Abstract
Background
Hypertensive emergencies, marked hypertension associated with acute end‐organ damage, are life‐threatening conditions. Many anti‐hypertensive drugs have been used in these clinical settings. The benefits and harms of such treatment and the best first‐line treatment are not known.
Objectives
To answer the following two questions using randomized controlled trials (RCTs): 1) does anti‐hypertensive drug therapy as compared to placebo or no treatment affect mortality and morbidity in patients presenting with a hypertensive emergency? 2) Does one first‐line antihypertensive drug class as compared to another antihypertensive drug class affect mortality and morbidity in these patients?
Search methods
Electronic sources: MEDLINE, EMBASE, Cochrane clinical trial register. In addition, we searched for references in review articles and trials. We attempted to contact trialists. Most recent search August 2007.
Selection criteria
All unconfounded, truly randomized trials that compare an antihypertensive drug versus placebo, no treatment, or another antihypertensive drug from a different class in patients presenting with a hypertensive emergency.
Data collection and analysis
Quality of concealment allocation was scored. Data on randomized patients, total serious adverse events, all‐cause mortality, non‐fatal cardiovascular events, withdrawals due to adverse events, length of follow‐up, blood pressure and heart rate were extracted independently and cross checked.
Main results
Fifteen randomized controlled trials (representing 869 patients) met the inclusion criteria. Two trials included a placebo arm. All studies (except one) were open‐label trials. Seven drug classes were evaluated in those trials: nitrates (9 trials), ACE‐inhibitors (7), diuretics (3), calcium channel blockers (6), alpha‐1 adrenergic antagonists (4), direct vasodilators (2) and dopamine agonists (1). Mortality event data were reported in 7 trials. No meta‐analysis was performed for clinical outcomes, due to insufficient data. The pooled effect of 3 different anti‐hypertensive drugs in one placebo‐controlled trial showed a statistically significant greater reduction in both systolic [WMD ‐13, 95%CI ‐19,‐7] and diastolic [WMD ‐8, 95%CI, ‐12,‐3] blood pressure with antihypertensive therapy.
Authors' conclusions
There is no RCT evidence demonstrating that anti‐hypertensive drugs reduce mortality or morbidity in patients with hypertensive emergencies. Furthermore, there is insufficient RCT evidence to determine which drug or drug class is most effective in reducing mortality and morbidity. There were some minor differences in the degree of blood pressure lowering when one class of antihypertensive drug is compared to another. However, the clinical significance is unknown. RCTs are needed to assess different drug classes to determine initial and longer term mortality and morbidity outcomes.
Plain language summary
Pharmacological interventions for hypertensive emergencies
Hypertensive emergencies occur when high blood pressure is associated with the presence of acute end organ damage, such as heart attack or stroke. There is controversy as to when and which blood pressure drugs to use in these situations. This review looked for all studies where patients were randomized to one or more treatments to measure the effects of such therapies. The questions of the review were to see whether drug treatments affected death or cardiovascular morbidity or whether there were differences between drug treatments. The available evidence was insufficient to answer these questions.
Background
A hypertensive emergency is the clinical setting where a marked elevation of blood pressure is associated with acute end organ damage e.g.. encephalopathy or aortic dissection. As such it is a life‐threatening condition. The goal of treatment is to reverse the end organ damage, prevent adverse outcomes and prolong life. This review focuses on blood pressure lowering drugs that are used in this emergency setting. The management of hypertension in these emergency situations represents a significant therapeutic challenge. Many antihypertensive drug classes have been used with the objective of rapidly reducing blood pressure, and the expectation of reducing adverse clinical outcomes. This approach was first recommended by Gifford in 1959 [Gifford 1959] based on a series of 8 cases with hypertensive encephalopathy that were treated with sodium nitroprusside. Based on this case series evidence this approach has become and remained the standard of care and is currently recommended by most if not all guideline committees [such as JNC‐7]. At issue in this review is whether RCT evidence supports this approach and which drug classes are the most effective. Two published systematic reviews have addressed these issues. One compares different antihypertensive drugs, but it pools hypertensive emergency and urgency trials [Cherney 2002]. Urgencies are defined as marked elevated blood pressure in an otherwise stable patient (i.e., without acute end organ damage). In our opinion the urgency setting is very different from that of emergencies and needs to be reviewed separately. The second systematic review, a Cochrane review of interventions [BASC 2001] that alter blood pressure after acute stroke, is not limited to RCTs studying drugs to reduce blood pressure and includes RCTs whether or not the patients had elevated blood pressure. Therefore, it also does not answer the question raised here.
Objectives
General To find and quantify the randomized controlled trial (RCT) evidence for antihypertensive drug treatment of patients with a hypertensive emergency, defined as marked hypertension associated with acute end organ damage.
Specific To answer the following two questions: Does anti‐hypertensive drug therapy as compared to placebo or no treatment affect mortality and morbidity in patients with a hypertensive emergency? Does one first‐line antihypertensive drug class offer a therapeutic advantage, in terms of mortality and morbidity, over another in patients with a hypertensive emergency?
Methods
Criteria for considering studies for this review
Types of studies
All unconfounded, truly randomized control trials that compare a first‐line antihypertensive drug class versus placebo, no treatment or another first‐line antihypertensive drug class. Crossover trials are excluded. There is no language restriction.
Types of participants
Participants must meet the following hypertensive emergency definition: any clinical setting where patients present with marked elevation of blood pressure in the presence of acute end organ damage. Examples of acute end organ damage are the following: myocardial infarction, unstable angina, acute left ventricular failure with pulmonary oedema, acute aortic dissection, encephalopathy, stroke, and life‐threatening bleeding (intracerebral haemorrhage, subarachnoid haemorrhage). Thus, patients with marked elevation of blood pressure but without acute end organ damage (defined as urgencies) are not included. There is no evidence as to what constitutes "marked blood pressure elevation". Therefore, we have chosen blood pressure level(s) commonly used in clinical practice to mandate the use of antihypertensive drugs (along with other acute therapy such as pain management) in relevant clinical settings. For example, for patients with acute myocardial infarction a SBP greater or equal to 180 and or DBP ≥ 110 mm Hg is the threshold above which thrombolysis is contraindicated [ACC/AHA‐Antman 2004].For patients with acute aortic dissection or with left ventricular failure and pulmonary oedema a SBP greater or equal to 120 mm Hg and or DBP ≥70 mm Hg is the threshold for therapy [Dalen 1979,Mattu 2005]. For patients with intracranial haemorrhage or subarachnoid haemorrhage a SBP ≥ 160 mm Hg is the threshold because of a higher incidence of re‐bleeding above this level [Wilson 2005]. For patients with any other acute end organ damage setting a SBP ≥ 180 and or DBP ≥ 110 mm Hg is the defined threshold. We included all RCTs that included patients with these minimum or higher thresholds. In the case that a RCT does not define blood pressure inclusion criteria but had included only one category of patients (patients with pulmonary oedema, for example), then the mean base‐line blood pressure had to be equal to or greater than these defined thresholds. In the event that an RCT had included patients with different end organ damage clinical settings, a mean base‐line blood pressure of SBP ≥180 and or DBP ≥ 110 mm Hg is acceptable for inclusion. Note: Pregnancy‐related hypertensive emergencies are excluded from this review.
Types of interventions
Intervention: A first‐line anti‐hypertensive drug class.* Control: placebo, no treatment or a different first‐line anti‐hypertensive drug class.
*First‐line anti‐hypertensive drug classes included: nitrates, beta blockers, ACE‐inhibitors, diuretics, calcium channel blockers, dopamine agonists, alpha‐adrenergic antagonists, and direct vasodilators (diazoxide, hydralazine) .
Types of outcome measures
Primary:
Total serious adverse events
All cause mortality
Composite of non‐fatal cardiovascular events including: myocardial infarction, unstable angina, dissection of aortic aneurysm, acute renal failure, stroke, and respiratory failure (necessitating mechanical ventilation).
Secondary:
Weighted mean change in systolic blood pressure (SBP), diastolic blood pressure (DBP) and in heart rate (HR), during the treatment period.
