Abstract
Background
Induction of labour is a common obstetric intervention. Amniotomy alone for induction of labour is reviewed separately and oxytocin alone for induction of labour is being prepared for inclusion in The Cochrane Library. This review will address the use of the combination of these two methods for induction of labour in the third trimester. This is one of a series of reviews of methods of cervical ripening and labour induction using standardised methodology.
Objectives
To determine, from the best available evidence, the efficacy and safety of amniotomy and intravenous oxytocin for third trimester induction of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, the Cochrane Controlled Trials Register and reference lists of articles were searched. Date of last search: May 2001. We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 21 September 2009 and added the results to the awaiting classification section of the review.
Selection criteria
Clinical trials comparing amniotomy plus intravenous oxytocin used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
Trial quality assessment and data extraction were done by both reviewers. A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction. The initial data extraction was done centrally, and incorporated into a series of primary reviews arranged by methods of induction of labour, following a standardised methodology. The data is to be extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
Main results
Seventeen trials involving 2566 women were included. Amniotomy and intravenous oxytocin were found to result in fewer women being undelivered vaginally at 24 hours than amniotomy alone (relative risk (RR) 0.03, 95% confidence intervals (CI) 0.001‐0.49). This finding was based on the results of a single study of 100 women. As regards secondary results amniotomy and intravenous oxytocin resulted in significantly fewer instrumental vaginal deliveries than placebo (RR 0.18, CI 0.05‐0.58). Amniotomy and intravenous oxytocin resulted in more postpartum haemorrhage than vaginal prostaglandins (RR 5.5, CI 1.26‐24.07). Significantly more women were also dissatisfied with amniotomy and intravenous oxytocin when compared with vaginal prostaglandins, RR 53, CI 3.32‐846.51.
Authors' conclusions
Data on the effectiveness and safety of amniotomy and intravenous oxytocin are lacking. No recommendations for clinical practice can be made on the basis of this review. Amniotomy and intravenous oxytocin is a combination of two methods of induction of labour and both methods are utilised in clinical practice. If their use is to be continued it is important to compare the effectiveness and safety of these methods, and to define under which clinical circumstances one may be preferable to another.
[Note: The three citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Amnion; Amnion/surgery; Injections, Intravenous; Labor, Induced; Labor, Induced/methods; Oxytocin; Oxytocin/administration & dosage; Treatment Outcome
Plain language summary
Amniotomy plus intravenous oxytocin for induction of labour
Intravenous oxytocin and amniotomy compares well with other forms used in the third trimester (full term) to bring on labour.
Sometimes it is necessary to help get labour started. There are several methods used and they either ripen the cervix or make the uterus start contracting. Oxytocin is a drug used to stimulate contractions of the uterus. Amniotomy (breaking the waters) helps bring on contractions. The review of trials found that oxytocin combined with amniotomy compares well with other forms of labour induction. However, adverse risks of amniotomy include pain and discomfort, bleeding, possible infection in the uterus and a decreased heart rate in the baby. The risk of infection following amniotomy is particularly important in areas where HIV is prevalent.
Background
Induction of labour is a common obstetric intervention which is usually undertaken for a clinical indication, however rightly or wrongly, it may also be undertaken for other reasons, such as a woman's request or clinician's convenience. This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2000). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix of the uterus and inducing labour.
Amniotomy alone for induction of labour and intravenous oxytocin alone for cervical ripening and induction of labour are reviewed separately (Bricker 2001; Tan 2001). This review will address the two in combination. Concomitant administration is regarded when the two are initiated within two hours of each other, irrespective of which is initiated first.
Objectives
To determine, from the best available evidence, the efficacy and safety of amniotomy plus oxytocin for third trimester induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing amniotomy plus oxytocin for labour induction, with placebo/no treatment or other methods; random allocation to treatment and comparison groups, reasonable measures to ensure allocation concealment; violations of allocated management not sufficient to materially affect outcomes.
Types of participants
Women due for third trimester induction of labour, with a viable fetus. Sub‐group analyses were performed for women regarding parity and subgroups of these for those with favourable, unfavourable or undefined cervices, as well as previous lower segment caesarean section.
Types of interventions
Amniotomy plus oxytocin compared with placebo/no treatment or other methods of induction of labour listed above it on a predefined list of methods of labour induction ‐ See Hofmeyr 2000.
Primary comparisons:
intravenous oxytocin and amniotomy versus placebo/no treatment;
intravenous oxytocin and amniotomy versus intra vaginal prostaglandins;
intravenous oxytocin and amniotomy versus intra cervical prostaglandins;
intravenous oxytocin and amniotomy versus oxytocin;
intravenous oxytocin and amniotomy versus amniotomy.
In the studies of oxytocin and amniotomy versus prostaglandins, the prostaglandins used were PGE2(1‐2mg) in a gel preparation; vaginal pessaries(3mg); PGE2 tablets(3mg); PGE2 in methyl hydroxyethyl cellulose gel(400ug); and PGF2 alpha(50mg).
The oxytocin dosage used varied between studies with a most common maximum dosage of 32 mU/min (16 mU/min‐40 mU/min), flow rate doubled half hourly, with 5% Dextrose in Water used as administration fluid.
Amniotomy and intravenous oxytocin were considered as concomitant if the amniotomy was performed within two hours from the start of the oxytocin infusion or vice versa. This time interval was determined before evaluation of studies for inclusion into the review was commenced and was agreed upon by both reviewers. In most studies the two interventions were commenced simultaneously but in five studies this was not specified (Saleh 1975; Thompson 1987; Martin 1978; Ratnam 1974; Kennedy 1978). In two studies failure to rupture the membranes occurred (Maclennan 1989, two women; Orhue 1995, nine women). Amniotomy was, however, successful after oxytocin administration for one to two hours prior to amniotomy.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). Differences were settled by discussion.
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. Sub‐group analyses were limited to the primary outcomes: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia.
Secondary outcomes relate to measures of effectiveness, complications and satisfaction:
Measures of effectiveness: (6) cervix unfavourable/unchanged after 12‐24 hours; (7) oxytocin augmentation.
Complications: (8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium stained liquor; (13) Apgar score < 7 at 5 minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction: (26) woman not satisfied; (27) caregiver not satisfied.
While all the above outcomes were sought, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we will use the term 'uterine hyperstimulation without FHR changes 'to include uterine tachysystole (> 5 contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short term variability).
Outcomes were included in the analysis: if reasonable measures were taken to minimise observer bias; missing data were insufficient to materially influence conclusions and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (May 2001). We updated this search on 21 September 2009 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
The original search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2000).
We search the reference lists of trial reports and reviews.
We did not apply any language restrictions.
Data collection and analysis
Trials under consideration were evaluated for methodological quality and appropriateness for inclusion according to the prestated selection criteria, without consideration of their results. Allocation concealment was scored as A: adequate (e.g. double blind, placebo controlled; envelopes administered centrally) B: unclear (e.g. numbered sealed envelopes not administered centrally) C: inadequate e.g. alternation). Individual outcome data were included in the analysis if they met the presented criteria in 'Types of outcome measures'. Included trial data were processed as described in Clarke 2000.
Data were extracted from the sources and entered onto the Review Manager computer software (RevMan 2000), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, relative risks and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed effects model. The predefined criteria for sensitivity analysis were: trial quality assessment and interval between amniotomy and commencement of oxytocin.
Primary analysis was limited to the prespecified outcomes and sub‐group analyses. In the event of differences in unspecified outcomes or sub‐groups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Results
Description of studies
See 'Characteristics of included studies'.
Intravenous oxytocin and amniotomy were compared with placebo/expectant management in one study (Martin 1978, 184 women ).
Comparisons were made with vaginal prostaglandin in ten studies (Orhue 1995; Dommisse 1987; Thompson 1987; Maclennan 1980; Parazzini 1998; Lamont 1991; Maclennan 1989; Kennedy 1982;Taylor 1993; Melchior 1989; 1169 women).
Comparisons were made with intracervical prostaglandin in one study (Kennedy 1978, 90 women).
Comparisons were made with oxytocin alone in two studies (Ratnam 1974, Mercer 1995, 416 women).
Comparisons were made with amniotomy alone in three studies (Saleh 1975, Patterson 1971, Moldin 1996, 707 women).
(Three reports from an updated search in September 2009 have been added to Studies awaiting classification.)
Risk of bias in included studies
The majority of the included studies were of good quality: Seven studies scored A: (Lamont 1991; Maclennan 1980; Mercer 1995; Moldin 1996; Orhue 1995; Parazzini 1998; Taylor 1993; with the rest of the studies scoring B: (Dommisse 1987; Kennedy 1978; Kennedy 1982; Patterson 1971; Ratnam 1974; Saleh 1975; Thompson 1987; Maclennan 1989; Martin 1978; Melchior 1989).
Allocation sequence generation was unclear in seven studies ( Kennedy 1978 'randomly allocated'; Kennedy 1982 'randomly allocated'; Lamont 1991 'random' stratified by parity; Patterson 1971; Saleh 1975 'randomly'; Thompson 1987 'randomised'; Melchior 1989, ' randomised in table of four').
Random number tables were used in five studies (Dommisse 1987; Maclennan 1989; Maclennan 1980; Martin 1978; Orhue 1995). Computer generated sequence was used in four studies (Mercer 1995; Moldin 1996; Taylor 1993; Parazzini 1998). In one study, allocation sequence was generated by lot drawing (Ratnam 1974).
Effects of interventions
Seventeen trials involving 2566 women were included.
Amniotomy and intravenous oxytocin versus placebo or no treatment ‐ all women.
(i) Primary outcomes: One study (Martin 1978), with 184 participants, evaluated serious neonatal morbidity or mortality. There was no serious neonatal morbidity or mortality in the amniotomy and intravenous oxytocin group and 1(1.08%) case in the placebo group, relative risk (RR) 0.33, confidence interval (CI) 0.01‐8.08. Although statistically there is no difference between the groups, the data should be interpreted with caution as this is a rare outcome and the confidence intervals are wide.
(ii) Other outcomes: A significant reduction in the amount of meconium stained liquor was found in the oxytocin and amniotomy group with 3 (3.3% ) cases in the amniotomy and oxytocin group and 13 (14.2% ) cases in the expectant group, RR 0.23, CI 0.068‐0.783. Amniotomy and intravenous oxytocin versus vaginal prostaglandins ‐ all women.
(i) Primary outcomes: One study (Taylor 1993) that included 42 participants, all of who had had previous caesarean sections, found that there was no difference in vaginal delivery not achieved in 24 hours. In the amniotomy and intravenous oxytocin group there were 9 (42.85%) women that had not delivered vaginally within 24 hours and in the vaginal prostaglandin group there were 10 (47.61%) cases, RR 0.9, CI 0.46‐1.75. Ten studies (Dommisse 1987; Kennedy 1982; Lamont 1991; Maclennan 1989; Maclennan 1980; Melchior 1989; Orhue 1995; Parazzini 1998; Taylor 1993; Thompson 1987) with 1140 participants, found no significant difference between the two groups as regards caesarean sections performed. Caesarean section was performed on 78 (13.6%) women in the amniotomy and intravenous oxytocin group and on 73 (12.9%) women in the vaginal prostaglandin group, RR 1.06, CI 0.79‐1.42. In four studies (Kennedy 1982; Maclennan 1989; Maclennan 1980; Parazzini 1998) with 739 women, there were no differences between the two groups as regards uterine hyperstimulation with fetal heart rate changes (3.4% versus 5.8%, RR 0.82, CI 0.47‐1.45). Five studies (Kennedy 1982; Lamont 1991; Maclennan 1989; Maclennan 1980; Melchior 1989), that included 612 participants reported no difference in serious neonatal morbidity or mortality in either group, RR 1, CI 0.2‐4.86. Two studies (Maclennan 1989; Orhue 1995) that included 378 women reported no serious maternal morbidity or death, RR 0.97, CI 0.06‐15.29.
(ii) Other outcomes: Three studies (Parazzini 1998; Taylor 1993; Thompson 1987 ), that included 414 participants, found that there was no difference between the two groups when evaluated for unchanged cervical status, with 29 (13.85%) cases in the amniotomy and intravenous oxytocin group reported as having an unchanged cervical status, compared with 39 (19.02%) cases in the vaginal prostaglandin group, RR 0.73, CI 0,47‐1.12.
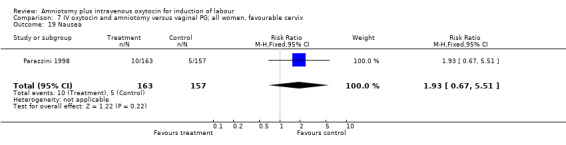
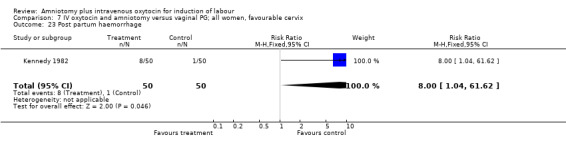
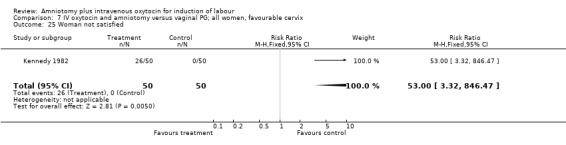
Two studies (Kennedy 1982; Orhue 1995) that included 160 women, found that there were statistically more postpartum haemorrhages in the amniotomy and intravenous oxytocin group with 11 (13.75%) cases compared with two (2.5%) cases in the vaginal prostaglandin group, RR 5.5, CI 1.26‐24.07. One study (Kennedy 1982) of 50 parturients reported that 26 (52%) women were not satisfied with amniotomy and intravenous oxytocin compared with no women reporting dissatisfaction with vaginal prostaglandins. Although based on a single study, this is a statistically significant difference, RR 53, CI 3.32‐846. Nine studies (Kennedy 1982; Lamont 1991; Maclennan 1989; Maclennan 1980; Melchior 1989; Orhue 1995; Parazzini 1998; Taylor 1993; Thompson 1987) that included 1086 women, found that there was no difference in the number of instrumental vaginal deliveries in the amniotomy and intravenous oxytocin group with 83 (15.12%) compared with 89(16,57%) in the vaginal prostaglandin group, RR 0.92, CI 0.70‐1.19. Two studies (Maclennan 1989; Parazzini 1998) that included 638 women, found no difference in the reporting of nausea, in the amniotomy and intravenous oxytocin group there were 12(3.67%) cases compared with 11(3.53%) cases in the vaginal prostaglandin group, RR 1.04, CI 0.47‐2.32.
Amniotomy and intravenous oxytocin versus cervical prostaglandins ‐ all women.
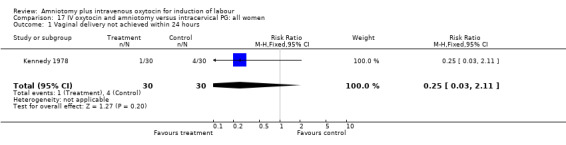
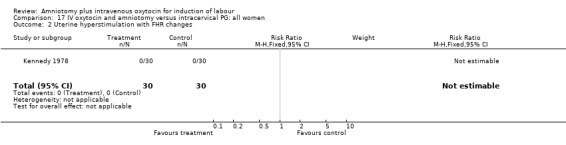
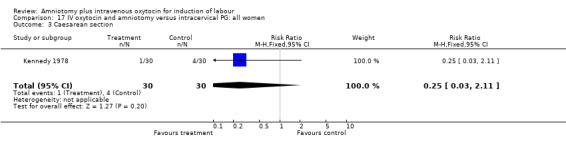
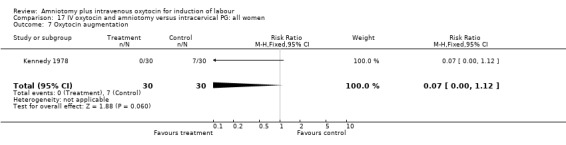
(i) Primary outcomes: All the findings are based on a single study (Kennedy 1978) with 60 participants. There was no significant difference between the two groups in women requiring caesarean section. One (3.3%) caesarean section was performed in the amniotomy and intravenous oxytocin group compared with four (13.3%) in the cervical prostaglandin group, RR 0.25, CI 0.03‐2.1 The same study reported no cases of uterine hyperstimulation and fetal heart rate changes in either group, RR 1, CI 0.02‐48.8. The impression that there are no differences between the two groups as regards these two outcomes must be interpreted with caution as the findings are based on data from a single study, with 60 participants.
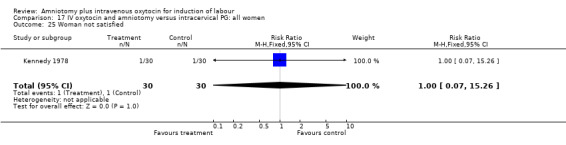
(ii) Other outcomes: The study reported the absence of meconium stained liquor in either groups. One woman in each group reported that she was not satisfied with the method of induction, RR 1, CI 0.07‐15.3.
Amniotomy and intravenous oxytocin versus oxytocin alone ‐ all women.
(i) Primary outcomes: Two studies (Mercer 1995; Ratnam 1974) that included 511 participants, found that there was no difference in caesarean section between these two groups with 27 (17.3%) caesarean sections performed in the amniotomy and intravenous oxytocin group, compared with 25 (16.3%) in the oxytocin alone group, RR 1.05, CI 0.64‐1.7. (ii) Other outcomes: One study (Mercer 1995) of 209 women reported 25 (23.6%) parturients in the amniotomy and intravenous oxytocin group had meconium stained liquor, compared with 15 (14.6%) in the oxytocin only group, RR 1.62, CI 0.91‐2.89.
