Abstract
Background and Purpose:
Cerebral edema (CED) develops in the hours to days after stroke; the resulting increase in brain volume may lead to midline shift (MLS) and neurologic deterioration. The time-course and implications of edema formation are not well-characterized across the spectrum of stroke. We analyzed displacement of CSF (ΔCSF) as a dynamic quantitative imaging biomarker of edema formation.
Methods:
We selected subjects enrolled in a stroke cohort study who presented within six hours of onset and had baseline and one or more follow-up brain computed tomography (CT) scans available. We applied a neural network-based algorithm to quantify hemispheric CSF volume at each imaging time point and modeled CSF trajectory over time (using a piece-wise linear mixed effects model). We evaluated ΔCSF within the first 24-hours as an early biomarker of CED (defined as developing MLS on CT beyond 24-hours) and poor outcome (modified Rankin scale 3–6).
Results:
We had serial imaging in 738 subjects with stroke, of whom 91 (13%) developed CED with MLS. Age did not differ (69 vs. 70 years) but baseline NIHSS was higher (16 vs. 7) and baseline CSF volume lower (132 vs. 161-ml, both p<0.001) in those with CED. CSF displacement (ΔCSF) was faster in those developing MLS, with the majority seen by 24-hours (36% vs. 11%, or 2.4 ml/hour vs. 0.8 ml/hour, p<0.0001). Risk of CED almost doubled for every 10% ΔCSF within 24-hours (OR 1.76, 95% CI: 1.46–2.14), adjusting for age, glucose, and NIHSS. Risk of neurologic deterioration (1.6 point increase in NIHSS at 24-hours) and poor outcome (aOR 1.34, 95% CI: 1.15–1.56) were also greater for every 10% increase in ΔCSF.
Conclusion:
CSF volumetrics provides quantitative evaluation of early edema formation. ΔCSF from baseline to 24-hour CT is a promising early biomarker for the development of MLS and worse neurologic outcome.
Keywords: acute stroke, brain imaging, edema, brain, volumetry
Subject Terms: Ischemic Stroke, Biomarkers, Computerized Tomography (CT)
Introduction:
Cerebral edema (CED) develops around regions of brain infarction within the first week after acute stroke.1 This pathologic increase in brain water and hemispheric volume can lead to mass effect with a rise in local compartmental pressure, ultimately precipitating subfalcine and transtentorial herniation in 30% or more of those with large hemispheric infarction (LHI).2, 3 Such brain swelling is responsible for the majority of neurologic deterioration after LHI and carries a mortality of nearly 80% without surgical decompression.4, 5 Malignant edema is clinically detected when mental status abruptly worsens (i.e. when cerebral herniation occurs, usually around 48 hours or more after stroke onset).6 However, the biologic cascades heralding edema likely begin in the first hours after stroke and evolve over the first few days until compensation for swelling has been exhausted. It is only at this decompensated stage that midline shift (MLS) develops and herniation may result from compartmental shifts.7 Imaging of the brain at this point will show a large hemispheric hypodensity that represents the combination of infarcted brain tissue and surrounding edema; this will be associated with MLS but also loss of the normal hemispheric sulci, compression of the ipsilateral (IL) lateral ventricle, and effacement of the basal cisterns. It is through progressive displacement of cerebrospinal fluid (CSF) from all these CSF-containing spaces (i.e. sulci, ventricles, cisterns) that compensation for edema-related brain expansion occurs and swelling is accommodated, at least initially.
In order to understand the kinetics and entire spectrum of this dynamic process and predict who will decompensate, we need to capture the critical early evolution of edema, prior to herniation. A major obstacle to this has been lack of a sensitive means of quantifying changes in edema severity over time, especially within the first few days. MLS is not well suited to this purpose as it develops later in the course of edema and may still be minimal at early stages despite significant edema.8 Measuring lesion volume either requires MRI (not feasible in many stroke patients) or more readily available CT imaging, in which stroke hypodensity may be subtle and hard to accurately quantify at early time points.9 Furthermore, total infarct-related hypodensity does not distinguish actual edema (i.e. amount of brain water increase) from the volume of the infarct itself; it is only the former that contributes to swelling and risk of herniation and so lesion volume only partially predicts risk of herniation.10
We have developed a CT-based technique to measure reduction in CSF volume (ΔCSF) in stroke patients and demonstrated that it reflects edema severity at peak swelling.11 As displacement of CSF reflects the earliest compensation for brain swelling, we propose that measuring ΔCSF may allow us to directly quantify degree of edema at earlier time points and across a broader spectrum of stroke patients. Furthermore, measurement of ΔCSF at serial time points may allow us to better understand the kinetics of this process before MLS and deterioration occur. Our primary hypothesis is that ΔCSF can serve as a quantitative imaging biomarker of early edema formation that is associated with midline shift, neurologic deterioration, and outcome.
