Key Points
Question
What is the incidence of hospital-onset Clostridium difficile infection (CDI) and its associated length of stay?
Findings
This systematic review and meta-analysis of 13 studies using patient-days as the denominator found that the incidence of hospital-onset CDI was 8.3 cases per 10 000 patient-days. Among propensity score–matched studies of the length of stay, the mean difference in length of stay between patients with and those without CDI varied from 3.0 to 21.6 days.
Meaning
Pooled estimates from currently available literature suggest that CDI is associated with a large burden on the US health care system.
This systematic review and meta-analysis of 13 studies analyzes the incidence of Clostridium difficile infection and its associated hospital length of stay in the United States.
Abstract
Importance
An understanding of the incidence and outcomes of Clostridium difficile infection (CDI) in the United States can inform investments in prevention and treatment interventions.
Objective
To quantify the incidence of CDI and its associated hospital length of stay (LOS) in the United States using a systematic literature review and meta-analysis.
Data Sources
MEDLINE via Ovid, Cochrane Library Databases via Wiley, Cumulative Index of Nursing and Allied Health Complete via EBSCO Information Services, Scopus, and Web of Science were searched for studies published in the United States between 2000 and 2019 that evaluated CDI and its associated LOS.
Study Selection
Incidence data were collected only from multicenter studies that had at least 5 sites. The LOS studies were included only if they assessed postinfection LOS or used methods accounting for time to infection using a multistate model or compared propensity score–matched patients with CDI with control patients without CDI. Long-term-care facility studies were excluded. Of the 119 full-text articles, 86 studies (72.3%) met the selection criteria.
Data Extraction and Synthesis
Two independent reviewers performed the data abstraction and quality assessment. Incidence data were pooled only when the denominators used the same units (eg, patient-days). These data were pooled by summing the number of hospital-onset CDI incident cases and the denominators across studies. Random-effects models were used to obtain pooled mean differences. Heterogeneity was assessed using the I2 value. Data analysis was performed in February 2019.
Main Outcomes and Measures
Incidence of CDI and CDI-associated hospital LOS in the United States.
Results
When the 13 studies that evaluated incidence data in patient-days due to hospital-onset CDI were pooled, the CDI incidence rate was 8.3 cases per 10 000 patient-days. Among propensity score–matched studies (16 of 20 studies), the CDI-associated mean difference in LOS (in days) between patients with and without CDI varied from 3.0 days (95% CI, 1.44-4.63 days) to 21.6 days (95% CI, 19.29-23.90 days).
Conclusions and Relevance
Pooled estimates from currently available literature suggest that CDI is associated with a large burden on the health care system. However, these estimates should be interpreted with caution because higher-quality studies should be completed to guide future evaluations of CDI prevention and treatment interventions.
Introduction
Clostridium difficile (also known as Clostridioides difficile) is the most common pathogen causing health care–associated infections in the United States, accounting for 15% of all such infections.1 A Centers for Disease Control and Prevention report on antibiotic resistance threats categorized C difficile as an urgent threat.2 Antibiotic treatment for C difficile infection (CDI) is often followed by recurrent infection, leading to nontraditional treatments, such as fecal transplant and oral administration of nontoxigenic C difficile spores.3,4
Information about the burden of CDI in the United States could inform investments in prevention and treatment interventions. This information should include the incidence of CDI, how this incidence has changed over time, and poor outcomes associated with CDI. Although prior studies have shown that CDI is associated with poor outcomes, such as recurrence, long hospital length of stay (LOS), mortality, and high treatment costs, these results vary by study location and patient population.2,5 In addition, many current estimates of the poor outcomes and costs associated with CDI do not take into account the underlying severity of illness among patients who develop CDI and may overestimate the true attributable outcomes.6
To address gaps in our understanding of the current burden associated with CDI in the United States, we conducted a systematic literature review of studies conducted in the United States and published after 2000 that evaluated the incidence of CDI and associated LOS. The goals were to describe the recent incidence of CDI and to evaluate LOS attributable to CDI.
Methods
Search Strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)7 and Meta-analysis of Observational Studies in Epidemiology (MOOSE)8 reporting guidelines. An experienced health sciences librarian (A.B.) conducted systematic searches in MEDLINE via Ovid, Cochrane Library Databases via Wiley, Cumulative Index of Nursing and Allied Health Complete via EBSCO Information Services, Scopus, and Web of Science to identify articles published from the inception of the database to February 2019. Citations published before 2000 were excluded. A combination of keywords and subject headings were used for “Clostridium difficile,” “length of stay,” and “incidence.” The full search strategies can be found in eAppendix 1 in the Supplement.
Inclusion and Exclusion Criteria
Publications were included if they evaluated the incidence of CDI or LOS associated with CDI. Studies were excluded if they did not contain original data, did not have a control group, were published outside the United States, were published in a language other than English, or were published before 2000. The year 2000 was chosen as the beginning of this systematic literature review because that was when the epidemic BI/NAP1/027 strain of C difficile emerged, after which CDI increased in prevalence and became less responsive to treatment.4 We excluded studies if they assessed only a specific subset of patients, unless that population could be categorized as 1 of the following subsets: immunocompromised patients, patients in the intensive care unit, patients with cancer, patients with end-stage renal disease, patients undergoing hemodialysis, surgical patients, solid-organ transplant recipients, patients with high-risk gastrointestinal conditions, or peripartum women. We excluded studies with a study period of less than 1 year. We also excluded studies of long-term care facilities. Incidence data were collected only from multicenter studies that had at least 5 sites, because single-site or small studies may be biased by outbreaks or other local conditions. We included incidence studies with denominators of patient-days or person-years, known timing of the CDI such as after surgery or after admission (ie, hospital onset [HO]), or exclusion of patients with a history of CDI.
Studies were included in the LOS analysis only if they provided data on postinfection LOS, if they used methods accounting for time to infection using a multistate model, or if propensity score–matched patients with CDI were compared with uninfected controls.5,9 Studies were excluded if they did not have an uninfected control group or a denominator that included patients without CDI.
Data Extraction and Quality Assessment
Titles and abstracts of all articles were screened to assess inclusion criteria. Two of 9 independent reviewers (M.L.S., M.A.W., M.F.K., H.-Y.C., M.L.C., L.A.H., D.J.D., A.R.M., and E.N.P.) abstracted data for each article. Reviewers resolved disagreements by consensus.
The reviewers abstracted data on study design, study population, setting and years, inclusion and exclusion criteria, number of patients included, description of control group, definition of CDI, outcomes (eg, incidence and LOS), and an assessment of the potential risk of bias. Risk of bias was assessed using the Downs and Black scale.10 Reviewers followed all questions from this scale as written except for question 27 (a single item on the Power subscale, which was scored 0-5), which was changed to a yes or no. Two of us (A.R.M. and M.L.S.) performed component quality analysis independently, reviewed all inconsistent assessments, and resolved disagreements by consensus.11
Statistical Analysis
Data analysis was performed in February 2019. Excel spreadsheet software version 2007 (Microsoft Corp) and RevMan statistical software version 5.3 (Cochrane Community) were used for statistical analysis. Incidence data were pooled only when the denominators used the same units (eg, patient-days). These data were pooled by summing the number of HO-CDI incident cases and the denominators across studies. Pooled incidence was reported as the number of incident cases per the given denominator (eg, 10 000 patient-days).12 No P values were calculated.
Results
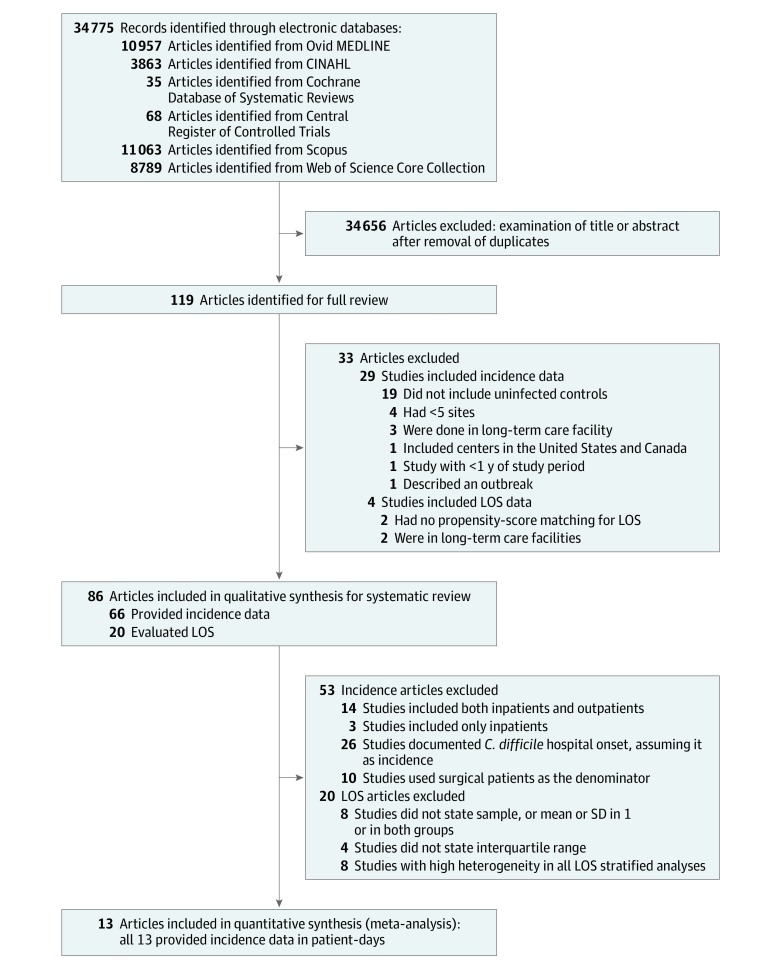
Of the 34 775 articles identified (Figure), 119 were full-text articles, and 86 (72.3%) of those articles met the selection criteria and were included in the systematic literature review.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93 Among these, 66 articles evaluated incidence,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78 and 20 articles evaluated LOS.16,54,66,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95 One-fifth of the studies that assessed LOS (4 studies)84,87,91,94 scored 18 or more points of the 28 points possible on the Downs and Black scale10 and, thus, were considered to be of higher quality.