Amniotomy and intravenous oxytocin versus amniotomy alone ‐ all women.
(i) Primary outcomes: Two studies (Moldin 1996; Saleh 1975), with 296 participants, found that there were significantly fewer women with vaginal delivery not achieved within 24 hours in the amniotomy and intravenous oxytocin group compared with the amniotomy alone group. There were three cases (2.1%) in the amniotomy and intravenous oxytocin group compared to 24 cases (16.3%) in the amniotomy alone group, RR 0.125, CI 0.038‐0.406. There was no statistically significant difference between the two groups as regards caesarean section (Patterson 1971; Saleh 1975) with five (1.97%) performed in the amniotomy and intravenous oxytocin group and 11 (4.28%) in the amniotomy alone group, RR 0.45, CI 0.16‐1.3. However, the power of this study to detect meaningful differences was low.
(ii) Other outcomes: Two studies (Patterson 1971; Saleh 1975) that included 510 participants found that there were statistically significantly fewer instrumental vaginal deliveries in the amniotomy and intravenous oxytocin group 57 (22.35%) compared with 88 (34.51%) performed in the amniotomy alone group, RR 0.65, CI 0.49‐0.85.
Discussion
Despite the fact that amniotomy and intravenous oxytocin appear to be widely used for induction of labour, surprisingly little research has been done in this area. Due to the paucity of information, firm conclusions cannot be drawn on the use of amniotomy and intravenous oxytocin for the induction of labour.
No single study addressed all the primary outcomes and no conclusions can be made as regards primary outcomes. Two studies that included 550 women, reported more postpartum haemorrhage in the amniotomy and intravenous oxytocin group compared with women induced with vaginal prostaglandins. One study that included 100 women, reported more women were not satisfied with amniotomy and intravenous oxytocin than vaginal prostaglandins. While interesting, the small sample sizes preclude a definitive conclusion.
This review did not evaluate comparisons between different methods of oxytocin administration and dosages and these studies have therefore been excluded (Mercer 1991; Arulkumaran 1987; Thomas 1974; Calder 1975; Chua 1991; Orhue 1993a; Orhue 1993b; Orhue 1994; Pavlou 1978; Pavlou 1978; Reid 1995; Steer 1985). It is however important to evaluate this in a separate review as the success of oxytocin induction may be dependant on the method of oxytocin administration, as it has not been standardised in the studies included in this review.
Authors' conclusions
Implications for practice.
Although amniotomy and intravenous oxytocin have been used widely in obstetric practice, the available literature does not clearly support or refute the value of using the combination instead of the separate methods individually.
Data on the effectiveness and safety of amniotomy and intravenous oxytocin are lacking. No recommendations for clinical practice can be made on the basis of this review. Cognizance however must be taken of the possibility of increased perinatal transmission of HIV following amniotomy (Biggar 1996) particularly in areas where the prevalence of HIV may be high and due to limited resources or other reasons HIV status of the woman is unknown.
Implications for research.
Despite the paucity of data, there is probably little role for further research into the use of the combination of amniotomy and intravenous oxytocin as a primary method of induction. In clinical settings where resources are limited, amniotomy alone may be the favoured method of induction.
Amniotomy may also be the favoured method of induction in women not keen on pharmacological intervention or in cases where avoiding uterine stimulation may be advantageous. Under these circumstances we concur with Bricker and Luckas (Bricker 2001) that it is reasonable to recommend that further research into the method of amniotomy alone for the induction of labour is needed, and would urge researchers to evaluate this method in the context of different time intervals between the primary (amniotomy) and secondary intervention (addition of a pharmaceutical agent and with reference to this review, the use of intravenous oxytocin).
This research should include assessment of women and caregiver satisfaction and economic analysis. The suggestion from this review that oxytocin may be associated with greater risk of postpartum haemorrhage than prostaglandin, warrants further research.
[Note: The three citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 29 January 2013 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 21 September 2009 | Amended | Search updated. Three new reports added to Studies awaiting classification (Chanrachakul 2003; Chua 1988; Selo‐Ojeme 2007) and a Published note added about the updating of this review. |
| 31 October 2008 | Amended | Converted to new review format. |
Notes
This review will be updated by a new review team following a new protocol, which is currently being prepared.
Acknowledgements
Justus Hofmeyr, Zarko Alfirevic, Tony Kelly, Sonja Henderson
Data and analyses
Comparison 1. IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.46, 35.11] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 10 Epidural analgesia | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.25] |
| 12 Meconium stained liquor | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.78] |
| 25 Woman not satisfied | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.77, 1.60] |
1.3. Analysis.
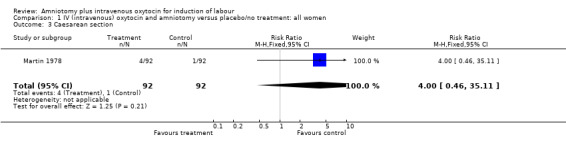
Comparison 1 IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women, Outcome 3 Caesarean section.
1.4. Analysis.
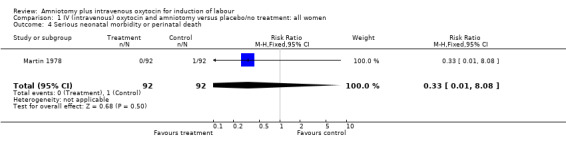
Comparison 1 IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
1.10. Analysis.
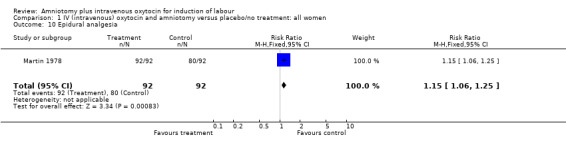
Comparison 1 IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women, Outcome 10 Epidural analgesia.
1.12. Analysis.
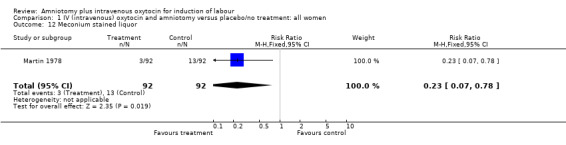
Comparison 1 IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women, Outcome 12 Meconium stained liquor.
1.25. Analysis.
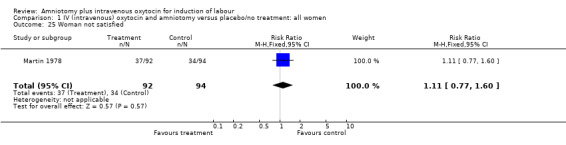
Comparison 1 IV (intravenous) oxytocin and amniotomy versus placebo/no treatment: all women, Outcome 25 Woman not satisfied.
Comparison 5. IV oxytocin and amniotomy versus vaginal PG:all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
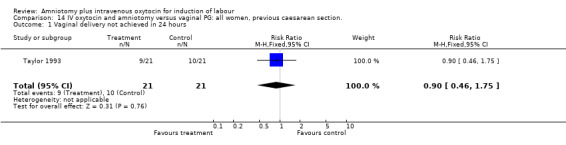
| 1 Vaginal delivery not achieved within 24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.46, 1.75] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.45] |
| 3 Caesarean section | 10 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.42] |
| 4 Serious neonatal morbidity or perinatal death | 5 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 5 Serious maternal morbidity or death | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.12] |
| 7 Oxytocin augmentation | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.62] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.51, 7.77] |
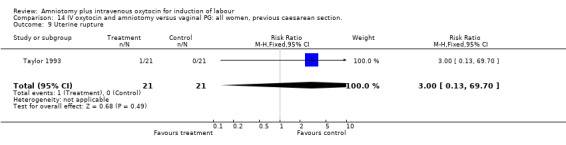
| 9 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 10 Epidural analgesia/opioid analgesia | 5 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.30] |
| 11 Instrumental vaginal delivery | 9 | 1086 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.19] |
| 12 Meconium stained liquor | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 13 Apgar score <7 at 5 minutes | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.44, 5.81] |
| 14 Neonatal intensive care unit admission | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.29] |
| 16 Perinatal death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Nausea | 2 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.47, 2.32] |
| 20 Vomiting | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.27, 2.47] |
| 21 Diarrhoea | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.70 [0.23, 97.06] |
| 23 Post partum haemorrhage | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.5 [1.26, 24.07] |
| 25 Woman not satisfied | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 53.0 [3.32, 846.47] |
| 27 Chorioamnionitis | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Retained placenta | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.36, 29.15] |
| 29 Precipitate labour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
5.1. Analysis.
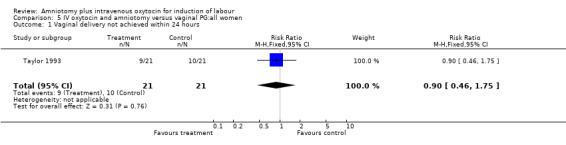
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
5.2. Analysis.
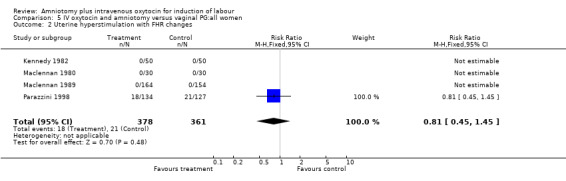
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 2 Uterine hyperstimulation with FHR changes.
5.3. Analysis.
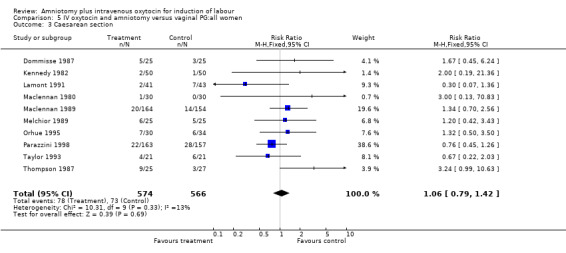
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 3 Caesarean section.
5.4. Analysis.
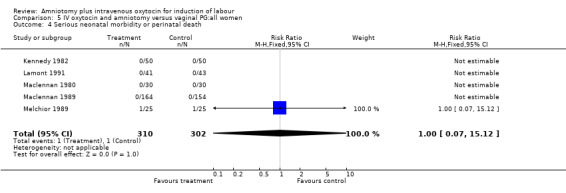
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 4 Serious neonatal morbidity or perinatal death.
5.5. Analysis.
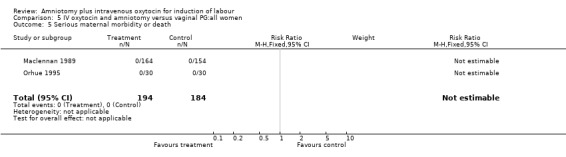
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 5 Serious maternal morbidity or death.
5.6. Analysis.
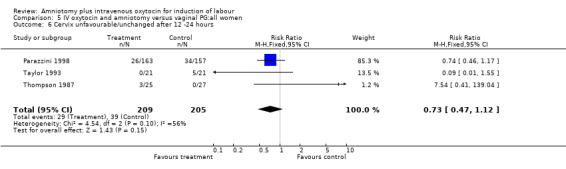
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours.
5.7. Analysis.
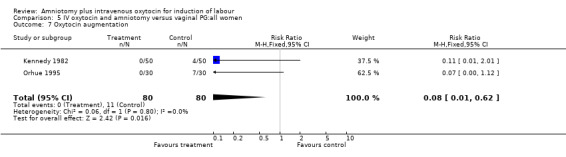
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 7 Oxytocin augmentation.
5.8. Analysis.
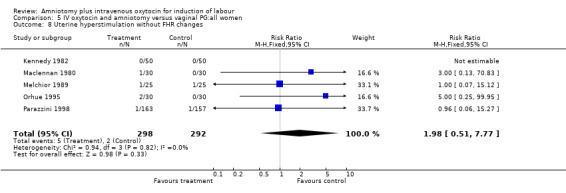
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 8 Uterine hyperstimulation without FHR changes.
5.9. Analysis.
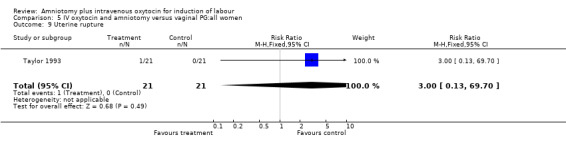
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 9 Uterine rupture.
5.10. Analysis.
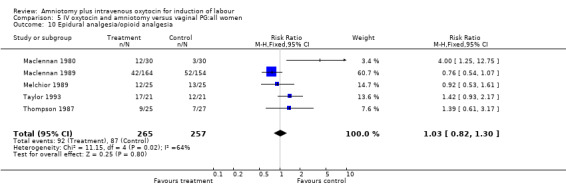
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 10 Epidural analgesia/opioid analgesia.
5.11. Analysis.
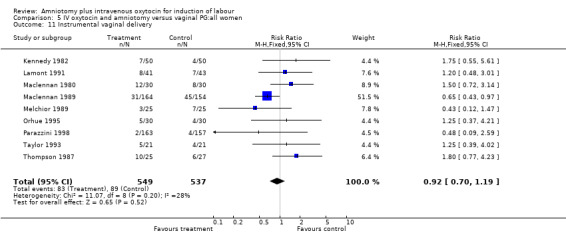
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 11 Instrumental vaginal delivery.
5.12. Analysis.
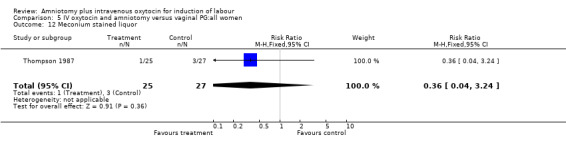
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 12 Meconium stained liquor.
5.13. Analysis.
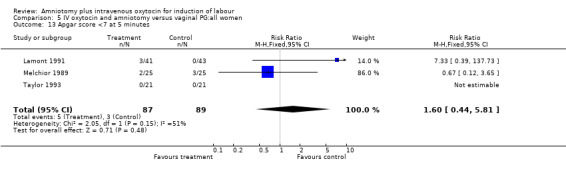
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 13 Apgar score <7 at 5 minutes.
5.14. Analysis.
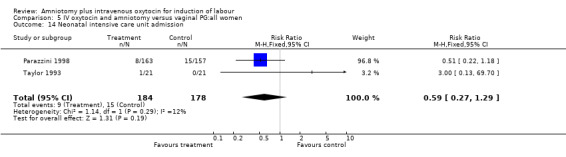
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 14 Neonatal intensive care unit admission.
5.16. Analysis.
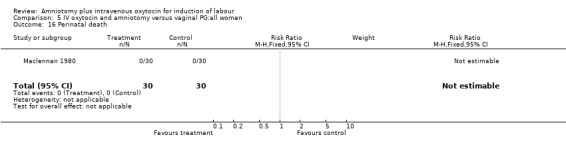
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 16 Perinatal death.
5.19. Analysis.
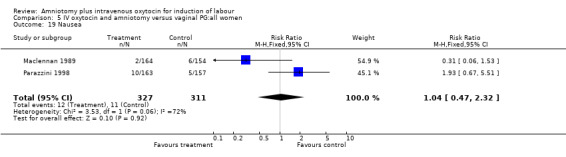
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 19 Nausea.
5.20. Analysis.
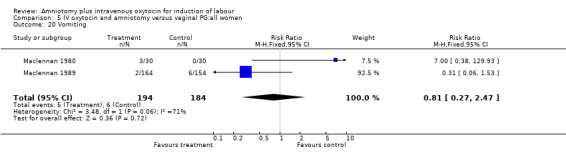
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 20 Vomiting.
5.21. Analysis.
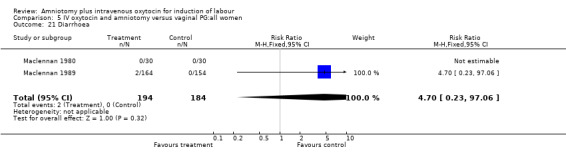
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 21 Diarrhoea.
5.23. Analysis.
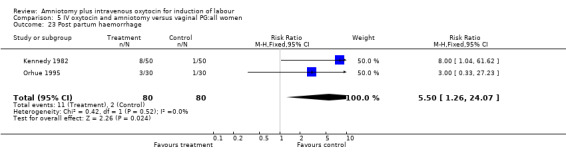
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 23 Post partum haemorrhage.
5.25. Analysis.
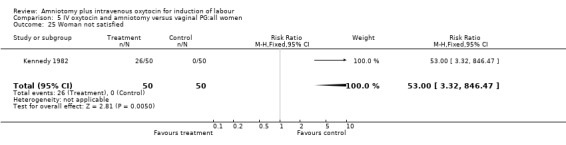
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 25 Woman not satisfied.
5.27. Analysis.
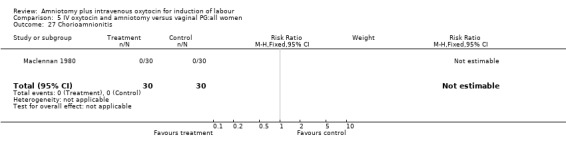
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 27 Chorioamnionitis.
5.28. Analysis.
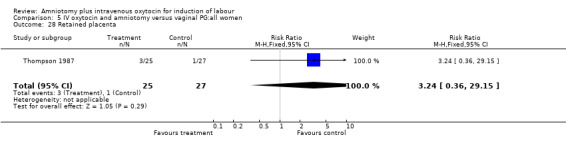
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 28 Retained placenta.