Methods:
Subject Selection
We selected patients from a multi-center prospective cohort study (GENISIS: Genetics of Early Neurological Instability after Ischemic Stroke) that enrolled patients with acute ischemic stroke presenting within six hours of onset. All participants provided informed consent for data collection including analysis of imaging. This imaging sub-study selected those subjects from three sites between 2008 and the start of 2017 with CT scans available at both baseline and one or more follow-up (FU) time points within seven days. The GENISIS study and this analysis were both approved by the institutional review board at the coordinating institution. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Image Analysis
All available non-contrast CT scans from eligible subjects were transferred to a central imaging repository for analysis.12 Image processing followed an established workflow.13 In brief, this included: 1) conversion of DICOM (Digital Imaging and Communications in Medicine) files into NIfTI (Neuroimaging Informatics Technology Initiative) format; 2) image normalization and brain extraction; 3) registration of baseline CT to supratentorial brain mask to exclude regions in the posterior fossa and provide consistent evaluation of changes within the cerebral hemispheres and superior basal cisterns; 4) co-registration of all FU CTs to baseline supratentorial mask; 5) segmentation of CSF regions (sulci and ventricles) from cerebral hemispheres, after exclusion of any visibly infarcted tissue. Cranial volume was the total supratentorial region registered between serial CT scans. CSF segmentation was performed using a fully convolutional neural network (based on the U-Net architecture)14 trained in a similar manner to described previously.15
Brain images were also manually reviewed and subjects excluded if the following were seen: 1) subacute stroke (i.e. clearly demarcated hypodensity) already visible on baseline CT; 2) acute stroke in cerebellum or brainstem. Subjects were also excluded if stroke onset time was unknown, if baseline CT was more than 12 hours from stroke onset, or if final hospital diagnosis was not a stroke. Specific imaging sessions were then excluded if the CT was: 1) beyond seven days from stroke onset; 2) performed after patient underwent hemicraniectomy; 3) unable to be segmented for technical reasons; 4) duplicate imaging session to another existing subject/time point.
Data Variables and Imaging Endpoints
From this automated imaging pipeline, we obtained CSF and cranial volumes at each time point. We also manually measured MLS at the level of the septum pellucidum and delineated region of hypodensity corresponding to infarcted brain tissue (if visible) on FU scans. We categorized each patient into CED grades based on a proposed classification scheme:16 CED-0 – no infarct seen; CED-1 – infarct seen occupying under one-third of hemisphere; CED-2 – infarct occupying over one-third of hemisphere but without midline shift; CED-3 – large hemispheric infarction with midline shift. If FU CT was not available beyond 24 hours then CED grade was coded as unable to be assessed. In those with subsequent CT scans beyond 24-hours, we also defined maximal infarct volume and MLS as the highest measured value. The GENISIS study collected stroke-related variables such as: age, demographics, baseline and FU NIHSS at 24-hours, treatment with tPA, and glucose on admission.
Statistical Modeling
We employed a random coefficients linear mixed effects model to predict average rate of change in CSF volume. This model assumes a linear relationship between CSF and time but allows both intercepts and slopes (hourly rate of change) to vary between patients and fits a separate regression line for each patient. CED grades were evaluated as predictors and their interaction with time was assessed (i.e. did slopes differ between groups). The spaghetti plots of CSF over time demonstrated that rate of change in CSF volume is dependent on time (i.e. slope is not constant in the days after stroke, but steeper in the first 24–48 hours, see Supplemental Figure I). Therefore, we refined our analysis by applying a two-piecewise random coefficients model that allows the separation of an early critical period from a later stable period, each with different slopes17. Aikake’s Information Criteria (AIC) was compared between piecewise models using various knots between 12 and 48 hours to determine the best fitting break point. The estimated rate of change for each segment could then be obtained across groups. All statistical analyses were conducted using a two-sided test at significance level of 0.05 with SAS 9.4 (SAS Institute, Cary, NC).
Biomarker Evaluation
We then selected participants with scans available both at baseline and within 12 hours of the inflection point, determined from piece-wise modeling, and calculated ΔCSF as the change in CSF volume over this critical early time period. We used linear regression to understand baseline variables that influenced extent of ΔCSF. In order to evaluate ΔCSF as a potential predictive biomarker of CED, we tested how increasing rates of CSF displacement predicted the development and degree of midline shift as well as need for hemicraniectomy. This was performed using receiver-operating-curve and logistic regression analyses, adjusting for baseline covariates of edema. We also studied the association of ΔCSF with two clinical outcomes: ΔNIHSS, the change from baseline to 24-hour NIHSS score (positive score indicating improvement), as a marker of early neurologic instability, and functional outcome using 90-day modified Rankin score.
