This randomized clinical trial examines the effectiveness of treatment with lemborexant therapy compared with placebo and with zolpidem tartrate extended release therapy in adults 55 years and older with insomnia disorder.
Key Points
Question
Does lemborexant therapy effectively treat insomnia disorder in patients 55 years and older compared with placebo and zolpidem tartrate extended release therapy?
Findings
In this randomized double-blind clinical trial of 1006 participants 55 years and older with insomnia disorder, lemborexant therapy significantly improved both latency to persistent sleep and sleep maintenance (wake-after-sleep onset and sleep efficiency) compared objectively via polysomnography with both placebo and zolpidem tartrate extended release therapy. Efficacy was also demonstrated on patient-reported end points of sleep onset, sleep efficiency, and wake-after-sleep onset for both doses of lemborexant compared with placebo.
Meaning
Lemborexant therapy may provide an additional option for sleep onset and sleep maintenance difficulties in older patients with insomnia disorder.
Abstract
Importance
Insomnia disorder is prevalent and associated with health risks in older adults; however, efficacy and safety issues with existing treatments create significant unmet needs in this patient population.
Objective
To compare treatment with the orexin receptor antagonist lemborexant with placebo and zolpidem tartrate extended release in participants with insomnia disorder.
Design, Setting, and Participants
The Study of the Efficacy and Safety of Lemborexant in Subjects 55 Years and Older With Insomnia Disorder (SUNRISE 1) clinical trial was a global randomized double-blind parallel-group placebo-controlled active-comparator phase 3 study conducted at 67 sites in North America and Europe from May 31, 2016, to January 30, 2018. Data analyses were conducted from January 31, 2018, to September 10, 2018. Participants were 55 years and older with insomnia disorder characterized by reported sleep maintenance difficulties and confirmed by sleep history, sleep diary, and polysomnography. Participants could have also had sleep onset difficulties.
Interventions
Participants received placebo, zolpidem tartrate extended release (6.25 mg), or lemborexant (5 mg or 10 mg) for 1 month at bedtime.
Main Outcomes and Measures
Paired polysomnograms were collected at baseline, the first 2 nights, and the last 2 nights of treatment. The primary end point was the change from baseline in latency to persistent sleep for lemborexant therapy vs placebo. Key secondary end points were changes from baseline in sleep efficiency and wake-after-sleep onset compared with placebo, and wake-after-sleep onset in the second half of the night compared with zolpidem therapy.
Results
Among 1006 participants randomized (placebo, n = 208; zolpidem, n = 263; lemborexant 5 mg, n = 266; and lemborexant 10 mg, n = 269), 869 (86.4%) were women and the median age was 63 years (range, 55-88 years). Both doses of lemborexant therapy demonstrated statistically significant greater changes from baseline on objective sleep onset as assessed by latency to persistent sleep (log transformed) that was measured using polysomnography at the end of 1 month of treatment (nights 29 and 30) compared with placebo (primary end point for least squares geometric means treatment ratio vs placebo: for lemborexant 5 mg, 0.77; 95% CI, 0.67-0.89; P < .001; for lemborexant 10 mg, 0.72; 95% CI, 0.63-0.83; P < .001). For nights 29 and 30, as measured using polysomnography, the mean change from baseline in sleep efficiency (LSM treatment difference vs placebo for lemborexant 5 mg, 7.1%; 95% CI, 5.6%-8.5%; P < .001 and for lemborexant 10 mg, 8.0%; 95% CI, 6.6%-9.5%; P < .001) and wake-after-sleep onset (least squares mean treatment ratio vs placebo for lemborexant 5 mg, −24.0 min; 95% CI, −30.0 to −18.0 min; P < .001 and for lemborexant 10 mg, −25.4 min; 95% CI, −31.4 to −19.3 min; P < .001) were significantly greater for both doses of lemborexant therapy compared with placebo. Also, for nights 29 and 30, wake-after-sleep onset in the second half of the night (least squares mean treatment difference vs zolpidem for lemborexant 5 mg, −6.7 min; 95% CI, −11.2 to −2.2 min; P = .004 and for lemborexant 10 mg, −8.0 min; 95% CI, −12.5 to −3.5 min; P < .001) was significantly greater for both doses of lemborexant therapy compared with zolpidem therapy measured using polysomnography. Six participants (4 in the zolpidem group and 2 in the lemborexant 5 mg group) reported serious adverse events; none were treatment-related. Other adverse events were mostly mild or moderate in severity.
Conclusions and Relevance
In this randomized clinical trial, lemborexant therapy significantly improved both sleep onset and sleep maintenance, including in the second half of the night, compared with both placebo and zolpidem measured objectively using polysomnography. Lemborexant therapy was well tolerated.
Trial Registrations
ClinicalTrials.gov identifier: NCT02783729; EudraCT identifier: 2015-001463-39
Introduction
Insomnia disorder, characterized by difficulty initiating and/or maintaining sleep 3 nights or more per week for 3 months or longer, is prevalent and associated with health risks in older adults.1 Cognitive behavior therapy for insomnia is a first-line insomnia therapy and has been shown to improve sleep in older patients with insomnia.2,3 However, pharmacologic treatment may be necessary in cases in which cognitive behavior therapy for insomnia is not effective or not accessible to the patient.2,4 Benzodiazepines and other sedative-hypnotic medications are prescribed frequently to treat insomnia in older adults, although many treatment options do not adequately address both sleep initiation and maintenance symptoms.5,6 This inadequacy is particularly problematic because older individuals tend to have relatively more difficulty maintaining sleep.7 Adverse effects from these medications may be associated with falls, hip fractures, and risk of unintentional injury.8,9 Thus, the American Academy of Sleep Medicine10 and the American Geriatric Society11 recommend restricting or foregoing use of sedative-hypnotic drugs in older adults.
Dual orexin receptor antagonists may provide an alternative to existing treatments. Orexins play a critical role in gating wakefulness and sleep/wake transitions. Dual orexin receptor antagonists block orexin receptors 1 and 2, dampening orexin activity and regulating sleep/wake function.12 Lemborexant (code name: E2006), an orally active investigational dual orexin receptor antagonist, is a reversible competitive antagonist that binds rapidly to both orexin receptors 1 and 2, with greater affinity for orexin receptor 2.13 In a randomized double-blind placebo-controlled phase 2 study with objective (polysomnography [PSG]) and subjective (self-reported sleep diary) end points, 5 mg and 10 mg of lemborexant therapy provided efficacy with minimal next-morning residual sleepiness in adults and elderly participants with insomnia disorder.14 The current study, Study of the Efficacy and Safety of Lemborexant in Subjects 55 Years and Older With Insomnia Disorder (SUNRISE 1), enrolled older patients with insomnia disorder and established the efficacy and tolerability of lemborexant therapy by comparison with both placebo and an active comparator, zolpidem tartrate extended release.