5.29. Analysis.
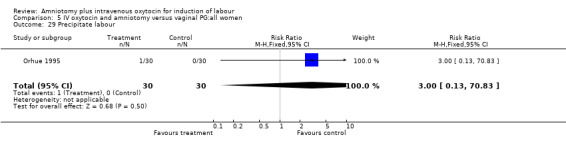
Comparison 5 IV oxytocin and amniotomy versus vaginal PG:all women, Outcome 29 Precipitate labour.
Comparison 6. IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.46, 1.75] |
| 3 Caesarean section | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.48, 2.03] |
| 5 Serious maternal morbidity or death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.20, 2.35] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.95] |
| 9 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 10 Epidural analgesia/opioid analgesia | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.35] |
| 11 Instrumental vaginal delivery | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.54, 2.90] |
| 13 Apgar score <7 at 5 minutes | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.33 [0.39, 137.73] |
| 14 Neonatal intensive care unit admission | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 23 Post partum haemorrhage | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 29 Precipitate labour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
6.1. Analysis.
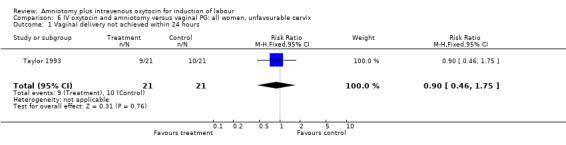
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
6.3. Analysis.
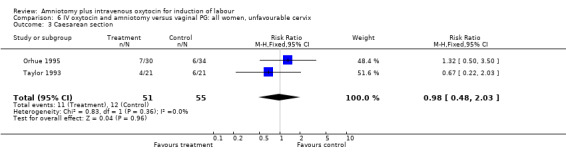
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 3 Caesarean section.
6.5. Analysis.
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
6.6. Analysis.
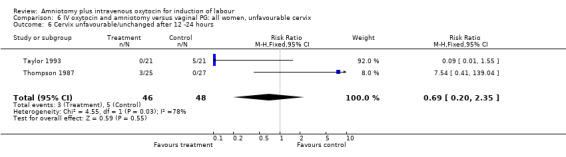
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours.
6.7. Analysis.
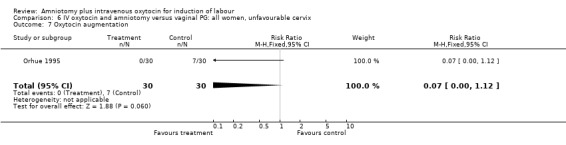
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 7 Oxytocin augmentation.
6.8. Analysis.
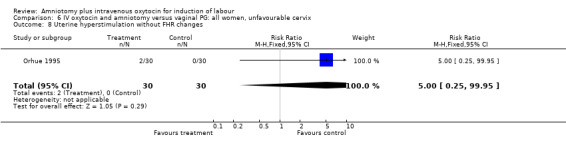
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
6.9. Analysis.
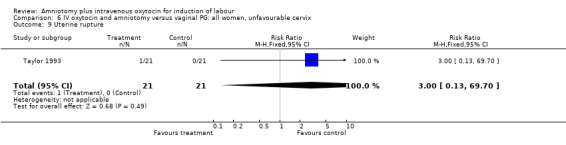
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 9 Uterine rupture.
6.10. Analysis.
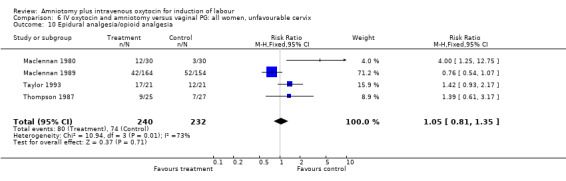
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
6.11. Analysis.
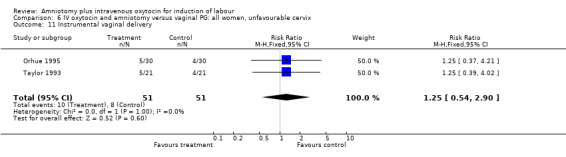
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
6.13. Analysis.
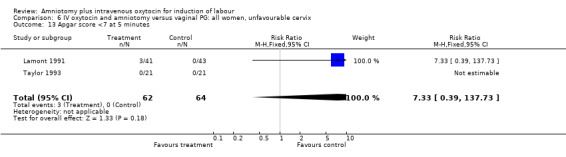
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 13 Apgar score <7 at 5 minutes.
6.14. Analysis.
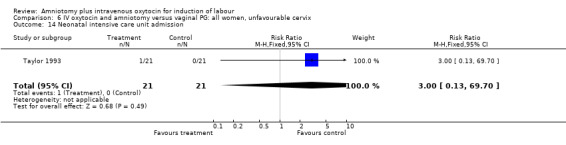
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
6.23. Analysis.
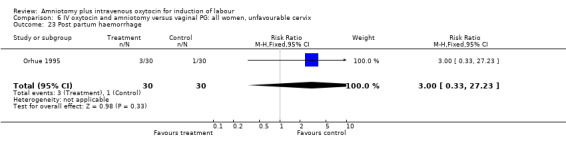
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 23 Post partum haemorrhage.
6.29. Analysis.
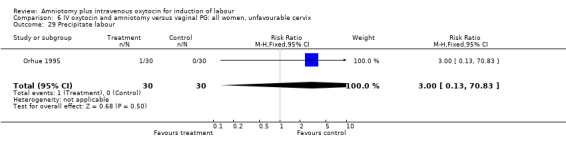
Comparison 6 IV oxytocin and amniotomy versus vaginal PG: all women, unfavourable cervix, Outcome 29 Precipitate labour.
Comparison 7. IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 5 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.41] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.30] |
| 7 Oxytocin augmentation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| 8 Uterine hyperstimulation without FHR changes | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.27] |
| 10 Epidural analgesia/opioid analgesia | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.61, 3.17] |
| 11 Instrumental vaginal delivery | 4 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| 12 Meconium stained liquor | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 13 Apgar score <7 at 5 minutes | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.33 [0.39, 137.73] |
| 14 Neonatal intensive care admission | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.18] |
| 19 Nausea | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.67, 5.51] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.04, 61.62] |
| 25 Woman not satisfied | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 53.0 [3.32, 846.47] |
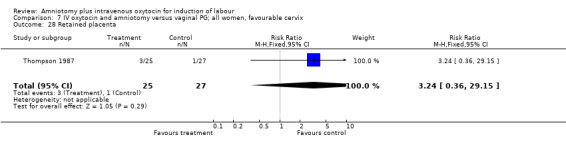
| 28 Retained placenta | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.36, 29.15] |
7.2. Analysis.
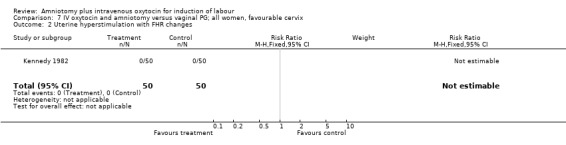
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
7.3. Analysis.
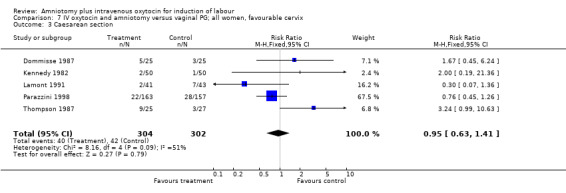
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 3 Caesarean section.
7.4. Analysis.
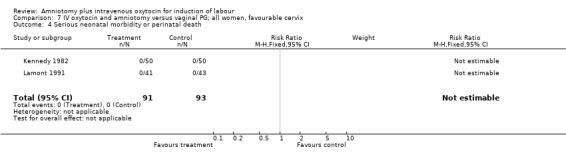
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death.
7.6. Analysis.
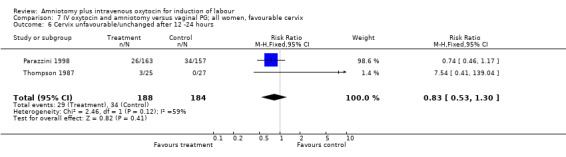
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours.
7.7. Analysis.
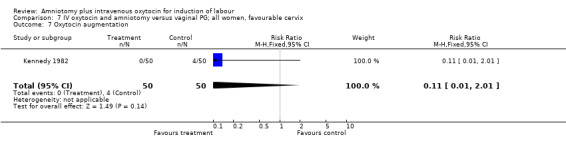
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 7 Oxytocin augmentation.
7.8. Analysis.
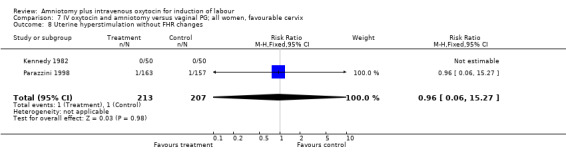
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
7.10. Analysis.
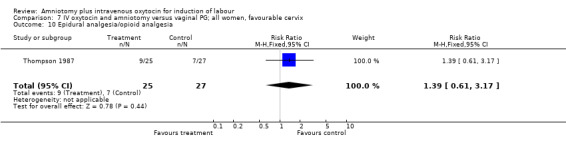
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
7.11. Analysis.
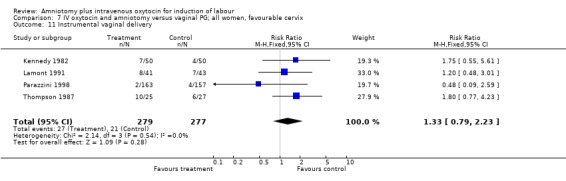
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
7.12. Analysis.
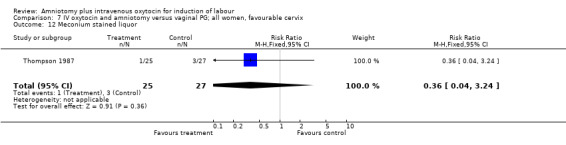
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 12 Meconium stained liquor.
7.13. Analysis.
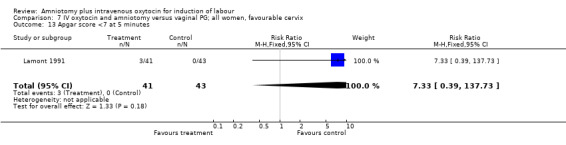
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 13 Apgar score <7 at 5 minutes.
7.14. Analysis.
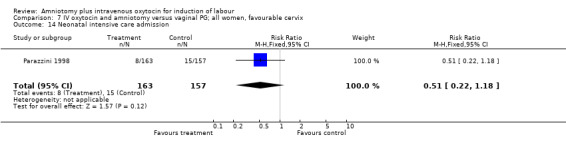
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 14 Neonatal intensive care admission.
7.19. Analysis.
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 19 Nausea.
7.23. Analysis.
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 23 Post partum haemorrhage.
7.25. Analysis.
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 25 Woman not satisfied.
7.28. Analysis.
Comparison 7 IV oxytocin and amniotomy versus vaginal PG; all women, favourable cervix, Outcome 28 Retained placenta.
Comparison 8. IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with FHR changes | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.70, 2.56] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia/opioid analgesia | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 11 Instrumental vaginal delivery | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.97] |
| 19 Nausea | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.53] |
| 20 Vomiting | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.53] |
| 21 Diarrhoea | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.70 [0.23, 97.06] |
8.2. Analysis.
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
8.3. Analysis.
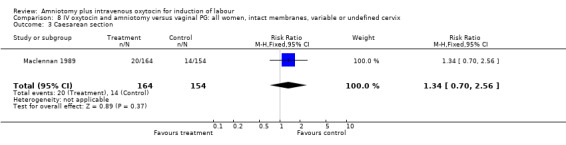
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 3 Caesarean section.
8.4. Analysis.
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 4 Serious neonatal morbidity or perinatal death.
8.5. Analysis.
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 5 Serious maternal morbidity or death.
8.10. Analysis.
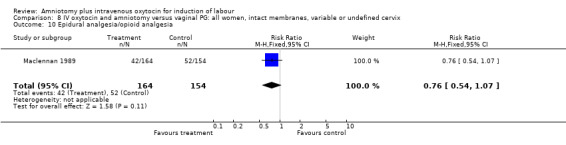
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 10 Epidural analgesia/opioid analgesia.
8.11. Analysis.
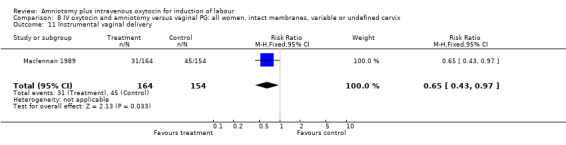
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
8.19. Analysis.
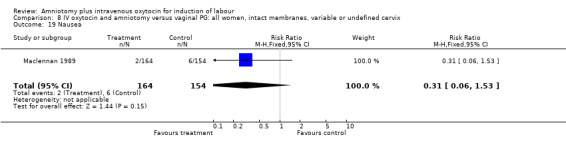
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 19 Nausea.
8.20. Analysis.
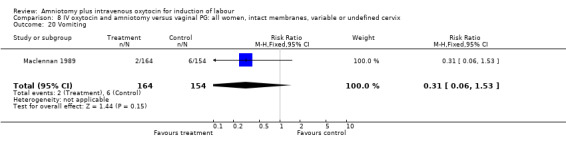
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 20 Vomiting.
8.21. Analysis.
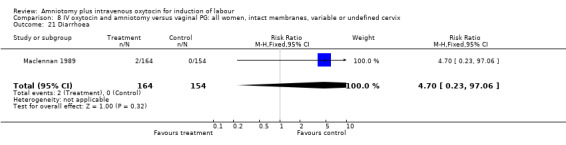
Comparison 8 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, variable or undefined cervix, Outcome 21 Diarrhoea.
Comparison 9. IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 3 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.68, 1.60] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.30] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.27] |
| 11 Instrumental vaginal delivery | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.60, 2.63] |
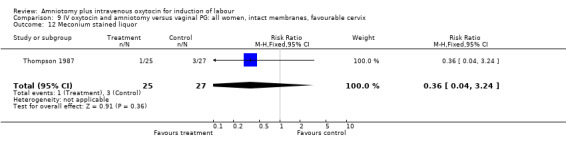
| 12 Meconium stained liquor | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
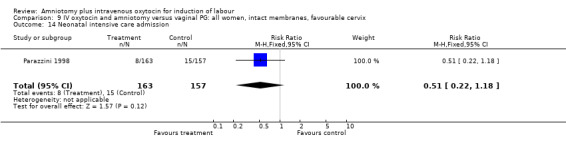
| 14 Neonatal intensive care admission | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.18] |
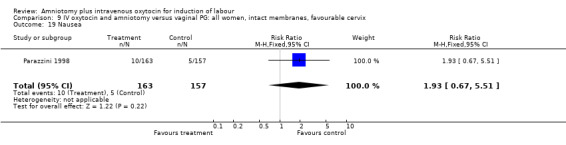
| 19 Nausea | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.67, 5.51] |
| 28 Retained placenta | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.36, 29.15] |
9.3. Analysis.
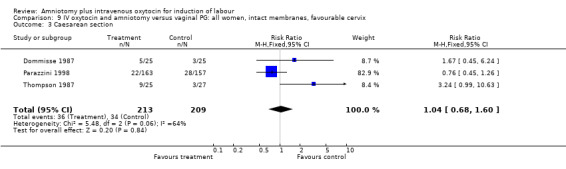
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 3 Caesarean section.
9.6. Analysis.
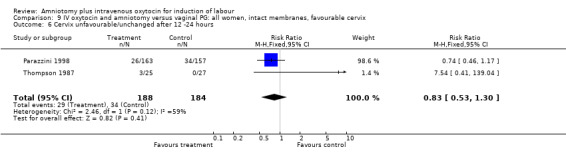
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours.
9.8. Analysis.
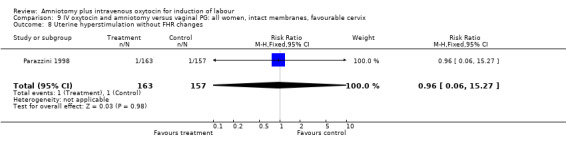
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
9.11. Analysis.
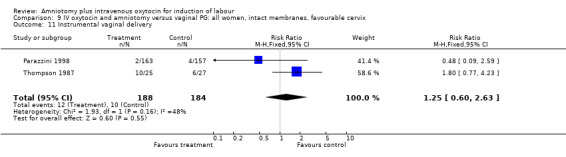
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
9.12. Analysis.
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 12 Meconium stained liquor.
9.14. Analysis.
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 14 Neonatal intensive care admission.
9.19. Analysis.
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 19 Nausea.
9.28. Analysis.
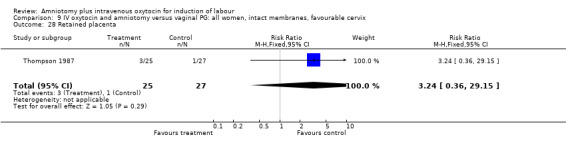
Comparison 9 IV oxytocin and amniotomy versus vaginal PG: all women, intact membranes, favourable cervix, Outcome 28 Retained placenta.
Comparison 10. IV oxytocin and amniotomy versus vaginal PG:all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.50, 3.50] |
| 5 Serious maternal morbidity or death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.95] |
| 11 Instrumental vaginal delivery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.21] |
| 23 Post partum haemorrhage | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 29 Precipitate labour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
10.3. Analysis.
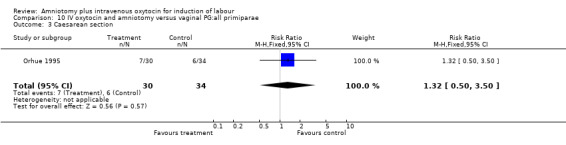
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 3 Caesarean section.
10.5. Analysis.
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 5 Serious maternal morbidity or death.
