Abstract
In spontaneously ovulating rodent species, the timing of the luteinizing hormone (LH) surge is controlled by the master circadian pacemaker in the suprachiasmatic nucleus (SCN). The SCN initiates the LH surge through the coordinated control of two, opposing neuropeptidergic systems that lie upstream of the gonadotropin-releasing hormone (GnRH) neuronal system, the stimulatory peptide, kisspeptin, and the inhibitory peptide, RFamide-related peptide-3 (RFRP-3; the mammalian ortholog of avian gonadotropin-inhibitory hormone (GnIH)). We have previously shown that the GnRH system exhibits time-dependent sensitivity to kisspeptin stimulation, further contributing to the precise timing of the LH surge. To examine whether this time-dependent sensitivity of the GnRH system is unique to kisspeptin, or a more common mechanism of regulatory control, we explored daily changes in the response of the GnRH system to RFRP-3 inhibition. Female hamsters were ovariectomized to eliminate estradiol (E2) negative feedback and RFRP-3 or saline were centrally administered in the morning or late afternoon. LH concentrations and Lhβ mRNA expression did not differ between morning RFRP-3-and saline-treated groups but were markedly suppressed by RFRP-3 administration in the afternoon. However, RFRP-3 inhibition of circulating LH at the time of the surge does not appear to act via the GnRH system as no differences in mPOA Gnrh or RFRP-3 receptor Gpr147 mRNA expression were observed. Rather, RFRP-3 suppressed arcuate nucleus Kiss1 mRNA expression and potentially impacted pituitary gonadotropes directly. Together, these findings reveal time-dependent responsiveness of the reproductive axis to RFRP-3 inhibition, potentially via variation in the sensitivity of arcuate nucleus kisspeptin neurons to this neuropeptide.
Keywords: Reproduction, Kiss1, HPG, circadian rhythms
Introduction
Circadian timing is critical for female reproduction with disruptions to circadian timing leading to pronounced deficits in female reproductive health. For example, women with irregular sleep or work cycles have decreased fertility and increased rates of miscarriages (1–4). In spontaneously ovulating species, the timing of the luteinizing hormone (LH) surge required for ovulation is under strict circadian regulation by the suprachiasmatic nucleus (SCN) of the hypothalamus, the master mammalian brain clock (4–7). The dependence of ovulation on circadian timing coordinates a limited time window of fertility with sexual motivation and activity to maximize reproductive success, with the preovulatory LH surge occurring during early mornings in women and diurnal rodents (8–10) and in late afternoon in nocturnal rodents (11–13). To ensure appropriate oocyte maturation at the time of ovulation, the neuroendocrine circuit initiating ovulation has an additional reliance on estradiol (E2) signaling from developing follicles. As maturing follicles develop during the follicular phase of the ovulatory cycle, increasing concentrations of E2 are secreted to maintain LH at low concentrations through negative feedback. However, just prior to ovulation, peak E2 concentrations act through positive feedback to initiate the LH surge that triggers ovulation (3, 6, 7, 14–17). Previous findings by our group and others suggest that the temporary shift from negative to positive feedback is coordinated by the SCN (11, 17–20).
The SCN modulates reproductive axis function via direct and indirect communication to the hypothalamic-pituitary-gonadal (HPG) axis. At the time of the LH surge, monosynaptic vasoactive intestinal peptide (VIP) projections from the SCN directly stimulate GnRH neurons, with FOS expression increased in GnRH neurons receiving VIP input around the time of the LH surge (21, 22). GnRH neurons do not express estrogen receptor α (ERα), the receptor subtype that mediates E2 positive and negative feedback. To modulate the balance of negative and positive E2 feedback, the SCN coordinates the activity of two opposing, ERα-expressing neuropeptidergic systems that lie upstream of the GnRH system, the stimulatory neuropeptide, kisspeptin, and the inhibitory neuropeptide, RFamide-related peptide-3 (RFRP-3; the mammalian ortholog of avian gonadotropin-inhibitory hormone (GnIH)) (4, 11, 18, 23–30). The SCN coordinates cellular activity of RFRP-3 neurons to suppress the reproductive axis outside the time window of the LH surge and allow for the transient suppression of E2 negative feedback around the time of the surge (11, 18, 23). RFRP-3 neurons are concentrated in the dorsomedial hypothalamus (DMH) and project broadly to hypothalamic loci that contain GnRH neurons and fibers (i.e., medial septum, diagonal band of Broca, preoptic area, anterior hypothalamus, and arcuate nucleus) in addition to the ventromedial nucleus of the hypothalamus and brainstem (24). RFRP-3 cell projections form direct contacts with GnRH neurons expressing the RFRP-3 receptor, GPR147 (24, 26, 31). Furthermore, RFRP-3 directly suppresses GnRH neuron activity and consequent LH release (18, 24, 29, 32–35). In some species, RFRP-3 neurons may also act on the anterior pituitary to mediate LH release as RFRP-3 neurons directly project to the median eminence and GPR147 is expressed in the pituitary (11, 34, 36–38). Finally, RFRP-3 may modulate the HPG axis via a subpopulation of arcuate nucleus (ARC) kisspeptin neurons that express GPR147 (39).
Concomitant with RFRP-3 suppression at the time of the LH surge, the SCN stimulates kisspeptin neurons located in anteroventral periventricular nucleus (AVPV) that, in turn, stimulate the GnRH system and the LH surge (22, 29, 40, 41). Whereas we have previously shown that kisspeptin neurons are indiscriminately sensitive to SCN signaling across the day in Syrian hamsters, GnRH neurons exhibit time-dependent sensitivity to kisspeptin stimulation, responding more robustly in the afternoon than in the morning (29). This additional mechanism of temporal control likely further ensures precision in the timing of the LH surge and ovulation. The present study examined whether this time-dependent sensitivity of the GnRH system is unique to kisspeptin or if daily changes in reproductive system sensitivity also occur in response to RFRP-3 inhibition to further precise timing of the LH surge.
Because reproductive axis inhibition is essential prior to ovulation, we hypothesized that the GnRH system is maximally responsive to RFRP-3 in the morning, prior to the LH surge. If true, then LH concentrations should be inhibited by RFRP-3 in the morning but not (or to a greater degree than) in the afternoon. However, it is also possible that the GnRH system is maximally responsive to RFRP-3 in the afternoon, because this is a time during which RFRP-3 neurons are typically transiently inactive (11, 18, 42). If it is the case that maximal responsiveness of the GnRH system occurs in the afternoon, then LH concentrations should be inhibited by RFRP-3 in the afternoon but not (or to a greater degree than) in the morning. RFRP-3 inhibition of LH occurs through changes in LH peptide secretion, which may or may not reflect changes in mRNA expression. Likewise, inhibition of LH is possibly accompanied by changes in GnRH peptide release which may or may not be reflected in changes in Gnrh mRNA expression. Finally, RFRP-3 may modify LH production and/or release via direct impact on pituitary gonadotropes, or indirectly via kisspeptin neurons in the ARC that control GnRH pulsatility (43–45). To select among these possibilities, we examined daily changes in HPG axis sensitivity to RFRP-3 inhibition in ovariectomized (OVX) female hamsters administered RFRP-3 or saline in the morning (prior to the LH surge) or late afternoon (around the time of the LH surge).
