Abstract
Background
Acute respiratory infections, mostly in the form of pneumonia, are the leading causes of death in children under five years of age in low‐income countries. Some clinical trials have demonstrated that vitamin A supplementation reduces the severity of respiratory infections and mortality in children with measles.
Objectives
To determine whether adjunctive vitamin A is effective in children diagnosed with non‐measles pneumonia.
Search methods
We searched The Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL 2010, issue 3) which contains the Acute Respiratory Infections Group's Specialised Regsiter, MEDLINE (1996 to July week 3, 2010), EMBASE (1990 to August 2010), LILACS (1985 to August 2010), CINAHL (1990 to August 2010), Biological Abstracts (1990 to August 2010), Current Contents (1990 to August 2010) and the Chinese Biomedicine Database (CBM) (1994 to June 2010).
Selection criteria
Only parallel‐arm, randomized controlled trials (RCTs) and quasi‐RCTs, in which children (younger than 15 years of age) with non‐measles pneumonia were treated with adjunctive vitamin A, were included.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality. Study authors were contacted for additional information.
Main results
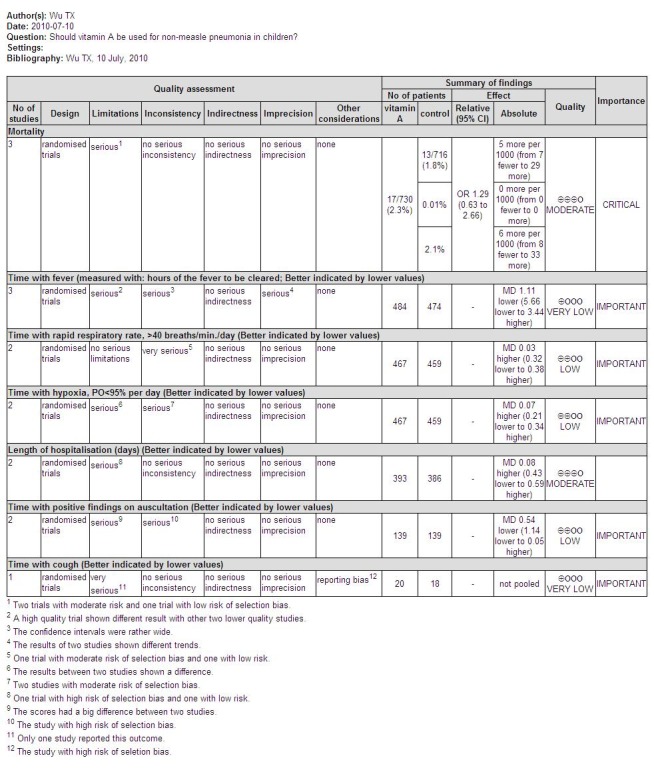
Six trials involving 1740 children were included. There was no significant reduction in mortality associated with pneumonia in children treated with vitamin A compared to those who were not (pooled odds ratio (OR) 1.29; 95% confidence interval (CI) 0.63 to 2.66). Also, there was no statistically significant difference in duration of hospital stay (mean difference (MD) 0.08; 95% CI ‐0.43 to 0.59). Disease severity after supplementary high‐dose vitamin A was significantly worse compared with placebo. However, low‐dose vitamin A significantly reduced the recurrence rate of bronchopneumonia (OR 0.12; 95% CI 0.03 to 0.46). Moderate vitamin A significantly reduced the time to remission of signs in children with normal serum retinol (> 200 ug/L).
Authors' conclusions
The evidence does not suggest a significant reduction in mortality, measures of morbidity, nor an effect on the clinical course of pneumonia with vitamin A adjunctive treatment in children with non‐measles pneumonia. However, not all studies measured all outcomes, which limited the number of studies that could be incorporated into the meta‐analyses, so that there may have been a lack of statistical power to detect statistically significant differences.
Plain language summary
Vitamin A for non‐measles pneumonia in children
Acute respiratory infections, mostly in the form of pneumonia, are the leading cause of death in children under five years of age living in low‐income countries. Vitamin A supplementation has been found to reduce mortality and the severity of respiratory infections in children with measles. This updated review was undertaken to assess the effectiveness of vitamin A adjunctive therapy in children with non‐measles respiratory infections, particularly pneumonia.
We found six trials (1740 participants) that used vitamin A adjunctive therapy in children with non‐measles pneumonia. There was no significant reduction in mortality or duration of hospital stay. Supplementary high‐dose vitamin A may result in a worsening of the disease, and low‐dose vitamin A significantly reduces the recurrence of bronchopneumonia. Moderate‐dose vitamin A significantly reduces the time to remission of signs in children with normal serum retinol. The possible reason of the lack of benefit of vitamin A in non‐measles pneumonia is that the effects of vitamin A may be disease‐specific, with vitamin A only being effective when pneumonia is complicated with measles. Further high‐quality research is required.
Background
Description of the condition
Acute respiratory tract infections (ARTIs) and vitamin A deficiency are important public health problems in many low‐income countries. Vitamin A deficiency is associated with impaired humoral and cellular immune function, keratinisation of the respiratory epithelium and decreased mucus secretion, which weaken barriers to infection (Ross 1996). In low‐income countries, ARTIs, mostly in the form of pneumonia, are the leading cause of death in children under five years of age. The incidence of clinical pneumonia in low‐income countries is estimated at 0.29 episodes per child per year. This equates to an annual incidence of 150.7 million new cases, 11 to 20 million (7% to 13%) of which are severe enough to require hospital admission. No comparable data are available for high‐income countries. However, from large population‐based studies, the incidence of community‐acquired pneumonia among children less than five years of age is approximately 0.026 episodes per child per year (Rundan 2004). Pneumonia is associated with and causes about 3.8 million childhood deaths annually; 30.3% of these are in children under the age of five (Kirkwood 1995).
Description of the intervention
Vitamin A functions in a very different way in human metabolism. It is needed by the retina of the eye's specific metabolite, the light‐absorbing molecule retinal; and as an irreversibly oxidized form retinoic acid, which is an important hormone‐like growth factor for epithelial and other cells. Lack of vitamin A will cause some severe problems such as night blindness, xerophthalmia and complete blindness. Vitamin A deficiency also contributes to maternal mortality and other poor outcomes in pregnancy and lactation (WHO 2010).
How the intervention might work
Community‐based clinical trials have been conducted in order to determine whether periodic high‐dose vitamin A supplementation reduces the incidence, severity or both of ARTIs in children. The association between vitamin A deficiency and child mortality was first observed in the 1930s when vitamin A supplementation significantly reduced mortality among measles patients (Ellison 1932). Two meta‐analyses (Fawzi 1993; Glasziou 1993) examined the relationship between vitamin A supplementation and infectious diseases. Glasziou (Glasziou 1993) reported that vitamin A reduced all‐cause mortality by one third in children in low‐income countries. However, the reduction in deaths from respiratory disease was seen only in the measles studies. Fawzi (Fawzi 1993) also reported that supplementation was protective against overall mortality in community‐based studies (odds ratio (OR) 0.70) and highly protective against mortality in hospitalised patients with measles (OR 0.39).
From the current evidence it appears that vitamin A supplementation reduces the severity of respiratory infection and other systemic complications of measles. However, the association between vitamin A and non‐measles ARTIs is unclear. The World Health Organization (WHO) Programme for the Control of Acute Respiratory Infections published a meta‐analysis to assess the impact of supplementation on pneumonia morbidity and mortality (VAPWG 1995). They reported no consistent overall protective or detrimental effect on pneumonia‐specific mortality and no effect on the incidence or the prevalence of pneumonia.
Why it is important to do this review
Hospital‐based clinical trials examining the effectiveness of high‐dose vitamin A, administered during an acute episode of non‐measles ARTI, in reducing morbidity or mortality, have been performed. However, no meta‐analyses have been carried out. Given the apparent effectiveness of vitamin A in reducing mortality in hospitalised patients with measles, and the inexpensiveness of the intervention, clarification of the association between vitamin A and non‐measles ARTIs is of some importance.
Objectives
The objective of this review was to assess the effectiveness and safety of vitamin A in children with diagnosed non‐measles pneumonia.
Methods
Criteria for considering studies for this review
Types of studies
We considered only parallel‐arm, randomized controlled trials (RCTs) and quasi‐RCTs in which children with diagnosed non‐measles pneumonia were treated with vitamin A (at the time of confirmed disease) for inclusion in this review. We did not consider studies that examined the effectiveness of vitamin A in preventing episodes of non‐measles pneumonia or studies including patients with measles.
Types of participants
We included children of either gender and under 15 years of age with non‐specific pneumonia that was uncomplicated by measles. The definition of pneumonia was a clinical case definition, radiological confirmation or both.
Types of interventions
Vitamin A plus standard medical treatment versus standard medical treatment with or without placebo.
Types of outcome measures
Primary outcomes
Mortality.
Secondary outcomes
Signs of pneumonia (for example, fever, tachypnoea, dyspnoea, chest X‐ray findings).
Clinical severity (for example, oxygen saturation, requirement for mechanical ventilation or need for supplemental oxygen, crepitations, bronchial breathing, duration of hospitalization, failure of first line treatment or change of antibiotic required).
Adverse events associated with the intake of vitamin A such as diarrhea and signs of toxicity, including vomiting and desquamation.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL 2010, issue 3) which contains the Acute Respiratory Infections Group's Specialised Regsiter, MEDLINE (1996 to July week 3, 2010), EMBASE (1990 to August 2010), LILACS (1985 to August 2010), CINAHL (1990 to August 2010), Biological Abstracts (1990 to August 2010), Current Contents (1990 to August 2010) and the Chinese Biomedicine Database (CBM) (1994 to June 2010).
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE:sensitivity‐ and precision‐ maximizing version (2008 revision); Ovid format (Lefebvre 2009). The search strategy was modified to search Embase (see Appendix 1), LILACS (see Appendix 2), CINAHL (see Appendix 3), Biological Abstracts (see Appendix 4) and Current Contents (see Appendix 5), The search strategy for the Chinese databases is presented in Figure 1. See Appendix 6 for details of original search.
1.

Search strategy in the Chinese databases.
MEDLINE (OVID) 1 exp Vitamin A/ ) 2 (vitamin a or retinol).tw,nm. 3 Dietary Supplements/ 4 or/1‐3 5 exp Pneumonia/ 6 pneumon*.tw. 7 Respiratory Tract Infections/ 8 (respiratory adj2 (infection* or disease* or acute)).tw.
In addition to electronic databases, we searched reference lists. There were no language or publication restrictions.
Searching other resources
We searched the WHO ICTRP for ongoing studies. This site contains records provided by nine primary registries (http://apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
One review author (JN) collated study citations into a single library, excluded duplicates and articles unlikely to be relevant by scanning the abstracts. We retrieved citations without abstracts in full text. Two review authors (JN, TW) independently reviewed a sample of the selection process. Two review authors (JN, WT) independently retrieved relevant articles in full text and applied the inclusion criteria.