Results
A total of 1799 subjects were enrolled in the GENISIS study from three contributing sites during the study period. Of these, we obtained 2379 CT scans from 1040 participants. After exclusion of those without serial CTs or those with non-hemispheric or subacute strokes, 738 stroke patients could be analyzed (see supplemental Table I for subject and CT-level exclusions). Those included for imaging analysis had more severe strokes deficits on presentation (NIHSS 8 vs. 5) and were more likely to receive tPA (supplemental Table II). We were able to analyze 1850 CT scans in our automated pipeline to obtain cranial and CSF volumes. Registration of cranial volume was successful with excellent correlation between baseline and follow-up cranial volumes (r=0.94, Supplemental Figure II).
Time from stroke onset to baseline CT was a median 1 hour 40 minutes (IQR 1–3 hours) and time to first FU scan was a median of 25 hours (IQR 13–31 hours). 678 patients (92%) had adequate FU imaging to permit CED grading, of whom 91 (13%) had developed midline shift (CED grade 3). This group had higher median baseline NIHSS than the remainder of the cohort (16 for CED-3 vs. 7 for CED 0–2), although it was comparable between large hemispheric strokes with and without edema (CED-2 vs. 3, Table 1). Age did not differ between CED groups but serum glucose was higher in those developing MLS. Etiology of stroke (by TOAST classification) was more often large-artery and cardioembolism in those with large hemispheric infarction (CED-2 and CED-3 vs. 0–1) while, as expected, no small vessel etiology strokes were found in these groups. Baseline CSF volume (as proportion of cranial volume) was correlated with subject age (r=0.69, Supplemental Figure III), explaining almost half of the variance in CSF volume at presentation. Furthermore, baseline CSF volume was lower in the CED-3 subgroup even though their age was comparable (median 132 vs. 161-ml for CED 0–2, p<0.0001).
Table 1:
Description of study cohort by cerebral edema (CED) group
| Variable | Total (n=738) | CED-3 (n=91) | CED-2 (n=47) | CED 0–1 (n=540) | p-value |
|---|---|---|---|---|---|
| Age | 70 (61–79) | 69 (58–77) | 72 (63–84) | 70 (61–79) | 0.14 |
| Gender, male | 375 (51%) | ||||
| Race, non-Caucasian | 83 (11%) | 17 (19%) | 7 (15%) | 41 (8%) | 0.01 |
| NIHSS, baseline | 8 (4–15) | 16 (13–19) | 18 (11–21) | 6 (4–12) | < 0.001 |
| tPA treatment | 538 (73%) | 64 (70%) | 31 (66%) | 389 (72%) | 0.12 |
| thrombectomy | 115 (16%) | 19 (21%) | 12 (26%) | 74 (14%) | 0.03 |
| NIHSS, 24hr | 5 (2–11) | 16 (10–21) | 15 (9–19) | 3 (1–8) | < 0.001 |
| CSF volume, baseline | 154 (109–205) | 132 (94–163) | 163 (111–198) | 160 (118–211) | < 0.001 |
| Glucose (mg/dl) | 121 (106–146) | 127 (115–157) | 123 (104–155) | 119 (104–144) | 0.02 |
| Blood pressure, systolic | 147±36 | 159±31 | 149±27 | 161±30 | 0.04 |
| Undetermined | 219 (30%) | 20 (23%) | 11 (23%) | 171 (31%) | |
| Outcomes | |||||
| MLS at 24-hour | 0 (0–0) | 2.8 (1.0–3.6) | 0 (0–0.2) | 0 (0–0) | < 0.001 |
| Peak MLS† | 0 (0–1.7) | 6.0 (3.8–10.0) | 0 (0–0.9) | 0 (0–0) | < 0.001 |
| Peak infarct volume (ml)† | 27 (0–81) | 238 (150–294) | 125 (90–154) | 2 (0–23) | < 0.001 |
| Hemicraniectomy | 17 (2%) | 17 (19%) | 0 | 0 | < 0.001 |
| In-Hospital Mortality | 32 (4%) | 16 (18%) | 5 (11%) | 6 (1%) | < 0.001 |
| Change in CSF % | 12% (5–23) | 36% (24–45) | 27% (18–40) | 10% (4–17) | < 0.001 |
| Rate of CSF change (ml/hr) | 1.0 (0.4–2.2) | 2.4 (1.4–3.4) | 2.0 (1.1–2.9) | 0.8 (0.3–1.4) | < 0.001 |
| Mrs 0–2 at 90d (n=670) | 392 (59%) | 19/82 (23%) | 11/40 (28%) | 335/499 (67%) | < 0.001 |
Continuous data are presented as medians (IQR); Kruskal-Wallis comparison for medians
Only for those with subsequent CTs available beyond 24-hours (n=211)
Piecewise linear mixed effects model
Spaghetti plots of serial CSF volumes are shown for all subjects separated by CED grades (supplemental Figure I). The best fitting piecewise model with the lowest AIC in our preliminary cohort of scans from our local site had a break point at 24 hours. Therefore, we constructed a piecewise mixed model with knot at 24 hours to model the full data set from all three sites, separating those with CED-3 from other groups to understand the trajectory of those developing significant cerebral edema. Slope of the reduction in CSF was much greater in the first 24-hours, while it was relatively flat beyond this time point. Furthermore, slope was significantly greater in the first 24-hours in those destined for edema with MLS compared to the other stroke categories (Figure 1), adjusting for age and baseline NIHSS. The estimated slope for CED-3 was −2.3 ml/hour compared to −0.96 for all other groups combined (p<0.0001) and −1.9 ml/hour for CED 2 (p=0.09). We also observed steeper CSF trajectories in those presenting with NIHSS above 12 (−1.8 ml/hour) compared to those with lower baseline stroke severities (−0.8 ml/hour, p<0.0001).
