Abstract
Background
There are a number of effective interventions for the treatment of depression. It is possible that the efficacy of these treatments will be improved further by the use of adjunctive therapies such as folate.
Objectives
1. To determine the effectiveness of folate in the treatment of depression 2. To determine the adverse effects and acceptability of treatment with folate.
Search methods
The Cochrane CENTRAL Register of Controlled Trials (CENTRAL), and the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Registers (CCDANCTR‐Studies and CCDANCTR‐References ‐ carried out on 12/5/2005) were searched. Reference lists of relevant papers and major textbooks of affective disorders were checked. Experts in the field and pharmaceutical companies were contacted regarding unpublished material.
Selection criteria
All randomised controlled trials that compared treatment with folic acid or 5'‐methyltetrahydrofolic acid to an alternative treatment, whether another antidepressant medication or placebo, for patients with a diagnosis of depressive disorder (diagnosed according to explicit criteria).
Data collection and analysis
Data were independently extracted from the original reports by two reviewers. Statistical analysis was conducted using Review Manager version 4.1.
Main results
Three trials involving 247 people were included. Two studies involving 151 people assessed the use of folate in addition to other treatment, and found that adding folate reduced Hamilton Depression Rating Scale scores on average by a further 2.65 points (95% confidence interval 0.38 to 4.93). Fewer patients treated with folate experienced a reduction in their HDRS score of less than 50% at ten weeks (relative risk (RR) 0.47, 95% CI 0.24 to 0.92) The number needed to treat with folate for one additional person to experience a 50% reduction on this scale was 5 (95% confidence interval 4 to 33). One study involving 96 people assessed the use of folate instead of the antidepressant trazodone and did not find a significant benefit from the use of folate. The trials identified did not find evidence of any problems with the acceptability or safety of folate.
Authors' conclusions
The limited available evidence suggests folate may have a potential role as a supplement to other treatment for depression. It is currently unclear if this is the case both for people with normal folate levels, and for those with folate deficiency.
Plain language summary
Folate for depression
This systematic review was undertaken to see if giving folate to people with depressive disorders reduced their depressive symptoms. Three randomized trials were identified, involving a total of 247 people. In all three trials, folate was well tolerated. In two of these trials, folate was added to other antidepressant drug treatment and there was limited evidence that folate helped. In the third trial, folate was compared to trazodone, an antidepressant drug. No difference was found. There is therefore limited evidence that adding folate to other antidepressant may be helpful, but larger trials are needed before patients and clinicians can be confident that it will be helpful.
Background
Depression is a major cause of worldwide disability. In the United States the one month prevalence of a major depressive episode has been estimated to be 2.2% (Regier 1988) and comparable figures have been found in the U.K. (Jenkins 1997). The Global Burden of Disease Study found unipolar depression to be the fourth leading cause of worldwide disability even after excluding deaths due to suicide (Murray 1997a). The prevalence of major depressive disorder may be on the increase (CNCG 1992) and the Global Burden of Disease Study predicted that, by 2020, unipolar major depression will be the second leading cause of disability worldwide (Murray 1997b).
There a number of effective interventions available for the acute treatment of depression including pharmacotherapy and psychotherapy (Geddes 2001). Since the introduction of antidepressants in the 1950s, the number of available pharmacological treatments has increased, but their efficacy has remained largely unchanged. A recent review reported a response rate of 50% with active drug compared to 32% with placebo (AHCPR 1999). One possible method of improving response to antidepressant medication is by using adjunctive agents such as amino acid precursors and cofactors. One such agent is folic acid, which is the parent compound of a number of naturally occurring folates. Dietary folates are absorbed and carried in the blood in the form of 5'‐methyltetrahydrofolate. Within the body, folates act as important methyl donors in the reactions of DNA synthesis and amino acid metabolism. Folate deficiency is a common finding in psychiatric patients, whether measured by serum folate (Carney 1967, Reynolds 1970, Reynolds 1976) or red blood cell folate (Reynolds 1971, Carney 1990). While low levels have not been found in all populations (Lee 1992, Lee 1998), lower folate levels have been linked to a worse response to pharmacological treatment (Fava 1997).
