Abstract
Context
Obesity-related insulin resistance (OIR) is one of the main contributors to type 2 diabetes and other metabolic diseases. Protein kinases are implicated in insulin signaling and glucose metabolism. Molecular mechanisms underlying OIR involving global kinase activities remain incompletely understood.
Objective
To investigate abnormal kinase activity associated with OIR in human skeletal muscle.
Design
Utilization of stable isotopic labeling-based quantitative proteomics combined with affinity-based active enzyme probes to profile in vivo kinase activity in skeletal muscle from lean control (Lean) and OIR participants.
Participants
A total of 16 nondiabetic adults, 8 Lean and 8 with OIR, underwent hyperinsulinemic-euglycemic clamp with muscle biopsy.
Results
We identified the first active kinome, comprising 54 active protein kinases, in human skeletal muscle. The activities of 23 kinases were different in OIR muscle compared with Lean muscle (11 hyper- and 12 hypo-active), while their protein abundance was the same between the 2 groups. The activities of multiple kinases involved in adenosine monophosphate–activated protein kinase (AMPK) and p38 signaling were lower in OIR compared with Lean. On the contrary, multiple kinases in the c-Jun N-terminal kinase (JNK) signaling pathway exhibited higher activity in OIR vs Lean. The kinase-substrate–prediction based on experimental data further confirmed a potential downregulation of insulin signaling (eg, inhibited phosphorylation of insulin receptor substrate-1 and AKT1/2).
Conclusions
These findings provide a global view of the kinome activity in OIR and Lean muscle, pinpoint novel specific impairment in kinase activities in signaling pathways important for skeletal muscle insulin resistance, and may provide potential drug targets (ie, abnormal kinase activities) to prevent and/or reverse skeletal muscle insulin resistance in humans.
Keywords: insulin resistance, quantitative proteomics, human skeletal muscle, protein kinase, active kinome, obesity
Obesity is a worldwide epidemic, with more than 1.2 billion adults overweight and 650 million adults with obesity in 2016, as reported by the World Health Organization, and it is a US epidemic, with nearly 40% of US adults being obese in 2015. Obesity-related insulin resistance (OIR) in the skeletal muscle, the main site for insulin-stimulated glucose uptake (1), is a common pathophysiological state contributing to the development of various human diseases, such as type 2 diabetes (T2D) (2) and cardiovascular diseases (3). It is known that individuals may have insulin resistance for decades before developing diabetes (4); however, early diagnosis and prevention of insulin resistance are critical for reducing the risks associated with hyperinsulinemia and impaired glucose tolerance, leading to T2D and other complications.
Protein kinases are the key enzymes catalyzing one of the most important posttranslational modifications: phosphorylation—which plays fundamental roles in the regulation of biological processes and molecular functions, such as insulin signal transduction and glucose disposal. Nonetheless, of the total 518 known protein kinases in the human kinome (5), it is still not completely known how many are involved in pathogenesis of OIR in skeletal muscle. Recent literature suggests approximately a dozen of protein kinases contribute to more than 90% of the kinase functional studies in skeletal muscle. Earlier work was focused on tyrosine kinase activity of the insulin receptor (6); later, multiple kinases that modulate insulin signaling in skeletal muscle were discovered, such as protein kinase C (7), protein kinase B (AKT) (8), adenosine monophosphate–activated protein kinase (AMPK) (9), p38 mitogen-activated protein kinase (p38 MAPK) (10), and c-Jun N-terminal kinases (JNK) (11), etc. The previous studies, however, have 2 limitations: first, most of them only addressed the kinase protein abundance or certain known phosphorylation sites of the kinase, but not the activity of the kinase; it is known that multiple kinases do not require phosphorylation for activation, such as casein kinase I (CK1), C-terminal Src kinase (CSK), cyclin dependent kinase 5 (CDK5) (12). Second, the majority of previous studies have been carried out in cell culture or animal models. Surprisingly, no large-scale profiling of active kinases, underlying the cellular mechanisms of insulin resistance, has been reported in human skeletal muscle from lean healthy control and OIR individuals.
Quantitative proteomics combining high-performance liquid chromatography electrospray ionization high resolution tandem mass spectrometry (HPLC-ESI-MS/MS) with affinity-based probe profiling (ABPP) has been developed to enrich and assess enzyme activities (13–15). Even though phosphoproteomics can profile a large number of phosphorylation sites of kinases, it measures activity of kinases indirectly. In addition, several kinases have multiple phosphorylation sites and some of them regulate kinase activity, while others do not. Furthermore, certain kinases do not require phosphorylation for activation, such as CK1, CSK, and CDK5 (12). ABPP provides more direct kinase activity assessment by selectively enriching the active kinases with conserved lysine residue in the adenosine triphosphate (ATP) binding domain and/or in the catalytic domain (13–15). We previously developed universal-stable isotope labeling with amino acids in cell culture (Universal-SILAC) to minimize experimental variations for quantification of phosphopeptides in the L6 skeletal muscle cell line (16). In the present study, we combined Universal-SILAC–based quantitative proteomics with ABPP to: 1) develop a high throughput and high selectivity platform to enrich active kinases in skeletal muscle; 2) assess the kinase activities by high sensitivity quantitative proteomics analysis; and 3) identify the abnormal kinase activity associated with OIR. We have compared the activities of more than 50 protein kinases enriched in the muscle biopsies from Lean and OIR participants, providing a useful resource for T2D early-detection markers and evaluating kinase activity in human skeletal muscle.
Materials and Methods
Overall experimental design
As illustrated in (17), the approach we utilized included extensive clinical and proteomics data acquisition and bioinformatic analysis. The clinical studies started with participant recruitment, which was followed by comprehensive screening tests and hyperinsulinemic-euglycemic clamp with muscle biopsies. The proteomics studies were conducted as follows: biopsy homogenization; spike-in standards; stable isotope-labeled protein lysates from primary human skeletal muscle cells obtained by SILAC to minimize experimental variation; ABPP pull-down to enrich the active kinases; in-solution trypsin digestion to generate tryptic peptides; and HPLC-ESI-MS/MS analysis to identify/quantify muscle proteome and enriched active kinases. Multiple biological comparisons were used to minimize false positives. Extensive bioinformatics and literature searches were used to integrate clinical and proteomics data and to identify pathways/functional categories in which identified active kinases were involved, that were impacted by OIR.
Human participants and hyperinsulinemic euglycemic clamp with muscle biopsy
All clinical procedures were approved by the institutional review board at Wayne State University and were performed as described in our recent publication (18). Participants were screened over the phone and were provided a brief explanation of the study. Prequalified subjects were scheduled for an onsite screening visit (Visit 1). Participants reported to the Clinical Research Center at Wayne State University from 8 to 9 am after an overnight fast. The details and potential risks of the study were discussed with the subjects, and the subjects were provided with a copy of the consent form. After written informed consent was obtained, comprehensive screening tests were performed such as vitals, height, weight, urinalysis, pregnancy test, electrocardiogram, body composition, medical/health history, complete blood chemistry, complete blood count, hemoglobin A1c (HbA1c), lipid profile, as well as waist and hip circumference. An oral glucose tolerance test (OGTT) was performed as follows: a catheter was placed in an antecubital vein, and baseline blood samples were drawn at −15 and 0 minutes. At time zero, each subject drank 75 grams of glucose. Plasma glucose was determined at 30-minute intervals for 2 hours following glucose ingestion. To be eligible for the study, the 2-hour OGTT plasma glucose had to be lower than 140 mg/dL. If the results of these screening tests indicated that the subject was eligible to participate in the study, a second visit (Visit 2) was scheduled and the participants were instructed to keep their diet consistent and not to eat anything after 10 pm the night before the visit.
Eligible subjects were admitted to the Clinical Research Center for Visit 2 from 8 to 9 am after the overnight fast. Subjects underwent a hyperinsulinemic-euglycemic clamp with muscle biopsies, described briefly as follows: an antecubital catheter was placed for infusion of insulin and glucose. A hand vein was catheterized for measurement of arterial glucose concentrations. Half an hour later, a percutaneous biopsy of the vastus lateralis muscle was performed as previously described (18). Biopsy specimens were quickly cleaned of connective tissue and fat and were frozen immediately in liquid nitrogen and stored in liquid nitrogen until they were needed for processing. Half an hour after the muscle biopsy, a primed-continuous insulin infusion was started and continued for 120 minutes to quantify the effects of insulin on glucose disposal (19, 20). Insulin infusion was started at 160 mU/(m2∙min∙) for 5 minutes, followed by 120 mU/(m2.∙min) for 5 minutes, and then maintained at constant 80 mU/(m2∙min). This ensured that steady-state levels were more rapidly achieved, minimizing the burden on the participants. Throughout the insulin infusion, an infusion of 20% glucose was adjusted to maintain euglycemia (targeted at 90 mg/dL) (18). Insulin-stimulated glucose disposal rate (M-value) was calculated as the average glucose infusion rate value during the final 30 min of insulin infusion.