10.7. Analysis.
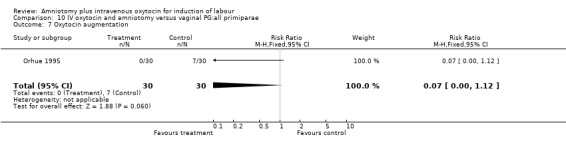
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 7 Oxytocin augmentation.
10.8. Analysis.
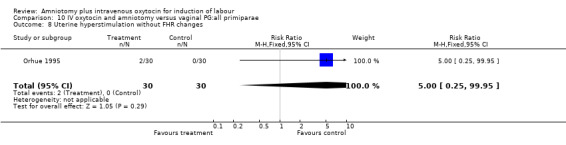
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
10.11. Analysis.
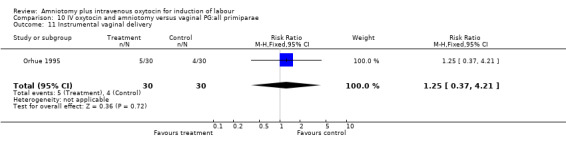
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 11 Instrumental vaginal delivery.
10.23. Analysis.
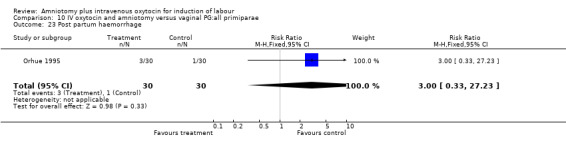
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 23 Post partum haemorrhage.
10.29. Analysis.
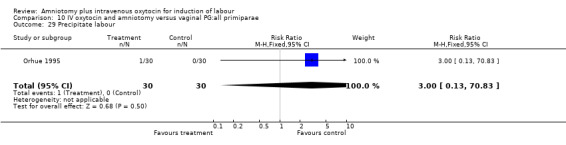
Comparison 10 IV oxytocin and amniotomy versus vaginal PG:all primiparae, Outcome 29 Precipitate labour.
Comparison 11. IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.50, 3.50] |
| 5 Serious maternal morbidity or death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.95] |
| 11 Instrumental vaginal delivery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.21] |
| 23 Post partum haemorrhage | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 29 Precipitate labour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
11.3. Analysis.
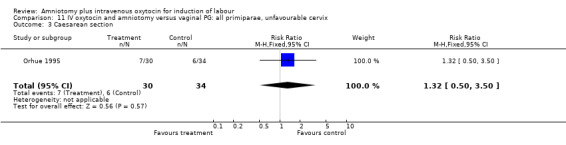
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 3 Caesarean section.
11.5. Analysis.
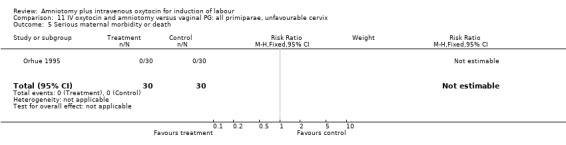
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
11.7. Analysis.
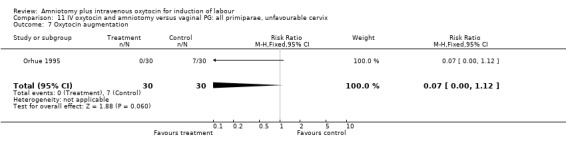
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 7 Oxytocin augmentation.
11.8. Analysis.
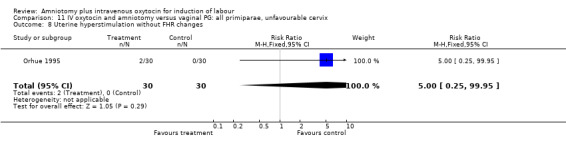
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
11.11. Analysis.
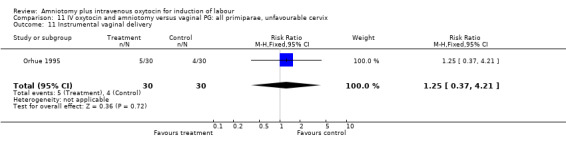
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
11.23. Analysis.
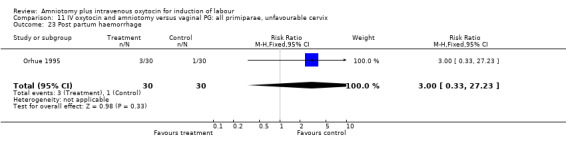
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 23 Post partum haemorrhage.
11.29. Analysis.
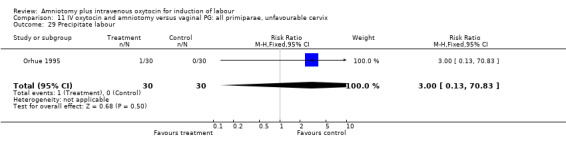
Comparison 11 IV oxytocin and amniotomy versus vaginal PG: all primiparae, unfavourable cervix, Outcome 29 Precipitate labour.
Comparison 12. IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.61] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.04, 61.62] |
| 25 Women not satisfied | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 53.0 [3.32, 846.47] |
12.2. Analysis.
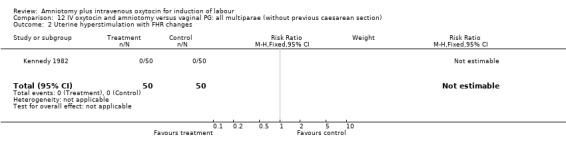
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 2 Uterine hyperstimulation with FHR changes.
12.3. Analysis.
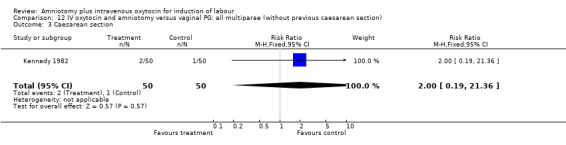
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 3 Caesarean section.
12.7. Analysis.
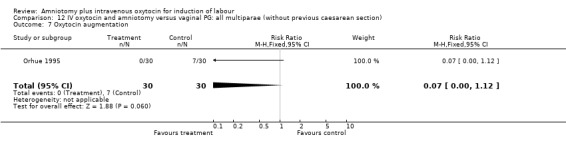
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 7 Oxytocin augmentation.
12.11. Analysis.
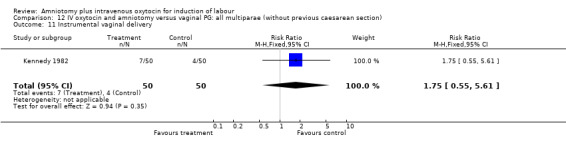
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 11 Instrumental vaginal delivery.
12.23. Analysis.
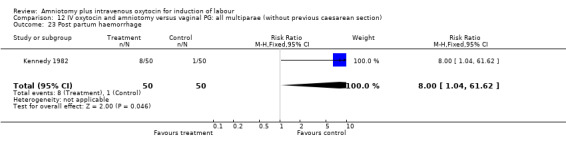
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 23 Post partum haemorrhage.
12.25. Analysis.
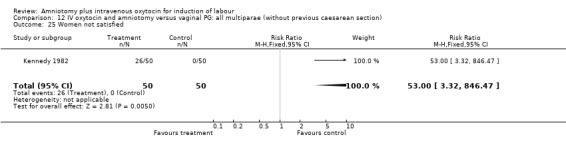
Comparison 12 IV oxytocin and amniotomy versus vaginal PG: all multiparae (without previous caesarean section), Outcome 25 Women not satisfied.
Comparison 13. IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.61] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.04, 61.62] |
| 25 Woman not satisfied | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 53.0 [3.32, 846.47] |
13.2. Analysis.
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
13.3. Analysis.
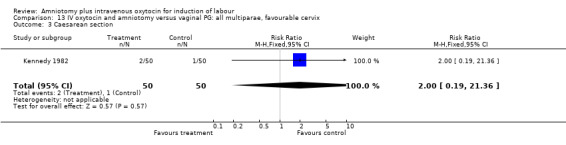
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 3 Caesarean section.
13.4. Analysis.
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death.
13.8. Analysis.
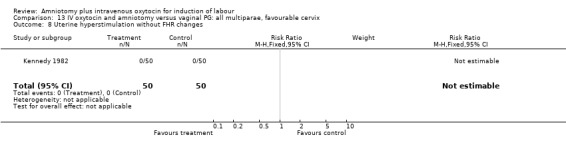
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
13.11. Analysis.
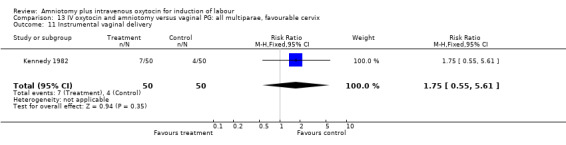
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 11 Instrumental vaginal delivery.
13.23. Analysis.
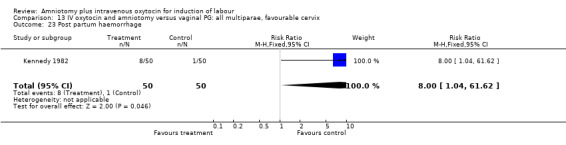
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 23 Post partum haemorrhage.
13.25. Analysis.
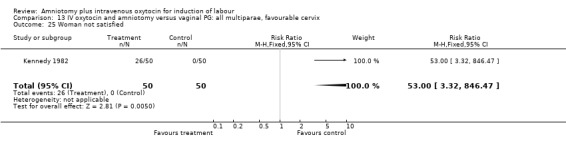
Comparison 13 IV oxytocin and amniotomy versus vaginal PG: all multiparae, favourable cervix, Outcome 25 Woman not satisfied.
Comparison 14. IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.46, 1.75] |
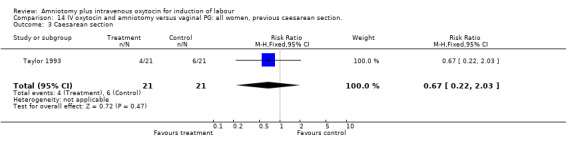
| 3 Caesarean section | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.03] |
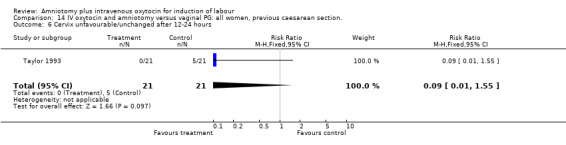
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.55] |
| 9 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 10 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.93, 2.17] |
| 11 Instrumental vaginal delivery | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.39, 4.02] |
| 13 Apgar score < 7 at 5 minutes | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
14.1. Analysis.
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 1 Vaginal delivery not achieved in 24 hours.
14.3. Analysis.
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 3 Caesarean section.
14.6. Analysis.
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
14.9. Analysis.
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 9 Uterine rupture.
14.10. Analysis.
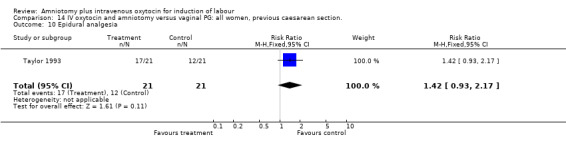
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 10 Epidural analgesia.
14.11. Analysis.
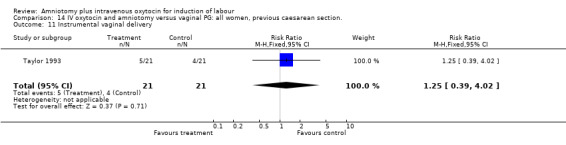
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 11 Instrumental vaginal delivery.
14.13. Analysis.
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 13 Apgar score < 7 at 5 minutes.
14.14. Analysis.
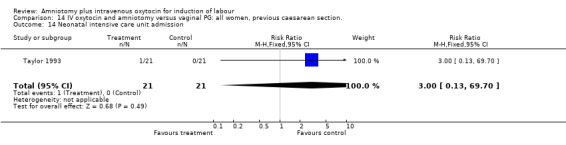
Comparison 14 IV oxytocin and amniotomy versus vaginal PG: all women, previous caesarean section., Outcome 14 Neonatal intensive care unit admission.
Comparison 15. IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.46, 1.75] |
| 3 Caesarean section | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.03] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.55] |
| 9 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 10 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.93, 2.17] |
| 11 Instrumental vaginal delivery | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.39, 4.02] |
| 13 Apgar score < 7 at 5 minutes | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
15.1. Analysis.
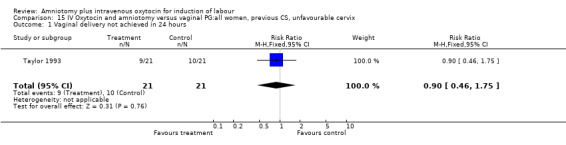
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
15.3. Analysis.
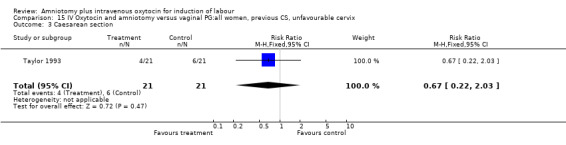
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 3 Caesarean section.
15.6. Analysis.
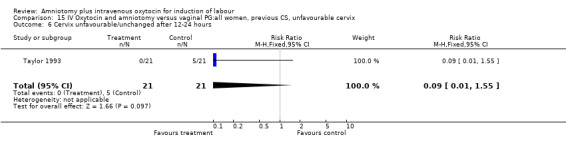
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
15.9. Analysis.
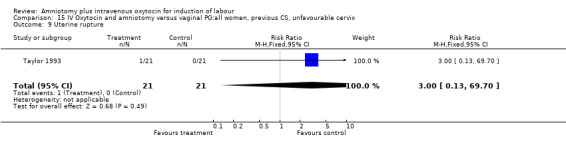
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 9 Uterine rupture.
15.10. Analysis.
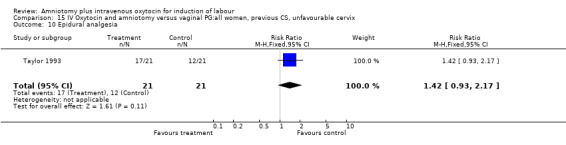
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 10 Epidural analgesia.
15.11. Analysis.
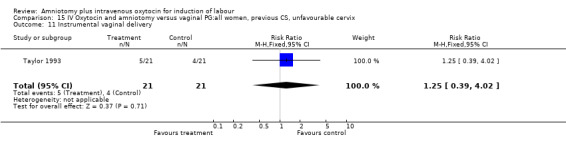
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
15.13. Analysis.
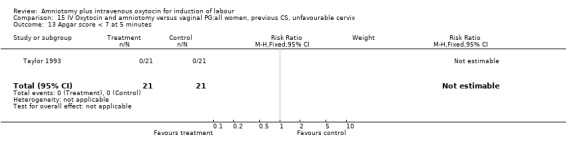
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
15.14. Analysis.
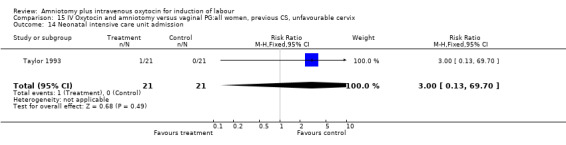
Comparison 15 IV Oxytocin and amniotomy versus vaginal PG:all women, previous CS, unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
Comparison 16. IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.46, 1.75] |
| 3 Caesarean section | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.03] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.55] |
| 9 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 10 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.93, 2.17] |
| 11 Instrumental vaginal delivery | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.39, 4.02] |
| 13 Apgar score < 7 at 5 minutes | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
16.1. Analysis.
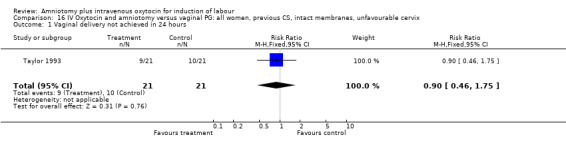
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
16.3. Analysis.
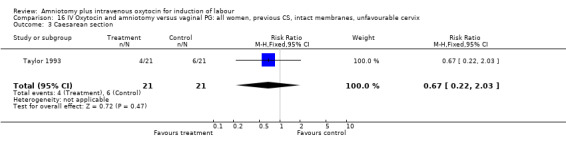
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
16.6. Analysis.
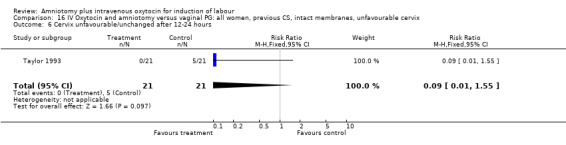
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
16.9. Analysis.
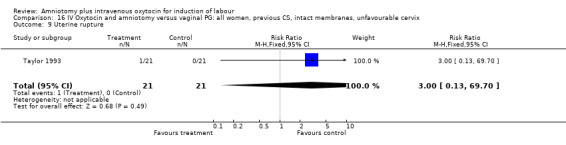
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 9 Uterine rupture.
16.10. Analysis.
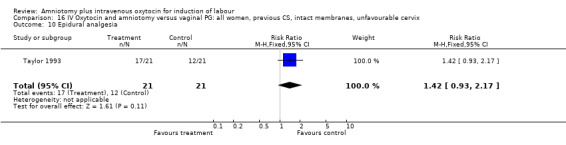
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 10 Epidural analgesia.