An association between folate and serotonin metabolism has been demonstrated in patients with neuropsychiatric disorders (Botez 1982) and patients with inborn errors of folate metabolism (Clayton 1986, Hyland 1988). The basis of this link may be the role played by folate in the methylation of homocysteine, which is necessary for its conversion to s‐adenosylmethionine (SAM), since SAM has itself been shown to influence serotonin metabolism (Bottiglieri 1984), and has been used in the treatment of depression (Agnoli 1976, Caruso 1984). Alternatively, it has also been shown that folates also play a role in the methylation reactions producing tetrahydrobiopterin (Kaufman 1991), which is an essential co‐factor for the hydroxylase enzymes which form the rate‐limiting step in the production of monoamines including serotonin (Kaufman 1981). There is some evidence of gender differences in tetrahydrobiopterin metabolism (Coppen 1989).
Dietary folate supplementation has appeared effective and reasonably tolerated in other contexts such as the treatment of the megaloblastic anaemia of folate deficiency, or the prevention of neural tube defects (MRC VSRG 1991, Czeizel 1992). This review aims to assess whether treatment with folate, either alone or as an adjunct to other antidepressant medication, is effective in the treatment of depression.
Objectives
1. To determine the effectiveness of folate in the treatment of depression 2. To determine the adverse effects and acceptability of treatment with folate.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials.
Types of participants
All patients suffering from a depressive disorder diagnosed according to explicit criteria, whether within a structured interview or otherwise, including major depressive disorder, bipolar affective disorder, and dysthymic disorder.
Types of interventions
1. Folic acid (or 5'‐methyltetrahydrofolic acid) compared to placebo, or 2. folic acid (or 5'‐methyltetrahydrofolic acid) compared to antidepressant medication, in the treatment of depressive disorder, whether as monotherapy or as an adjunct to other treatment.
Types of outcome measures
A. Primary outcome measure The primary outcome measure is the proportion of study participants whose depressive episode resolves within a defined period of observation. Resolution is often measured using depressive symptom rating scales, when possible these are analysed as continuous data. These continuous data, however, are often dichotomised at an arbitrary cut point (e.g. Hamilton Depression Rating Scale score <8, or a >50% reduction on the HDRS) because this facilitates clinical interpretation. Accordingly such data are analysed as dichotomous data.
B. Secondary outcome measures Where possible the following outcomes are also investigated using continuous and dichotomous data as appropriate: 1. The overall drop‐out rate as a proxy measure of overall acceptability of treatment. 2. The drop‐out rate due to side‐effects. 3. Quality of life. 4. Global clinical impression rated by the clinician. 5. Global clinical impression rated by the patient. 6. Admission to hospital. 7. Social functioning. 8. Occupational functioning. 9. The number of patients reporting at least one adverse event i.e. troublesome side effects. 10. Mortality due to all causes and specifically suicides and verdicts of undetermined deaths.
Search methods for identification of studies
See Collaborative Review Group search strategy.
1. Electronic databases were searched: The twin Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Registers were searched using the following search strategies : CCDANCTR‐Studies ‐ carried out on 12/5/2005 Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Intervention = folate* or "folic acid" or methylfolate or methyltetrahydrofolate or 5‐methyltetrahydrofolate or MTHF or "5'‐methyltetrahydrofolate" or tetrahydrofolate
CCDANCTR‐References ‐ carried out on 12/5/2005 Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Free‐text = folate* or "folic acid" or methylfolate or methyltetrahydrofolate or 5‐methyltetrahydrofolate or MTHF or "5'‐methyltetrahydrofolate" or tetrahydrofolate
The Cochrane Central Register of Controlled Trials (CENTRAL) was also searched using the same terms as for CCDANCTR excluding references tagged with sr‐depress* as these have come from CCDANCTR.
2. Reference checking The reference lists of all selected studies were inspected for more published reports and citations of unpublished research. In addition other relevant papers, and major textbooks which cover affective disorder, were checked.
3. Personal communications To ensure that as many randomised controlled trials as possible were identified, the authors of significant papers in the field , including all included trials, were contacted.
4. Pharmaceutical Companies Pharmaceutical companies manufacturing other antidepressant medication, and companies manufacturing folate, were contacted via CCDAN to find out if they knew of any published or unpublished randomised controlled trials relevant to this review.
Data collection and analysis
1. Selection of trials Studies generated by the search strategies were checked independently by two reviewers (MT and SC) to ensure that they met the previously defined inclusion criteria.
2. Quality assessment Two reviewers (MT and JG) independently assessed the methodological quality of the included studies. The reviewers were not kept blind to the authorship and source of the papers because the beneficial effect of these measures is uncertain (Berlin 1997). Quality was assessed according to the Cochrane criteria for quality assessment (Sackett 1997). These criteria pay particular attention to the quality of the randomisation procedure including allocation concealment. On this basis studies were given a quality rating of A (adequate), B (unclear), and C (inadequate). If the raters disagreed, the final rating was made by consensus with the involvement, if necessary, of another of the review group. In addition, aspects of quality such as whether the trial was of a double blind design and reporting of withdrawals and dropouts were described. Where adequate details of randomisation and other characteristics of trials were not provided the authors were contacted in order to obtain further information.
