Key Points
Question
What is the available evidence for the efficacy of vitamin D with or without calcium supplementation for reducing the risk of fracture?
Findings
This systematic review and meta-analysis of randomized clinical trials of vitamin D alone (11 randomized clinical trials with 34 243 participants) showed no significant association with risk of any fracture or of hip fracture. In contrast, daily supplementation with both vitamin D and calcium (6 randomized clinical trials with 49 282 participants) was associated with a 16% reduced risk of hip fracture.
Meaning
In this study, neither intermittent nor daily dosing with standard doses of vitamin D alone was associated with reduced risk of fracture, but daily treatment with both vitamin D and calcium was a more promising strategy.
This systematic review and meta-analysis assesses the risk of fracture associated with differences in concentrations of 25-hydroxyvitamin D (25[OH]D) in observational studies and the effects of supplementation with vitamin D alone or in combination with calcium on fracture in randomized clinical trials.
Abstract
Importance
Vitamin D and calcium supplements are recommended for the prevention of fracture, but previous randomized clinical trials (RCTs) have reported conflicting results, with uncertainty about optimal doses and regimens for supplementation and their overall effectiveness.
Objective
To assess the risks of fracture associated with differences in concentrations of 25-hydroxyvitamin D (25[OH]D) in observational studies and the risks of fracture associated with supplementation with vitamin D alone or in combination with calcium in RCTs.
Data Sources
PubMed, EMBASE, Cochrane Library, and other RCT databases were searched from database inception until December 31, 2018. Searches were performed between July 2018 and December 2018.
Study Selection
Observational studies involving at least 200 fracture cases and RCTs enrolling at least 500 participants and reporting at least 10 incident fractures were included. Randomized clinical trials compared vitamin D or vitamin D and calcium with control.
Data Extraction and Synthesis
Two researchers independently extracted data according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and assessed possible bias. Rate ratios (RRs) were estimated using fixed-effects meta-analysis. Data extraction and synthesis took place between July 2018 and June 2019.
Main Outcomes and Measures
Any fracture and hip fracture.
Results
In a meta-analysis of 11 observational studies (39 141 participants, 6278 fractures, 2367 hip fractures), each increase of 10.0 ng/mL (ie, 25 nmol/L) in 25 (OH)D concentration was associated with an adjusted RR for any fracture of 0.93 (95% CI, 0.89-0.96) and an adjusted RR for hip fracture of 0.80 (95% CI, 0.75-0.86). A meta-analysis of 11 RCTs (34 243 participants, 2843 fractures, 740 hip fractures) of vitamin D supplementation alone (daily or intermittent dose of 400-30 000 IU, yielding a median difference in 25[OH]D concentration of 8.4 ng/mL) did not find a reduced risk of any fracture (RR, 1.06; 95% CI, 0.98-1.14) or hip fracture (RR, 1.14; 95% CI, 0.98-1.32), but these trials were constrained by infrequent intermittent dosing, low daily doses of vitamin D, or an inadequate number of participants. In contrast, a meta-analysis of 6 RCTs (49 282 participants, 5449 fractures, 730 hip fractures) of combined supplementation with vitamin D (daily doses of 400-800 IU, yielding a median difference in 25[OH]D concentration of 9.2 ng/mL) and calcium (daily doses of 1000-1200 mg) found a 6% reduced risk of any fracture (RR, 0.94; 95% CI, 0.89-0.99) and a 16% reduced risk of hip fracture (RR, 0.84; 95% CI, 0.72-0.97).
Conclusions and Relevance
In this systematic review and meta-analysis, neither intermittent nor daily dosing with standard doses of vitamin D alone was associated with reduced risk of fracture, but daily supplementation with both vitamin D and calcium was a more promising strategy.
Introduction
Osteoporosis is characterized by reduced bone mass and fragmentation of bone architecture, resulting in an increased risk of fracture.1,2 Approximately 1 in 2 women and 1 in 5 men aged 50 years or older will experience an osteoporotic fracture in their remaining lifetime.1,2,3 Hip fracture is the most serious type of osteoporotic fracture, with an approximate 30% risk of death in the year following hip fracture.4 The incidence of hip fracture increases exponentially with age, particularly among women 60 years or older and men 70 years or older (eFigure 1 in the Supplement), highlighting the high absolute risks of hip fracture in extreme old age.5
Vitamin D is essential for optimal musculoskeletal health because it promotes calcium absorption, mineralization of osteoid tissue formation in bone, and maintenance of muscle function.6 Low vitamin D status causes secondary hyperparathyroidism, bone loss, and muscle weakness.6,7,8,9 Observational studies6,10 have reported that lower blood concentrations of 25-hydroxyvitamin D (25[OH]D) are associated with higher risks of falls and fractures. A mendelian randomization study11 reported no beneficial effects of vitamin D on fracture, but the study had a weak instrument bias and evaluation in populations with a low overall risk of fracture.
Combined supplementation with 800 IU of vitamin D per day and 1200 mg of calcium per day has been recommended for prevention of fractures in older adults living in institutions and in those with low vitamin D status.8,12,13 However, previous randomized clinical trials (RCTs) and meta-analyses of vitamin D alone or in combination with calcium for the prevention of fracture in either community-dwelling or general population settings reported conflicting results, with some reporting protective effects against fractures14,15,16 and others demonstrating no beneficial effects.17,18,19 However, most previous RCTs had only limited power to detect differences in risk of fracture projected by the observational studies, largely because of a combination of small sample size, low daily doses of vitamin D, intermittent dosing regimens (ie, >1 month), and short duration of treatment. In addition, interpretation of the results of previous meta-analyses of such RCTs was complicated by the use of variable inclusion criteria, inappropriate statistical methods, inclusion of multiple small RCTs with very few fracture events, and a failure to report achieved differences in blood 25(OH)D concentrations.16,18,19
To summarize the available evidence and guide clinical practice, we conducted parallel meta-analyses of the following: (1) observational studies of risks of fracture associated with prolonged differences in blood concentrations of 25(OH)D; (2) RCTs of vitamin D alone vs placebo or no treatment for the prevention of fracture; and (3) RCTs of vitamin D plus calcium vs placebo or no treatment for the prevention of fracture. In addition, we reviewed the design of ongoing RCTs assessing the effects of higher doses of vitamin D alone or in combination with calcium for prevention of fracture.
