journal of Clinical Psychiatry 1994;55(5):179‐83. [PubMed] [Google Scholar]
- Koran LM, Agras WS, Rossiter EM, Arnow B, Schneider JA, Telch CF, et al. Comparing the cost effectiveness of psychiatric treatment: Bulimia nervosa. Psychiatry Research 1995;58(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Rossiter EM, Agras WS, Telch CF, Schneider JA. Cluster B personality disorder characteristics predict outcome in the treatment of bulimia nervosa. International Journal of Eating Disorders 1993;13(4):349‐57. [DOI] [PubMed] [Google Scholar]
Agras 1994 {published data only}
- Agras WS, Telch CF, Arnow B, Eldredge K, Detzer MJ, Henderson J, et al. Does interpersonal therapy help patients with binge eating disorder who fail to respond to cognitive‐behavioral therapy?. Journal of Consulting and Clinical Psychology 1995;63(3):356‐60. [DOI] [PubMed] [Google Scholar]
- Agras WS, Telch CF, Arnow B, Eldredge K, Wilfley DE, Raeburn SD, et al. Weight loss, cognitive‐behavioral, and desipramine treatments in binge eating disorder. An additive design. Behavior Therapy 1994;25:225‐38. [Google Scholar]
- Eldredge KL, Locke KD, Horowitz LM. Patterns of interpersonal problems associated with binge eating disorder. International Journal of Eating Disorders 1998;23(4):383‐9. [DOI] [PubMed] [Google Scholar]
Alger 1991 {published data only}
- Alger AS, Shwalberg MD, Bigaouette JM, Michalek AV, Howard LJ. Effect of a tricyclic antidepressant and opiate antagonist on binge‐eating behavior in normoweight bulimic and obese, binge‐eating subjects. American Journal of Clinical Nutrition 1991;53(4):865‐71. [DOI] [PubMed] [Google Scholar]
Barlow 1988 {published data only}
- Barlow J, Blouin J, Blouin A, Perez E. Treatment of bulimia with desipramine: a double‐blind crossover study. Canadian Journal of Psychiatry 1988;33(2):129‐33. [DOI] [PubMed] [Google Scholar]
- Blouin J, Blouin A, Perez E, Barlow J. Bulimia: Independence of antibulimic and antidepressant properties of desipramine. Canadian Journal of Psychiatry 1989;34(1):24‐9. [DOI] [PubMed] [Google Scholar]
Blouin 1988 {published data only}
- Blouin AG, Blouin JH, Perez EL, Bushnik T, Zuro C, Mulder E. Treatment of bulimia with fenfluramine and desipramine. Journal of Clinical Psychopharmacology 1988;8(4):261‐9. [PubMed] [Google Scholar]
Box 1983 {published data only}
- Box J, Arnold LE, Smeltzer DJ. Protriptyline weight loss in compulsive eaters: a placebo‐controlled study. Journal of Psychiatric Treatment and Evaluation 1983;5(4):387‐91. [Google Scholar]
Fichter 1991 {published data only}
- Fichter MM, Leibl K, Rief W, Brunner E, Schmidt‐Auberger S, Engel RR. Fluoxetine versus placebo: a double blind study with bulimic inpatients udergoing intensive psychotherapy. Pharmacopsychiatry 1991;24(1):1‐7. [DOI] [PubMed] [Google Scholar]
Fichter 1996 {published data only}
- Fichter MM. Fluvoxamine in relapse prevention of bulimia nervosa. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans; LA, USA. 2001.
- Fichter MM. Psychopharmacological relapse prevention and maintenance treatment of bulimia nervosa. XXIst Collegium Internationale Neuro psychopharmacologicum, Glasgow, Scotland. 12th 16th July. 1998.
