Abstract
Background:
Dietary interventions are implicated in the development of atopic dermatitis, psoriasis, and acne.
Objective:
To investigate the effect of diet and the bile acid (BA) receptors, such as TGR5 (Takeda G protein receptor 5) and S1PR2 (sphingosine-1-phosphate receptor 2) in the development of dermatitis.
Methods:
C57BL/6 mice were fed a control diet (CD) or Western diet (WD) since weaning until they were 10 months old followed by analyzing histology, gene expression, and BA profiling.
Results:
Mice developed dermatitis as they aged and the incidence was higher in females than males. Additionally, WD intake substantially increased the incidence of dermatitis. Cutaneous antimicrobial peptide genes S100A8, S100A9, and Defb4 were reduced in WD-fed mice, but increased when mice developed skin lesions. In addition, Tgr5 and TGR5-regulated Dio2 and Nos3 were reduced in WD intake but induced in dermatitic lesions. Trpa1 and Trpv1, which mediate itch, were also increased in dermatitic lesions. The expression of S1pr2 and genes encoding sphingosine kinases, S1P phosphatases, binding protein, and transporter were all reduced by WD intake but elevated in dermatitic lesions. Furthermore, dermatitis development increased total cutaneous BA with an altered profile, which may change TGR5 and S1PR2 activity. Moreover, supplementation with BA sequestrant cholestyramine reduced epidermal thickening as well as cutaneous inflammatory cytokines.
Conclusion:
In summary, activation of TGR5 and S1PR2, which regulate itch, keratinocyte proliferation, metabolism, and inflammation, may contribute to WD-exacerbated dermatitis with Th2 and Th17 features. In addition, elevated total BA play a significant role in inducing dermatitis and cutaneous inflammation.
Keywords: inflammation, bile acid, bile acid receptor, TGR5, S1PR2, psoriasis, atopic dermatitis
INTRODUCTION
Psoriasis and atopic dermatitis have been linked to a variety of systemic inflammatory disorders and are associated with increased risk of cardiovascular disease, obesity, hypertension, and diabetes [1]. Psoriasis is an immune-mediated disease characterized by inflammation, epidermal hyperplasia, parakeratosis, and mild spongiosis that affects about 2-3% of the population [2]. There is no question that diet also influences the development of obesity. The so-called Western diet containing moderate-to-high levels of fat and high levels of simple sugars has been thought to contribute to obesity in the Western world [3]. Interestingly, high fat diet-fed mice exhibit exacerbated imiquimod (IMQ)-induced dermatitis in the auricula skin, whereas obese (ob/ob) mice fed a normal diet do not show enhanced susceptibility to IMQ-induced dermatitis, indicating that obesity alone may be insufficient to enhance susceptibility to psoriasiform dermatitis [4, 5]. Supporting this concept in our recent report demonstrating that more obese high-fat diet-fed mice did not have enhanced susceptibility to IMQ-induced dermatitis whereas less obese WD-fed mice did, suggesting that dietary components, rather than obesity alone, may influence susceptibility to IMQ dermatitis [6]. Atopic dermatitis is another chronic inflammatory skin disease that is driven by terminal keratinocyte differentiation abnormalities, and displays heterogeneity in the sense that Th2, Th22, Th17/IL-23, and Th1 cytokine pathways can be activated, depending on the disease subtype. Interestingly, recent clinical studies suggest that obesity is also likely to be a co-morbidity of atopic disease [7]. In the current study, we ask if a WD can influence the development of spontaneous dermatitis in mice.
Bile acids (BAs) are signaling molecules with diverse effects on gut microbiota as well as host immunity and metabolism [8, 9]. BAs are synthesized in the liver correlate with pruritus severity while cholestyramine reduces BA levels and relieves pruritus [10]. Pruritus affects about 60-90% of patients with psoriasis. Intriguingly, small clinical studies conducted several decades ago suggest that cholestyramine can ameliorate psoriatic skin disease [11] Thus, we hypothesize that BA may serve as an intrinsic link between diet, obesity, and the development of dermatitis.
BA signaling is mediated via nuclear receptors FXR (farnesoid x receptor) as well as membrane receptors TGR5 (Takeda G protein receptor 5) and sphingosine-1-phosphate (S1P) receptor 2 (S1PR2) [12-14]. While FXR is primarily found in the liver and intestine to regulate BA synthesis and homeostasis, TGR5 and S1PR2 are ubiquitously expressed [13, 15]. TGR5 is highly expressed in cells as monocytes and macrophages; its activation confers potent antiinflammatory property at the systemic level [16]. Moreover, by regulating the expression of cation channels such as TRPA1 and TRPV1, BA-activated TGR5 regulates sensations of itch and analgesia [17]. In addition, S1PR2 also has a pivotal role in regulating inflammation, immunity, as well as metabolism [18]. In contrast to the anti-inflammatory effect of TGR5, S1PR2 is essential for lipopolysaccharides to induce systemic inflammation [19]. Sphingomyelin in the cell membrane can be converted to S1P, which activates S1PR2 to stimulate mast cell degranulation, leading to increased inflammatory cytokine production and circulating S1P [20, 21]. Additionally, higher levels of circulating S1P promotes Th2 response and increase the number of Th2 and Th17 cells as well as the ratio of M2 to M1 macrophages [22]. Due to the significant role of TGR5 and S1PR2 in regulating systemic inflammation and metabolism, we hypothesize that BAs, acting through these BA receptors, may contribute to the development of diet-exacerbated dermatitis.