Figure 1:
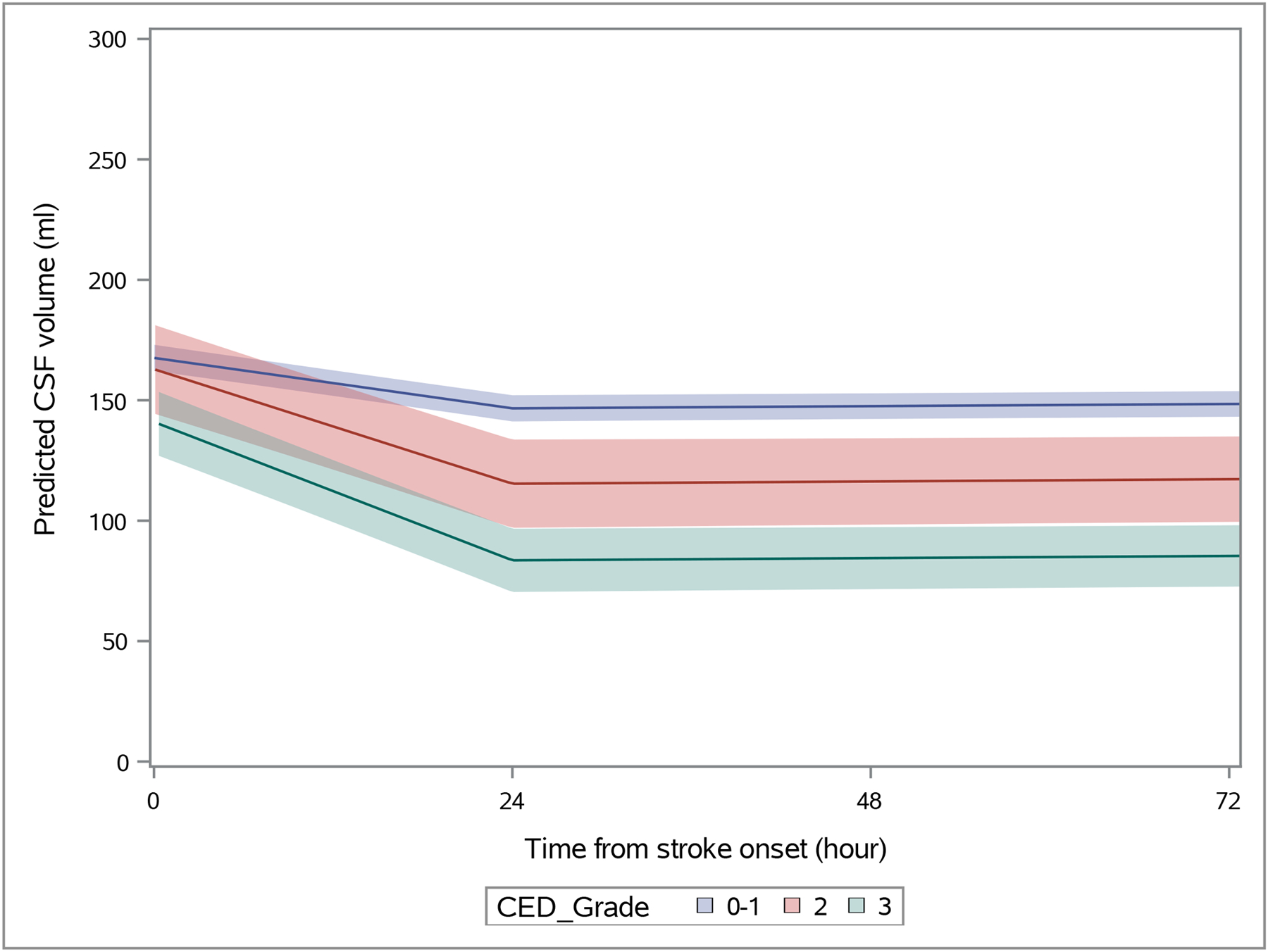
Predicted CSF volume over time by CED groups, modeled using piece-wise linear mixed effects model with break-point at 24-hours
Change in CSF volume within 24 hours
The change in CSF volume between baseline and 24-hours (ΔCSF24hr) was explored as a quantitative biomarker of early cerebral edema in the 474 subjects with scans available at both baseline and within 12-hours of 24-hours (median time between scans: 24 hours, IQR 20–25). Absolute and percent ΔCSF24hr exhibited relatively broad distributions with median ΔCSF of 21 ml (IQR 9 to 36-ml) and %ΔCSF24hr of 13% (IQR 6 to 25%, Supplemental Figure IV). In comparison, midline shift was greater than zero in only 74 (15%) patients at 24-hours and skewed to values below 3-mm even in those with measurable MLS.