Methods
Clinical Trial Oversight and Study Participants
The SUNRISE 1 clinical trial was a global randomized double-blind parallel-group placebo-controlled active-comparator study sponsored by Eisai Inc. The study was conducted at 67 sites in North America and Europe from May 31, 2016, to January 30, 2018. Data were collected by site investigators, analyzed by statisticians employed by Eisai Inc, and Firma Clinical Research, and interpreted by the authors. Data analyses were conducted from January 31, 2018, to September 10, 2018. The protocol was approved by the relevant institutional review boards or ethics committees and conducted in accordance with the principles of the Guideline for Good Clinical Practice,15 the Declaration of Helsinki,16 and any applicable local regulations (trial protocol is available in Supplement 1). Protocol amendments or revisions were resubmitted to the respective institutional review boards or independent ethics committees for review and approval (eAppendix 1 in Supplement 2). After an explanation of study procedures, risks, and benefits, written informed consent was obtained from each participant. This report follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized clinical trials.
Women 55 years and older and men 65 years and older were eligible for participation if they met the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) criteria for insomnia disorder.17 Participants were required to have a history of subjective wake-after-sleep onset (sWASO) of 60 minutes or more at least 3 nights per week in the previous 4 weeks, regular time spent in bed (7-9 hours), evidence of sleep maintenance insomnia, and an Insomnia Severity Index (ISI) score of 13 or greater. Participants could also have had sleep onset difficulties, but this criterion was not required. Criteria were confirmed by sleep history, sleep diary, and PSG (wake-after-sleep onset [WASO] mean ≥60 minutes on 2 consecutive PSGs, with neither night <45 minutes). Additional details of key inclusion and exclusion criteria are provided in eAppendix 2 in Supplement 2. Participants were compensated for time and travel to study visits.
Clinical Trial Procedures
Following the initial screening period, eligible participants received placebo for approximately 2 weeks (run-in period with baseline PSG) to rule out placebo responders and to identify participants who did not adhere to sleep diary instructions. Participants were then treated for 30 nights followed by a follow-up period of approximately 14 days but not more than 18 days before an end-of-study visit. Participants were randomized in a double-blinded double-dummy manner to receive 5 mg of lemborexant (lemborexant 5 mg), 10 mg of lemborexant (lemborexant 10 mg), 6.25 mg of zolpidem, or placebo in a 5:5:5:4 ratio. The 6.25 mg dose of zolpidem was chosen based on zolpidem prescribing information, which stipulates this dose for patients older than 65 years.18 Randomization was stratified by country and age group (55-64 years and ≥65 years). Randomization to study drugs was based on a computer-generated randomization scheme that was reviewed and approved by an independent statistician. Randomization was performed centrally by an interactive voice and web response system.
During the run-in period of the prerandomization phase, single blinding was in effect such that the participant was blinded to the study drug, but the study personnel were not blinded. During the randomization phase, participants and all personnel involved with the conduct and interpretation of the study, including investigators, site personnel, and sponsor staff, were blinded to the treatment codes. Randomization data were kept strictly confidential, filed securely by an appropriate group with the sponsor or contract research organization, and accessible only to authorized persons until the time of unblinding.
Participants completed an electronic sleep diary within 1 hour of wake time every morning from screening until the end-of-study visit (additional details regarding sleep diary procedures are provided in eAppendix 2 in Supplement 2). Failure to adhere to requirements for completion of the sleep diary required discussion with clinical site staff and may have resulted in discontinuation of the participant from the study.
Outcomes
Efficacy outcomes included change from baseline in PSG measures of sleep onset and maintenance at the beginning and end of treatment. The primary end point was to demonstrate using PSG that lemborexant 10 mg and lemborexant 5 mg therapies were superior to placebo with regard to objective sleep onset as assessed by latency to persistent sleep (LPS; defined as minutes from lights off to the first epoch of 20 consecutive 30-second epochs of nonwakefulness) after the last 2 nights (nights 29 and 30) of 1 month of treatment. Key secondary end points included sleep maintenance outcomes of sleep efficiency (proportion of time spent asleep per time in bed, calculated as total sleep time/interval from lights off until lights on [standardized at 8 hours]), minutes of wake from LPS until lights on (WASO), and WASO in the second half of the night (WASO2H; minutes of wake from 240 minutes after lights off until lights on).
The PSG parameters were obtained separately for each PSG and averaged across the pairs of consecutive PSGs (ie, the average of night 1 and night 2 and the average of night 29 and night 30). Centralized PSG scorers scored PSG records in 30-second epochs according to standard criteria.
Additional secondary end points included subjective patient-reported measures of sleep onset and maintenance as determined by sleep diaries. The patient-reported measure of sleep onset was subjective sleep onset latency (sSOL), reported as minutes from the time the participant attempted to sleep until sleep onset. Patient-reported measures of sleep maintenance included subjective sleep efficiency (derived from the proportion of total sleep time per time in bed) and sWASO (defined as the sum of estimated minutes of wake after initial sleep onset until the participant got out of bed for the day). Each sleep diary parameter was calculated as the mean of the first 7 (week 1) and last 7 (week 4) nights of treatment.
Disease severity and daily functioning were assessed using the patient-rated ISI.19 Change from baseline on ISI total scores and ISI daily functioning (items 4–7) scores were analyzed at the end of the treatment period (day 31).
Safety Assessments
Safety assessments were collected at every clinic visit throughout the study and at the end of study. These assessments consisted of monitoring and recording all adverse events (AEs); regular laboratory evaluation for hematology, chemistry, and urine values; periodic measurement of vital signs, weight, and electrocardiograms; ratings on the Columbia-Suicide Severity Rating Scale (10 categories measuring the presence or absence of suicidal ideation and behavior); and performance of physical evaluations.
Potential withdrawal symptoms were assessed by the Tyrer Benzodiazepine Withdrawal Symptom Questionnaire,20 which asks participants to rate the severity of 20 symptoms using 3 possible responses (0 indicates no symptoms, 1 indicates moderate symptoms, and 2 indicates severe symptoms). Scores range from 0 to 40, with a total score of 20 or higher considered clinically important. This questionnaire was administered at the end-of-study visit. Rebound insomnia was assessed using sleep diary variables comparing the 2-week follow-up period to pretreatment. Additional details of rebound insomnia can be found in eAppendix 2 in Supplement 2.
Statistical Analysis
Sample size was estimated on the mean change from baseline comparison of LPS for lemborexant therapy vs placebo at month 1 using a 2-sided, 2-sample t test at the 5% significance level. Based on the phase 2 proof-of-concept/dose-finding study,14 at nights 14 and 15, the SD of change from baseline for log-transformed LPS was assumed to be 0.9. The estimated least squares mean (LSM) treatment difference at nights 14 and 15 for log-transformed LPS of lemborexant 5 mg and lemborexant 10 mg therapies compared with placebo was 0.75 and −1.15, respectively. Therefore, a planned sample size of 950 participants (250 participants each for the lemborexant 5 mg, lemborexant 10 mg, and zolpidem therapy groups, and 200 participants for the placebo group) had at least 95% power to detect a statistically significant difference between lemborexant therapy and placebo. For the key secondary objectives, the planned sample size had at least 95% power to detect a statistically significant difference between lemborexant therapy and placebo for change from baseline for sleep efficiency, and at least 80% power to detect a statistically significant difference between lemborexant 10 mg therapy and zolpidem therapy or placebo for change from baseline for WASO2H and WASO based on a 2-sided, 2-sample t test at the 5% significance level.