Methods
This study was registered on PROSPERO (CRD42019126568). This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.20
Meta-analysis of the Observational Studies
We searched PubMed and EMBASE databases using terms for vitamin D, 25-hydroxyvitamin D, 25(OH)D, 25(OH) vitamin D, cholecalciferol, and fracture to identify published observational studies of 25(OH)D and risk of fracture in English language that were reported until December 31, 2019 (eTable 1 in the Supplement). We restricted studies to those that included at least 200 fracture events (to minimize random error); used a prospective, nested case-control, or case-cohort design; and reported blood 25(OH)D concentrations and risk estimates with 95% CIs for fracture (eAppendix 1 in the Supplement). The risk of bias was assessed by 2 of us (P.Y. and R.C.) using the Risk of Bias in Nonrandomized Studies of Interventions tool.21
For each study, 2 of us (P.Y. and R.C.) transformed category-specific risk estimates into estimates of rate ratios (RRs) associated with an increase of 10.0 ng/mL (ie, 25.0 nmol/L) in blood 25(OH)D concentration (to convert to nanomoles per liter, multiply by 2.496) using a previously reported method.22 Subsequently, we pooled the results of individual studies using inverse-variance weighted fixed-effects meta-analysis.
Meta-analysis of RCTs
Randomized clinical trials were identified by literature searches of the relevant English language reports published before January 1, 2019, through PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov, using search terms vitamin D, calcium, randomized trial, and fracture. Reference lists of studies included in previous systematic reviews were also reviewed to identify any additional RCTs (eTable 1 in the Supplement).
Randomized clinical trials of vitamin D alone were eligible for inclusion if they met the following criteria: (1) compared the effects of vitamin D supplementation with a placebo or no treatment, (2) reported at least 10 incident fractures, and (3) included at least 500 participants (to minimize random error and publication bias). Randomized clinical trials of calcium plus vitamin D were selected based on the following inclusion criteria: (1) compared calcium plus vitamin D supplements with a placebo or no treatment group, (2) reported at least 10 incident fractures, and (3) included at least 500 participants. Each RCT was assessed for bias by 2 of us (P.Y. and R.C.) using the Cochrane Collaboration risk of bias tool (eAppendix 2 in the Supplement).23
Any fracture was defined as a fracture that occurred at any site, but if an RCT only reported hip fracture events, these were also counted as any fracture. The study-specific RRs and 95% CIs were estimated using the Peto 1-step method.24,25,26 Data from individual RCTs were pooled using inverse variance-weighted fixed-effects meta-analysis. Heterogeneity was assessed using the I2 statistic, with I2 greater than 50% considered significant heterogeneity. Contour-enhanced funnel plots were constructed to assess publication bias.27 Prespecified subgroup analyses included age, residential status, geographic region, open-label RCT design, daily supplementation level, concurrent calcium supplementation, and mean treatment differences in blood 25(OH)D concentrations. R version 3.4.2 (R Project for Statistical Computing) was used for statistical analyses, and a 2-tailed P < .05 was considered statistically significant.
Results
Meta-analysis of the Observational Studies of 25(OH)D Concentration and Risk of Fracture
We identified 618 published reports of observational studies of blood 25(OH)D concentrations and risk of fracture (eFigure 2 in the Supplement). After an initial review of titles and abstracts, 59 studies were selected for detailed assessment, yielding 11 eligible observational studies, with 39 141 participants,6278 fractures, and 2367 hip fractures.28,29,30,31,32,33,34,35,36,37,38 Of these 11 studies, 5 (45%) were nested case-control or case-cohort studies (8052 participants with 3469 fracture events and 1575 hip fracture events),32,34,35,36,37 and 6 (55%) were prospective studies (31 089 participants with 2809 fracture events and 792 hip fracture events).28,29,30,31,33,38 Selected characteristics of these 11 observational studies are shown in the Table28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 and in eTable 2 in the Supplement. The sample size of individual studies varied from 800 to 14 624 individuals, and the mean (range) age was 68.6 (52.6-76.7) years. The weighted mean (range) blood 25(OH)D concentration was 23.7 (21.4-25.2) ng/mL, with the exception of 1 UK study37 involving much younger adults, who had a mean blood 25(OH)D concentration of 32.5 ng/mL. The results of quality assessments of individual studies are provided in eTable 3 in the Supplement. Among the 11 observational studies included in the meta-analysis, 7 (64%) had serious risk of bias, including selection bias (3 [27%]),28,31,32 missing data (2 [18%]),29,30 or incomplete measurement of outcomes (2 [18%]),36,37 but none had a critical risk of bias.
Table. Selected Characteristics of the Observational Studies and RCTs.
| Source | Design | Treatment | Participants, No. | Age, Mean, y | Follow-up, y | Any Fractures, No. | Hip Fractures, No. |
|---|---|---|---|---|---|---|---|
| Observational studies | |||||||
| Looker,28 2013 | Cohort | NA | 4749 | 73.6 | 7.0 | 525 | 287 |