Figure. Literature Search for Articles That Evaluated Incidence and Length of Stay (LOS) Associated With Clostridium difficile Infection.
CINAHL indicates Cumulative Index of Nursing and Allied Health.
Incidence of CDI Calculated Using Patient-Days (13 Studies)
Sixty-six studies13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78 measured CDI incidence. Thirteen of those 66 studies13,14,15,16,17,18,19,20,21,22,23,24,25 used patient-days as the denominator (Table 1). Among these studies, the CDI incidence varied from 2.8 CDI cases per 10 000 patient-days22 to 15.8 CDI cases per 10 000 patient-days.20 Three studies13,17,23 were conducted by the Centers for Disease Control and Prevention. Three studies17,18,21 were done in New York State. One study24 from Southern California found that the incidence of community-onset, health care facility (HCF)–associated CDI (11.1 cases per 10 000 patient-days) was almost 2-fold higher than that for HO, HCF-associated CDI (6.8 cases per 10 000 patient-days). The pooled incidence of HO-CDI among the 13 studies13,14,15,16,17,18,19,20,21,22,23,24,25 (Table 1) that used patient-days as the denominator was 8.3 CDI cases per 10 000 patient-days. Four studies13,15,18,21 included more than 100 facilities.
Table 1. Multicenter Studies (≥5 Sites) That Evaluated Clostridium difficile Infection Incidence Calculated Using Patient-Days.
| Source | Data Set | Study Period | Facilities or Hospitals, No. | C difficile Definition | Incidence |
|---|---|---|---|---|---|
| Archibald et al,13 2004 | CDC’s National Nosocomial Infections Surveillance | 1987-2001 | 90-340 Hospitals depending on year | CDC definition | Teaching hospital intensive care units, 5.1 cases/10 000 patient-days; nonteaching hospital intensive care units, 4.4 cases/10 000 patient-days |
| Burger et al,14 2006 | Veterans’ Health Administration East Coast Infection Control Council hospitals | Q3 1999 to Q4 2002 | 32 | CDC definition | 7.9 Cases/10 000 patient-days |
| Campbell et al,15 2009 | State of Ohio | January 1, 2006, to December 31, 2006 | 210 | ICD-9 code, laboratory tests, clinical findings | 6.4-7.9 Cases/10 000 patient-days |
| Dubberke et al,16 2010 | Hospitals in CDC Epicenter Network | July 2000 to June 2006 | 5 | C difficile toxin assay results and the ICD-9 code | HO, HCF–associated cases: 7.0 cases/10 000 patient-days in 2001 and 8.5 cases/10 000 patient-days in 2006 |
| Gase et al,17 2013 | New York State National Healthcare Safety Network | July-December 2009 | 30 | Clinical findings, laboratory tests | 9.66 Cases/10 000 patient-days (95% CI, 9.21-10.1 cases/10 000 patient-days) |
| Haley et al,18 2014 | New York hospital discharge billing records | 2010 | 124 | Clinical findings, laboratory tests | 11.6 Cases/10 000 patient-days |
| Kim et al,19 2008 | Pediatric Health Information System Database | 2001-2006 | 22 | ICD-9 code, billing charge for C difficile toxin assay, and an initial dose of C difficile antibiotic therapy in the period of 1 d before to 2 d after C difficile toxin assay | 2001, 4.4 Cases/10 000 patient-days; 2006, 6.5 cases/10 000 patient-days |
| Kamboj et al,20 2012 | Comprehensive Cancer Center’s Infection Control Group Network of Patients with Cancer or Hematopoietic Stem Cell Transplant | 2010-2011 | 11 | Laboratory tests and C difficile surveillance | HO C difficile infection, 15.8 cases/10 000 patient-days |
| McDonald et al,21 2012 | 3 State-led programs (Illinois, Massachusetts, New York) | 2008-2011 | 711 | Clinical findings, laboratory tests | 7.4 Cases/10 000 patient-days |
| Miller et al,22 2011 | Duke Infection Control Outreach Network | 2005-2009 | 28 | Infection preventionist evaluated surveillance or diagnosis | 2.8 Cases/10 000 patient-days |
| Sohn et al,23 2005 | Hospitals in CDC Epicenter Network | 2000-2003 | 7 | Clinical findings, laboratory tests, and CDC surveillance of C difficile | 12.1 Cases/10 000 patient-days (mean range, 3.1-25.1 cases/patient-days); 7.4 cases/1000 admissions (mean range, 3.1-13.1 cases/1000 admissions) |
| Tartof et al,24 2014 | Kaiser Permanente Southern California health care system | 2011-2012 | 14 | Laboratory tests: polymerase chain reaction | Community-onset, HCF-associated, 11.1 cases/10 000 patient-days; HO, HCF-associated, 6.8 cases/10 000 patient-days |
| Zilberberg et al,25 2011 | CareFusion clinical research database | January 2007 to June 2008 | 85 | Laboratory tests | 6.3 Cases/10 000 patient-days |
Abbreviations: CDC, Centers for Disease Control and Prevention; HCF, health care facility; HO, hospital onset; ICD-9, International Classification of Diseases, Ninth Revision; Q, quarter.
The definitions of C difficile used to identify cases varied. Three studies17,18,21 used clinical findings and results of laboratory tests for C difficile, 3 studies13,14,23 used the Centers for Disease Control and Prevention surveillance definition to identify C difficile, 2 studies20,22 applied infection preventionist evaluations for C difficile surveillance, and 2 studies24,25 used only results of laboratory tests for C difficile. The remaining studies used a variety of ways to identify CDI, including International Classification of Diseases, Ninth Revision (ICD-9) codes or other billing codes,15,16,19 laboratory test results,15,16,20,23 clinical findings,15,23 and initial doses of C difficile antibiotic therapy.19 When we examined incidence by time period, we found that the early studies from 2000 to 2008 had a range from 2.8 to 12.2 CDI cases per 10 000 patient-days, studies from 2008 to 2009 had a range from 6.3 to 9.6 CDI cases per 10 000 patient-days, and the later studies after 2010 reported a range from 6.8 to 15.8 CDI cases per 10 000 patient-days (Table 1).
Incidence of CDI Calculated Using Person-Years (17 Studies)
Fourteen studies26,27,28,29,30,31,32,33,34,35,36,37,38,39 included both inpatients and outpatients (Table 2), reflected in a denominator of person-years in 8 studies.27,28,29,30,32,34,36,39 Seven of those 14 studies27,28,29,30,32,34,39 used only ICD-9 codes to define CDI. In a study36 of adult and adolescent patients with HIV/AIDS that included more than 100 hospitals, during 10 years of study, the peak incidence of CDI was 9.59 cases per 1000 person-years among patients with clinical AIDS. A study28 of the Armed Forces Health Surveillance Center in Maryland over the course of 12 years found the incidence of community-associated CDI to be 5.5 cases per 100 000 person-years. In a study29 evaluating the annual incidence of CDI and multiply recurrent CDI per 1000 person-years, the incidences increased by 42.7% and 188.8%, respectively, during a decade (2001-2012) in the United States. In another study30 with 12 years of data from 5 administrative databases, elderly people (ie, aged >65 years) had a CDI rate of 677 cases per 100 000 person-years. In contrast, a managed-care organization in Colorado found that the CDI incidence in 2007 was 14.9 CDI cases per 10 000 patient-years.32 These studies were too diverse to pool together into 1 estimate.
Table 2. Multicenter Studies (≥5 Sites) That Evaluated Clostridium difficile Infection Incidence Calculated Using Person-Years.