Baseline NIHSS accounted for 17% of the variance in %ΔCSF24hr. Age and serum glucose on admission had small but significant contributions to %ΔCSF24hr (2% and 1%, respectively, p=0.0007 and p=0.01) while sex, blood pressure, and tPA treatment did not. However, when adjusting for baseline CSF volume, only baseline NIHSS and glucose remained significantly associated with %ΔCSF24hr in multivariate regression. Furthermore, %ΔCSF24hr was correlated not only with MLS measured on the 24-hour scan (r=0.49) but also maximal MLS measured on subsequent scans, performed at a median of 90 hours after stroke (r=0.52, both p<0.0001). %ΔCSF24hr was able to explain over one-quarter of the variation in maximal MLS (adjusted R2 27%) while baseline NIHSS accounted for only 7%.
The risk of CED increased as %ΔCSF24hr increased by logistic regression (Figure 2) and was significantly greater in those with CED 3 (36%) compared with other edema categories (CED 0–1 11%, p<0.001, CED-2 27%, p=0.02, Supplemental Figure V). Odds of developing MLS almost doubled for every 10% reduction in CSF within 24 hours, adjusting for age, glucose and NIHSS (adjusted OR 1.76, 95% CI: 1.46–2.14, p<0.0001). The area-under-curve (AUC) for %ΔCSF24hr to predict CED was 0.83 (95% CI 0.76–0.89) with a cut-off of 24% providing 77% sensitivity and 83% specificity. In addition, risk for hemicraniectomy increased with every 10% ΔCSF (aOR 1.86, 95% CI 1.35–2.64, p=0.0002), with AUC of 0.91 (0.84–0.98) and optimal cutoff of 34% (86% sensitivity, 87% specificity).
Figure 2:
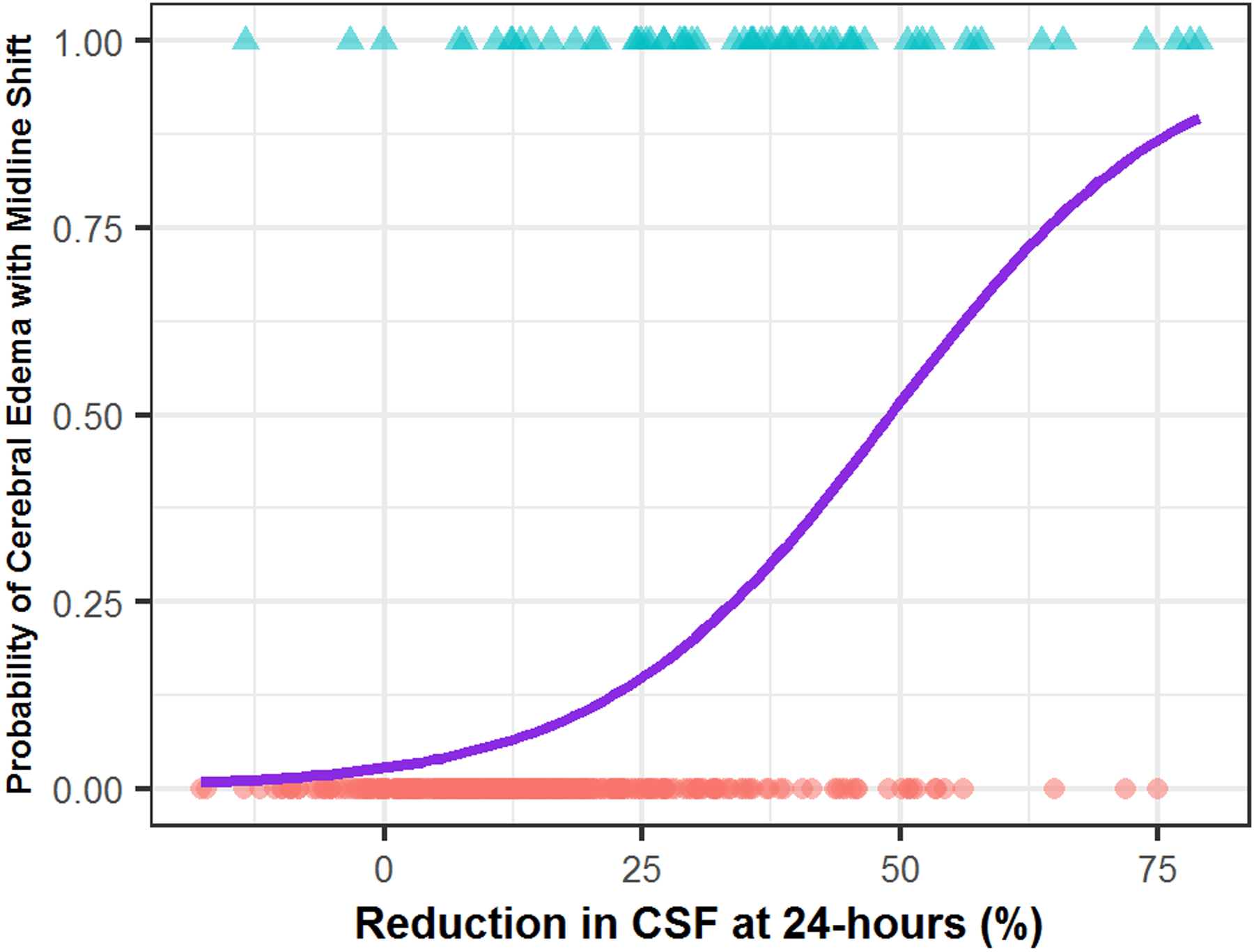
Risk of developing cerebral edema with midline shift (CED-3) as ΔCSF within 24-hours increases. The logistic regression curve represents the estimated probability depending on percent ΔCSF.
Median ΔNIHSS was +3 for those with CED-0 or 1, +2 for CED-2 and +1 for CED-3. Adjusting for baseline NIHSS, %ΔCSF24hr was strongly associated with worse ΔNIHSS (estimate of −1.6 points for each 10% CSF reduction, p<0.0001), together explaining 28% of the variance in this endpoint of early neurologic instability. Functional outcome at 90-days was available in 439 (93%) of these subjects. Greater reduction in CSF within 24-hours was an independent predictor of poor outcome (mRS 3–6), adjusting for age and baseline NIHSS (OR 1.34, 95% CI 1.15–1.56 per 10% reduction from baseline CSF volume, p=0.0001).