3. Data extraction Data were extracted from the studies about participant characteristics, intervention details, study design, and outcome measures from the included studies. Any disagreements were resolved by consensus discussions with a third member of the review team.
4. Data analysis Data were entered into Review Manager 4.1 software. For binary efficacy outcomes, a relative risk (with 95% confidence intervals) was calculated using a fixed effects model. For continuously distributed outcomes the weighted mean difference (WMD) was calculated when the same scale was used in a similar manner across studies, otherwise, the standardised weighted mean difference was used. Heterogeneity between studies was assessed using the chi‐ squared test. Random effects models were used routinely to investigate the sensitivity of results to the choice of statistical method. We used intention‐to‐treat data when available and where this was not possible, endpoint data for trial completers was used. Non‐quantitative data is presented descriptively. Subgroup analyses were performed where feasible to assess the extent to which the results differed 1. between male and female subjects and 2. between dysthymia and other depressive disorders. Sensitivity analyses were performed excluding studies of lower methodological quality to assess the robustness of the results.
Results
Description of studies
Three randomised controlled trials satisfied the inclusion criteria for this review.
Participants Two trials (151 patients randomised) were conducted in patients over the age of 18 (Coppen 2000, Godfrey 1990). Both required a DSM‐III or DSM‐IIIR diagnosis of major depression and in addition, a score of 20 or more on the HDRS was required to be included in the Coppen study. A low folate level (less than 200 µg/litre) was necessary for inclusion in the study by Godfrey. However, abnormal laboratory tests (including megaloblastic anaemia), were an exclusion criterion in the Coppen study. The third trial (Passeri 1991) included 96 patients who were all aged at least 65. Only patients with a DSM diagnosis of dementia, MMSE of 12‐23, and depression with a Hamilton Depression Rating Scale score of greater than 18 were included. All participants had red blood cell folate in the normal range. In this trial patients who responded to a two week placebo run‐in period were excluded.
Setting All three trials were conducted in Europe, two in the UK (Coppen 2000, Godfrey 1990) and one in Italy (Passeri 1991). Godfrey et al recruited both outpatients and inpatients while in the Coppen trial the sample was selected predominantly from outpatients. The study in older adults with comorbid depression and dementia was conducted in a nursing home.
Interventions used Two studies compared treatment with folate to placebo in the context of continued use of other psychotropic medication. Of these, one used 500µg folic acid (Coppen 2000), and the other 15mg methyltetrahydrofolate (Godfrey 1990). One trial compared the use of 50mg methyltetrahydrofolate once daily to 50mg trazodone twice daily (Passeri 1991).
Duration of follow‐up The durations of treatment in the studies were eight weeks (Passeri 1991), ten weeks (Coppen 2000) and six months (Godfrey 1990).
Outcome measures All studies used a Hamilton Depression Rating Scale (either 17 or 21 item) to measure depressive symptoms. Information on drop‐out rates was also included. Two studies reported the number of subjects reporting adverse event (Coppen 2000, Passeri 1991), and one reported admissions to hospital (Coppen 2000). One study (Godfrey 1990) reported a clinical outcome scale combining clinician impressions of improvement in both symptoms and social functioning.
Risk of bias in included studies
Allocation All the studies are described as randomised, but generally little information was given in the published reports regarding the methods used to achieve random allocation or to maintain concealment of allocation. The studies received a "B" rating according to the Cochrane criteria (Sackett 1997).
Blinding All the studies report being 'double blind'. This implies that both patients and investigators were blind to which treatment was being received during the course of the study. However relatively little information was provided as to how this was achieved in practice.
Reporting of withdrawals and drop‐outs Two of the included studies (Godfrey 1990, Passeri 1991) had no withdrawals or drop‐outs. In the third study (Coppen 2000) only those patients completing six weeks of treatment were included in the analyses.
Effects of interventions
Resolution of a depressive episode Continuous rating scales (Hamilton Depression Rating Scale (HDRS)) were used in all studies allowing comparison of scores between different treatment groups after defined periods.