To test the proposed hypotheses, this study examines the impact of a WD on dermatitis development and BA receptor-regulated signaling. Our data showed that C57BL/6 mice spontaneously develop dermatitis as they aged and that WD substantially increased disease incidence. Interestingly, the lesional skin of mice that developed the WD-exacerbated dermatitis showed cytokine mRNA expression patterns that contained Th2 as well as Th17 cytokines. In addition, BA-regulated TGR5 and S1PR2 pathways were dysregulated in a tissue-specific manner based on differential dietary feeding and the development of skin lesion. Our data suggest that activated TGR5 and S1PR2 in the skin are implicated in WD-exacerbated dermatitis.
MATERIALS AND METHODS
Mice
Specific pathogen-free C57BL/6 wild type mice (Jackson Laboratory, Sacramento, CA, USA) were housed in steel microisolator cages at 22°C with a 12-hour light/dark cycle. Mice were given a CD that contained 5.2% fat, 12% sucrose, and 0.01% cholesterol or a WD that contained 21.2% fat, 34% sucrose, and 0.2% cholesterol (w/w, Envigo, Indianapolis, IN, USA) after weaning. Another WD used has high fat as well as sugar content (29% fat, 34% sucrose, 1.25% cholesterol, 23.1 g glucose and 18.9 g fructose per liter of drinking water). For cholestyramine treatment, mice were supplemented with 2% cholestyramine diet for 3 months. All the studied mice were euthanized when they were 10 months old. Experiments were conducted in accordance with the National Institutes of Health Guidelines and approved protocols by the Institutional Animal Care and Use Committee of the University of California, Davis.
Skin histopathology
Hematoxylin and eosin (H&E) stained unaffected and lesional dorsal skin sections from formalin-fixed paraffin-embedded tissue were reviewed by a board certified dermatopathologist and were measured using Olympus Cellsens imaging software V1.18.
Gene expression profiling
RNA was isolated from dorsal skin and abdominal white adipose tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) followed by reverse transcription into cDNA. qRT-PCR was performed on an ABI 7900HT Fast real-time PCR system using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The mRNA levels were normalized to the level of Gapdh mRNA. Primers sequences are available in Supplementary Table S1.
Protein extraction and Western blot
Dorsal skin protein (40 μg) was subjected to polyacrylamide gel electrophoresis under reducing conditions followed by transferring to polyvinylidene difluoride membranes. The membranes were incubated with 5% nonfat milk followed by an antibody. The following primary antibodies were used: TGR5 (Lifespan Biosciences, WA, USA), IL-6 and TNFα (Bioss Antibodies, MA, USA), IL-17A and IL-4 (eBiosciences, San Diego, CA, USA), and β-ACTIN (Sigma Chemical Co, St Louis, MO, USA). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies. The signals were detected using an ECL-enhanced chemiluminescence system with Pierce SuperSignal West Pico chemiluminescent substrates (Thermo Fisher Scientific, Rockford, IL, USA).
Quantification of bile acids
Hepatic and cutaneous BAs were quantified based on published methods [23, 24]. The detection of BAs was carried out on a Prominence™ UFLC system (Shimadzu, Kyoto, Japan) coupled to an API 4000 QTRAP™ mass spectrometer (AB Sciex, Redwood City, CA, USA) operated in the negative ionization mode. Chromatography was performed on a Kinetex C18 column (50 mm X 2.1 mm, 2.6 μm) maintained at 40°C preceded by a high pressure column prefilter. The mobile phase consisted of a gradient of methanol delivered at a flow rate of 0.4 ml/min. Mass Spectrometer parameters were described in our previous publication [25].
Statistical analysis
Data are expressed as mean ± SD. All comparisons were calculated by two-tailed Student’s t test or one-way ANOVA followed by Tukey’s test using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA). The p-values are adjusted for multiple comparisons using false discovery rate, p < 0.05 was considered statistically significant.
RESULTS
Incidence of WD-exacerbated dermatitis
At 10 months of age, CD-fed C57BL/6 mice spontaneously developed dermatitis. The incidence of dermatitis in CD-fed male and female mice was 5% and 28%, respectively, indicating increased dermatitis susceptibility in female mice. Long-term intake of WD increased dermatitis incidence in both sexes. Dermatitis incidence in WD-fed male and female mice was 28% and 39%, respectively (Figure 1A, n = 80 mice per group). Thus, female mice consistently had higher incidence of dermatitis than males, and the remainders of the experiments were done using female mice.
Figure 1.
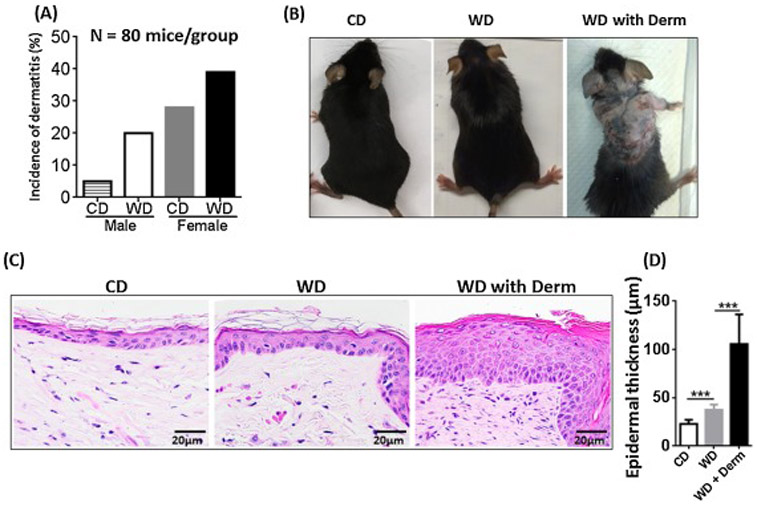
The incidence of dermatitis lesions in CD- and WD-fed male and female mice, n = 80 mice per group (A). The morphology (B), histopathology (C), and epidermal skin thickness (C) of CD- and WD-fed female mice with and without dermatitis. Original magnification bar (20 μm). *** p<0.001.