Discussion
Although the clinical consequences of cerebral edema are usually seen between two and five days after stroke,1, 4 the biologic processes resulting in brain swelling start within hours of stroke onset. A combination of cytotoxic, ionic, and vasogenic edema contribute to an increase in brain volume after cerebral ischemia.18 Furthermore, this edema likely occurs in a majority of hemispheric strokes, not just those with malignant edema where compensation is exhausted and midline shift develops. Brain edema volume (measured using paired MRI at baseline and at three to five days) has recently been shown to be associated with poor outcome, adjusting for baseline NIHSS, DWI volume, and glucose.10 In that cohort, measurable swelling was seen in 67% of follow-up MRI scans and a threshold of 11-ml best distinguished those with good versus poor outcomes (AUC 0.8). However, serial MRI is not feasible in large scale studies and so this biomarker of edema is limited in its broad applicability.
While CT is readily available and often performed serially in routine care, there are few accurate quantifiable means of estimating edema with this modality. Midline shift has long been established as a marker of severe edema and is known to correlate with clinical deterioration.7 However, MLS only captures the most severe end of the edema spectrum (i.e. malignant edema) and is also only a later reflection of swelling, with the majority of MLS occurring beyond 24-hours.19 It is not suited to capture the broad spectrum of edema as a continuous biologic process, as most hemispheric strokes with even moderate degrees of edema will still have no MLS measurable. MLS develops once compensatory mechanisms for brain volume increase have been exhausted, chief amongst these is the displacement of CSF out of the cranial vault.