| Buchebner et al,29 2014 | Cohort | NA | 1044 | 75.5 | 13.1 | 349 | NA |
| Barbour et al,30 2012 | Cohort | NA | 2614 | 74.7 | 6.4 | 247 | NA |
| Robinson-Cohen et al,31 2011 | Cohort | NA | 2294 | 73.9 | 13.0 | 244 | 244 |
| Holvik et al,32 2013 | Case-cohort | NA | 2613 | 73.1 | 10.7 | 1175 | 1175 |
| Steingrimsdottir et al,33 2014 | Cohort | NA | 5764 | 76.7 | 5.4 | 261 | 261 |
| Cauley et al,34 2011a | Nested case-control | NA | 2264 | 64.1 | 8.6 | 1132 | NA |
| Cauley et al,35 2008a | Nested case-control | NA | 800 | 71.0 | 7.1 | NA | 400 |
| Swanson et al,36 2015 | Case-cohort | NA | 1000 | 74.6 | 5.1 | 432 | NA |
| Roddam et al,37 2007 | Nested case-control | NA | 2175 | 52.6 | 5.0 | 730 | NA |
| Julian et al,38 2016 | Cohort | NA | 14 624 | 63.3 | 15.0 | 1183 | NA |
| Subtotalb | NA | NA | 39 141 | 68.6 | 10.4 | 6278 | 2367 |
| RCTs of vitamin D alone | |||||||
| Glendenning et al,39 2012 | RCT | 150 000 IU/3 mo | 686 | 76.7 | 0.8 | 20 | |
| Larsen et al,40 2018 | RCT | 20 000 IU/wk | 511 | 61.8 | 5.0 | 28 | |
| Law et al,41 2006 | RCT | 100 000 IU/3 mo | 3717 | 85.0 | 0.8 | 119 | 44 |
| Meyer et al,42 2002 | RCT | 400 IU/d | 1144 | 84.7 | 2.0 | 145 | 97 |
| Lips et al,43 1996 | RCT | 400 IU/d | 2578 | 80.0 | 3.5 | 257 | 106 |
| Trivedi et al,44 2003 | RCT | 100 000 IU/4 mo | 2686 | 74.8 | 5.0 | 268 | 45 |
| Sanders et al,45 2010 | RCT | 500 000 IU/y | 2258 | 76.1 | 4.0 | 306 | 34 |
| Khaw et al,46 2017c | RCT | 100 000 IU/mo | 5108 | 65.9 | 3.4 | 292 | |
| Grant et al,47 2005 | RCT | 800 IU/d | 2675 | 77.0 | 3.8 | 400 | 88 |
| Lyons et al,48 2007 | RCT | 100 000 IU/4 mo | 3440 | 84.0 | 3.0 | 423 | 216 |
| Smith et al,49 2007 | RCT | 300 000 IU/y | 9440 | 79.1 | 3.0 | 585 | 110 |
| Subtotalb | NA | Approximately 833 IU/d | 34 243 | 77.1 | 3.1 | 2843 | 740 |
| RCTs of calcium plus vitamin D | |||||||
| Chapuy et al,50 2002 | RCT | 800 IU/d vitamin D; 1200 mg/d calcium | 583 | 85.2 | 2.0 | 105 | 48 |
| Porthouse et al,51 2005 | RCT | 800 IU/d vitamin D; 1000 mg/d calcium | 3314 | 76.8 | 2.1 | 149 | 25 |
| Salovaara et al,52 2010 | RCT | 800 IU/d vitamin D; 1000 mg/d calcium | 3195 | 67.3 | 3.0 | 189 | 6 |
| Grant et al,47 2005 | RCT | 800 IU/d vitamin D; 1000 mg/d calcium | 2638 | 77.1 | 3.8 | 371 | 87 |
| Chapuy et al,53 1992 | RCT | 800 IU/d vitamin D; 1200 mg/d calcium | 3270 | 84.0 | 1.5 | 375 | 190 |
| Jackson et al,54 2006 | RCT | 400 IU/d vitamin D; 1000 mg/d calcium | 36 282 | 62.4 | 7.0 | 4260 | 374 |
| Subtotalb | NA | 800 IU/d vitamin D; 1000 mg/d calcium | 49 282 | 66.2 | 5.9 | 5449 | 730 |
Abbreviations: NA, not applicable; RCT, randomized clinical trial.
Cauley et al (2008)35 and Cauley et al (2011)34 were based on 1 study, and hip fractures reported in Cauley et al (2008)35 were also reported in Cauley et al (2011).34
Reported as median of equivalent daily dose, weighted mean of age and follow-up or duration, and sum of participants, any fracture events, and hip fracture events.
Participants in vitamin D group received an initial oral dose of 200 000 IU followed by 100 000 IU every month.
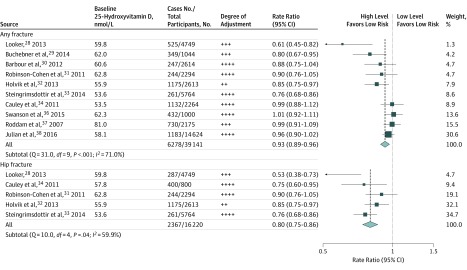
Figure 1 shows that an increase of 10.0 ng/mL in blood 25(OH)D concentration was associated with 7% lower risk of any fracture (RR, 0.93; 95% CI, 0.89-0.96) and 20% lower risk of hip fracture (RR, 0.80; 95% CI, 0.75-0.86). However, there was significant heterogeneity between the results of individual studies for both fracture outcomes (any fracture: Q = 31.0; df = 9; P < .001; I2 = 71.0%; hip fracture: Q = 10.0; df = 4; P = .04; I2 = 59.9%). Likewise, there was some evidence of asymmetry in the contour-enhanced funnel plots for both fracture outcomes (eFigure 3 in the Supplement). The prespecified subgroup analyses for an increase of 10.0 ng/mL in blood 25(OH)D concentration and risk of any or hip fracture are shown in eFigure 4 in the Supplement. The RRs for any fracture were lower in prospective studies than in nested case-control or case-cohort studies (0.89 [95% CI, 0.85-0.93] vs 0.97 [95% CI, 0.92-1.03]; P for heterogeneity = .01) and among older participants than younger participants (0.88 [95% CI, 0.83-0.93] vs 0.96 [95% CI, 0.92-1.00]; P for heterogeneity = .01). For hip fracture, no significant differences were observed between subgroups by study design, age, region, duration of follow-up, or baseline 25(OH)D concentrations.
Figure 1. Meta-analysis of Observational Studies of Risk of Any Fracture or of Hip Fracture Associated With an Increase of 25.0 nmol/L in Blood 25-Hydroxyvitamin D Concentration.
The size of each square corresponds to the precision of the estimates in each observational study. Degree of adjustment for confounders denoted as ++, age and sex plus body mass index; +++, age, sex, and body mass index plus other standard fracture risk factors; and ++++, age, sex, body mass index, and standard fracture risk factors plus markers of season or latitude. To convert 25-hydroxyvitamin D to nanograms per milliliter, divide by 2.496.
Meta-analysis of RCTs of Vitamin D Alone for Prevention of Fracture
Of the 1262 published reports identified for initial assessment (eFigure 5 in the Supplement), full texts were retrieved from 52 RCTs, but 36 RCTs were excluded for reasons listed in eTable 4 in the Supplement. A total of 11 RCTs of vitamin D39,40,41,42,43,44,45,46,47,48,49 and 6 RCTs of calcium plus vitamin D47,50,51,52,53,54 were included in the meta-analysis, and 1 factorial-design RCT47 was also included in the meta-analysis.