Material and Methods
Animals
Thirty-four >8-week-old female Syrian hamsters (Mesocricetus auratus) were purchased from Charles River (Wilmington, MA) and maintained on a 14:10 light:dark cycle (lights on at 06:00, lights off at 20:00) at 23 ± 1°C with food and water available ad libitum. A 14:10 light:dark cycle was employed to create a “long day” light regimen, as Syrian hamsters are seasonal breeders that breed under long day conditions. All procedures were approved by the Animal Care and Use Committee at the University of California, Berkeley and conformed to principles enumerated in the NIH guide for the use and care of laboratory animals.
Experimental Procedure
Surgical Procedures
After a 2 wk acclimation period, all hamsters were ovariectomized (OVX) to eliminate E2 negative feedback. Surgeries were conducted under isoflurane anesthesia with buprenorphine (s.c., 0.1 mg/kg) provided for analgesia. After a 2 wk recovery, a guide cannula (22GA, 6 mm; PlasticsOne, San Diego, CA, USA) was stereotaxically implanted under deep anesthesia (ketamine-xylazine cocktail (i.p., 60/5 mg/kg) directed at the lateral ventricle. For cannular implantation, the head was shaved, prepared for surgery, and animals were placed in a stereotaxic apparatus (Kopf, Tujunga, CA). Guide cannulae were placed at the following coordinates relative to bregma: 1.3 mm mediolateral, 1.1 mm posterior, and 3 mm ventral from the surface of the dura mater. Following surgery, a dummy cannula (6.5 mm; PlasticsOne, San Diego, CA, USA) was inserted into each guide cannula to prevent obstruction. Buprenorphine was administered before and after the surgeries for analgesia (s.c., 0.1 mg/kg). Following the procedure, hamsters were singly housed for the remainder of the study. Animals were given 1 wk to recover before assessing cannula placement via injections of angiotensin-II (5 ng angiotensin-II in 2 μL sterile 0.9% saline) and examination of subsequent drinking behavior. Immediate drinking exhibited by hamsters confirmed the location of the cannula in the lateral ventricle.
Pharmacological manipulations and sample collection
Five μl of saline or RFRP-3 (100 or 500 ng in saline) (Syrian hamster RFRP: ILSRVPSLPQRF-NH2, purchased from Phoenix Pharmaceuticals, CA, USA) were injected (i.c.v.) in the morning (3 h after lights on, n=6/group) or in the afternoon (3h before lights off, n=6–7/group), at a rate of 0.5 μl per 30s, while the animals were freely moving about their home cage. Blood samples were collected from the retro-orbital sinus 20 mins following injection and centrifuged at 1400 RCF for 15 mins. Serum was collected and stored at −20˚C until assayed. 2 weeks later, animals were injected again with RFRP-3 (100 ng/5 μl saline) or saline (5 μl) in the morning or afternoon (n=6–9/group) and animals were sacrificed 2h later. Brains and pituitaries were flash frozen and brains were sectioned at 300 μm and transferred to RNAlater (AMBION, AM7021, Grand Island, NY, USA) for one night at 4˚C and −20˚C thereafter until further processed. A 3 mm biopsy punch was used to microdissect the DMH and ARC in a single punch, and a 2 mm biopsy punch was used to microdissect the mPOA and AVPV bilaterally (Figure 1). RNA was extracted using ISOLATEII RNA mini kit (Bioline, BIO-52073, Memphis, TN, USA) and reversed transcribed for RT-PCR (iScript RT supermix, BIO-RAD, 170–8841, Hercules, CA, USA). A random, representative sample of RNA (for each tissue n=8) was assessed for RNA quality on an Agilent Technologies Bioanalyzer and yielded an average RNA integrity number (RIN) of 7.3 or higher. To confirm LH suppression and further validate the detection levels of the LH ELISA at low concentrations, 5 hamsters were injected with estradiol benzoate (EB, 100 μg in 200 μl sesame oil) and retro-orbital blood samples were collected 90 min later as described above.
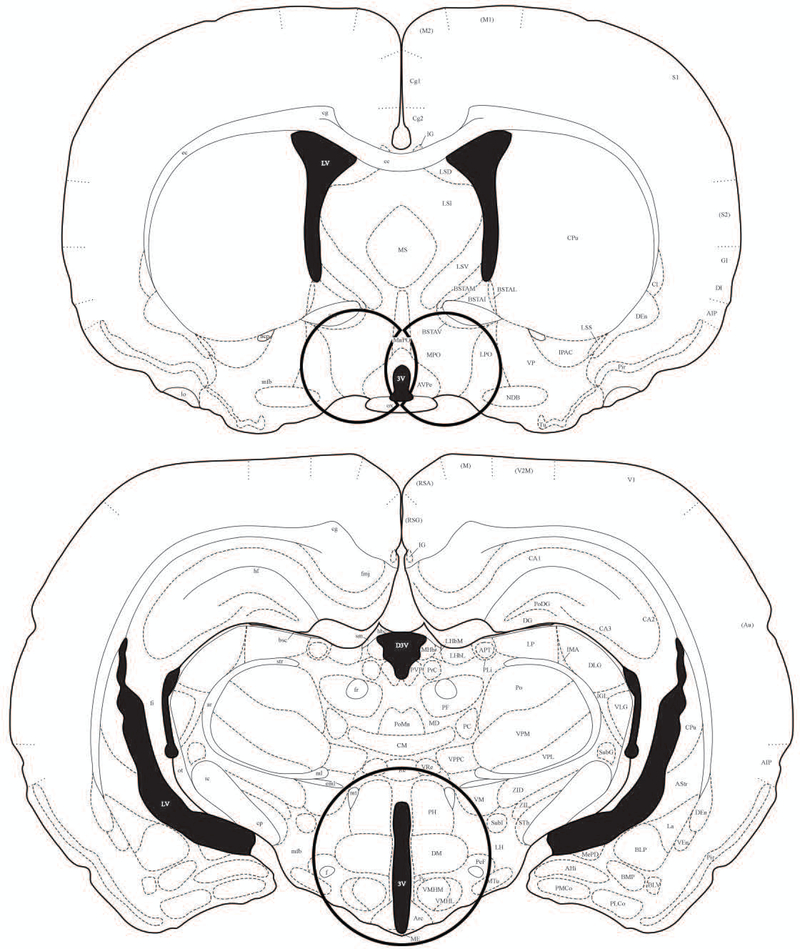
Figure 1.