Primary human skeletal muscle (HSkM) cell culture and isotopic metabolic labeling
The primary HSkM cells, with proteome similar to that of skeletal muscle tissue, were generated right after the biopsy procedure, and were labeled by SILAC (21) for 6 doublings to ensure almost complete incorporation of the “heavy” isotope-labeled arginine and lysine analogs. Briefly, a fresh muscle biopsy specimen was minced finely and digested in 0.05% trypsin EDTA for 1 hour at 37 °C, followed by isolation and maintenance of primary skeletal muscle cells in growth medium (1.0 g/L glucose Dulbecco’s Modified Eagle Medium [DMEM] containing 20% fetal bovine serum [FBS], 1% penicillin-streptomycin-glutamine [PSG], 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/mL fetuin, 10 ng/mL epidermal growth factor and 0.4 μg/mL dexamethasone). At the third generation, the HSkM cells were transferred to labeling medium, containing arginine/lysine-free DMEM supplemented with 20% dialyzed FBS, 0.4 mM 13C615N4 L-arginine (Arg10) and 0.8 mM 4,4,5,5-D4 L-lysine (Lys4). Finally, equal amounts of HSkM cell lysates from 2 Lean and 2 OIR participants were pooled to represent the skeletal muscle proteome. Heavy SILAC labeled primary HSkM cells from 1 woman and 1 man from each group (ie, Lean, OIR) were selected and mixed for serving as spike-in universal standard. We evaluated the SILAC incorporation rate after 6 doublings with 1 μg tryptic peptides from HSkM protein mixture using HPLC-MS/MS. The results indicated that among the 6971 MS/MS scans assigned to a peptide, only 336 were assigned to peptides without Arg10 and/or Lys4 incorporation, while 6635 were assigned to peptides with Arg10 and/or Lys4 incorporation, ie, > 95% of the total number of MS/MS scans were assigned to Arg10 and/or Lys4 incorporated peptides.
Active kinome enrichment
ABPP was used to enrich the active kinases or the kinases with active conformation; subsequently, kinase activity was assessed using the enriched kinase peptides. For each sample, 1.0 mg muscle lysate and 0.5 mg SILAC cell lysate were mixed. One of the disadvantages of adding “heavy” labeled standards is the increased sample complexity, resulting in decreased identification of the endogenous peptides of interest using data-dependent tandem mass spectrometry. On the other hand, if the amount of “heavy” labeled protein standards added into the sample is too low, insufficient number of “heavy” labeled peptides may be identified, which may not serve as a proper standard for quantification purposes. We have performed preliminary experiments using different protein concentration ratios for muscle lysates and “heavy” SILAC-labeled protein standards, and the results indicated that a protein concentration ratio of 2:1 offered a good balance between the number of identified proteins and active kinase derived from muscle biopsies and from the standards. The mixture of the muscle lysates and the SILAC-labeled cell lysates was desalted to remove endogenous ATP; subsequently, the mixed lysate proteins were labeled with the ActivX Desthiobiotin-ATP Probe, which specifically targets 2 different conserved lysine residues either in the ATP binding pocket (Lys1) or in the catalytic loop (Lys2) (13, 22). Then, the probe-labeled proteins underwent reduction and alkylation, and were subjected to trypsin digestion at 37 °C on a thermomixer overnight. We used 1% of the resulting peptides (without probe labeling) for total proteome analysis, and the rest of peptides were used for streptavidin affinity precipitation. The pulled-down kinase peptides were washed, eluted, and desalted by ZipTip Pipette tips.
MS-based quantitative proteomics analysis
The kinase peptides were separated on a 75 cm × 75 µm analytical column with 3 µm ReproSil-Pur C18 beads using a 2% to 35% solvent B (0.1% formic acid in acetonitrile) 4-hour linear gradient. Subsequently, the eluted peptides were analyzed by an Orbitrap Elite in the data-dependent acquisition (the top 20 peaks were selected for MS/MS). Database search of the MS raw files was conducted by MaxQuant software package (version 1.5.4.7) (23) against UniProt human reference proteome database (version April, 2016) with a false discovery rate for protein and peptide identification at 0.01.
Statistical analysis
SILAC-based quantitative proteomics is a reliable approach for relative quantification. For the universal-SILAC reported in Reference (16), an equal amount of SILAC-labeled lysate proteins is spiked into each experimental sample and is used as a universal standard for quantification purpose. The normalized peak areas for nonlabeled peptides are calculated by normalizing the peak area of a peptide (PAi) against the sum of the peak area of the stable isotope-labeled peptides in the same sample:
The total proteome was also normalized by universal SILAC where the peak area of a nonlabeled protein (PAj) was normalized against the sum of peak area of labeled proteins in the same sample:
It may be germane to note that, to qualify active kinase abundance, we excluded other probe-labeled peptides without a well-defined kinase core, such as ATPase, lipid kinases or atypical protein kinases, but only included the labeled protein kinases with known conserved lysine residues, Lys1 and Lys2. Fold change was calculated by dividing the SILAC normalized ratio of OIR by that of Lean. Even though many proteins or active kinases were identified in the study, to achieve more reliable comparison, only the proteins or active kinases satisfying 2 relatively strict criteria were considered for statistical significance between Lean and OIR groups: 1) identified in at least half of the 16 independent biopsy samples (≥ 8 biopsies), 2) with a fold change > 1.5 or < 0.67 when comparing OIR vs Lean. Proteome and active kinome data were Log2 transformed to be normally distributed and the two-sided independent t-test was performed. If a protein or an active kinase was only identified either in Lean or OIR with at least half of the time (ie, ≥ 4 samples), this kinase was considered as significantly different with an infinite or infinitesimal fold change.
Bioinformatics analysis
Canonical pathway analysis of the active protein kinome were carried out by 2 software packages: (1) MetaCore (version 6.36 build 69400) which consists of the GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) libraries (24) (2) Ingenuity Pathway Analysis (IPA) which consists of a manually curated knowledge base of biological functions and molecular networks (25). A significantly enriched pathway needed to have P < 0.05. To identify Lys1 and Lys2 sites of the protein kinases in the UniProt database (http://www.uniprot.org/), we conducted a Boolean search: keyword: “ATP-binding [KW-0067]” AND organism: “Homo sapiens (Human) [9606].” The corresponding XML file was downloaded programmatically for each of the UniProt database entries matching this search. Each XML file was parsed to retrieve the location and number of lysine residues for proteins with ATP binding sites. The source code used is available under an MIT open-source license at https://github.com/griffincalme/ATP-binding_lysine_Yue_Qi.
Results
Clinical characteristics of participants
As can be seen from Table 1, no significant differences in sex, race, ethnicity, age, and fasting low-density lipoprotein (LDL) cholesterol were observed between the 8 Lean and 8 OIR participants. All the subjects were nondiabetic with fasting plasma glucose, 2-hour OGTT glucose, and HbA1C within the normal ranges, according to American Diabetes Association’s criteria for diabetes diagnosis. On the other hand, body mass index (BMI), percentage body fat, fasting plasma insulin, and fasting triglycerides, were significantly higher in OIR participants. The glucose infusion rate (M-value), a direct measure of in vivo insulin sensitivity, was significantly lower in the OIR group.
Table 1.
Clinical Characteristics of the 8 Lean and 8 OIR Participants in the Study
| Lean | OIR | Significance | |
|---|---|---|---|
| (n = 8) | (n = 8) | ||
| Sex (male/female) | 4/4 | 4/4 | NS |
| Race (White/Black) | 5/3 | 4/4 | NS |
| Ethnicity (Hispanic/Non-Hispanic) | 1/7 | 0/8 | NS |
| Age (years) | 27.6 ± 3.1 | 29.1 ± 3.9 | NS |
| BMI (kg m-2) | 22.1 ± 0.7 | 32.2 ± 1.5 | P < 0.001 |
| Body fat (%) | 16.7 ± 3.1 | 27.9 ± 3.2 | P < 0.05 |
| 2-hour OGTT glucose (mg dL-1) | 100 ± 5.3 | 104.7 ± 6.4 | NS |
| HbA1c (%) | 5.1 ± 0.1 | 5.3 ± 0.1 | NS |
| Fasting plasma glucose (mg dL-1) | 86.7 ± 1.0 | 91.2 ± 2.3 | NS |
| Fasting plasma insulin (pmol L-1) | 20.6 ± 3.2 | 40.8 ± 9.7 | P < 0.05 |
| Total Cholesterol (mg dL-1) | 144.8 ± 13.8 | 169.9 ± 10.4 | NS |
| Triglycerides (mg dL-1) | 49.1 ± 5.0 | 89.3 ± 15.3 | P < 0.05 |
| HDL Cholesterol (mg dL-1) | 57.4 ± 5.2 | 43.9 ± 7.2 | NS |
| LDL Cholesterol (mg dL-1) | 77.6 ± 10.9 | 104.5 ± 9.8 | NS |
| M-value (mg kg-1 min-1)* | 10.1 ± 0.8 | 5.5 ± 0.5 | P < 0.001 |
Data are given as mean ± SEM. Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant; OGTT, oral glucose tolerance test. *Insulin-stimulated glucose disposal rates (M-value), the average glucose infusion value during the last 30 minute of clamp study.
Skeletal muscle proteome profiling
The proteome profiling of the skeletal muscle of the 16 participants resulted in the identification of 2573 proteins, of which 45 were protein kinases (Fig. 1a) (17). The normalized SILAC ratios for each of identified proteins in the biopsies for the 8 Lean and 8 OIR participants are listed in reference (17). Among the 2573 proteins, 847 had no SILAC ratios in any of the 16 muscle biopsies because there were no corresponding heavy-labeled peptides identified, which is a phenomenon commonly observed in Super-SILAC experiments (26). Therefore, these 847 proteins cannot be quantified by traditional SILAC/Super-SILAC. However, using Universal-SILAC as described in our manuscript (16) and in the Method section above, we were able to obtain quantitative information for all 2573 identified proteins, including these 847 proteins.