Data extraction and management
Two review authors (JN, WJ) independently extracted data from included studies using standardized forms. They obtained a copy of the original article from Dr. Quinlan and telephoned the original authors of Chinese articles. All data were extracted from published papers and differences were resolved by discussion among the review authors.
Assessment of risk of bias in included studies
We assessed study quality using an adaptation of the method specified in the Cochrane Handbook for Systematic Reviews of Interventions (Julian 2009). The following characteristics were assessed.
Adequacy of the randomisation process
Adequate sequence generation was reported using one of following approaches: random number tables, computer‐generated random numbers, coin tossing, or shuffling.
Yes: with low risk of selection bias. Unclear: did not specify one of the adequate methods outlined above but only mentioned 'random' in which situation moderate risk of selection bias existed. No: other methods of allocation that appeared to be biased with high risk.
Adequacy of the allocation concealment process
Adequate measures to conceal allocations such as central randomisation; serially numbered, opaque, sealed envelopes; or another description that contained convincing elements of concealment. Yes: with low risk of selection bias. Unclear: did not report an allocation concealment approach at all in which situation moderate risk of selection bias existed. No: inadequately concealed allocation that reported an approach that did not fall into one of the categories described above, and did not conceal allocation. In which situation a high risk of selection bias existed.
Level of masking
Low risk of both performance and detection bias: the healthcare providers and outcome assessors were masked to know what interventions were received in each participants. Moderate risk of both performance and detection bias: single blinding used only. High risk of both performance and detection bias: blinding not been used.
Free of other bias
Yes: it was specified that without any potential bias, for example, conflict of interest. No: there was conflict of interest or other risk of bias.
Measures of treatment effect
Dichotomous data were expressed as an odds ratio (OR) with 95% confidence intervals (CI). We converted continuous data to mean differences (MD). All meta‐analyses were based on a fixed‐effect model.
Unit of analysis issues
Only individual participants data were included and assessed.
Dealing with missing data
Original investigators of Chinese studies were contacted by telephone to request missing data include randomisation method and allocation concealment.
Incomplete outcome data bias were assessed depending on the percentage of missing data: Low risk: only few withdraw or losses to follow up occurred. Moderate risk: exclusions were about 10%. High risk: exclusions of at least 10%, or wide differences in exclusions between groups.
Assessment of heterogeneity
We were able to perform only limited pooled analyses. We presented a statistical summary of treatment effects only in the absence of significant clinical or statistical heterogeneity. We tested heterogeneity using the Cochran Q statistic (Cochran 1954) with significance at P = < 0.10 (Boissel 1989; Fleiss 1986).
Assessment of reporting biases
We planed to assess publication bias by using funnel plots. However, due to the small number of included studies we did not perform this assessment. Free of selective reporting was assessed depends on whether all the outcomes were reported or not:
Yes: all outcomes were addressed and reported. No: some of outcomes appeared in protocol but not reported in the article.
Data synthesis
We statistically combined data when it appropriate. Some data were analyzed separately using Review Manager 5 software (RevMan 2008). We expressed the effects as OR with 95% confidence intervals (CI) for dichotomous data. However, as mortality was an unlikely event, we used Peto OR with 95% CI. For continuous outcomes mean differences (MD) with 95% CI were calculated.
Subgroup analysis and investigation of heterogeneity
We intend to explore the following potential sources of heterogeneity using subgroup analyses or meta‐regression. However there was insufficient data. 1. Different doses (low, medium, high). 2. Duration of treatment.
Sensitivity analysis
We tested the robust of evidence by sensitivity analysis when pooling analysis takes place.
1. Repeating the analysis excluding unpublished studies (if any). 2. Repeating the analysis excluding poor quality studies, as specified above. 3. Comparing the results of fixed‐effect models to random‐effects models. Robust evidence should not be reversed by changing these models.
Results
Description of studies
Results of the search
After full‐text examination of the search results, we identified 72 papers. No new trials were identified for inclusion or exclusion in our updated searches.
Included studies
Although we failed to contact the authors of two Chinese studies, we identified their trials as a randomized controlled trial (RCT) (Liu 1997) and a quasi‐RCT (Zhang 1999) based on the information provided in the studies. One study (Fawzi 1998) conducted in Africa, and three studies conducted in South America (Nacul 1997; Rodriguez 2005; Stephensen 1998) were identified as RCTs. Finally, a total of six studies were included in the review (Fawzi 1998; Liu 1997; Nacul 1997; Rodriguez 2005; Stephensen 1998; Zhang 1999).
Participants
A total of 1740 infants and children participated in the six studies. One study (Fawzi 1998) included 687 participants; three studies (Nacul 1997; Rodriguez 2005; Stephensen 1998) included 854 participants; and two studies (Liu 1997; Zhang 1999) conducted in China included 119 participants, of which 80 children had recurrent bronchopneumonia. All participants were hospitalised children with ages ranging from one month to 14 years.
Interventions
Two studies (Fawzi 1998; Nacul 1997) administered 200,000 IU of vitamin A to children aged one year and older, and 100,000 IU to infants under the age of one year, daily for two days. One study (Rodriguez 2005) administered 100,000 IU of vitamin A to children aged one year and older, and 50,000 IU to infants under the age of one year. One study (Liu 1997) administered 150,000 IU of vitamin A. One study (Stephensen 1998) used a different dosing scheme: children younger than one year old received 100,000 IU of vitamin A on admission to the study and 50,000 IU on the second day of hospitalization; children one year of age or more received 200,000 IU on the first day and 100,000 IU on the second day. The final study (Zhang 1999) administered 10,000 IU twice daily for the first six days; following this, 1500 IU/day was administered for the next 20 days. All of the studies used a placebo control.
Outcomes
Three studies (Fawzi 1998; Nacul 1997; Rodriguez 2005) reported mortality, two studies (Fawzi 1998; Stephensen 1998) reported the duration of hospitalization, and one study (Nacul 1997) reported a change in antibiotic use. One study (Stephensen 1998) reported clinical severity scores with eight individual components that included, for example, oxygen saturation, requirement of supplemental oxygen and signs of pneumonia from chest X‐ray results. Three studies (Fawzi 1998; Nacul 1997; Stephensen 1998) reported the frequency of children requiring supplemental oxygen. We contacted the trial authors for further information but as yet no response has been forthcoming. One study (Nacul 1997) reported on adverse outcomes including vomiting, diarrhea, irritability and bulging fontanelle. Two studies (Liu 1997; Rodriguez 2005) reported variable outcomes and we only extracted data for outcomes which matched the inclusion criteria of this review: time to resolution of fever and cough, time until positive auscultation and positive chest X‐ray results. The study by Zhang (Zhang 1999) reported on a lack of improvement of bronchopneumonia at short‐term follow up and the recurrence rate of bronchopneumonia at long‐term follow up.
Excluded studies
One trial author (Quinlan 1996) kindly sent us her paper. After careful consideration it was excluded. Ten Chinese studies seemed to meet the inclusion criteria and we contacted eight authors by telephone. We discovered that the allocation methods they had used were not actually randomized, thus these studies were excluded.
Risk of bias in included studies
One study (Rodriguez 2005) was identified as low risk of bias and four studies (Fawzi 1998; Liu 1997; Nacul 1997; Stephensen 1998) as moderate risk. One study (Zhang 1999) was identified as high risk of bias. An overview of the study quality can be found in the Characteristics of included studies table and Figure 2 and Figure 3.
2.
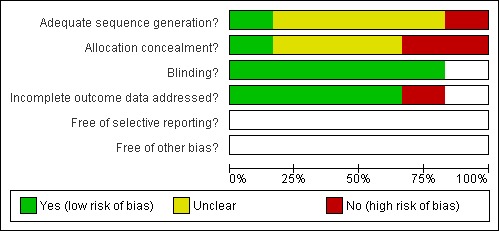
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
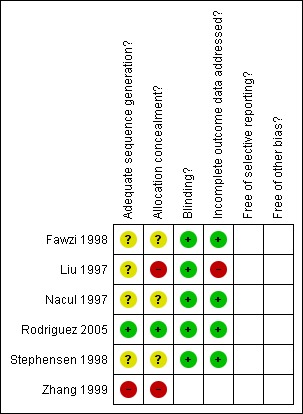
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Four studies (Fawzi 1998; Nacul 1997; Rodriguez 2005; Stephensen 1998) used block randomisation design and two studies (Liu 1997; Zhang 1999) use individual randomisation. All studies except for Rodriguez 2005 did not mention the randomisation method and allocation concealment in detail. Four studies (Fawzi 1998; Liu 1997; Nacul 1997; Stephensen 1998) were judged to have a moderate risk of selection bias and one study (Zhang 1999) had a high risk of selection bias.
Rodriguez 2005 used block randomisation to allocate the participants and the sequence was generated by using tables of random numbers and was converted into a sequence of envelopes that contained the regimen assignments for all children in the trial. The Ethical Committee of the Corporacion Ecuatoriana de Biotecnología held the blinded randomisation codes in a secure place. The study code was not broken until all the data were entered and the initial analyses performed. It was judged to have a low risk of selection bias.
Blinding
Five studies (Fawzi 1998; Liu 1997; Nacul 1997; Rodriguez 2005; Stephensen 1998) performed double‐blinding by masking the containers, they were judged to have a low risk of both performance and detection bias. One study (Zhang 1999) did not perform blinding and was thus judged to have a high risk of performance and detection bias.
Incomplete outcome data
Four studies (Fawzi 1998; Nacul 1997; Rodriguez 2005; Stephensen 1998) addressed the number of withdraws or loss of follow‐up. In the study by Rodriguez 2005, 48 children (16.7%) were lost to follow‐up during the course of the study. There was a moderate risk of incomplete outcome data bias. Two studies (Liu 1997; Zhang 1999) did not address the incomplete outcome data.
Selective reporting
All studies were judged as unclear for selective reporting.
Other potential sources of bias
All studies were judged as unclear for other potential sources of bias.
Effects of interventions
The outcome measures included in the meta‐analysis were 1. mortality; 2. time period with signs of pneumonia, indicators of disease severity including duration of hospitalization, time with fever, time with rapid respiratory rate, time with hypoxia, time with positive findings on auscultation, time with cough, time with positive at X‐ray findings, requirement for mechanical ventilation, failure of first line antibiotic treatment, slight improvement at short‐term follow up, and recurrent rate of bronchopneumonia (no improvement) at long‐term follow up; 3. adverse events including vomiting, diarrhea and bulging fontanelles.
We did not perform a sensitivity analysis as there were no low quality studies included in a pooled analysis.
Primary outcomes
Mortality
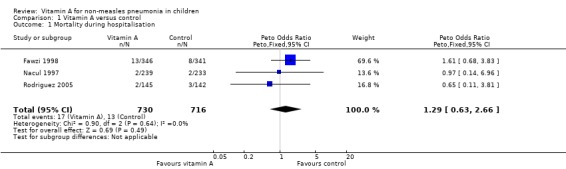
Pooled analysis across three studies (Fawzi 1998; Nacul 1997; Rodriguez 2005) showed no statistically significant difference in deaths during hospitalization (OR 1.29; 95% CI 0.63 to 2.66) between the vitamin A and placebo groups. Two studies used the same high dose of vitamin A (Fawzi 1998; Nacul 1997); the other study (Rodriguez 2005) used a moderate dose of vitamin A. There was no statistically significant heterogeneity (P = 0.64, I2 statistic = 0%). (Figure 4).