An illustration of the location of samples punched for RT-PCR analysis. Brains were flash frozen and cut at 300μm and then transferred to RNAlater for one night. A 2mm biopsy punch was used to microdissect the mPOA and AVPV bilaterally (left), and a 3mm biopsy punch was used to microdissect the DMH and ARC in a single punch (right). Illustrations adapted and modified from the Stereotaxic Atlas of the Golden Hamster Brain by L.P Morin and R.I Wood (2000) (68).
qRT-PCR
Analysis of relative gene expression via qRT-PCR was performed using SSOAdvanced SYBR Green supermix (BIO-RAD, 1725272, Hercules, CA, USA). Samples were run on a BIO-RAD CFX384 machine with 10μl reaction volumes with a 2-step amplification for 40 cycles followed by a melt curve. Primers were designed from published sequences for Syrian hamsters using NCBI Primer BLAST software (Table 1). Primer sets were validated for specificity using positive, negative, no reverse transcriptase, and no template controls, and confirmed with a single-peak melt curve and correct product length. Efficiency of each primer set was determined by standard curve; primers were 94.7–105.4% efficient with R2 values above 0.99. All samples were run in triplicate. Replicate sets in which Cq values varied beyond 0.5 cycles were excluded from analysis and resulting data were analyzed in Microsoft Excel following the delta delta Cq method (46). The geometric mean of 2 housekeeping genes’ expression was used for reference. Because the expression of housekeeping genes was found to vary with time of day or treatment between brain regions, samples from different brain regions were analyzed with different reference genes. Gapdh and Actb were used as reference genes for the pituitary (Cq ranges were 20.3–24.26 and 20.6–24.64, respectively), whereas Hmbs and Tbcc were used as reference genes for the DMH and ARC (Cq ranges were 23.9–26.8 and 21.35–24.24, respectively), and B2m and Rplp16 were used as their reference genes for AVPV and POA samples (Cq ranges were 20.78–23.88 and 18.77–21.53, respectively). Housekeeping genes were not significantly different between all groups, and in all gene replicate groups Cq Standard deviation was smaller than 0.2. Whereas Kiss1 mRNA expression was measured in the DMH and ARC, it was not assessed in the AVPV and POA due to late and unstable amplification, indicating low mRNA expression, possibly as a result of the OVX. All data are expressed as a fold-change over morning, saline hamsters. Some samples did not have sufficient cDNA to quantify the expression of all genes, thus sample sizes vary for different genes measured.
Table 1.
Primers used for qRT-PCR
| Primer | Forward | Reverse | Product Size |
|---|---|---|---|
| Lhβ | CGGCTACTGTCCTAGCATGG | AGGCGGACAGATGTGAAGTG | 102 |
| Gnrh-r | TCATCTTCACCCTCACACG | GTGGCAAATGCGACTGTCAT | 121 |
| Gnrh | AGGGACCTTCGAGGAGTTCT | TGTGGATCCTTTGGTGCTGAT | 88 |
| Kiss1 | TGGTTATCTTTGACCTCCGGC | TGCCAAGAAGCCAATGTGGT | 105 |
| Gpr147 | CCGGTTGGCCTTTTGACAAT | CAGCTTCTCACGGAAAGGGT | 140 |
| Gapdh | ACAGTCAAGGCTGAGAACGG | TCCACAACATACTCGGCACC | 116 |
| Actb | GACCCAGATCATGTTTGAGACCT | TCCGGAGTCCATCACAATGC | 112 |
| B2m | TGGCCGTGGTCTTTCTGATG | TGGAACTGCGACACATAGCA | 139 |
| Rplp16 | ATCTACTCCGCCCTCATCCT | GCAGATGAGGCTTCCAATGT | 159 |
| Hmbs | TATCCTGGATGTTGCACGGC | TCTCAACACCCAGTGGTTCA | 165 |
| Tbcc | CAGTGGGACTGAGCACTAGC | TAGCAAAAGCCCCGGGTTAG | 156 |
Assessment of LH levels
LH concentrations were quantified with an ELISA, using a modified protocol that was kindly provided by Jens D. Mikkelsen (Copenhagen University Hospital, Denmark) (47), and all samples were run in duplicate. Briefly, 96-well microtiter plates were coated with 50 μl of bovine LHβ 518B7 monoclonal antibody (kindly provided by Lillian E Sibley, UC Davis, CA, USA) and incubated overnight at 4˚C. Excess antibody was removed, and plates were washed 3 times with 200 μl of 10mM PBS with 0.05% Tween 20 (PBS-T). Plates were blocked for 1h at room temperature using 5% skim milk powder in PBS-T. Following washes, 50 μl of each sample and standards (mouse RIA kit, AF Parlow, National Hormone and Pituitary Program, University of California, Harbor Medical Center, Los Angeles, CA, USA) diluted in assay buffer was added to each well and incubated for 2 h at room temperature. Plates were then washed and 50μl of rabbit polyclonal LH antibody (AFP240580Rb, AF Parlow, National Hormone and Pituitary Program, University of California, Harbor Medical Center, Los Angeles, CA, USA) were added into each well and incubated for 90 mins at room temperature. After washing, a 1:2000 dilution of polyclonal goat anti-rabbit IgG conjugated to horseradish peroxidase (DAKO Cytomation, catalog # P0448, Santa Clara, CA, USA) was added to each well and incubated for 1 h at room temperature. After washing, o-Phenylenediamine (OPD, Invitrogen, catalog # 00–2003, Camarillo, CA, USA) in citrate buffer was added to each well and the reaction was allowed to proceed for 30 mins at room temperature in darkness before being stopped by the addition of 3M HCl to each well. Light absorbance was immediately read at 490 nm with a reference of 655 nm. Representative random serum samples were assessed by the Center for Research in Reproduction at the University of Virginia (UVA), and Pearson’s r=0.97 correlation was found between the LH values obtained at UVA and the values generated by the ‘in-house’ LH ELISA. The assay was also validated by assessing parallelism with the standard curve as well as blood samples collected 90 min following E2 benzoate (EB) administration used to suppress LH concentrations. Assay sensitivity was 0.002 ng/ml and intra-and inter-assay variability were 1.1% and 3.4%, respectively.