Withdrawals due to adverse effects.
Search methods for identification of studies
See: Collaborative Review Group search strategy. We searched randomized controlled trials of all antihypertensive drugs used for hypertensive emergencies through the following databases of articles published from 1966 to August 2007: MEDLINE, EMBASE, COCHRANE clinical trial register. A comprehensive search strategy was used to identify all relevant articles. Review articles, and trials reference lists were also checked. Key words: controlled clinical trial, randomized controlled trials, meta‐analysis, severe/ accelerated/ crisis (es), hypertension, antihypertensive, emergencies: hypertensive encephalopathy, myocardial infarction, unstable angina, acute left ventricular failure, pulmonary oedema, stroke, subarachnoid / intracranial haemorrhage, aortic dissection ; nitrates: nitroglycerine, isosorbide, nitroprusside; beta‐adrenergic antagonist: acebutolol, atenolol, bisoprolol, carvedilol, esmolol, labetalol, metoprolol, nadolol, practolol, propranolol, sotalol, timolol; calcium channel blockers: Amlodipine, ranidipine, Azelnidipine, Barnidipine, Bencyclane, Benidipine, Bepridil, Cilnidipine, Cinnarizin, Clentiazem, Darodipine, Diltiazem, Efonidipine, Elgodipine, Etafenone, Fantofarone, Felodipine, Fendiline, Flunarizine, Gallopamil, Isradipine, Lacidipine, Lercanidipine, Lidoflazine, Lomerizine, Manidipine, Mibefradil, Nicardipine, Nifedipine, Niguldipine, Nilvadipine, Nimodipine, Nisoldipine, Nitrendipine, Perhexiline, Prenylamine, Semotiadil, Terodiline, Tiapamil, verapamil. ; angiotensin converting enzyme inhibitors: alacepril, benazepril, captopril, ceronapril, cilazapril, delapril, derapril, enalapril, fosinopril, idapril, Imidapril, Lisinopril, moexipril, moveltopril, perindopril, quinapril, ramipril, spirapril, temocapril, trandolapril, zofenopril; diuretics: hydrochlorothiazide, chlortalidone, furosemide, dopamine agonists: fenoldopam; alfa‐adrenergic antagonists: urapidil, ketanserine, phentolamine, prazosin, direct vasodilators: diazoxide, hydralazine. 1randomized controlled trial.pt. 2randomized controlled trials.mp. 3randomized controlled trial.mp. 4controlled clinical trial.pt. 5controlled clinical trials.mp. 6controlled clinical trial.mp. 7random allocation.mp. 8exp double‐blind method/ 9double‐blind.mp. 10exp single‐blind method/ 11single‐blind.mp. 12or/1‐11 13exp animal/ 1412 not 13 15clinical trial.pt. 16clinical trials.mp. 17clinical trial.mp. 18exp clinical trials/ 19(clin$ adj25 trial$).mp. 20((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp. 21random$.mp. 22exp research design/ 23research design.mp. 24or/15‐23 2524 not 13 2625 not 14 27comparative studies.mp. 28comparative study.mp. 29exp evaluation studies/ 30evaluation studies.mp. 31evaluation study.mp. 32follow up studies.mp. 33follow up study.mp. 34prospective studies.mp. 35prospective study.mp. 36(control$ or prospective$ or volunteer$).mp. 37or/27‐36 3837 not 13 3938 not (14 or 26) 4014 or 26 or 39 41Alacepril.mp. 42Benazepril.mp. 43captopril.mp. 44ceronapril.mp. 45cilazapril.mp. 46derapril.mp. 47enalapril.mp. 48enalaprilat.mp. 49fosinopril.mp. 50idapril.mp. 51imidapril.mp. 52Lisinopril.mp. 53moexipril.mp. 54moveltopril.mp. 55perindopril.mp. 56quinapril.mp. 57ramipril.mp. 58spirapril.mp. 59temocapril.mp. 60trandolapril.mp. 61zofenopril.mp. 62angiotensin converting enzyme inhibitor.mp. or Angiotensin‐Converting Enzyme Inhibitors/ 63acebutolol.mp. 64atenolol.mp. 65Bisoprolol.mp. 66esmolol.mp. 67labetalol.mp. 68metoprolol.mp. 69nadolol.mp. 70practolol.mp. 71propranolol.mp. 72sotalol.mp. 73timolol.mp. 74carvedilol.mp. 75Adrenergic beta‐Antagonists.mp. 76Amlodipine.mp. 77Aranidipine.mp. 78Azelnidipine.mp. 79Barnidipine.mp. 80Bencyclane.mp. 81Benidipine.mp. 82Bepridil.mp. 83Cilnidipine.mp. 84Cinnarizine.mp. 85Clentiazem.mp. 86Darodipine.mp. 87Diltiazem.mp. 88Efonidipine.mp. 89Elgodipine.mp. 90Etafenone.mp. 91Fantofarone.mp. 92Felodipine.mp. 93Fendiline.mp. 94Flunarizine.mp. 95Gallopamil.mp. 96Isradipine.mp. 97Lacidipine.mp. 98Lercanidipine.mp. 99Lidoflazine.mp. 100Lomerizine.mp. 101Manidipine.mp. 102Mibefradil.mp. 103Nicardipine.mp. 104Nifedipine.mp. 105Niguldipine.mp. 106Nilvadipine.mp. 107Nimodipine.mp. 108Nisoldipine.mp. 109Nitrendipine.mp. 110Perhexiline.mp. 111Prenylamine.mp. 112Semotiadil.mp. 113Terodiline.mp. 114Tiapamil.mp. 115verapamil.mp. 116calcium channel blocker.mp. or Calcium Channel Blockers/ 117nitroprusside.mp. 118nitroglycerine.mp. 119Nitroglycerin/ or nitroglycerine.mp. or Isosorbide Dinitrate/ 120nitrates.mp. or Nitrates/ 121urapidil.mp. 122Trimethaphan/ or trimethaphan camsylate.mp. 123reserpine.mp. 124phentolamine.mp. 125methyldopa.mp. 126labetalol.mp. 127ketanserine.mp. 128hydralazine.mp. 129guanethidine.mp. 130fenoldopam.mp. or FENOLDOPAM/ 131diazoxide.mp. 132clonidine.mp. 133thiazide$.mp. 134hydrochlorothiazide.mp. 135chlorthalidone.mp. or Chlorthalidone/ 136furosemide.mp. or Furosemide/ 137or/41‐136 13840 and 137 139myocardial infarction.mp. 140unstable angina.mp. 141acute left ventricular failure.mp. 142Pulmonary Edema/ or pulmonary oedema.mp. 143stroke.mp. 144life‐threatening bleeding.mp. 145Aneurysm, Dissecting/ or aortic dissection.mp. 146Intracranial Hemorrhages/ or Cerebral Hemorrhage/ or intracranial haemorrhage.mp. 147Intracranial Aneurysm/ or Subarachnoid Hemorrhage/ or subarachnoid haemorrhage.mp. 148or/139‐147 149hypertension.ti,ab. 150high blood pressure.ti,ab. 151blood pressure.ti,ab. 152or/149‐151 153pulmonary artery hypertension.mp. 154pulmonary hypertension.mp. 155portal hypertension.mp. 156or/153‐155 157152 not 156 158148 and 157 159hypertensive emergencies.ti,ab. 160hypertensive emergency.ti,ab. 161hypertensive urgency.ab,ti. 162hypertensive urgencies.ti,ab. 163hypertensive crisis.ti,ab. 164hypertensive crises.ti,ab. 165acute end organ damage.mp. 166or/158‐165 167138 and 166
Data collection and analysis
Data abstraction: Two reviewers (MIP & VM) independently decided whether a trial was included. They also independently extracted and entered the data from the included studies. Discrepancies were resolved by discussion. Absence of consensus was resolved by a third reviewer (JMW). A modified Cochrane quality scoring system was used for concealment of allocation and blinding: A (adequate & double‐blind), B (unclear & single‐blind or open label), C (clearly inadequate & open‐label). The two reviewers (MIP & VM) also independently assessed the quality of studies. Authors were contacted in case of missing information.