Morphologic and histologic appearance of WD-exacerbated dermatitis
At all of the frequencies noted (Figure 1A), CD-fed mice and a larger fraction of WD-fed mice developed patches of alopecia and ultimately, dermatitis (Figure 1B). Certain WD-fed female mice developed increased scratching behavior confined to certain anatomical sites, mainly the cervicodorsal head and neck region as well as the frontosternal chest region. Histologically, lesional skin biopsies taken from the backs of WD-fed mice tended to show acanthosis with hypergranulosis (as opposed to hypogranulosis which is classic for psoriasis) [26] and overlying compact orthokeratosis and parakeratosis. Sections also showed moderate spongiosis, which has been reported as an atypical feature in clinically confirmed human psoriasis [26], but is typically a hallmark of atopic dermatitis and contact dermatitis. Foci of prominent neutrophils as well as sparse eosinophils in the upper dermis were present in many histologic sections with overlying ulceration and hemorrhagic crust. Overall, mild-to-moderate neutrophil (vs. lymphocyte) predominant leukocytic infiltration was found in lesional areas away from erosions. Within erosions, dense neutrophil infiltration was also observed. Foci of non-specific neutrophilic granulomatous inflammation associated with follicular rupture were seen in both lesional and non-lesional skin of WD-fed mice (Figure 1C). Sections from lesional skin showed extensive dermal fibrosis with abundant fibroblasts, reduction of density of hair follicles, mild miniaturization of hair follicles, and eccentric follicular epithelial atrophy features found in some forms of human alopecia. Moreover, WD-fed mice had increased dorsal skin epidermal thickness in the non-lesional skin, which was further increased in lesional skin of WD-fed mice with dermatitis (Figure 1D).
Effect of diet and dermatitis development on inflammatory signaling in cutaneous and adipose tissues
Because of the marked epidermal hyperplasia in WD-exacerbated dermatitis that was reminiscent of psoriasis-like inflammation, we studied Th17 cytokine expression patterns that are known to be elevated in human psoriasis and a number of murine models of psoriasis [27-29]. WD-induced both protein and mRNA level of IL-17A in non-lesional skin, which was further increased in lesional skin of affected WD-fed mice (Figure 2A, B). In addition, the expression of ∥-23, which induces Th17 cell differentiation and promotes chronic inflammation [30], was increased in dermatitis lesions (Figure 2A). Protein and mRNA levels of IL-6 and TNFα were increased due to WD intake and elevated in lesional skin. The expression of ∥-1β, Nlrp3 (inflammasome), Ccl17, and Cc/20, which mediate inflammatory cell migration, were also elevated in WD-exacerbated dermatitis lesions (Figure 2A). As noted previously, we also observed moderate spongiosis in lesional skin (Supplemental Figure S2), a feature that is more characteristic in allergic Th2-driven skin disease such as atopic dermatitis. Thus, we also analyzed lesional skin for expression of Th2 markers. Interestingly, lesional skin from WD-fed mice showed elevated IL-4 protein and increased mRNA expression of Th2 associated cytokines and itch mediators including ∥-4, ∥-4r and ∥-31 (Figure 2A, B) [31]. However, IMQ-treated mice showed unchanged or reduced Th2 associated cytokine ∥-4, ∥-4r, ∥-31, ∥-31r at mRNA level. However, IMQ-treatment increased ∥-17a and ∥-6 mRNA expression significantly (Supplemental figure S2). Thus, the expression pattern of cytokines in WD-exacerbated dermatitis differed from Th17-dominant cytokine pattern in IMQ-induced dermatitis, suggesting that lesional skin of WD-fed mice had both Th2 and Th17 cytokine expression patterns.
Figure 2.
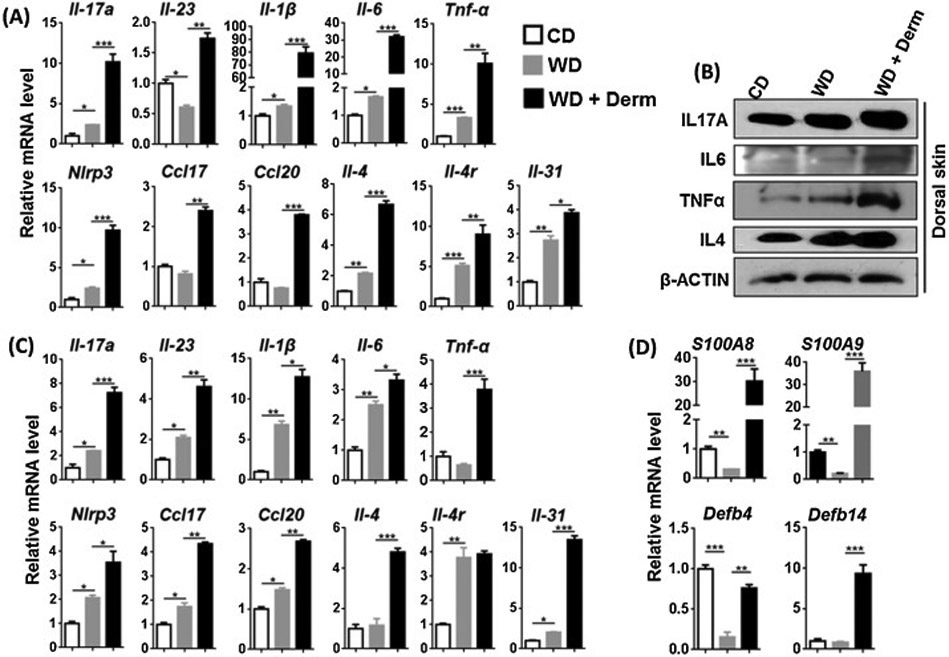
The effect of diet and dermatitis development in inflammatory signaling in CD- and WD-fed female mice with or without dermatitis. The mRNA and protein level was studied in cutaneous (A, B), mRNA level of white visceral adipose tissues (C) and mRNA level of antimicrobial peptide from cutaneous tissue (D) obtained from CD-and WD-fed with or without dermatitis lesions. Data expressed as mean ± SD. n > 6 per group. *p<0.05, **p<0.01, and *** p<0.001.