Of the 11 RCTs of supplementation with vitamin D alone included in the meta-analysis (Table), the sample size varied from 686 to 9440 participants, and mean age varied from 65.9 to 85.0 years. Among a total of 34 243 participants, there were 2843 fracture events (8.3%) and 740 hip fracture events (2.2%) during a mean (range) duration of approximately 3 years (9 months to 5 years). Among these 11 RCTs, 9 (82%) had a high risk of bias, 1 (9%) had an uncertain risk of bias, and only 1 (9%) had a low risk of bias (eFigure 6 in the Supplement). Selected characteristics of the included RCTs of vitamin D and fracture are provided in eTable 5 in the Supplement. Among the 11 RCTs, 3 (27%) used daily dosing with vitamin D,42,43,47 but dosing regimens in the 8 other RCTs varied from weekly (1 [9%]),40 monthly (1 [9%]),46 quarterly (2 [18%]),39,41 every 4 months (2 [18%]),44,48 to annually (2 [18%]).45,49 Moreover, only 2 of 11 RCTs (18%) assessed the effects of equivalent daily doses of vitamin D greater than 2000 IU.40,46 Two RCTs45,49 assessed very high annual doses of vitamin D, which appeared to increase the risk of fractures and falls among those allocated to the vitamin D group. All RCTs used placebo controls except for 1 (9%) that had an open-label design, and all assessed the effects of either ergocalciferol or cholecalciferol (except for 1 [9%] that assessed the effects of 5 mL of cod liver oil containing 400 IU of cholecalciferol).42 Among the 34 243 participants, 20 511 (59.9%) were women, the mean age was 77.1 years, and baseline blood 25(OH)D concentration varied from 10.6 to 26.3 ng/mL. Among 22 803 participants in 6 RCTs42,43,45,46,47,49 7895 participants (34.6%) reported a history of fracture. A total of 6 RCTs40,42,43,44,46,47 (55%) reported that adherence to supplementation with vitamin D varied between 80% and 99%.
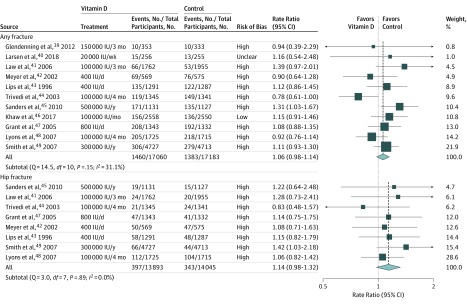
Figure 2 shows that supplementation with vitamin D alone was not associated with risk for any fracture (RR, 1.06; 95% CI, 0.98-1.14) or hip fracture (RR, 1.14; 95% CI, 0.98-1.32). There was no significant heterogeneity between RCTs for the associations of treatment with risk of any fracture (Q = 14.5; df = 10; P = .15; I2 = 31.1%) or hip fracture (Q = 3.0; df = 7; P = .89; I2 = 0.0%). There was some asymmetry in the contour-enhanced funnel plots of vitamin D for hip fracture, consistent with publication bias (eFigure 7 in the Supplement). Subgroup analyses did not demonstrate any significant differences by age, residential status, geographic region, open-label design, daily supplementation, or duration for any fracture or hip fracture (eFigure 8 in the Supplement). However, while there was no heterogeneity in the associations of vitamin D with risk of any fracture or hip fracture by baseline 25(OH)D concentrations (<20.0 vs ≥20.0 ng/mL), there was some heterogeneity in the associations of treatment with any fracture in baseline 25(OH)D concentrations of 8.0 ng/mL or higher vs less than 8.0 ng/mL (P for heterogeneity = .02). The results of a metaregression analysis of associations of risks of fracture by achieved treatment differences in 25(OH)D concentration are summarized in eFigure 9 in the Supplement. Among the 11 RCTs, vitamin D supplementation was associated with a median difference in blood 25(OH)D concentrations of 8.4 ng/mL, and 8 of 11 RCTs (73%) were associated with median differences in blood 25(OH)D concentrations of less than 10.0 ng/mL. Blood concentrations of 25(OH)D were only available for a small subset of participants in each trial, but the metaregression of RRs by achieved differences in blood 25(OH)D concentration suggested that each 0.4-ng/mL difference in blood 25(OH)D concentration was associated with an RR of 1.00 (95% CI, 0.99-1.01) for any fracture and of 0.98 (95% CI, 0.96-1.00) for hip fracture.
Figure 2. Meta-analysis of Randomized Clinical Trials of Supplementation With Vitamin D Alone vs Placebo or No Treatment for Prevention of Any Fracture or of Hip Fracture.
The size of each square corresponds to the precision of the estimates in each randomized clinical trial.
Meta-analysis of RCTs of Vitamin D Plus Calcium for Prevention of Fracture
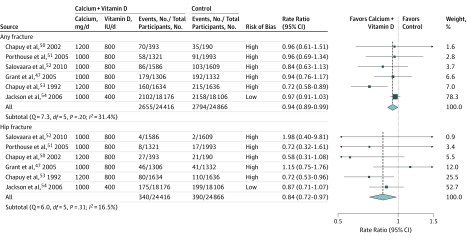
A total of 6 RCTs47,50,51,52,53,54 compared the effects of allocation to treatment with both vitamin D and calcium vs control (placebo or no treatment) on risk of fracture (Table; eTable 6 in the Supplement). Among these 6 RCTs, 5 (83%)47,50,51,52,53 had a high risk of bias, and 1 (17%)54 had a low risk of bias (eFigure 6 in the Supplement). Two RCTs (33%) had an open-label design, and all RCTs used either 800 or 400 IU of vitamin D per day and 1200 or 800 mg of calcium per day. Participants had a mean age of 66.2 years and a mean treatment duration of 5.9 years. Among a total of 49 282 participants, there were 5449 (11.1%) fracture events and 730 (1.5%) hip fracture events. Figure 3 demonstrates that daily supplementation with both vitamin D and calcium (for approximately 6 years) was associated with a 6% reduced risk of any fracture (RR, 0.94; 95% CI, 0.89-0.99) and a 16% reduced rate of hip fracture (RR, 0.84; 95% CI, 0.72-0.97). There was no significant heterogeneity between the RCTs for the associations of calcium plus vitamin D with the risk of any fracture (Q = 7.3; df = 5; P = .20; I2 = 31.4%) or risk of hip fracture (Q = 6.0; df = 5; P = .31; I2 = 16.5%). There was also some evidence of asymmetry in the contour-enhanced funnel plots of calcium plus vitamin D for any fracture and for hip fracture (eFigure 10 in the Supplement). Furthermore, eFigure 11 in the Supplement shows that the combined supplementation of calcium and vitamin D was associated with more extreme changes in risk of any fracture in the RCTs of older participants (ie, aged ≥80 years) living in an institution than those younger than 80 years living in the community (P for heterogeneity = .02) and in the RCTs that achieved greater treatment differences in blood 25(OH)D concentrations (P for heterogeneity = .04). Marginally significant lower risks of hip fracture were also observed in RCTs among older participants living in institutions (P for heterogeneity = .07) and in those achieving greater treatment differences in 25(OH)D concentration (P for heterogeneity = .08). Metaregression analysis based on a random sample of participants indicated that each 0.4-ng/mL difference in blood 25(OH)D concentration was associated with an RR of 0.99 (95% CI, 0.98-1.00) for any fracture and 0.98 (95% CI, 0.97-0.99) for hip fracture (eFigure 12 in the Supplement). Overall, the results of RCTs of calcium with vitamin D were consistent with the reported risk reductions associated with differences in 25(OH)D concentrations projected by the observational studies (eFigure 13 in the Supplement).