Analyses: For the synthesis and analysis of the data Cochrane Review Manager 4.2.9 was used. Relative and absolute risk differences (with 95% confidence interval) were calculated for dichotomous outcomes for each trial on an intention to treat basis. Heterogeneity between trials results was tested using chi‐squared test, where p less than 0.05 was taken to indicate significant heterogeneity. The fixed effect model was used when there was homogeneity and the random effect model was used to test for statistical significance where there was heterogeneity. Trials were not sub‐classified according to dose or dosing regimen. Data for blood pressure was combined using a weighted mean difference method, whereby the trials are weighted according to the number of subjects in the trial and the within‐study variance. Some of the trials did not report a within‐study variance for blood pressure reduction. In these studies standard deviation (SD) was imputed using the following hierarchy: 1. Pooled standard deviation calculated either from the t‐statistic corresponding to an exact p‐value reported or from the 95% confidence interval of the mean difference between treatment group and comparative group. 2. Standard deviation of blood pressure/heart rate at the end of treatment. 3. Standard deviation of blood pressure/heart rate at baseline (except if this measure is used for entry criteria). 4. Weighted mean standard deviation of change in blood pressure/heart rate calculated from at least 3 other trials using the same drug and dose regimen. 5. Weighted mean standard deviation of change in blood pressure/heart rate calculated from other trials using the same drug. 6. Weighted mean standard deviation of change in blood pressure/heart rate calculated from all other trials (any drug and dose). Several sensitivity analyses were pre‐planned to test robustness including the use of both fixed and random effects models, 95 and 99% confidence intervals, and quality of trials. Also sensitivity analyses were pre‐planned according to the clinical setting and to the class of drug.
Results
Description of studies
Fifteen randomized controlled trials (869 patients) were found that satisfied the inclusion criteria. Two trials were placebo‐controlled[Hamilton 1996, Pastorelli 1991]. Only one trial [Hamilton 1996] was confirmed to be double‐blind, while the rest were open‐label. No trial was designed for or had the power to detect differences in clinical outcomes. The largest trial consisted of 133 patients [Schreiber 1998]. The longest trial [Elliott 1990] lasted 10 days. Most of the trials reported data for only 2 to 6 hours. Seven drug classes were evaluated: nitrates (9 trials), ACE‐inhibitors (7), calcium channel blockers (6), peripheral alpha‐1 blockers (4), diuretics (3), direct vasodilators (2) and dopamine agonists (1).
All trials had patients with elevated blood pressure in the presence of acute end organ damage. Blood pressure entry criteria differed among trials. Four trials were included on the basis of their mean blood pressure values at baseline [Beltrame 1998, Hamilton 1996, Nelson 1983, Pastorelli 1991] .Seven trials included exclusively patients with acute pulmonary edema [Beltrame 1998; Hamilton 1996; Hirschl 1999; Nelson 1983; Schreiber 1998; Verma 1987; Yang 2004]. One trial included exclusively patients with hypertensive encephalopathy [DANISH II 1986]. There was no trial that included exclusively patients with acute aortic dissection or acute myocardial infarction. Thus, the rest of 7 trials included a diverse population with different acute end organ damage. Only two trials [Angeli 1991; Marigliano 1988] reported the standard deviation of the change of blood pressure. In the rest of the trials this measure of variability was imputed from the standard deviation at endpoint. Additional information was required and requested from all included trials. One trialist [Angeli 1991] provided missing information in the original publication. The rest of the trialists did not reply to our request.
We excluded 27 clinical trials for several reasons:
Several trials mixed patients with and without acute end organ damage in the same RCT (12 trials ‐Bussmann 1992; Conen 1988; Dadkar 1993; Marghli 1997; Moritz 1989; Neutel 1994; Nielsen 1980; Panacek 1995; Perez 1991; Risler 1998; Rohr 1994; Spah 1988).
Other trials included patients without explicitly stating whether patients had acute end organ damage or not (7 trials‐ Ceyhan 1990; Guerrera 1990; Pascale 1992; Pilmer 1993; Pujadas 1987; Reisin 1990; Zampaglione 1994).
Some trials included non‐randomized participants in the trial's results (1 trial ‐ Franklin 1986).
One trial did not report any of the outcomes of interest (1 trial ‐ Bertel 1983).
Two trials did not fulfilling blood pressure threshold criteria (Borghi 1999; Lisk 1993).
One was a cross‐over trial (Nelson 1984).
Two trials had wrong comparators (1 compared different doses of the same combination therapy ‐ Cotter 1998; 1 compared two drugs of the same class ‐ Yoshida 1998).
One RCT only included responders to a previously given antihypertensive therapy (Annane 1996).
Two out of 27 excluded trials involved a beta‐blocker arm and 18 / 27 excluded trials involved a calcium channel blocker arm. One excluded trial studied exclusively patients with acute aortic dissection (Yoshida 1998).
Risk of bias in included studies
All studies, except one [Hamilton 1996] were open‐label trials. The method of randomization was not reported in 8 trials. The method to achieve concealment of allocation was reported in only two trials [DANISH II 1986; Hamilton 1996].
Effects of interventions
Total serious adverse events: No trial reported total serious adverse events.
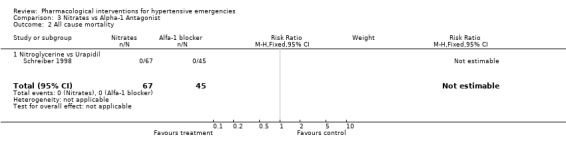
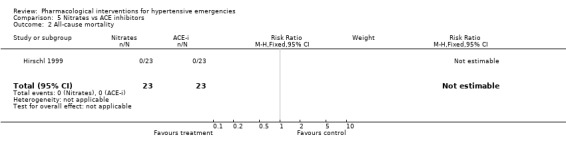
All‐cause mortality: Mortality was reported in 7 trials [Angeli 1991; Beltrame 1998; Hirschl 1999; DANISH II 1986; Nelson 1983; Verma 1987; Schreiber 1998] and totalled 6 deaths in 3 RCTs. The group to which the dead patients were originally allocated was not reported for 5 of the deaths. In one RCT, a patient treated with hydralazine died of a rupture of the inter‐ventricular septum [Verma 1987]. In 4 trials mortality was reported as nil. In 8 trials there was no mention of mortality. It is possible that there were no deaths during the short range of follow‐up (6‐24 hours), but it is impossible to be certain.
Non‐fatal cardiovascular events: Composite Cardiovascular events were reported in 5 trials [Beltrame 1998, Hamilton 1996, Hirschl 1999, DANISH II 1986, Schreiber 1998]. No trial reported cardiovascular events as a composite. It was not possible to extract events from the original trials and analyze them as a composite due to a risk of double‐counting the events.
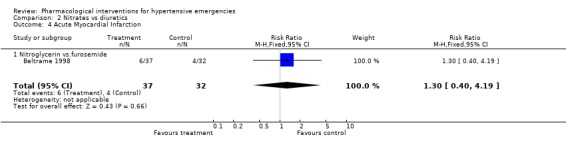
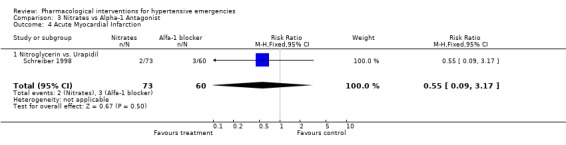
Myocardial Infarction One placebo‐controlled trial [Hamilton 1996] reported this outcome. There was no statistically significant difference between ACEi and placebo (RR 0.72, 95%CI 0.31 ‐1.72). Three head to head trials reported this outcome. There was no statistical difference in myocardial infarctions between nitrates (2.7%) and alfa‐adrenergic antagonist (5%) [RR 0.55, 95%CI 0.09‐3.17, Schreiber 1998]; or nitrates (16%) vs. diuretics (12.5%) [RR 1.30,95%CI 0.40‐4.19, Beltrame 1998]; or between diazoxide (3.5%) vs. dihydralazine (4%), [RR 0.86, 95%CI 0.06‐12.98, DANISH II 1986].