Statistical analysis
Group comparisons were examined using a two-way analysis of variance (ANOVA). In instances which assumptions of normality and/or equal group variance were violated, data was analyzed by planned contrasts for comparisons between specific groups was made on the basis on a priori hypotheses and corrected for multiple comparison with Bonferroni’s inequality test. Statistical analyses were performed in SPSS (Armonk, New York, USA) and Prism (San Diego, CA, USA). All data are reported as mean ± standard error of the mean (SEM) with p < 0.05 considered statistically significant. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Circulating LH
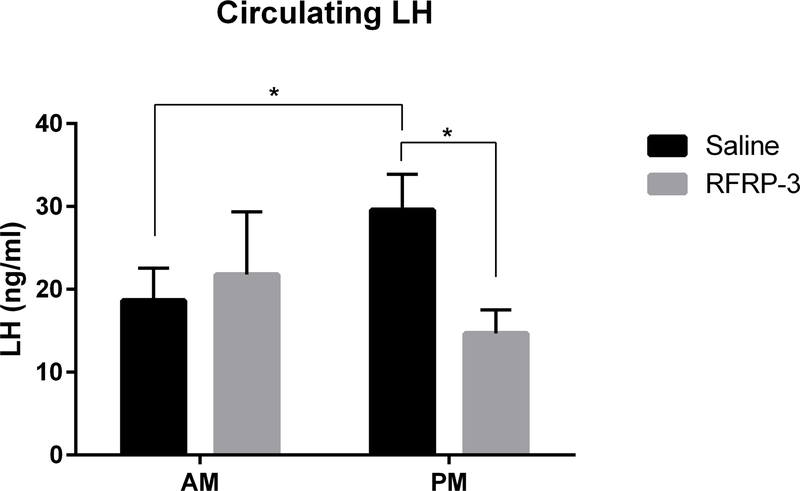
LH concentrations were measured to examine whether the GnRH system exhibits daily changes in sensitivity to RFRP-3 inhibition. Consistent with the timing of the LH surge to the afternoon, baseline LH concentrations (saline groups) were significantly different across the day, increasing from 18.65 ng/ml ± 3.8 in the morning to 29.6 ng/ml ± 4.3 in the afternoon (p<0.044, 95% confidence interval: −1.998 to 23.87) (Figure 2, n=6–7/group). 100 ng RFRP-3 significantly decreased circulating LH concentrations in the afternoon 20 mins after administration (from 29.6 ng/ml ± 4.3 to 14.7 ng/ml ± 2.8 (p<0.006, 95% confidence interval: −25.89 to −3.883). At this same dose, no differences were found between saline and RFRP-3 administration in the morning (p>0.05). No effects were observed with the 500 ng dose of RFRP-3 (p>0.05 in all cases; data not shown). Finally, EB markedly suppressed LH concentrations 90 mins post administration (decreasing to 5.47 ng/ml ± 0.9, t=2.808, p<0.01, data not shown), further validating the LH assay.
Figure 2.
Central administration of RFRP-3 (100 ng) inhibits circulating LH in the afternoon 20 min post administration. n = 6, 6, 6, 7, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively. Data are presented as mean ± standard error of the mean. * p < 0.05.
Pituitary gene expression
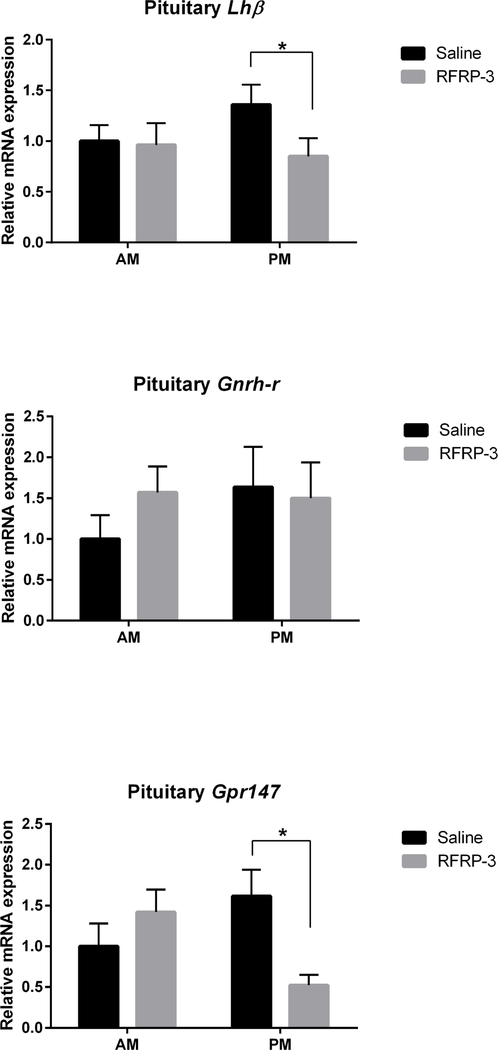
Pituitary gene expression was measured to examine whether changes in LH concentrations are accompanied by changes at the mRNA level and whether pituitary cells exhibit the potential for direct inhibition by RFRP-3 (i.e., changes at the level of the pituitary independent of changes in the GnRH or kisspeptin systems) (Figure 3, n=6–9/group). Within each treatment (saline or RFRP-3), no differences in Lhβ mRNA expression were found across time of day. However, RFRP-3 significantly decreased pituitary Lhβ subunit mRNA expression in the afternoon (p<0.04, 95% confidence interval: −1.092 to 0.07406) but not in the morning (p>0.05), compared to saline, consistent with the impact of this peptide on circulating LH. In contrast, pituitary Gnrh-r mRNA expression did not differ at any time point regardless of treatment. However, a significant time X treatment interaction was found for pituitary Gpr147 mRNA expression (F(1,24)=2.427, p<0.019), with RFRP-3 significantly decreasing pituitary Gpr147 mRNA expression in the afternoon (p<0.016, 95% confidence interval: −1.702 to −0.08974) but not in the morning (p>0.05).
Figure 3.