4.
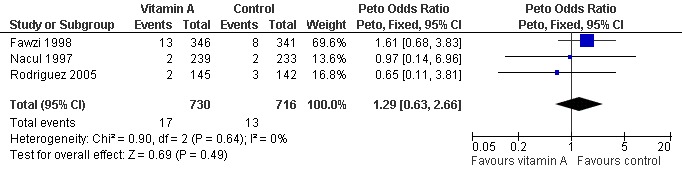
Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.1 Mortality during hospitalisation.
Secondary outcomes
Time with fever
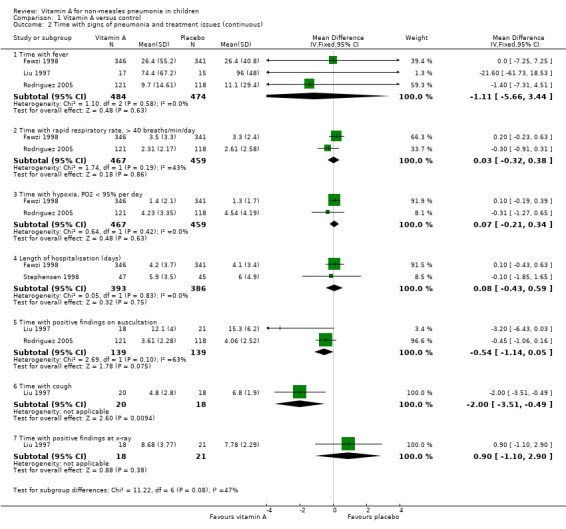
Pooled analysis across three studies (Fawzi 1998; Liu 1997; Rodriguez 2005) showed no statistical significance (MD ‐1.11, 95% CI ‐5.66 to 3.44) and no heterogeneity was detected (P = 0.58, I2 statistic = 0%) (Figure 5)
5.
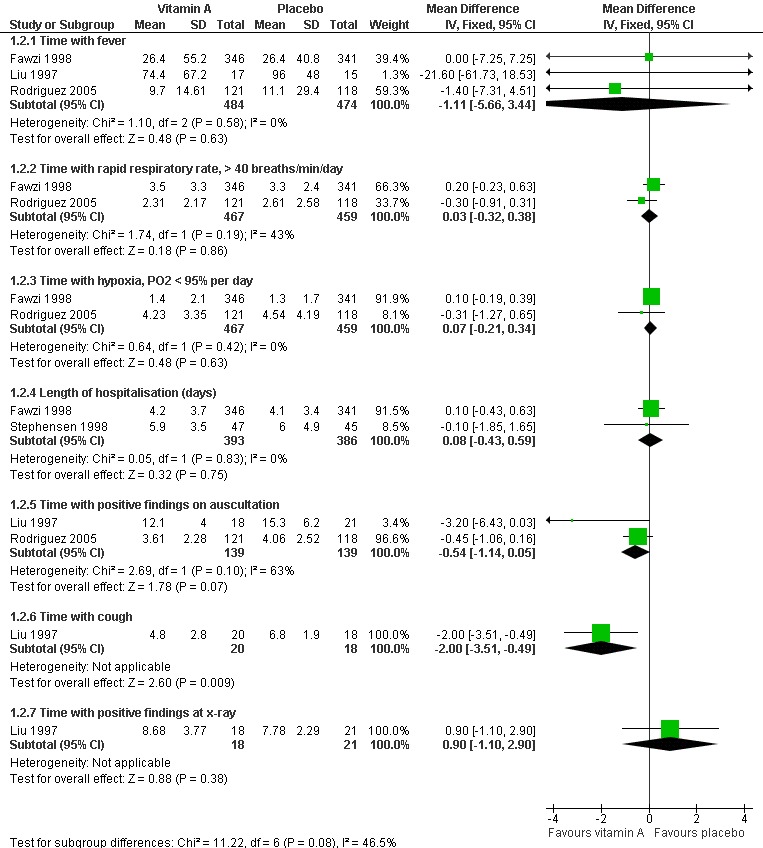
Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.2 Time with signs of pneumonia and treatment issues (continuous).
Time with respiratory rate greater than 40 breaths/minute/day
Two studies (Fawzi 1998; Rodriguez 2005) reported this outcome; no differences were found (MD 0.03; 95% CI ‐0.32 to 0.38) and no heterogeneity was detected (P = 0.19, I2 statistic = 42.5%) (Figure 5).
Time with positive finding on auscultation
Two studies (Liu 1997; Rodriguez 2005) reported the time with positive findings on auscultation; there was no difference between the two groups (MD ‐0.54; 95% CI ‐1.14 to 0.05) and no heterogeneity was detected (P = 0.10, I2 statistic = 62.8%) (Figure 5).
Time with positive finding on chest X‐ray
Two studies (Liu 1997; Stephensen 1998) reported the signs of pneumonia in chest X‐ray examinations after giving high doses of vitamin A. There was no statistically significant difference between the vitamin A and placebo groups (MD 0.90; 95% CI ‐1.10 to 2.90) (Liu 1997). There were also no positive findings on hyperinflation (in two of 41 children receiving vitamin A versus one of 31 receiving placebo), perihilar infiltrate (three of 41 versus six of 31), interstitial infiltrate (three of 41 versus none of 31), consolidation (seven of 41 versus three of 31), atelectasis (three of 41 versus none of 31), or effusions (three of 41 versus one of 31) (Stephensen 1998) (Figure 5).
Time with cough
One study (Liu 1997) reported that the time with cough was shortened by vitamin A (MD ‐2.00; 95% CI ‐3.51 to ‐0.49) (Figure 5).
No improvement at short‐term follow up and recurrence rate at long‐term follow up
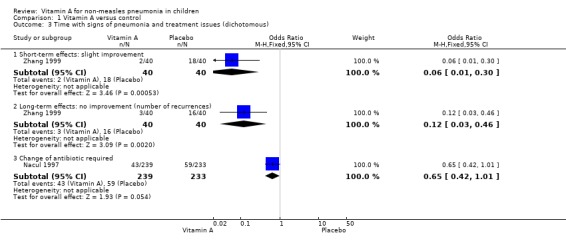
One study (Zhang 1999) reported lower rates of no improvement at both short‐term follow up and of recurrence rate at long‐term follow up in the low‐dose vitamin A supplement group than in the control group. There were statistically significant differences (OR 0.06, 95% CI 0.01 to 0.30 and OR 0.12, 95% CI 0.03 to 0.46, respectively). These results suggest that vitamin A can promote the recovery rate at short‐term follow up and decrease the recurrence rate of recurrent bronchopneumonia at long‐term follow up (Figure 6).
6.
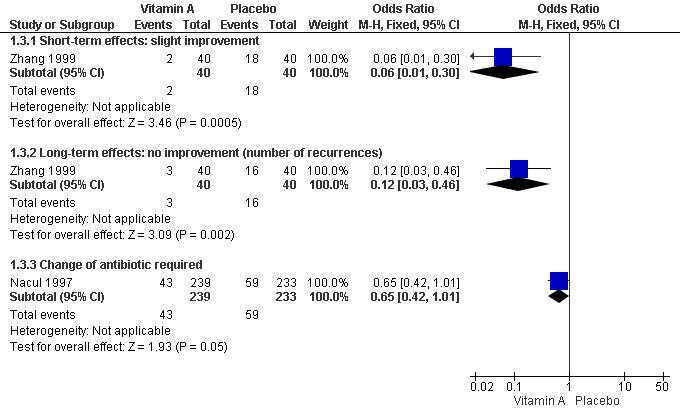
Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.3 Time with signs of pneumonia and treatment issues (dichotomous).
Time with fever, tachypnoea and hypoxaemia in children with basal serum retinol concentration > 200 ug/L
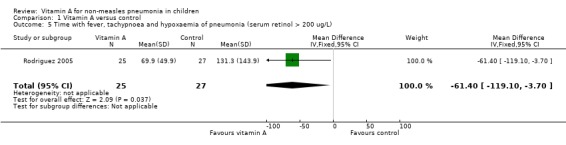
One study (Rodriguez 2005) reported the time with fever, tachypnoea and hypoxaemia in children with basal serum retinol concentration > 200 ug/L. There was a significant difference between the two groups (MD ‐61.40; 95% CI ‐119.10 to ‐3.7). This result suggests that vitamin A can shorten the time to remission of signs in children whose basal serum retinol was > 200 ug/L (Figure 7).
7.
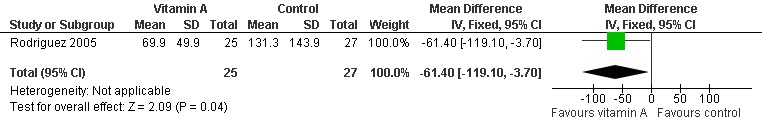
Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.5 Time with fever, tachypnoea and hypoxaemia of pneumonia (serum retinol > 200 ug/L).
Indicators of disease severity
Duration of hospitalization
Duration of hospitalization was reported in two studies (Fawzi 1998; Stephensen 1998). There was no statistically significant heterogeneity (P = 0.83 and I2 statistic = 0%) between the studies. The summary OR indicated that vitamin A treatment did not have a significant effect on hospital stay duration (MD 0.08; 95% CI ‐0.43 to 0.59) (Figure 5).
Time with hypoxia, less than 95% oxygen saturation per day
One study (Fawzi 1998) analyzed time with hypoxia; there was no difference (MD 0.07; 95% CI ‐0.21 to 0.34) to be found between the vitamin A and placebo groups (Figure 5).
Requirement for mechanical ventilation
None of the studies reported on requirements for mechanical ventilation.
Failure of first line antibiotic treatment
Children that required a change of antibiotic due to failure of first line treatment were reported in only one paper (Nacul 1997). This study reported no statistically significant reduction in the odds of first line antibiotic failure (OR 0.65; 95% CI 0.42 to 1.01) in children receiving vitamin A compared to those receiving placebo (Figure 6).
Disease severity
One study (Stephensen 1998) assessed disease severity during hospitalization by using a clinical severity score that consisted of 12 variables. Commencing measurement at 24 hours post‐treatment, the mean blood oxygen saturation levels of the placebo group increased to a higher level than the mean levels for the vitamin A group, and this difference persisted throughout hospitalization (P = 0.003 for treatment period). Significantly more children in the vitamin A group than in the placebo group eventually required supplementary oxygen (P = < 0.0005 for the treatment period). The mean respiratory rate (P = < 0.0005 for the treatment period) and heart rate (P = 0.001 for the treatment period) were significantly higher in the vitamin A group than in the placebo group when compared over time. The prevalence of auscultatory evidence of consolidation was initially the same in both groups but the prevalence decreased steadily in the placebo group while staying nearly constant in the vitamin A group, resulting in a significant difference between the groups (P = 0.0014 for the treatment period).