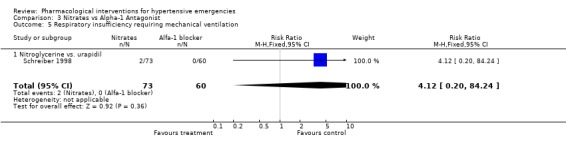
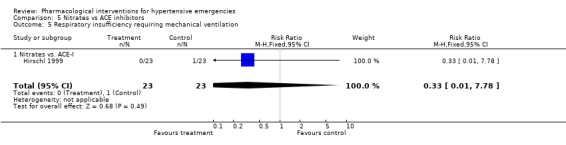
Pulmonary edema requiring mechanical ventilation Three trials [Hamilton 1996; Hirschl 1999; Schreiber 1998] reported this outcome. There was no meta‐analysis performed since there was only one trial for each comparison. There was no statistically significant difference between captopril and placebo (RR 0.40, 95%CI 0.09 ‐1.86), nitrates and alfa‐adrenergic antagonist (RR 4.12, 95%CI 0.20‐84.24) or between nitrates and ACE‐Inhibitor (RR 0.33, 95%CI 0.01‐7.78).
Other than the above, the trials did not report any of our list of CV events (unstable angina, dissection of aortic aneurysm, acute renal failure, or stroke). An additional cardiovascular event was reported that was not on our list: asystole, which happened in one patient randomized to an ACE inhibitor [Hirschl 1999].
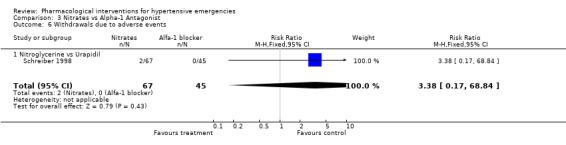
Withdrawals due to adverse events: Only one trial comparing an alpha‐blocker with nitroglycerine reported withdrawal due to adverse events [Schreiber 1998]. There were no significant differences between these two drugs classes (5% vs 2.7%; [RR 3.38, 95%CI 0.17‐68.84]).
Weighted mean change in blood pressure and heart rate during treatment: For this secondary outcome all trials provided some data and we were able to pool this data (see meta view). Drug vs. placebo or no treatment Although we included two placebo‐controlled trials, only one provided systolic or diastolic blood pressure (BP) data [Pastorelli 1991] and this was limited to one hour of follow‐up. In this trial, 3 classes of antihypertensives were included : calcium channel blockers, angiotensin converting enzyme inhibitors, and alpha‐1 adrenergic antagonists. The pooled effect showed a statistically significant greater reduction in both systolic [WMD ‐13.14, 95%CI ‐19.48,‐6.80] and diastolic [WMD ‐8.03, 95%CI ‐12.61,‐3.45] blood pressure with antihypertensive therapy. There was no data on heart rate. It was not possible to extract BP data from the other placebo‐controlled trial [Hamilton 1996]. In addition to not reporting any measurement of variability, this trial reported BP data as change in mean arterial pressure (MAP).
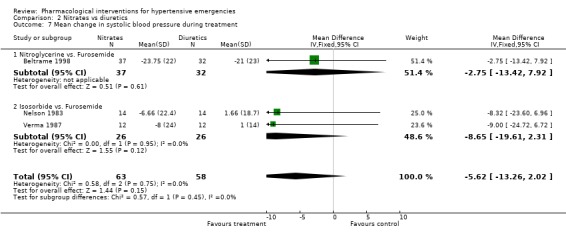
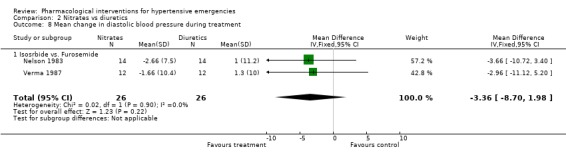
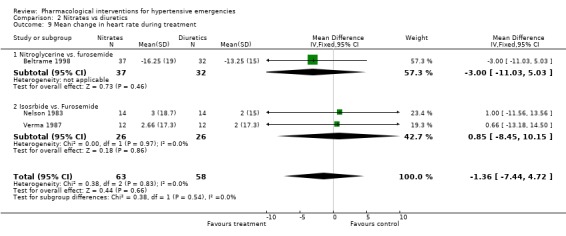
Nitrates vs. diuretics Three trials compared nitrates to diuretics [Beltrame 1998; Nelson 1983; Verma 1987]. Furosemide was the common diuretic used in all of them with two nitrates, nitroglycerine and isosorbide as comparators. Neither systolic nor diastolic blood pressure lowering effect was statistically different between the two classes of drugs. However, in Beltrame 1998, the systolic blood pressure lowering effect of both drugs was greater (‐21 mm Hg for furosemide; ‐23.75 mm Hg for nitroglycerin) than that reported in the other two trials [+1.0, +1.6 mm Hg for furosemide groups; and ‐6,‐8 mm Hg for isosorbide groups, respectively ]. The reasons for that difference across trials are not clear. Despite these differences, heterogeneity was not present when pooling all these three trials. Heart rate change was also not significantly different for both classes of drugs.
Nitrates vs. alpha‐1 antagonist Two trials compared the alpha‐1 adrenergic antagonist (A1A), urapidil, with nitrates [Hirschl 1997; Schreiber 1998]. The first trial used nitroprusside and the second used nitroglycerine as comparator. The systolic blood pressure lowering effect of the two nitrates was similar (‐58.4 mmHg for nitroprusside and ‐59.5 mmHg for nitroglycerine). However, the effect of urapidil (administrated at the same dose in both trials) was very different (‐37.6 mmHg and ‐73.5 mmHg). A similar discrepancy was seen for diastolic blood pressure. This heterogeneity precluded the pooling of these trials in a meta‐analysis for these outcomes.
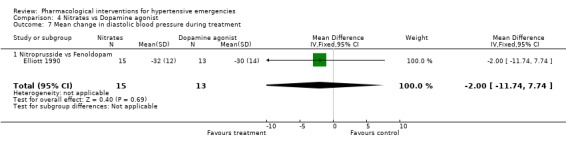
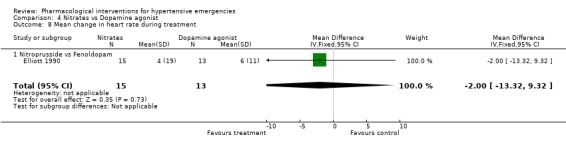
Nitrates vs. dopamine agonist For this comparison one trial was included [Elliott 1990]. During 4 hours of treatment, nitrates were associated with a statistically significant greater reduction in systolic blood pressure as compared with a dopamine agonist (WMD ‐14.00, 95%CI ‐27.72, ‐0.28). There were no differences between these classes in diastolic blood pressure or heart rate.
Nitrates vs. ACE‐inhibitors One trial compared a nitrate with an ACE inhibitor [Hirschl 1999]. No statistically significant difference was found between the two groups in systolic or diastolic blood pressure or heart rate.
Nitrates vs. calcium channel blockers In two trials [Rubio‐G 1999; Yang 2004] calcium channel blockers were not associated with statistically significant differences in systolic or diastolic blood pressure as compared to nitrates. Using the fixed effect model, CCBs were associated with statistically significant increase in heart rate as compared to the nitrates (WMD 11.76, 95%CI 4.45,19.07). However there was significant heterogeneity across trials and this increase was no longer statistically significant when a random effect model was used.
Nitrates vs. direct vasodilator For this comparison one trial was included [Verma 1987]. There was no statistical difference in systolic or diastolic blood pressure reduction between the two drugs. There was also no significant difference between these classes in heart rate change.