Central administration of RFRP-3 suppresses pituitary Lhβ subunit mRNA expression in the afternoon but not the morning (top; n = 6, 6, 6, 7, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively). Pituitary Gnrh-r mRNA expression is not affected by either RFRP-3 or time of day (middle; n = 6, 8, 7, 7, for AM saline, AM RFRP-3, PM saline, PM RFRP-r, respectively). RFRP-3 suppresses pituitary Gpr147 mRNA expression in the afternoon but not the morning (bottom; n = 6, 8, 9, 5, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively). Data are presented as mean ± standard error of the mean. * p < 0.05.
mPOA gene expression
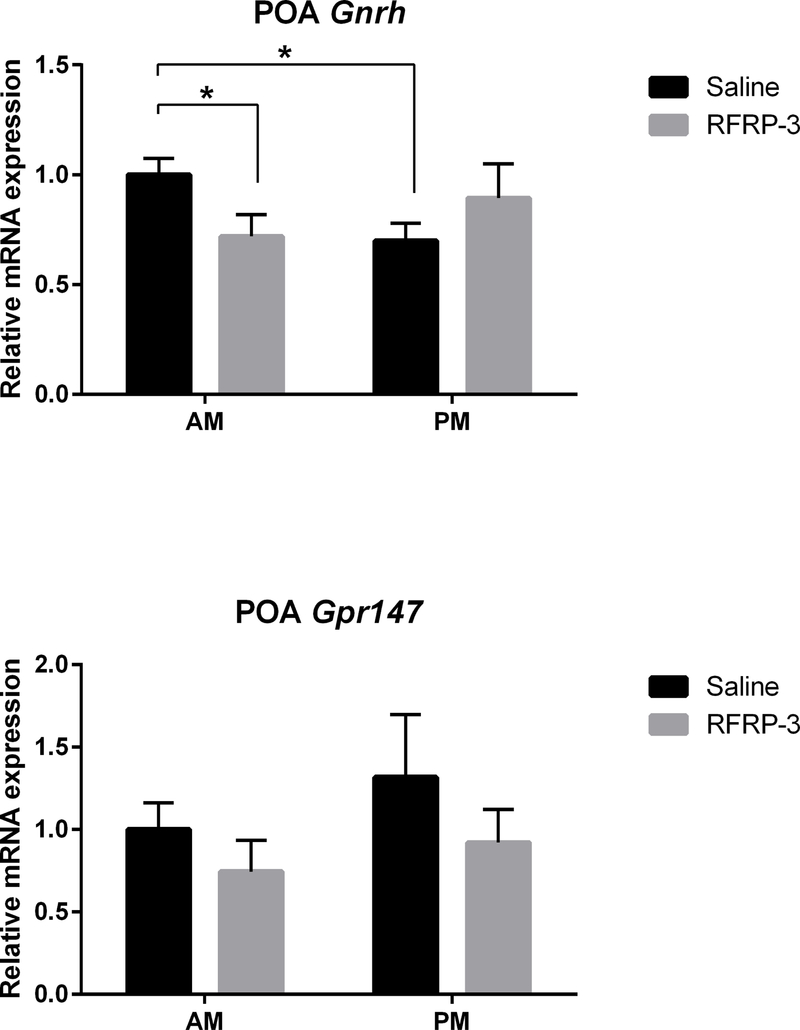
To examine whether changes in LH concentrations are mediated via the classic GnRH-LH pathway, mPOA Gnrh and Gpr147 mRNA expression were assessed (Figure 4, n=5–9/group). Baseline mPOA Gnrh expression (i.e., saline groups) was significantly reduced in the afternoon relative to morning (p<0.01, 95% confidence interval: −0.5464 to −0.05362). Additionally, RFRP-3 significantly decreased mPOA Gnrh mRNA expression in the morning (p<0.024, 95% confidence interval: −0.5581 to −0.002333) but not in the afternoon (p>0.05). mPOA Gpr147 mRNA expression did not differ at any time point for either treatment (p>0.05 in all cases).
Figure 4.
mPOA Gnrh mRNA expression is suppressed by central administration of RFRP-3 in the morning but not in the afternoon (top; n = 6, 6, 7, 9, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively). RFRP-3 does not alter mPOA Gpr147 mRNA expression (bottom; n = 7, 5, 7, 8, for AM saline, AM RFRP-3, PM saline, PM RFRP-r, respectively). Data are presented as mean ± standard error of the mean. * p < 0.05.
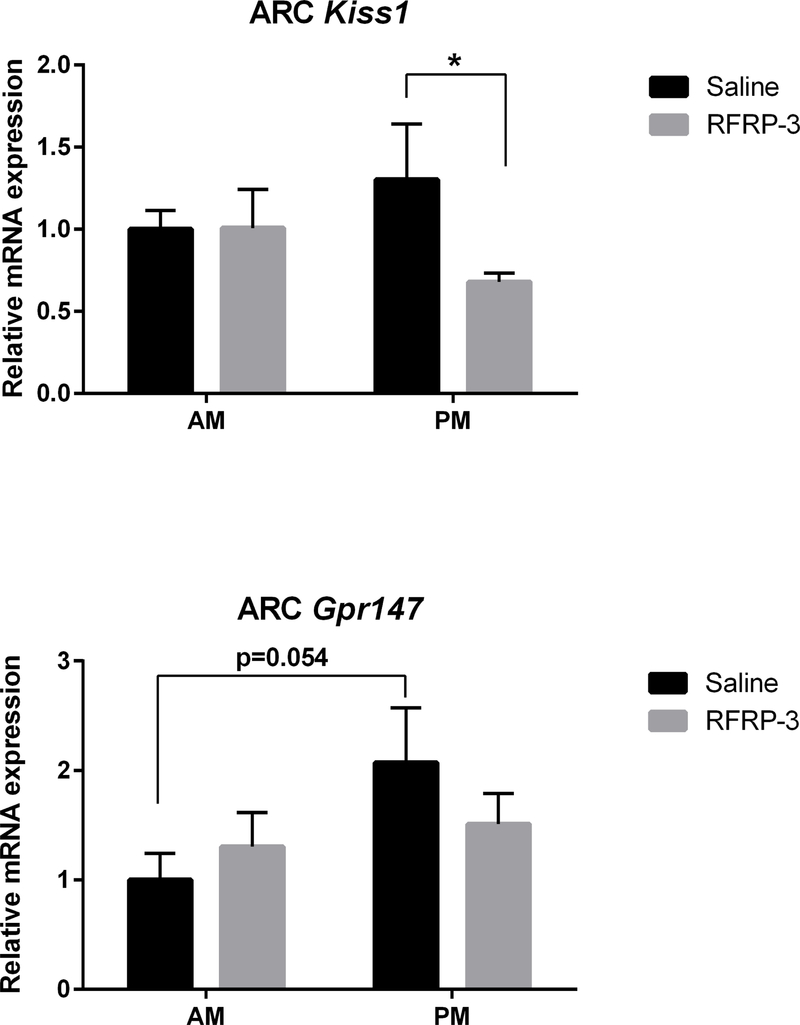
ARC gene expression
RFRP-3 may modify LH production and/or release indirectly via kisspeptin neurons in the ARC that control GnRH pulsatility (48). Thus, we examined the expression of Kiss1 and Gpr147 mRNA in the ARC under RFRP-3 and saline treatments (Figure 5, n=7–9/group). Within each treatment (saline or RFRP-3), no difference in mRNA expression was found across time of day. However, RFRP-3 significantly decreased ARC Kiss1 mRNA expression in the afternoon (p<0.022, 95% confidence interval: −1.229 to −0.01701) but not in the morning (p>0.05), compared to saline controls. In the ARC, Gpr147 mRNA baseline expression (saline groups) exhibited a non-significant trend in which afternoon levels were reduced compared to morning (p<0.054, 95% confidence interval: −0.2739 to 2.418). No effect of RFRP-3 was observed for ARC Gpr147 mRNA expression at either time point (p>0.05 in each case).