We have proposed that reduction in CSF volume would act as a quantitative biomarker of brain volume increase and demonstrated that, in large hemispheric strokes, degree of CSF displacement correlates with MLS.11 Other groups have also suggested that CSF volumetric analysis could provide insights into edema in other disease states.20, 21 Reduction in CSF volume from baseline to subacute (5 day) imaging using MRI provided a quantitative estimate of edema volume in another study.22 The median ΔCSF was 13-ml in their small cohort of 20 stroke patients, which is comparable to the 21-ml we observed in our large heterogenous cohort and suggests that our CT-based metric provides adequate sensitivity to detect meaningful volumetric shifts.
Importantly, we have demonstrated that the majority of CSF displacement occurs within 24-hours of stroke onset and is greater and more rapid in those destined to develop edema with midline shift (although there is a measurable reduction in CSF across a broad range of stroke severities). The magnitude of CSF reduction was a biomarker of edema development, assessed using the CED grading schema.16 Use of CSF volumetrics is important because it can be assessed on non-contrast CT at serial early time points after stroke, without waiting for midline shift to develop or for clear hypodensity to appear on CT. An alternate CT-based biomarker, lesional water uptake, has also shown promise in quantifying edema on CT scans (using density of tissue within the infarct lesion as a surrogate of water uptake) and distinguishing those who may develop a malignant trajectory.23 A recent analysis measured water uptake on serial CT scans from large hemispheric stroke patients and confirmed that it increased rapidly within the first 24-hours after stroke, corroborating our own findings with CSF-based measures of edema.24
We believe that CSF-based volumetric evaluation of edema captures a biologically relevant biomarker of the volume increase due to edema, rather than water uptake within the stroke itself. It is a quantitative trait that captures a broad spectrum of severities at various time points, being measurable even before a lesion is visible. We demonstrate that ΔCSF (measurable on FU CT around 24 hours after stroke) can serve as an explanatory biomarker (i.e. biologic endophenotype) of cerebral edema, making this response to brain injury accessible for large-scale studies evaluating efficacy of therapeutic interventions to reduce edema as well as genetic studies seeking to uncover markers and biologic pathways most relevant to edema formation. Similar quantitative endophenotypes have proven useful in uncovering novel targets relevant to the biology of neurodegenerative diseases.25
We have also presented preliminary data that ΔCSF can be used as a predictor of development of midline shift and need for hemicraniectomy (i.e. malignant edema). The risk of MLS almost doubles for every ten percent reduction in CSF volume at 24-hours. Interestingly, the baseline CSF volume was also lower in these patients (independent of age), suggesting (as two other studies have) that this compartment serves as a crucial intracranial reserve for edema development.26, 27. This aligns with Harvey Cushing’s view of the CSF as the brain’s critical “third circulation.”28 Finally, ΔCSF24hr was associated with both early (ΔNIHSS) and late (modified Rankin scale) clinical endpoints, independent of stroke severity, suggesting that it is capturing a clinically relevant edema phenotype.
This study has several limitations and caveats in its design. We recruited subjects from a large stroke cohort but had to limit our analysis to those patients who had at least two CT scans performed as part of clinical care. This likely biased our sample to more severe strokes with a higher incidence of edema and poor outcomes than a truly unselected cohort. On the other hand, we did not restrict our analysis to only large hemispheric strokes, wanting to understand how edema develops in a broad stroke cohort. Further focused studies should evaluate ΔCSF in LHI and validate its ability to predict deterioration and even need for surgery as early as 12–24 hours after onset without the need for MRI.9 We limited evaluation of CSF displacement to the cerebral hemispheres in order to ensure consistency of brain regions being compared across time points and focus on where we believed most stroke-related edema would develop. However, we may have excluded some of the basal cisterns (where effacement may occur with herniation) and so intend to specifically evaluate volumetric changes within these regions in future studies. In addition, our CSF segmentation, while automated, still requires some manual quality control and inspection of results. We are now in the process of fully automating the processing pipeline so that CSF volumes can be obtained on thousands of patients in a much quicker time-frame. This will facilitate large-scale genetic studies of edema that can attempt to uncover novel targets to mitigate this important stroke complication.