ACE inhibitors vs. calcium channel blockers Four trials [Angeli 1991; Marigliano 1988; Pastorelli 1991; Wu 1993] compared an ACE‐Inhibitor with a CCB. The pooled data shows that CCBs were associated with a significantly greater reduction in diastolic blood pressure as compared with ACE‐I (WMD 7.86, 95% CI [4.92, 10.81]. No statistically significant difference was found between the two groups in the reduction of systolic blood pressure. In 3 trials that reported heart rate changes [Angeli 1991; Marigliano 1988; Wu 1993] CCBs were associated with a significant increase in heart rate as compared with ACE‐Inhibitors (WMD 22.91, 95%CI 19.8, 26.01). However there was significant heterogeneity across trials and this increase was no longer significant when a random effect model was used.
ACE inhibitors vs. alpha‐1 adrenergic antagonist Two trials [Pastorelli 1991; Wu 1993] compared an ACE‐Inhibitor with an alpha‐1 adrenergic antagonist (A1A). Both trials used captopril as comparator but one trial used prazosin and the other used ketanserin. The pooled data shows that ACE‐I were associated with a significantly greater reduction in both systolic and diastolic blood pressure as compared with A1A (SBP WMD ‐20, 95% CI [‐22.85,‐17.39; DBP WMD ‐3.70, 95% CI [‐7.08,‐0.31]). For SBP outcome there was statistically significant heterogeneity across trials. However the difference was still significant when the random effects model was used. No statistically significant difference was found between the two groups in the heart rate change in the only trial reporting that outcome [Wu 1993].
Diazoxide vs. hydralazine For this comparison one trial [DANISH II 1986], which dealt with exclusively hypertensive encephalopathy patients, was included. During 4 hours of treatment, hydralazine was associated with a statistically significant greater reduction in both systolic (WMD 13.56, 95%CI 3.06, 24.06) and diastolic (WMD 14.67, 95%CI 8.01, 21.33) blood pressure as compared with diazoxide (WMD ‐14.00, 95%CI ‐27.72, ‐0.28). It is important to mention, though, that there was no measure of variability reported in this trial. Therefore, we imputed the standard deviation of the change according to our hierarchy from other trials (Last option: weighted mean standard deviation of change from all trials; any drug any dose). There was no heart rate data reported.
Discussion
This is the first systematic review investigating mortality and morbidity outcomes for all RCTs of drug treatment for hypertensive emergencies. A systematic review that combined hypertensive emergencies and urgencies [Cherney 2002] did not include 11 trials included in our systematic review. Furthermore, Cherney's review mixed randomized with non‐randomized trials. The only other relevant systematic review in relation to hypertensive emergencies is that conducted for acute stroke by BASC 2001. We excluded one trial [Lisk 1993; n =16 patients] that the BASC 2001 systematic review had included. The reason for excluding it was because the blood pressure criteria in this trial (>170/95 mmHg) did not meet our blood pressure threshold criteria (SBP≥ 180 and or DBP ≥ 110 mm Hg). This exclusion does not affect our conclusion for clinical outcomes as this trial did not report clinical outcomes. The other BASC 2001 trials were not included because blood pressure at baseline was not elevated. Thus, these clinical trials did not include hypertensive emergency patients, as we have defined it. One of the limitations in our review is that most of the included trials were small (average 58 patients per trial). Furthermore, with the exception of Hamilton 1996 all trials were of poor quality. Three included trials deserve further discussion. Hamilton 1996, the only double‐blind trial, includes patients with acute pulmonary edema and high blood pressure, and it compared captopril vs. placebo. It demonstrates that this high quality and double‐blind trial was ethical and feasible. The DANISH II 1986 trial was the only trial that included patients exclusively with hypertensive encephalopathy. This was a well organized multicentre trial, conducted in Denmark, comparing diazoxide vs. dihydralazine. Due to its study design, the ethical committee accepted that the informed consent could not be obtained from patients as all of them had symptoms of hypertensive encephalopathy. A downside of this study is the fact that the trialists reported their results in duplicate publications that did not cite the other publications [The original publication, Krogsgaard 1983, is not cited in the other duplicate publications, McNair 1985‐D, McNair 1986; Krogsgaard 1986‐D]. In addition, blood pressure values were not the same in the different publications, and none of the publications measures blood pressure variability. The largest trial, Schreiber 1998, included 133 patients with acute pulmonary edema plus high blood pressure, in an out‐of‐hospital setting, who were randomized to receive either nitroglycerin or urapidil. The ethical committee (Vienna, Austria) agreed that no informed consent had to be obtained at the time of inclusion for randomization. However, 16% of all randomized patients were excluded from the analyses which could potentially bias the results. Consistent with this, there was significant heterogeneity when this trial was combined with another trial studying the same comparison groups. In 19 of the excluded trials it was not possible to determine how many patients had acute end organ damage or merely had elevation of blood pressure. We believe that it would be misleading to include these trials in this review as the impact of antihypertensive drugs is potentially different. If individual patient data could be obtained, the patients with acute end organ damage could be added to our review. It was perhaps surprising and definitely disappointing that we could find no randomized controlled trial evidence to answer the first question we have posed: Does antihypertensive therapy as compared to placebo or no treatment change mortality and morbidity in patients with hypertensive emergencies? The one available placebo‐controlled trial demonstrated that blood pressure was reduced with drugs as compared to the control treatment, however, it was too small and of too short duration to assess morbidity and mortality. We feel it is important for physicians to know that this is one of the clinical settings where treatment is not supported by RCT evidence. Despite the lack of evidence it is not hard to accept the necessity of lowering blood pressure in those clinical settings where the excessive increases in blood pressure are the cause of the end organ damage. However, this is not necessarily the best approach in settings where the excessive elevations of blood pressure are probably caused by end organ damage such as high BP in the presence of a cerebrovascular accident. The presently accepted approach for the immediate treatment of hypertensive emergencies in clinical practice is primarily based on a series of cases published in 1959 [Gifford 1959]. In this study carried out over a period of 18 months the author demonstrated the ability to reduce blood pressure with nitroprusside, within minutes, in 8 patients with hypertensive emergencies (mostly patients with hypertensive encephalopathy) whose blood pressures had remained elevated after treatment with reserpine or hydralazine. However, he did not report clinical outcomes so we do not know whether these patients did better as a result of the blood pressure lowering. Gifford recommended prompt blood pressure reduction in clinical settings other than hypertensive encephalopathy such as intracerebral or subarachnoid hemorrhage or acute left ventricular failure. The lack of RCT evidence leaves the distinct possibility that in some clinical settings defined as hypertensive emergencies immediate antihypertensive therapy could be doing more harm than good. There is a hypertensive emergency not included in the present systematic review, eclampsia. Due to its pathophysiology and the involvement of the infant as well as the mother , we felt this clinical entity must be studied separately from other hypertensive emergencies and include outcomes in the infant as well as the mother. There is a Cochrane systematic review [Duley 2006] that has studied the drugs for treatment of very high blood pressure during pregnancy. However, Duley's SR was not limited to patients with eclampsia and did not separately report outcomes in the eclampsia patients. To the best of our knowledge there is no systematic review dealing exclusively with eclampsia and anti‐hypertensive treatment. Thus, a systematic review in this specific area is currently needed. The present review also does not provide any mortality and morbidity evidence from RCTs to inform clinicians as to which first‐line antihypertensive drug class provides more benefit than harm in hypertensive emergencies. This lack of evidence was due to the fact that the trials were too small, did not follow the patients for a long enough period of time and frequently failed to report all important outcomes. In addition all the RCTs except one were open‐label trials and therefore concealment of allocation was not possible in most cases. Although, these shortcomings of the trials would not likely affect mortality and morbidity outcomes, they could bias blood pressure and heart rate data. Neither did we find RCTs that compared different strategies to reduce blood pressure. Thus, how fast or how much blood pressure should be lowered in hypertensive emergencies remains unknown. Although it is unproven, it is highly likely that antihypertensive therapy is an overall benefit in a hypertensive emergency and therefore a placebo controlled trial to prove this would be unethical. What is clear is that this is a clinical area where properly conducted randomised trials are badly needed. At the present time RCTs could be conducted to compare different drug classes and treatment strategies e.g.. aggressive rapid lowering of blood pressure to a target versus lowering the blood pressure slowly at a defined rate such as 5‐10% every 2 hours. What is also clear from this review is that any trial must follow patients long‐term and document mortality and morbidity. One of the best examples of an adequate RCT in an emergency setting is the CRASH trial [Roberts 2004] where 10,000 patients with acute head injury were randomized to intravenous steroids or placebo. Its approach to handle ethical issues could serve as model when conducting a trial with hypertensive emergency patients.