Adverse events
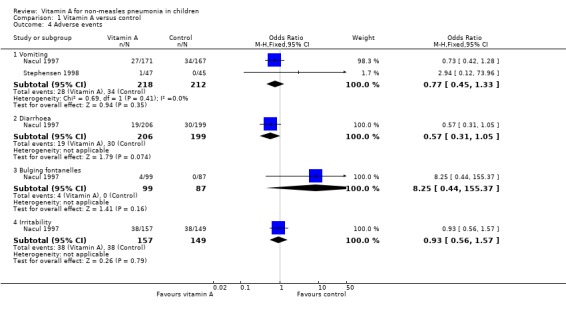
Three studies reported adverse effects of vitamin A intake. Four children (four out of 99) were observed with bulging fontanelle in Nacul 1997; none in the placebo group. The difference was not statistically significant (OR 8.25; 95% CI 0.44 to 155.37). The incidence of vomiting was 27 of 171 in the vitamin A group and 34 of 167 in the placebo group of one study (Nacul 1997); and one of 47 in the vitamin A group and none of 45 in the placebo group in a second study (Stephensen 1998). There was no statistical significance in the pooled analysis across these two studies (OR 0.77; 95% CI 0.45 to 1.33). The incidence of diarrhea was 19 of 206 in the vitamin A group and 30 of 199 in the placebo group (Nacul 1997); there was no statistical significance (OR 0.57; 95% CI 0.31 to 1.05). The incidence of irritability (Nacul 1997) was 38 of 157 in the vitamin A group and 38 of 149 in the placebo group. No statistical significance was apparent (OR 0.93; 95% CI 0.56 to 1.57). Nausea was observed in one child from each group (Stephensen 1998) (Figure 8).
8.
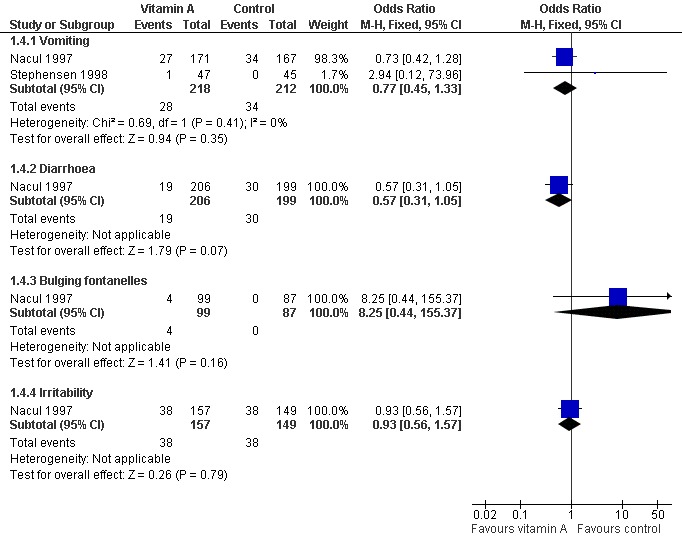
Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.4 Adverse events.
Discussion
Vitamin A supplementation appears to have had little effect on the clinical course of non‐measles pneumonia in children. The lack of a clear beneficial effect after vitamin A treatment is a little surprising given the protective effects of vitamin A in pneumonia associated with measles.
Summary of main results
Headaches, loss of appetite, vomiting and bulging fontanelles in infants are some of the adverse effects known to occasionally occur with the administration of high doses of vitamin A. These symptoms are minor and transitory, with no known long‐term effects, and require no special treatment (Yang 2009). From three included studies in this review (Fawzi 1998; Nacul 1997; Stephensen 1998), the incidence of toxicity as an adverse event was not statistically different between the vitamin A and placebo‐treated groups.
There was a borderline relative increase in the placebo group of children who failed to respond satisfactorily to first line antibiotic treatment. However, only one study reported on this and whether it represents a consistent effect of vitamin A, or is the result of chance, needs further investigation.
Rodriguez (Rodriguez 2005) conducted a subgroup analysis according to the serum retinol concentration, and found vitamin A can shorten the duration of signs in children with normal serum retinol (> 200 ug/L) but no benefit in children with vitamin A deficiency. However, this trial did not report on the nutritional status and severity of illness, which may be confounding factors. This study also found the time to remission of respiratory signs did not differ significantly between the groups of normal weight children and underweight children. However, data regarding this issue were unavailable to review.
Supplemental vitamin A in a lower dose seems to result in a beneficial effect on recurrent bronchopneumonia. This finding was from one study of poor methodological quality and lacking nutritional information about the vitamin A status of included children (Zhang 1999). More well‐designed, large RCTs are needed to support this positive finding.
Fawzi (Fawzi 1998) observed a trend towards a higher incidence of death in the vitamin A group (13 of 346 participants versus eight of 341 in the placebo group) although this was not statistically significant (OR 1.63; 95% CI 0.66 to 3.97). Stephensen (Stephensen 1998) reported more severe clinical scores in children receiving vitamin A than those who received a placebo. We expect that studies in the future will provide more conclusive evidence.
The possible explanation for the lack of benefit of vitamin A in non‐measles pneumonia is that the effects of vitamin A may be disease‐specific, with vitamin A only being effective when pneumonia is complicated with measles.
Overall completeness and applicability of evidence
The benefit of vitamin A as an adjunct to the treatment of non‐measles pneumonia was not clarified, but vitamin A might be beneficial to children with high basal serum retinol. Further RCTs, possibly with measured vitamin A levels and varying vitamin A doses, may provide sufficient evidence to clarify the role of vitamin A in non‐measles pneumonia.
Quality of the evidence
The quality of the evidence of outcomes, which include mortality and adverse events (rates of diarrhea, bulging fontanelles and irritability), have a higher quality (moderate); the quality of other evidence ranks from very low to low (see Figure 9; Figure 10; Figure 11).
9.
Primary outcomes
10.
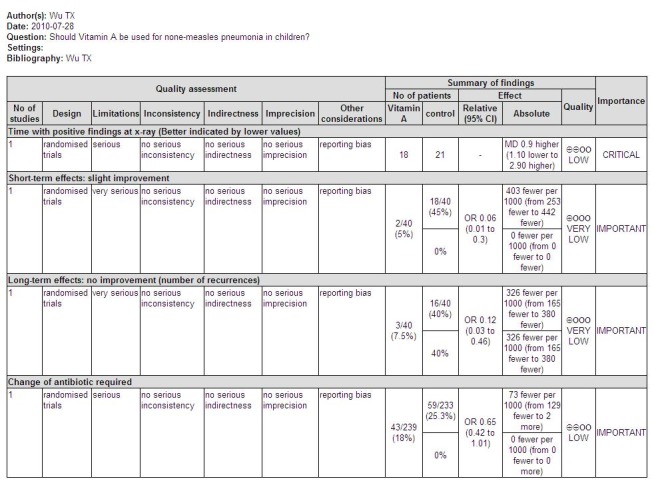
Secondary outcomes
11.
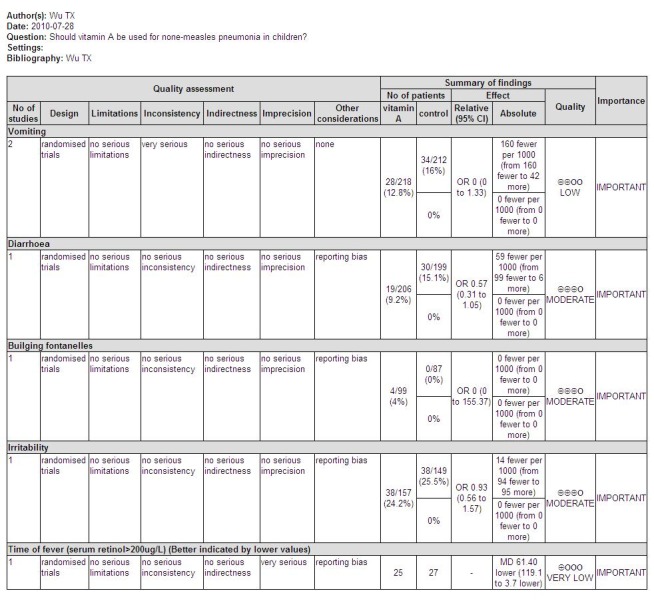
Adverse events and secondary outcome
Potential biases in the review process
We included English language and Chinese language articles only as we were unable to search articles in other languages. This might result in selection bias.
Agreements and disagreements with other studies or reviews
Similar findings were reported in a meta analysis (Brown 2004) which reviewed vitamin A for children aged from one month to six years with acute respiratory infections in low‐income countries. Five studies including a total of 2177 children (1067 intervention, 1110 controls) were included in this meta analysis. There were no significant differences in any of the recovery measures or mortality between the intervention and control groups. Pooled results showed no statistical significant differences between the vitamin A group and placebo group: fever: 0.03 (‐0.10 to 0.17); oxygen requirement: ‐0.08 (‐0.31 to 0.16); raised respiratory rate: ‐0.09 (‐0.38 to 0.19); and hospital stay: ‐0.06 (‐0.52 to 0.40). Mortality was below 2% in both groups, with a non‐significant higher risk in the intervention group (odds ratio 1.16, 95% CI: 0.61 to 2.21).
Authors' conclusions
Implications for practice.
From the evidence available at this time and given the lack of clinical benefit associated with vitamin A treatment in children with non‐measles pneumonia, it is difficult to recommend vitamin A as an adjunctive therapy in this patient group, particularly if the risk of vitamin A deficiency is low.
Implications for research.
Even though six studies met the inclusion criteria, the variability in the outcomes reported and measured meant that only results from a few studies were eligible for inclusion in the meta‐analyses for each of the outcomes. This limited the power of the meta‐analyses to detect statistically significant differences. Large RCTs reporting clinically important outcomes are needed to increase the likelihood of obtaining a true and precise estimate of the effect. However, dosage of vitamin A could also be important and RCTs with a risk‐stratified population, that is children at high risk and low risk of vitamin A deficiency, and varying administered dosage may provide evidence of the effectiveness of vitamin A treatment for non‐measles pneumonia in children.
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2010 | New search has been performed | Searches conducted. No new trials were included or excluded in this update. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 9 January 2008 | Amended | Converted to new review format. |
| 9 August 2007 | New search has been performed | Searches conducted. Contact details updated. In this updated review, we included one new trial (Rodriguez 2005) and excluded five trials (Chen 2005; Fu 2005; Long 2006; Long 2007; Winkler 2005). The included trial reported that moderate vitamin A was associated with a significant reduction in the time to remission of signs in children with normal serum retinol (> 200 ug/L). The conclusions remain unchanged. |
Notes
1. We used new standards of quality assessment described in new Cochrane Handbook of Systematic Reviews of Interventions. 2. We included a summary findings table result using GRADEprofiler to assess the quality of evidence.