Figure 5.
Central administration of RFRP-3 suppresses ARC Kiss1 mRNA expression in the afternoon but not in the morning (top; n = 7, 8, 7, 9, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively). No effect of RFRP-3 on ARC Gpr147 mRNA expression was observed (bottom; n = 6, 8, 7, 9, for AM saline, AM RFRP-3, PM saline, PM RFRP-3, respectively). Data are presented as mean ± standard error of the mean. * p < 0.05.
Discussion
The present findings indicate that the reproductive axis responds to RFRP-3 in a time-dependent manner, with central RFRP-3 administration in the afternoon, but not the morning, reducing circulating LH and downregulating pituitary Lhβ subunit mRNA expression. These findings support the notion that the reproductive axis is most sensitive to RFRP-3 inhibition around the time of the LH surge (i.e., late afternoon). As previous studies have established that administration of RFRP-3 around the time of ovulation suppresses the GnRH/LH surge (49) and sexual motivation (50), this finding further underscores the importance of RFRP-3 cellular inhibition at this time as we and others have previously shown (11, 18, 23). Additionally, consistent with previous findings in this species (29), the present findings further establish daily changes in the reproductive axis that are coordinated with the timing of the LH surge, even in the absence of estrogen. Together, these outcomes underscore the importance of circadian-controlled RFRP-3 system inhibition to permit the LH surge and coordinate maximal fertility with sexual motivation.
To explore where daily changes in sensitivity to RFRP-3 are mediated, we examined the expression of Gpr147, the cognate receptor for RFRP-3. In the brain, Gpr147 is expressed in GnRH cells (26, 32, 33, 51), in the pituitary (11, 34, 36, 38), and in kisspeptin neurons (26, 39), providing three potential loci at which such changes may occur. Specifically for kisspeptin neurons, 12–15% of AVPV kisspeptin cells express Gpr147 and 25% of KNDy neurons express Gpr147 in both male and female mice (26, 39). Likewise, ~35% of ARC kisspeptin neurons receive RFRP-3 immunoreactive fiber contacts (39). In the present study, RFRP-3 had no effect on the expression of Gpr147 in the mPOA and AVPV, suggesting that enhanced RFRP-3 signaling via Gpr147 in these regions is not responsible for increased responsiveness to RFRP-3 inhibition in the afternoon. Likewise, hypothalamic Gnrh mRNA levels were not reduced in the afternoon by infusion of RFRP-3. Furthermore, RFRP-3 did not influence Gnrh-r mRNA expression in the pituitary. These findings suggest that changes in the sensitivity of hypothalamic GnRH neurons, or reduced pituitary sensitivity to GnRH, do not underlie the enhanced suppression of LH by RFRP-3 in the afternoon. Whether or not the enhanced suppression of LH in the afternoon by RFRP-3 is a result of inhibition of GnRH peptide release, post transcriptional/translational events regarding GPR147 (e.g., more GRP147 receptors are available/translated in the afternoon), or the specific time intervals between RFRP-3 administration and sampling represents an important area for future inquiry.
Although the present findings do not support a role for altered GnRH cell sensitivity to RFRP-3 signaling or changes in pituitary sensitivity to GnRH across the day, the findings suggest that daily changes in the suppressive actions of RFRP-3 might occur at the level of ARC kisspeptin cells. Specifically, we observed a substantial reduction in Kiss1 mRNA expression in the ARC following afternoon, but not morning, RFRP-3 administration. These findings point to the possibility that ARC kisspeptin cells may act on GnRH terminals to modulate their output across the day in response to upstream mediators. GnRH neurons possess unique axonal projections to the median eminence that also exhibit dendritic functions (43, 52, 53). These so-called ‘dendrons’ allow for synaptic input and the integration of information to control the release of GnRH. In several species, ARC kisspeptin neurons exhibit axo-axonal contacts with GnRH neurons (54, 55) as well as projections to the internal and external layer of the median eminence (43). Our results show that the expression of ARC Kiss1 mRNA co-varies with circulating LH levels, with RFRP-3 acting to reduce both Kiss1 mRNA expression and circulating concentrations of LH in the afternoon but not in the morning, consistent with this pathway of control. In support of this possibility, ablation of ARC KNDy neurons leads to atypical LH surge amplitude (56, 57). The present findings are also in agreement with a recent study demonstrating RFRP-3 suppression of ARC kisspeptin expression in free cycling Syrian hamsters maintained in long photoperiods (23). This same study found that hamsters injected with RFRP-3 in the afternoon, but not in the morning, exhibit suppression of LH concentrations when in proestrus. Furthermore, ARC kisspeptin neurons receive monosynaptic input from RFRP-3 neurons and express the RFRP-3 receptors (39). Together, the present and previous findings support the notion that RFRP-3 cells are in a position to modify LH secretion through actions on ARC kisspeptin cells and these cells differ in their response to RFRP-3 across the day.
In addition to actions on the ARC kisspeptin cell population, daily changes in RFRP-3 sensitivity may also be mediated at the level of the pituitary, as pituitary Gpr147 and Lhβ mRNA expression are reduced following RFRP-3 treatment in the afternoon but not in the morning. Future studies in which RFRP-3 are administered peripherally in the morning and afternoon are necessary to examine this possibility as it is unclear whether or not injections of RFRP-3 in the present study enter the hypophyseal portal system. Across species (e.g., sheep, mice, hamsters, macaques, and humans), RFRP-3 projections to the median eminence and RFRP-3 receptor expression in the pituitary have been reported (11, 34, 36, 58, 59). In contrast, neither RFRP-3 projections to the median eminence nor its receptor are found in some species (60–63). In cultured pituitaries across species, RFRP-3 administration inhibits gonadotropin production and release (64–66), suggesting the potential for inhibition in vivo. Although our study did not assess this pathway directly, the expression of pituitary Gpr147 mRNA exhibited a similar pattern to that of LH and Lhβ, with RFRP-3 inhibition of Grp147 mRNA expression in the afternoon but not in the morning. These findings suggest potential actions of RFRP-3 that ultimately affect pituitary level responsiveness to this neuropeptide.