| Source | Data Set | Study Period | Facilities or Hospitals, No. | C difficile Definition | Incidence |
|---|---|---|---|---|---|
| Denominator: geographic population (inpatient and outpatient) | |||||
| Chernak et al,26 2005 | Philadelphia, Pennsylvania, and surrounding 4 counties | 2004-2005 | Not stated | Clinical diagnosis | Community-associated, 7.6 cases/100 000 population |
| Dubberke et al,27 2016 | Medicare Chronic Condition Warehouse (5% random sample) | 2009 | 5% Random sample | ICD-9 | Overall incidence of CDI, 677 cases/100 000 persons |
| Gutiérrez et al,28 2013 | Defense Medical Surveillance Center, Armed Forces Health Surveillance Center, US Department of Defense, Silver Spring, Maryland | 1998-2010 | Not stated | ICD-9 | C difficile–associated disease incidence, 13.2 cases/100 000 person-years; community-associated, 5.5 cases/100 000 person-years; health care C difficile–associated disease, 1.3 cases/1000 hospitalizations |
| Ma et al,29 2017 | OptumInsight Clinformatics Database | 2001-2012 | 38 911 718 Commercially insured patients | ICD-9 | Annual incidence of CDI and multiply recurrent CDI per 1000 person-years increased by 42.7% (from 0.4408 to 0.6289 case) and 188.8% (from 0.0107 to 0.0309 case), respectively |
| Olsen et al,30 2016 | 5 Databases: Medicare 5% Sample, Healthcare Cost and Utilization Project State Inpatient Databases and the National Inpatient Sample, OptumInsight Retrospective Database, and Premier Perspective | 2000-2012 | Not stated | ICD-9 | Adults aged <65 y, 66.0 cases/100 000 person-years for OptumInsight Retrospective Database and 37.5 cases/100 000 person-years for State Inpatient Databases; adults aged >65 y, 677 cases/100 000 person-years for Medicare and 383 cases/100 000 person-years for State Inpatient Databases |
| Rabatsky-Ehr et al,31 2008 | Connecticut Department of Health reportable conditions surveillance system | 2006 | 28 Hospitals and US Census for Connecticut | Clinical findings, laboratory tests | 6.9 Cases/100 000 population |
| Kuntz et al,32 2012 | Kaiser Permanente Colorado and Kaiser Permanente Northwest (both inpatient and outpatient) | 2007 | Not stated | ICD-9 code and positive test result needing antibiotic dispensation | 14.9 Cases/10 000 patient-years; for women, 213 cases/100 000 enrollees aged 60-69 y, 420 cases/100 000 enrollees aged 70-79 y, and 795 cases/100 000 enrollees aged ≥80 y; for men, 167 cases/100 000 enrollees aged 60-69 y, 311 cases/100 000 enrollees aged 70-79 y, and 871 cases/100 000 enrollees aged ≥80 y |
| Lessa et al,33 2014 | Centers for Disease Control and Prevention Emerging Infections Program | 2010 | CDI surveillance in selected counties across 7 US states | Laboratory test (nucleic acid amplification) | Crude incidence varied by geographic area; community-associated, 30.7-41.3 cases/100 000 population; health care–associated, 58.5-94.8 cases/100 000 population |
| Reveles et al,34 2017 | Veterans Affairs Informatics and Computing Infrastructure | 2002-2014 | 150 VHA hospitals and 820 VHA clinics | ICD-9 and positive test result for CDI | Overall, 3.1 cases/10 000 VHA enrollees; 2002, 1.6 cases/10 000 VHA enrollees; 2013, 5.1 cases/10 000 VHA enrollees; 2014, 4.6 cases/10 000 VHA enrollees |
| Rhee et al,35 2014 | Centers for Disease Control and Prevention Emerging Infections Program | 2010-2011 | CDI surveillance in Monroe County, New York | Clinical diagnosis plus laboratory tests; enzyme immunoassay toxin or glutamate dehydrogenase with enzyme immunoassay toxin or nucleic acid amplification test | 2010, 33.8 cases/100 000 population; 2011, 45.8 cases/100 000 population |
| Sanchez et al,36 2005 | Adult or adolescent spectrum of HIV disease project (inpatient and outpatient) | 1992-2002 | >100 Hospitals | Clinical findings, laboratory tests | All patients with HIV or AIDS, 4.12 cases/1000 person-years; patients with immunologic AIDS, 2.10 cases/1000 person-years; patients with clinical AIDS, 9.59 cases/1000 person-years |
| Troppy et al,37 2019 | 3 Sources of data: Massachusetts Virtual Epidemiology Network, National Healthcare Safety Network, and 2010 US Census data in Massachusetts | 2016 | Not stated | Laboratory tests | 132.5 Cases/100 000 population |
| Wendt et al,38 2014 | Centers for Disease Control and Prevention Emerging Infections Program in selected counties in 10 US states (California, Colorado, Connecticut, Georgia, Minnesota, New York, Oregon, Tennessee, Maryland, and New Mexico) | 2010-2011 | Not stated | Infection preventionist evaluated surveillance or diagnosis | Of 944 pediatric CDI cases identified, 71% were in California; CDI incidence children was highest among children aged 1 y (66.3 cases/per 100 000) |
| Young-Xu et al,39 2015 | VHA health care records | 2009-2013 | 152 Hospitals | ICD-9 and positive test for CDI | Overall CDI rate increased by 8.4% from 193 episodes/100 000 patient-years in 2009 to 209 episodes/100 000 patient-years in 2013 |
| Denominator: geographic population (only inpatient) | |||||
| Argamany et al,40 2015 | US National Hospital Discharge Survey | 2001-2010 | National Hospital Discharge Survey data are collected manually or automatically by trained hospital staff, US Census Bureau staff, or National Center for Health Statistics staff | ICD-9 | Pediatric population: 1.2 CDI discharges/1000 total discharges |
| Zilberberg et al,41 2008 | AHRQ National Inpatient Sample infant patients | 2000-2005 | Not stated | ICD-9 | 2000, 2.8 Cases/10 000 hospitalizations in infants; 2005, 5.1 cases/10 000 hospitalizations in infants |
| Zilberberg et al,42 2008 | AHRQ National Inpatient Sample adult patients | 2000-2005 | Not stated | ICD-9 | 2000, 5.5 Cases/10 000 hospitalizations in adults; 2005, 11.2 cases/10 000 hospitalizations in adults |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CDI, Clostridium difficile infection; ICD-9, International Classification of Diseases, Ninth Revision; VHA, Veterans Health Administration.
Three studies40,41,42 included only inpatients (Table 2). Two of these studies41,42 assessed the Agency for Healthcare Research and Quality (AHRQ) National Inpatient Sample (NIS). One evaluated infant patients from the AHRQ NIS cohort,41 and the other study evaluated adult patients from the AHRQ NIS cohort.42 Both studies documented substantial increases in CDI incidence between 2000 and 2005, from 2.8 to 5.1 cases per 10 000 hospitalizations, and from 5.5 to 11.2 cases per 10 000 hospitalizations, respectively.41,42 The third study,40 which was from the US National Hospital Discharge Survey between 2001 and 2010, found that the incidence of CDI in the pediatric population was 1.2 CDI discharges per 1000 total discharges.
Incident Cases of CDI (36 Studies)
Twenty-six studies43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68 documented HO-CDIs, which we assumed were incident cases (Table 3). Of these studies, the AHRQ NIS was the main data set, represented by 10 included studies.43,45,47,50,51,56,58,59,61,68 These studies assessed diverse patient populations with different comorbidities, including peripartum women68 and patients with inflammatory bowel disease,43 lymphoma,45 leukemia,58 subarachnoid hemorrhage treated with microsurgical or endovascular aneurysm repair,47 chronic liver disease,50 hematopoietic stem cell transplant,51 megacolon,56 or heart failure.59 Thus, the results of these studies were also too diverse to pool together. One study68 found that the CDI incidence among peripartum women increased from 0.36 cases per 10 000 in 1998 to 0.70 cases per 10 000 in 2006. The US National Hospital Discharge Survey database was represented in 6 included studies.49,52,53,55,64,65 These studies also assessed diverse patient populations, including children52 and adults with different comorbidities, such as cancer49,52 and inflammatory bowel disease.65 In 1 of these studies,65 the overall incidence of HO-CDI was 369.8 cases per 10 000 hospitalizations for inflammatory bowel disease. In that same study,65 the HO-CDI incidence was 445.6 cases per 10 000 hospitalizations for ulcerative colitis and 220.3 cases per 10 000 hospitalizations for Crohn disease.
Table 3. Multicenter Studies (≥5 Sites) That Evaluated Clostridium difficile Infection Incidence Using Incident Cases.