Efficacy analyses were conducted on the group of randomized participants who received at least 1 dose of randomized study drug and had at least 1 postdose primary efficacy measurement (full analysis set). For each of the primary (LPS) and key secondary (sleep efficiency, WASO, and WASO2H) efficacy end points, the changes from baseline values on nights 1 and 2 and nights 29 and 30 were analyzed separately using the mixed-effect model for repeat measurement (MMRM), with the factors of age group (55-64 years and ≥65 years), region (North America and Europe), treatment, visit (nights 1 and 2 and nights 29 and 30), and treatment-by-visit interaction as fixed effects and the baseline value as a covariate. Because LPS is known to be nonnormally distributed, a log transformation was used in the LPS analysis, and statistical comparisons were made based on the least squares geometric means (LSGMs).
The primary analysis of change from baseline on nights 1 and 2 and nights 29 and 30 in the primary end point and key secondary end points was based on the full analysis set. Missing values were imputed using pattern-mixture model multiple imputation, assuming the missing values were missing not at random using the complete-case missing value pattern (ie, missing data at any day were assumed to have a similar distribution as the study completers within the respective treatment group, in which completers were defined as having no missing assessments for any postbaseline visits). Of the 1006 study participants, data were missing from approximately 35 (3.5%), most of whom withdrew from the study early. Missing data were generally balanced across treatment groups (8 of 208 participants [3.8%] in the placebo group, 13 of 263 [4.9%] in the zolpidem group, 6 of 266 [2.3%] in the lemborexant 5 mg group, and 9 of 269 [3.3%] in the lemborexant 10 mg group).
To control for the overall type 1 error at the 5% significance level, a sequential gate-keeping procedure was used for the primary (LPS) and the key secondary (sleep efficiency, WASO, and WASO2H, in that order) end point comparisons (eFigure 1 in Supplement 2). Within each end point, the comparison with lemborexant 10 mg therapy was tested first. If the lemborexant 10 mg comparison was found to be statistically significant at the 5% significance level, then testing for that variable proceeded to the lemborexant 5 mg comparison; otherwise, testing was stopped. The key secondary end points were only tested if both primary analyses were statistically significant at the P < .05 level. No multiplicity adjustment was performed on other efficacy analyses.
For sSOL, subjective sleep efficiency, and sWASO, changes from baseline were analyzed with the same MMRM model used for the primary analysis, which assumed missing values to be missing at random (no missing value imputation). As a result of nonnormal distribution, sSOL values were log transformed, and statistical comparisons were made based on the LSGMs.
Changes from baseline for ISI total score and ISI daily functioning score were analyzed using an analysis of covariance model, with baseline ISI total score or baseline ISI daily functioning score as a covariate, and age group, region, and treatment as factors. Missing values were not imputed.
Safety analyses were summarized using descriptive statistics for the group of randomized participants who received at least 1 dose of randomized study drug and had at least 1 postdose safety assessment (safety population).
Results
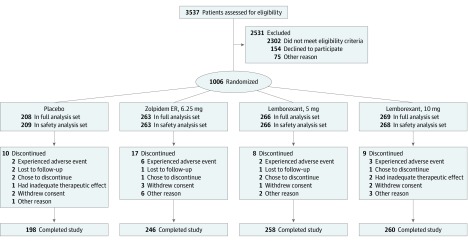
Of 3537 participants screened, 1006 were randomized to receive placebo (n = 208), zolpidem therapy (n = 263), lemborexant 5 mg therapy (n = 266), and lemborexant 10 mg therapy (n = 269; Figure 1). Among the 1006 participants randomized, 869 (86.4%) were women, 727 (72.3%) were white, and 256 (25.4%) were black, with a median age of 63 years (range, 55-88 years) (Table 1). The number of randomized participants exceeded the planned sample size of 950 as a result of an influx of participants screened near the end of the enrollment period. Very few participants discontinued the study, with 962 participants (95.6%) completing the study (198 [95.2%] in the placebo group, 246 [93.5%] in the zolpidem group, 258 [97.0%] in the lemborexant 5 mg group, and 260 [96.7%] in the lemborexant 10 mg group). Similarly, small numbers of participants discontinued the study as a result of AEs (2 [1.0%] in the placebo group, 6 [2.3%] in the zolpidem group, 2 [0.8%] in the lemborexant 5 mg group, and 3 [1.1%] in the lemborexant 10 mg group).
Figure 1. CONSORT Flow Diagram of Participants Through the Trial.
The full analysis set included all randomized participants who received at least 1 dose of the study drug and had at least 1 postdose primary efficacy measurement. The safety analysis set included all participants who received at least 1 dose of the study drug and had a postdose safety assessment. ER indicates extended release.
Table 1. Baseline Characteristics of Participants.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Total (N = 1006) | Placebo (n = 208) | Zolpidem ER 6.25 mg (n = 263) | Lemborexant 5 mg (n = 266) | Lemborexant 10 mg (n = 269) | ||
| Age, y | ||||||
| Mean (SD) | 63.9 (6.8) | 63.4 (6.4) | 64.3 (7.1) | 63.7 (6.8) | 64.2 (6.9) | |
| Median (range) | 63 (55-88) | 62 (55-82) | 63 (55-83) | 63 (55-88) | 64 (55-85) | |
| ≥55 to <65 | 553 (55.0) | 115 (55.3) | 143 (54.4) | 148 (55.6) | 147 (54.6) | |
| ≥65 | 453 (45.0) | 93 (44.7) | 120 (45.6) | 118 (44.4) | 122 (45.4) | |
| Sex | ||||||
| Male | 137 (13.6) | 24 (11.5) | 37 (14.1) | 37 (13.9) | 39 (14.5) | |
| Female | 869 (86.4) | 184 (88.5) | 226 (85.9) | 229 (86.1) | 230 (85.5) | |
| Race | ||||||
| White | 727 (72.3) | 153 (73.6) | 173 (65.8) | 199 (74.8) | 202 (75.1) | |
| Black | 256 (25.4) | 51 (24.5) | 80 (30.4) | 63 (23.7) | 62 (23.0) | |
| Japanese | 2 (0.2) | 1 (0.5) | 1 (0.4) | 0 | 0 | |
| Chinese | 2 (0.2) | 1 (0.5) | 0 | 0 | 1 (0.4) | |
| Other Asian | 10 (1.0) | 0 | 4 (1.5) | 2 (0.8) | 4 (1.5) | |
| American Indian or Alaskan Native | 0 | 0 | 0 | 0 | 0 | |
| Native Hawaiian or other Pacific Islander | 2 (0.2) | 0 | 2 (0.8) | 0 | 0 | |
| Other | 7 (0.7) | 2 (1.0) | 3 (1.1) | 2 (0.8) | 0 | |
| Polysomnography sleep variables, mean (SD) | ||||||
| Latency to persistent sleep, min | 44.5 (35.5) | 43.9 (33.6) | 44.5(38.3)a | 44.9 (36.5) | 44.6 (33.0) | |
| Sleep efficiency, % | 68.3 (10.9) | 68.9 (9.6) | 68.1 (11.4)a | 68.4 (11.3) | 67.9 (10.8) | |
| Wake-after-sleep onset, min | 113.7 (39.1) | 111.8 (37.2) | 114.31 (39.9)a | 113.4 (39.0) | 114.8 (40.0) | |
| Wake-after-sleep onset in second half of night, min | 76.6 (32.4) | 74.4 (30.1) | 78.0 (33.8)a | 76.6 (32.9) | 76.9 (32.1) | |
| ISI total score, mean (SD) | 19.1 (3.5) | 19.4 (3.6) | 19.2 (3.5) | 18.9 (3.5) | 19.0 (3.3) | |
Abbreviations: ER, extended release; ISI, Insomnia Severity Index.