16.11. Analysis.
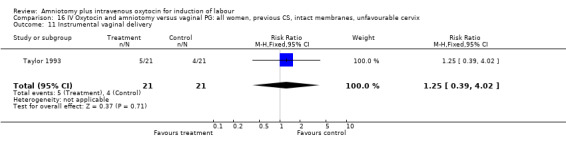
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
16.13. Analysis.
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
16.14. Analysis.
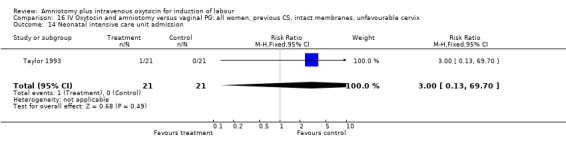
Comparison 16 IV Oxytocin and amniotomy versus vaginal PG: all women, previous CS, intact membranes, unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
Comparison 17. IV oxytocin and amniotomy versus intracervical PG: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
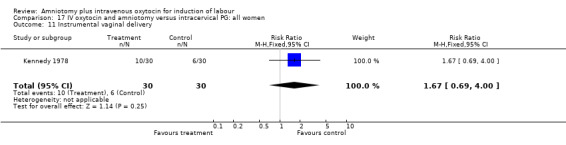
| 11 Instrumental vaginal delivery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.69, 4.00] |
| 12 Meconium stained liquor | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Woman not satisfied | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
17.1. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
17.2. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
17.3. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 3 Caesarean section.
17.7. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 7 Oxytocin augmentation.
17.8. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
17.11. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 11 Instrumental vaginal delivery.
17.12. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 12 Meconium stained liquor.
17.25. Analysis.
Comparison 17 IV oxytocin and amniotomy versus intracervical PG: all women, Outcome 25 Woman not satisfied.
Comparison 18. IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 7 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.12] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.69, 4.00] |
| 12 Meconium stained liquor | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Women not satisfied | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
18.1. Analysis.
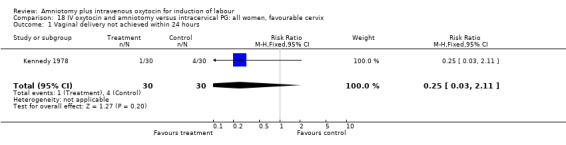
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
18.2. Analysis.
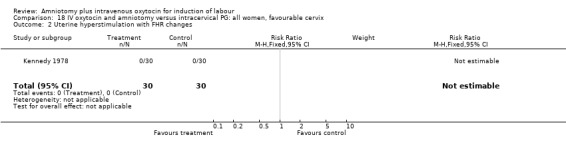
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
18.3. Analysis.
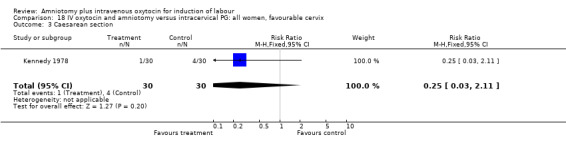
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 3 Caesarean section.
18.7. Analysis.
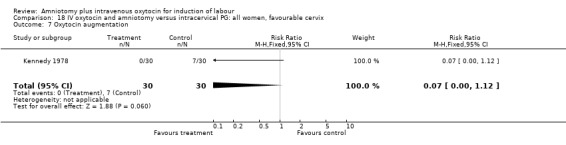
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 7 Oxytocin augmentation.
18.8. Analysis.
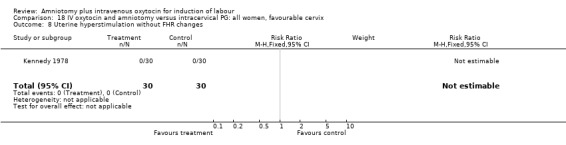
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
18.11. Analysis.
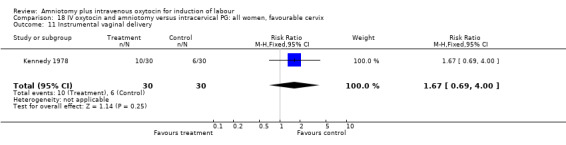
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
18.12. Analysis.
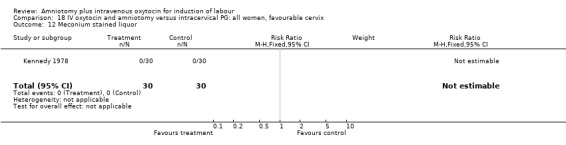
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 12 Meconium stained liquor.
18.25. Analysis.
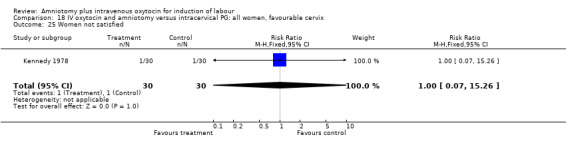
Comparison 18 IV oxytocin and amniotomy versus intracervical PG: all women, favourable cervix, Outcome 25 Women not satisfied.
Comparison 19. IV oxytocin and amniotomy versus oxytocin alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 2 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.71] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.89, 2.75] |
| 10 Epidural analgesia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.24] |
| 12 Mecomium stained liquor | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.89] |
| 13 Apgar score <7 at 5 minutes | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.89 [0.44, 34.19] |
19.3. Analysis.
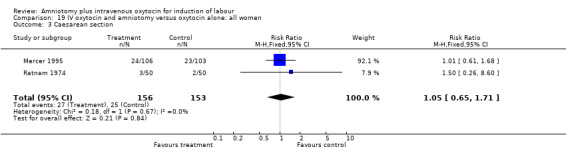
Comparison 19 IV oxytocin and amniotomy versus oxytocin alone: all women, Outcome 3 Caesarean section.
19.8. Analysis.
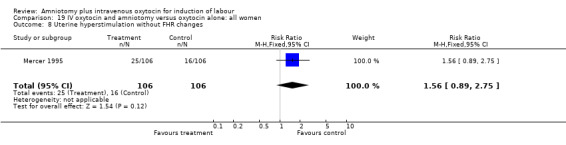
Comparison 19 IV oxytocin and amniotomy versus oxytocin alone: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
19.10. Analysis.
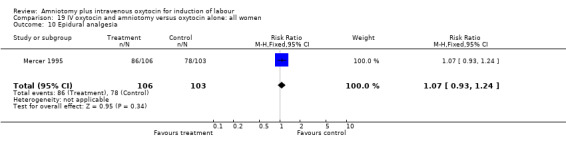
Comparison 19 IV oxytocin and amniotomy versus oxytocin alone: all women, Outcome 10 Epidural analgesia.
19.12. Analysis.
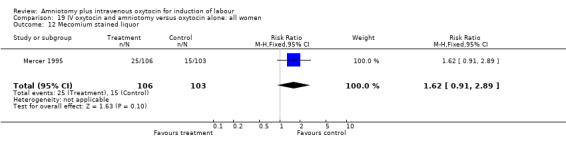
Comparison 19 IV oxytocin and amniotomy versus oxytocin alone: all women, Outcome 12 Mecomium stained liquor.
19.13. Analysis.
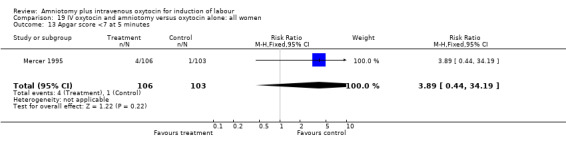
Comparison 19 IV oxytocin and amniotomy versus oxytocin alone: all women, Outcome 13 Apgar score <7 at 5 minutes.
Comparison 20. IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.61, 1.68] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.89, 2.75] |
| 10 Epidural analgesia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.24] |
| 12 Mecomium stained liquor | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.89] |
| 13 Apgar score <7 at 5 minutes | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.89 [0.44, 34.19] |
20.3. Analysis.
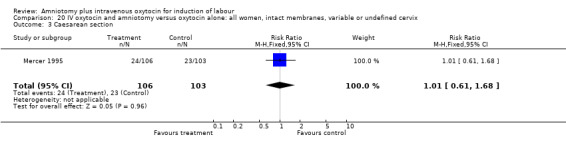
Comparison 20 IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix, Outcome 3 Caesarean section.
20.8. Analysis.
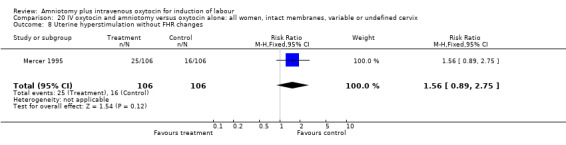
Comparison 20 IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
20.10. Analysis.
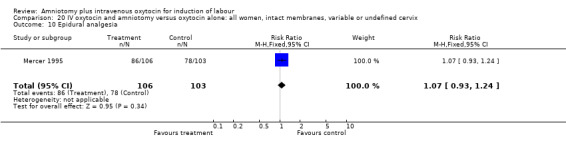
Comparison 20 IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix, Outcome 10 Epidural analgesia.
20.12. Analysis.
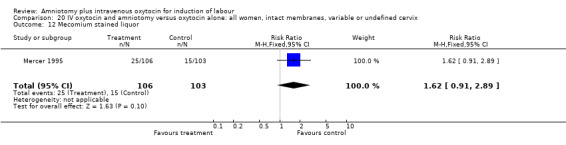
Comparison 20 IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix, Outcome 12 Mecomium stained liquor.
20.13. Analysis.
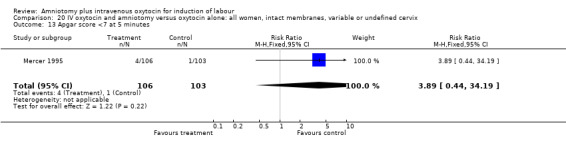
Comparison 20 IV oxytocin and amniotomy versus oxytocin alone: all women, intact membranes, variable or undefined cervix, Outcome 13 Apgar score <7 at 5 minutes.
Comparison 21. IV oxytocin and amniotomy versus amniotomy alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.41] |
| 3 Caesarean section | 2 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.30] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.49, 0.85] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 1.00] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
21.1. Analysis.
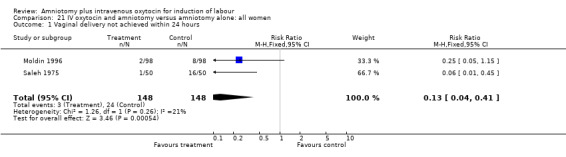
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
21.3. Analysis.
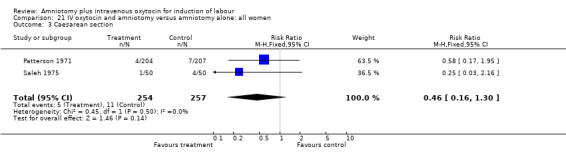
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 3 Caesarean section.
21.10. Analysis.
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 10 Epidural analgesia/opioid analgesia.
21.11. Analysis.
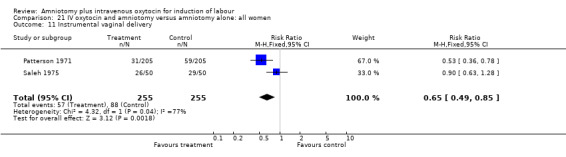
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 11 Instrumental vaginal delivery.
21.16. Analysis.
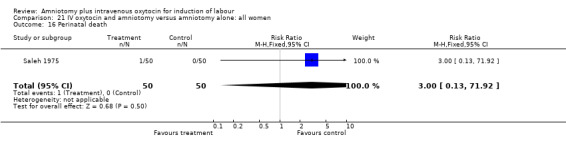
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 16 Perinatal death.
21.23. Analysis.
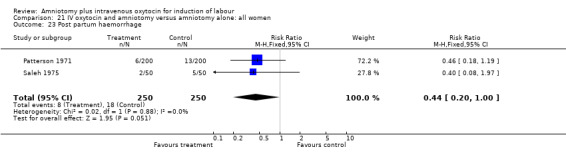
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 23 Post partum haemorrhage.
21.30. Analysis.
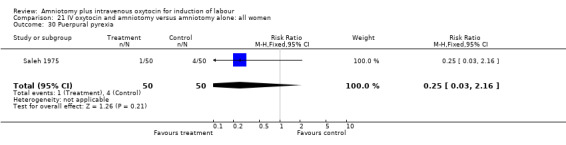
Comparison 21 IV oxytocin and amniotomy versus amniotomy alone: all women, Outcome 30 Puerpural pyrexia.
Comparison 22. IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.41] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.28] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.97] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
22.1. Analysis.
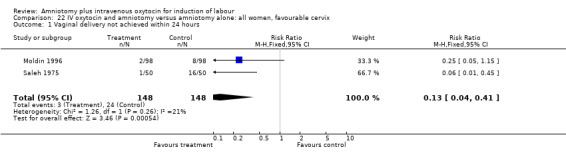
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
22.3. Analysis.
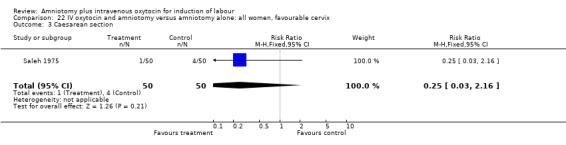
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 3 Caesarean section.
22.10. Analysis.
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
22.11. Analysis.
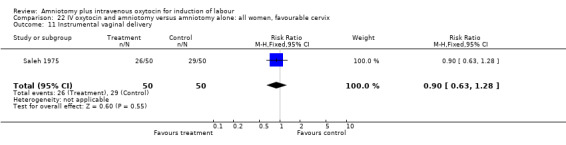
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
22.16. Analysis.
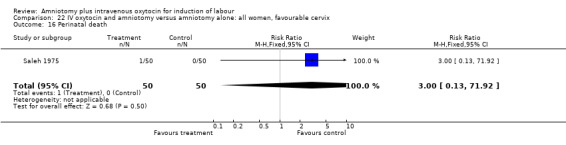
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 16 Perinatal death.
22.23. Analysis.
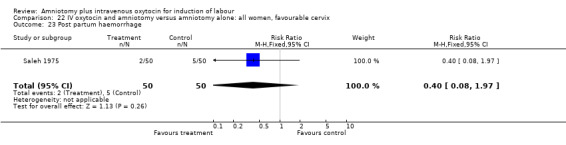
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 23 Post partum haemorrhage.
22.30. Analysis.
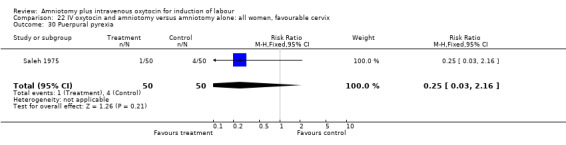
Comparison 22 IV oxytocin and amniotomy versus amniotomy alone: all women, favourable cervix, Outcome 30 Puerpural pyrexia.
Comparison 23. IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.41] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.28] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.97] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
23.1. Analysis.
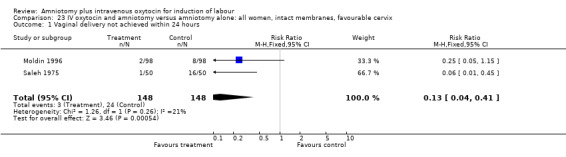
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
23.3. Analysis.
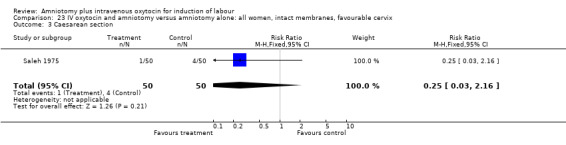
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 3 Caesarean section.
23.10. Analysis.
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
23.11. Analysis.
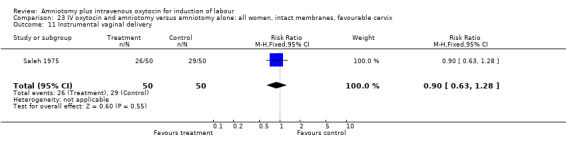
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
23.16. Analysis.
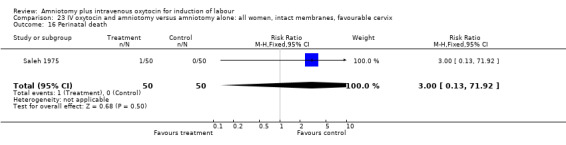
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 16 Perinatal death.
23.23. Analysis.
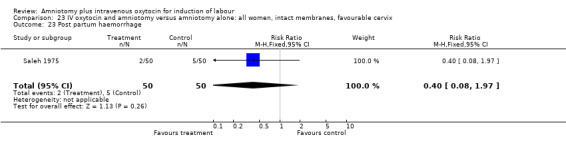
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 23 Post partum haemorrhage.
23.30. Analysis.
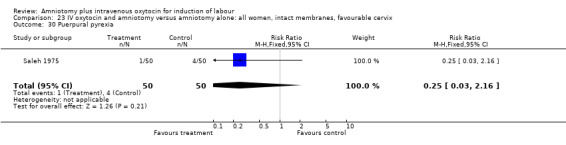
Comparison 23 IV oxytocin and amniotomy versus amniotomy alone: all women, intact membranes, favourable cervix, Outcome 30 Puerpural pyrexia.
Comparison 24. IV oxytocin and amniotomy versus amniotomy alone: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.45] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.28] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.97] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
24.1. Analysis.
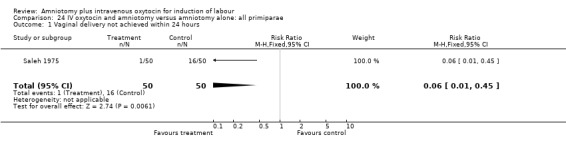
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
24.3. Analysis.
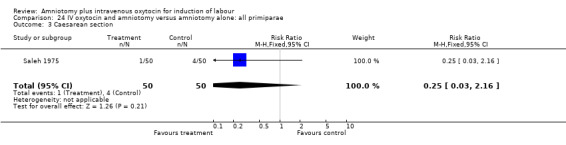
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 3 Caesarean section.