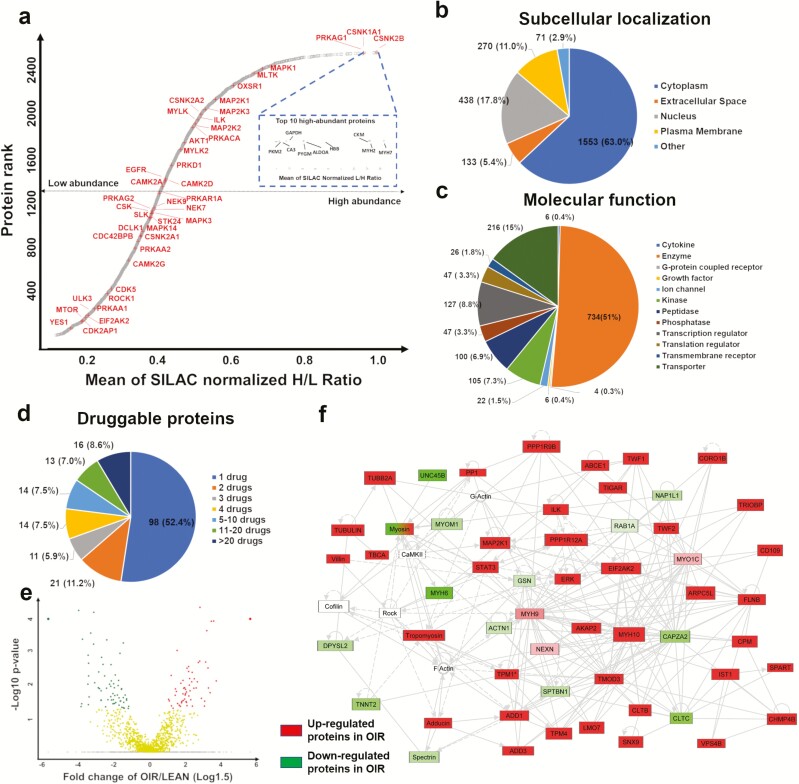
Figure 1.
Proteome profiling of human skeletal muscle a) Dynamic range of protein abundance in skeletal muscle tissue. b) Subcellular localization. c) Molecular function. d) Proteins that are potential targets for FDA approved medications. e) Volcano plots of Log1.5-fold change (cutoff <−1 or >1) and −Log10 P value (cutoff >1.33), where red circles (upper left) represent upregulated proteins in obesity-related insulin resistance (OIR), green circles (upper right) represent downregulated proteins in OIR and yellow circles (center) represent unchanged proteins between Lean and OIR. Note that proteins only identified in OIR are assigned with a cap ratio of a 10-fold change and P = 0.0001 whereas proteins only identified in Lean are assigned with a cap ratio of a 0.1-fold change and P= 0.0001. f) IPA-based networking of significantly changed proteins, green color-coding represents lower abundance and red color-coding represents higher abundance in OIR vs Lean, non–color-coded proteins are not included in the current dataset.
As expected, the mean of SILAC-normalized ratios across all participants showed that muscle housekeeping proteins, such as myosin heavy chain 2 (MYH2), myosin heavy chain 7 (MYH7), hemoglobin subunit beta (HBB), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were among the most abundant proteins. Notably, creatine kinase muscle (CKM) and pyruvate kinase muscle (PKM2), nonprotein kinases that contribute to energy transduction and glycolysis, were also on the top list. Among the 45 protein kinases was the casein kinase family (eg, CK1 and casein kinase II subunit beta), an upstream kinase family regulating many proteins in the WNT pathway. AKT1 and several mitogen-activated protein kinases (MAPK1, dual specificity mitogen-activated protein kinase kinase 1 [MAP2K1], dual specificity mitogen-activated protein kinase kinase 3 [MAP2K3]) appeared in the upper tail of the graph for dynamic range of the muscle protein abundance; on the other hand, mammalian target of rapamycin kinase (mTOR) and protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1, also known as AMPK1) were in the lower end. Fig. 1b shows that the majority of the proteins are localized in the cytoplasm (63%), followed by the nucleus (17.8%) and plasma membrane (11%). Interestingly, we identified 734 enzymes, 105 kinases (including 45 protein kinases and 60 nonprotein kinases) and 47 phosphatases in skeletal muscle (Fig. 1c (17)). Fig. 1d indicates that 189 identified proteins are targets of FDA-approved antidiabetic drugs (eg, metformin), and among those, 98 are targets for 1 drug, and 16 proteins are targets for > 20 drugs, such as epidermal growth factor receptor (EGFR), proteasome subunits, and tubulin (17). One antidiabetic drug target in our data is AMPK1, a reported primary target for metformin, reducing blood glucose by activating AMPK and stimulating GLUT4-mediated skeletal muscle glucose uptake (27, 28).
Next, we sought to compare the protein abundance between the Lean and OIR groups to identify the potential proteins and signaling pathways associated with OIR. To achieve a highly reliable comparison, we only included those proteins 1) identified in at least half of the 16 biopsy samples (≥ 8 biopsies), and 588 out of the 2573 proteins satisfied this criterion; 2) with a > 1.5- or < 0.67-fold change when comparing OIR vs Lean, and 232 out of the 588 proteins above satisfied this criterion; 3) with P value < 0.05 when comparing OIR vs Lean, and 68 out of the 232 proteins above satisfied this criterion. In addition, if a protein was identified only in 1 group (Lean or OIR), and was present in at least half of the samples in that group (≥ 4 biopsies), we assumed the protein in the other group was too low to be detected, and those proteins were considered to have higher abundance in the detected group by default, and 441 proteins satisfied this criterion. As a result, in total, the protein abundance for 509 (68 + 441 = 509) proteins were significantly different (460 upregulated and 49 downregulated) in OIR compared with Lean muscle (Fig. 1e (17)). Among these 509 proteins, 147 were reported in our 2 previous publications (20, 29). In addition, only 5 out of the 509 proteins are kinases, for example, MAPK1 and MAP2K1 are upregulated in OIR. Of interest, eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2; also known as interferon-induced, double-stranded RNA-activated protein kinase) showed significantly higher protein expression in OIR. We first report here the identification of EIF2AK2 in human skeletal muscle, and this kinase has been known for inhibitory phosphorylation of insulin receptor substrate 1 (IRS1) at Ser312 (30) and AKT at Ser473 (31); therefore, it is likely that upregulated EIF2AK2 might be a upstream regulator of insulin resistance in subjects with obesity.
The top scored IPA network of the 509 proteins with differential abundance in OIR vs Lean muscle are shown in Fig. 1f. Upregulated ERK/MAPK1, MAP2K1, EIF2AK2, signal transducer and activator of transcription 3 (STAT3), protein phosphatase 1 regulatory subunit 12A (PPP1R12A) served as major nodes in the network. The significantly enriched canonical pathways (17) included integrin signaling, eukaryotic translation initiation factor 2 (EIF2) signaling, integrin linked kinase (ILK) signaling, actin cytoskeleton signaling, and mitochondrial dysfunction.
Active protein kinome profiling
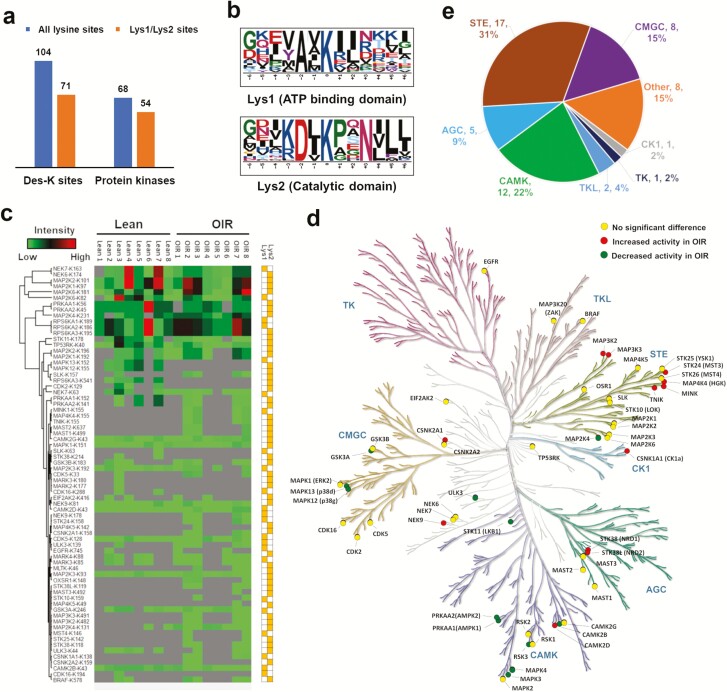
Although the protein abundance of only 5 out of 45 identified kinases in skeletal muscle proteome is significantly different between OIR and Lean, the activity of additional kinases may be different. ABPP is designed to bind to and enrich active protein kinases with a suitable ATP binding pocket where a conserved lysine either in the ATP-binding domain (Lys1) or in the catalytic domain (Lys2) binds to the probe. To parse out the conserved lysine sites in the UniProt human proteome reference database, we wrote a Python script to establish a “ATP binding site” and “active site” library in the 518 protein kinases, then matched our data to that library. We identified 71 conserved Lys1 or Lys2 sites assigned to 54 kinases (ie, 54 active kinases) out of the total 104 identified Lys sites assigned to 68 kinases (Fig. 2a (17)), which indicated a ~20% off-target enrichment rate for this ABPP. Binding motifs for Lys1 and Lys2 were determined by the specific enriched active kinase peptides, which implied that an alanine (A) or an aspartic acid (D) at the −2 position was also highly conserved (Fig. 2b). The 71 conserved Lys1 or Lys2 sites were sorted by hierarchical clustering to visualize the kinase activity pattern between Lean and OIR groups (Fig. 2c).