| Source | Data Set | Study Period | Facilities or Hospitals, No. | C difficile Definition | Incidence |
|---|---|---|---|---|---|
| HO infections | |||||
| Barber et al,43 2018 | AHRQ NIS patients with inflammatory bowel disease | 1998-2014 | Approximately 1000 hospitals | ICD-9 | Incidence of HO-CDI, 7.8 cases/1000 hospitalizations in 1998 and 32.1 cases/ 1000 hospitalizations in 2014 among patients with Crohn disease, and 23.0 cases/1000 hospitalizations in 1998 and 84.7 cases/1000 hospitalizations in 2014 among patients with ulcerative colitis |
| Barlam et al,44 2018 | Truven Health Marketscan Commercial Claims and Encounters database | 2011-2013 | This database represents approximately 50 million covered lives (annually) for employed subscribers aged <65 y and their dependents | ICD-9 | 4 080 597 Unique individuals aged 1-64 y were admitted to the hospital in 2011; 12 025 had ≥1 C difficile diagnosis and complete enrollment information for 2011 (12 025 / 4 080 597 = 0.29%) |
| Bhandari et al,45 2018 | AHRQ NIS database | 2007-2011 | 20% Stratified sample of US community hospitals | ICD-9 | Incidence of HO-CDI was 2.13% among patients with lymphoma and 0.8% among patients without lymphoma |
| Brown et al,46 2017 | VA health care system | January 2006- December 2012 | 131 Acute care facilities | Laboratory tests | 15.6 CDI cases/10 000 person-days |
| Dasenbrock et al,47 2016 | AHRQ NIS patients with subarachnoid hemorrhage who underwent microsurgical or endovascular aneurysm repair | 2002-2011 | Approximately 1000 hospitals | ICD-9 | Incidence of HO-CDI was 1.9% |
| Davis et al,48 2018 | Electronic medical record of the health system | 2014-2016 | 5-Hospital health system in Houston, Texas | Laboratory tests | Incidence of HO-CDI was 1.52% |
| Delgado et al,49 2017 | US NHDS | 2001-2010 | Not stated | ICD-9 | Incidence of HO-CDI was 8.6 cases/1000 cancer discharges |
| Dotson et al,50 2018 | AHRQ NIS patients with chronic liver disease | 2009 | Approximately 1000 hospitals | ICD-9 | Incidence of HO-CDI was 189.4 cases/10 000 discharges |
| Guddati et al,51 2014 | AHRQ NIS database | 2000-2009 | 20% Stratified sample of US community hospitals | ICD-9 | Incidence of HO-CDI among hematopoietic stem cell transplant recipients was 4.7%; nontransplant discharges were 0.86 cases/100 hospitalized patients |
| Gupta et al,52 2016 | US NHDS | 2005-2009 | Not stated | ICD-9 | Overall HO-CDI incidence in children was 33.5 cases/10 000 hospitalizations |
| Gupta et al,53 2017 | US NHDS | 2001-2010 | 100 Hospitals | ICD-9 | Incidence of HO-CDI in patients with cancer was 64.7 cases/10 000 discharges in 2001-2002 and 109.1 cases/10 000 discharges in 2009-2010 |
| Jiang et al,54 2013 | Rhode Island Hospital Discharge Database | 2010-2011 | 11 Hospitals | ICD-9 excluding present on admission code | HO-CDI, 1211 infections among 225 999 discharges = 53.5 cases/10 000 discharges |
| Khanna et al,55 2016 | US NHDS | 2005-2009 | 100 Hospitals | ICD-9 | HO-CDI incidence was 77.8 cases/10 000 hospitalizations |
| Kuy et al,56 2016 | AHRQ NIS patients with both C difficile and megacolon | 2000-2010 | Approximately 1000 hospitals | ICD-9 | Overall incidence of megacolon among all hospitalized patients was 0.02% from 2000 to 2010; percentage of cases of megacolon due to CDI was 3.61% in 2000 and 9.39% in 2010 |
| Lessa et al,57 2015 | Centers for Disease Control and Prevention Emerging Infections Program | 2011 | 10 Program sites across 34 counties | Laboratory tests | 453 000 Incident infections |
| Luo et al,58 2015 | AHRQ NIS patients with CDI with leukemia | 2005-2011 | Approximately 1000 hospitals | ICD-9 | Overall incidence of CDI among patients with leukemia, 3.4%; incidence of CDI among all hospitalized patients, 0.85%; incidence of CDI among patients with leukemia in 2005, 3.0%; incidence of CDI among patients with leukemia in 2011, 3.5% |
| Mamic et al,59 2016 | AHRQ NIS database | 2012 | 20% Stratified sample of US community hospitals | ICD-9 | HO-CDI incidence among patients with a discharge diagnosis of heart failure, 3.5% |
| Miller et al,60 2016 | Healthcare Cost and Utilization Project State Inpatient Database for California | 2005-2011 | 480 Hospitals | ICD-9 | Overall incidence of HO-CDI, 0.15 cases/100 patients |
| Miller et al,61 2016 | AHRQ NIS database | 2009-2011 | 480 Hospitals | ICD-9 | HO-CDI incidence, 0.85 cases/100 patients in 2009, 0.89 cases/100 patients in 2010, and 0.99 cases/100 patients in 2011 |
| Pant et al,62 2016 | Kids’ Inpatient Database (Healthcare Cost and Utilization Project) | 2003-2012 | Contains data from a variety of hospitals, including nonfederal, short-term, general, and special hospitals (including children’s hospitals) accessible by the general public | ICD-9 | Incidence rate of CDI increased from 24.0 to 58.0 cases/10 000 discharges per year (P < .001) across all age groups, with the greatest increase in children aged ≥15 y |
| Pant et al,63 2016 | 2012 | Rate of CDI infection in children without solid-organ transplant was 0.6% and was greater (3.6%) in children with solid-organ transplant | |||
| Reveles et al,64 2014 | US NHDS of hospitalized adults | 2001-2010 | 100 Hospitals | ICD-9 | Incidence of HO-CDI, 4.5 cases/1000 adult discharges in 2001 and 8.2 cases/1000 adult discharges in 2010 |
| Saffouri et al,65 2017 | US NHDS inflammatory bowel disease hospitalizations | 2005-2009 | 100 Hospitals | ICD-9 | Overall incidence of HO-CDI was 369.8 cases/10 000 inflammatory bowel disease hospitalizations; HO-CDI incidence was 445.6 cases/10 000 ulcerative colitis hospitalizations and 220.3 cases/10 000 Crohn disease hospitalizations |
| Sammons et al,66 2013 | Pediatric Health Information System Database | 2006-2011 | 41 Pediatric hospitals | ICD-9 and positive test for CDI | 5107 Cases/693 516 patients; 73.6 cases/10 000 patients |
| Murphy et al,67 2012 | California hospital discharge data | 2000-2007 | 29 Hospitals | ICD-9 | 28.7 Cases/10 000 admissions in 2000 and 52.2 cases/10 000 admissions in 2007 |
| Kuntz et al,68 2010 | AHRQ NIS women hospitalized for childbirth and delivery | 1998-2006 | 20% Stratified sample of discharges from nonfederal acute care hospitals | ICD-9 | CDI incidence ranged from 0.36 CDI cases/10 000 peripartum women in 1998 to 0.70 CDI cases/10 000 peripartum women in 2006 |
| Denominator: surgical patients | |||||
| Aquina et al,69 2016 | Statewide Planning and Research Cooperative System (a hospital discharge database by the New York Department of Health) | 2005-2013 | Patient-level data for all hospital admissions, ambulatory surgery procedures, and emergency department visits within New York State | ICD-9 | 22 Cases of CDI/1000 discharges |
| Bovonratwet et al,70 2018 | American College of Surgeons National Surgical Quality Improvement Program database | 2015 | 500 Institutions | Clinical findings, laboratory tests | 0.11% of the population had postoperative CDI |
| Bovonratwet et al,71 2018 | American College of Surgeons National Surgical Quality Improvement Program database | 2015 | 500 Institutions | Clinical findings, laboratory tests | A total of 73 patients had C difficile colitis, generating an incidence of 1.05% (adult elderly, surgical patients [hip fracture]) |
| Bovonratwet et al,72 2018 | The incidence of C difficile colitis was 0.10% (adult nonelderly and elderly, surgical patients [hip and knee arthroplasty]) | ||||
| Delanois et al,73 2018 | AHRQ NIS database | 2009-2013 | Not stated | ICD-9 | After revision total hip arthroplasty, 1.7% of patients had postoperative CDI |
| Englesbe et al,74 2010 | Michigan Surgical Quality Collaborative and American College of Surgeons-National Surgical Quality Improvement Program on colectomy operations | 2007-2009 | 24 Hospitals | Not stated | Among patients undergoing colectomies who received nonabsorbable antibiotics for bowel preparation, 1.9% had postoperative CDI; among patients undergoing colectomies who did not receive nonabsorbable antibiotics for bowel preparation, 3% had postoperative CDI |
| Lesperance et al,75 2011 | AHRQ NIS patients who underwent elective colon resections | 2004-2006 | Approximately 1000 hospitals | ICD-9 | Overall, 1.4%; 2004, 1.31%; 2005, 1.45%; 2006, 1.67% |
| Guzman et al,76 2016 | AHRQ NIS patients who underwent cervical spine surgery | 2002-2011 | Approximately 1000 hospitals | ICD-9 | Overall incidence of CDI in postoperative cervical spine surgery hospitalizations, 0.08%; in 2011, 0.14% |
| Gwam et al,77 2018 | AHRQ NIS database | 2009-2013 | Not stated | ICD-9 | Incidence of CDI after revision total knee arthroplasty, 1.0% |
| Maltenfort et al,78 2013 | AHRQ NIS database | 2002-2010 | Not stated | ICD-9 | Incidence of C difficile remained <0.6% during the study period |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CDI, Clostridium difficile infection; HO, hospital onset; ICD-9, International Classification of Diseases, Ninth Revision; NHDS, National Hospital Discharge Survey; NIS, National Inpatient Sample; VA, Veterans Affairs.
Ten studies69,70,71,72,73,74,75,76,77,78 evaluated surgical patients (Table 3), and, thus, we assumed that the CDI cases were incident cases. Five studies73,75,76,77,78 used data from AHRQ NIS. These AHRQ NIS studies analyzed a variety of surgical procedures, including spine surgery76; hip,73 knee,77 or lower-extremity78 arthroplasty; and elective colon resections.75 One of them had CDI occurring in 1.4% of patients, for a rate of 144.99 cases of C difficile colitis per 10 000 elective colon resections, and the incidence increased from 1.31% in 2004 to 1.67% in 2006.75
LOS Associated With CDI (20 Studies)
Twenty studies16,54,66,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94 (Table 4) evaluated CDI-associated LOS. Sixteen studies54,66,79,80,81,82,83,84,85,86,87,88,89,92,94,95 used propensity score matching to evaluate LOS associated with CDI, 2 studies16,93 used postinfection LOS, 1 study90 matched on LOS from admission until either positive C difficile test results or discharge, and 1 study91 accounted for time to infection using a multistate model. Also, one of the propensity score matched–studies applied multistate modeling to account for timing of infection.88 Pediatric patients were included in 3 of these studies.66,86,87
Table 4. Length of Stay Associated With Clostridium difficile Infection Among Studies That Used Appropriate Methodsa.