Sample size was 262 participants.
The 4 treatment groups were balanced with respect to baseline demographics (Table 1). Insomnia severity was considered moderate based on the baseline ISI score in all treatment groups.
Efficacy Outcomes
Sleep Onset by PSG
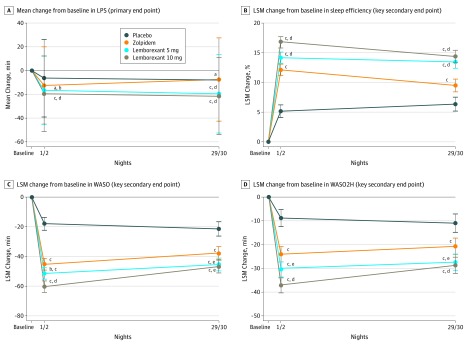
The mean decrease from baseline in the log-transformed LPS (primary end point) was significantly larger for both doses of lemborexant therapy at nights 1 and 2 compared with placebo (LSGM treatment ratio vs placebo for lemborexant 5 mg, 0.85; 95% CI, 0.75-0.96; P = .009 and for lemborexant 10 mg, 0.80; 95% CI, 0.70-0.90; P < .001) and zolpidem therapy (LSGM ratio vs zolpidem for lemborexant 5 mg, 0.87; 95% CI, 0.78-0.98; P = .02 and for lemborexant 10 mg, 0.82; 95% CI, 0.73-0.92; P < .001) (Figure 2A and Table 2).
Figure 2. Sleep Onset and Sleep Maintenance Outcomes Assessed by Polysomnography, by Treatment Group.
Outcomes were assessed at the beginning (nights 1 and 2) and end (nights 29 and 30) of treatment. A total of 208 participants received placebo, 263 received 6.5 mg of zolpidem tartrate extended release, 266 received 5 mg of lemborexant, and 269 received 10 mg of lemborexant. A, Mean change from baseline in latency to persistent sleep (LPS) (primary end point). As a result of the nonnormal distribution of LPS, the values were log transformed, and the geometric mean ratio was used to test for statistically significant treatment differences. B, The least squares mean (LSM) change from baseline in sleep efficiency (key secondary end point). C, The LSM change from baseline in wake-after sleep onset (WASO) (key secondary end point). D, The LSM change from baseline in WASO in the second half of the night (WASO2H) (key secondary end point).
aP < .01 vs placebo.
bP < .05 vs zolpidem.
cP < .001 vs placebo.
dP ≤ .001 vs zolpidem.
eP < .01 vs zolpidem.
Table 2. Sleep Onset and Sleep Maintenance End Points by Treatment Groupa.
| End Point | Placebo (n = 208) | Zolpidem ER 6.25 mg (n = 263) | Lemborexant 5 mg (n = 266) | Lemborexant 10 mg (n = 269) |
|---|---|---|---|---|
| Latency to Persistent Sleep, min | ||||
| Nights 1 and 2, mean (SD)b | 37.4 (32.5) | 31.9 (23.7) | 28.3 (24.4) | 25.1 (16.7) |
| Change from baseline, mean (SD) | –6.5 (32.6) | –12.6 (32.5) | –16.6 (28.7) | –19.5 (31.8) |
| LSGM treatment ratio vs placebo (95% CI) | NA | 0.97 (0.86 to 1.10) | 0.85 (0.75 to 0.96) | 0.80 (0.70 to 0.90) |
| P valuec | NA | .66 | .009 | <.001 |
| LSGM treatment ratio vs zolpidem (95% CI) | NA | NA | 0.87 (0.78 to 0.98) | 0.82 (0.73 to 0.92) |
| P valuec | .02 | <.001 | ||
| Nights 29 and 30, mean (SD)d | 36.0 (32.1) | 37.1 (28.4) | 25.8 (24.3) | 22.8 (17.5) |
| Change from baseline, mean (SD) | –7.9 (32.0) | –7.5 (35.1) | –19.5 (33.1) | –21.5 (32.4) |
| LSGM treatment ratio vs placebo (95% CI) | NA | 1.22 (1.06 to 1.40) | 0.77 (0.67 to 0.89) | 0.72 (0.63 to 0.83) |
| P valuec | NA | .006e | <.001 | <.001 |
| LSGM treatment ratio vs zolpidem (95% CI) | NA | NA | 0.63 (0.56 to 0.72) | 0.59 (0.52 to 0.68) |
| P valuec | NA | NA | <.001 | <.001 |
| Sleep Efficiency, % | ||||
| Nights 1 and 2, mean (SD)b | 73.1 (10.8) | 79.9 (8.5) | 82.0 (8.4) | 84.3 (7.6) |
| Change from baseline, mean (SD) | 4.2 (9.0) | 11.7 (9.7) | 13.6 (9.7) | 16.5 (9.6) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | 7.0 (5.7 to 8.3) | 9.0 (7.7 to 10.3) | 11.6 (10.3 to 12.9) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active zolpidem | NA | NA | 2.1 (0.8 to 3.3) | 4.6 (3.4 to 5.9) |
| P valuef | NA | NA | .001 | <.001 |
| Nights 29 and 30, mean (SD)d | 74.5 (9.8) | 77.2 (10.2) | 81.3 (8.8) | 82.0 (8.8) |
| Change from baseline, mean (SD) | 5.4 (9.9) | 9.1 (11.2) | 12.9 (9.7) | 14.1 (10.5) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | 3.2 (1.7 to 4.6) | 7.1 (5.6 to 8.5) | 8.0 (6.6 to 9.5) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active zolpidem | NA | NA | 3.9 (2.5 to 5.3) | 4.9 (3.5 to 6.3) |
| P valuef | NA | NA | <.001 | <.001 |
| Wake-After-Sleep Onset, min | ||||
| Nights 1 and 2, mean (SD)b | 96.7 (41.3) | 69.9 (33.5) | 63.5 (31.5) | 55.2 (30.5) |
| Change from baseline, mean (SD) | –15.1 (36.9) | –44.4 (38.1) | –50.0 (39.6) | –59.6 (37.7) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | –27.2 (–32.6 to –21.9) | –33.4 (–38.7 to –28.1) | –42.3 (–47.6 to –37.0) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active zolpidem | NA | NA | –6.2 (–11.2 to –1.2) | –15.0 (–20.0 to –10.1) |
| P valuef | NA | NA | .02 | <.001 |
| Nights 29 and 30, mean (SD)d | 92.1 (41.0) | 77.7 (39.9) | 69.1 (34.5) | 68.6 (35.2) |
| Change from baseline, mean (SD) | –18.6 (41.9) | –36.5 (43.4) | –43.9 (39.3) | –46.4 (39.6) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | –16.3 (–22.3 to –10.2) | –24.0 (–30.0 to –18.0) | –25.4 (–31.4 to –19.3) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active zolpidem | NA | NA | –7.7 (–13.4 to –2.1) | –9.1 (–14.8 to –3.5) |
| P valuef | NA | NA | .007 | .002 |
| Wake-After-Sleep Onset in Second Half of Night, min | ||||
| Nights 1 and 2, mean (SD)b | 67.4 (32.9) | 53.3 (27.7) | 46.3 (25.6) | 39.8 (23.7) |