Figure 3. Meta-analysis of Randomized Clinical Trials of Supplementation With Calcium Plus Vitamin D vs Placebo or No Treatment for Prevention of Any Fracture or of Hip Fracture.
The size of each square corresponds to the precision of the estimates in each randomized clinical trial.
Ongoing Trials of Vitamin D for Prevention of Fracture
A total of 7 ongoing large RCTs, involving a total of 62 857 participants, are expected to report the effects of supplementation with higher daily doses of vitamin D on risk of fracture (eTable 7 in the Supplement).55,56,57,58,59,60,61 The weighted mean daily dose (or equivalent daily dose) of vitamin D in the ongoing RCTs is 2094 IU, which would be expected to yield an increase of approximately 20.0 ng/mL in blood 25(OH)D concentration, with full adherence by allocated treatment, and perhaps 12.0 to 16.0 ng/mL in an intention-to-treat analysis.62,63 Based on the findings of the present meta-analysis of observational studies, the ongoing RCTs are expected to yield a 9% reduction in risk of any fracture and 19% reduction in risk of hip fracture, assuming that half the effect is reversible within the scheduled treatment duration of the trial. However, assuming an annual event rate of 2% for any fracture and of 0.5% for hip fracture, none of the individual RCTs alone is likely to have sufficient power to detect a significant reduction in risk of any fracture or hip fracture of this magnitude (eAppendix 3 and eFigure 14 in the Supplement).
Discussion
The present meta-analysis of the observational studies of blood 25(OH)D concentration and risk of fracture (11 studies with 39 141 participants) demonstrated that higher blood 25(OH)D concentrations were associated with lower risks of any fracture and hip fracture. An increase of 10.0 ng/mL in 25(OH)D concentration was associated with a 7% lower risk of any fracture and a 20% lower risk of hip fracture. In contrast, the present meta-analysis of RCTs of vitamin D alone (11 RCTs with 34 243 participants) demonstrated no beneficial association of supplementation with vitamin D alone with risk of fracture. However, interpretation of the results of these RCTs is constrained by their small sample size, short treatment duration, high risk of bias (chiefly because of incomplete ascertainment of outcomes), intermittent dosing regimens of vitamin D, and failure to achieve adequate differences in 25(OH)D concentrations. Furthermore, 2 RCTs that assessed very high annual doses of vitamin D both showed an increase in the risk of fractures and falls among those allocated to the vitamin D group,45,49 reinforcing the conclusion that intermittent dosing regimens with high doses of vitamin D can cause toxic effects.
In contrast, a meta-analysis of the RCTs of daily supplementation with vitamin D and calcium (6 RCTs with 49 282 participants) demonstrated a marginally significant reduction in the risk of any fracture of 6% and hip fracture of 16%. However, the 95% CIs indicated some uncertainty for these estimates. As with the RCTs of vitamin D alone, these RCTs also had a high risk of bias. The risk reductions achieved in the RCTs of calcium plus vitamin D were somewhat greater in RCTs among older participants living in institutions and in RCTs that achieved greater differences in blood 25(OH)D concentrations between the allocated treatment groups. However, given these uncertainties, further large RCTs of combined treatment with vitamin D and calcium are needed before advocating vitamin D and calcium supplements or fortified foods with vitamin D and calcium for prevention of hip fracture.
Previous meta-analyses also reported that vitamin D supplementation alone was not associated with reduction of risks of any fracture or of hip fracture in community-dwelling older adults (14 RCTs with 13 106 participants)18 or in the general older population (24 RCTs with 39 485 participants).19 The present meta-analysis differed from previous meta-analyses by excluding small RCTs (ie, <500 participants) or those including few fracture events (ie, <10 events) to minimize the risks of bias. The doses of vitamin D used in most previous RCTs were too low to achieve the differences in blood 25(OH)D concentrations that the observational studies estimated would significantly reduce the risk of fracture. For example, 400 IU of vitamin D daily typically increases 25(OH)D concentration by only 2.8 to 4.0 ng/mL,64 and doses of 2000 IU per day are required to increase 25(OH)D concentration by 20.0 ng/mL. The extent to which intermittent dosing of vitamin D increases blood 25(OH)D concentration depends on the dose and dosing interval, but given that the half-life of 25(OH)D is 2 to 3 weeks,65 intermittent dosing with intervals longer than 1 month between doses is likely to result in substantial fluctuations in blood 25(OH)D concentration and may not achieve adequate differences in blood 25(OH)D concentration for a sustained duration.45,66,67 Significantly lower risks of falls and fracture have also been reported in RCTs assessing the effects of higher daily doses of vitamin D (ie, 1000 IU) administered in combination with calcium supplements66 but not in RCTs using intermittent dosing regimens of vitamin D (ie, intervals >1 month between doses) or those using extreme doses of vitamin D at even longer intervals between doses.45,67 One participant-level meta-analysis of RCTs14 reported effect modification by daily doses of vitamin D for prevention of fracture and highlighted the need to assess the effects of higher daily doses of vitamin D. Of 11 RCTs of vitamin D alone included in the present study, 9 RCTs allocated individuals to vitamin D supplementation with an equivalent dose of at least 800 IU per day, but 8 RCTs used intermittent doses (intramuscular or oral) administered monthly, quarterly, or even annually, and none assessed the effects of vitamin D administered in daily doses greater than 800 IU per day.
Other meta-analyses of RCTs assessing the effects of both vitamin D and calcium vs placebo have reported inconsistent results14,16,18 owing to differences in inclusion criteria and use of inappropriate random-effects meta-analysis16,18 (which inappropriately assigns undue weight to smaller RCTs). Likewise, some previous meta-analyses18 were restricted to RCTs among community-dwelling participants and excluded RCTs conducted among residents in nursing homes. Another,14 which assessed the effects of treatment in fewer RCTs (ie, 4 RCTs involving 54 493 participants, 5764 fractures, and 486 hip fractures), reported a borderline significant reduction in risk of any fracture (hazard ratio, 0.92; 95% CI, 0.86-0.99) and hip fracture (hazard ratio, 0.84; 95% CI, 0.70-1.01). In contrast, the present meta-analysis differed from the previous meta-analyses by providing comparisons of the observational studies of blood concentrations of 25(OH)D and risk of fracture, stratifying the results of RCTs by achieved differences in 25(OH)D concentration, and providing a detailed assessment of the risk of bias. Importantly, the present meta-analysis of 6 RCTs of vitamin D combined with calcium vs placebo or no treatment demonstrated that combined treatment with both vitamin D and calcium was associated with a 16% (95% CI, 3%-28%) reduction in the risk of hip fracture. However, concerns have been raised about the safety of combining calcium and vitamin D for cardiovascular disease and higher risks of kidney stones associated with calcium supplements.17,68
A total of 7 ongoing large RCTs involving 62 857 participants are expected to report the effects of supplementation with higher doses of vitamin D on risk of fracture, but none of the individual RCTs are likely to have sufficient power to detect a significant reduction on risk of any fracture or hip fracture. However, a further meta-analysis of all such RCTs should provide a reliable summary of the available evidence. Moreover, further large RCTs of vitamin D plus calcium are needed among older individuals with frailty or among other high-risk groups with low vitamin D status to clarify the relevance of combined treatment of vitamin D plus calcium for prevention of hip and other fragility fractures.