| Source | Data Set | Study Period | Patient Population | Facilities or Hospitals, No. | LOS | Method | Downs and Black Scoreb |
|---|---|---|---|---|---|---|---|
| Campbell et al,79 2013 | Cerner Health Facts Electronic Health Record Database | 2005-2011 | Hospitalized adults at high risk for poor outcomes including those aged >65 y, those with complex conditions or chronic diseases (renal disease, cancer, inflammatory bowel disease) and those with concomitant antibiotic use | 74 | Among patients aged >65 y with HO-CDI, mean 19.10 d; among patients without CDI aged >65 y, mean, 16.06 d; mean difference, 3.04 d (95% CI, 1.44-4.63 d) | Propensity score matched including matching on preinfection LOS | 17 |
| Drozd et al,80 2015 | Medicare Standard Analytic Files | 2009-2010 | Inpatients | 5% Random sample of Medicare | Among patients with CDI, mean, 7.0 d; among patients without CDI, mean, 3.8 d; mean difference, 3.2 d | Propensity score matched | 17 |
| Dubberke et al,81 2008 | Barnes-Jewish Hospital | 2003 | Inpatients | 1 | Among patients with CDI, median, 9.6 d; among patients without CDI, median, 5.8 d; attributable median difference, 2.8 d | Propensity score matched | 15 |
| Dubberke et al,16 2010 | Hospitals in Centers for Disease Control and Prevention Epicenter Network | July 2000 to June 2006 | Hospitalized adults | 5 Hospitals | Community-onset, patients with community-associated CDI, median, 5 d; patients with community-onset HCF-associated CDI (study hospital), median, 6 d; patients with community-onset HCF-associated CDI (other hospital), median, 8 d | Postinfection LOS | 13 |
| Egorova et al,82 2015 | AHRQ NIS database | 2000-2011 | Patients included in the Nationwide Inpatient Sample | 20% of US Hospitals | Among patients with CDI, median (IQR), 15 (9-25) d; among patients without CDI, median (IQR), 8.3 (4.6-13.6) d; attributable median difference, 6.7 d | Propensity score matched | 17 |
| Gabriel et al,83 2018 | University of California Irvine Trauma Database | 2014-2016 | CDI in hospitalized adult trauma patients | 1 | Odds ratio, 1.39; 95% CI, 1.16-1.66 | Propensity score matched | 15 |
| Jiang et al,54 2013 | Rhode Island Hospital Discharge Database | 2010-2011 | Hospitalized adults; evaluated health care–onset CDI | 11 | Among patients with CDI, mean (SD), 18.9 (21.7) d; among patients without CDI, mean (SD), 8.6 (11.3) d; mean difference, 10.3 d | Propensity score matched | 15 |
| Li et al,84 2016 | Veterans Affairs Surgical Quality Improvement Program database and Decision Support System pharmacy | 2009-2013 | Postoperative adult patients | 134 | Among patients with CDI, mean (SD), 15.6 (19.5) d; among patients without CDI, mean (SD), 8.1 (12.6) d; mean difference, 7.5 d | Propensity score matched | 18 |
| Magee et al,85 2015 | Discharges from Premier database | 2009-2011 | Inpatients | Geographically diverse hospitals | Among patients with CDI mean (SD), 14.4 (18.3) d; among patients without CDI, mean (SD), 8.7 (15.6) d; mean difference, 5.7 d | Propensity score matched | 17 |
| Mehrotra et al,86 2017 | AHRQ Kids’ Inpatient Database | 2012 | Pediatric inpatients | 2500-4100 Hospitals/y | Among patients with CDI mean, 9.4 d (95% CI, 9.1-9.6 d); among patients without CDI, mean, 5.4 d (95% CI, 5.3-5.6 d); mean difference, 3.9 d | Propensity score matched | 17 |
| Nylund et al,87 2011 | Healthcare Cost and Utilization Project Kids’ Inpatient Database | 1997, 2000, 2003, 2006 | Pediatric patients | Not stated | Odds ratio, 4.34; 95% CI, 3.97-4.83 | Propensity score matched | 19 |
| Pak et al,88 2017 | Mount Sinai Hospital Electronic Medical Record | 2009-2015 | Adult inpatients | 1 | Median difference by case definition: ICD-9 code, 3.1 d (95% CI, 2.2-3.9 d); positive toxin enzyme immunoassay, 10.1 d (95% CI, 7.3-12.2 d); positive toxin polymerase chain reaction, 6.6 d (95% CI, 5.0-8.1 d); either toxin assay, 7.2 d (95% CI, 5.8-8.3 d); by any of these, 5.7 d (95% CI, 4.5-6.6 d); stratification by time to first positive toxin assay, 3.1 d (95% CI, 1.7-4.4 d); under the same case definition, the multistate model averaged an excess LOS of 3.3 d (95% CI, 2.6-4.0 d) | Propensity score matched plus multistate modeling to account for timing of infection | 14 |
| Radcliff et al,89 2016 | Texas Health Care Information Collection Inpatient Public Use Data Files | 2007-2011 | Inpatients | Texas hospitals | For 2007, among patients with CDI, mean, 19.0 d; among patients without CDI, mean, 9.7 d; mean difference: 9.3 d; for 2011, among patients with CDI, mean, 16.5 d; among patients without CDI, mean, 9.2 d; mean difference, 7.4 d | Propensity score matched | 12 |
| Sammons et al,66 2013 | Pediatric Health Information System Database | 2006-2011 | Hospitalized children at 41 children’s hospitals | 41 | Among patients with HO-CDI, median (IQR), 23 d (12-44 d); among patients without CDI matched to patients with HO-CDI, median (IQR not stated), 4 d; median difference, 19 d; adjusted mean difference, 21.6 d (95% CI, 19.29-23.90 d) | Propensity score matched | 15 |
| Song et al,90 2008 | Johns Hopkins Hospital | January 2000 to October 2005 | Hospitalized adults patients | 1 | Among patients with CDI, median, 19 d; among patients without CDI, median, 18 d; adjusted difference, 13% increased LOS among patients with CDI | Matched on LOS from admission to either positive C difficile test or discharge | 15 |
| Stevens et al,91 2015 | VA Healthcare System | January 2005 to December 2012 | Hospitalized adults patients | 120 Acute care facilities | Among patients with CDI, mean (SD), 19.4 (31.7) d; among patients without CDI, mean (SD), 5.4 (8.4) d; mean difference, 14 d; multistate modeling estimated an attributable LOS of only 2.27 d (95% CI, 2.14-2.40 d) | Multistate modeling to account for timing of infection | 19 |
| Stewart et al,92 2011 | AHRQ NIS database | 2007 | Patients included in the Nationwide Inpatient Sample; age unknown, assumed all ages | 20% of US hospitals | Among patients with CDI, mean (SD), 13.0 (14) d; among patients without CDI mean (SD), 7.9 (9) d; mean difference, 5.1 d | Propensity score matched | 17 |
| Stewart et al,93 2012 | Pennsylvania State College of Medicine | 2004-2009 | Patients with and without hematologic malignancies who acquired CDI | 1 | Postinfection LOS for patients with CDI with malignancies and receiving chemotherapy, mean (SD), 22.4 (23.2) d; postinfection LOS for patients with CDI without malignancies, mean (SD), 10.2 (10) d | Postinfection LOS | 14 |
| Tabak et al,94 2013 | CareFusion database of 6 Pennsylvania hospitals | 2007-2008 | Hospitalized patients | 6 | Among patients with CDI, mean (SD), 16.3 (14.2) d; among patients without CDI, mean (SD), 14.0 (11.9) d; attributable days, 2.4 (95% CI, 0.7-4.4; P < .01) | Propensity score matched | 18 |
| Zilberberg et al,95 2009 | AHRQ NIS database | 2005 | Hospitalized patients | Approximately 1000 hospitals | Patients with CDI had an independent increase in the hospital LOS by 6.1 d (95% CI, 4.9-7.4 d) | Propensity score matched | 16 |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CDI, Clostridium difficile infection; HCF, health care facility; HO, hospital onset; ICD-9, International Classification of Diseases, Ninth Revision; IQR, interquartile range; LOS, length of stay; NIS, National Inpatient Sample; VA, Veterans’ Affairs.
Methods include propensity score matching or postinfection LOS or matched on preinfection LOS or multistate modeling.
The Downs and Black scale measures study quality, with a score of 18 or higher indicating higher quality, and a maximum score of 28 possible.10
Among the 13 propensity score–matched studies of adults, the CDI-associated mean difference in LOS (in days) between patients with CDI and patients who did not have CDI varied greatly from 3.0 days (95% CI, 1.44-4.63 days)79 to 10.3 days.54 Among the 3 pediatric propensity score–matched studies,66,86,87 the highest CDI-associated mean difference in LOS (in days) was 21.6 days (95% CI, 19.29-23.90 days).66
Among the studies that used multistate models to account for timing of infection, a study91 performed in the Veterans Affairs health care system found that the magnitude of its estimated impact was smaller when methods were used to account for the time-varying nature of infection. That study estimated a CDI-attributable LOS of only 2.27 days (95% CI, 2.14-2.40 days).91 The other study88 that performed propensity score matching and used a multistate model converged on similar excess LOS estimates of 3.1 days (95% CI, 1.7-4.4 days) and 3.3 days (95% CI, 2.6-4.0 days), respectively.
Four studies84,87,91,94 that evaluated LOS earned 18 or more points on the Downs and Black scale.10 One study91 also used multistate modeling. Another was also performed in the Veterans Affairs health care system84,91 and found a mean difference between patients with and without CDI of 7.5 days.84 One study87 of pediatric patients found that those with CDI had a longer LOS (adjusted odds ratio, 4.34; 95% CI, 3.97-4.83). Another study94 of adult patients in Pennsylvania hospitals showed an attributable hospital LOS difference of 2.4 days (95% CI, 0.7-4.4 days; P < .01) between patients with and without CDI.
Discussion
National epidemiological investigations have demonstrated recent marked increases in CDI in the United States.34 Thus, a national public health response to this increase requires current estimates of the CDI incidence.96,97,98 Our systematic review of the literature found that the CDI incidence varied by study and that the investigators used different denominators when they calculated the incidence for specific study populations. In our meta-analysis of studies that used patient-days as the denominator, we estimated the incidence of CDI in the United States to be 8.3 CDI cases per 10 000 patient-days.