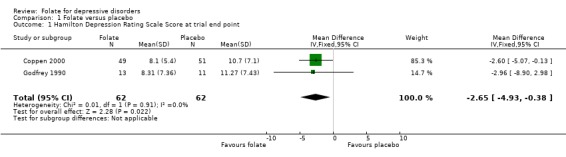
Pooling the results of the two trials that compared folate with placebo in addition to other psychotropic medication, the weighted mean difference (WMD) in HDRS score between the groups at trial end point favoured treatment with folate, (WMD ‐2.65; 95% CI ‐4.93 to ‐0.38; heterogeneity chi‐square=0.01, p = 0.91). Although this result is statistically significant, the confidence interval is wide. When folate was compared with trazodone for patients with comorbid dementia and depression (Passeri 1991) the WMD was ‐1.00 (95% CI ‐3.21 to 1.21).
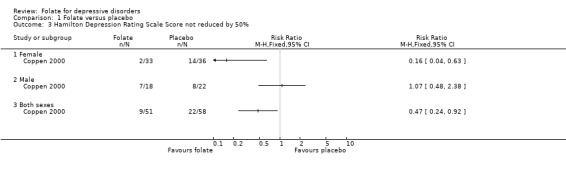
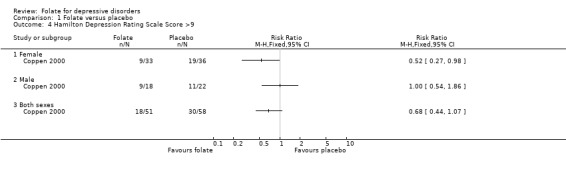
Dichotomised outcomes were also reported by one of the studies comparing folate with placebo (Coppen 2000). Compared to placebo, fewer patients treated with folate experienced a reduction in HDRS of less than 50% at ten weeks (relative risk (RR) 0.47, 95% CI 0.24 to 0.92), the number needed to treat (NNT) with folate to cause one additional subject to experience a reduction on the HDRS of at least 50% was 5 (95% CI 4 to 33). Folate treated patients were also less likely to have HDRS >19 at ten weeks (RR 0.28, 95% CI 0.08 to 0.95; NNT 7, 95% CI 6 to 97). However, when compared to trazodone (Passeri 1991), treatment with folate was no more likely to avoid a reduction in HDRS of less than 50% (RR 0.97, 95% CI 0.84 to 1.12).
Drop‐out rate Only one study reported that any participants withdrew from the trial (Coppen 2000). The overall drop‐out rates were similar, RR 0.97 (95% CI 0.50 to 1.90), as were drop‐outs attributed to side‐effects, RR 0.52 (95% CI 0.14 to 2.01).
Adverse events reported There were no clear differences in the numbers of patients reporting at least one side‐effect on folate compared to placebo, RR 0.76 (95% CI 0.55 to 1.05) (Coppen 2000), or trazodone, RR 0.35 (95% CI 0.01 to 8.32) (Passeri 1991).
Global clinical impression and social functioning One study (Godfrey 1990) comparing folate with placebo reported a clinician‐rated outcome scale combining symptom resolution and social functioning. On this scale, the results favoured treatment with folate, WMD ‐0.90 (95% CI ‐1.45 to ‐0.35).
Admission to hospital. One study (Coppen 2000) reported that one subject, from the placebo arm, required admission to hospital.
Mortality No deaths were reported by any of the included studies .
Other outcomes No data was available on differences in quality of life, clinical global impression rated by patient or occupational functioning.
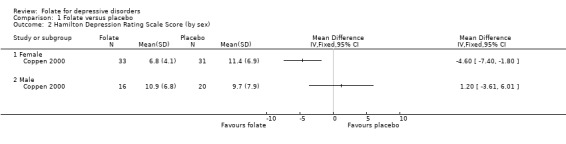
Subgroup Analyses Male versus Female Only one trial (Coppen 2000) published separate data for male and female subjects. There was no statistically significant difference in HDRS score for male subjects treated with either folate or placebo, WMD 1.20 (95% CI ‐3.61 to 6.01). In contrast, HDRS score for female subjects was improved with folate, WMD ‐4.60 (95% CI ‐7.40 to ‐1.80). Although the 95% CIs of male and female subjects overlap, there was statistically significant heterogeneity between the two groups on this measure (chi‐square=4.18, p=0.041). Similarly, considering the outcome of less than 50% reduction in HDRS, in the male subgroup there was no difference between folate and placebo, RR 1.07 (95% CI 0.48 to 2.38), while for the female group results favoured folate, RR 0.16 (95% CI 0.04 to 0.63); there was also statistically significant heterogeneity on this outcome (chi‐square=6.36, p=0.012). If data regarding the relationship between treatment response and gender becomes available from the authors of other studies, it will be included in subsequent versions of this review.