Authors' conclusions
Implications for practice.
There is no evidence from RCTs that anti‐hypertensive drugs reduce mortality or morbidity in patients with hypertensive emergencies, defined as marked hypertension associated with acute end organ damage. Furthermore, there is insufficient RCT evidence to determine which drug or drug class is most effective in reducing mortality and morbidity. There were some minor differences in degree of blood pressure lowering between drug classes. However, the clinical significance is unknown. This review demonstrates a blood pressure lowering efficacy for: nitrates, ACE inhibitors, diuretics, alpha‐adrenergic antagonist, calcium channel blockers and dopamine agonists. Nitrates (including nitroprusside) have been studied the most. Therefore, if a hypertensive emergency patient cannot be treated as part of an RCT and a nitrate is available, it is a reasonable choice of therapy.
Implications for research.
Randomized controlled trials are needed to assess different blood pressure lowering strategies and different first‐line drug classes in patients with hypertensive emergencies. Outcomes in such trials must be mortality and total serious adverse events at different times of follow‐up such as 7 days, 1 month and including at least 6 months of follow‐up of all patients.
What's new
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Contact details updated |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
| 19 October 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to acknowledge help and advice from Dr Ken Bassett, from Stephen Adams for retrieving trials, and Benji Heran, Michelle Wong and Jenny Chen for comments on a draft. We also acknowledge the trialists who provided us with additional information from their studies.
Data and analyses
Comparison 1. Antihypertensive vs. Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.31, 1.72] |
| 4.1 ACE‐inhibitors vs. placebo | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.31, 1.72] |
| 5 Respiratory insufficiency requiring mechanical ventilation | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.86] |
| 5.1 ACE‐I vs placebo | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.86] |
| 6 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Mean change in systolic blood pressure during treatment | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐13.14 [‐19.48, ‐6.80] |
| 7.1 CCB vs. placebo | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐19.46 [‐31.17, ‐7.75] |
| 7.2 ACE‐I vs. placebo | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐15.36 [‐26.53, ‐4.19] |
| 7.3 A1A vs. placebo | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐6.46 [‐16.69, 3.77] |
| 8 Mean change in diastolic blood pressure during treatment | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐8.03 [‐12.61, ‐3.45] |
| 8.1 CCB vs. Placebo | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐10.36 [‐18.39, ‐2.33] |
| 8.2 ACE‐I vs. placebo | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐6.94 [‐14.65, 0.77] |
| 8.3 A1A vs. placebo | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐6.86 [‐14.93, 1.21] |
| 9 Mean change in heart rate during treatment | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.4. Analysis.
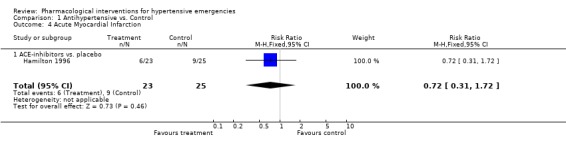
Comparison 1 Antihypertensive vs. Control, Outcome 4 Acute Myocardial Infarction.
1.5. Analysis.
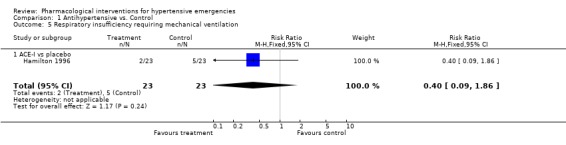
Comparison 1 Antihypertensive vs. Control, Outcome 5 Respiratory insufficiency requiring mechanical ventilation.
1.7. Analysis.
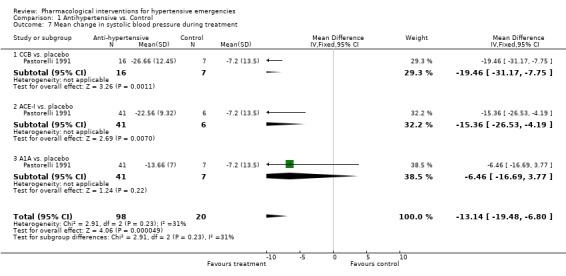
Comparison 1 Antihypertensive vs. Control, Outcome 7 Mean change in systolic blood pressure during treatment.
1.8. Analysis.
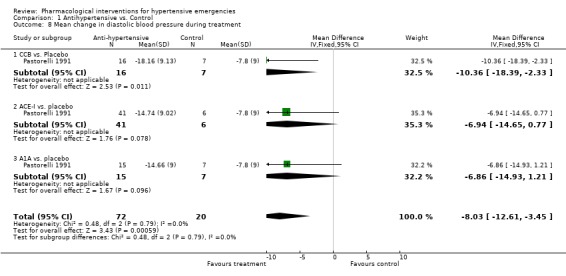
Comparison 1 Antihypertensive vs. Control, Outcome 8 Mean change in diastolic blood pressure during treatment.
Comparison 2. Nitrates vs diuretics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.40, 4.19] |
| 4.1 Nitroglycerin vs.furosemide | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.40, 4.19] |
| 5 Respiratory insufficiency requiring mechanical ventilation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Mean change in systolic blood pressure during treatment | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐5.62 [‐13.26, 2.02] |
| 7.1 Nitroglycerine vs. Furosemide | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐2.75 [‐13.42, 7.92] |
| 7.2 Isosorbide vs. Furosemide | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐8.65 [‐19.61, 2.31] |
| 8 Mean change in diastolic blood pressure during treatment | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐8.70, 1.98] |
| 8.1 Isosrbide vs. Furosemide | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐8.70, 1.98] |
| 9 Mean change in heart rate during treatment | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐7.44, 4.72] |
| 9.1 Nitroglycerine vs. furosemide | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐11.03, 5.03] |
| 9.2 Isosrbide vs. Furosemide | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [‐8.45, 10.15] |
2.4. Analysis.
Comparison 2 Nitrates vs diuretics, Outcome 4 Acute Myocardial Infarction.
2.7. Analysis.
Comparison 2 Nitrates vs diuretics, Outcome 7 Mean change in systolic blood pressure during treatment.
2.8. Analysis.
Comparison 2 Nitrates vs diuretics, Outcome 8 Mean change in diastolic blood pressure during treatment.
2.9. Analysis.
Comparison 2 Nitrates vs diuretics, Outcome 9 Mean change in heart rate during treatment.
Comparison 3. Nitrates vs Alpha‐1 Antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All cause mortality | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Nitroglycerine vs Urapidil | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.09, 3.17] |
| 4.1 Nitroglycerin vs. Urapidil | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.09, 3.17] |
| 5 Respiratory insufficiency requiring mechanical ventilation | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.20, 84.24] |
| 5.1 Nitroglycerine vs. urapidil | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.20, 84.24] |
| 6 Withdrawals due to adverse events | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.17, 68.84] |
| 6.1 Nitroglycerine vs Urapidil | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.17, 68.84] |
| 7 Mean change in systolic blood pressure during treatment | 2 | 193 | Mean Difference (IV, Fixed, 95% CI) | ‐3.94 [‐9.31, 1.43] |
| 7.1 Nitroprusside vs Urapidil | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐20.80 [‐28.27, ‐13.33] |
| 7.2 Nitroglycerine vs Urapidil | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [6.29, 21.71] |
| 8 Mean change in diastolic blood pressure during treatment | 2 | 193 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐2.70, 4.23] |
| 8.1 Nitroprusside vs Urapidil | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐10.80 [‐16.27, ‐5.33] |
| 8.2 Nitroglycerine vs Urapidil | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 8.5 [4.03, 12.97] |
| 9 Mean change in heart rate during treatment | 2 | 193 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.93, 3.72] |
| 9.1 Nitroprusside vs Urapidil | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐6.64, 8.64] |
| 9.2 Nitroglycerine vs Urapidil | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐8.73, 3.73] |
3.2. Analysis.
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 2 All cause mortality.