| Change from baseline, mean (SD) | –7.1 (31.1) | –24.6 (33.3) | –30.3 (32.1) | –37.1 (30.8) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | –15.2 (–19.6 to –10.8) | –21.7 (–26.0 to –17.3) | –28.3 (–32.7 to –24.0) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active zolpidem | NA | NA | –6.5 (–10.6 to –2.4) | –13.1 (–17.2 to –9.0) |
| P valuef | NA | NA | .002 | <.001 |
| Nights 29 and 30, mean (SD)d | 64.4 (32.4) | 56.7 (31.1) | 49.1 (28.2) | 48.2 (27.8) |
| Change from baseline, mean (SD) | –8.9 (31.9) | –21.4 (36.3) | –27.2 (33.0) | –28.8 (33.1) |
| LSM treatment difference (95% CI) | ||||
| Active placebo | NA | –9.8 (–14.6 to –4.9) | –16.4 (–21.2 to –11.6) | –17.8 (–22.6 to –13.0) |
| P valuef | NA | <.001 | <.001 | <.001 |
| Active | NA | NA | –6.7 (–11.2 to –2.2) | –8.0 (–12.5 to –3.5) |
| P valuef | NA | NA | .004 | <.001 |
Abbreviations: ER, extended release; LSGM, least squares geometric mean; LSM, least squares mean; NA, not applicable.
Measured by polysomnography at the beginning (nights 1 and 2) and end (nights 29 and 30) of treatment.
Sample size was 262 participants for zolpidem ER 6.25 mg.
P values were based on mixed-effects model repeated measurements model with log transformation of latency to persistent sleep and factors for age group (55-64 years and ≥65 years), region (North America and Europe), treatment, visit (nights 1 and 2 and nights 29 and 30), and treatment-by-visit interaction as fixed effects and the baseline persistent sleep as a covariate. Missing values were imputed using multiple imputation and assumed to be missing not at random.
Sample sizes were 200 participants for placebo, 250 for zolpidem ER 6.25 mg, 260 for lemborexant 5 mg, and 260 for lemborexant 10 mg.
Increases with placebo were greater and significantly different from zolpidem.
P values were based on a mixed-effects model repeated measurements model, with factors of age group (55-64 years and ≥65 years), region (North America and Europe), treatment, visit (nights 1 and 2 and nights 29 and 30), and treatment-by-visit interaction as fixed effects and the baseline value of the variable as a covariate. Missing values were imputed using multiple imputation and assumed to be missing not at random.
This effect of lemborexant therapy was maintained after 1 month of treatment, with a significant mean decrease from baseline in the log-transformed LPS observed for both doses of lemborexant therapy compared with placebo (LSGM treatment ratio vs placebo for lemborexant 5 mg, 0.77; 95% CI, 0.67-0.89; P < .001 and for lemborexant 10 mg, 0.72; 95% CI, 0.63-0.83; P < .001) and zolpidem therapy (LSGM treatment ratio vs zolpidem for lemborexant 5 mg, 0.63; 95% CI, 0.56-0.72; P < .001 and for lemborexant 10 mg, 0.59; 95% CI, 0.52-0.68; P < .001) at nights 29 and 30 (Figure 2A and Table 2).
Sleep Maintenance by PSG
For nights 1 and 2, mean changes from baseline in sleep efficiency were significantly larger for both lemborexant 5 mg and lemborexant 10 mg therapies compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, 9.0%; 95% CI, 7.7%-10.3%; P < .001 and for lemborexant 10 mg, 11.6%; 95%, 10.3%-12.9%; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, 2.1%; 95% CI, 0.8%-3.3%; P = .001 and for lemborexant 10 mg, 4.6%; 95% CI, 3.4%-5.9%; P < .001) (Figure 2B and Table 2). Mean changes from baseline in sleep efficiency at nights 29 and 30 were also significantly larger for both lemborexant 5 mg and lemborexant 10 mg therapies compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, 7.1; 95% CI, 5.6-8.5; P < .001 and for lemborexant 10 mg, 8.0; 95% CI, 6.6-9.5; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, 3.9; 95% CI, 2.5-5.3; P < .001 and for lemborexant 10 mg, 4.9; 95% CI, 3.5-6.3; P < .001) (Figure 2B and Table 2). The increases in sleep efficiency in both lemborexant treatment groups translated into more than 60 minutes more sleep per night than before treatment.
Mean decreases from baseline WASO at nights 1 and 2 were significantly larger for both doses of lemborexant therapy compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, −33.4 min; 95% CI, −38.7 to −28.1 min; P < .001 and for lemborexant 10 mg, −42.3 min; 95% CI, −47.6 to −37.0 min; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, −6.2 min; 95% CI, −11.2 to −1.2 min; P = .02 and for lemborexant 10 mg, −15.0 min; 95% CI, −20.0 to −10.1 min; P < .001) (Figure 2C and Table 2). As measured by PSG, both doses of lemborexant therapy reduced WASO by more than 45 minutes relative to baseline. Mean decreases from baseline WASO at nights 29 and 30 were also significantly larger for both doses of lemborexant therapy compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, −24.0 min; 95% CI, −30.0 to −18.0 min; P < .001 and for lemborexant 10 mg, −25.4 min; 95% CI, −31.4 to −19.3 min; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, −7.7 min; 95% CI, −13.4 to −2.1 min; P = .007 and for lemborexant 10 mg, −9.1 min; 95% CI, −14.8 to −3.5 min; P = .002) (Figure 2C and Table 2).