Dysthymia versus Other Diagnosis No trials were identified assessing the efficacy of folate in dysthymia, so this subgroup analysis could not be performed.
Sensitivity Analyses Due to the small number of included trials, no sensitivity analyses were performed.
Discussion
Use of folate as augmentation There is some evidence that augmentation of antidepressant treatment with folate may improve outcome. The existence of one or two relatively small neutral ‐ or negative ‐ trials would have a substantial effect on the pooled estimate. As there are few studies, indirect methods of identiifying publication bias such as funnel plots are of very limited value. The limited evidence also limits the precision of the results ‐ this means that the size of any potential benefit is uncertain, and may be clinically insignificant. When the results are expressed as a continuous variable, the difference in Hamilton Depression scale score lies between 0.4 and 4.9. Similarly the NNT for dichotomous outcomes may be as few as 4 or as many as 97.
Use as alternative to other antidepressant Only one trial was identified that examined the use of folate as an alternative to conventional antidepressant therapy (Passeri 1991) and this identified no clear benefit from the use of folate. However, it was underpowered to measure a moderate treatment difference between folate and trazodone. The dosage of trazodone used was relatively low, and it was in a group of patients with comorbid dementia, which may limit generalisability.
Acceptability/adverse events The included trials did not find evidence that the use of folate supplementation or folate compared to trazodone was associated with any statistically significant problems of acceptability or adverse events. However, the small total number of patients randomised mean that infrequent but serious adverse events cannot be excluded.
Subgroup effects There are insufficient data to draw clear conclusions about potentially important subgroup differences. Only one study (Coppen 2000) has examined male/female differences in the response to folate augmentation. This trial carried out a secondary analysis which found a clear benefit of folate augmentation in female patients but not in male patients. There was statistically significant heterogeneity between the subgroups. Effects of augmentation of similar magnitude were seen both for folate deficient subjects and those with normal blood results, although of note the treatment of the folate deficient population was over a longer time period ‐ six months rather than twelve weeks.
Authors' conclusions
Implications for practice.
The currently available evidence suggests that folate supplementation may be effective when used in addition to conventional antidepressant medication. The evidence does not support the use of folate as a replacement for antidepressant medication in the treatment of depression. There is no evidence that supplementation is only effective in those with low folate results. There is as yet not enough evidence of a qualitative difference in response by sex to make clear cut conclusions about the absence of an effect of folate supplementation in male patients.
Implications for research.
The available evidence raises the possibility that folate may have therapeutic potential as an augmentation strategy in the treatment of depressive disorder. Further randomised trials are required to establish the exact magnitude of the main effect and these will need to be adequately powered to investigate the possiblity of subgroup effects, such as differences by gender, or by the presence or absence of folate deficiency. The benefits of addition of folate in cases of non‐response to conventional antidepressants have yet to be investigated.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 22 February 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the CCDAN Editorial Team for their support, information and advice.
Data and analyses
Comparison 1. Folate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hamilton Depression Rating Scale Score at trial end point | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐2.65 [‐4.93, ‐0.38] |
| 2 Hamilton Depression Rating Scale Score (by sex) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Female | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Male | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Hamilton Depression Rating Scale Score not reduced by 50% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Female | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Male | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Both sexes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Depression Rating Scale Score >9 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Female | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Male | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Both sexes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Hamilton Depression Rating Scale Score >19 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Female | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Male | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Both sexes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Folate versus placebo, Outcome 1 Hamilton Depression Rating Scale Score at trial end point.
1.2. Analysis.
Comparison 1 Folate versus placebo, Outcome 2 Hamilton Depression Rating Scale Score (by sex).
1.3. Analysis.
Comparison 1 Folate versus placebo, Outcome 3 Hamilton Depression Rating Scale Score not reduced by 50%.
1.4. Analysis.
Comparison 1 Folate versus placebo, Outcome 4 Hamilton Depression Rating Scale Score >9.
1.5. Analysis.
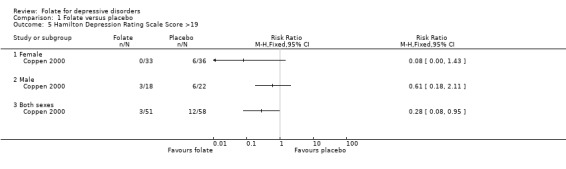
Comparison 1 Folate versus placebo, Outcome 5 Hamilton Depression Rating Scale Score >19.