24.10. Analysis.
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 10 Epidural analgesia/opioid analgesia.
24.11. Analysis.
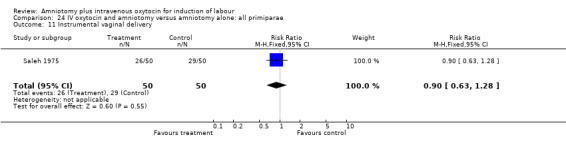
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 11 Instrumental vaginal delivery.
24.16. Analysis.
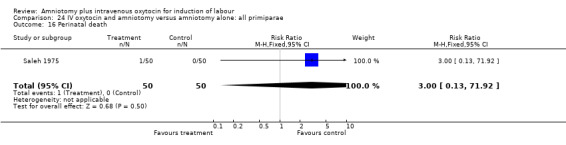
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 16 Perinatal death.
24.23. Analysis.
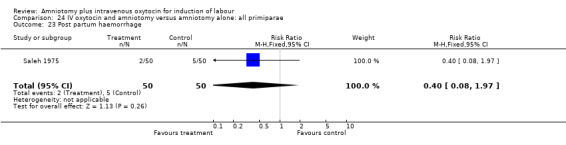
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 23 Post partum haemorrhage.
24.30. Analysis.
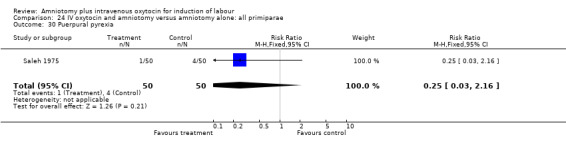
Comparison 24 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, Outcome 30 Puerpural pyrexia.
Comparison 25. IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.45] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.28] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.97] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
25.1. Analysis.
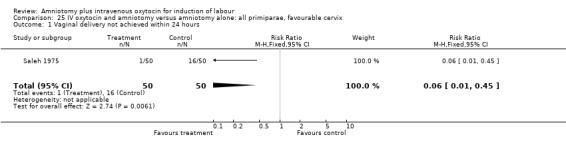
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
25.3. Analysis.
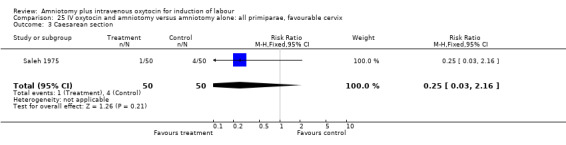
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 3 Caesarean section.
25.10. Analysis.
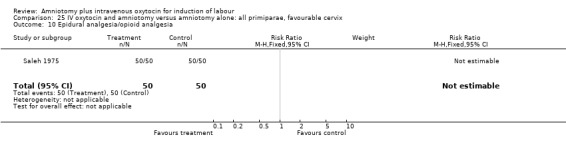
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
25.11. Analysis.
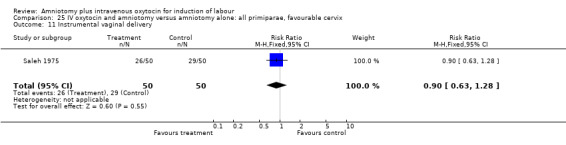
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 11 Instrumental vaginal delivery.
25.16. Analysis.
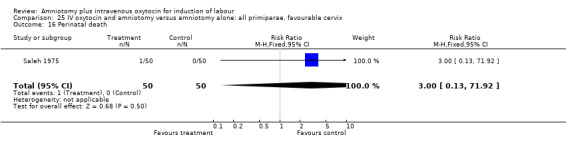
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 16 Perinatal death.
25.23. Analysis.
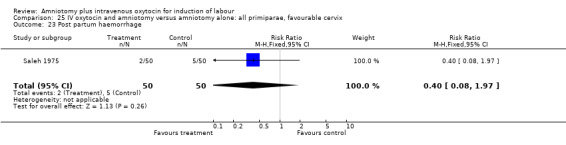
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 23 Post partum haemorrhage.
25.30. Analysis.
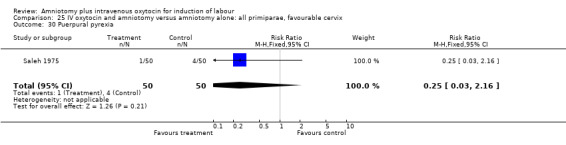
Comparison 25 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, favourable cervix, Outcome 30 Puerpural pyrexia.
Comparison 26. IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.45] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 10 Epidural analgesia/opioid analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.28] |
| 16 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 23 Post partum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.97] |
| 30 Puerpural pyrexia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
26.1. Analysis.
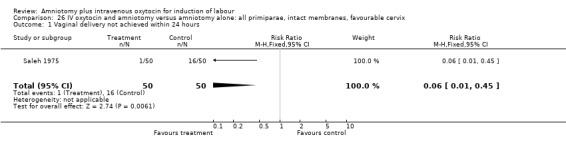
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
26.3. Analysis.
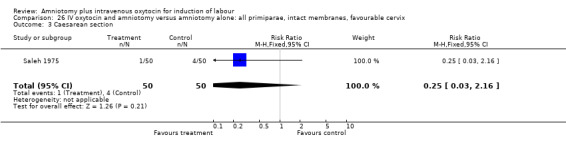
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 3 Caesarean section.
26.10. Analysis.
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 10 Epidural analgesia/opioid analgesia.
26.11. Analysis.
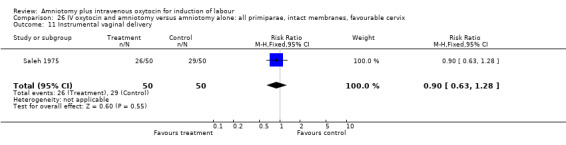
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
26.16. Analysis.
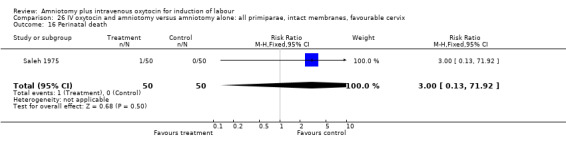
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 16 Perinatal death.
26.23. Analysis.
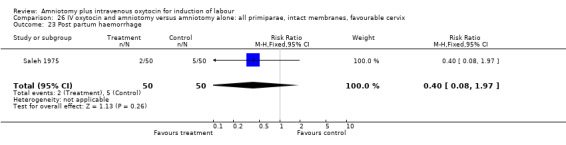
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 23 Post partum haemorrhage.
26.30. Analysis.
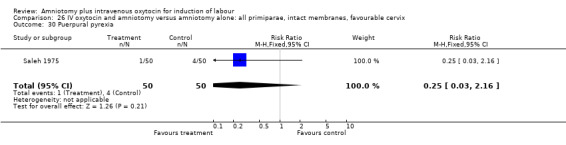
Comparison 26 IV oxytocin and amniotomy versus amniotomy alone: all primiparae, intact membranes, favourable cervix, Outcome 30 Puerpural pyrexia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dommisse 1987.
| Methods | Study design: parallel with possible crossover. Written consent. Setting: University Hospital. | |
| Participants | Participants: 50. Inclusion criteria: Intact membranes; Bishop score >5; gestation 37 weeks or more; acceptable indication for induction. Exclusion criteria: Previous caesarean section; vaginal bleeding; ruptured membranes; patients in labour before admission; history of asthma, glaucoma. | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (2U in 1000 ml 5% dextrose in water; titration dose 2mU/min, dose doubled every 30 min till max 16 mU/min). Intervention 2: PGE2 gel (Prepidil gel‐Upjohn Limited) Dosage: 1 mg in posterior fornix, reassessed in 6 hours; if not 3cm dilatation, 2 mg inserted. If not in labour after 12 hours, cross‐over to intervention 1. | |
| Outcomes | Included outcomes: Caesarean section. | |
| Notes | Crossover unclear. Prepidil used only intravaginally, not intracervical. Adequate allocation generation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kennedy 1978.
| Methods | Random allocation to one of three groups. Crossover design. Amniotomy performed in control and study group but not in all study group participants. Favourable cervical score. | |
| Participants | Participants: 90 patients (60 patients' data relevant to review). Primigravid and multigravid. Inclusion criteria: Gestational age >36 weeks; Bishop score >4. | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (dose range;1‐32 mu/min) (Intervention 2: Amniotomy + oral PGE2 (Prostin E2; Upjohn), 0.5mg hourly. If not adequate progression after 6 hours, intravenous oxytocin). Intervention 3: Endocervical PGE2 400 microgram single application; AROM after 3 hours ‐ if not adequate progression after 6 hours, intravenous oxytocin. | |
| Outcomes | Included outcomes: Vaginal delivery not achieved in 24 hours; uterine hyperstimulation with FHR changes; caesarean section; uterine hyperstimulation without FHR changes; instrumental vaginal delivery; meconium stained liquor; women not satisfied. | |
| Notes | Allocation sequence generation unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kennedy 1982.
| Methods | Study design: Parallel study. Randomization into two arms. | |
| Participants | Participants: 100 patients. Multigravid patients of low parity. Inclusion criteria: Singleton pregnancies; cephalic presentations; gestation 38‐42 weeks; parity 1‐2; Bishop score >4. | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (controlled semi‐ automatic infusion system; max dose 32 mU/min). Intervention 2: PGE2 3mg in posterior fornix; amniotomy after 6 hours or sooner if regular uterine activity and cervical dilatation >3cm. If not adequate progression in second group, intravenous oxytocin was added (augmentation). | |
| Outcomes | Included outcomes: Uterine hyperstimulation with FHR changes; caesarean section; perinatal death; uterine hyperstimulation without FHR changes; epidural/narcotic analgesia; instrumental vaginal delivery (forceps); post partum haemorrhage; women not satisfied. | |
| Notes | Allocation sequence generation unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lamont 1991.
| Methods | Study design: Parallel study. Patients in induction group randomized into two groups with stratification to ensure similar numbers of nulliparous and parous women in each group. Patient fall out did occur due to wrong intervention/lost data. | |
| Participants | Participants: 93 (84 patients' data relevant to review). Inclusion criteria: Singleton pregnancies; cephalic presentations; gestation >36 weeks; live fetus; Bishop score >4. Exclusion criteria: Previous caesarean section; parity 4 or more. | |
| Interventions | Intervention 1: Intravenous oxytocin + amniotomy (dose 2 mU/min rate adjustment according to attendants discretion; max dose 32 mU/min) Intervention 2: PGE2 gel (Prostin gel‐ Upjohn) 1 mg intravaginal; repeated after 4 hours if not established labour (max 3 applications). | |
| Outcomes | Included outcomes: Caesarean section; neonatal/perinatal death; instrumental vaginal delivery; Apgar score <7 at 5 minutes. | |
| Notes | Main outcome of study: intra uterine pressure differences between spontaneous and induced labours. Regarding the main outcome fall out did occur but analysis of induction method valid. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Maclennan 1980.
| Methods | Two trials reported as one, comparing PG F2 alpha and intravenous oxytocin in subgroups of patients with spontaneous rupture of membranes without onset of labour and artificial amniotomy. | |
| Participants | Participants: 83 (23 + 60). Inclusion criteria: Singleton pregnancies; cephalic presentations; maternal height >150 cm. Exclusion criteria: Previous uterine surgery; history of asthma; signs of labour. | |
| Interventions | Intervention 1: Intravenous oxytocin (dose 3.33 mU/min, increasing dose every 15 min; max 40 mU/min). Subgroups: (a) Spontaneous rupture of membranes without labour (PROM). (b) Artificial rupture of membranes (amniotomy).
Intervention 2: Intravaginal PG F2alpha (mixed with 700 mg tylose granules). Subgroups: (a) Spontaneous rupture of membranes without labour (PROM). (b) Artificial rupture of membranes (amniotomy). If patients in study group was not in labour in 4 hours, intravenous oxytocin was given. |
|
| Outcomes | Included outcomes: Uterine hyperstimulation with FHR changes; caesarean section; serious neonatal morbidity/perinatal death; uterine hyperstimulation without FHR changes; epidural analgesia; instrumental vaginal delivery; perinatal death; maternal side effects: vomiting, diarrhoea, chorioamnionitis. Other outcomes: Patient satisfaction. |
|
| Notes | Only means of gestation given, uncertainty whether all patients were third trimester inductions. Crossover to oxytocin was done only 4 hours post insertion of PG if not established labour, thus inducing labour, not augmentation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Maclennan 1989.
| Methods | Multicentre open label trial. Parallel randomized design. 2 arms: PGE2 gel (1‐2 mg) compared to amniotomy and intravenous oxytocin. Setting: 6 teaching hospitals. | |
| Participants | Participants: 320 Women, near term pregnancies. Multigravid and primigravid inclusions. Exclusion criteria: Evidence of labour; ruptured membranes; previous attempts at labour induction in this pregnancy; previous caesarean section; contraindication for labour or vaginal delivery; vaginal bleeding; known hypersensitivity to PG; glaucoma; asthma. | |
| Interventions | Intervention 1: Amniotomy and intravenous oxytocin (dose: 1mU/min; 15‐30 min escalating doses; max 16mU/min. Intervention 2: PGE2 (Prepidil gel‐Upjohn) posterior vaginal fornix. Repeated after 6 hours if not spontaneous ROM. As soon as established labour, artificial rupture of membranes. If not in established labour within 12 hours, intravenous oxytocin + amniotomy (depending on treating physician). |
|
| Outcomes | Included outcomes: Uterine hyperstimulation with FHR changes; epidural analgesia; instrumental vaginal delivery; nausea; vomiting; caesarean section; perinatal deaths; maternal death. | |
| Notes | Study too small to detect maternal death/perinatal death. Protocol violations: Exclusions after randomization‐ 1 from each group (spontaneous labour before induction); 4 patients received other treatment than randomized to; 2 patients included with previous caesarean sections; 3 twin pregnancies; 3 breech presentations. Some patients in PGE2 group received oxytocin before 12 hours. Protocol violations not regarded as significant as to data interpretation. Uneven totals randomised to two groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Martin 1978.
| Methods | Prospective, randomized trial. Study design: parallel study. Crossover did occur in expectant group. 2 arms: planned delivery versus expectant management. Induction at 39 weeks gestation; control awaiting spontaneous labour till 42 weeks, then induction. | |
| Participants | 264 uncomplicated pregnancies randomized, 184 analysed. Inclusion criteria: 38 weeks gestation according to accurate gestational dating; uncomplicated pregnancies. Patient fall out did occur but reasons given. | |
| Interventions | Intervention 1: Planned delivery: Amniotomy + intravenous oxytocin (started at 2.5 mU/min; doubled at 30 min intervals until a satisfactory uterine response. Intervention 2: Awaiting spontaneous onset of labour till 42 weeks gestation. If necessary, augmentation of labour by amniotomy + intravenous oxytocin. |
|
| Outcomes | Outcomes included: Caesarean section; perinatal death; analgesia; meconium stained liquor; women not satisfied. | |
| Notes | Multiple exclusions which may have had effect on outcome regarding risk of expectant management beyond 40 weeks gestation. The way in which patients were monitored during labour differed in the two groups: ('When possible, these patients also were monitored') ‐ control. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Melchior 1989.
| Methods | Patients randomized into two groups, comparing artificial rupture of membranes and intravenous oxytocin with vaginal prostaglandin. | |
| Participants | Participants: 50. Inclusion criteria: Primiparous as well as multiparous women; gestational age more than 36 weeks; alive fetus. Exclusion criteria: not mentioned. | |
| Interventions | Intervention 1: PG E2 vaginal gel, 1 mg administered vaginally and repeated in 6 hours (dose 1‐2 mg, depending on previous response). Intervention 2: Amniotomy and intravenous oxytocin, amniotomy was performed within 30 minutes after starting the oxytocin infusion (starting dose 2 mU/min; maximum dose 30 mU/min). | |
| Outcomes | Included outcomes: Caesarean section; serious neonatal morbidity/perinatal death; uterine hyperstimulation without FHR changes; epidural analgesia; instrumental vaginal delivery; Apgar score < 8 at 5 minutes. | |
| Notes | French study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mercer 1995.
| Methods | Patients randomized into two groups of timing of amniotomy with subgroup interval oxytocin dose increments of 30 min compared to 60 min. | |
| Participants | Participants: 209. Inclusion criteria: Intact membranes; gestation >36 weeks (37‐42). Exclusion criteria: Patients with intrauterine infection; prior cervical ripening with PG. | |
| Interventions | Intervention 1: Early amniotomy and two dose interval groups of intravenous oxytocin (30 min/ 60 min). Intervention 2: Amniotomy at 5 cm cervical dilatation and two groups of intravenous oxytocin dose intervals (30 min/60 min). | |
| Outcomes | Included outcomes: Caesarean section; uterine hyperstimulation without FHR changes; epidural analgesia; meconium stained liquor; Apgar score <7 at 5 minutes. Other outcomes: ROM till delivery; chorioamnionitis; time to delivery; time to active phase. | |
| Notes | Intravenous oxytocin stopped when in established labour; outcomes only reported for vaginal deliveries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Moldin 1996.
| Methods | Patients randomized into two groups comparing the combination of intravenous oxytocin and amniotomy with amniotomy alone. | |
| Participants | Participants: 196 Inclusion criteria: Singleton pregnancies, cephalic presentations, intact membranes, >36 weeks gestation, Bishop score > 6. | |
| Interventions | Intervention 1: Amniotomy and intravenous oxytocin started within one hour (3 mU/min and increasing by 3 mU/min every 30 min). Intervention 2: Amniotomy done and patient observed for 24 hours, if not in labour, intravenous oxytocin was started. | |
| Outcomes | Included outcomes: Vaginal delivery not achieved in 24 hours. | |
| Notes | 12 % of patients in group A and 10 % of patients in group B received intracervical PGE2 prior to amniotomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Orhue 1995.
| Methods | Study design: Parallel study, with crossover between two arms. University hospital setting. Patients randomised into 3 arms. | |
| Participants | Primigravid patients only. Participants: 94 (64 patients' data included in review). Duration of study: 18 months. Inclusion criteria: Singleton pregnancies; cephalic presentations; primiparous; gestation >36 weeks (37‐42); Bishop score >3; adequate pelvis on clinical assessment; minimal maternal height 155cm. Exclusion criteria: Known or suspected fetal anomalies; previous caesarean section; recent IOL attempt; dead fetus; scarred uterus; maternal age >35 years; placenta praevia, abruptio placentae; haemoglobinopathies, anaemia; polyhydramnios; hypersensitivity to PG (glaucoma, asthma) | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (5mU/ml of 5% dextrose at 2mU/min. Dose doubled every 30 min to max 32 mU/min or until regular contractions). Intervention 2: Vaginal PGE2 pessary (Prostin E2, Upjohn). 3 mg in posterior fornix, subsequent doses of 3mg at 6 and 12 hours depending on cervical dilatation, number of uterine contractions. (Intervention 3: Overnight extra‐amniotic Foley catheter with 30 ml bulb(17FG). If not in spontaneous labour the next morning, amniotomy + intravenous oxytocin begun). | |
| Outcomes | Included outcomes: Caesarean section; serious maternal morbidity/death; oxytocin augmentation; uterine hyperstimulation without FHR changes; instrumental vaginal delivery; post partum haemorrhage. Other (included): Precipitate labour (3 hours). | |
| Notes | 4 Patients in PGE2 group excluded after randomization due to failed induction of labour (no change in cervical status after 12 hours). Reanalysis possible for caesarean section as outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Parazzini 1998.
| Methods | Multicenter study, parallel with cross over. Central telephonic randomization into two arms. | |
| Participants | Participants: 320.