Supplementary Material
Sources of Funding:
JML received funding from NIH (R01NS085419); RD received funding from NIH (K23NS099440); LH received funding from NIH K23NS099487 and the American Heart Association Mentored Clinical and Population Research award
Disclosures: JML received funding unrelated to this work from Biogen and Regenera; LH received speaker fees unrelated to this work from Genentech
References
- 1.Silver FL, Norris JW, Lewis aJ, Hachinski VC. Early mortality following stroke: A prospective review. Stroke. 1984;15:492–496 [DOI] [PubMed] [Google Scholar]
- 2.Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology. 1995;45:1286–1290 [DOI] [PubMed] [Google Scholar]
- 3.Kasner SE, Demchuk aM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32:2117–2123 [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Archives of neurology. 1996;53:309–315 [DOI] [PubMed] [Google Scholar]
- 5.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurology. 2007;6:215–222 [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MF, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: A multicenter review. Crit Care Med. 2003;31:272–277 [DOI] [PubMed] [Google Scholar]
- 7.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–958 [DOI] [PubMed] [Google Scholar]
- 8.Gerriets T, Stolz E, Modrau B, Fiss I, Seidel G, Kaps M. Sonographic monitoring of midline shift in hemispheric infarctions. Neurology. 1999;52:45–49 [DOI] [PubMed] [Google Scholar]
- 9.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt F-G, Köhrmann M, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Annals of Neurology. 2010;68:435–445 [DOI] [PubMed] [Google Scholar]
- 10.Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar R, Yuan K, Kulik T, Chen Y, Heitsch L, An H, et al. Csf volumetric analysis for quantification of cerebral edema after hemispheric infarction. Neurocrit Care. 2016;24:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurney J, Olsen T, Flavin J, Ramaratnam M, Archie K, Ransford J, et al. The washington university central neuroimaging data archive. Neuroimage. 2017;144:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhar R, Chen Y, An H, Lee JM. Application of machine learning to automated analysis of cerebral edema in large cohorts of ischemic stroke patients. Front Neurol. 2018;9:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronneberger O, Fisher P, Brox T. U-net: Convolutional neural networks for biomedical image segmentation In: Navab N, Hornegger J, Well W, Frangi A, eds. Medical image computing and computer-assisted intervention - miccai 2015. Lecture notes in computer science, vol 9351 Springer; 2015. [Google Scholar]
- 15.Chen Y, Dhar R, Heitsch L, Ford A, Fernandez-Cadenas I, Carrera C, et al. Automated quantification of cerebral edema following hemispheric infarction: Application of a machine-learning algorithm to evaluate csf shifts on serial head cts. Neuroimage Clin. 2016;12:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strbian D, Meretoja A, Putaala J, Kaste M, Tatlisumak T. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. International Journal of Stroke. 2013;8:529–534 [DOI] [PubMed] [Google Scholar]
- 17.Naumova EN, Must A, Laird NM. Tutorial in biostatistics: Evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–1341 [DOI] [PubMed] [Google Scholar]
- 18.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurology. 2007;6:258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerriets T, Stolz E, Konig S, Babacan S, Fiss I, Jauss M, et al. Sonographic monitoring of midline shift in space-occupying stroke: An early outcome predictor. Stroke. 2001;32:442–447 [DOI] [PubMed] [Google Scholar]
- 20.Liotta EM, Lizza BD, Romanova AL, Guth JC, Berman MD, Carroll TJ, et al. 23.4% saline decreases brain tissue volume in severe hepatic encephalopathy as assessed by a quantitative ct marker. Crit Care Med. 2016;44:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi HA, Bajgur SS, Jones WH, Savarraj JP, Ko SB, Edwards NJ, et al. Quantification of cerebral edema after subarachnoid hemorrhage. Neurocrit Care. 2016;25:64–70 [DOI] [PubMed] [Google Scholar]
- 22.Tipirneni-Sajja A, Christensen S, Straka M, Inoue M, Lansberg MG, Mlynash M, et al. Prediction of final infarct volume on subacute mri by quantifying cerebral edema in ischemic stroke. J Cereb Blood Flow Metab. 2017;37:3077–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broocks G, Flottmann F, Scheibel A, Aigner A, Faizy TD, Hanning U, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke. 2018;49:1906–1912 [DOI] [PubMed] [Google Scholar]
- 24.Vorasayan P, Bevers MB, Beslow LA, Sze G, Molyneaux BJ, Hinson HE, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. [published online September 20, 2019]. Stroke. 2019. https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.119.026036. Accessed September 28, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruchaga C, Kauwe JSK, Harari O, Jin SC, Cai Y, Karch CM, et al. Gwas of cerebrospinal fluid tau levels identifies risk variants for alzheimer’s disease. Neuron. 2013;78:256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minnerup J, Wersching H, Ringelstein EB, Heindel W, Niederstadt T, Schilling M, et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke. 2011;42:3403–3409 [DOI] [PubMed] [Google Scholar]
- 27.Kauw F, Bennink E, de Jong H, Kappelle LJ, Horsch AD, Velthuis BK, et al. Intracranial cerebrospinal fluid volume as a predictor of malignant middle cerebral artery infarction. [published online May 16, 2019]. Stroke. 2019. https://www.ahajournals.org/doi/10.1161/STROKEAHA.119.024882. Accessed September 21, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cushing H The third circulation in studies in intracranial physiology and surgery. 1926:1–51
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