Figure 2.
Active kinome profiling of human skeletal muscle a) Number of specific binding sites and kinases. b) Binding motif analysis of conserved Lys1 and Lys2. c) Hierarchical clustering of 71 identified active kinase sites. d) Color-coded kinome tree according to the differential fold changes of the 54 identified active kinases in Lean and OIR groups, where kinases highlighted in yellow did not show significant change; kinases highlighted in red showed significant increased activity in OIR; kinases highlighted in green showed significant decreased activity. e) Protein kinases classification of the 54 identified active kinases according to the human KinBase (http://kinase.com/human/kinome/).
Among the 54 active kinases, the STE family contained the largest number of identified kinases, many of which are upstream regulators of the MAP kinase pathway (eg, MAP2K1, MAP3K2, MAP3K5, MAP4K5), and EGFR was the only tyrosine kinase identified (Fig. 2d).
Next, we set stricter criteria to define hyperactivity or hypoactivity of the kinases: 1) identified in at least half of the 16 biopsy samples (≥ 8 biopsies), and 31 out of the 71 conserved Lys1 or Lys2 sites satisfied this criterion; 2) with a > 1.5- or < 0.67-fold change when comparing OIR vs Lean, and 23 out of the 31 conserved Lys1 or Lys2 sites above satisfied this criterion; 3) with P value < 0.05 when comparing OIR vs Lean, and 9 out of the 23 conserved Lys1 or Lys2 sites above satisfied this criterion. In addition, if a conserved Lys1 or Lys2 site was identified only in 1 group (Lean or OIR), and was present in at least half of the samples in that group (≥ 4 biopsies), we assumed kinase activity in the other group was too low to be detected, and those kinases were considered to have higher activity in the detected group by default, and 14 conserved Lys1 or Lys2 sites satisfied this criterion. As a result, in total, 23 kinases with a conserved Lys1 or Lys2 site showed significantly different activity (11 hyperactive and 12 hypoactive in OIR) between the 2 groups (Fig. 2e, Table 2 (1)).
Table 2.
Twenty-Two Kinases With a Significant Difference in Their Activity in Skeletal Muscle Tissue in OIR Compared With Lean
| Gene Name | Protein Name | Fold Change OIR/Lean | Main Pathway/Function |
|---|---|---|---|
| MAPK12 | Mitogen-activated protein kinase 12 | Infinitesimala | p38 MAPK |
| MAPK13 | Mitogen-activated protein kinase 13 | Infinitesimal | p38 MAPK |
| RPS6KA3 | Ribosomal protein S6 kinase alpha-3 | Infinitesimal | MAPK/ERK; mTOR |
| CAMK2B | Calcium/calmodulin-dependent protein kinase type II subunit beta | 0.06 ± 0.04 | Calcium signaling |
| STK11 (LKB1) | Serine/threonine-protein kinase STK11 | 0.07 ± 0.04 | AMPK; mTOR |
| PRKAA1(AMPK1) | 5’-AMP-activated protein kinase catalytic subunit alpha-1 | 0.14 ± 0.04 | AMPK; mTOR |
| PRKAA2 (AMPK2) | 5’-AMP-activated protein kinase catalytic subunit alpha-2 | 0.14 ± 0.04 | AMPK; mTOR |
| MARK3 | MAP/microtubule affinity-regulating kinase 3 | 0.14 ± 0.09 | AMPK |
| MARK4 | 0.14 ± 0.09 | AMPK; mTOR | |
| GSK3A | Glycogen synthase kinase-3 alpha | 0.22 ± 0.09 | Glucose Homeostasis |
| ULK3 | Serine/threonine-protein kinase ULK3 | 0.44 ± 0.13 | SHH signaling |
| MAP2K4(MKK4) | Dual specificity mitogen-activated protein kinase kinase 4 | 0.20 ± 0.06 | JNK |
| CSNK1A1(CK1) | Casein kinase I isoform alpha | Infiniteb | Wnt/NF-κB |
| CSNK2A1(CK2) | Casein kinase II subunit alpha | Infinite | Wnt/NF-κB |
| MAP3K2 (MEKK2) | Mitogen-activated protein kinase kinase kinase 2 | Infinite | JNK |
| MAP3K3 (MEKK3) | Mitogen-activated protein kinase kinase kinase 3 | Infinite | JNK |
| MAP4K4 (HGK) | Mitogen-activated protein kinase kinase kinase kinase 4 | Infinite | JNK |
| MINK1 | Misshapen-like kinase 1 | Infinite | JNK |
| NEK9 | Serine/threonine-protein kinase Nek9 | Infinite | Cell Division |
| STK24 (MST3) | Serine/threonine-protein kinase 24 | Infinite | JNK |
| STK38 (NDR1) | Serine/threonine-protein kinase 38 | Infinite | JNK |
| STK38L (NDR2) | Serine/threonine-protein kinase 38-like | Infinite | JNK |
| TNIK | TRAF2 and NCK-interacting protein kinase | Infinite | JNK/Wnt |
aIndicates the active kinase is only identified in Lean.
bIndicates the active kinase is only identified in OIR.
Altered molecular function and cell signaling in the insulin resistant skeletal muscle from adults with obesity
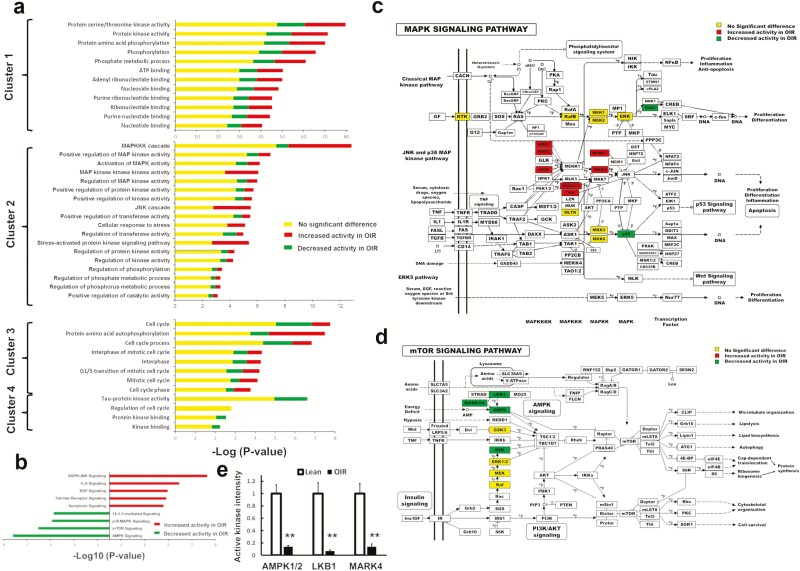
The molecular function and biological process analysis of the 54 active kinases demonstrated 4 significantly enriched clusters (Fig. 3a). Of interest, the cluster 1 was highly related to kinase activity, phosphorylation and nucleotide binding. The cluster 2 included MAPKKK and JNK cascade as well as cellular response to stress; importantly, they contained more kinases with higher activity than the kinases with similar or lower activity in OIR. Meanwhile, the kinases in cluster 3 were involved in cell cycle and protein autophosphorylation, but no significance trend in their activity was observed in OIR compared with Lean. In the cluster 4, activities of kinases involved in protein kinase binding and tau-protein kinases activity were lower in OIR; however, those in regulation of cell cycle exhibited no difference.
Figure 3.
Significantly enriched pathways and biofunctions analysis a) Functional annotation clustering of the 54 identified kinases using biological process and molecular function of Gene Ontology. The clustering annotation was generated by David bioinformatics (version 6.7), where the kinases with unchanged activities between groups were colored in yellow; the kinases with increased activity in obesity-related insulin resistance (OIR) were colored in red; the kinases with decreased activity in OIR were colored in green. Each cluster involved at least 3 kinases with P < 0.05. b) Pathway Analysis of the 23 kinases with different activity between Lean and OIR subjects. The pathways color-coded in red only involved the 11 hyperactive kinases while the pathways color-coded in green only involved the 12 hypoactive kinases, and each pathway contained at least 3 kinases with P < 0.0001. c) 23 kinases involved in the modified KEGG MAPK signaling pathway including EGFR, MAPK1, MLTK, BRAF, MAP2K1, MAP2K2, MAP2K3, MAP2K4, MAP4K4, RPS6KA3, RPS6KA2, RPS6KA1, MAPK12, MAPK13, MINK1, STK24 (MST3), STK38 (NDR1), STK38L (NDR2), TNIK, MAP3K3 (aka, MEKK3), MAP3K2 (aka, MEKK2) and MAP2K6 (aka, MKK6). d) 11 kinases involved in modified KEGG mTOR signaling pathway including STK11, AMPK1, AMPK2, MARK3, MARK4, GSK3B, RPS6KA3, MAPK1, MAP2K1, MAP2K2, BRAF. The color coding of each kinases was based on their differential fold changes between groups, where no significantly changed kinases were colored in yellow, elevated kinases in OIR were colored in red and suppressed kinases were colored in green. Note that some kinases have multiple subunits which are not differentiated in KEGG pathway; for example, both AMPK alpha and AMPK beta subunits were labeled as AMPK in the pathway. e) Fold changes of AMPK1/2, LKB1, and MARK4 between Lean and OIR group.