Multigravid and primigravid patients.
Inclusion criteria: Singleton pregnancies; cephalic presentations; intact membranes; gestation >36 weeks (>40 weeks); Bishop score >4 (5‐7); parity 0‐3; less than six uterine contractions/hour. Exclusion criteria: Previous caesarean section; diabetes; hypertension/PET; ruptured membranes. |
|
| Interventions | Intervention 1: Intravenous oxytocin followed in 1 hour by amniotomy (dose 1 mU/m min; rate doubled every 30 min to max 32 mU/min.
Intervention 2: Vaginal PGE2 (Prepidil gel, Upjohn 2mg ); two doses at six hourly intervals. Crossover did occur after 12 hours if patients were not in established labour. |
|
| Outcomes | Included outcomes: Caesarean section. Uterine hyperstimulation; instrumental vaginal delivery; neonatal intensive care admission; nausea; fever. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Patterson 1971.
| Methods | Study design: Parallel study. Two arms with crossover if labour not established after 24 hours. Patients excluded after initial randomization due to obstetrical factors. | |
| Participants | Participants: Initial randomization: 441 41 patients excluded thereafter. Primigravid and multigravid patients. Reason for induction: Medical conditions (PET, Eclampsia, Rhesus disease, other); obstetrical (prolonged pregnancy, malpresentations, multiple pregnancy, antepartum haemorrhage, disproportion, other). No information analyses given of patients with previous caesarean sections. No information given on gestational age ( assumed to be third trimester inductions). | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (0.5 IU/1000 ml dextrose and water; increased half hourly to 20 drops per minute. Thereafter 2 IU/1000 ml and 4 IU/1000 ml respectively, max 60 drops/min). Intervention 2: Amniotomy. If not in labour by 24 hours; Intravenous oxytocin added. |
|
| Outcomes | Included outcomes: Caesarean section; instrumental vaginal delivery; post partum haemorrhage. Other outcomes: Patients not in labour after 24 hours (given as mean; sedatives required during labour (given as number of doses required); bacteriological investigations (groups not specified). |
|
| Notes | Intra uterine deaths/ intrapartum deaths included in data but subgroup analyses not possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ratnam 1974.
| Methods | Randomised study, parallel design. Patients randomised into 4 groups. Indications for induction include pre eclampsia and may therefore be pre term inductions. | |
| Participants | Participants: 207. (100 Patients data included in review). Inclusion criteria: No evidence of chronic or acute fetal distress. Exclusion criteria: Malpresentations; uncertain dates; suspected cephalo‐pelvic disproportion. | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin ('physiological dose' increased every 30 min until satisfactory contractions, then dose maintained). Intervention 2: Intravenous oxytocin (dose as in 1). Intervention 3: Oral PGE2 (0.5 mg capsules, repeated hourly until labour established; dosage doubled every hour with max single dose 2,0 mg, + amniotomy. Intervention 4: Oral PGE2 (dose as in 3). | |
| Outcomes | Included outcomes: Caesarean section; cervix unfavourable/unchanged after 12‐24 hours. Other outcomes: (data not given with subgroup analyses): Hypertension, diarrhoea, post partum haemorrhage, perinatal death. | |
| Notes | All patients delivered within 24 hours. Patients with intact membranes also underwent caesarean sections with no information why regarded as failed induction without opting for second attempt next day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saleh 1975.
| Methods | Prospective, randomized trial with 2 arms. Study design: parallel. | |
| Participants | Participants: 100 patients
Inclusion criteria: Primigravid, age 15‐25; at or near term; not in labour; membranes intact; live singleton pregnancies; longitudinal lie; cephalic presentation; engaged presenting part; favourable cervix. Exclusion criteria: Severe PET/antepartum haemorrhage. |
|
| Interventions | Intervention 1: Amniotomy followed by intravenous oxytocin.
Dose 2‐4 mU/min; i U in 1000 ml 5% D+W. Intervention 2: Amniotomy: Oxytocin infusion commenced after 24 hours if not in active labour. All patients had epidural analgesia. |
|
| Outcomes | Included outcomes: Vaginal delivery not achieved in 24 hours; caesarean section; epidural analgesia; instrumental vaginal delivery; perinatal death; puerpural pyrexia; post partum haemorrhage. | |
| Notes | Perinatal death in oxytocin and amniotomy group had multiple congenital abnormalities. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Taylor 1993.
| Methods | Setting: Academic Hospital. Prospective randomised trial comparing vaginal prostaglandin with amniotomy and intravenous oxytocin in patients previously delivered by caesarean section. | |
| Participants | Participants: 42 women. Inclusion criteria: Singleton pregnancy, cephalic presentation, gestational age more than 36 weeks. Multiparous women with one previous pregnancy delivered by caesarean section. Bishop score less than 9. All indications for induction due to pre‐eclampsia or postterm gestation. | |
| Interventions | Intervention 1: Low amniotomy and immediate intravenous oxytocin titration. Intervention 2: Vaginal administration of prostaglandin E2 2.5 mg (witepsol pessary) followed by amniotomy after 3 hours and intravenous oxytocin augmentation after 6 hours if labour not established. |
|
| Outcomes | Included outcomes: Vaginal delivery not achieved within 24 hours; caesarean section; cervix unfavourable/unchanged after 12‐24 hours; uterine rupture; epidural analgesia; instrumental vaginal delivery; Apgar score < 7 at 5 minutes; neonatal intensive unit admission. | |
| Notes | Also published by Sellers 1988. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Thompson 1987.
| Methods | Report on 2 studies. 1: Randomized trial: Intravaginal PGE2 gel and intravenous oxytocin with amniotomy. 2: Questionnaire sent to women included in study, one year later to evaluate patient satisfaction with method of induction. | |
| Participants | Participants: 52 women. Inclusion criteria: Bishop score 4+. Exclusion criteria: Asthma; glaucoma; raised intraocular pressure; vaginal bleeding of uncertain aetiology; ruptured membranes; previous caesarean section; failed induction of labour in this pregnancy, labour; known hypersensitivity to PG. | |
| Interventions | Intervention 1: Amniotomy + intravenous oxytocin (Dose: 0.7‐1.4 mU/min infusion rate increased at intervals of 30 min; max 10 mu/min). Intervention 2: 1 mg PGE2(Prepidil ‐Upjohn) intravaginal ‐posterior fornix. Dose repeated if labour not established and membranes intact; dosage 1‐2mg. Amniotomy if indicated. |
|
| Outcomes | Included outcomes: Caesarean section; cervix unfavourable/unchanged after 12‐24 hours; epidural analgesia; instrumental vaginal delivery; meconium stained liquor. Other outcomes: Retained placenta. | |
| Notes | Patients excluded from trial if not in labour by 12 hours ‐ managed as failed induction. Data has been included for this review. Protocol violations: Multiple violations, inconsistent denominators. Second study: Information on patient satisfaction not included: 40 women questioned, 23 responders; non responders valuable in interpretation of results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Amniotomy regarded as augmentation of labour if applied more than two hours after induction was started.
AROM = intentional rupturing of the membranes to stimulate labour or inspect the amniotic fluid CTG = electronic heart rate monitoring of the fetus D+W = a solution of sterile water with sugar (dextrose) for intravenous use in labour FHR = fetal heart rate IOL = induction of labour IUPC = intra uterine pressue catheter, which is a soft plastic/celastic catheter system that is inserted through the cervix into the uterus during labour to accurately measure the strength of contractions min = minutes max = maximum PET = positron emission tomography PG = prostaglandin ROM = rupture of membranes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arulkumaran 1987 | Intervention and control group received the same induction agent with different methods of administration. |
| Augensen 1987 | Both groups received the same intervention. |
| Bakos 1987 | Inadequate randomization . Relevance to Review: Cross over trial comparing amniotomy with intravenous oxytocin for induction of labour. Subgroup analyses not possible from available data. Patients who received oxytocin and amniotomy only started with both interventions 4 hours after commencing induction with either one of the two interventions. (Possibility does exist that it may be augmentation.) |
| Breart 1982 | Deals with management of labour, not specified as induction Timing study. Same intervention both arms. |
| Breart 1991 | Deals with management of labour, not specified as induction of labour. '... choice of policies to be evaluated is based on policies applied and the results observed in Dublin ...' active management of labour. |
| Bremme 1984 | Compares amniotomy with oral PGE2. |
| Calder 1975 | Compares intravenous oxytocin + amniotomy with intravenous PGE2 + amniotomy. |
| Cameron 1985 | No primary outcomes reported. |
| Cameron 1988 | No numerators/denominators given. Data sought from authors. |
| Casey 1993 | All patients in control group did not receive amniotomy and intravenous oxytocin. |
| Chia 1993 | IUPC monitoring. Fetal monitoring with different methods. Both arms same induction agent. |
| Chua 1991 | Both groups received the same intervention/ induction agent: methods of administration differed. |
| Cole 1975 | Same intervention both arms. Randomization on timing. |
| D'Souza 1986 | Neonatal effects of oxytocin induction. Outcome of labour not investigated. |
| Engleman 1979 | Same intervention both arms. Compares spontaneous versus induced labour. Cost analysis study. |
| Gihwala 1987 | Amniotomy augmentation of labour. One patient included with an intrauterine death. |
| Goeree 1995 | Cost effectiveness study. Methods of induction not investigated. |
| Heden 1991 | Method of randomization not accepted (folder number). |
| Henry 1969 | Too much uncertainty regarding randomization, oxytocin administration and whether amniotomy was performed or not. |
| Katz 1983 | Same intervention both arms of study. |
| Leijon 1979 | No primary outcomes reported. Investigation of effect of induction of labour, not methods for induction. |
| Leijon 1980 | No primary outcomes reported. |
| Lo 1994 | Not a randomized controlled trial (patient allocation by alternation). |
| Lykkesfeldt 1979 | Intravenous oxytocin + amniotomy compared to oral PG. |
| Mahmood 1992 | Compares PGE2 with amniotomy(alone). Cross over occurred. Oxytocin augmentation, not induction. |
| Mancuso 1996 | No primary outcomes reported. |
| Mercer 1991 | Both groups received the same intervention. 'Priming of cervix' prior to induction. |
| Misra 1994 | Randomization unclear and amniotomy only performed after uterine contractions were present (augmentation). |
| Nilsson 1984 | No primary outcomes reported. Compares oral PGE2 with oxytocin. |
| Orhue 1993a | Both groups received the same intervention. |
| Orhue 1993b | Both groups received the same intervention. |
| Orhue 1994 | Both groups received the same intervention. |
| Pavlou 1978 | Both groups received the same intervention. |
| Reid 1995 | Both groups received the same intervention. Patients included in study with prior PG administration: no subgroup analyse in order to exclude them. |
| Sande 1983 | Large number of discrepancies without mentioning reasons, unspecified randomization. |
| Secher 1981 | Inadequate randomization with a large discrepancy in numbers. |
| Sellers 1988 | Uncertainty whether it is an abstract from the study published by Taylor in 1993. |
| Sivasuriya 1978 | No primary outcomes reported. Effect of various induction methods of labour on neonate given as means. |
| Steer 1976 | Some participants not randomly selected. |
| Steer 1985 | Both groups received the same intervention. |
| Suikkari 1983 | Allocation on parity. |
| Thomas 1974 | Both groups received the same intervention. Compares method of administration. |
| Tylleskar 1978 | No primary outcomes reported. |
| Tylleskar 1979 | No primary outcomes reported. |
| Varma 1981 | Not a randomized controlled trial. |
| Ward 1991 | No primary outcomes reported. |
| Westergaard 1983 | Compares oral PGE2 with oral oxytocin. |
| Witter 1987 | Same intervention both arms. Amniotomy possibly as augmentation. |
| Witter 1989 | Prediction trial. |
IUPC = intra uterine pressue catheter PG = prostaglandin
Contributions of authors
Both reviewers prepared the text. Danie Botha entered the data into RevMan and Graham Howarth checked the data.
Sources of support
Internal sources
No sources of support supplied
External sources
University of Pretoria and the South African MRC Unit for Maternal and Infant Care Strategies, South Africa.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Dommisse 1987 {published data only}
- Dommisse J, Wild JM. Assessment of a new prostaglandin E2 gel in labour induction. S Afr Med J 1987;71:506‐7. [PubMed] [Google Scholar]
Kennedy 1978 {published data only}
- Kennedy JH, Quinn MA, Howie PW, Calder AA. Single shot prostaglandin gel for labor induction. Prostaglandins 1978;15:169‐73. [DOI] [PubMed] [Google Scholar]
Kennedy 1982 {published data only}
- Kennedy JH, Stewart P, Barlow DH, Hillan E, Calder AA. Induction of labour: a comparison of a single prostaglandin E2 vaginal tablet with amniotomy and intravenous oxytocin. Br J Obstet Gynaecol 1982;89:704‐7. [DOI] [PubMed] [Google Scholar]
Lamont 1991 {published data only}
- Lamont RF, Neave S, Baker AC, Steer PJ. Intrauterine pressures in labours induced by amniotomy and oxytocin or vaginal prostaglandin gel compared with spontaneous labour. Br J Obstet Gynaecol 1991;98:441‐7. [DOI] [PubMed] [Google Scholar]
Maclennan 1980 {published data only}
- MacLennan AH, Green RC. The effect of intravaginal prostaglandin F2alpha on labour after spontaneous and artificial rupture of the membranes. Aust NZ J Obstet Gynaecol 1980;20:87‐90. [DOI] [PubMed] [Google Scholar]
Maclennan 1989 {published data only}
- MacLennan AH, Fraser I, Jakubowicz DL, Murray‐Arthur F, Quinn MA, Trudinger BJ. Labour induction with PGE2 vaginal gel: results of an Australian multicentre randomised trial. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:119.
- MacLennan AH, Fraser I, Jakubowicz DL, Murray‐Arthur F, Quinn MA, Trudinger BJ. Labour induction with low dose PGE2 vaginal gel: result of an Australian multicentre randomized trial. Aust NZ J Obstet‐Gynaecol 1989;29:124‐8. [DOI] [PubMed] [Google Scholar]
Martin 1978 {published data only}
- Martin DH, Thompson W, Pinkerton JHM, Watson JD. A randomized controlled trial of selective planned delivery. Br J Obstet Gynaecol 1978;85:109‐13. [DOI] [PubMed] [Google Scholar]
Melchior 1989 {published data only}
- Melchior J, Bernard N, Andre‐David F. Artificial inducement of labor, at term, for medical reasons. Comparison between two labor inducement techniques: pitocin + artificial rupture of the membranes at an early stage vs E2 vaginal gel. An open controlled randomized study [translation]. Rev Fr Gynecol Obstet 1989;84:747‐52. [PubMed] [Google Scholar]
Mercer 1995 {published data only}
- Mercer BM, McNanley T, O'Brien JM, Randal L, Sibai BM. Early vs late amniotomy for labor induction: a randomized trial. Am J Obstet Gynecol 1995;173:1321‐5. [DOI] [PubMed] [Google Scholar]
Moldin 1996 {published data only}
- Moldin PG, Sundell G. Induction of labour: a randomised clinical trial of amniotomy vs amniotomy with oxytocin infusion. Br J Obstet Gynaecol 1996;103:306‐12. [DOI] [PubMed] [Google Scholar]
Orhue 1995 {published data only}
- Orhue, AAE. Induction of labour at term in primigravidae with low Bishop's score: a comparison of three methods. Eur J Obstet‐Gynecol Reprod Biol 1995;58:119‐25. [DOI] [PubMed] [Google Scholar]
Parazzini 1998 {published data only}
- Parazzini F, Benedetto C, Danti L, Zanini A, Facchinetti F, Ettore G, et al. A randomized comparison of vaginal prostaglandin E2 with oxytocin plus amniotomy for induction of labour in women with intermediately ripe cervices. Eur J Obstet Gynecol Reprod Biol 1998;81:15‐20. [DOI] [PubMed] [Google Scholar]
Patterson 1971 {published data only}
- Patterson WM. Amniotomy, with or without simultaneous oxytocin infusion. J Obstet Gynaecol Br Cmmwlth 1971;78:310‐6. [DOI] [PubMed] [Google Scholar]
Ratnam 1974 {published data only}
- Ratnam SS, Khew KS, Chen C, Lim TC. Oral prostaglandin E2 in induction of labour. Aust NZ J Obstet Gynaecol 1974;14:26‐30. [Google Scholar]
Saleh 1975 {published data only}
- Saleh YZ. Surgical induction of labour with and without oxytocin infusion. A prospective study. Aust NZ J Obstet Gynaecol 1975;15:80‐3. [Google Scholar]
Taylor 1993 {published data only}
- Taylor AVG, Sellers S, Ah‐Moye M, MacKenzie IZ. A prospective random allocation trial to compare vaginal prostaglandin E2 with intravenous oxytocin for labour induction in women previously delivered by caesarean section. Journal of Obstetrics and Gynaecology 1993;13:333‐6. [Google Scholar]
Thompson 1987 {published data only}
- Thompson JH. Induction of labour: a comparison between intravaginal prostaglandin E2 gel and oxytocin infusion with low amniotomy. Proceedings of 21st International Congress of International Confederation of Midwives; 1987; The Hague, Netherlands. 1987.