Acknowledgements
We thank Drs. George Swingler and Nelcy Rodriguez and the ARI Group editorial team for advice in writing this review. We wish to acknowledge Janet Grant, Amy Zelmer, David Ross, Bhavneet Bharti, Heather Zar, and Chanpen Choprapawon for commenting on drafts of this review. We also thank Alexandra Raulli, Elmer Villanueva and Renae Johnston for their prior involvement with this review. Finally, we thank Dr. Kyran P. Quinlan, from the Department of Pediatrics at the University of Chicago, for kindly providing her published paper for assessment.
Appendices
Appendix 1. Embase search strategy
14. #10 AND #13 13. #11 OR #12 12. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti 11. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 10. #4 AND #9 9. #5 OR #6 OR #7 OR #8 8. (respiratory NEAR/2 (acute OR infection OR disease)):ab,ti 7. 'respiratory tract infection'/de OR 'lower respiratory tract infection'/exp 6. pneumon*:ab,ti 5. 'pneumonia'/exp 4. #1 OR #2 OR #3 3. 'diet supplementation'/exp 2. 'vitamin a':ab,ti OR retinol:ab,ti 1. 'retinol'/exp
Appendix 2. LILACS search strategy
Mh vitamin a OR Tw vitamin a OR Tw vitamina a OR Tw retinol OR Mh dietary supplements OR Tw dietary supplement$ OR Tw suplementos dieteticos [Words] and Mh pneumonia OR Mh aspiration pneumonia or Mh bronchiolitis obliterans organizing pneumonia or Mh cryptogenic organizing pneumonia or Mh eosinophilic pneumonia or Mh lobar pneumonia or Mh mycoplasma pneumonia or Mh pneumocystis pneumonia or Mh staphylococcal pneumonia or Mh ventilator‐associated pneumonia or Mh pneumonia aspiration or Mh pneumonia, bacterial or Mh pneumonia, eosinophilic or Mh pneumonia, interstitial or Mh pneumonia, interstitial plasma cell or Mh pneumonia, lipid or Mh pneumonia, lobar or Mh pneumonia, mycoplasma or Mh pneumonia, pneumococcal or Mh pneumonia, pneumocystis or Mh pneumonia, primary atypical or Mh pneumonia, radiation or Mh pneumonia, staphylococcal or Mh pneumonia, ventilator‐associated or Mh pneumonia, viral or Mh chlamydia pneumoniae or Mh chlamydophila pneumoniae or Mh diplococcus pneumoniae or Mh klebsiella pneumoniae or Mh meningitis, streptococcus pneumoniae or Mh mycoplasma pneumoniae or Mh streptococcus pneumonia or Mh streptococcus pneumoniae infections or Mh idiopathic interstitial pneumonias OR Tw pneumon$ OR Tw neumonS [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR w aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
Appendix 3. CINAHL search strategy
S22 S11 and S21 S21 S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 TI ( random* or placebo* ) or AB ( random* or placebo* ) S19 (MH "Quantitative Studies") S18 (MH "Placebos") S17 (MH "Random Assignment") S16 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S15 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S14 TI clinic* W1 trial* or AB clinic* W1 trial* S13 PT clinical trial S12 (MH "Clinical Trials+") S11 S5 and S10 S10 S6 or S7 or S8 or S9 S9 TI ( respiratory N2 infection OR respiratory N2 acute OR respiratory N2 disease ) or AB ( respiratory N2 infection OR respiratory N2 acute OR respiratory N2 disease ) S8 (MH "Respiratory Tract Infections") S7 TI pneumon* or AB pneumon* S6 (MH "Pneumonia+") S5 S1 or S2 or S3 or S4 S4 TI ( vitamin a or retinol ) or AB ( vitamin a or retinol ) S3 (MH "Dietary Supplements") S2 (MH "Vitamin A Deficiency") S1 (MH "Vitamin A+")
Appendix 4. Biosis Previews (Thomson ISI) search strategy
Topic=("vitamin a" or retinol or dietary supplement*) AND Topic=(pneumon*) Refined by: Topic=(random* or placebo* or clinical trial* or singl* blind* or doubl* blind*) Timespan=2007‐2010.
Appendix 5. Current Contents (Thomson ISI) search strategy
Topic=("vitamin a" or retinol or dietary supplement*) AND Topic=(pneumon*) Refined by: Topic=(random* or placebo* or clinical trial* or singl* blind* or doubl* blind*) Timespan=2007‐2010.
Appendix 6. Original search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 3); MEDLINE (1996 to August Week 2, 2007); EMBASE (1990 to January 2007); LILACS (6 May 2007); CINAHL (1990 to August 2007); Biological Abstracts (1990 to July 2007); Current Contents (1990 to May 2007); and the Chinese Biomedicine Database (CBM) (1994 to June 2007). We ran the following search strategy in CENTRAL and MEDLINE in combination with the highly sensitive search strategy developed by the Cochrane Collaboration for identifying randomised controlled trials (Dickersin 1994). The search strategy was modified to search the other electronic databases. MEDLINE (OVID) 1. exp vitamin A/ 2. vitamin A.mp 3. retinol.mp 4. exp dietary supplements/ 5. or/1‐4 6. exp pneumonia/ 7. pneumonia$.mp 8. exp pneumonia, bacterial/ 9. exp pneumonia, lipid/ 10. exp pneumonia, mycoplasma/ 11. exp pneumonia, pneumococcal/ 12. exp pneumonia, rickettsial/ 13. exp pneumonia, staphylococcal/ 14. exp pneumonia, viral/ 15. exp respiratory tract infections/ 16. acute adj respiratory.mp 17. respiratory adj infection.mp 18. respiratory adj disease.mp 19. or/6‐18 20. 5 and 19 In addition to electronic databases, we searched reference lists. There were no language or publication restrictions.
Data and analyses
Comparison 1. Vitamin A versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality during hospitalisation | 3 | 1446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.63, 2.66] |
| 2 Time with signs of pneumonia and treatment issues (continuous) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Time with fever | 3 | 958 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐5.66, 3.44] |
| 2.2 Time with rapid respiratory rate, > 40 breaths/min/day | 2 | 926 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.32, 0.38] |
| 2.3 Time with hypoxia, PO2 < 95% per day | 2 | 926 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.21, 0.34] |
| 2.4 Length of hospitalisation (days) | 2 | 779 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.43, 0.59] |
| 2.5 Time with positive findings on auscultation | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.14, 0.05] |
| 2.6 Time with cough | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.51, ‐0.49] |
| 2.7 Time with positive findings at x‐ray | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.10, 2.90] |
| 3 Time with signs of pneumonia and treatment issues (dichotomous) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term effects: slight improvement | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.30] |
| 3.2 Long‐term effects: no improvement (number of recurrences) | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.46] |
| 3.3 Change of antibiotic required | 1 | 472 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 4 Adverse events | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vomiting | 2 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.33] |
| 4.2 Diarrhoea | 1 | 405 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.05] |
| 4.3 Bulging fontanelles | 1 | 186 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.44, 155.37] |
| 4.4 Irritability | 1 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.57] |
| 5 Time with fever, tachypnoea and hypoxaemia of pneumonia (serum retinol > 200 ug/L) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐61.40 [‐119.10, ‐3.70] |
1.1. Analysis.
Comparison 1 Vitamin A versus control, Outcome 1 Mortality during hospitalisation.
1.2. Analysis.
Comparison 1 Vitamin A versus control, Outcome 2 Time with signs of pneumonia and treatment issues (continuous).
1.3. Analysis.
Comparison 1 Vitamin A versus control, Outcome 3 Time with signs of pneumonia and treatment issues (dichotomous).
1.4. Analysis.
Comparison 1 Vitamin A versus control, Outcome 4 Adverse events.
1.5. Analysis.
Comparison 1 Vitamin A versus control, Outcome 5 Time with fever, tachypnoea and hypoxaemia of pneumonia (serum retinol > 200 ug/L).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fawzi 1998.
| Methods | Randomised block design, placebo controlled trial. Double‐blind. Baseline was similar | |
| Participants | Children between 6 and 60 months of age admitted to hospital with pneumonia as diagnosed by a paediatrician on the presence of cough and one or more of the following signs: respiratory rates >= 40 breaths (50 breaths for infants between the ages of 6 and 11 months) per minute, chest retractions, inability to eat or drink, the presence of decreased air entry, crackling sounds or dullness to percussion | |
| Interventions | Children in vitamin A group received a dose of vitamin A at admission according to the age. Children > 1 year and infants were administered 1 ml (200,000 IU) and 0.5 ml (100,000 IU) of a vitamin A solution, respectively | |
| Outcomes | Clinical outcomes: mortality, duration of hospitalization, time with rapid respiratory rate (> 40 breaths/minute), time with fever (> 37.8 C), time with hypoxia (PO2 < 90%) | |
| Notes | Setting: Tanzania | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | The randomisation was described as "eligible children were randomly assigned in blocks of 20 to receive a dose of vitamin A or placebo", but how the allocation sequence was generated did not described in detail |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes except mortality | Low risk | The treatment regimens were prepared especially for the study, and the batch code was retained by the manufacturer until the end of the study |
| Incomplete outcome data addressed? All outcomes except mortality | Low risk | |
Liu 1997.
| Methods | Both 'completed random method' and double‐blind was mentioned | |
| Participants | 4 groups with different interventions (selenium) were selected. Data in vitamin A group and placebo group were extracted. 18 children in vitamin A group and 21 in placebo group. All children were younger than 14 years old | |
| Interventions | Children in vitamin A group received a dose of vitamin A 150,000 IU orally | |
| Outcomes | Variate outcomes were used; we only extracted data from those that matched the inclusion criteria for this review: 1. clearance time of fever 2. remission time of cough 3. remission time of positive finding of auscultation 4. remission days of positive finding at chest X‐ray | |
| Notes | Setting: Youyi Hospital, Beijing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Completed random method" was mentioned but lacked a detailed description |
| Allocation concealment? | High risk | Not concealment mentioned |
| Blinding? All outcomes except mortality | Low risk | Double‐blinding |
| Incomplete outcome data addressed? All outcomes except mortality | High risk | |
Nacul 1997.
| Methods | Block randomisation, placebo controlled; double‐blind. The baseline was broadly similar | |
| Participants | Children aged 6 to 59 months with a clinical diagnosis either admitted to hospital or treated as outpatients. The diagnosis of pneumonia was made after taking a detailed history and looking for signs of lung infection such as fever, tachypnoea, signs of dyspnoea and consolidation | |
| Interventions | Children received a dose of vitamin A on admission to the trial and then again the following day. Children > 1 year and infants were administered 200,000 IU and 100,000 IU of vitamin A respectively. Children who received vitamin A supplements in the preceding month received a capsule (100,000 IU) on admission only | |
| Outcomes | Clinical outcomes: mortality, episode of pneumonia, fever, tachypnoea, lung complications, other complications, hospital admission (for outpatients only), antibiotic change, vomiting, diarrhea, irritability, bulging fontanelle | |
| Notes | Setting: Recife, north east Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Children were individually randomized into one of the two treatment groups. The randomisation was blocked into groups of four". There was no description for how the sequence was generated |
| Allocation concealment? | Unclear risk | No description about allocation concealment |
| Blinding? All outcomes except mortality | Low risk | The vitamin A or placebo capsules were put into small brown envelopes labelled with identification numbers |
| Incomplete outcome data addressed? All outcomes except mortality | Low risk | |
Rodriguez 2005.