- Fichter MM, Kruger R, Rief W, Holland R, Dohne J. Fluvoxamine in prevention of relapse in bulimia nervosa:effects on eating specific psychopathology. Journal of Clinical Psychopharmacology 1996;16(1):9‐18. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Leibl C, Krüger R, Rief W. Effects of fluvoxamine on depression, anxiety and other areas of general psychopathology in bulimia nervosa. Pharmacopsychiatry 1997;30(3):85‐92. [DOI] [PubMed] [Google Scholar]
Goldbloom 1996 {published data only}
- Goldbloom D, Garfinkel P. Pharmacotherapy and psychotherapy in bulimia nervosa. XXth Collegium Internationale Neuro psychopharmacologicum, Melbourne, Australia. 1996.
- Goldbloom DS, Olmsted MP, Davies R, Shaw B. A randomized control trial of fluoxetine and cognitive behavioural therapy for bulimia nervosa: short‐term outcome. Behavioral Research and Therapeutics 1997;35(9):803‐11. [DOI] [PubMed] [Google Scholar]
Hahn 1989 {published data only}
- Hahn WK. Therapeutic effectiveness of bupropion for bulimia: a possible artifact. Journal of Clinical Psychiatry 1989;50(5):188. [PubMed] [Google Scholar]
Hughes 1986a {published data only}
- Hughes PL, Wells LA, Cunningham CJ, Ilstrup DM. Treating bulimia with desipramine. A double‐blind, placebo‐controlled study. Archives of General Psychiatry 1986;43(2):82‐6. [DOI] [PubMed] [Google Scholar]
Kennedy 1986 {published data only}
- Kennedy SH, Piran N, Garfinkel PE. Isocarboxazid in the treatment of bulimia. American Journal of Psychiatry 1986;143(11):1495‐6. [DOI] [PubMed] [Google Scholar]
Koran 1995 {published data only}
- Koran L, McElroy S, Davidson J, Rasmussen S, Hollander E, Jenike M. Double‐blind comparison of fluvoxamine and clomipramine in obsessive compulsive disorder in the United States. 8th ECNP (European College of Neuropsychopharmacology) Congress, Venice, Italy. 1995.
- Koran LM, McElroy SL, Davidson JR, Rasmussen SA, Hollander E, Jenike MA. Fluvoxamine versus clomipramine for obsessive‐compulsive disorder: a double‐blind comparison. Journal of Clinical Psychopharmacology 1996;16(2):121‐9. [DOI] [PubMed] [Google Scholar]
Leitenberg 1994 {published and unpublished data}
- Leitenberg H, Rosen JC, Wolf J, Vara LS, Detzer MJ, Srebnik D. Comparison of cognitive‐behavior therapy and desipramine in the treatment of bulimia nervosa. Behaviour Research and Therapy 1994;32(1):37‐45. [DOI] [PubMed] [Google Scholar]
Marcus 1990 {published and unpublished data}
- Marcus MD, Wing RR, Ewing L, Kern E, McDermott M, Gooding W. A double‐blind placebo‐controlled trial of fluoxetine plus behavioral modification in the treatment of obese binge‐eaters. American Journal of Psychiatry 1990;147(7):876‐81. [DOI] [PubMed] [Google Scholar]
Margittai 1987 {published data only}
- Margittai KJ, Blouin A, Perez E. A study of drop‐outs in psychopharmacological research with bulimics. International Journal of Psychiatry in Medecine 1987;16(4):297‐304. [DOI] [PubMed] [Google Scholar]
Mitchell 1987a {published data only}
- Mitchell JE, Pyle RL, Eckert ED, Pomeroy C, Hatsukami D, Zimmerman R. Antidepressants vs group therapy in the treatment of bulimia. Psychopharmacology Bulletin 1987;23(1):41‐4. [PubMed] [Google Scholar]
- Pyle RL, Mitchell JE, Eckert ED, Hatsukami D, Pomeroy C, Zimmerman R. Maintenance treatment and 6‐month outcome for bulimic patients who respond to initial treatment. American Journal of Psychiatry 1990;147(7):871‐5. [DOI] [PubMed] [Google Scholar]
Price 1987 {published data only}
- Price WA, Babai MR. Antidepressant drug therapy for bulimia: current status revisited. Journal of Clinical Psychiatry 1987;48(9):385. [PubMed] [Google Scholar]
Russell 1995b {published data only}
- Beumont P, Russell J, Tobyz S, Johnson G. Fluoxetine and nutritional counselling in bulimia nervosa. Xth World Congress of Psychiatry, Madrid, Spain. 1996. [DOI] [PubMed]
- Beumont P, Russell J, Touyz S, Johnson G. Fluoxetine and intensive nutritional counselling in the treatment of bulimia nervosa. XXth Collegium Internationale Neuro psychopharmacologicum, Melbourne, Australia. 1996.