3.4. Analysis.
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 4 Acute Myocardial Infarction.
3.5. Analysis.
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 5 Respiratory insufficiency requiring mechanical ventilation.
3.6. Analysis.
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 6 Withdrawals due to adverse events.
3.7. Analysis.
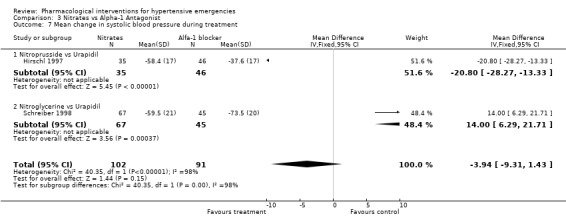
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 7 Mean change in systolic blood pressure during treatment.
3.8. Analysis.
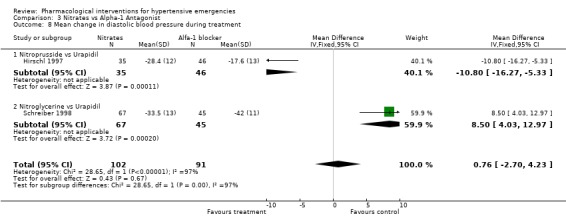
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 8 Mean change in diastolic blood pressure during treatment.
3.9. Analysis.
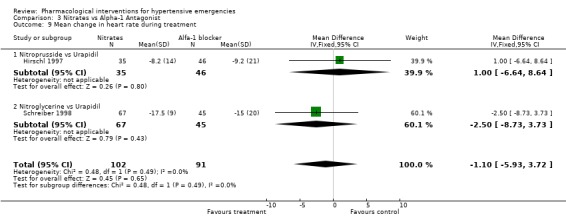
Comparison 3 Nitrates vs Alpha‐1 Antagonist, Outcome 9 Mean change in heart rate during treatment.
Comparison 4. Nitrates vs Dopamine agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute myocardial infarction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean change in systolic blood pressure during treatment | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐14.00 [‐27.72, ‐0.28] |
| 6.1 Nitroprusside vs Fenoldopam | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐14.00 [‐27.72, ‐0.28] |
| 7 Mean change in diastolic blood pressure during treatment | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐11.74, 7.74] |
| 7.1 Nitroprusside vs Fenoldopam | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐11.74, 7.74] |
| 8 Mean change in heart rate during treatment | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐13.32, 9.32] |
| 8.1 Nitroprusside vs Fenoldopam | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐13.32, 9.32] |
4.6. Analysis.
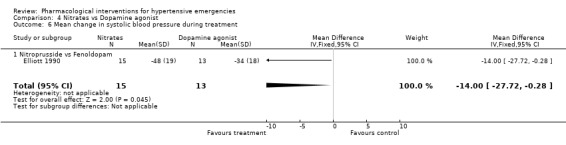
Comparison 4 Nitrates vs Dopamine agonist, Outcome 6 Mean change in systolic blood pressure during treatment.
4.7. Analysis.
Comparison 4 Nitrates vs Dopamine agonist, Outcome 7 Mean change in diastolic blood pressure during treatment.
4.8. Analysis.
Comparison 4 Nitrates vs Dopamine agonist, Outcome 8 Mean change in heart rate during treatment.
Comparison 5. Nitrates vs ACE inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Myocardial Infarction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Respiratory insufficiency requiring mechanical ventilation | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 5.1 Nitrates vs. ACE‐I | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 6 Withdrawals due to adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Mean change in systolic blood pressure during treatment | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 3.33 [‐7.37, 14.03] |
| 7.1 Nitrogycerine vs Enalaprilat | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 3.33 [‐7.37, 14.03] |
| 8 Mean change in diastolic blood pressure during treatment | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐6.69, 6.03] |
| 8.1 Nitrogycerine vs Enalaprilat | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐6.69, 6.03] |
| 9 Mean change in heart rate during treatment | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 4.5 [‐1.18, 10.18] |
| 9.1 Nitrogycerine vs Enalaprilat | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 4.5 [‐1.18, 10.18] |
5.2. Analysis.
Comparison 5 Nitrates vs ACE inhibitors, Outcome 2 All‐cause mortality.
5.5. Analysis.
Comparison 5 Nitrates vs ACE inhibitors, Outcome 5 Respiratory insufficiency requiring mechanical ventilation.
5.7. Analysis.
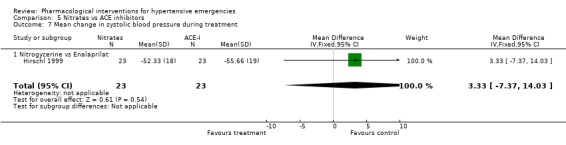
Comparison 5 Nitrates vs ACE inhibitors, Outcome 7 Mean change in systolic blood pressure during treatment.
5.8. Analysis.
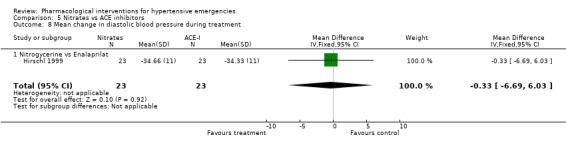
Comparison 5 Nitrates vs ACE inhibitors, Outcome 8 Mean change in diastolic blood pressure during treatment.
5.9. Analysis.
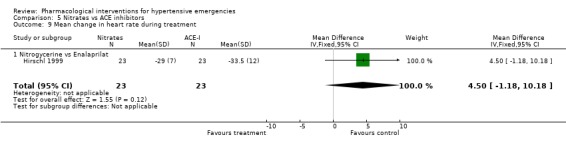
Comparison 5 Nitrates vs ACE inhibitors, Outcome 9 Mean change in heart rate during treatment.
Comparison 6. Nitrates vs. CCB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawals due to adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean change in systolic blood pressure during treatment | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.67 [‐3.97, 13.32] |
| 6.1 Isosorbide dinitrate vs nifedipine | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.74, 13.74] |
| 6.2 Nitroprusside vs. Nicardipine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 7.75 [‐6.82, 22.32] |
| 7 Mean change in diastolic blood pressure during treatment | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐2.44, 3.59] |
| 7.1 Isosorbide dinitrate vs nifedipine | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.30, 3.30] |
| 7.2 Nitroprusside vs. Nicardipine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.5 [‐3.94, 10.94] |
| 8 Mean change in heart rate during treatment | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐11.76 [‐19.07, ‐4.45] |
| 8.1 Isosorbide dinitrate vs nifedipine | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐23.0 [‐32.64, ‐13.36] |
| 8.2 Nitroprusside vs. Nicardipine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.5 [‐7.72, 14.72] |
6.6. Analysis.
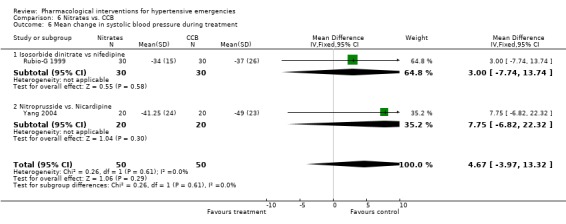
Comparison 6 Nitrates vs. CCB, Outcome 6 Mean change in systolic blood pressure during treatment.
6.7. Analysis.
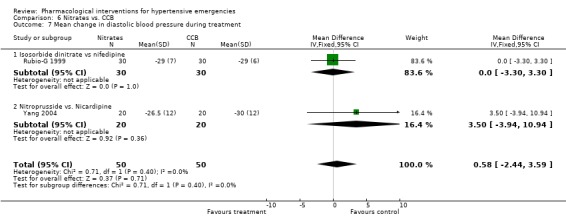
Comparison 6 Nitrates vs. CCB, Outcome 7 Mean change in diastolic blood pressure during treatment.