| Methods | Randomised block design, placebo controlled trial. Double‐blind | |
| Participants | Children between 2 and 59 months of age admitted to hospital with clinical pneumonia (confirmed by X‐ray). Clinical pneumonia was defined as the presence of elevated respiratory rate (> 40/minute in children aged > 12 to 59 months; > 50/minute in children aged 2 to 12 months), fever (axial temperature > 37.5 C), cough or chest indrawing or both, and low oxygen saturation (pulse oximetry level < 90%), and at least one clinical sign by auscultation (for example, rales, wheezing, diminished breath sounds, bronchial breath sounds, or pleural rub) | |
| Interventions | Children in vitamin A group received a dose of vitamin A 50,000 IU orally in children aged 2 to 12 months; 100,000 IU orally in children aged > 12 to 59 months | |
| Outcomes | Clinical outcomes: mortality, time to remission of signs, the duration of respiratory sign, resolution of focal infiltrates at 72 hours | |
| Notes | Setting: Baca Ortiz Children's Hospital, Quito, Ecuador | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation sequence was generated by a random number table |
| Allocation concealment? | Low risk | The Ethical Committee of the Corporacio´n Ecuatoriana de Biotecnología held the blinded randomisation codes in a secure place. The study code was not broken until all the data were entered and the initial analyses performed |
| Blinding? All outcomes except mortality | Low risk | Double‐blinding |
| Incomplete outcome data addressed? All outcomes except mortality | Low risk | 48 children (16.7%) were lost to follow‐up during the course of the study |
Stephensen 1998.
| Methods | Randomised block design, placebo controlled trial. Double‐blind | |
| Participants | Children between 3 months and 10 years of age with a principal diagnosis of pneumonia (confirmed by X‐ray) admitted as inpatients to the paediatrics ward. A purified protein derivative (PPD) skin test was applied at the time of admission to the paediatrics ward and was read 48 hours later. 47 in vitamin A group and 48 in placebo group | |
| Interventions | Children < 1 year received 100,000 IU (2 ml of water‐miscible preparation) of vitamin A on admission to the study and 50,000 IU (1 ml) on the second day of hospitalization. Children >= 1 year of age received 200,000 IU (4 ml) on the first day and 100,000 (2 ml) on the second day | |
| Outcomes | Clinical outcomes: temperature, heart rate, respiratory rate, presence or absence of retractions, occurrence of central cyanosis, percent of blood oxygen saturation, use of supplemental oxygen Other: food consumed, mother's impression of child's appetite | |
| Notes | Setting: Lima, Peru | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Did not mention any means of sequence generation, but block randomisation was mentioned |
| Allocation concealment? | Unclear risk | Did not mention any means of allocation concealment |
| Blinding? All outcomes except mortality | Low risk | Double‐blinding |
| Incomplete outcome data addressed? All outcomes except mortality | Low risk | In placebo group 3 participants were excluded after randomisation due to positive PPD tests, 2 participants were withdrawn from additional data collection because of complications of their infection at day 10 (placebo) and day 14 (vitamin A). One participant was withdrawn from the placebo group during the first hospital day because of medical complications |
Zhang 1999.
| Methods | Quasi‐randomised parallel design, placebo‐controlled trial. Children were allocated into two groups by the order of admission. No blinding | |
| Participants | 80 children between 1 and 3 years old with recurrent bronchopneumonia were equally allocated to two groups. Diagnosis of pneumonia was confirmed by X‐ray | |
| Interventions | Same routine therapy, including antibiotics, anti‐symptoms drugs, given in both groups. Oxygen was given for children with hypoxic and digitalis was given for children with heart failure. In addition to these, vitamin A was added for the vitamin A group with 10,000 IU, b.i.d. orally for 6 days, then 1500 IU/d for 20 days | |
| Outcomes | Short‐term effect: 1. Marked improvement: all symptoms of pneumonia improved within 6 days, whilst no positive finding at chest X‐ray 2. Improvement: 7 to 9 days after treatment, all of symptoms in remission and no positive finding at chest X‐ray 3. Slight improvement: symptoms in remission after more than 10 days, but no change at chest X‐ray Long‐term effects: 1. Marked improvement: no recurrence of bronchopneumonia during 1 year post‐treatment 2. Improvement: bronchopneumonia recurred 1 to 2 times during 1 year post‐treatment 3. No improvement: bronchopneumonia recurred 3 times or more during 1 year post‐treatment | |
| Notes | Setting: Sihong County, Jiangsu, China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Quasi‐randomisation |
| Allocation concealment? | High risk | D ‐ Not used |
IU = international units PO2 = blood oxygen level b.i.d. = twice daily /d = per day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1991 | Patients had pneumonia complicated by measles |
| Barreto 1994 | Vitamin A administered prior to pneumonia diagnosis |
| Basu 2003 | Vitamin A administered prior to pneumonia diagnosis |
| Bhandari 1997 | Vitamin A administered prior to pneumonia diagnosis |
| Biesalski 2001 | Not a RCT |
| Biswas 1994 | Vitamin A administered prior to pneumonia diagnosis |
| Bresee 1996 | Participants included were children with lower respiratory tract diseases not just pneumonia |
| Buyukgebiz 1990 | Clinical outcomes not reported |
| Chen 2005 | The study compared the efficacy of vitamin A administered intravenously and orally |
| Coles 2001 | Vitamin A administered prior to pneumonia diagnosis |
| Coutsoudis 2000 | Vitamin A administered prior to pneumonia diagnosis |
| Daulaire 1992 | Vitamin A administered prior to pneumonia diagnosis |
| Dibley 1996 | Vitamin A administered prior to pneumonia diagnosis |
| Donnen 1998 | Vitamin A administered prior to pneumonia diagnosis |
| Dowell 1996 | Participants included were children with lower respiratory tract diseases not just pneumonia |
| Dudley 1997 | Not a RCT |
| Fauveau 1992 | Not a RCT |
| Fawzi 1995 | Vitamin A administered prior to pneumonia diagnosis |
| Fawzi 1999 | Duplicate patient groups |
| Fawzi 2000 | Duplicate patient groups |
| Fu 2005 | Not a RCT |
| Ghana VAST team 1993 | Vitamin A administered prior to pneumonia diagnosis |
| Hadi 1999 | Vitamin A administered prior to pneumonia diagnosis |
| Humphrey 1996 | Vitamin A administered prior to pneumonia diagnosis |
| Julien 1999 | Paticipants included were children with lower respiratory tract diseases not just pneumonia |
| Junhong 1997 | Not a RCT |
| Kao 1996 | Although 'randomized allocation' was mentioned, by asking the trial author we understand that the method of randomisation was 'convenience' allocation; not a real RCT |
| Kartasasmita 1995 | Vitamin A administered prior to pneumonia diagnosis |
| Khandait 2000 | Not a RCT |
| Kjolhede 1995 | Subjects included were children with lower respiratory tract diseases ‐ not just pneumonia but also bronchiolitis |
| Li 2004 | Not a real RCT, although 'randomisation' was mentioned |
| Lie 1993 | Vitamin A administered prior to pneumonia diagnosis |
| Lin 1999 | 'Random' was mentioned, but by asking the trial author we discovered that 'random' meant 'sampling' so this was not a RCT |
| Liu 2002 | Not a RCT |
| Lloyd 1991 | Not a RCT |
| Long 2006 | Vitamin A administered prior to pneumonia diagnosis |
| Long 2007 | Vitamin A administered prior to pneumonia diagnosis |
| Ma 2000 | 'Randomised allocation' was mentioned, but by asking the trial author we discovered they performed a convenience allocation |
| Mahalanabis 2001 | Not a RCT |
| Qin 1999 | 'Randomised' was mentioned. We asked the trial authors and understood that the method of randomisation was 'convenience' allocation, not a real RCT |
| Quinlan 1996 | Participants included were children with lower respiratory tract disease rather than pneumonia |
| Rahman 2001 | Vitamin A administered prior to pneumonia diagnosis |
| Rahmathullah 1991 | Vitamin A administered prior to pneumonia diagnosis |
| Ramakrishnan 1998 | Not a RCT |
| Roy 1997 | Vitamin A administered prior to pneumonia diagnosis |
| Ruz 1995 | Vitamin A administered prior to pneumonia diagnosis |
| Semba 1993 | Vitamin A administered prior to pneumonia diagnosis |
| Semba 1995 | Not a RCT |
| Semba 1999 | Not a RCT |
| Sempertegui 1999 | Vitamin A administered prior to pneumonia diagnosis |
| Shah 1994 | Not a RCT |
| Si 1997 | Patients had pneumonia complicated by measles |
| Stansfield 1993 | Vitamin A administered prior to pneumonia diagnosis |
| Stephensen 2002 | Clinical outcomes not reported |
| Tomkins 2000 | Not a RCT |
| Velasquez 1995 | Clinical outcomes not reported |
| Vijayaraghavan 1990 | Vitamin A administered prior to pneumonia diagnosis |
| Villamor 2000a | Not a RCT |
| Villamor 2000b | Duplicate patient groups |
| Wang 2003a | Not a real RCT |
| Wang 2003b | Duplicate publication of Wang 2003a, and not a real RCT |
| West 1991 | Vitamin A administered prior to pneumonia diagnosis |
| Willumsen 1997 | Not a RCT |
| Winkler 2005 | Vitamin A administered prior to pneumonia diagnosis |
| Wu 2000 | 'Randomised' was mentioned, but by asking the trial author we understand that the method of randomisation was 'convenience' allocation; not a real RCT |
| Yang 2002 | Non‐randomised controlled study |
| Ying 1998 | Although 'randomized' was mentioned, we asked the trial author and understand that the method of randomisation was 'convenience' allocation; not a real RCT |
| Zhen 2003 | 'Randomised' was mentioned, we asked the trial author and understand that the method of randomisation was 'convenience' allocation; not a real RCT |
Contributions of authors
Taixiang Wu (TW) was responsible for drafting, editing, commenting, making amendments and updating this review. Juan Ni (JN) was responsible for searching for studies, data extraction and analysis and drafting the review. Jiafu Wei (JW) was responsible for searching for studies and data extraction.
Sources of support
Internal sources
Chinese Cochrane Center, Chinese Centre of Evidence‐Based Medicine, West China Hospital of Sichuan University, China.