Limitations
The present meta-analysis has several limitations. First, there was heterogeneity between the results of the observational studies as well as among the assays used to measure 25(OH)D concentration. These assays were not standardized. Furthermore, there was possible publication bias in the results of the individual RCTs, and we were not able to assess the effects of treatment separately by sex.
Conclusions
In this systematic review and meta-analysis, the available evidence from completed RCTs provided no support for the effects of vitamin D alone on prevention of fracture, but most of these RCTs were constrained by methodological problems. Meta-analyses of ongoing RCTs assessing the effects of higher daily doses of vitamin D on fracture risk are needed before making recommendations on the use of vitamin D for prevention of fracture. Further RCTs are needed to assess the efficacy and safety of higher daily doses of vitamin D with calcium in high-risk individuals for prevention of fracture.
eAppendix 1. Meta-analysis of the Observational Studies
eAppendix 2. Meta-analysis of the Randomized Clinical Trials
eAppendix 3. Use of Observational Evidence to Estimate the Power for Future Trials
eFigure 1. Age and Sex-Specific Incidence Rates of Any Fracture and Relative Frequency of Selected Fragility Fractures Among Older People Living in the United Kingdom
eFigure 2. Flow Chart for the Literature Search for Studies Investigating the Associations of Blood 25(OH)D Concentrations with Risk of Fracture
eFigure 3. Contour-Enhanced Funnel Plot for the Meta-analysis of Cohort Studies of Blood 25(OH)D Concentrations and Risk of Fracture
eFigure 4. Rate Ratios (95% CIs) for Any Fracture and Hip Fracture Associated With 10 ng/mL Higher Blood 25(OH)D Concentrations in Cohort Studies by Baseline Characteristics
eFigure 5. Flow Chart of Literature Search for Trials Investigating the Effects of Vitamin D Alone or in Combination With Calcium for Prevention of Fracture
eFigure 6. Assessment of the Risk of Bias and Proportions of Randomized Clinical Trials That Met Each Criteria for Bias in the 16 Included Randomized Clinical Trials
eFigure 7. Contour-Enhanced Funnel Plot for the Meta-analysis of Randomized Clinical Trials of Vitamin D and Risk of Fracture
eFigure 8. Effects of Vitamin D Supplements on Risk of Any Fracture or Hip Fracture by Baseline Characteristics
eFigure 9. Rate Ratios (95% CIS) for Any Fracture and for Hip Fracture by Treatment Differences in Blood 25(OH)D Concentrations in the Vitamin D Randomized Clinical Trials
eFigure 10. Contour-Enhanced Funnel Plot for the Meta-analysis of Randomized Clinical Trials of Calcium Plus Vitamin D Supplements and Risk of Fracture
eFigure 11. Effects of Combined Vitamin D and Calcium Supplementation on Risk of Any Fracture or Hip Fracture by Baseline Characteristics
eFigure 12. Rate Ratios (95% CIs) for Any Fracture or for Hip Fracture by Treatment Differences in Blood 25(OH)D Concentrations in the Calcium Plus Vitamin D Randomized Clinical Trials
eFigure 13. Overall Effects of Supplementation of Vitamin D Alone or in Combination With Calcium on Risk of Any Fracture or of Hip Fracture in Meta-analyses of Randomized Clinical Trials in Their Epidemiological Context
eFigure 14. Estimated Power for a Meta-analysis of Randomized Clinical Trials of Vitamin D for Prevention of Fracture Associated With a 20 ng/mL Difference in Blood 25(OH)D Concentrations for 5 Years
eTable 1. Search Strategy
eTable 2. Characteristics of Studies Included in the Meta-analysis of Observational Studies of Blood Vitamin D Concentrations and Risk of Fracture
eTable 3. Assessment of the Risk of Bias in the Observational Studies Included in the Meta-analysis of the Observational Studies
eTable 4. Excluded Randomized Clinical Trials and Reasons for Their Exclusion
eTable 5. Summary of Included Randomized Clinical Trials of Vitamin D Alone vs Placebo or No Treatment
eTable 6. Summary of Included Randomized Clinical Trials of Vitamin D Plus Calcium vs Placebo or No Treatment
eTable 7. Ongoing Large Randomized Clinical Trials of Supplementation With Vitamin D Alone or in Combination With Calcium for Prevention of Fracture or Other Disease Outcomes
eReferences
References
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):-. doi: 10.1016/S0140-6736(06)68891-0 [DOI] [PubMed] [Google Scholar]
- 2.Watts NB, Manson JE. Osteoporosis and fracture risk evaluation and management: shared decision making in clinical practice. JAMA. 2017;317(3):253-254. doi: 10.1001/jama.2016.19087 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Tao Y, Hyman ME, Li J, Chen Y. Osteoporosis in China. Osteoporos Int. 2009;20(10):1651-1662. doi: 10.1007/s00198-009-0925-y [DOI] [PubMed] [Google Scholar]
- 4.Lund CA, Møller AM, Wetterslev J, Lundstrøm LH. Organizational factors and long-term mortality after hip fracture surgery: a cohort study of 6143 consecutive patients undergoing hip fracture surgery. PLoS One. 2014;9(6):e99308. doi: 10.1371/journal.pone.0099308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis EM, van der Velde R, Moon RJ, et al. . Epidemiology of fractures in the United Kingdom, 1988-2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19-26. doi: 10.1016/j.bone.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 7.Yao P, Sun L, Lu L, et al. . Effects of genetic and nongenetic factors on total and bioavailable 25(OH)D responses to vitamin D supplementation. J Clin Endocrinol Metab. 2017;102(1):100-110. [DOI] [PubMed] [Google Scholar]
- 8.Ross AC, Manson JE, Abrams SA, et al. . The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. doi: 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao P, Lu L, Hu Y, et al. . A dose-response study of vitamin D3 supplementation in healthy Chinese: a 5-arm randomized, placebo-controlled trial. Eur J Nutr. 2016;55(1):383-392. doi: 10.1007/s00394-015-0859-4 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Cheng G, Wang H, Chen B. The associations between serum 25-hydroxyvitamin D level and the risk of total fracture and hip fracture. Osteoporos Int. 2017;28(5):1641-1652. doi: 10.1007/s00198-017-3955-x [DOI] [PubMed] [Google Scholar]
- 11.Trajanoska K, Morris JA, Oei L, et al. ; GEFOS/GENOMOS Consortium and the 23andMe Research Team . Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. 2018;362:k3225. doi: 10.1136/bmj.k3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 13.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(15):1592-1599. doi: 10.1001/jama.2018.3185 [DOI] [PubMed] [Google Scholar]
- 14.DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. doi: 10.1136/bmj.b5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. . A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40-49. doi: 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 16.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi: 10.1002/14651858.CD000227.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahwati LC, Weber RP, Pan H, et al. . Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(15):1600-1612. doi: 10.1001/jama.2017.21640 [DOI] [PubMed] [Google Scholar]