A similar expression pattern was noted in visceral adipose tissues. The mRNA expression level of almost all studied genes was induced in WD and further increased due to dermatitis development except for Tnf-α, ∥-4, and ∥-4r (Figure 2C). Taken together, WD increased inflammatory signaling in both skin and visceral adipose tissues, with the highest inflammatory activity was found in mice that developed dermatitis.
S100A8 and S100A9 (calprotectin) belong to the damage associated molecular pattern molecules and their abundance in myeloid cells has been attributed to their role in inflammatory cells [32]. Lesional skin of WD-fed mice had dramatically increased expression of S100A8 and S100A9 mRNA (Figure 2D). However, their expression levels were markedly increased in lesional skin when mice developed dermatitis (Figure 2D). In addition, antimicrobial peptides Defb4 (β -Defensin 3) and Defb14 (β -Defensin 3) were reduced or unchanged in WD-fed non-lesional skin of WD-fed mice but, increased in lesional skin (Figure 2D).
Effect of diet and dermatitis development on TGR5 signaling
To monitor TGR5 activity, we studied the expression of the Tgr5 gene as well as its downstream target. Activation of TGR5 induces type II iodothyronine deiodinase (Dio2) that generates thyroxine, a key player in basal metabolism [33]. In addition, BA-activated TGR5 stimulates nitric oxide production by inducing Nos3 in vascular endothelial cells [34]. TGR5 activation also induces Trpa1 and Trpv1 to cause itch in mice [35]. Cutaneous expression of TGR5 protein and mRNA level and its downstream target Dio2, and Nos3 at mRNA level was consistently reduced by WD intake, but induced in dermatitis lesions (Figure 3A, B). In addition, cutaneous expression of Trpa1 and Trpv1 mRNA was induced or unchanged in WD, but consistently increased in dermatitis lesions (Figure 3A).
Figure 3.
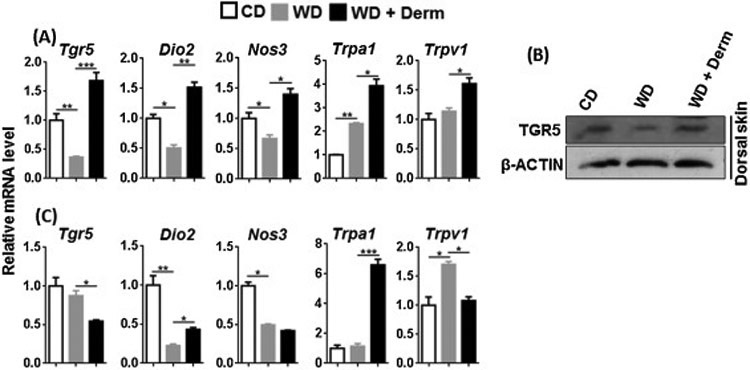
The effect of diet and dermatitis development in TGR5 signaling. The mRNA and protein level was studied in cutaneous (A, B) and visceral adipose tissues (C) obtained from CD- and WD-fed with or without dermatitis lesions. Data expressed as mean ± SD. n > 6 per group. *p<0.05, **p<0.01, and *** p<0.001.
In contrast to cutaneous findings, TGR5 signaling appeared to be reduced in white visceral adipose tissue. Expression levels of Tgr5, Dio2, Nos3, and Trpv1 in mice with dermatitis were lower than that of CD and/or WD-fed mice (Figure 4C). Only Trpa1 mRNA was elevated in the visceral adipose tissue of dermatitis mice (Figure 4C). Overall, TGR5-regulated signaling appears specifically up-regulated in in lesional skin of WD-fed mice.
Effect of diet and dermatitis development on S1PR2 signaling
We studied another BA receptor S1PR2, which regulates lipid and sterol metabolism in the liver and also modulates inflammatory skin disease [36]. To monitor S1PR2 signaling, we assessed the expression of S1pr2, sphingosine kinase isoenzymes (Sphk1, Sphk2), S1P phosphatase 1 and 2 (Sgpp1, Sgpp2), S1P binding protein, i.e., prohibitin 2 (Phb2), as well as S1P transporter (Spns2) in the cutaneous and adipose tissues. Whereas WD intake reduced mRNA expression of most of these genes, we observed elevated expression of these genes in the lesional skin of mice with dermatitis (Figure 4A). However, this expression pattern was not noted in visceral adipose tissue (Figure 4B). Thus, the alteration in S1PR2 signaling was specific to lesional dermatitis skin.
Figure 4.