We next proceeded to identify which signaling pathways were significantly different in the OIR participants. Pathway analysis of the 23 kinases with different activity in OIR vs Lean showed that JNK, IL-6, EGF, Toll-like receptor, and apoptosis signaling were upregulated while AMPK, mTOR, p38 MAPK and 14-3-3-mediated signaling pathways were downregulated in OIR (Fig. 3b). Furthermore, we implanted active kinases in the MAPK signaling pathway (Fig. 3c): 23 kinases were color-coded according to their activity differences between Lean and OIR, where MAPK contains several sub-pathways, such as the classical ERK pathway as well as the JNK and p38 MAPK pathway. As can be seen from Table 2, the majority of kinases involved in the JNK cascade, an inflammatory and stress-activated kinase signaling pathway, had higher activity in OIR compared with the Lean group: 8 of 9 kinases directly or indirectly involved in upstream of JNK signaling were hyperactivated in the OIR group, which are mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4, also known as HGK) (32), mitogen-activated protein kinase kinase kinase 2 (MAP3K2, also known as MEKK2) (33), MEKK3, serine/threonine-protein kinase 24 (STK24, also known as MST3) (34), misshapen-like kinase 1 (MINK1) (35), TRAF2 and NCK-interacting protein kinase (TNIK) (36), STK38 (NDR1) (37, 38) and STK38L (NDR2) (39). Only 1 kinase, dual specificity mitogen-activated protein kinase kinase 4 (MKK4) (40), a JNK and p38 MAPK kinase, showed decreased activity. Two p38 MAPKs (MAPK12 and MAPK13) and MKK4 were hypoactivated in OIR; however, 2 upstream kinases (MKK3 and MKK6) did not display significant changes.
Similarly, 11 kinases were mapped into the mTOR signaling pathway (Fig. 3d), where 3 of them belonged to the AMPK pathway, and 6 involved in the MAPK pathway, where the kinase activity was 7-fold lower for PRKAA1/2 (AMPK1/2), 14-fold lower for LKB1 and 7-fold lower for MAP/microtubule affinity-regulating kinase 4 (MARK4) in OIR subjects compared with Lean (Fig. 3e), which indicates a potential attenuated AMPK signaling.
Comparison of previous reports and kinase-substrate prediction
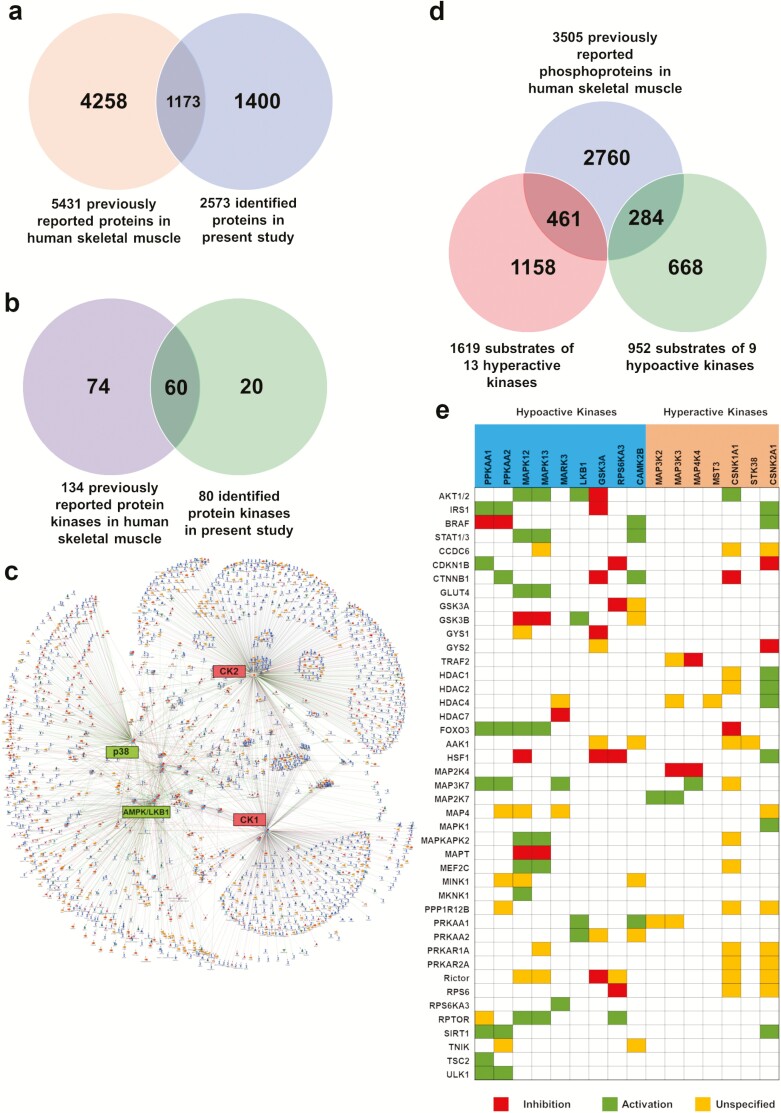
Gonzalez-Freire et al reviewed 38 peer-reviewed publications of muscle proteome profiling and function, and they summarized 5431 proteins that have been reported in human skeletal muscle (41). We compared our proteome data to the proteins reported in this comprehensive review in 2017. Notably, we found 1400 proteins in the present study that were not reported in the review, increasing the total number of proteins identified in human skeletal muscle to 7025 (Fig. 4a (17)). Furthermore, we compared the 82 kinases (combined proteome and ABPP data) to the 134 kinases reported in previous studies (41). The results indicated that 20 kinases were identified only in our dataset (Fig. 4b (17)), including B-Raf (BRAF), EGFR, protein kinase cyclic AMP-activated catalytic subunit gamma (PRKACG), cyclin dependent kinase 2 (CDK2).
Figure 4.
Comparison with previous studies and kinase-substrate prediction a) Comparison of current identified proteins with those in the comprehensive review in 2017 (41) in human skeletal muscle. b) Comparison of identified kinases with previous literature. c) Prediction of 23 significantly changed kinase-substrate proteins based on experimental data using MetaCore. d) Potential substrates that were previously identified as phosphoproteins in the literature (41–44). e) Kinase-substrate function based heatmap of highly frequent substrates in the prediction.
Next, we sought to predict the downstream substrate proteins. Three most comprehensive phosphoproteome studies in human skeletal muscle reported a total of 3505 phosphoproteins (42–44). We predicted the substrate proteins of the 23 significantly changed kinases (1619 substrates for hyperactive kinases and 952 substrates for the hypoactive kinases, respectively) using a protein-protein interaction prediction algorithm (MetaCore database) based on the experimental data (Fig. 4c), where CK1/2, p38 MAPK, and AMPK/LKB1 complex demonstrated the most protein substrates. Of interest, 461 predicted substrates for the 11 hyperactive kinases and 284 predicted substrates for the 12 hypoactive kinases were reported as phosphoproteins previously (Fig. 4d). Pathway analysis of the 745 (461 + 284; see above) predicted substrate proteins showed AMPK signaling, insulin signaling and ErbB signaling were significantly enriched. Based on the kinase-substrate interaction—inhibition, activation, or unspecified—we constructed a heatmap to visualize the potential abnormal kinase downstream signaling (Fig. 4e (17)). IRS1 and AKT1/2 are the key mediators of insulin signaling, and as expected, lower activity of AMPK1/2 may reduce IRS1 activity; similarly, lower activity of p38 MAP kinases might decrease AKT1/2 and further STAT3 (45) phosphorylation in insulin resistant adults with obesity.
Discussion
OIR is one of the main factors leading to metabolic syndrome and T2D. Skeletal muscle is responsible for the majority of insulin-stimulated glucose uptake; therefore, understanding the cellular mechanisms of insulin resistance in skeletal muscle may provide a key to prevention of T2D and metabolic syndrome in the insulin-resistant population with obesity. Protein kinases play important roles in cell signaling and molecular pathogenesis for many diseases, such as cancer and T2D. Previous reports primarily focused on expression (mRNA and protein) and function of kinases in cell or animal models. In addition, due to the large dynamic range of protein abundance of muscle tissue, a relatively small number of proteins have been identified in skeletal muscle tissue when compared with other organs/tissues (43). Furthermore, the challenge of active kinome enrichment still exists. Therefore, in the present study, we profiled the global proteome, and more importantly assessed the kinase activity in human skeletal muscle in vivo in an unbiased fashion for 54 kinases. Among them, 23 displayed abnormal activities in the OIR group.
To ensure that the selected participants reflected the physiological feature (ie, insulin resistance) of this current study, we employed the hyperinsulinemic-euglycemic clamp technique to assess the insulin sensitivity in vivo. The insulin infusion rate at 80 mU/(m2∙min) was chosen because existing evidence suggests that hepatic glucose production is completely, or nearly completely suppressed at this level of exposure to insulin (46). Thus, hepatic insulin resistance would have minimal effect on the M-value during the clamp used in the study. To identify potential abnormal kinase targets that might help with the prevention of metabolic diseases, all the included 16 participants were nondiabetic and had normal glucose tolerance (2-hour OGTT < 140 mg/dL). The skeletal muscle biopsies were performed before the insulin infusion started, so that kinase activity was measured at basal level without insulin stimulation. The OIR group displayed significantly lower insulin sensitivity and higher fasting plasma insulin compared with the Lean group. On the other hand, there were some variations among the OIR participants such as BMI andfasting plasma glucose/insulin (Table 1). It may be worthwhile to follow these participants to check whether the variability in the insulin resistance group is related to the later development of diabetes.