External sources
China Medical Board of New York, USA.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Fawzi 1998 {published data only}
- Fawzi W, Chalmers T, Herrera M, Mosteller F. Vitamin A supplementation and severity of pneumonia in children admitted to the hospital in Dar es Salaam, Tanzania. American Journal of Clinical Nutrition 1998;68:187‐92. [DOI] [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu XH, Yin SA, Li G, Gao HY, Xu QM. A clinical observation on supplementing selenium and vitamin A for mycoplasma pneumonia in children. Journal of Chinese Children Health Care 1997;5(4):239‐40. [Google Scholar]
Nacul 1997 {published data only}
- Nacul L, Kirkwood B, Arthur P, Morris S, Magalhaes M, Fink MC. Randomised, double blind, placebo controlled clinical trial of efficacy of vitamin A treatment in non‐measles childhood pneumonia. BMJ 1997;315(7107):505‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2005 {published data only}
- Rodriguez A, Hamer DH, Rivera J, Acosta M, Salgado G, Gordillo M. Effects of moderate doses of vitamin A as an adjunct to the treatment of pneumonia in underweight and normal‐weight children: a randomized, double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 2005;82(5):1090‐6. [DOI] [PubMed] [Google Scholar]
Stephensen 1998 {published data only}
- Stevensen C, Franchi L, Hernandez H, Campos M, Gilman R, Alvarez J. Adverse effects of high‐dose vitamin A supplements in children hospitalized with pneumonia. Pediatrics 1998;101:1‐8. [DOI] [PubMed] [Google Scholar]
Zhang 1999 {published data only}
- Zhang J. Observation for vitamin A as a complementary agent in the treatment of bronchopneumonia. Journal of Jining Medical College 1999;22(2):55. [Google Scholar]
References to studies excluded from this review
Anonymous 1991 {published data only}
- Anonymous. Vitamin A administration reduces mortality and morbidity from severe measles in populations nonendemic for hypovitaminosis. Nutrition Reviews 1991;49:89‐91. [DOI] [PubMed] [Google Scholar]
Barreto 1994 {published data only}
- Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, et al. Effect of vitamin A supplementation on diarrhoea and acute lower‐respiratory‐tract infections in young children in Brazil. Lancet 1994;344:228‐31. [DOI] [PubMed] [Google Scholar]
Basu 2003 {published data only}
- Basu S, Sengupta B, Paladhi PK. Single megadose vitamin A supplementation of Indian mothers and morbidity in breast fed young infants. Postgraduate Medical Journal 2003;79(933):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 1997 {published data only}
- Bhandari N, Bhan MK, Sazawal S. Impact of massive dose of vitamin A given to preschool children with acute diarrhoea on subsequent respiratory and diarrhoeal morbidity. BMJ 1994;309:1404‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biesalski 2001 {published data only}
- Biesalski HK. Importance of vitamin a for lung development. Monatsschrift fur Kinderheilkunde 2001;149(Suppl):25‐32. [Google Scholar]
Biswas 1994 {published data only}
- Biswas R, Biswas AB, Manna B, Bhattacharya SK, Dey R, Sarkar S. Effect of vitamin A supplementation on diarrhoea and acute respiratory tract infection in children. A double blind placebo controlled trial in a Calcutta slum community. European Journal of Epidemiology 1994;10:57‐61. [DOI] [PubMed] [Google Scholar]
Bresee 1996 {published data only}
- Bresee J, Fischer M, Dowell S, Johnston B, Biggs V, Levine, R, et al. Vitamin A therapy for children with respiratory syncytial virus infection: a multicenter trial in the United States. Pediatric Infectious Diseases Journal 1996;15:777‐82. [DOI] [PubMed] [Google Scholar]
Buyukgebiz 1990 {published data only}
- Buyukgebiz B, Ozalp I, Oran O. Investigation of serum vitamin A levels of children who had a history of recurrent diarrhoea and acute respiratory infections in Ankara. Journal of Tropical Pediatrics 1990;36:251‐5. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen Y, Hua Z, Di P, Xu T. Effect of intravenously administered liposomal vitamin A in neonatal pneumonia. Journal of Pediatric Phamacy 2005;11(6):11‐3. [Google Scholar]
Coles 2001 {published data only}
- Coles CL, Rahmathullah L, Kanungo R, Thulasiraj RD, Katz J, Santhosham M, et al. Vitamin A supplementation at birth delays pneumococcal colonization in South Indian infants. Journal of Nutrition 2001;131:255‐61. [DOI] [PubMed] [Google Scholar]
Coutsoudis 2000 {published data only}
- Coutsoudis A, Adhikari M, Pillay K, Kuhn L, Coovadia HM. Effect of vitamin A supplementation on morbidity of low‐birth‐weight neonates. South African Medical Journal 2000;90:730‐6. [PubMed] [Google Scholar]
Daulaire 1992 {published data only}
- Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. British Medical Journal 1992;304:207‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dibley 1996 {published data only}
- Dibley MJ, Sadjimin T, Kjolhede CL, Moulton LH. Vitamin A supplementation fails to reduce incidence of acute respiratory illness and diarrhea in preschool‐age Indonesian children. Journal of Nutrition 1996;126:434‐42. [DOI] [PubMed] [Google Scholar]
Donnen 1998 {published data only}
- Donnen P, Dramaix M, Brasseur D, Bitwe R, Vertongen F, Hennart P. Randomized placebo‐controlled clinical trial of the effect of a single high dose or daily low doses of vitamin A on the morbidity of hospitalized, malnourished children. American Journal of Clinical Nutrition 1998;68:1254‐60. [DOI] [PubMed] [Google Scholar]
Dowell 1996 {published data only}
- Dowell S. Treatment of respiratory syncytial virus infection with vitamin A: a randomized, placebo‐controlled trail in Santiago, Chile. Pediatrics Infectious Diseases 1996;15:782‐6. [DOI] [PubMed] [Google Scholar]
Dudley 1997 {published data only}
- Dudley L, Hussey G, Huskissen J, Kessow G. Vitamin A status, other risk factors and acute respiratory infection morbidity in children. South African Medical Journal 1997;87:65‐70. [PubMed] [Google Scholar]
Fauveau 1992 {published data only}
- Fauveau V, Stewart MK, Chakraborty J, Khan SA. Impact on mortality of a community‐based programme to control acute lower respiratory tract infections. Bulletin of the World Health Organization 1992;70:109‐16. [PMC free article] [PubMed] [Google Scholar]
Fawzi 1995 {published data only}
- Fawzi WW, Herrera MG, Willett WC, Nestel P, Amin A, Mohamed KA. Dietary vitamin A intake and the incidence of diarrhea and respiratory infection among Sudanese children. Journal of Nutrition 1995;125:1211‐21. [DOI] [PubMed] [Google Scholar]
Fawzi 1999 {published data only}
- Fawzi WW, Mbise RL, Hertzmark E, Fataki MR, Herrera MG, Ndossi G, et al. A randomized trial of vitamin A supplements in relation to mortality among human immunodeficiency virus‐infected and uninfected children in Tanzania. Pediatric Infectious Disease Journal 1999;18:127‐33. [DOI] [PubMed] [Google Scholar]
Fawzi 2000 {published data only}
- Fawzi WW, Mbise R, Spiegelman D, Fataki M, Hertzmark E, Ndossi G. Vitamin A supplements and diarrheal and respiratory tract infections among children in Dar es Salaam, Tanzania. Journal of Pediatrics 2000;137:660‐7. [DOI] [PubMed] [Google Scholar]
Fu 2005 {published data only}
- Fu Y. The usage of Vitamin A for respiratory tract infection in children. Central Plains Medical Journal 2005;32(10):25‐6. [Google Scholar]
Ghana VAST team 1993 {published data only}
- Ghana VAST Study Team. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342:7‐12. [PubMed] [Google Scholar]
Hadi 1999 {published data only}
- Hadi H, Stoltzfus RJ, Moulton LH, Dibley MJ, West KP Jr. Respiratory infections reduce the growth response to vitamin A supplementation in a randomized controlled trial. International Journal of Epidemiology 1999;28:874‐81. [DOI] [PubMed] [Google Scholar]
Humphrey 1996 {published data only}
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. Journal of Pediatrics 1996;128:489‐96. [DOI] [PubMed] [Google Scholar]
Julien 1999 {published data only}
- Julien M, Gomes A, Varandas L, Lerberghe W, Rodrigues P, Malveiro F. A randomised, double‐blind, placebo‐controlled clinical trial of vitamin A in Mozambican children hospitalized with non‐measles acute lower respiratory tract infections. Tropical Medicine and International Health 1999;4:794‐800. [DOI] [PubMed] [Google Scholar]
Junhong 1997 {published data only}
- Junhong Z, Youqing Q, Deyuan Z. The study of the effect of massive dose vitamin A intervention to recurrent respiratory infection. Acta Nutrimenta Sinica 1997;19:291‐3. [Google Scholar]
Kao 1996 {published data only}
- Kao Y, Yu X, Li Z. Vitamin A as a complementary agent in the treatment of pneumonia in children. Journal of Zhejiang Prevention Medicine 1996;8(6):39‐40. [Google Scholar]
Kartasasmita 1995 {published data only}
- Kartasasmita CB, Rosmayudi O, Deville W, Demedts M. Plasma retinol level, vitamin A supplementation and acute respiratory infections in children of 1‐5 years old in a developing country. Respiratory Diseases Working Group. Tubercle and Lung Disease 1995;76:563‐9. [DOI] [PubMed] [Google Scholar]
Khandait 2000 {published data only}
- Khandait DW, Vasudeo ND, Zodpey SP, Kumbhalkar DT. Risk factors for subclinical vitamin A deficiency in children under the age of 6 years. Journal of Tropical Pediatrics 2000;46:239‐41. [DOI] [PubMed] [Google Scholar]
Kjolhede 1995 {published data only}
- Kjolhede C, Chew F, Gadomski A, Marroquin D. Clinical trial of vitamin A as adjuvant treatment for lower respiratory tract infections. Journal of Pediatrics 1995;126:807‐12. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li Y. Observation for vitamin A as a complementary agent in the treatment of new born baby with pneumonia. Central Plains Medical Jounal 2004;31(9):40‐1. [Google Scholar]
Lie 1993 {published data only}
- Lie C, Ying C, Wang EL, Brun T, Geissler C. Impact of large‐dose vitamin A supplementation on childhood diarrhoea, respiratory disease and growth. European Journal of Clinical Nutrition 1993;47:88‐96. [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin L, Lu X. Observation on curative of vitamin AD as an adjuvant for premature delivered infant infectious pneumonia. Journal of Huangshi Polytechnic College 1999;15(2):36‐7. [Google Scholar]
Liu 2002 {published data only}
- Liu JM, Gu XY. Effect of vitamin A as an adjuvant drug in the treatment of bronchopneumonia. Modern Practice Medicine 2002;14(6):302. [Google Scholar]
Lloyd 1991 {published data only}
- Lloyd‐Puryear MA, Mahoney J, Humphrey JH, Mahoney F, Siren N, Moorman C, et al. Vitamin A deficiency in Micronesia: A statewide survey in Chuuk. Nutrition Research 1991;11:1101‐10. [Google Scholar]
Long 2006 {published data only}
- Long KZ, Montroya Y, Hertzmark E, Santos JI, Rosado JL. A double‐blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693‐700. [MEDLINE: ; ISSN:0002‐9165] [DOI] [PubMed] [Google Scholar]