- 18.Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318(24):2466-2482. doi: 10.1001/jama.2017.19344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847-858. doi: 10.1016/S2213-8587(18)30265-1 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301-1309. doi: 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225-234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baigent C, Peto R, Gray R, Parish S, Collins R. Large-scale randomized evidence: trials and meta-analyses of trials In: Warrell DA, Cox TM, Firth JD, eds. Oxford Textbook of Medicine. 5th ed Oxford, UK: Oxford University Press; 2010:31-45. doi: 10.1093/med/9780199204854.003.020303_update_002 [DOI] [Google Scholar]
- 26.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335-371. doi: 10.1016/S0033-0620(85)80003-7 [DOI] [PubMed] [Google Scholar]
- 27.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 28.Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997-1006. doi: 10.1002/jbmr.1828 [DOI] [PubMed] [Google Scholar]
- 29.Buchebner D, McGuigan F, Gerdhem P, Malm J, Ridderstråle M, Akesson K. Vitamin D insufficiency over 5 years is associated with increased fracture risk: an observational cohort study of elderly women. Osteoporos Int. 2014;25(12):2767-2775. doi: 10.1007/s00198-014-2823-1 [DOI] [PubMed] [Google Scholar]
- 30.Barbour KE, Houston DK, Cummings SR, et al. ; Health ABC Study . Calciotropic hormones and the risk of hip and nonspine fractures in older adults: the Health ABC Study. J Bone Miner Res. 2012;27(5):1177-1185. doi: 10.1002/jbmr.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson-Cohen C, Katz R, Hoofnagle AN, et al. . Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96(7):2186-2193. doi: 10.1210/jc.2010-2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holvik K, Ahmed LA, Forsmo S, et al. . Low serum levels of 25-hydroxyvitamin D predict hip fracture in the elderly: a NOREPOS study. J Clin Endocrinol Metab. 2013;98(8):3341-3350. doi: 10.1210/jc.2013-1468 [DOI] [PubMed] [Google Scholar]
- 33.Steingrimsdottir L, Halldorsson TI, Siggeirsdottir K, et al. . Hip fractures and bone mineral density in the elderly: importance of serum 25-hydroxyvitamin D. PLoS One. 2014;9(3):e91122. doi: 10.1371/journal.pone.0091122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauley JA, Danielson ME, Boudreau R, et al. . Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res. 2011;26(10):2378-2388. doi: 10.1002/jbmr.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cauley JA, Lacroix AZ, Wu L, et al. . Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250. doi: 10.7326/0003-4819-149-4-200808190-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson CM, Srikanth P, Lee CG, et al. ; Osteoporotic Fractures in Men MrOS Study Research Group . Associations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D with bone mineral density, bone mineral density change, and incident nonvertebral fracture. J Bone Miner Res. 2015;30(8):1403-1413. doi: 10.1002/jbmr.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roddam AW, Neale R, Appleby P, Allen NE, Tipper S, Key TJ. Association between plasma 25-hydroxyvitamin D levels and fracture risk: the EPIC-Oxford study. Am J Epidemiol. 2007;166(11):1327-1336. doi: 10.1093/aje/kwm210 [DOI] [PubMed] [Google Scholar]
- 38.Julian C, Lentjes MA, Huybrechts I, et al. . Fracture risk in relation to serum 25-hydroxyvitamin D and physical activity: results from the EPIC-Norfolk cohort study. PLoS One. 2016;11(10):e0164160. doi: 10.1371/journal.pone.0164160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012;27(1):170-176. doi: 10.1002/jbmr.524 [DOI] [PubMed] [Google Scholar]
- 40.Larsen AU, Grimnes G, Jorde R. The effect of high-dose vitamin D3 supplementation on bone mineral density in subjects with prediabetes. Osteoporos Int. 2018;29(1):171-180. doi: 10.1007/s00198-017-4222-x [DOI] [PubMed] [Google Scholar]
- 41.Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing. 2006;35(5):482-486. doi: 10.1093/ageing/afj080 [DOI] [PubMed] [Google Scholar]
- 42.Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? a randomized controlled trial. J Bone Miner Res. 2002;17(4):709-715. doi: 10.1359/jbmr.2002.17.4.709 [DOI] [PubMed] [Google Scholar]
- 43.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons: a randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400-406. doi: 10.7326/0003-4819-124-4-199602150-00003 [DOI] [PubMed] [Google Scholar]
- 44.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders KM, Stuart AL, Williamson EJ, et al. . Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822. doi: 10.1001/jama.2010.594 [DOI] [PubMed] [Google Scholar]
- 46.Khaw K-T, Stewart AW, Waayer D, et al. . Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5(6):438-447. doi: 10.1016/S2213-8587(17)30103-1 [DOI] [PubMed] [Google Scholar]
- 47.Grant AM, Avenell A, Campbell MK, et al. ; RECORD Trial Group . Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621-1628. doi: 10.1016/S0140-6736(05)63013-9 [DOI] [PubMed] [Google Scholar]
- 48.Lyons RA, Johansen A, Brophy S, et al. . Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int. 2007;18(6):811-818. doi: 10.1007/s00198-006-0309-5 [DOI] [PubMed] [Google Scholar]
- 49.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women: a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46(12):1852-1857. doi: 10.1093/rheumatology/kem240 [DOI] [PubMed] [Google Scholar]
- 50.Chapuy MC, Pamphile R, Paris E, et al. . Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257-264. doi: 10.1007/s001980200023 [DOI] [PubMed] [Google Scholar]
- 51.Porthouse J, Cockayne S, King C, et al. . Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. doi: 10.1136/bmj.330.7498.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salovaara K, Tuppurainen M, Kärkkäinen M, et al. . Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial: the OSTPRE-FPS. J Bone Miner Res. 2010;25(7):1487-1495. doi: 10.1002/jbmr.48 [DOI] [PubMed] [Google Scholar]
- 53.Chapuy MC, Arlot ME, Duboeuf F, et al. . Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637-1642. doi: 10.1056/NEJM199212033272305 [DOI] [PubMed] [Google Scholar]
- 54.Jackson RD, LaCroix AZ, Gass M, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683. doi: 10.1056/NEJMoa055218 [DOI] [PubMed] [Google Scholar]
- 55.LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2015;41:259-268. doi: 10.1016/j.cct.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neale RE, Armstrong BK, Baxter C, et al. . The D-Health Trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83-90. doi: 10.1016/j.cct.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 57.Joseph P, Pais P, Dans AL, et al. ; TIPS-3 Investigators . The International Polycap Study-3 (TIPS-3): Design, baseline characteristics and challenges in conduct. Am Heart J. 2018;206:72-79. doi: 10.1016/j.ahj.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov Finnish Vitamin D Trial (FIND). https://clinicaltrials.gov/ct2/show/NCT01463813. Accessed April 1, 2019.