Variation in CDI incidence may be due, in part, to advances in diagnostic technology and variations in diagnostic practices.99,100,101 Nucleic acid amplification tests are more sensitive than traditional C difficile stool tests (eg, toxin enzyme immunoassay). Nucleic acid amplification tests have been used more frequently in clinical practice since 2009, when the first commercial polymerase chain reaction was approved by the US Food and Drug Administration.102 The topic of CDI testing methods and risk adjustment is complex.103,104 Concerns have been expressed about the adequacy of risk adjustment to account for different CDI testing methods (toxin enzyme immunoassay alone, polymerase chain reaction alone, toxin enzyme immunoassay plus glutamate dehydrogenase followed by polymerase chain reaction for discrepancies, polymerase chain reaction followed by toxin enzyme immunoassay, and other diagnostic options) across HCFs. The choice of testing methods substantially affects the performance of these testing algorithms.99,100,101
In addition, the CDI incidence found by these studies likely varied because of the different database structures adopted by the various hospitals.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78 Some analyses were based on health care systems databases, but most used large infection control surveillance, state, or national discharge databases.13,14,15,16,17,18,19,20,21,22,23,24,25 Beginning in January 2013, the Centers for Medicare & Medicaid Services began requiring public reporting of CDI rates via the National Healthcare Safety Network for those hospitals participating in the Inpatient Prospective Payment System.64 Specifically, 1 study29 demonstrated an increase in the annual incidence of CDI and multiply recurrent CDI per 1000 person-years by 42.7% and 188.8%, respectively, between 2001 and 2012. Another CDI surveillance study33 in 7 US states reported an increase not only in community-associated CDI incidence rates but also an increase in health care–associated CDI incidence rates. Furthermore, CDI can complicate comorbid conditions and result in the need for additional hospital resources.34 Included studies detected an increase in the CDI incidence in patients with inflammatory bowel disease,43 patients with cancer,52 those undergoing surgery,75,76 and even infants.41 The results of our systematic review of literature and meta-analysis emphasize the need to perform C difficile surveillance and direct resources to the prevention of CDI in order to reduce the incidence across the United States.
Limitations
This systematic literature review has some limitations. First, the results of systematic literature reviews and meta-analyses are only as valid as the results of the studies evaluated. Most studies included in this systematic literature review were of moderate-to-low quality and may have overestimated the outcomes. We need more high-quality studies so that we can accurately determine postinfection LOS, because LOS before the infection should not be attributed to C difficile.5 Second, we included studies that used ICD-9 codes to define CDI. The ICD-9 codes are used for billing purposes and are not ideal for surveillance. However, a prior meta-analysis105 found that the ICD-9 code for C difficile had good sensitivity, specificity, positive predictive value, and negative predictive value compared with clinical definitions. Third, we only included studies conducted in the United States and published in English, which limits the external validity of this research. We used these inclusion criteria because our goal was to evaluate the burden of CDI in the United States. Future systematic literature reviews should be performed to evaluate this burden in other countries. Fourth, we found heterogeneity in all LOS-stratified analyses (eAppendix 2 and eTable in the Supplement). We found that the higher-quality studies that used advanced statistical methods to attempt to account for time-dependent bias found lower CDI-attributable LOS compared with other studies that did not use advanced methods. In addition, our incidence estimates were derived from multicenter studies only. Incidence rates in small studies may be variable and subject to bias; thus, this criterion was established a priori to determine representative incidence rates. From incident cases of CDI (36 studies), we were unable to exclude recurrent and multiply recurrent CDI cases if the study did not exclude those cases. For this meta-analysis, we decided to calculate the incidence rate with studies with a similar denominator (patient-days), with a result of 8.3 CDI cases per 10 000 patient-days.
Conclusions
Pooled estimates from the currently available literature suggest that C difficile is associated with a large burden on the US health care system. However, these estimates should be used with caution, and higher-quality studies should be completed to guide future evaluations of C difficile prevention and treatment interventions.
eAppendix 1. Search Methods
eAppendix 2. Statistical Methods
eReferences.
eTable. Subset Analyses Evaluating Hospital Length of Stay Attributable to Clostridium difficile Infection (8 Studies)
References
- 1.Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team . Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):-. doi: 10.1056/NEJMoa1801550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Published April 23, 2013. Accessed November 7, 2019.
- 3.Gerding DN, Meyer T, Lee C, et al. . Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA. 2015;313(17):1719-1727. doi: 10.1001/jama.2015.3725 [DOI] [PubMed] [Google Scholar]
- 4.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313(4):398-408. doi: 10.1001/jama.2014.17103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States: a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. doi: 10.1186/s12879-016-1786-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RE, Nelson SD, Khader K, et al. . The magnitude of time-dependent bias in the estimation of excess length of stay attributable to healthcare-associated infections. Infect Control Hosp Epidemiol. 2015;36(9):1089-1094. doi: 10.1017/ice.2015.129 [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 9.van Kleef E, Green N, Goldenberg SD, et al. . Excess length of stay and mortality due to Clostridium difficile infection: a multi-state modelling approach. J Hosp Infect. 2014;88(4):213-217. doi: 10.1016/j.jhin.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderson PGS, Higgins JPT. Assessment of Study Quality: Cochrane Reviewer’s Handbook 4.2.3. Chichester, UK: John Wiley & Sons, Ltd; 2004. [Google Scholar]
- 12.Chan KY, Wang W, Wu JJ, et al. ; Global Health Epidemiology Reference Group (GHERG) . Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 2013;381(9882):2016-2023. doi: 10.1016/S0140-6736(13)60221-4 [DOI] [PubMed] [Google Scholar]
- 13.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987-2001. J Infect Dis. 2004;189(9):1585-1589. doi: 10.1086/383045 [DOI] [PubMed] [Google Scholar]
- 14.Burger T, Fry D, Fusco R, et al. . Multihospital surveillance of nosocomial methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, and Clostridium difficile: analysis of a 4-year data-sharing project, 1999-2002. Am J Infect Control. 2006;34(7):458-464. doi: 10.1016/j.ajic.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Campbell RJ, Giljahn L, Machesky K, et al. . Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6):526-533. doi: 10.1086/597507 [DOI] [PubMed] [Google Scholar]
- 16.Dubberke ER, Butler AM, Yokoe DS, et al. . Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31(10):1030-1037. doi: 10.1086/656245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gase KA, Haley VB, Xiong K, Van Antwerpen C, Stricof RL. Comparison of 2 Clostridium difficile surveillance methods: National Healthcare Safety Network’s laboratory-identified event reporting module versus clinical infection surveillance. Infect Control Hosp Epidemiol. 2013;34(3):284-290. doi: 10.1086/669509 [DOI] [PubMed] [Google Scholar]
- 18.Haley VB, DiRienzo AG, Lutterloh EC, Stricof RL. Quantifying sources of bias in National Healthcare Safety Network laboratory-identified Clostridium difficile infection rates. Infect Control Hosp Epidemiol. 2014;35(1):1-7. doi: 10.1086/674389 [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics. 2008;122(6):1266-1270. doi: 10.1542/peds.2008-0469 [DOI] [PubMed] [Google Scholar]
- 20.Kamboj M, Son C, Cantu S, et al. . Hospital-onset Clostridium difficile infection rates in persons with cancer or hematopoietic stem cell transplant: a C3IC network report. Infect Control Hosp Epidemiol. 2012;33(11):1162-1165. doi: 10.1086/668023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald LC, Lessa F, Sievert D, et al. ; Centers for Disease Control and Prevention (CDC) . Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61(9):157-162. [PubMed] [Google Scholar]
- 22.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390. doi: 10.1086/659156 [DOI] [PubMed] [Google Scholar]
- 23.Sohn S, Climo M, Diekema D, et al. ; Prevention Epicenter Hospitals . Varying rates of Clostridium difficile-associated diarrhea at prevention epicenter hospitals. Infect Control Hosp Epidemiol. 2005;26(8):676-679. doi: 10.1086/502601 [DOI] [PubMed] [Google Scholar]
- 24.Tartof SY, Yu KC, Wei R, Tseng HF, Jacobsen SJ, Rieg GK. Incidence of polymerase chain reaction-diagnosed Clostridium difficile in a large high-risk cohort, 2011-2012. Mayo Clin Proc. 2014;89(9):1229-1238. doi: 10.1016/j.mayocp.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 25.Zilberberg MD, Tabak YP, Sievert DM, et al. . Using electronic health information to risk-stratify rates of Clostridium difficile infection in US hospitals. Infect Control Hosp Epidemiol. 2011;32(7):649-655. doi: 10.1086/660360 [DOI] [PubMed] [Google Scholar]
- 26.Chernak E, Johnson CC, Weltman A, et al. Severe Clostridium difficile–associated disease in populations previously at low risk: four states, 2005. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5447a1.htm. Published December 2, 2005. Accessed November 7, 2019.
- 27.Dubberke ER, Olsen MA, Stwalley D, et al. . Identification of Medicare recipients at highest risk for Clostridium difficile infection in the US by population attributable risk analysis. PLoS One. 2016;11(2):e0146822. doi: 10.1371/journal.pone.0146822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutiérrez RL, Riddle MS, Porter CK. Epidemiology of Clostridium difficile infection among active duty United States military personnel (1998-2010). BMC Infect Dis. 2013;13:609. doi: 10.1186/1471-2334-13-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med. 2017;167(3):152-158. doi: 10.7326/M16-2733 [DOI] [PubMed] [Google Scholar]
- 30.Olsen MA, Young-Xu Y, Stwalley D, et al. . The burden of Clostridium difficile infection: estimates of the incidence of CDI from U.S. Administrative databases. BMC Infect Dis. 2016;16:177. doi: 10.1186/s12879-016-1501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabatsky-Ehr T, Purviance K, Mlynarski D, Mshar P, Hadler J, Sosa L Surveillance for community-associated Clostridium difficile: Connecticut, 2006. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5713a3.htm. Published April 4, 2008. Accessed November 7, 2019.