The human skeletal muscle proteome profiling shows 460 upregulated versus 49 downregulated proteins in OIR compared with Lean, which suggests an increased protein synthesis and protein overexpression in general in individuals with high BMI and excessive body fat. Our data indicate ERK/MAPK signaling activation with 2 key overexpressed kinases, MAPK1 and MAP2K1, in OIR. Interestingly, the upregulated EIF2AK2 protein expression in OIR indicates a potentially enhanced inflammation via activated JNK and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling (47), even though EIF2AK2 was reported to have no effect on high-fat–diet-induced obesity in mice (48). Our results support the notion that this kinase may play a role in obesity-induced insulin resistance in humans. We reported an increased interaction of PPP1R12A with IRS1 upon insulin stimulation in muscle cells (49), and PPP1R12A knockdown resulted in significantly altered phosphorylation events in L6 muscle cell line (16). The current finding that PPP1R12A protein abundance is higher in OIR further supports the role of PPP1R12A in skeletal muscle insulin signaling.
It is noteworthy that the proteome coverage is low compared with what was reported in the paper reported by Lang et al (50). However, in that paper, each protein lysate sample for mouse muscle proteome measurement was separated first by SDS-PAGE and each lane of the gel was cut into ten slices, followed by digestion and HPLC-ESI-MS/MS, which indicates that 10 HPLC-ESI-MS/MS analyses were performed for each sample. On the other hand, in our experiment, each protein lysate sample for human muscle proteome measurement was digested directly without protein separation, followed by HPLC-ESI-MS/MS, which means that only 1 HPLC-ESI-MS/MS analysis was performed for each sample. Therefore, it is likely Lang et al would identify more proteins since approximately 10 times more HPLC-ESI-MS/MS analyses were performed.
In the present study, muscle tissue samples from well-characterized live human participants were used, which provide direct information for human physiology/pathophysiology. Nonetheless, main limitations of human tissue studies include the limited amount of tissues available for further analysis in the same study samples, descriptive nature and lack of functional characterization. Additional participants will be needed to collect tissues to confirm the findings by proteomics using CO-IP followed by western blotting analysis. Moreover, additional mechanistic experiments such as immunoblotting regulatory kinases and substrate phosphorylation sites of active and/or inactive kinases are warranted.
A common link between obesity and inflammation has been well demonstrated. Some evidence suggests that there is accumulation of immune cells (ie, T lymphocytes, macrophages, etc.) in skeletal muscle cells (myotubes) in human and rodent obesity with insulin resistance and T2D (51, 52). It is established that pro-inflammatory cytokines (eg, tumor necrosis factor-α, interleukin-1 [IL-1], and IL-6) lead to inflammation via inflammatory response pathways, such as p38 MAPK signaling, JNK signaling, NF-κB signaling and IL-6 signaling, etc (53, 54). Interestingly, pathway analysis of our findings demonstrated that multiple inflammatory response signaling pathways have been altered in OIR. Among the 3 major inflammatory and stress activated pathways, p38 MAPK was attenuated, but JNK and NF-κB pathways were likely elevated in obesity with insulin resistance. Emerging evidence implies the JNK pathway is upregulated in insulin resistance models via the phosphorylation on Ser307 of IRS1 (55, 56). Even though we did not observe active JNK directly (this might be due to low abundance of JNK), our data strongly support an increased JNK signaling in skeletal muscle insulin resistance in obesity; more importantly, our data pinpointed the kinases (ie, STK24, MINK1, STK38L) that might be responsible for the increased JNK signaling. Another key regulator, casein kinase II (CK2), which directly phosphorylates multiple transcription factors, such as IκB and NF-κB, was also activated in OIR participants (57). Our findings are consistent with activated IκB/NF-κB pathway in nondiabetic insulin resistant subjects (58). On the other hand, 2 p38 MAPKs, MAPK12 (p38γ) and MAPK13 (p38δ) and its upstream regulator MKK4, showed decreased activities in OIR. One reported study directly addressed the p38γ function in glucose uptake in skeletal muscle, reporting that overexpressed p38γ enhanced basal glucose uptake, but lowered the 2,4-dinitrophenol (DNP)-stimulated glucose uptake in L6 rat skeletal muscle cells and contraction-stimulated glucose uptake in mouse skeletal muscle (59). To date, no study has reported that p38δ is involved in glucose uptake and insulin resistance in human skeletal muscle. To the best of our knowledge, we provided the first experimental evidence of abnormal kinase activities of p38γ and p38δ in skeletal muscle in human participants. Previous studies suggest that inhibition of p38α and p38β may result in impaired insulin-dependent glucose uptake in skeletal muscle cells and adipocytes (60). Furthermore, Somwar et al (61) observed significantly decreased GLUT4 translocation by p38 inhibitor in L6 rat skeletal muscle cells, which suggests that p38 inhibitors impairs insulin stimulated glucose uptake through a GLUT4 dependent manner. It should be noted, however, that the 2 pharmacological inhibitors used (SB203580 and SB202190) in these studies (60, 61) specifically inhibit distinct isoforms of p38 (ie, α and β), which are distinct from p38γ and p38δ isoforms that we report in this study. However, observations reported in these studies may be of significance in the overall context of the current proteomics findings. Furthermore, it may be somewhat difficult to extrapolate the inhibitory effects (and specificity) of the inhibitors used in those studies to our proteomics findings. Future studies will be needed to further address potential alterations in the expression and activation of individual isoforms of p38 in skeletal muscle preparations derived from different participant populations.
Exercise can activate the AMPK pathway and subsequently boost insulin sensitivity (62, 63). Of interest, we discovered that the activity of AMPK1/2 and LKB1, which can directly activate AMPK, was reduced in OIR. Lower activity of MARK4 in OIR was also observed; Lizcano et al (64) reported that LKB1 phosphorylates MARK4 which activates AMPK. Published evidence indicates AMPK activity is largely attenuated in insulin resistance, obesity, and T2D, with reduced glucose uptake via AMPK-GLUT4 signaling (65, 66). Our results are also consistent with the defective AMPK-mediated fatty acid oxidation in insulin-resistant mice (67). Furthermore, metformin, the first-line drug for the treatment of T2D, has been shown to increase insulin sensitivity (ie, decreasing insulin resistance) in skeletal muscle, possibly through increasing Thr172 phosphorylation and activity of AMPK (27).
It is noted that the activity of the beta subunit of CaMKII, another exercise-responsive kinase that maintains calcium homeostasis (68, 69), showed an approximately 16-fold decrease, while the gamma subunit had no change in OIR compared with Lean. Our findings suggest differential changes of CaMKII subunits in sedentary participants with obesity and insulin resistance.
Even though mTOR cannot be enriched by ABPP because it is an atypical kinase without a classic kinase core, we identified several hypoactivated upstream regulators of mTOR, such as LKB1, AMPK1/2, and ribosomal protein S6 kinase alpha-3 (RSK2). Multiple studies suggested that the LKB1/AMPK complex is a negative regulator of mTOR (70); nonetheless, RSK2, a downstream kinase of ERK1/2, inactivates glycogen synthase kinase 3 β (GSK3β) and inhibits glycogen synthesis and serves as a positive regulator for mTOR and insulin sensitivity (71). Recent mTOR studies reported conflicting roles for mTOR in insulin resistance and diabetes (72). Our current findings indicate that mTOR signaling might remain unchanged because LKB1/AMPK upregulates, yet RSK2 downregulates, mTOR activity.
It is noted that there are missing data points when comparing samples from different human donors. However, this is a common phenomenon in bottom-up proteomics using label-free or SILAC quantification. This is why we required a protein or active kinase to be present in at least half of the samples in either Lean or obese insulin resistant (OIR) group (≥ 4 participants out of the 8 participants in each group) to increase the reproducibility and reliability of the results. While isobaric mass tags can reduce the missing data points in HPLC-ESI-MS/MS experiments, since they are introduced after the protein digestion, any experimental variations before and during protein digestion cannot be accounted for. In contrast, using Universal-SILAC, the SILAC-labelled protein lysates are mixed with the unlabeled protein lysates before any subsequent protein treatment; therefore, experimental variations before and during protein digestion can be also accounted for. On the other hand, OIR-induced changes to skeletal muscle may affect the ability to retrieve and/or detect the OIR sample proteins or kinases following the muscle biopsy sample preparation workflow. This limitation could not be accounted for even by Universal-SILAC. Moreover, deeper analysis with targeted detection of missing data points will undoubtedly increase the quantifiable proteins; however, it will be very time consuming to set up the target list and perform the targeted LC-MS/MS analyses. Furthermore, the absence of detection does not necessarily indicate evidence of absence of a protein. However, if a protein is detected in ≥ 4 participants out of the 8 participants in the Lean group, but not detected in any of the 8 participants in the OIR group, it provides strong evidence that this protein has higher abundance in the Lean group compared with the OIR group, and vice versa.