In the current study, hamsters were ovariectomized to eliminate E2 negative feedback. In the absence of E2, the pattern of LH in the saline (control) groups resembled the expected pattern, with LH concentrations being higher in the afternoon than in the morning (11). However, this daily change is not reflected in Gnrh mRNA expression. Also contrary to expectation, RFRP-3 suppressed Gnrh expression in the mPOA in the morning but not in the afternoon, contrasting with patterns of circulating LH that are not inhibited by morning RFRP-3 treatment. These unexpected relationships between the pattern of Gnrh mRNA and daily change in LH is possibly due to the disparity between the time at which blood and brain samples were collected (i.e., blood samples were collected 20 min post treatment, whereas brains were collected 2 h post treatment). It is also possible, that post transcription/translation modifications lead to differential GnRH peptide release (67). Finally, we cannot exclude the possibility that the removal of ovarian hormones alters the typical hypothalamic response to RFRP-3 communication. Future studies examining the time course of gene transcription/translation and the association with peptide release will help to select among these possibilities.
The present findings suggest that the mechanisms driving LH secretion differ depending on the time of day and neurochemical environment. Specifically, in the absence of RFRP-3 administration (i.e., saline conditions), or during times that RFRP-3 is administered when it is typically released (i.e., the morning), the mechanism driving LH secretion converge at the level of GnRH neurons. However, when RFRP-3 is administered in the afternoon, a time during which it is not typically released, it appears to bypass direct communication with the GnRH system, instead acting through ARC kisspeptin cells and/or directly on the pituitary. This latter circumstance might result in the case of circadian disrupted individuals, including women who are jetlagged, have irregular shift work hours, or are exposed to light at nighttime (e.g., from electronic devices), conditions associated with marked deficits in ovulatory cycling (1, 2).
In conclusion, the present findings indicate that time-dependent sensitivity to regulators of the HPG axis is not unique to kisspeptin stimulation of the GnRH system, at least in Syrian hamsters. The reproductive axis is maximally responsive to RFRP-3 administration in the afternoon, with no effect in the morning, even in the absence of estrogen. During the afternoon, RFRP-3 appears to inhibit LH secretion through actions on ARC kisspeptin cells and the pituitary rather than the GnRH system. These findings further highlight the importance of timed suppression of the RFRP-3 system at the appropriate time of day to allow for the LH surge and ovulation. These findings raise the possibility that, in cases of circadian disruption (e.g., irregular sleep patterns, nighttime exposure to light-emitting devices, shift work), mistimed RFRP-3 release may be responsible for compromised fertility seen across species, including humans.
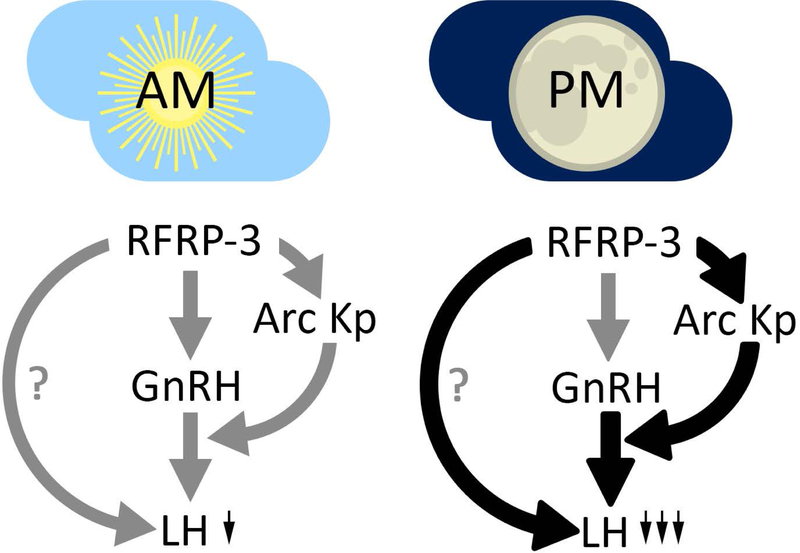
Figure 6.
Proposed model by which RFRP-3 leads to greater suppression of LH in the afternoon relative to morning injections. In the morning, RFRP-3 is proposed to have actions via GnRH cells, and potentially pituitary gonadotropes, to suppress LH. In the afternoon, based on the present findings, it is suggested that RFRP-3 acts to more potently suppress LH via additional suppression of ARC kisspeptin cells that mediate GnRH release, potentially in combination with actions on GnRH soma and/or pituitary gonadotropes. Black and thicker lines indicate points of proposed increased RFRP-3 suppression of the reproductive axis in the PM relative to AM conditions.
Acknowledgements
We thank Kim Jennings, Veronica Kim, and Pooja Srinivas for technical assistance. We also thank Benjamin Smarr for assistance with figure artwork. This study was supported by NSF grant IOS-1257638 and NIH grant HD-050470.
Footnotes
The authors of the manuscript have no conflicts of interest to declare.
References
- 1.Gamble KL, Resuehr D, Johnson CH. Shift work and circadian dysregulation of reproduction. Frontiers in endocrinology. 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol 2010;2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotlieb N, Moeller J, Kriegsfeld LJ. Circadian control of neuroendocrine function: implications for health and disease. Current Opinion in Physiology. 2018;5:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonneaux V, Bahougne T, Angelopoulou E. Daily rhythms count for female fertility. Best Pract Res Clin Endocrinol Metab. 2017;31(5):505–19. [DOI] [PubMed] [Google Scholar]
- 5.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. [DOI] [PubMed] [Google Scholar]
- 6.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. [DOI] [PubMed] [Google Scholar]
- 7.Kriegsfeld LJ. Circadian regulation of kisspeptin in female reproductive functioning. Adv Exp Med Biol 2013;784:385–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertility and sterility. 1998;70(1):56–9. [DOI] [PubMed] [Google Scholar]
- 9.Mahoney MM, Sisk C, Ross HE, Smale L. Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biology of reproduction. 2004;70(4):1049–54. [DOI] [PubMed] [Google Scholar]
- 10.McElhinny TL, Sisk CL, Holekamp KE, Smale L. A morning surge in plasma luteinizing hormone coincides with elevated Fos expression in gonadotropin-releasing hormone-immunoreactive neurons in the diurnal rodent, Arvicanthis niloticus. Biology of reproduction. 1999;61(4):1115–22. [DOI] [PubMed] [Google Scholar]
- 11.Gibson EM, Humber SA, Jain S, Williams WP 3rd, Zhao S, Bentley GE, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turek FW, Swann J, Earnest DJ. Role of the circadian system in reproductive phenomena. Recent progress in hormone research. 1984;40:143–83. [DOI] [PubMed] [Google Scholar]
- 14.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocrine reviews. 2010;31(4):544–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147(3):1148–53. [DOI] [PubMed] [Google Scholar]
- 16.Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17beta-estradiol in preoptic area kisspeptin neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(26):10828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonneaux V, Piet R. Neuroendocrine pathways driving daily rhythms in the hypothalamic pituitary gonadal axis of female rodents. Current Opinion in Physiology. 2018;5:99–108. [Google Scholar]
- 18.Russo KA, La JL, Stephens SB, Poling MC, Padgaonkar NA, Jennings KJ, et al. Circadian Control of the Female Reproductive Axis Through Gated Responsiveness of the RFRP-3 System to VIP Signaling. Endocrinology. 2015;156(7):2608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams WP 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Frontiers in endocrinology. 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. Journal of neuroendocrinology. 2012;24(1):131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134(6):2636–44. [DOI] [PubMed] [Google Scholar]