The reduction in time spent awake was observed mostly in the latter half of the sleep period (Figure 2D). For nights 1 and 2, mean decreases in WASO2H were significantly larger for both doses of lemborexant therapy compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, −21.7 min; 95% CI, −26.0 to −17.3 min; P < .001 and for lemborexant 10 mg, −28.3 min; 95% CI, −32.7 to −24.0 min; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, −6.5 min; 95% CI, −10.6 to −2.4 min; P = .002 and for lemborexant 10 mg, −13.1 min; 95% CI, −17.2 to −9.0 min; P < .001) (Figure 2D and Table 2). For nights 29 and 30, mean decreases in WASO2H were also significantly larger for both doses of lemborexant therapy compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg, −16.4 min; 95% CI, −21.2 to −11.6 min; P < .001 and for lemborexant 10 mg, −17.8 min; 95% CI, −22.6 to −13.0 min; P < .001) and zolpidem therapy (LSM treatment difference vs zolpidem for lemborexant 5 mg, −6.7 min; 95% CI, −11.2 to −2.2 min; P = .004 and for lemborexant 10 mg, −8.0 min; 95% CI, −12.5 to −3.5 min; P < .001) (Figure 2D and Table 2).
Decreases for WASO in each quarter of the night (analyzed post hoc) at nights 1 and 2 and nights 29 and 30 were numerically larger in both lemborexant treatment groups compared with placebo; similar decreases were observed in the zolpidem group for the first 3 quarters of the night (eFigure 2 in Supplement 2). In the final quarter of the night, participants in the zolpidem group experienced placebo-like effects, but decreases for both lemborexant groups were sustained.
Sleep Onset and Sleep Maintenance by Sleep Diary
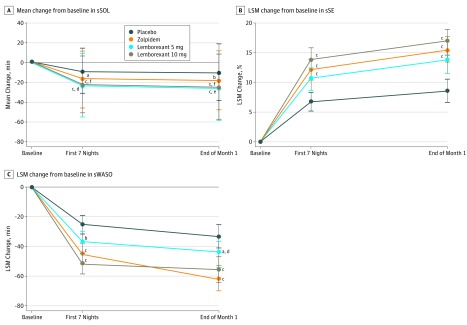
Mean decreases from baseline in log-transformed sSOL were significantly larger with lemborexant 5 mg and lemborexant 10 mg therapies compared with placebo during the first 7 nights of treatment and at the end of month 1 (LSGM treatment ratio vs placebo for first 7 nights for lemborexant 5 mg, 0.82; 95% CI, 0.75-0.89; P < .001 and for lemborexant 10 mg, 0.75; 95% CI, 0.69-0.82; P < .001; LSGM treatment ratio vs placebo for end of month 1 for lemborexant 5 mg, 0.75; 95% CI, 0.67-0.84; P < .001 and for lemborexant 10 mg, 0.69; 95% CI, 0.62-0.77; P < .001 for all comparisons) (Figure 3A and eTable in Supplement 2). In addition, during the first 7 nights of treatment and at the end of month 1, mean decreases from baseline were significantly larger with lemborexant 5 mg therapy (LSGM treatment ratio vs zolpidem for lemborexant 5 mg for the first 7 nights, 0.90; 95% CI, 0.83-0.98; P = .01 and at the end of month 1, 0.88; 95% CI, 0.80-0.98; P = .02) and lemborexant 10 mg therapy (LSGM treatment ratio vs zolpidem for lemborexant 10 mg for the first 7 nights, 0.83; 95% CI, 0.76-0.90; P < .001 and at end of month 1, 0.81; 95% CI, 0.73-0.90; P < .001) vs zolpidem (Figure 3A and eTable in Supplement 2).
Figure 3. Sleep Onset and Sleep Maintenance Outcomes Assessed by Sleep Diary, by Treatment Group.
Outcomes were assessed at the beginning (first 7 nights) and end (end of month 1) of treatment. A total of 208 participants received placebo, 263 received 6.5 mg of zolpidem tartrate extended release, 266 received 5 mg of lemborexant, and 269 received 10 mg of lemborexant. A, Mean change from baseline in subjective sleep onset latency (sSOL). As a result of nonnormal distribution of sSOL latency, values were log transformed, and the geometric mean ratio was used to test for statistically significant treatment differences. B, The least squares mean (LSM) change from baseline in subjective sleep efficiency (sSE). C, The LSM change from baseline in subjective wake-after-sleep onset (sWASO).
aP < .05 vs placebo.
bP < .01 vs placebo.
cP < .001 vs placebo.
dP ≤ .01 vs zolpidem.
eP < .05 vs zolpidem.
fP < .001 vs zolpidem.
Mean increases from baseline in subjective sleep efficiency during the first 7 nights and at the end of month 1 were larger and significantly different for lemborexant 5 mg and lemborexant 10 mg therapies (P < .001 for both time points) compared with placebo (LSM treatment difference vs placebo for lemborexant 5 mg during the first 7 nights, 3.8%; 95% CI, 1.6%-6.0%; P < .001 and at the end of month 1, 4.6%; 95% CI, 2.0%-7.2%; P < .001; LSM treatment difference vs placebo for lemborexant 10 mg during the first 7 nights, 6.8%; 95% CI, 4.6%-9.0%; P < .001 and at the end of month 1, 7.2%; 95% CI, 4.6%-9.8%; P < .001); comparisons with zolpidem therapy were not statistically significant for lemborexant 5 mg or lemborexant 10 mg therapies at either time point (Figure 3B and eTable in Supplement 2).
Mean decreases from baseline in sWASO during the first 7 nights and at the end of month 1 were larger and significantly different with lemborexant 5 mg (LSM treatment difference vs placebo for lemborexant 5 mg during the first 7 nights, −12.4 min; 95% CI, −21.8 to −3.1 min; P = .009 and at the end of month 1, −11.5 min; 95% CI, −22.4 to −0.6 min; P = .04) and lemborexant 10 mg (LSM treatment difference vs placebo for lemborexant 10 mg during the first 7 nights, −26.3 min; 95% CI, −35.7 to −17.0 min; P < .001 and at the end of month 1, −20.6 min; 95% CI, −31.5 to −9.6 min; P < .001) compared with placebo. Significant treatment differences compared with zolpidem therapy were not observed for lemborexant 5 mg or lemborexant 10 mg during the first 7 nights or for lemborexant 10 mg at the end of month 1. However, the mean decrease from baseline in sWASO observed with zolpidem therapy was larger and significantly different compared with lemborexant 5 mg therapy at the end of month 1 (LSM treatment difference vs zolpidem for lemborexant 5 mg, 14.5 min; 95% CI, 4.2-24.7 min; P = .006) (Figure 3C and eTable in Supplement 2).