To minimize the experimental variation during sample preparation and HPLC-ESI-MS/MS data acquisition, Super-SILAC has been developed, in which spike-in stable isotope–labeled proteins or protein lysates are used as exogenous standards (26). Furthermore, we have developed a modified Super-SILAC approach (we now term it as Universal-SILAC), in which SILAC-labeled protein lysates were spiked into each experimental sample and were used as a universal standard for quantification (16). It is noted that traditional SILAC/spike-in Super-SILAC requires both light peptides and corresponding heavy-labeled peptides to be identified for quantification. Unfortunately, identification of only the light or heavy-labeled peptide is a phenomenon commonly observed in traditional SILAC/Super-SILAC experiments (26), which leads to fewer quantifiable peptides. Our Universal-SILAC approach, which does not require both light peptides and corresponding heavy-labeled peptides to be identified for quantification, therefore provides more quantitative information than the traditional SILAC/Super-SILAC quantification, especially for differential tissue proteome or phosphoproteome studies.
One constraint of spike-in SILAC is the limit of detection when there are large differences in the abundance between heavy and light peptides, which would be an issue for muscle proteome studies since there are numerous highly abundant proteins in muscle. However, since we used Universal-SILAC, in which SILAC-labeled protein lysates were used as a universal standard for quantification purposes and both light peptides and corresponding heavy-labeled peptides are not required to be identified, this was not an issue.
Phosphoproteomics is a powerful approach to study phosphorylation sites of kinases, however, phosphoproteomics assess activity of kinases indirectly. In addition, many kinases have multiple phosphorylation sites and some of them regulate kinase activity, while others do not. Moreover, multiple kinases do not require phosphorylation for activation, such as CK1, CSK, and CDK5 (12). ABPP has been developed to enrich and assess kinase activities (13–15). ABPP selectively labels 1 of 2 lysine residues in the catalytic site of active protein kinases, providing a more direct measure of kinase activity than phosphorylation of kinases.
Quantitative proteomics is primarily used to assess protein abundance or posttranslational modifications in the cells or tissues; however, it has become possible to assess protein (enzyme) activities by coupling with activity-based enzyme enrichment. Researchers have developed various activity-based probes to assess enzyme activities (15, 73–75). For example, Liu et al synthesized the activity-based chemical probes for enrichment of serine hydrolases; a biotinylated fluorophosphonate (FP-biotin) was designed specifically for the active sites of serine hydrolases (76). Similarly, the kinase activity–based probe was synthesized with novel nucleotide acyl phosphates that selectively bind to the ATP binding pocket (in the kinase core) of active eukaryotic protein kinases (13).
It is noted that a small percentage of maximal activity of kinases may be sufficient to fully phosphorylate its downstream substrates. Therefore, the relative activity threshold of each kinase is likely to influence the present kinome data to downstream functional cellular consequences of substrate phosphorylation.
Conclusion
This study reports the first profiling of 54 active kinases in human skeletal muscle from lean and obese insulin-resistant participants using ABPP and Universal-SILAC quantitative proteomics with HPLC-ESI-MS/MS. The activities of 23 kinases demonstrated significant differences in obese insulin-resistant versus lean healthy participants, pinpointing novel specific impairment in kinase activities in multiple signaling pathways important for skeletal muscle insulin signaling and insulin resistance, such as p38 MAPK signaling, AMPK signaling, JNK signaling, NF-κB signaling, ERK1/2 signaling and mTOR signaling. These results may provide novel insights into the molecular mechanism of OIR and may provide new drug targets to restore aberrant kinase activation and prevent or reverse OIR and metabolic diseases.
Acknowledgments
We thank study participants, and the staff at the clinical research center and other research assistants for carrying out clinical study related tasks.
Financial Support: This work was supported by NIH/NIDDK R01DK081750 & R01DK107666 (ZYi), Wayne State University faculty start-up, faculty competition for post-doctoral fellow award, Diabetes and Obesity Team Science, bridge funding, and grant boost (ZYi), Diabetes and Obesity scholar (AK/ZYi), and T32DK080657 (AA-S). AK is supported by National Eye Institute (MIH/NEI R01EY022230) and the Department of VA MERIT (101 BX002801-01) and Senior Research Career Scientist awards.
Author contributions: Y.Q. designed and performed clinical and proteomic experiments, analyzed data, generated figures, and wrote the manuscript. B.S., Z.M., A.Ma., D.D., M.C., W.A., and M.A. carried out muscle biopsy and hyperinsulinemic-euglycemic clamp experiments and performed other clinical related tasks. X.Z., A.A-S., and A.K. helped with experimental design and data interpretation. D.M. and A.Me. helped with proteomic experiments. R.T., G.C, and S.D. assisted with computer-aided data analysis. Z.Yi is the guarantor of this work and, as such, supervised the project, designed the clinical and proteomic experiments, analyzed data, helped with data interpretation, and wrote the manuscript.
Glossary
Abbreviations
- ABPP
affinity-based probe profiling
- AMPK
adenosine monophosphate–activated protein kinase
- AMPK1
adenosine monophosphate–activated catalytic subunit alpha 1
- CK1
casein kinase I
- CDK
cyclin dependent kinase
- CSK
C-terminal Src kinase
- EIF2AK2
interferon-induced, double-stranded RNA-activated protein kinase
- HbA1c
hemoglobin A1c
- HPLC-ESI-MS/MS
high-performance liquid chromatography electrospray ionization high resolution tandem mass spectrometry
- HSkM cells
human skeletal muscle cells
- IRS1
insulin receptor substrate-1
- JNK
c-Jun N-terminal kinase
- MAP3K2
mitogen-activated protein kinase kinase kinase 2 (also known as MEKK2)
- MAP3K3
mitogen-activated protein kinase kinase kinase 3 (also known as MEKK3)
- MAP4K4
mitogen-activated protein kinase kinase kinase kinase 4 (also known as HGK)
- MINK1
misshapen-like kinase 1
- MKK4
dual specificity mitogen-activated protein kinase kinase 4
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- OGTT
oral glucose tolerance test
- OIR
obesity-related insulin resistance
- p38 MAPK
p38 mitogen-activated protein kinase
- PPP1R12A
protein phosphatase 1 regulatory subunit 12A
- PRKAA1
adenosine monophosphate–activated catalytic subunit alpha 1
- SILAC
stable isotope labeling with amino acids in cell culture
- STAT3
signal transducer and activator of transcription 3
- STK24
serine/threonine-protein kinase 24 (also known as MST3)
- STK38
serine/threonine-protein kinase 24
- T2D
type 2 diabetes
- TNIK
TRAF2 and NCK-interacting protein kinase.
Additional Information
Disclosure Summary: The authors declare no conflict of interests and have nothing to disclose.
Data Availability: The supplemental figure and tables are available at Qi, Yue (2019): Global kinome profiling reveals abnormal active kinases in human skeletal muscle from insulin resistant participants with obesity. figshare dataset. https://doi.org/10.6084/m9.figshare.8202314.v4 (17). The MS raw data that support the findings of this study have been deposited in ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD011909. The data is currently private, and can only be accessed with a single reviewer account that has been created, Username: reviewer26985@ebi.ac.uk, Password: BXfB7ji9.
References
- 1. Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43(7):821–835. [DOI] [PubMed] [Google Scholar]
- 2. Liu XM, Liu YJ, Zhan J, He QQ. Overweight, obesity and risk of all-cause and cardiovascular mortality in patients with type 2 diabetes mellitus: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2015;30(1):35–45. [DOI] [PubMed] [Google Scholar]
- 3. Rippe JM, Angelopoulos TJ. Sugars, obesity, and cardiovascular disease: results from recent randomized control trials. Eur J Nutr. 2016;55(Suppl 2):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn CR. Banting lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43(8):1066–1084. [DOI] [PubMed] [Google Scholar]
- 5. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. [DOI] [PubMed] [Google Scholar]
- 6. Damm P, Handberg A, Kühl C, Beck-Nielsen H, Mølsted-Pedersen L. Insulin receptor binding and tyrosine kinase activity in skeletal muscle from normal pregnant women and women with gestational diabetes. Obstet Gynecol. 1993;82(2):251–259. [PubMed] [Google Scholar]
- 7. Itani SI, Pories WJ, Macdonald KG, Dohm GL. Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism. 2001;50(5):553–557. [DOI] [PubMed] [Google Scholar]
- 8. Hakonen E, Ustinov J, Eizirik DL, Sariola H, Miettinen PJ, Otonkoski T. In vivo activation of the PI3K-Akt pathway in mouse beta cells by the EGFR mutation L858R protects against diabetes. Diabetologia. 2014;57(5):970–979. [DOI] [PubMed] [Google Scholar]
- 9. Rutter GA, Leclerc I. The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol. 2009;297(1-2):41–49. [DOI] [PubMed] [Google Scholar]
- 10. Liu Z, Cao W. p38 mitogen-activated protein kinase: a critical node linking insulin resistance and cardiovascular diseases in type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9(1):38–46. [DOI] [PubMed] [Google Scholar]
- 11. Yang R, Trevillyan JM. c-Jun N-terminal kinase pathways in diabetes. Int J Biochem Cell Biol. 2008;40(12):2702–2706. [DOI] [PubMed] [Google Scholar]
- 12. Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell. 2004;15(5):661–675. [DOI] [PubMed] [Google Scholar]
- 13. Patricelli MP, Szardenings AK, Liyanage M, et al. Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry. 2007;46(2):350–358. [DOI] [PubMed] [Google Scholar]
- 14. Patricelli MP, Nomanbhoy TK, Wu J, et al. In situ kinase profiling reveals functionally relevant properties of native kinases. Chem Biol. 2011;18(6):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okerberg ES, Wu J, Zhang B, et al. High-resolution functional proteomics by active-site peptide profiling. Proc Natl Acad Sci U S A. 2005;102(14):4996–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Ma D, Caruso M, Lewis M, Qi Y, Yi Z. Quantitative phosphoproteomics reveals novel phosphorylation events in insulin signaling regulated by protein phosphatase 1 regulatory subunit 12A. J Proteomics. 2014;109:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi Y, Zhang X, Seyoum B, et al. doi:10.6084/m9.figshare.8202314.v4.2019.