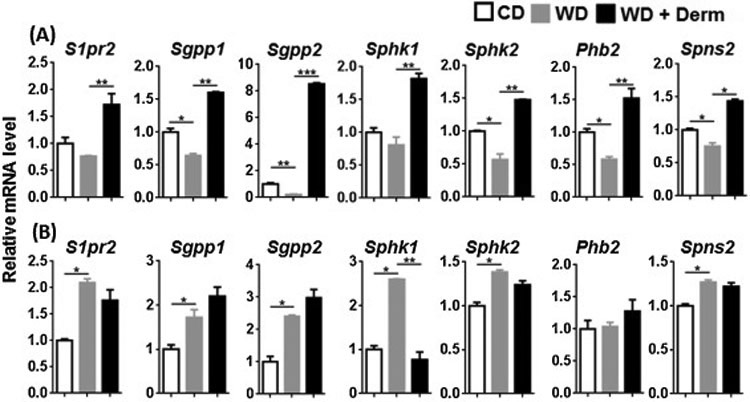
The effect of diet and dermatitis development in S1PR2 signaling. The mRNA level was studied in cutaneous (A) and visceral adipose tissues (B) obtained from CD- and WD-fed with or without dermatitis lesions. Data expressed as mean ± SD. n > 6 per group. *p<0.05, **p<0.01, and *** p<0.001.
Effect of diet and dermatitis development on BA profile
Because dietary intake is known to affect BA profile, we next quantified BA levels in the skin and liver by liquid chromatography–mass spectrometry. WD intake did not change total BA in non-lesional skin. However, dermatitis lesions had increased total BAs. In addition, WD increased free α-and β-muricholic acid (α- and β-MCA), hydrophobic secondary BA deoxycholic acid (DCA), and taurine-conjugated lithocholic acid (TLCA) (Figure 5A). In addition, β-MCA, DCA, chenodeoxycholic acid (CDCA), taurine-conjugated DCA (TDCA), and taurine-conjugated α, β-MCA (T-α, β-MCA) were higher in dermatitis lesions compared to non-lesional skin of WD-fed mice (Figure 5A).
Figure 5.
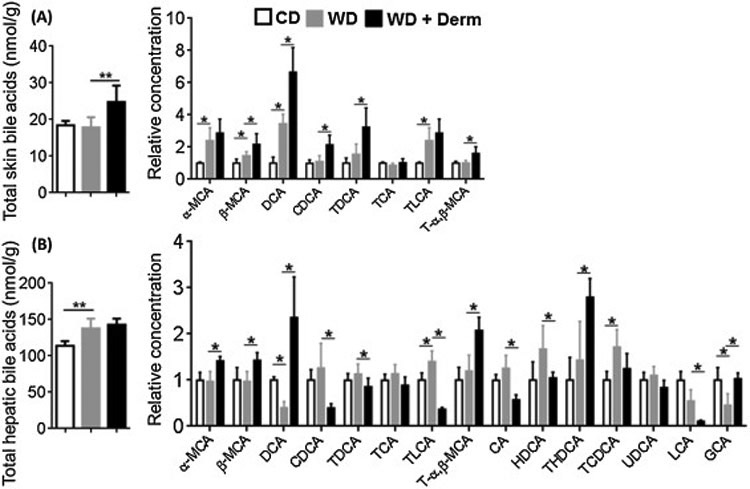
The effect of diet and dermatitis development in shifting BA profile. Cutaneous (A) and hepatic (B) total and individual BA profile of CD- and WD-fed female mice with or without dermatitis lesions. Data expressed as mean ± SD. n > 6 per group. *p<0.05, **p<0.01.
Hepatic total BAs were increased due to WD consumption, but did not further increase with dermatitis development (Figure 6). More individual BAs were detectable in the liver than skin. WD intake had no effect on altering α, β-MCA and T-α, β-MCA, but dermatitis development increased those BAs (Figure 5B). Other apparent changes due to dermatitis development were reductions in hepatic CDCA, CA, and hyodeoxycholic acid (HDCA) as well as LCA and TLCA. However, mice with dermatitis had increased taurine-conjugated-hyodeoxycholic acid (THDCA) and glycine-conjugated CA (GCA) (Figure 5B).
Figure 6.
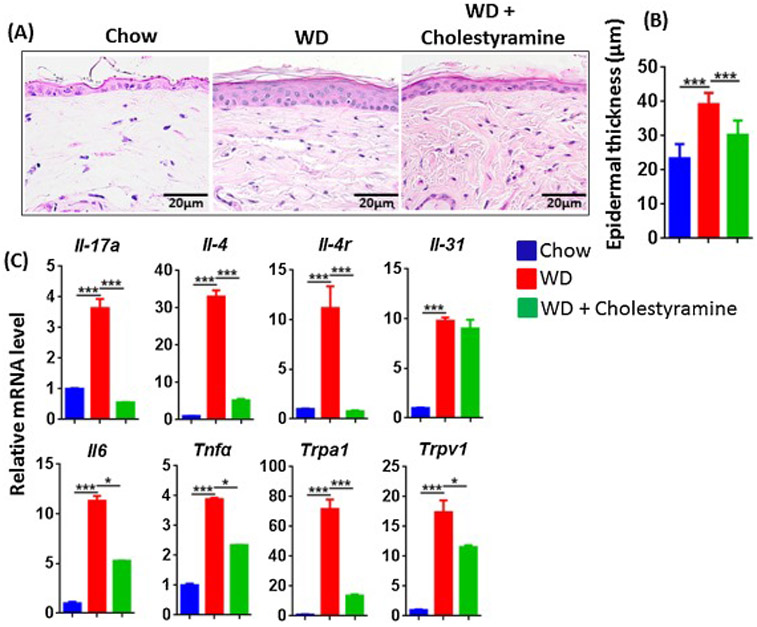
The effect of BA sequestration cholestyramine in WD-fed mice. Histopathology (A), epidermal skin thickness (B), and mRNA level studied in the cutaneous tissue (C) of CD, WD-fed mice with or without cholestyramine. Original magnification bar (20 μm). Data expressed as mean ± SD. n > 6 per group. *p<0.05 and *** p<0.001.