References to studies excluded from this review
Arulkumaran 1987 {published data only}
- Arulkumaran S, Ingemarsson I, Ratnam SS. Oxytocin titration to achieve preset active contraction area values does not improve the outcome of induced labour. Br J Obstet Gynaecol 1987;94:242‐48. [DOI] [PubMed] [Google Scholar]
Augensen 1987 {unpublished data only}
- Augensen K, Bergsjo P, Eikeland T, Askvik K, Carlsen J. Induction of labour in prolonged pregnancy. A prospective, randomized study. Proceedings of 10th European Congress of Perinatal Medicine; 1986 Aug 12‐16; Leipzig, Germany. 1986:242.
- Augensen K, Bergsjo P, Eikeland T, Askvik K, Carlsen J. Randomised comparison of early vs late induction of labour in post‐term pregnancy. BMJ 1987;294:1192‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakos 1987 {published data only}
- Bakos O, Backstrom T. Induction of labor: a prospective, randomized study into amniotomy and oxytocin as induction methods in a total unselected population. Acta Obstet Gynecol Scand 1987;66:537‐41. [DOI] [PubMed] [Google Scholar]
Breart 1982 {published data only}
- Breart G, Goujard J, Maillard F, Chavigny C, Rumeau‐Rouquette C, Sureau C. Comparison of two obstetrical policies with regard to artificial induction of labour at term. A randomised trial [translation]. J Gynecol Obstet Biol Reprod (Paris) 1982;11:107‐12. [PubMed] [Google Scholar]
Breart 1991 {published data only}
- Breart G, Du Mazaubrun C, Maillard F, Garel M. Comparison of two policies of management of labour for primiparous women: effects of early rupture of membranes and use of oxytocin. Results of randomized controlled trial. Proceedings of International Conference on Primary Care, Obstetrics and Perinatal Health; 1991; Utrecht, Netherlands. 1991:49.
Bremme 1984 {published data only}
- Bremme K, Eneroth P, Samuelson K. Estriol and cholic acid in maternal serum in induced labor. Gynecol Obstet Invest 1984;17:120‐6. [DOI] [PubMed] [Google Scholar]
Calder 1975 {published data only}
- Calder AA, Embrey MP. Comparison of intravenous oxytocin and prostaglandin E2 for induction of labour using automatic and non‐automatic infusion techniques. Br J Obstet Gynaecol 1975;82:728‐33. [DOI] [PubMed] [Google Scholar]
Cameron 1985 {published data only}
- Cameron A. High Bishop score and labour induction. In: Wood C editor(s). The role of prostaglandins in labour. London: RSM Services Limited, 1985:61‐7. [Google Scholar]
Cameron 1988 {published data only (unpublished sought but not used)}
- Cameron AD, Calder AA, Walker JJ. Randomised comparison of PGE2 vaginal gel vs amniotomy and intravenous oxytocin in favourable induction. Proceedings of 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988:157.
Casey 1993 {published data only}
- Casey C, Kehoe J, Mylotte MJ. Vaginal prostaglandins for the ripe cervix. Int J Gynecol Obstet 1993;44:21‐6. [DOI] [PubMed] [Google Scholar]
Chia 1993 {published data only}
- Chia YT, Arulkumaran S, Soon SB, Norshida S, Ratnam SS. Induction of labour: does internal tocography result in better obstetric outcome than external tocography. Aust NZ J Obstet Gynaecol 1993;33(2):159‐61. [DOI] [PubMed] [Google Scholar]
Chua 1991 {published data only}
- Chua S, Arulkumaran S, Kurup A, Tay D, Ratnam SS. Oxytocin titration for induction of labour: a prospective randomized study of 15 vs 30 minute dose increment schedules. Aust NZ J Obstet Gynaecol 1991;31:134‐7. [DOI] [PubMed] [Google Scholar]
Cole 1975 {published data only}
- Cole RA, Howie PW, MacNaughton MC. Elective induction of labour. A randomised prospective trial. Lancet 1975;1:767‐70. [DOI] [PubMed] [Google Scholar]
D'Souza 1986 {published data only}
- D'Souza SW, Lieberman B, Cadman J, Richards B. Oxytocin induction of labour: hyponatraemia and neonatal jaundice. Eur J Obstet Gynecol Reprod Biol 1986;22:309‐17. [DOI] [PubMed] [Google Scholar]
Engleman 1979 {published data only}
- Engleman SR, Hilland MA, Howie PW, McIlwaine GM, McNay MB. An analysis of the economic implications of elective induction of labour at term. Community Medicine 1979;1:191‐8. [DOI] [PubMed] [Google Scholar]
Gihwala 1987 {published data only}
- Gihwala N. A comparison of prostaglandin E2 and oxytocin for the induction of labour ‐ a randomised trial. Proceedings of 23rd Congress of Obstetrics and Gynaecology; 1986 September 23‐26; South Africa. 1986:52.
- Gihwala N, Moodley J, Hansen J, Naicker SN. Prostaglandin E2 vaginal gel ‐ a new formulation for the induction of labour. S Afr Med J 1987;72:615‐7. [PubMed] [Google Scholar]
Goeree 1995 {published data only}
- Goeree R, Hannah M, Hewson S. Cost‐effectiveness of induction of labour vs serial antenatal monitoring in the Canadian Multicentre Postterm Pregnancy Trial. Can Med Assoc J 1995;152:1445‐50. [PMC free article] [PubMed] [Google Scholar]
Heden 1991 {published data only}
- Heden L, Ingemarsson I, Ahlstrom H, Solum T. Induction of labor vs conservative management in prolonged pregnancy: controlled study. Int J Feto Maternal Med 1991;4(4):148‐52. [Google Scholar]
Henry 1969 {published data only}
- Henry GR. A controlled trial of surgical induction of labour and amnioscopy in the management of prolonged pregnancy. J Obstet Gynaecol Br Cmmwlth 1969;76:795‐8. [DOI] [PubMed] [Google Scholar]
Katz 1983 {published data only}
- Katz Z, Yemini M, Lancet M, Mogilner BM, Ben‐Hur H, Caspi B. Non‐aggressive management of post‐date pregnancies. Eur J Obstet Gynecol Reprod Biol 1983;15:71‐9. [DOI] [PubMed] [Google Scholar]
Leijon 1979 {published data only}
- Leijon I, Finnstrom O, Hedenskog S, Ryden G, Tylleskar J. Spontaneous labour and elective induction ‐ a prospective randomised study. Behavioural assessment and neurological examination in the newborn period. Acta Paediatr Scand 1979;68:553‐60. [DOI] [PubMed] [Google Scholar]
Leijon 1980 {published data only}
- Leijon I, Finnstrom O, Hedenskog S, Ryden G, Tylleskar J. Spontaneous labor and elective induction ‐ a prospective randomized study. II Bilirubin levels in the neonatal period. Acta Obstet Gynecol Scand 1980;59:103‐6. [DOI] [PubMed] [Google Scholar]
Lo 1994 {published data only}
- * Lo L, Ho MW, Leung P. Comparison of prostaglandin E2 vaginal tablet with amniotomy and intravenous oxytocin for induction of labour. Aust NZ J Obstet Gynaecol 1994;34(2):149‐53. [DOI] [PubMed] [Google Scholar]
- Lo L, Ho MW, Leung P. Comparison of prostaglandin E2 vaginal tablet with amniotomy and intravenous oxytocin for induction of labour. The Second International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1993; Hong Kong. 1993:155.
Lykkesfeldt 1979 {published data only}
- Lykkesfeldt G, Osler M. A comparison of three methods for inducing labor: oral prostaglandin E2, buccal desaminooxytocin, intravenous oxytocin. Acta Obstet Gynecol Scand 1979;58:321‐5. [DOI] [PubMed] [Google Scholar]
Mahmood 1992 {published data only}
- Mahmood TA, Reyner A, Smith NC. A prospective randomized study of induction of labour with favourable cervix at term: a comparison between PGE2 gel single dose vs forewater amniotomy and delayed oxytocin infusion. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:403.
Mancuso 1996 {published data only}
- Mancuso S, Ferrazzani S, Carolis S, Carducci B, Santis L, Caruso A. Term and postterm low‐risk pregnancies: management schemes for the reduction of high rates of cesarean section. Minerva Ginecol 1996;48:95‐8. [PubMed] [Google Scholar]
Mercer 1991 {published data only}
- Mercer B, Pilgram P, Sibai B. Low dose oxytocin vs a routine induction protocol for the induction of labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:21.
- Mercer B, Pilgrim P, Sibai B. Labor induction with continuous low‐dose oxytocin infusion: a randomized trial. Obstet Gynecol 1991;77:659‐63. [PubMed] [Google Scholar]
Misra 1994 {published data only}
- Misra M, Vavre S. Labour induction with intracervical prostaglandin E2 gel and intravenous oxytocin in women with a very unfavourable cervix. Aust NZ J Obstet Gynaecol 1994;34:511‐5. [Google Scholar]
Nilsson 1984 {published data only}
- Nilsson B, Bremme K. Prediction of start of contractions in labor induced with oral prostaglandin E2 or oxytocin: a life table analysis approach. Int J Gynecol Obstet 1984;22:145‐50. [DOI] [PubMed] [Google Scholar]
Orhue 1993a {published data only}
- Orhue A. A randomized trial of 45 minutes and 15 minutes incremental oxytocin infusion regimes for the induction of labour in women of high parity. Br J Obstet Gynaecol 1993;100:126‐9. [DOI] [PubMed] [Google Scholar]
Orhue 1993b {published data only}
- Orhue AAE. A randomized trial of 30‐min and 15‐min oxytocin infusion regimen for induction of labor at term in women of low parity. Int J Gynecol Obstet 1993;40:219‐25. [DOI] [PubMed] [Google Scholar]
Orhue 1994 {published data only}
- Orhue AAE. Incremental increases in oxytocin infusion regimens for induction of labor at term in primigravidas: a randomized controlled trial. Obstet Gynecol 1994;83:229‐3. [PubMed] [Google Scholar]
Pavlou 1978 {published data only}
- Pavlou C, Barker GH, Roberts A, Chamberlain GVP. Pulsed oxytocin infusion in the induction of labour. Br J Obstet Gynaecol 1978;85:96‐100. [DOI] [PubMed] [Google Scholar]
Reid 1995 {published data only}
- Reid G, Helewa M. Pulsatile vs continuous oxytocin infusion for induction of labour. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993:14.
- Reid GJ, Helewa ME. A trial of pulsatile vs continuous oxytocin administration for the induction of labor. J Perinatol 1995;15:364‐66. [PubMed] [Google Scholar]
Sande 1983 {published data only}
- Sande HA, Tuveng J, Fonstelien T. A prospective randomized study of induction of labor. Int J Gynecol Obstet 1983;21:333‐6. [DOI] [PubMed] [Google Scholar]
Secher 1981 {published data only}
- Secher NJ, Lange AP, Hassing Nielsen F, Thomson Pedersen G, Westergaard JG. Induction of labor with and without primary amniotomy. A randomized study of prostaglandin E2 tablets and intravenous oxytocin. Acta Obstet Gynecol Scand 1981;60:237‐41. [DOI] [PubMed] [Google Scholar]
Sellers 1988 {published data only}
- Sellers SM, Ah‐Moye M, MacKenzie IZ. Comparison of vaginal prostaglandin E2 and intravenous oxytocin for induction of labour in women previously delivered by caesarean section. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:128.
Sivasuriya 1978 {published data only}
- Sivasuriya M, Tan KL, Salmon YM, Karim SMM. Neonatal serum bilirubin levels in spontaneous and induced labour. Br J Obstet Gynaecol 1978;85:619‐3. [DOI] [PubMed] [Google Scholar]
Steer 1976 {published data only}
- Steer PJ, Little DJ, Lewis NL, Kelly MCME, Beard RW. The effect of membrane rupture on fetal heart rate in induced labour. Br J Obstet Gynaecol 1976;83:454‐59. [DOI] [PubMed] [Google Scholar]
Steer 1985 {published data only}
- Steer PJ, Carter MC, Choong K, Hanson M, Gordon AJ, Pradhan P. A multicentre prospective randomized controlled trial of induction of labour with an automatic closed‐loop feedback controlled oxytocin infusion system. Br J Obstet Gynaecol 1985;92:1127‐33. [DOI] [PubMed] [Google Scholar]
Suikkari 1983 {published data only}
- Suikkari AM, Jalkanen M, Heiskala H, Koskela O. Prolonged pregnancy: induction or observation. Acta Obstet Gynecol Scand Suppl 1983;116:58. [Google Scholar]
Thomas 1974 {published data only}
- Thomas G, Blackwell RJ. A controlled trial of the Cardiff automatic infusion system in the management of induced labour. Br J Clin Pract 1974;28:203‐6. [PubMed] [Google Scholar]
Tylleskar 1978 {published data only}
- Tylleskar J, Finnstrom O, Hedenskog S, Leijon I, Ryden G. Spontaneous delivery‐elective induction for convenience, a comparative study [Abstract no 345]. Proceedings of 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1; Vienna, Austria. 1978.
Tylleskar 1979 {published data only}
- Tylleskar J, Finnstrom O, Leijon I, Hedenskog S, Ryden G. Spontaneous labor and elective induction ‐ a prospective randomized study. I Effects on mother and fetus. Acta Obstet Gynecol Scand 1979;58:513‐8. [DOI] [PubMed] [Google Scholar]
Varma 1981 {published data only}
- Varma R, Norman J, Cowell L. Induction of labour with vaginal prostaglandin E2 pessaries. J Obstet Gynaecol 1981;2:65‐70. [Google Scholar]
Ward 1991 {published data only}
- Ward SJ. Induction of labour using prostaglandin gel in patients with a favourable cervix. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:143.
Westergaard 1983 {published data only}
- Westergaard JG, Lange AP, Pedersen GT, Secher NJ. Oral oxytocics for induction of labor. Acta Obstet Gynecol Scand 1983;62:103‐10. [DOI] [PubMed] [Google Scholar]
Witter 1987 {published data only}
- Witter FR, Weitz CM. A randomized trial of induction at 42 weeks gestation vs expectant management for postdates pregnancies. Am J Perinatol 1987;4:206‐11. [DOI] [PubMed] [Google Scholar]
Witter 1989 {published data only}
- Witter FR, Weitz CM. Cervical examination prior to induction in postdate pregnancies. Surg Gynecol Obstet 1989;168:214‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Chanrachakul 2003 {published data only}
- Chanrachakul B, Herabutya Y. Postterm with favorable cervix: is induction necessary?. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:154‐7. [DOI] [PubMed] [Google Scholar]
Chua 1988 {published data only}
- Chua SM, Lee KW, Phua SM. Comparative study between prostaglandin E2 vaginal tablet and intravenous oxytocin in induction of labour. Singapore Medical Journal 1988;29(4):379‐82. [PubMed] [Google Scholar]
Selo‐Ojeme 2007 {published data only}
Additional references
Biggar 1996
- Biggar RJ, Miotti PG, Taha TE. Perinatal intervention trial in Africa: effect of a birth canal cleansing intervention to prevent HIV transmission. Lancet 1996;347(June 15):1647‐50. [DOI] [PubMed] [Google Scholar]
Bricker 2001
- Bricker L, Luckas M. Amiotomy alone for induction of labour (Cochrane Review). The Cochrane Library 2001, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 Oxford, England: The Cochrane Collaboration, 2000.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. J Reprod Med 1987;32:91‐5. [PubMed] [Google Scholar]
Hofmeyr 2000
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, Neilson JP. Methods for cervical ripening and labour induction in late pregnancy: generic protocol (Cochrane Review). The Cochrane Library, Issue 2, 2000. [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Tan 2001
- Tan B, Kelly AJ. Intravenous oxytocin alone for cervical ripening and induction of labour (Cochrane Review). The Cochrane Library 2001, Issue 3. [DOI] [PubMed] [Google Scholar]