- Beumont PJ, Russell JD, Touyz SW, Buckley C, Lowinger K, Talbot P, et al. Intensive nutritional counselling in bulimia nervosa: a role for supplementation with fluoxetine?. Australian and New Zealand Journal of Psychiatry 1997;31(4):514‐24. [DOI] [PubMed] [Google Scholar]
- Russell J, Beumont P, Touyz S, Buckley C, Lowinger K, Talbot P, et al. The effects of fluoxetine in patients receiving nutritional counselling for bulimia nervosa. 8th ECNP (European College of Neuropsychopharmacology) Congress, Venice, Italy. 1995.
Scrimali 1994 {published data only}
- Scrimali T, Grimali L, Corriere A, Grasso F, Frisone MF. Fluoxetine in the pharmacological treatment of bulimia nervosa [La fluoxetina nel tratamento farmacologico della bulimia nervosa]. Rivista di Psichiatria 1994;29(3):171‐7. [Google Scholar]
Walsh 1984 {published data only}
- Walsh BT, Gladis M, Roose SP, Stewart JW, Glassman AH. A controlled trial of phenelzine in bulimia. Psychopharmacology Bulletin 1987;23(1):49‐51. [PubMed] [Google Scholar]
- Walsh BT, Gladis M, Roose SP, Stewart JW, Stetner F, Glassman AH. Phenelzine vs placebo in 50 patients with bulimia. Archives of General Psychiatry 1988;45(5):471‐5. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Stewart JW, Roose SP, Gladis M, Glassman AH. A double‐blind trial of phenelzine in bulimia. Journal of Psychiatric Research 1985;19(2‐3):485‐9. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Stewart JW, Roose SP, Gladis M, Glassman AH. Treatment of bulimia with phenelzine. A double‐blind, placebo‐controlled study. Archives of General Psychiatry 1984;41(11):1105‐9. [DOI] [PubMed] [Google Scholar]
Walsh 1992 {published data only}
- Walsh BT, Colleen MH, Wong LM. Increased pulse and blood pressure associated with desipramine treatment of bulimia nervosa. Journal of Clinical Psychopharmacology 1992;12(3):163‐8. [PubMed] [Google Scholar]
Walsh 1997 {published data only}
- Walsh BT, Wilson GT, Loeb KL, Devlin MJ, Pike KM, Roose SP, et al. Medication and psychotherapy in the treatment of bulimia nervosa. American Journal of Psychiatry 1997;154(4):523‐31. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Loeb KL, Walsh BT, Labouvie E, Petkova E, Liu X, et al. Psychological versus pharmacological treatments of bulimia nervosa: predictors and processes of change. Journal of Consulting and Clinical Psychology 1999;67(4):451‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Advokat 1995
- Advokat C, Kutlesic V. Pharmacotherapy of the eating disorders: a commentary. Neuroscience and Biobehavioral Reviews 1995;19(1):59‐66. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistics Manual of mental disorders (DSM‐III). 3rd Edition. Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistics Manual of mental disorders (DSM‐III‐R). 3rd Edition. Washington DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistics Manual of mental disorders (DSM‐IV). 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Bacaltchuk 2003
- Bacaltchuk J, Hay P, Trefiglio R. Antidepressants versus psychological treatments and their combination for bulimia nervosa. The Cochrane Library 2003, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchan 1998 [Computer program]
- Buchan IE. Arcus QuickStat Biomedical for windows. Version 1. Cambridge, UK: Addison Wesley Longman Limited Trading as Research Solutions, 1998.