Effect of cholestyramine in WD-fed mice
Cholestyramine is a BA sequestrant that reduces serum cholesterol and improves cholestatic pruritus associated with liver disease [37]. To determine if BAs were critical for the increased inflammatory genes following long term WD, we supplemented WD-fed mice with 2% cholestyramine. We observed that mice without dermatitis following WD-feeding for 3 months had mild epidermal hyperplasia as well as obvious elevations of mRNA levels of Th17, Th2 cytokines and other inflammatory markers (Figure 6A-C). Strikingly, cholestyramine-supplemented WD-fed mice had reduced epidermal hyperplasia and generally reduced expression of ∥-17a, ∥-4 and other inflammatory markers, except ∥-31, compared to mice on WD alone (Figure A-C).
DISCUSSION
C57BL/6 mice are known to develop patches of poorly characterized dermatitis with age [38]. Even with a control healthy diet, female mice had a higher incidence of dermatitis, but both male and female mice showed higher incidence of dermatitis on a WD, which has moderate amount of fat and high sugar. Similarly, in a mouse model of atopic dermatitis, female mice by a 4:1 gender ratio had increased scratching behavior and skin disease [39]. Moreover, females in general have higher incidence of autoimmune disease in humans [40]. We also reported that WD-fed mice had increased susceptibility to IMQ-induced psoriasiform dermatitis whereas mice on high fat diet alone did not [6] suggesting that obesity alone may be insufficient to increase the propensity of mice to cutaneous inflammation. Histologically, the dermatitis we observed in WD-fed mice had epidermal hyperplasia with hypergranulosis and spongiosis typical of atopic dermatitis but also displayed intraepidermal neutrophils that are expected in classic psoriatic lesions in humans [26]. Thus, the collection of histopathologic changes seen in lesional skin showed overlapping features with psoriasis and atopic dermatitis.
In analyzing the cytokine expression pattern in lesional skin, we showed that both Th2 and Th17 cytokines were up-regulated in lesional skin, consistent with the overlapping histopathologic features. The presence of Th2 cytokines may help explain the observation of spongiosis in affected skin since patient s with atopic dermatitis (a Th2-driven disease in the acute phase) usually show epidermal spongiosis. Th17 cytokines are known to be pathogenic in human psoriasis. While epidermal hyperplasia, intraepidermal neutrophils, and protypical Th17 cytokines such as IL-17A were found in the lesional skin of WD-fed mice, there was also evidence of increased Th2 cytokines such as ∥-4 and ∥-31 at mRNA level in WD-exacerbated dermatitis in mice. Together, these results suggest that activation of both Th17 and Th2 pathways may contribute to the pathogenesis of dermatitis development in WD-fed mice. Previous studies showed that high fat diets exacerbated IMQ responses [4]. We hypothesize that IMQ drives skin that is “primed” for inflammation following chronic feeding with the WD toward a Th17 phenotype. The direction of cutaneous immune response may very well be influenced by topical application of IMQ whereas without further exogenous stimulation, the dermatitis that develops in WD-fed mice may have both Th2 and Th17 components. One limitation of our studies is that we have not tested the intriguing hypothesis that WD-preconditioned skin may drive toward either exacerbated Th2 or Th17 responses depending on specific external triggers. This hypothesis will be the topic of future studies.
Because of the known effects of a WD on BA metabolism, we investigated the expression and downstream signaling of two major BA receptors: TGR5 and S1PR2. Sphingosine-1-phosphate (S1P) produced in allergically stimulated mast cells mediates degranulation, potent bioactive sphingolipid and involved in a variety of cellular processes, including cell proliferation, differentiation, motility, angiogenesis, inflammation, malignant transformation and immune response [15, 41]. S1PR2 is a receptor for S1P as well as conjugated BAs [15]. Our data suggest that WD specifically activated TGR5 signaling in dermatitic lesions. Activated TGR5 in the dermatitis mice increased Nos3 expression, which likely leads to increased production of nitric oxide, a potent regulator for the growth and differentiation of keratinocytes and is implicated in the pathogenesis of and risk for developing psoriasis [42, 43]. TGR5 activation in dermatitic lesions was also demonstrated by the induction of Trpa1 and Trpv1, which are the downstream mediators of many pruritogenic pathways. Because it is thought that activation of TGR5 has an anti-inflammatory effect [44], we believe that the increased inflammation is mainly due to activation of S1PR2 in dermatitis lesions whereas TGR5 may be activating the itching behavior in the affected mice.
Our data showed that total BA in the skin was increased in mice with dermatitis with β-MCA and DCA being consistently increased in both the liver and skin of these mice. Our published data suggested that increased production of these two BAs is implicated in the development of nonalcoholic steatohepatitis and WD-fed mice dysregulated BA synthesis which promote systemic inflammation involving the brain, liver, ileum, and adipose tissue [24, 45, 46]. LCA, DCA, CDCA, CA, and TLCA are endogenous agonists for TGR5 [47], whereas conjugated BAs such as GCA, TCA, GDCA, TDCA, and TUDCA can activate S1PR2 [15]. It is apparent that there is an association between mice with dermatitis and dysregulated BAs in the skin and we speculate that other factors, such as skin or gut microbiome, help to determine which mice go on to develop dermatitis lesion .
Lastly, we supplemented the WD with 2% cholestyramine and observed that the increase in epidermal thickness and, ∥-4 and ∥-17a mRNA expression were largely ameliorated; suggesting BAs may have a critical role in inducing the moderate increases in Th2 and Th17 cytokines in the skin following long term WD feeding. Skinner et al. reported rapid improvement of psoriasis after oral administration of cholestyramine resin in five patients with psoriasis [11]. Given these findings, larger clinical studies should be conducted to assess the efficacy of cholestyramine in treatment of psoriasis, particularly in patients who regularly eat a WD.