Long 2007 {published data only}
- Long KZ, Rosado JL, DuPont HL, Hertzmark E, Santos JI. Supplementation with vitamin A reduces watery diarrhoea and respiratory infections in Mexican children. British Journal of Nutrition 2007;97(2):337‐43. [DOI] [PubMed] [Google Scholar]
Ma 2000 {published data only}
- Ma C. Effects observation of vitamin A in the complementary treatment of respiratory syncytial virus pneumonia. Clinical Medicine 2000;20(3):45‐6. [Google Scholar]
Mahalanabis 2001 {published data only}
- Mahalanabis D, Bhan MK. Micronutrients as adjunct therapy of acute illness in children: Impact on the episode outcome and policy implications of current findings. British Journal of Nutrition 2001;85(Suppl):151‐8. [DOI] [PubMed] [Google Scholar]
Qin 1999 {published data only}
- Qin XL. Observation of vitamin A as an adjuvant in the treatment of infant pneumonia. Jounral of Guangxi Medical University 1999;16(4):492‐3. [Google Scholar]
Quinlan 1996 {published data only}
- Quinlan K, Hayani K. Vitamin A and Respiratory Syncytial Virus Infection.Serum levels and supplementation trial. Archives of Pediatric and Adolescent Medicine 1996;150:25‐30. [DOI] [PubMed] [Google Scholar]
Rahman 2001 {published data only}
- Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: Randomised double blind controlled trial. BMJ 2001;323:314‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahmathullah 1991 {published data only}
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC. Diarrhea, respiratory infections, and growth are not affected by a weekly low‐dose vitamin A supplement: a masked, controlled field trial in children in southern India. American Journal of Clinical Nutrition 1991;54:568‐77. [DOI] [PubMed] [Google Scholar]
Ramakrishnan 1998 {published data only}
- Ramakrishnan U, Martorell R. The role of vitamin A in reducing child mortality and morbidity and improving growth. Salud Publica de Mexico 1998;40:189‐98. [DOI] [PubMed] [Google Scholar]
Roy 1997 {published data only}
- Roy SK, Islam A, Molla A, Akramuzzaman SM, Jahan F, Fuchs G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. European Journal of Clinical Nutrition 1997;51:302‐7. [DOI] [PubMed] [Google Scholar]
Ruz 1995 {published data only}
- Ruz M, Solomons NW, Mejia LA, Chew F. Alteration of circulating micronutrients with overt and occult infections in anaemic Guatemalan preschool children. International Journal of Food Sciences and Nutrition 1995;46:257‐65. [DOI] [PubMed] [Google Scholar]
Semba 1993 {published data only}
- Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Archives of Internal Medicine 1993;153:2149‐54. [PubMed] [Google Scholar]
Semba 1995 {published data only}
- Semba RD, Caiaffa WT, Graham NM, Cohn S, Vlahov D. Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus‐infected injection drug users. Journal of Infectious Diseases 1995;171:1196‐202. [DOI] [PubMed] [Google Scholar]
Semba 1999 {published data only}
- Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proceedings of the Nutrition Society 1999;58:719‐27. [DOI] [PubMed] [Google Scholar]
Sempertegui 1999 {published data only}
- Sempertegui F, Estrella B, Camaniero V, Betancourt V, Izurieta R, Ortiz W, et al. The beneficial effects of weekly low‐dose vitamin A supplementation on acute lower respiratory infections and diarrhea in Ecuadorian children. Pediatrics 1999;104:e1. [DOI] [PubMed] [Google Scholar]
Shah 1994 {published data only}
- Shah N, Ramankutty V, Premila PG, Sathy N. Risk factors for severe pneumonia in children in south Kerala: a hospital‐based case‐control study. Journal of Tropical Pediatrics 1994;40:201‐6. [DOI] [PubMed] [Google Scholar]
Si 1997 {published data only}
- Si NV, Grytter C, Vy NN, Hue NB, Pedersen FK. High dose vitamin A supplementation in the course of pneumonia in Vietnamese children. Acta Paediatrica 1997;86:1052‐5. [DOI] [PubMed] [Google Scholar]
Stansfield 1993 {published data only}
- Stansfield SK, Pierre‐Louis M, Lerebours G, Augustin A. Vitamin A supplementation and increased prevalence of childhood diarrhoea and acute respiratory infections. Lancet 1993;342:578‐82. [DOI] [PubMed] [Google Scholar]
Stephensen 2002 {published data only}
- Stephensen CB, Franchi LM, Hernandez H, Campos M, Colarossi A, Gilman RH, et al. Assessment of vitamin A status with the relative dose response test in Peruvian children recovering from pneumonia. American Journal of Clinical Nutrition 2002;76:1351‐7. [DOI] [PubMed] [Google Scholar]
Tomkins 2000 {published data only}
- Tomkins A. Malnutrition, morbidity and mortality in children and their mothers. Proceedings of the Nutrition Society 2000;59:135‐46. [DOI] [PubMed] [Google Scholar]
Velasquez 1995 {published data only}
- Velasquez‐Melendez G, Okani ET, Kiertsman B, Roncada MJ. Vitamin A status in children with pneumonia. European Journal of Clinical Nutrition 1995;49:379‐84. [PubMed] [Google Scholar]
Vijayaraghavan 1990 {published data only}
- Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet 1990;336:1342‐5. [DOI] [PubMed] [Google Scholar]
Villamor 2000a {published data only}
- Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. Journal of Infectious Diseases 2000;182(Suppl):122‐33. [DOI] [PubMed] [Google Scholar]
Villamor 2000b {published data only}
- Villamor E, Mbise R, Spiegelman D, Ndossi G, Fawzi WW. Vitamin A supplementation and other predictors of anemia among children from Dar Es Salaam, Tanzania. American Journal of Tropical Medicine and Hygiene 2000;62:590‐7. [DOI] [PubMed] [Google Scholar]
Wang 2003a {published data only}
- Wang W, Xie S. Vitamin A as an adjuvant in the treatment of persistent pneumonia. Herald of Medicine 2003;22(4):258‐9. [Google Scholar]
Wang 2003b {published data only}
- Wang W, Xie S. Vitamin A as an adjuvant in the treatment of persistent pneumonia. Journal of Nanhua University (Medical Edition) 2003;31(1):103‐4. [Google Scholar]
West 1991 {published data only}
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338:67‐71. [DOI] [PubMed] [Google Scholar]
Willumsen 1997 {published data only}
- Willumsen JF, Simmank K, Filteau SM, Wagstaff LA, Tomkins AM. Toxic damage to the respiratory epithelium induces acute phase changes in vitamin A metabolism without depleting retinol stores of South African children. Journal of Nutrition 1997;127:1339‐43. [DOI] [PubMed] [Google Scholar]
Winkler 2005 {published data only}
- Winkler P, Vrese M, Laue C, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. International Journal of Clinical Pharmacology & Therapeutics 2005;43(7):318‐26. [MEDLINE: ; ISSN: 0946‐1965] [DOI] [PubMed] [Google Scholar]
Wu 2000 {published data only}
- Wu Y. Effect observation of Vitamin A, Vitamin E as an adjuvant in the treatment of bronchopneumonia. Heilongjiang Medicine and Pharmacy 2000;23(4):25‐6. [Google Scholar]
Yang 2002 {published data only}
- Yang LL, Jia J. Effect of vitamin A as adjuvant drug in the treatment of pneumonia. Journal of Changzhi Medical College 2002;16(2):139‐40. [Google Scholar]
Ying 1998 {published data only}
- Ying GY. Effect of vitamin A in the treatment of pneumonia. Journal of Shanxi Medical University 1998;29(3):262‐3. [Google Scholar]
Zhen 2003 {published data only}
- Zhen B. Effect analysis of vitamin A as a complementary agent in the treatment of new born baby infectious pneumonia. Journal of Pediatric Pharmacy 2003;9(1):48‐9. [Google Scholar]
Additional references
Boissel 1989
- Boissel JP, Blanchard J, Panak E, Peyrieux JC, Sacks H. Considerations for the meta‐analysis of randomised clinical trials. Summary of a panel discussion. Controlled Clinical Trials 1989;10:254‐81. [DOI] [PubMed] [Google Scholar]
Brown 2004
- Brown N, Roberts C. Vitamin A for acute respiratory infection in developing countries: a meta‐analysis. Acta Paediatrics 2004;93(11):1437‐42. [DOI] [PubMed] [Google Scholar]
Cochran 1954
- Cochran W. The combination of estimates from different experiments. Biometrics 1954;10:101‐29. [Google Scholar]
Ellison 1932
- Ellison J. Intensive vitamin therapy in measles. British Medical Journal 1932;II:708‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawzi 1993
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality, a meta‐analysis. Journal of the American Medical Association 1993;269:899‐903. [PubMed] [Google Scholar]
Fleiss 1986
- Fleiss JL. Analysis of data from multi clinical trials. Controlled Clinical Trials 1986;7:267‐75. [DOI] [PubMed] [Google Scholar]
Glasziou 1993
- Glasziou PP, Mackerras DEM. Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ 1993;306:366‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Julian 2009
- Julian PT Higgins, Douglas G Altman. Chapter 8: Assessing risk of bias in included studies. In: Julian PT Higgins, Sally Green editor(s). Cochrane Handbook for Systematic Reivews of Interventions. Chichester, UK: John Wiley & Sons, Ltd, September 2009. [Google Scholar]
Kirkwood 1995
- Kirkwood BR, Gove S, Rodgers S, Lob‐Levyt J, Arthur P, Campbell H. Potential interventions for the prevention of childhood pneumonia in developing countries: a systematic review. Bulletin of the World Health Organization 1995;73:793‐8. [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 (updated September 2009). The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2009. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Ross 1996
- Ross A. In: Sommer A, West K editor(s). Vitamin A deficiency: health, survival and vision. New York: Oxford University Press, 1996:251‐73. [Google Scholar]
Rundan 2004
- Rudan I, Tomaskovic L, Boschi‐Pinto C, Campbell H (on behalf of WHO Child Health Epidemiology Reference Group). Bulletin of the World Health Organization (Print ISSN 0042‐9686): Global estimate of the incidence of clinical pneumonia among children under five years of age. http://www.scielosp.org/scielo.php?pid=S0042‐96862004001200005&script=sci_arttext&tlng=en (accessed 30 March 2005). [PMC free article] [PubMed]
VAPWG 1995
- The Vitamin A and Pneumonia Working Group. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta‐analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. Bulletin of the World Health Organization 1995;73:609‐19. [PMC free article] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Micronutrient deficiencies: Vitamin A deficiency. http://www.who.int/nutrition/topics/vad/en/ (Accessed 10 July 2010) 2010.
Yang 2009
- Yang HM, Mao M, Wan CM. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [Google Scholar]
References to other published versions of this review
Ni 2005
- Ni J, Wei J, Wu T. Vitamin A for non‐measles pneumonia in children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003700.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2008
- Ni J, Wei J, Wu T. Vitamin A for non‐measles pneumonia in children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003700.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