Comparison 2. Folate versus antidepressant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hamilton Depression Rating Scale Score at trial end point | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Hamilton Depression Rating Scale score not reduced by 50% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Hamilton Depression Rating Scale score not reduced by 25% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
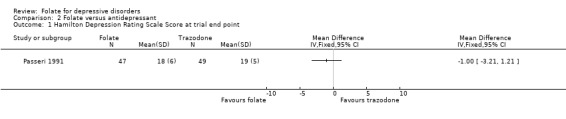
Comparison 2 Folate versus antidepressant, Outcome 1 Hamilton Depression Rating Scale Score at trial end point.
2.2. Analysis.
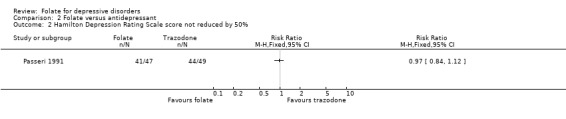
Comparison 2 Folate versus antidepressant, Outcome 2 Hamilton Depression Rating Scale score not reduced by 50%.
2.3. Analysis.
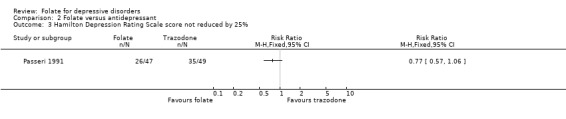
Comparison 2 Folate versus antidepressant, Outcome 3 Hamilton Depression Rating Scale score not reduced by 25%.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coppen 2000.
| Methods | Double‐blind parallel arm RCT, central randomisation stratified by sex | |
| Participants | 127 ‐ new episode major depression (DSM‐III‐R) and HDRS >20 | |
| Interventions | 20mg fluoxetine plus either (1)500ug folic acid or (2)placebo capsule | |
| Outcomes | Hamilton Depression Rating Scale (17 item). Drop‐outs. Adverse event rate. Admissions. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Godfrey 1990.
| Methods | Double‐blind, parallel arm RCT. Randomisation stratified by diagnosis (schizophrenia or depression) ‐ only depressed patients included in this review. | |
| Participants | 24 ‐ major depression (DSM III) and RBC folate < 200 ug/litre | |
| Interventions | (1)15mg methyltetrahydrofolate or (2)placebo in addition to standard psychotropic treatments | |
| Outcomes | Hamilton Depression Rating Scale (17‐item). Clinical rating Scale. Drop‐outs. Clinical Outcome Scale. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Passeri 1991.
| Methods | Double‐blind, parallel arm RCT. Multi‐centre. Placebo run‐in. | |
| Participants | 96 ‐ age >65, dementia (DSM‐III‐R), MMSE 12‐23, HDRS >17, RBC folate 175‐700 ng/ml | |
| Interventions | (1)50mg methyltetrahydrofolate plus placebo or (2)trazodone 50mg twice daily | |
| Outcomes | Hamilton Depression Rating Scale (21‐item). Drop‐outs. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RCT ‐ randomised controlled trial DSM ‐ Diagnosic and Statistical Manual of American Psychiatric Association; DSM‐III 3rd edition; DSM‐III‐R 3rd edition revised MMSE ‐ Mini mental state examination HDRS ‐ Hamilton depression rating scale RBC ‐ red blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bell 1992 | not a study using folate |
| Carney 1970 | not a randomised controlled trial |
| Coppen 1986 | diagnostic criteria not explicit |
| Guaraldi 1993 | not a randomised controlled trial |
| Procter 1991 | review of Godfrey 1990 |
Characteristics of ongoing studies [ordered by study ID]
Reynolds.
| Trial name or title | unknown |
| Methods | |
| Participants | unknown |
| Interventions | methyltetrahydrofolate monotherapy versus comparator |
| Outcomes | unknown |
| Starting date | unknown |
| Contact information | Dr E H Reynolds |
| Notes | Submitted for publication 2002 |
Contributions of authors
JG and GG conceived the review. MT coordinated the review and will act as guarantor. MT, SC, and JG performed data collection and analysis. All reviewers contributed to the design of the review, and interpretation of results.
Sources of support
Internal sources
Department of Psychiatry, University of Oxford, UK.
Oxfordshire Mental Healthcare NHS Trust, UK.
External sources
No sources of support supplied
Declarations of interest
JG has received research funding and support from Sanofi‐Aventis and GlaxoSmithKline and is currently in discussion with several other companies that manufacture SSRIs about collaboration on planned independent trials and systematic reviews.