Overall Insomnia Severity, Daily Functioning, and Rebound Insomnia During Follow-up
At the end of month 1, all active doses significantly decreased (ie, improved) the ISI total score and the ISI daily functioning score compared with placebo (Table 3); comparisons with zolpidem therapy were not significantly different for either dose of lemborexant therapy. There was no evidence of rebound insomnia during the 2 weeks after 1 month of treatment with either lemborexant or zolpidem therapies.
Table 3. Insomnia Severity and Daily Functioning End Points at the End of Month 1a.
| End Point | Placebo (n = 208) | Zolpidem ER 6.25 mg (n = 263) | Lemborexant 5 mg (n = 266) | Lemborexant 10 mg (n = 269) |
|---|---|---|---|---|
| ISI Total Score (Items 1-7) | ||||
| Baseline, mean (SD)b | 19.4 (3.6) | 19.2 (3.5) | 18.9 (3.5) | 19.0 (3.3) |
| Month 1, mean (SD)c | 13.3 (5.4) | 11.0 (5.4) | 11.2 (5.4) | 11.1 (5.6) |
| Change from baseline, mean (SD)c | –6.1 (5.5) | –8.3 (6.0) | –7.8 (5.5) | –7.9 (5.9) |
| LSM treatment difference vs placebo (95% CI)c | NA | –2.3 (–3.3 to –1.3) | –1.9 (–2.9 to –1.0) | –2.1 (–3.1 to –1.1) |
| P valued | NA | <.001 | <.001 | <.001 |
| LSM treatment difference vs zolpidem (95% CI)c | NA | NA | 0.4 (–0.6 to 1.3) | 0.2 (–0.7 to 1.2) |
| P valued | NA | NA | .45 | .64 |
| ISI Daytime Functioning (Items 4-7) | ||||
| Baseline, mean (SD)b | 11.2 (2.4) | 11.1 (2.5) | 10.9 (2.4) | 10.8 (2.3) |
| Month 1, mean (SD)c | 7.3 (3.6) | 5.9 (3.4) | 6.1 (3.5) | 6.1 (3.6) |
| Change from baseline, mean (SD)c | –3.9 (3.6) | –5.2 (3.8) | –4.8 (3.6) | –4.8 (3.7) |
| LSM treatment difference vs placebo (95% CI)c | NA | –1.4 (–2.1 to –0.8) | –1.1 (–1.7 to –0.5) | –1.1 (–1.7 to –0.5) |
| P valued | NA | <.001 | .001 | .001 |
| LSM treatment difference vs zolpidem (95% CI)c | NA | NA | 0.3 (–0.3 to 0.9) | 0.3 (–0.3 to 0.9) |
| P valued | NA | NA | .23 | .27 |
Abbreviations: ER, extended release; ISI, Insomnia Severity Index; LSM, least squares mean; NA, not applicable.
Measured by the ISI.
Sample sizes were 208 for placebo, 263 for zolpidem ER 6.25 mg, 266 for lemborexant 5 mg, and 269 for lemborexant 10 mg.
Sample sizes were 198 for placebo, 244 for zolpidem ER 6.25 mg, 257 for lemborexant 5 mg, and 253 for lemborexant 10 mg.
P values were based on an analysis of covariance model, with age group (55-64 years and ≥65 years), region (North America and Europe), and treatment as factors and baseline ISI value as a covariate.
Safety
The overall incidence of treatment-emergent AEs (TEAEs) was similar among treatment groups (Table 4). Nonserious AEs were mostly mild or moderate in severity. Although 6 participants (4 in the zolpidem group and 2 in the lemborexant 5 mg group) reported 8 serious AEs, none were treatment related; all AEs were transient and resolved completely. Fall was reported as a TEAE by 4 participants, all of whom received lemborexant 5 mg therapy, but none were considered related to the study drug by the investigator. Sleep paralysis was reported by 1 participant receiving lemborexant 5 mg and 3 participants receiving lemborexant 10 mg therapies. All TEAEs of sleep paralysis were determined to be of mild severity. No deaths occurred during the study. No AEs were adjudicated as cataplexy, as determined by the AE adjudication committee, and no complex sleep-related behaviors were reported. No evidence of suicidality was found, and no notable findings were reported for clinical laboratory tests, vital signs, weight, or electrocardiograms.
Table 4. Treatment-Emergent Adverse Events During Treatment and Follow-up Periods, Safety Analysis Seta.
| Event | No. (%) | |||
|---|---|---|---|---|
| Placebo (n = 209) | Zolpidem ER 6.25 mg (n = 263) | Lemborexant 5 mg (n = 266) | Lemborexant 10 mg (n = 268) | |
| TEAE | 53 (25.4) | 93 (35.4) | 74 (27.8) | 82 (30.6) |
| Treatment related | 16 (7.7) | 41 (15.6) | 30 (11.3) | 39 (14.6) |
| Severe | 3 (1.4) | 8 (3.0) | 1 (0.4) | 2 (0.7) |
| Serious | 0 | 4 (1.5) | 2 (0.8) | 0 |
| Leading to study discontinuation | 2 (1.0) | 7 (2.7) | 2 (0.8) | 3 (1.1) |
| Leading to interruption of study drug | 1 (0.5) | 2 (0.8) | 1 (0.4) | 0 |
| Death | 0 | 0 | 0 | 0 |
| TEAE reported in >2% of participants in any active treatment group | ||||
| Headache | 13 (6.2) | 14 (5.3) | 17 (6.4) | 13 (4.9) |
| Somnolence | 4 (1.9) | 4 (1.5) | 11 (4.1) | 19 (7.1) |
| Urinary tract infection | 2 (1.0) | 2 (0.8) | 3 (1.1) | 9 (3.4) |
| Nasopharyngitis | 3 (1.4) | 1 (0.4) | 7 (2.6) | 1 (0.4) |
| Upper respiratory tract infection | 4 (1.9) | 2 (0.8) | 6 (2.3) | 1 (0.4) |
| Dizziness | 4 (1.9) | 8 (3.0) | 3 (1.1) | 2 (0.7) |
Abbreviations: ER, extended release; TEAE, treatment-emergent adverse event.
A TEAE was defined as an adverse event with an onset date on or after the first dose of study drug was administered until 14 days after the last dose of study drug was administered. The follow-up period of at least 14 days (and ≤18 days) began when participants completed the 30-night treatment period.
Analyses of change in Tyrer Benzodiazepine Withdrawal Symptom Questionnaire scores provided no evidence of withdrawal. At the end of the study, mean (SD) scores were 1.1 (2.5) in the placebo group, 1.2 (2.7) in the zolpidem group, 0.8 (1.6) in the lemborexant 5 mg group, and 0.8 (1.6) in the lemborexant 10 mg group.