Taken together, increased incidence of dermatitis is accompanied by the induction of skin TGR5 and S1PR2 signaling. TGR5 and S1PR2 have differential roles in regulating inflammation, pruritus, and metabolism, which are implicated in the pathogenesis of dermatitis. Thus, targeting those signaling pathways may potentially provide options for treating and preventing the progression of dermatitis. Reducing BAs using agents such as cholestyramine may be a novel strategy for treating patients with psoriasis without the need to use potent immunosuppressive medication.
Supplementary Material
Highlights.
Western diet (WD) facilitated the development of dermatitis in C57BL/6 mice.
WD-exacerbated dermatitis is sex-dependent with higher incidence in female mice.
Reduced TGR5 and S1PR2 signaling was found in the dermis of WD-fed mice.
WD-induced lesional skin has activated TGR5 and S1PR2 signaling.
Cholestyramine supplementation reduced dermatitis in WD-fed mice.
ACKNOWLEDGEMENTS:
The authors thank Michelle Nguyen for her contributions in preparing this manuscript.
FUNDING: This study is supported by grants funded by National Institutes of Health CA179582 and CA222490 to YJW and a National Psoriasis Foundation Discovery Grant to STH and YJW.
Funding Source: This study is supported by grants funded by National Institutes of Health CA179582 and CA222490 to YJW and a National Psoriasis Foundation Discovery Grant to STH and YJW.
Footnotes
CONFLICT OF INTEREST: The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Kwa MC, Silverberg JI, Association Between Inflammatory Skin Disease and Cardiovascular and Cerebrovascular Co-Morbidities in US Adults: Analysis of Nationwide Inpatient Sample Data, American journal of clinical dermatology 18(6) (2017) 813–823. [DOI] [PubMed] [Google Scholar]
- [2].Carlstrom M, Ekman AK, Petersson S, Soderkvist P, Enerback C, Genetic support for the role of the NLRP3 inflammasome in psoriasis susceptibility, Experimental dermatology 21(12) (2012) 932–7. [DOI] [PubMed] [Google Scholar]
- [3].Blüher M, Obesity: global epidemiology and pathogenesis, Nature Reviews Endocrinology (2019). [DOI] [PubMed] [Google Scholar]
- [4].Nakamizo S, Honda T, Adachi A, Nagatake T, Kunisawa J, Kitoh A, Otsuka A, Dainichi T, Nomura T, Ginhoux F, Ikuta K, Egawa G, Kabashima K, High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing gammadelta T cells, Scientific reports 7(1) (2017) 14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vasseur P, Serres L, Jegou JF, Pohin M, Delwail A, Petit-Paris I, Levillain P, Favot L, Samson M, Yssel H, Morel F, Silvain C, Lecron JC, High-Fat Diet-Induced IL-17A Exacerbates Psoriasiform Dermatitis in a Mouse Model of Steatohepatitis, Am J Pathol 186(9) (2016) 2292–301. [DOI] [PubMed] [Google Scholar]
- [6].Yu S, Wu X, Zhou Y, Sheng L, Jena PK, Han D, Wan Y-JY, Hwang ST, A Western diet, but not high fat and low sugar diet, predisposes mice to enhanced susceptibility to imiquimod-induced psoriasiform dermatitis, Journal of Investigative Dermatology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ali Z, Suppli Ulrik C, Agner T, Thomsen SF, Is atopic dermatitis associated with obesity? A systematic review of observational studies, Journal of the European Academy of Dermatology and Venereology : JEADV (2018). [DOI] [PubMed] [Google Scholar]
- [8].Chiang JYL, Bile acid metabolism and signaling in liver disease and therapy, Liver Research 1(1) (2017) 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chiang JY, Bile acid metabolism and signaling, Comprehensive Physiology 3(3) (2013) 1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herndon JH Jr, Pathophysiology of pruritus associated with elevated bile acid levels in serum, Archives of Internal Medicine 130(4) (1972) 632–637. [PubMed] [Google Scholar]
- [11].Skinner RB Jr, Rosenberg EW, Belew PW, Marley WM, Improvement of Psoriasis With Cholestyramine, Archives of Dermatology 118(3) (1982) 144–144. [PubMed] [Google Scholar]
- [12].Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, Xu W, Liu X, Bohdan P, Zhang L, Zhou H, Hylemon PB, Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes, Hepatology (Baltimore, Md.) 55(1) (2012) 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K, TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis, Cell Metabolism 10(3) (2009) 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang H, Chen J, Hollister K, Sowers LC, Forman BM, Endogenous bile acids are ligands for the nuclear receptor FXR/BAR, Molecular cell 3(5) (1999) 543–53. [DOI] [PubMed] [Google Scholar]
- [15].Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, Hylemon PB, Zhou H, Takabe K, Wakai T, The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases, Journal of lipid research 57(9) (2016) 1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K, TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading, Cell Metab 14(6) (2011) 747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU, The TGR5 receptor mediates bile acid-induced itch and analgesia, The Journal of clinical investigation 123(4) (2013) 1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K, Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential, Mediators of inflammation 2016 (2016) 8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang G, Yang L, Kim GS, Ryan K, Lu S, O'Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T, Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation, Blood 122(3) (2013) 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J, The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis, Immunity 26(3) (2007) 287–97. [DOI] [PubMed] [Google Scholar]
- [21].Nguyen M, Pace AJ, Koller BH, Age-Induced Reprogramming of Mast Cell Degranulation, 175(9) (2005) 5701–5707. [DOI] [PubMed] [Google Scholar]