- 59.LeBlanc ES, Pratley RE, Dawson-Hughes B, et al. ; D2d Research Group . Baseline characteristics of the Vitamin D and Type 2 Diabetes (D2d) Study: a contemporary prediabetes cohort that will inform diabetes prevention efforts. Diabetes Care. 2018;41(8):1590-1599. doi: 10.2337/dc18-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ClinicalTrials.gov Vitamin D3 Omega3 Home Exercise Healthy Ageing and Longevity Trial (DO-HEALTH). https://clinicaltrials.gov/ct2/show/NCT01745263. Accessed April 1, 2019.
- 61.Lappe J, Watson P, Travers-Gustafson D, et al. . Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234-1243. doi: 10.1001/jama.2017.2115 [DOI] [PubMed] [Google Scholar]
- 62.Ng K, Scott JB, Drake BF, et al. . Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr. 2014;99(3):587-598. doi: 10.3945/ajcn.113.067777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hin H, Tomson J, Newman C, et al. . Optimum dose of vitamin D for disease prevention in older people: BEST-D Trial of vitamin D in primary care. Osteoporos Int. 2017;28(3):841-851. doi: 10.1007/s00198-016-3833-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-210. doi: 10.1093/ajcn/77.1.204 [DOI] [PubMed] [Google Scholar]
- 65.Jones KS, Assar S, Harnpanich D, et al. . 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373-3381. doi: 10.1210/jc.2014-1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flicker L, MacInnis RJ, Stein MS, et al. . Should older people in residential care receive vitamin D to prevent falls? results of a randomized trial. J Am Geriatr Soc. 2005;53(11):1881-1888. doi: 10.1111/j.1532-5415.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 67.Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS One. 2015;10(1):e0115850. doi: 10.1371/journal.pone.0115850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199-210. doi: 10.1177/2042098613499790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Meta-analysis of the Observational Studies
eAppendix 2. Meta-analysis of the Randomized Clinical Trials
eAppendix 3. Use of Observational Evidence to Estimate the Power for Future Trials
eFigure 1. Age and Sex-Specific Incidence Rates of Any Fracture and Relative Frequency of Selected Fragility Fractures Among Older People Living in the United Kingdom
eFigure 2. Flow Chart for the Literature Search for Studies Investigating the Associations of Blood 25(OH)D Concentrations with Risk of Fracture
eFigure 3. Contour-Enhanced Funnel Plot for the Meta-analysis of Cohort Studies of Blood 25(OH)D Concentrations and Risk of Fracture
eFigure 4. Rate Ratios (95% CIs) for Any Fracture and Hip Fracture Associated With 10 ng/mL Higher Blood 25(OH)D Concentrations in Cohort Studies by Baseline Characteristics
eFigure 5. Flow Chart of Literature Search for Trials Investigating the Effects of Vitamin D Alone or in Combination With Calcium for Prevention of Fracture
eFigure 6. Assessment of the Risk of Bias and Proportions of Randomized Clinical Trials That Met Each Criteria for Bias in the 16 Included Randomized Clinical Trials
eFigure 7. Contour-Enhanced Funnel Plot for the Meta-analysis of Randomized Clinical Trials of Vitamin D and Risk of Fracture
eFigure 8. Effects of Vitamin D Supplements on Risk of Any Fracture or Hip Fracture by Baseline Characteristics
eFigure 9. Rate Ratios (95% CIS) for Any Fracture and for Hip Fracture by Treatment Differences in Blood 25(OH)D Concentrations in the Vitamin D Randomized Clinical Trials
eFigure 10. Contour-Enhanced Funnel Plot for the Meta-analysis of Randomized Clinical Trials of Calcium Plus Vitamin D Supplements and Risk of Fracture
eFigure 11. Effects of Combined Vitamin D and Calcium Supplementation on Risk of Any Fracture or Hip Fracture by Baseline Characteristics
eFigure 12. Rate Ratios (95% CIs) for Any Fracture or for Hip Fracture by Treatment Differences in Blood 25(OH)D Concentrations in the Calcium Plus Vitamin D Randomized Clinical Trials
eFigure 13. Overall Effects of Supplementation of Vitamin D Alone or in Combination With Calcium on Risk of Any Fracture or of Hip Fracture in Meta-analyses of Randomized Clinical Trials in Their Epidemiological Context
eFigure 14. Estimated Power for a Meta-analysis of Randomized Clinical Trials of Vitamin D for Prevention of Fracture Associated With a 20 ng/mL Difference in Blood 25(OH)D Concentrations for 5 Years
eTable 1. Search Strategy
eTable 2. Characteristics of Studies Included in the Meta-analysis of Observational Studies of Blood Vitamin D Concentrations and Risk of Fracture
eTable 3. Assessment of the Risk of Bias in the Observational Studies Included in the Meta-analysis of the Observational Studies
eTable 4. Excluded Randomized Clinical Trials and Reasons for Their Exclusion
eTable 5. Summary of Included Randomized Clinical Trials of Vitamin D Alone vs Placebo or No Treatment
eTable 6. Summary of Included Randomized Clinical Trials of Vitamin D Plus Calcium vs Placebo or No Treatment
eTable 7. Ongoing Large Randomized Clinical Trials of Supplementation With Vitamin D Alone or in Combination With Calcium for Prevention of Fracture or Other Disease Outcomes
eReferences