Discussion
This phase 3 study of lemborexant therapy in participants 55 years and older with insomnia disorder achieved its primary and key secondary objectives, demonstrating objectively that treatment with lemborexant therapy led to greater improvements in both PSG-based sleep onset and sleep maintenance outcomes compared with both placebo and zolpidem therapy. Improvements were also observed on self-reported measures of sleep onset and maintenance compared with placebo, as assessed by sleep diaries. Although lemborexant therapy provided a statistically significant benefit compared with zolpidem therapy on sSOL, lemborexant therapy did not provide a statistically significant benefit compared with zolpidem therapy on subjective sleep efficiency or sWASO. Lemborexant therapy was generally well tolerated, with rates of discontinuation as a result of TEAEs comparable with placebo.
The decreases in sleep-onset latency with lemborexant therapy were notable, as most participants fell asleep in less than 20 minutes, which is typical for those without insomnia.21 Participants receiving lemborexant therapy also gained more than 60 minutes of sleep per night than they had before treatment. Both doses were effective on the first 2 and last 2 nights of treatment, indicating that lemborexant therapy works immediately and over time. This finding is in contrast to zolpidem therapy, which improved sleep onset initially (nights 1 and 2) but became less effective over time, with no difference from placebo on LPS after the month of treatment. In a previous study, the effects of zolpidem therapy on LPS appeared to diminish over the 3-week testing interval.22 Although LPS was still significantly shorter with zolpidem therapy than placebo at nights 15 and 16, by the second week of treatment, self-reported sleep latency was no longer different from placebo in that study.22
The reduction in time spent awake during the night (>45 minutes overall for lemborexant therapy) occurred mostly in the latter half of the sleep period, which is the time when most people with sleep maintenance insomnia have difficulty staying asleep.5 Walsh et al22 reported that the mean reduction of WASO in the zolpidem group was approximately 32 minutes on nights 1 and 2 and 18 minutes on nights 15 and 16, with most of the improvements occurring in the first 6 hours.
Self-rated severity of insomnia symptoms was reduced with both doses of lemborexant therapy at the end of treatment. This improvement was seen in all active treatment groups and was not unexpected, as there was some benefit on sleep from zolpidem treatment.
In this study, lemborexant therapy was generally well tolerated over 1 month, and nearly all participants completed the treatment period. As expected from an earlier phase 2 study of lemborexant therapy, a dose response in somnolence was observed.14 Similar rates of somnolence were reported in a pooled analysis of phase 3 clinical trials of suvorexant therapy (6.7% for 20/15 mg)23 and in a clinical trial of zolpidem therapy (6%),22 both of which were conducted with older patients.
Limitations
Several considerations limit the interpretation and applicability of these findings. The study was restricted to participants 55 years and older; therefore, a fixed, albeit age-appropriate and sex-appropriate, dose of zolpidem was used. Because the study period was 1 month, the effects of long-term use of lemborexant therapy are not yet known. Participants enrolled were required to have sleep maintenance insomnia. However, to our knowledge, this was the first phase 3 clinical trial to compare a dual orexin receptor antagonist with a commonly prescribed sedative-hypnotic medication using objective and subjective measures of sleep, and it provides important information about the use of lemborexant therapy for the treatment of insomnia in older patients.
In general, participants in the zolpidem group had larger decreases from baseline in sWASO than were observed in PSG-based WASO, and participants in the lemborexant 5 mg group had smaller decreases in sWASO than were observed in PSG-based WASO. Possible reasons for the disagreement between the objective and subjective measures of WASO may include effects on memory and recall related to zolpidem treatment.
Conclusions
In this study, lemborexant therapy provided more sleep promotion than placebo and zolpidem therapy based on objective and subjective patient-reported measures of sleep onset and maintenance in participants 55 years and older. Effects were observed at the start of treatment and were maintained at 1 month. Lemborexant therapy was well tolerated and effective, especially in the latter half of the night, in these older individuals with sleep maintenance difficulties. Results of this first head-to-head phase 3 clinical trial are encouraging and support continued development of lemborexant therapy for the treatment of insomnia disorder.
Trial Protocol
eAppendix 1. Trial Protocol Amendments
eAppendix 2. Supplemental Methods
eTable. Summary of Sleep Onset and Sleep Maintenance End Points Measured by Sleep Diary at the Beginning (First 7 Nights) and End (Month 1) of Treatment by Treatment Group in Subjects Aged >55 Years With Insomnia Disorder
eFigure 1. Flowchart of the Gate-Keeping Procedure for Handling Multiple Comparisons
eFigure 2. Change From Baseline WASO by Quarter of the Night
eReferences.
Data Sharing Statement
References
- 1.Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015;99(2):-. doi: 10.1016/j.mcna.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504. [PMC free article] [PubMed] [Google Scholar]
- 3.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991-999. doi: 10.1001/jama.281.11.991 [DOI] [PubMed] [Google Scholar]
- 4.Buenaver LF, Townsend D, Ong JC. Delivering cognitive behavioral therapy for insomnia in the real world: considerations and controversies. Sleep Med Clin. 2019;14(2):275-281. doi: 10.1016/j.jsmc.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 5.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98:653-665. doi: 10.1016/B978-0-444-52006-7.00041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35. [PubMed] [Google Scholar]
- 7.Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening—temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17(6):551-554. [PubMed] [Google Scholar]
- 8.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. doi: 10.1136/bmj.38623.768588.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372. doi: 10.1016/j.clinthera.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 10.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Wang Q, Ji B, et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beuckmann CT, Suzuki M, Ueno T, Nagaoka K, Arai T, Higashiyama H. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287-295. doi: 10.1124/jpet.117.241422 [DOI] [PubMed] [Google Scholar]
- 14.Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289-1299. doi: 10.5664/jcsm.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1). https://apps.who.int/medicinedocs/documents/s22154en/s22154en.pdf. Published June 10, 1996. Accessed November 8, 2019.
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed January 7, 2019.
- 18.Ambien CR tablets [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2014.
- 19.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 20.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19(1):53-61. doi: 10.1016/0165-0327(90)90009-W [DOI] [PubMed] [Google Scholar]
- 21.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleeP values across the human lifespan. Sleep. 2004;27(7):1255-1273. doi: 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 22.Walsh JK, Soubrane C, Roth T. Efficacy and safety of zolpidem extended release in elderly primary insomnia patients. Am J Geriatr Psychiatry. 2008;16(1):44-57. doi: 10.1097/JGP.0b013e3181256b01 [DOI] [PubMed] [Google Scholar]
- 23.Herring WJ, Connor KM, Snyder E, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12(9):1215-1225. doi: 10.5664/jcsm.6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Trial Protocol Amendments
eAppendix 2. Supplemental Methods
eTable. Summary of Sleep Onset and Sleep Maintenance End Points Measured by Sleep Diary at the Beginning (First 7 Nights) and End (Month 1) of Treatment by Treatment Group in Subjects Aged >55 Years With Insomnia Disorder
eFigure 1. Flowchart of the Gate-Keeping Procedure for Handling Multiple Comparisons
eFigure 2. Change From Baseline WASO by Quarter of the Night
eReferences.
Data Sharing Statement