- 32.Kuntz JL, Johnson ES, Raebel MA, et al. . Clostridium difficile infection, Colorado and the northwestern United States, 2007. Emerg Infect Dis. 2012;18(6):960-962. doi: 10.3201/eid1806.111528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessa FC, Mu Y, Winston LG, et al. . Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis. 2014;1(2):ofu048. doi: 10.1093/ofid/ofu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reveles KR, Lawson KA, Mortensen EM, et al. . National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014. PLoS One. 2017;12(12):e0189227. doi: 10.1371/journal.pone.0189227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee SM, Tsay R, Nelson DS, van Wijngaarden E, Dumyati G. Clostridium difficile in the pediatric population of Monroe County, New York. J Pediatric Infect Dis Soc. 2014;3(3):183-188. doi: 10.1093/jpids/pit091 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez TH, Brooks JT, Sullivan PS, et al. ; Adult/Adolescent Spectrum of HIV Disease Study Group . Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis. 2005;41(11):1621-1627. doi: 10.1086/498027 [DOI] [PubMed] [Google Scholar]
- 37.Troppy TS, Mishra T, Barton K, et al. . Using public health surveillance data to measure Clostridium difficile infection population burden in Massachusetts. Am J Infect Control. 2019;47(2):211-212. doi: 10.1016/j.ajic.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Wendt JM, Cohen JA, Mu Y, et al. . Clostridium difficile infection among children across diverse US geographic locations. Pediatrics. 2014;133(4):651-658. doi: 10.1542/peds.2013-3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young-Xu Y, Kuntz JL, Gerding DN, et al. . Clostridium difficile infection among Veterans Health Administration patients. Infect Control Hosp Epidemiol. 2015;36(9):1038-1045. doi: 10.1017/ice.2015.138 [DOI] [PubMed] [Google Scholar]
- 40.Argamany JR, Aitken SL, Lee GC, Boyd NK, Reveles KR. Regional and seasonal variation in Clostridium difficile infections among hospitalized patients in the United States, 2001-2010. Am J Infect Control. 2015;43(5):435-440. doi: 10.1016/j.ajic.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000-2005. Pediatr Infect Dis J. 2008;27(12):1111-1113. doi: 10.1097/INF.0b013e31817eef13 [DOI] [PubMed] [Google Scholar]
- 42.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14(6):929-931. doi: 10.3201/eid1406.071447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barber GE, Hendler S, Okafor P, Limsui D, Limketkai BN. Rising incidence of intestinal infections in inflammatory bowel disease: a nationwide analysis. Inflamm Bowel Dis. 2018;24(8):1849-1856. doi: 10.1093/ibd/izy086 [DOI] [PubMed] [Google Scholar]
- 44.Barlam TF, Soria-Saucedo R, Ameli O, Cabral HJ, Kaplan WA, Kazis LE. Retrospective analysis of long-term gastrointestinal symptoms after Clostridium difficile infection in a nonelderly cohort. PLoS One. 2018;13(12):e0209152. doi: 10.1371/journal.pone.0209152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhandari S, Pandey RK, Dahal S, et al. . Risk, outcomes, and predictors of Clostridium difficile infection in lymphoma: a nationwide study. South Med J. 2018;111(10):628-633. doi: 10.14423/SMJ.0000000000000872 [DOI] [PubMed] [Google Scholar]
- 46.Brown KA, Daneman N, Jones M, et al. . The drivers of acute and long-term care Clostridium difficile infection rates: a retrospective multilevel cohort study of 251 facilities. Clin Infect Dis. 2017;65(8):1282-1288. doi: 10.1093/cid/cix532 [DOI] [PubMed] [Google Scholar]
- 47.Dasenbrock HH, Bartolozzi AR, Gormley WB, Frerichs KU, Aziz-Sultan MA, Du R. Clostridium difficile infection after subarachnoid hemorrhage: a nationwide analysis. Neurosurgery. 2016;78(3):412-420. doi: 10.1227/NEU.0000000000001065 [DOI] [PubMed] [Google Scholar]
- 48.Davis ML, Sparrow HG, Ikwuagwu JO, Musick WL, Garey KW, Perez KK. Multicentre derivation and validation of a simple predictive index for healthcare-associated Clostridium difficile infection. Clin Microbiol Infect. 2018;24(11):1190-1194. doi: 10.1016/j.cmi.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 49.Delgado A, Reveles IA, Cabello FT, Reveles KR. Poorer outcomes among cancer patients diagnosed with Clostridium difficile infections in United States community hospitals. BMC Infect Dis. 2017;17(1):448. doi: 10.1186/s12879-017-2553-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dotson KM, Aitken SL, Sofjan AK, Shah DN, Aparasu RR, Garey KW. Outcomes associated with Clostridium difficile infection in patients with chronic liver disease. Epidemiol Infect. 2018;146(9):1101-1105. doi: 10.1017/S0950268818001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guddati AK, Kumar G, Ahmed S, et al. . Incidence and outcomes of Clostridium difficile-associated disease in hematopoietic cell transplant recipients. Int J Hematol. 2014;99(6):758-765. doi: 10.1007/s12185-014-1577-z [DOI] [PubMed] [Google Scholar]
- 52.Gupta A, Pardi DS, Baddour LM, Khanna S. Outcomes in children with Clostridium difficile infection: results from a nationwide survey. Gastroenterol Rep (Oxf). 2016;4(4):293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta A, Tariq R, Frank RD, et al. . Trends in the incidence and outcomes of hospitalized cancer patients with Clostridium difficile infection: a nationwide analysis. J Natl Compr Canc Netw. 2017;15(4):466-472. doi: 10.6004/jnccn.2017.0046 [DOI] [PubMed] [Google Scholar]
- 54.Jiang Y, Viner-Brown S, Baier R. Burden of hospital-onset Clostridium difficile infection in patients discharged from Rhode Island hospitals, 2010-2011: application of present on admission indicators. Infect Control Hosp Epidemiol. 2013;34(7):700-708. doi: 10.1086/670993 [DOI] [PubMed] [Google Scholar]
- 55.Khanna S, Gupta A, Baddour LM, Pardi DS. Epidemiology, outcomes, and predictors of mortality in hospitalized adults with Clostridium difficile infection. Intern Emerg Med. 2016;11(5):657-665. doi: 10.1007/s11739-015-1366-6 [DOI] [PubMed] [Google Scholar]
- 56.Kuy S, Jenkins P, Romero RA, Samra N, Kuy S. Increasing incidence of and increased mortality associated with Clostridium difficile-associated megacolon. JAMA Surg. 2016;151(1):85-86. doi: 10.1001/jamasurg.2015.2677 [DOI] [PubMed] [Google Scholar]
- 57.Lessa FC, Mu Y, Bamberg WM, et al. . Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. doi: 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo R, Greenberg A, Stone CD. Outcomes of Clostridium difficile infection in hospitalized leukemia patients: a nationwide analysis. Infect Control Hosp Epidemiol. 2015;36(7):794-801. doi: 10.1017/ice.2015.54 [DOI] [PubMed] [Google Scholar]
- 59.Mamic P, Heidenreich PA, Hedlin H, Tennakoon L, Staudenmayer KL. Hospitalized patients with heart failure and common bacterial infections: a nationwide analysis of concomitant Clostridium difficile infection rates and in-hospital mortality. J Card Fail. 2016;22(11):891-900. doi: 10.1016/j.cardfail.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 60.Miller AC, Polgreen LA, Cavanaugh JE, Polgreen PM. Hospital Clostridium difficile infection (CDI) incidence as a risk factor for hospital-associated CDI. Am J Infect Control. 2016;44(7):825-829. doi: 10.1016/j.ajic.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller AC, Polgreen LA, Cavanaugh JE, Polgreen PM. Hospital Clostridium difficile infection rates and prediction of length of stay in patients without C. difficile infection. Infect Control Hosp Epidemiol. 2016;37(4):404-410. doi: 10.1017/ice.2015.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pant C, Deshpande A, Gilroy R, Olyaee M, Donskey CJ. Rising incidence of Clostridium difficile related discharges among hospitalized children in the United States. Infect Control Hosp Epidemiol. 2016;37(1):104-106. doi: 10.1017/ice.2015.234 [DOI] [PubMed] [Google Scholar]
- 63.Pant C, Deshpande A, Desai M, et al. . Outcomes of Clostridium difficile infection in pediatric solid organ transplant recipients. Transpl Infect Dis. 2016;18(1):31-36. doi: 10.1111/tid.12477 [DOI] [PubMed] [Google Scholar]
- 64.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42(10):1028-1032. doi: 10.1016/j.ajic.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 65.Saffouri G, Gupta A, Loftus EV Jr, Baddour LM, Pardi DS, Khanna S. The incidence and outcomes from Clostridium difficile infection in hospitalized adults with inflammatory bowel disease. Scand J Gastroenterol. 2017;52(11):1240-1247. doi: 10.1080/00365521.2017.1362466 [DOI] [PubMed] [Google Scholar]
- 66.Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis. 2013;57(1):1-8. doi: 10.1093/cid/cit155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy CR, Avery TR, Dubberke ER, Huang SS. Frequent hospital readmissions for Clostridium difficile infection and the impact on estimates of hospital-associated C. difficile burden. Infect Control Hosp Epidemiol. 2012;33(1):20-28. doi: 10.1086/663209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuntz JL, Yang M, Cavanaugh J, Saftlas AF, Polgreen PM. Trends in Clostridium difficile infection among peripartum women. Infect Control Hosp Epidemiol. 2010;31(5):532-534. doi: 10.1086/652454 [DOI] [PubMed] [Google Scholar]