Corcos 1996
- Corcos M, Flament M, Atger F, Jeammet P. Pharmacological treatment of bulimia [Traitement pharmacologique de la boulimie]. Encephale 1996;22(2):133‐42. [PubMed] [Google Scholar]
Cucherat 1997
- Cucherat M. Meta‐analyse des essais therapeutiques. Paris: Masson, 1997. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Fichter 1996b
- Fichter MM, Krüger R, Rief W, Holland R, Döhne J. Fluvoxamine in prevention of relapse in bulimia nervosa: effects on eating specific psychopathology. Journal of Clinical Psychopharmacology 1996;6(1):9‐18. [DOI] [PubMed] [Google Scholar]
Hay 2001
- Hay P, Bacaltchuk J. Bulimia Nervosa. Clinical Evidence 2001;5:0‐10. [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical methods for meta‐analysis. San Diego CA: Academic Press, 1985. [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, Jenkinson C, Reynolds DJ, Gavanagh DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Johnson 1987
- Johnson C, Connors ME. The etiology and treatment of bulimia nervosa. New Jersey: Jason Aronson Inc, 1994. [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing up: The Science of Revieweing Research. Cambridge MA: Harvard University Press, 1984. [Google Scholar]
Lima 1998
- Lima MS, Montcrieff J. A comparison of drugs versus placebo for the treatment of dysthymia: a systematic review. Cochrane Library 1998, Issue 2. [DOI] [PubMed] [Google Scholar]
Mulrow 1996
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. Cochrane Library. Oxford: Update Software, 1996. [Google Scholar]
Rosenthal 1979
- Rosenthal R. The "file drawer problem" and the tolerance for null results. Psychological Bulletin 1979;86:638‐41. [Google Scholar]
Russell 1979
- Russell GF. Bulimia Nervosa: an ominous variant of anorexia nervosa. Psychological Medicine 1979;9:429‐48. [DOI] [PubMed] [Google Scholar]
Russell 1997
- Russell GF. The history of bulimia nervosa. In: Garner DM, Garfinkel PE editor(s). Handbook of treatment for eating disorders. 2nd Edition. New York: Guilford Press, 1997:11‐24. [Google Scholar]
Sackett 1994
- Sackett DL. Cochrane Collaboration Handbook. Oxford: Update Software, 1994. [Google Scholar]
Sackett 1997
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB. Evidence‐based medicine. How to practice and teach EBM. Edinburgh: Churchill Livingstone, 1997. [Google Scholar]
Schultz 1995
- Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐412. [DOI] [PubMed] [Google Scholar]
Shaw 1990
- Shaw B, Garfinkel PE. Problems of research on eating disorders. International Journal of Eating Disorders 1990;5:545‐55. [Google Scholar]
Thomson 1995
- Thomson SG. Why sources of heterogeneity in meta‐analysis should be investigated. In: Chalmers I, Altman DG editor(s). Systematic Reviews. London: BMJ Publishing Group, 1995:48‐63. [Google Scholar]
Wheadon 1992a
- Wheadon DE, Rampey AH Jr, Thompson VL, Potvin JH, Masica DN, Beasley CM Jr. Lack of an association between fluoxetine and suicidality in bulimia nervosa. Journal of Clinical Psychiatry 1992;53(7):235‐41. [PubMed] [Google Scholar]
Wilson 1998
- Wilson GT. The clinical utility of randomized controlled trials. International Journal of Eating Disorders 1998;24(1):13‐29. [DOI] [PubMed] [Google Scholar]
Wolfe 1995
- Wolfe BE. Dimensions of response to antidepressant in bulimia nervosa: a review. Archives of Psychiatric Nursing 1995;9(3):111‐21. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bacaltchuk 2000
- Bacaltchuk J, Hay P, Mari JJ. Antidepressants versus placebo for the treatment of bulimia nervosa: a systematic review. Australian and New Zealand Journal of Psychiatry 2000;34(2):310‐7. [DOI] [PubMed] [Google Scholar]