Edited (no change to conclusions)
References
References to studies included in this review
Coppen 2000 {published data only}
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. Journal of Affective Disorders 2000;60(2):121‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Godfrey 1990 {published data only}
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990;336(8712):392‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Procter A. Enhancement of recovery from psychiatric illness by methylfolate. British Journal of Psychiatry 1991;159:271‐2. [DOI] [PubMed] [Google Scholar]
Passeri 1991 {published data only}
- Passeri M, Cucinotta D, Abate G, Senin U, Ventura A, Stramba BM, et al. Oral 5'‐methyltetrahydrofolic acid in senile organic mental disorders with depression: Results of a double‐blind multicenter study. Aging 1993;5(1):63‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Passeri M, Ventura S, Abate G, Cucinotta D, et al. Oral 5‐methyltetrahydrofolate (MTHF) in depression associated with senile organic mental disorders (OMDs): a double‐blind muticenter study vs trazodone (TRZ). European Journal of Clinical Investigation 1991;21(2 Pt II):24. [Google Scholar]
References to studies excluded from this review
Bell 1992 {published data only}
- Bell IR, Edman JS, Morrow FD, Marby DW, Perrone G, Kayne HL, et al. Brief communication. Vitamin B1, B2, and B6 augmentation of tricyclic antidepressant treatment in geriatric depression with cognitive dysfunction. Journal of the American College of Nutrition 1992;11(2):159‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Carney 1970 {published data only}
- Carney MW, Sheffield BF. Associations of subnormal serum folate and vitamin B12 values and effects of replacement therapy. Journal of Nervous & Mental Disease 1970;150(5):404‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coppen 1986 {published data only}
- Coppen A, Chaudhry S, Swade C. Folic acid enhances lithium prophylaxis. Journal of Affective Disorders 1986;10(1):9‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guaraldi 1993 {published data only}
- Guaraldi GP, Fava M, Mazzi F, Greca P. An open trial of methyltetrahydrofolate in elderly depressed patients. Annals of Clinical Psychiatry 1993;5(2):101‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Procter 1991 {published data only}
- Procter A. Enhancement of recovery from psychiatric illness by methylfolate. British Journal of Psychiatry 1991;159:271‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Reynolds {published data only}
- unknown. Ongoing study unknown.
Additional references
Agnoli 1976
- Agnoli A, Andreoli V, Casachia M, Cerbo R. Effect of S‐adenosyl L‐methionine (SAMe) upon depressive symptoms. Journal of Psychiatric Research 1976;13(1):43‐54. [DOI] [PubMed] [Google Scholar]
AHCPR 1999
- Agency for Health Care Policy and Research. Treatment of Depression ‐ Newer Pharmacotherapies. Summary, Evidence Report/Technology Assessment: Number 7. http://www.ahcpr.gov/clinic/deprsumm.htm 1999. [AHCPR Publication No. 99‐E013 AHCPR Publication No. 99‐E013 AHCPR Publication No. 99‐E013 AHCPR Publication No. 99‐E013]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350(9072):185‐6. [DOI] [PubMed] [Google Scholar]
Botez 1979
- Botez MI, Young SN, Bachevalier J, Gauthier S. Folate deficiency decreased brain 5‐hydroxytryptamine synthesis in man and rat. Nature 1979;278(5700):182‐3. [DOI] [PubMed] [Google Scholar]
Botez 1982
- Botez MI, Young SN, Bachevalier J, Gauthier S. Effect of folic acid and vitamin B12 deficiencies on 5‐hydroxyindole acetic acid in human cerebrospinal fluid. Annals of Neurology 1982;12(5):479‐84. [DOI] [PubMed] [Google Scholar]
Bottiglieri 1984
- Bottiglieri T, Laundy M, Martin R, Carney MW, Nissenbaum H, Toone BK. S‐adenosylmethionine influences monoamine metabolism. Lancet 1984;2(8396):224. [DOI] [PubMed] [Google Scholar]
Carney 1967
- Carney MW. Serum folate values in 423 psychiatric patients. BMJ 1967;4(578):512‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 1990
- Carney MW, Chary TK, Laundy M, Bottiglieri T, Chanarin I, Reynolds EH, et al. Red‐cell folate concentrations in psychiatric patients. Journal of Affective Disorders 1990;19(3):207‐13. [DOI] [PubMed] [Google Scholar]
Caruso 1984