- 22.Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant Neuropeptide Cotransmission in Kisspeptin-GABA Regulation of GnRH Neuron Firing Driving Ovulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38(28):6310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henningsen JB, Ancel C, Mikkelsen JD, Gauer F, Simonneaux V. Roles of RFRP-3 in the Daily and Seasonal Regulation of Reproductive Activity in Female Syrian Hamsters. Endocrinology. 2017;158(3):652–63. [DOI] [PubMed] [Google Scholar]
- 24.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 2006;103(7):2410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piet R, Fraissenon A, Boehm U, Herbison AE. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(17):6881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, et al. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology. 2012;153(8):3770–9. [DOI] [PubMed] [Google Scholar]
- 27.Schafer D, Kane G, Colledge WH, Piet R, Herbison AE. Sex-and sub region-dependent modulation of arcuate kisspeptin neurones by vasopressin and vasoactive intestinal peptide. Journal of neuroendocrinology. 2018;30(12):e12660. [DOI] [PubMed] [Google Scholar]
- 28.Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. Journal of neuroendocrinology. 2010;22(9):1032–9. [DOI] [PubMed] [Google Scholar]
- 29.Williams WP 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152(2):595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriegsfeld LJ, Jennings KJ, Bentley GE, Tsutsui K. Gonadotrophin-inhibitory hormone and its mammalian orthologue RFamide-related peptide-3: Discovery and functional implications for reproduction and stress. Journal of neuroendocrinology. 2018;30(7):e12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubuka T, Son YL, Bentley GE, Millar RP, Tsutsui K. Gonadotropin-inhibitory hormone (GnIH), GnIH receptor and cell signaling. General and comparative endocrinology. 2013;190:10–7. [DOI] [PubMed] [Google Scholar]
- 32.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–804. [DOI] [PubMed] [Google Scholar]
- 33.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153(1):373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, et al. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PloS one. 2009;4(12):e8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. The Journal of physiology. 2009;587(Pt 7):1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–21. [DOI] [PubMed] [Google Scholar]
- 37.Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, et al. Gonadotrophin-inhibitory hormone: a multifunctional neuropeptide. Journal of neuroendocrinology. 2009;21(4):276–81. [DOI] [PubMed] [Google Scholar]
- 38.Smith JT, Young IR, Veldhuis JD, Clarke IJ. Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology. 2012;153(7):3368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. Journal of neuroendocrinology. 2013;25(10):876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao S, Kriegsfeld LJ. Daily changes in GT1–7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chassard D, Bur I, Poirel VJ, Mendoza J, Simonneaux V. Evidence for a Putative Circadian Kiss-Clock in the Hypothalamic AVPV in Female Mice. Endocrinology. 2015;156(8):2999–3011. [DOI] [PubMed] [Google Scholar]
- 42.Ancel C, Inglis MA, Anderson GM. Central RFRP-3 Stimulates LH Secretion in Male Mice and Has Cycle Stage-Dependent Inhibitory Effects in Females. Endocrinology. 2017;158(9):2873–83. [DOI] [PubMed] [Google Scholar]
- 43.Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional Viral Tract Tracing Delineates the Projections of the Distinct Kisspeptin Neuron Populations to Gonadotropin-Releasing Hormone (GnRH) Neurons in the Mouse. Endocrinology. 2015;156(7):2582–94. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocrine journal. 2009;56(6):729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Frontiers in endocrinology. 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ancel C, Bentsen AH, Sebert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153(3):1352–63. [DOI] [PubMed] [Google Scholar]
- 48.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy Cells Revisited. Endocrinology. 2018;159(9):3219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–40. [DOI] [PubMed] [Google Scholar]
- 50.Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, et al. Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus). Hormones and behavior. 2013;64(3):501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(31):12689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iremonger KJ, Herbison AE. Multitasking in Gonadotropin-Releasing Hormone Neuron Dendrites. Neuroendocrinology. 2015;102(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 54.Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, et al. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology. 2011;94(4):323–32. [DOI] [PubMed] [Google Scholar]
- 55.Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. Journal of neuroendocrinology. 2011;23(10):863–70. [DOI] [PubMed] [Google Scholar]
- 56.Helena CV, Toporikova N, Kalil B, Stathopoulos AM, Pogrebna VV, Carolino RO, et al. KNDy Neurons Modulate the Magnitude of the Steroid-Induced Luteinizing Hormone Surges in Ovariectomized Rats. Endocrinology. 2015;156(11):4200–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Ablation of KNDy Neurons Results in Hypogonadotropic Hypogonadism and Amplifies the Steroid-Induced LH Surge in Female Rats. Endocrinology. 2016;157(5):2015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(45):15060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukhbaatar U, Kanasaki H, Mijiddorj T, Oride A, Miyazaki K. Expression of gonadotropin-inhibitory hormone receptors in mouse pituitary gonadotroph LbetaT2 cells and hypothalamic gonadotropin-releasing hormone-producing GT1–7 cells. Endocrine journal. 2014;61(1):25–34. [DOI] [PubMed] [Google Scholar]
- 60.Harbid AA, McLeod BJ, Caraty A, Anderson GM. Seasonal changes in RFamide-related peptide-3 neurons in the hypothalamus of a seasonally breeding marsupial species, the brushtail possum (Trichosurus vulpecula). The Journal of comparative neurology. 2013;521(13):3030–41. [DOI] [PubMed] [Google Scholar]
- 61.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–20. [DOI] [PubMed] [Google Scholar]
- 62.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biology of reproduction. 2010;83(4):568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res 2003;982(2):156–67. [DOI] [PubMed] [Google Scholar]
- 64.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, et al. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: in vivo and in vitro studies. American journal of physiology Endocrinology and metabolism. 2010;299(1):E39–46. [DOI] [PubMed] [Google Scholar]
- 65.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, et al. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol 2009;36(4):219–24. [DOI] [PubMed] [Google Scholar]
- 66.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150(12):5549–56. [DOI] [PubMed] [Google Scholar]
- 67.Kim T, Do MH, Lawson MA. Translational control of gene expression in the gonadotrope. Molecular and cellular endocrinology. 2014;385(1–2):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain 2000. [Google Scholar]