- 69.Aquina CT, Probst CP, Becerra AZ, et al. . High variability in nosocomial Clostridium difficile infection rates across hospitals after colorectal resection. Dis Colon Rectum. 2016;59(4):323-331. doi: 10.1097/DCR.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 70.Bovonratwet P, Bohl DD, Russo GS, Ondeck NT, Singh K, Grauer JN. Incidence, risk factors, and impact of Clostridium difficile colitis after spine surgery. Spine (Phila Pa 1976). 2018;43(12):861-868. doi: 10.1097/BRS.0000000000002430 [DOI] [PubMed] [Google Scholar]
- 71.Bovonratwet P, Bohl DD, Russo GS, et al. . How common—and how serious—is Clostridium difficile colitis after geriatric hip fracture? findings from the NSQIP dataset. Clin Orthop Relat Res. 2018;476(3):453-462. doi: 10.1007/s11999.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bovonratwet P, Bohl DD, Malpani R, Nam D, Della Valle CJ, Grauer JN. Incidence, risk factors, and impact of Clostridium difficile colitis following primary total hip and knee arthroplasty. J Arthroplasty. 2018;33(1):205-210. doi: 10.1016/j.arth.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 73.Delanois RE, George NE, Etcheson JI, Gwam CU, Mistry JB, Mont MA. Risk factors and costs associated with Clostridium difficile colitis in patients with prosthetic joint infection undergoing revision total hip arthroplasty. J Arthroplasty. 2018;33(5):1534-1538. doi: 10.1016/j.arth.2017.11.035 [DOI] [PubMed] [Google Scholar]
- 74.Englesbe MJ, Brooks L, Kubus J, et al. . A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Ann Surg. 2010;252(3):514-519. doi: 10.1097/SLA.0b013e3181f244f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lesperance K, Causey MW, Spencer M, Steele SR. The morbidity of Clostridium difficile infection after elective colonic resection: results from a national population database. Am J Surg. 2011;201(2):141-148. doi: 10.1016/j.amjsurg.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 76.Guzman JZ, Skovrlj B, Rothenberg ES, et al. . The burden of Clostridium difficile after cervical spine surgery. Global Spine J. 2016;6(4):314-321. doi: 10.1055/s-0035-1562933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gwam CU, George NE, Etcheson JI, et al. . Clostridium difficile infection in the USA: incidence and associated factors in revision total knee arthroplasty patients. Eur J Orthop Surg Traumatol. 2019;29(3):667-674. doi: 10.1007/s00590-018-2319-3 [DOI] [PubMed] [Google Scholar]
- 78.Maltenfort MG, Rasouli MR, Morrison TA, Parvizi J. Clostridium difficile colitis in patients undergoing lower-extremity arthroplasty: rare infection with major impact. Clin Orthop Relat Res. 2013;471(10):3178-3185. doi: 10.1007/s11999-013-2906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campbell R, Dean B, Nathanson B, Haidar T, Strauss M, Thomas S. Length of stay and hospital costs among high-risk patients with hospital-origin Clostridium difficile-associated diarrhea. J Med Econ. 2013;16(3):440-448. doi: 10.3111/13696998.2013.770749 [DOI] [PubMed] [Google Scholar]
- 80.Drozd EM, Inocencio TJ, Braithwaite S, et al. . Mortality, hospital costs, payments, and readmissions associated with Clostridium difficile infection among Medicare beneficiaries. Infect Dis Clin Pract (Baltim Md). 2015;23(6):318-323. doi: 10.1097/IPC.0000000000000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dubberke ER, Butler AM, Reske KA, et al. . Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14(7):1031-1038. doi: 10.3201/eid1407.070867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egorova NN, Siracuse JJ, McKinsey JF, Nowygrod R. Trend, risk factors, and costs of Clostridium difficile infections in vascular surgery. Ann Vasc Surg. 2015;29(4):792-800. doi: 10.1016/j.avsg.2014.10.031 [DOI] [PubMed] [Google Scholar]
- 83.Gabriel V, Grigorian A, Phillips JL, et al. . A propensity score analysis of Clostridium difficile infection among adult trauma patients. Surg Infect (Larchmt). 2018;19(7):661-666. doi: 10.1089/sur.2018.110 [DOI] [PubMed] [Google Scholar]
- 84.Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2016;151(4):314-322. doi: 10.1001/jamasurg.2015.4263 [DOI] [PubMed] [Google Scholar]
- 85.Magee G, Strauss ME, Thomas SM, Brown H, Baumer D, Broderick KC. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: a multicenter retrospective study of inpatients, 2009-2011. Am J Infect Control. 2015;43(11):1148-1153. doi: 10.1016/j.ajic.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 86.Mehrotra P, Jang J, Gidengil C, Sandora TJ. Attributable cost of Clostridium difficile infection in pediatric patients. Infect Control Hosp Epidemiol. 2017;38(12):1472-1477. doi: 10.1017/ice.2017.240 [DOI] [PubMed] [Google Scholar]
- 87.Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med. 2011;165(5):451-457. doi: 10.1001/archpediatrics.2010.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pak TR, Chacko KI, O’Donnell T, et al. . Estimating local costs associated with Clostridium difficile infection using machine learning and electronic medical records. Infect Control Hosp Epidemiol. 2017;38(12):1478-1486. doi: 10.1017/ice.2017.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Radcliff TA, Lorden AL, Zhao H. Clostridium difficile infection in Texas Hospitals, 2007-2011. Infect Control Hosp Epidemiol. 2016;37(3):357-359. doi: 10.1017/ice.2015.291 [DOI] [PubMed] [Google Scholar]
- 90.Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828. doi: 10.1086/588756 [DOI] [PubMed] [Google Scholar]
- 91.Stevens VW, Khader K, Nelson RE, et al. . Excess length of stay attributable to Clostridium difficile infection (CDI) in the acute care setting: a multistate model. Infect Control Hosp Epidemiol. 2015;36(9):1024-1030. doi: 10.1017/ice.2015.132 [DOI] [PubMed] [Google Scholar]
- 92.Stewart DB, Hollenbeak CS. Clostridium difficile colitis: factors associated with outcome and assessment of mortality at a national level. J Gastrointest Surg. 2011;15(9):1548-1555. doi: 10.1007/s11605-011-1615-6 [DOI] [PubMed] [Google Scholar]
- 93.Stewart DB, Yacoub E, Zhu J. Chemotherapy patients with C. difficile colitis have outcomes similar to immunocompetent C. difficile patients. J Gastrointest Surg. 2012;16(8):1566-1572. doi: 10.1007/s11605-012-1930-6 [DOI] [PubMed] [Google Scholar]
- 94.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013;34(6):588-596. doi: 10.1086/670621 [DOI] [PubMed] [Google Scholar]
- 95.Zilberberg MD, Nathanson BH, Sadigov S, Higgins TL, Kollef MH, Shorr AF. Epidemiology and outcomes of Clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136(3):752-758. doi: 10.1378/chest.09-0596 [DOI] [PubMed] [Google Scholar]
- 96.Shah DN, Aitken SL, Barragan LF, et al. . Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect. 2016;93(3):286-289. doi: 10.1016/j.jhin.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 97.Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and costs in Clostridium difficile infection among the elderly in the United States. Infect Control Hosp Epidemiol. 2016;37(11):1331-1336. doi: 10.1017/ice.2016.188 [DOI] [PubMed] [Google Scholar]
- 98.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88(1):12-21. doi: 10.1016/j.jhin.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 99.Polage CR, Gyorke CE, Kennedy MA, et al. . Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175(11):1792-1801. doi: 10.1001/jamainternmed.2015.4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moehring RW, Lofgren ET, Anderson DJ. Impact of change to molecular testing for Clostridium difficile infection on healthcare facility-associated incidence rates. Infect Control Hosp Epidemiol. 2013;34(10):1055-1061. doi: 10.1086/673144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Planche TD, Davies KA, Coen PG, et al. . Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis. 2013;13(11):936-945. doi: 10.1016/S1473-3099(13)70200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burnham CA, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013;26(3):604-630. doi: 10.1128/CMR.00016-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kufelnicka AM, Kirn TJ. Effective utilization of evolving methods for the laboratory diagnosis of Clostridium difficile infection. Clin Infect Dis. 2011;52(12):1451-1457. doi: 10.1093/cid/cir201 [DOI] [PubMed] [Google Scholar]
- 104.Thompson ND, Edwards JR, Dudeck MA, Fridkin SK, Magill SS. Evaluating the use of the case mix index for risk adjustment of healthcare-associated infection data: an illustration using Clostridium difficile infection data from the National Healthcare Safety Network. Infect Control Hosp Epidemiol. 2016;37(1):19-25. doi: 10.1017/ice.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goto M, Ohl ME, Schweizer ML, Perencevich EN. Accuracy of administrative code data for the surveillance of healthcare-associated infections: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(5):688-696. doi: 10.1093/cid/cit737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Methods
eAppendix 2. Statistical Methods
eReferences.
eTable. Subset Analyses Evaluating Hospital Length of Stay Attributable to Clostridium difficile Infection (8 Studies)