- Caruso I, Fumagalli M, Boccassini L, Puttini S, Ciniselli G, Cavallari G. Antidepressant activity of S‐adenosylmethionine. Lancet 1984;1(8382):904. [DOI] [PubMed] [Google Scholar]
Clayton 1986
- Clayton PT, Smith I, Harding B, Hyland K, Leonard JV, Leeming RJ. Subacute combined degeneration of the cord, dementia and Parkinsonism due to an inborn error of folate metabolism. Journal of Neurology, Neurosurgery & Psychiatry 1986;49(8):920‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
CNCG 1992
- Cross‐National Collaborative Group. The changing rate of major depression: cross‐national comparisons. JAMA 1992;268(21):3098‐105. [DOI] [PubMed] [Google Scholar]
Coppen 1989
- Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: The folate connection. Journal of Affective Disorders 1989;16(2‐3):103‐7. [DOI] [PubMed] [Google Scholar]
Czeizel 1992
- Czeizel AE, Dudas I. Prevention of the first occurence of neural‐tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327(26):1832‐5. [DOI] [PubMed] [Google Scholar]
Fava 1997
- Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. American Journal of Psychiatry 1997;154(3):426‐8. [DOI] [PubMed] [Google Scholar]
Geddes 2001
- Geddes JR, Butler R, Warner J. Depressive disorders in adults. Clinical Evidence. London: BMJ Publishing Group, 2001. [Google Scholar]
HLTC 1998
- Homocysteine Lowering Trialists' Collaboration. Lowering blood homocysteine with folic acid based supplements: Meta‐analysis of randomised trials. BMJ 1998;316(7135):894‐8. [PMC free article] [PubMed] [Google Scholar]
Hyland 1988
- Hyland K, Smith I, Bottiglieri T, Perry J, Wendel U, Clayton PT, et al. Demyelination and decreased S‐adenosylmethionine in 5,10‐methylenetetrahydrofolate reductase deficiency. Neurology 1988;38(3):459‐62. [DOI] [PubMed] [Google Scholar]
Jenkins 1997
- Jenkins R, Lewis G, Bebbington P, Brugha P, Farrell M, Gill B, et al. The national psychiatric morbidity surveys of Great Britain ‐ initial findings from the household survey. Psychological Medicine 1997;27(4):775‐89. [DOI] [PubMed] [Google Scholar]
Kaufman 1981
- Kaufman S. Regulatory properties of pterin‐dependant hydroxylases: Variations on a theme. In: Kaufman S, Usdin E, Weiner N, Yondin MB editor(s). Function and regulation of monoamine enzymes. New York, NY: Macmillan, 1981:165‐73. [Google Scholar]
Kaufman 1991
- Kaufman S. Some metabolic relationships between biopterin and folate: Implications for the "methyl trap hypothesis". Neurochemical Research 1991;16(9):1031‐6. [DOI] [PubMed] [Google Scholar]
Lee 1992
- Lee S, Chow CC, Shek CC, Wing YK, Chen CN. Folate concentrations in Chinese psychiatric outpatients on long‐term lithium treatment. Journal of Affective Disorders 1992;24(4):265‐70. [DOI] [PubMed] [Google Scholar]
Lee 1998
- Lee S, Wing YK, Fong S. A controlled study of folate levels in Chinese inpatients with major depression in Hong Kong. Journal of Affective Disorders 1998;49(1):73‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MRC VSRG 1991
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991;338(8760):131‐7. [PubMed] [Google Scholar]
Murray 1997a
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease study. Lancet 1997;349(9063):1436‐42. [DOI] [PubMed] [Google Scholar]
Murray 1997b
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020. Lancet 1997;349(9064):1498‐504. [DOI] [PubMed] [Google Scholar]
Regier 1988
- Regier D, Boyd J, Burke J, Rae D, Myers J, Kramer M, et al. One month prevalence of mental disorders in the United States. Archives of General Psychiatry 1988;45(11):977‐86. [DOI] [PubMed] [Google Scholar]
Reynolds 1970
- Reynolds EH, Preece JM, Bailey J, Coppen A. Folate deficiency in depressive illness. British Journal of Psychiatry 1970;117(538):287‐92. [PubMed] [Google Scholar]
Reynolds 1971
- Reynolds EH, Preece J, Johnson AL. Folate metabolism in epileptic and psychiatric patients. Journal of Neurosurgery & Psychiatry 1971;34(6):726‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 1976
- Reynolds EH. Neurological aspects of folate and vitamin B12 metabolism. In: Hoffbrand AV editor(s). Clinics in Haematology. Vol. 5, London: Saunders, 1976:661‐96. [PubMed] [Google Scholar]
Sackett 1997
- Sackett D. Cochrane Collaboration Handbook. Oxford: Update Software, 1997. [Google Scholar]