- [22].Rivera J, Proia RL, Olivera A, The alliance of sphingosine-1-phosphate and its receptors in immunity, Nature reviews. Immunology 8(10) (2008) 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jena PK, Sheng L, Lucente JD, L.W J, Maezawa I, Wan YJ, Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet–induced systemic inflammation, microglial activation, and reduced neuroplasticity, The FASEB Journal 32(5) (2018) 2866–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jena PK, Sheng L, Liu H-X, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, Krishnan VV, Mills DA, Wan YJ, Western Diet–Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment, The American Journal of Pathology 187(8) (2017) 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu HX, Rocha CS, Dandekar S, Wan YJ, Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration, Journal of hepatology 64(3) (2016) 641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chau T, Parsi KK, Ogawa T, Kiuru M, Konia T, Li CS, Fung MA, Psoriasis or not? Review of 51 clinically confirmed cases reveals an expanded histopathologic spectrum of psoriasis, Journal of cutaneous pathology 44(12) (2017) 1018–1026. [DOI] [PubMed] [Google Scholar]
- [27].Dainichi T, Hanakawa S, Kabashima K, Classification of inflammatory skin diseases: a proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity, Journal of dermatological science 76(2) (2014) 81–9. [DOI] [PubMed] [Google Scholar]
- [28].Tan Q, Yang H, Liu EM, Wang H, Establishing a Role for Interleukin-17 in Atopic Dermatitis-Related Skin Inflammation, Journal of cutaneous medicine and surgery 21(4) (2017) 308–315. [DOI] [PubMed] [Google Scholar]
- [29].Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y, Possible pathogenic role of Th17 cells for atopic dermatitis, The Journal of investigative dermatology 128(11) (2008) 2625–30. [DOI] [PubMed] [Google Scholar]
- [30].McKenzie BS, Kastelein RA, Cua DJ, Understanding the IL-23-IL-17 immune pathway, Trends in immunology 27(1) (2006) 17–23. [DOI] [PubMed] [Google Scholar]
- [31].Guttman-Yassky E, Krueger JG, Lebwohl MG, Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment, Experimental dermatology 27(4) (2018) 409–417. [DOI] [PubMed] [Google Scholar]
- [32].Kerkhoff C, Voss A, Scholzen TE, Averill MM, Zanker KS, Bornfeldt KE, Novel insights into the role of S100A8/A9 in skin biology, Experimental dermatology 21(11) (2012) 822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo C, Chen WD, Wang YD, TGR5, Not Only a Metabolic Regulator, Frontiers in physiology 7 (2016) 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T, Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells, Arteriosclerosis, thrombosis, and vascular biology 33(7) (2013) 1663–9. [DOI] [PubMed] [Google Scholar]
- [35].Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU, Bunnett NW, The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice, Gastroenterology 147(6) (2014) 1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thieme M, Zillikens D, Sadik CD, Sphingosine-1-phosphate modulators in inflammatory skin diseases - lining up for clinical translation, Experimental dermatology 26(3) (2017) 206–210. [DOI] [PubMed] [Google Scholar]
- [37].Datta DV, Sherlock S, Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis, Gastroenterology 50(3) (1966) 323–32. [PubMed] [Google Scholar]
- [38].Hampton AL, Hish GA, Aslam MN, Rothman ED, Bergin IL, Patterson KA, Naik M, Paruchuri T, Varani J, Rush HG, Progression of ulcerative dermatitis lesions in C57BL/6Crl mice and the development of a scoring system for dermatitis lesions, Journal of the American Association for Laboratory Animal Science : JAALAS 51(5) (2012) 586–93. [PMC free article] [PubMed] [Google Scholar]
- [39].Gutermuth J, Ollert M, Ring J, Behrendt H, Jakob T, Mouse models of atopic eczema critically evaluated, International archives of allergy and immunology 135(3) (2004) 262–76. [DOI] [PubMed] [Google Scholar]
- [40].Ngo ST, Steyn FJ, McCombe PA, Gender differences in autoimmune disease, Frontiers in neuroendocrinology 35(3) (2014) 347–69. [DOI] [PubMed] [Google Scholar]
- [41].Maceyka M, Harikumar KB, Milstien S, Spiegel S, Sphingosine-1-phosphate signaling and its role in disease, Trends in cell biology 22(1) (2012) 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhan R, Yang S, He W, Wang F, Tan J, Zhou J, Yang S, Yao Z, Wu J, Luo G, Nitric oxide enhances keratinocyte cell migration by regulating Rho GTPase via cGMP-PKG signaling, PloS one 10(3) (2015) e0121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coto-Segura P, Coto E, Mas-Vidal A, Morales B, Alvarez V, Diaz M, Alonso B, Santos-Juanes J, Influence of endothelial nitric oxide synthase polymorphisms in psoriasis risk, Archives of dermatological research 303(6) (2011) 445–9. [DOI] [PubMed] [Google Scholar]
- [44].Duboc H, Tache Y, Hofmann AF, The bile acid TGR5 membrane receptor: from basic research to clinical application, Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 46(4) (2014) 302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sheng L, Jena PK, Hu Y, Liu H-X, Nagar N, Kalanetra KM, French SW, French SW, Mills DA, Wan YJ, Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation, The Journa of Pathology 243(4) (2017) 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jena PK, Sheng L, Di Lucente J, Jin LW, Maezawa I, Wan YY, Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32(5) (2018) 2866–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M, A G protein-coupled receptor responsive to bile acids, J Biol Chem 278(11) (2003) 9435–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