- 18. Caruso M, Ma D, Msallaty Z, et al. Increased interaction with insulin receptor substrate 1, a novel abnormality in insulin resistance and type 2 diabetes. Diabetes. 2014;63(6):1933–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 20. Hwang H, Bowen BP, Lefort N, et al. Proteomics analysis of human skeletal muscle reveals novel abnormalities in obesity and type 2 diabetes. Diabetes. 2010;59(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gruhler A, Olsen JV, Mohammed S, et al. Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol Cell Proteomics. 2005;4(3):310–327. [DOI] [PubMed] [Google Scholar]
- 22. Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. [DOI] [PubMed] [Google Scholar]
- 23. Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–1372. [DOI] [PubMed] [Google Scholar]
- 24. Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiménez-Marín Á, Collado-Romero M, Ramirez-Boo M, Arce C, Garrido JJ. Biological pathway analysis by ArrayUnlock and ingenuity pathway analysis. BMC Proc. 2009;3(Suppl 4):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilmore JM, Milloy JA, Gerber SA. SILAC surrogates: rescue of quantitative information for orphan analytes in spike-in SILAC experiments. Anal Chem. 2013;85(22):10812–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. [DOI] [PubMed] [Google Scholar]
- 28. Lee JO, Lee SK, Jung JH, et al. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. J Cell Physiol. 2011;226(4):974–981. [DOI] [PubMed] [Google Scholar]
- 29. Lefort N, Glancy B, Bowen B, et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes. 2010;59(10):2444–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang X, Nath A, Opperman MJ, Chan C. The double-stranded RNA-dependent protein kinase differentially regulates insulin receptor substrates 1 and 2 in HepG2 cells. Mol Biol Cell. 2010;21(19):3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blalock WL, Grimaldi C, Fala F, et al. PKR activity is required for acute leukemic cell maintenance and growth: a role for PKR-mediated phosphatase activity to regulate GSK-3 phosphorylation. J Cell Physiol. 2009;221(1):232–241. [DOI] [PubMed] [Google Scholar]
- 32. Yao Z, Zhou G, Wang XS, et al. A novel human STE20-related protein kinase, HGK, that specifically activates the c-Jun N-terminal kinase signaling pathway. J Biol Chem. 1999;274(4):2118–2125. [DOI] [PubMed] [Google Scholar]
- 33. Kesavan K, Lobel-Rice K, Sun W, et al. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004;199(1):140–148. [DOI] [PubMed] [Google Scholar]
- 34. Stegert MR, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol. 2005;25(24):11019–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu H, Su YC, Becker E, Treisman J, Skolnik EY. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr Biol. 1999;9(2):101–104. [DOI] [PubMed] [Google Scholar]
- 36. Fu CA, Shen M, Huang BC, Lasaga J, Payan DG, Luo Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J Biol Chem. 1999;274(43):30729–30737. [DOI] [PubMed] [Google Scholar]
- 37. Enomoto A, Kido N, Ito M, et al. Negative regulation of MEKK1/2 signaling by serine-threonine kinase 38 (STK38). Oncogene. 2008;27(13):1930–1938. [DOI] [PubMed] [Google Scholar]
- 38. Tamaskovic R, Bichsel SJ, Rogniaux H, Stegert MR, Hemmings BA. Mechanism of Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J Biol Chem. 2003;278(9):6710–6718. [DOI] [PubMed] [Google Scholar]
- 39. Devroe E, Erdjument-Bromage H, Tempst P, Silver PA. Human Mob proteins regulate the NDR1 and NDR2 serine-threonine kinases. J Biol Chem. 2004;279(23):24444–24451. [DOI] [PubMed] [Google Scholar]
- 40. Lin A, Minden A, Martinetto H, et al. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268(5208):286–290. [DOI] [PubMed] [Google Scholar]
- 41. Gonzalez-Freire M, Semba RD, Ubaida-Mohien C, et al. The human skeletal muscle proteome project: a reappraisal of the current literature. J Cachexia Sarcopenia Muscle. 2017;8(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Højlund K, Bowen BP, Hwang H, . et al. In vivo phosphoproteome of human skeletal muscle revealed by phosphopeptide enrichment and HPLC-ESI-MS/MS. J Proteome Res. 2009;8(11):4954–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lundby A, Secher A, Lage K, et al. Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun. 2012;3:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffman NJ, Parker BL, Chaudhuri R, et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015;22(5):922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moh A, Zhang W, Yu S, et al. STAT3 sensitizes insulin signaling by negatively regulating glycogen synthase kinase-3 beta. Diabetes. 2008;57(5):1227–1235. [DOI] [PubMed] [Google Scholar]
- 46. Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981;240(6):E630–E639. [DOI] [PubMed] [Google Scholar]
- 47. Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF. PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol. 2000;20(13):4532–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lancaster GI, Kammoun HL, Kraakman MJ, Kowalski GM, Bruce CR, Febbraio MA. PKR is not obligatory for high-fat diet-induced obesity and its associated metabolic and inflammatory complications. Nat Commun. 2016;7:10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geetha T, Langlais P, Caruso M, Yi Z. Protein phosphatase 1 regulatory subunit 12A and catalytic subunit δ, new members in the phosphatidylinositide 3 kinase insulin-signaling pathway. J Endocrinol. 2012;214(3):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lang F, Aravamudhan S, Nolte H, et al. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis Model Mech. 2017;10(7):881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan IM, Perrard XY, Brunner G, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond). 2015;39(11):1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fink LN, Costford SR, Lee YS, et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring). 2014;22(3):747–757. [DOI] [PubMed] [Google Scholar]
- 53. Zhong J, Gavrilescu LC, Molnár A, et al. GCK is essential to systemic inflammation and pattern recognition receptor signaling to JNK and p38. Proc Natl Acad Sci U S A. 2009;106(11):4372–4377. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Hou T, Tieu BC, Ray S, et al. Roles of IL-6-gp130 signaling in vascular inflammation. Curr Cardiol Rev. 2008;4(3):179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henstridge DC, Bruce CR, Pang CP, et al. Skeletal muscle-specific overproduction of constitutively activated c-Jun N-terminal kinase (JNK) induces insulin resistance in mice. Diabetologia. 2012;55(10):2769–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sabio G, Kennedy NJ, Cavanagh-Kyros J, et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kato T Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol Cell. 2003;12(4):829–839. [DOI] [PubMed] [Google Scholar]
- 58. Friedrichsen M, Ribel-Madsen R, Wojtaszewski J, et al. Dissociation between skeletal muscle inhibitor-kappaB kinase/nuclear factor-kappaB pathway activity and insulin sensitivity in nondiabetic twins. J Clin Endocrinol Metab. 2010;95(1):414–421. [DOI] [PubMed] [Google Scholar]
- 59. Ho RC, Alcazar O, Fujii N, Hirshman MF, Goodyear LJ. p38gamma MAPK regulation of glucose transporter expression and glucose uptake in L6 myotubes and mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R342–R349. [DOI] [PubMed] [Google Scholar]
- 60. Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274(15):10071–10078. [DOI] [PubMed] [Google Scholar]
- 61. Somwar R, Kim DY, Sweeney G, et al. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J. 2001;359(Pt 3):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee-Young RS, Griffee SR, Lynes SE, et al. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284(36):23925–23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lizcano JM, Göransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23(4):833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamada E, Lee TW, Pessin JE, Bastie CC. Targeted therapies of the LKB1/AMPK pathway for the treatment of insulin resistance. Future Med Chem. 2010;2(12):1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gauthier MS, O’Brien EL, Bigornia S, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404(1):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553(Pt 1):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Combes A, Dekerle J, Webborn N, Watt P, Bougault V, Daussin FN. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol Rep. 2015;3(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. [DOI] [PubMed] [Google Scholar]
- 71. Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vergès B, Cariou B. mTOR inhibitors and diabetes. Diabetes Res Clin Pract. 2015;110(2):101–108. [DOI] [PubMed] [Google Scholar]
- 73. Bogyo M, Verhelst S, Bellingard-Dubouchaud V, Toba S, Greenbaum D. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem Biol. 2000;7(1):27–38. [DOI] [PubMed] [Google Scholar]
- 74. Greenbaum D, Baruch A, Hayrapetian L, et al. Chemical approaches for functionally probing the proteome. Mol Cell Proteomics. 2002;1(1):60–68. [DOI] [PubMed] [Google Scholar]
- 75. Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt BF. Activity-based probes for the proteomic profiling of metalloproteases. Proc Natl Acad Sci U S A. 2004;101(27):10000–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: the serine hydrolases. Proc Natl Acad Sci U S A. 1999;96(26):14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]