6.8. Analysis.
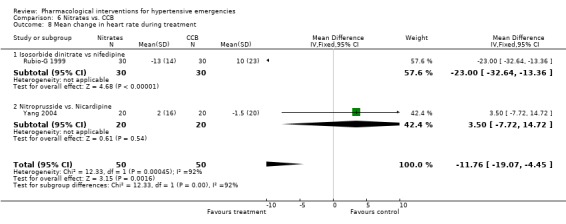
Comparison 6 Nitrates vs. CCB, Outcome 8 Mean change in heart rate during treatment.
Comparison 7. Nitrates vs Direct Vasodilator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Respiratory insufficiency requiring mechanical ventilation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Mean change in systolic blood pressure during treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐20.41, 13.07] |
| 7.1 Nitroglycerine vs. Hydralazine | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐20.41, 13.07] |
| 8 Mean change in diastolic blood pressure during treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐4.82, 11.50] |
| 8.1 Nitroglycerine vs. hydralazine | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐4.82, 11.50] |
| 9 Mean change in heart rate during treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐5.34 [‐20.65, 9.97] |
| 9.1 Nitroglycerine vs. hydralazine | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐5.34 [‐20.65, 9.97] |
7.7. Analysis.
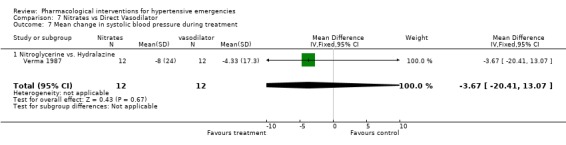
Comparison 7 Nitrates vs Direct Vasodilator, Outcome 7 Mean change in systolic blood pressure during treatment.
7.8. Analysis.
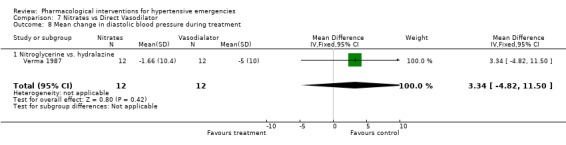
Comparison 7 Nitrates vs Direct Vasodilator, Outcome 8 Mean change in diastolic blood pressure during treatment.
7.9. Analysis.
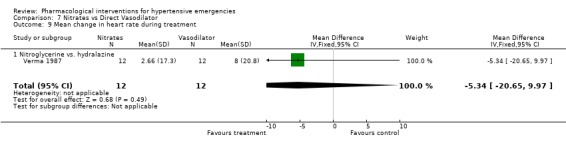
Comparison 7 Nitrates vs Direct Vasodilator, Outcome 9 Mean change in heart rate during treatment.
Comparison 8. ACE‐I vs CCB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.67] |
| 4.1 Captopril vs Nifedipine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.67] |
| 5 Withdrawals due to adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean change in systolic blood pressure during treatment | 4 | 183 | Mean Difference (IV, Fixed, 95% CI) | 1.68 [‐1.78, 5.14] |
| 6.1 Captopril vs Nifedipine | 4 | 183 | Mean Difference (IV, Fixed, 95% CI) | 1.68 [‐1.78, 5.14] |
| 7 Mean change in diastolic blood pressure during treatment | 4 | 183 | Mean Difference (IV, Fixed, 95% CI) | 7.86 [4.92, 10.81] |
| 7.1 Captopril vs Nifedipine | 4 | 183 | Mean Difference (IV, Fixed, 95% CI) | 7.86 [4.92, 10.81] |
| 8 Mean change in heart rate during treatment | 3 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐15.79 [‐18.00, ‐11.59] |
| 8.1 Captopril vs Nifedipine | 3 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐15.79 [‐18.00, ‐11.59] |
8.4. Analysis.
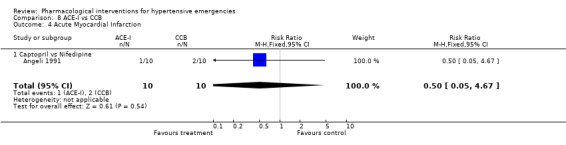
Comparison 8 ACE‐I vs CCB, Outcome 4 Acute Myocardial Infarction.
8.6. Analysis.
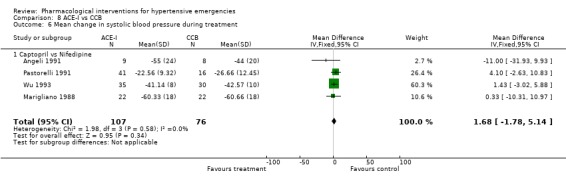
Comparison 8 ACE‐I vs CCB, Outcome 6 Mean change in systolic blood pressure during treatment.
8.7. Analysis.
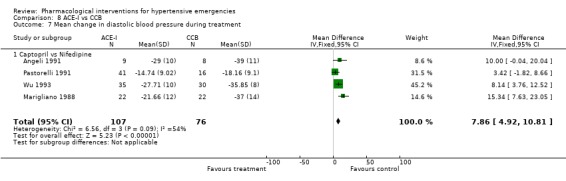
Comparison 8 ACE‐I vs CCB, Outcome 7 Mean change in diastolic blood pressure during treatment.
8.8. Analysis.
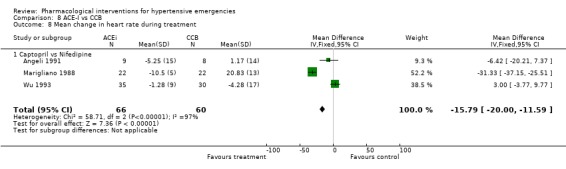
Comparison 8 ACE‐I vs CCB, Outcome 8 Mean change in heart rate during treatment.
Comparison 9. ACE‐I vs Alfa1‐Antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Serious Adverse Events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawals due to adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean change in systolic blood pressure during treatment | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐20.12 [‐22.85, ‐17.39] |
| 6.1 Captopril vs prazosin | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐26.43 [‐29.84, ‐23.02] |
| 6.2 Captopril vs ketanserine | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐8.90 [‐13.45, ‐4.35] |
| 7 Mean change in diastolic blood pressure during treatment | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐7.08, ‐0.31] |
| 7.1 Captopril vs prazosin | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐6.14 [‐10.52, ‐1.76] |
| 7.2 Captopril vs Ketanserin | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐5.41, 5.25] |
| 8 Mean change in heart rate during treatment | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐4.81, 3.97] |
| 8.1 Captopril vs Prazosin | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐4.81, 3.97] |
9.6. Analysis.
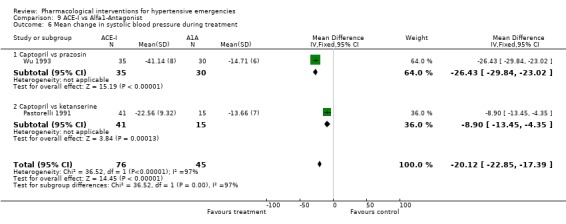
Comparison 9 ACE‐I vs Alfa1‐Antagonist, Outcome 6 Mean change in systolic blood pressure during treatment.
9.7. Analysis.
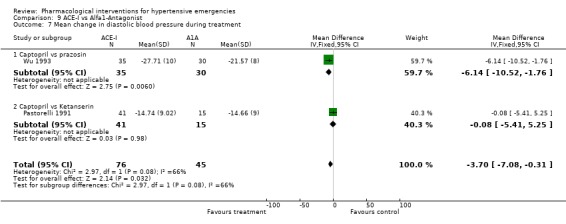
Comparison 9 ACE‐I vs Alfa1‐Antagonist, Outcome 7 Mean change in diastolic blood pressure during treatment.
9.8. Analysis.
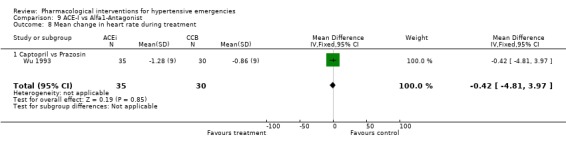
Comparison 9 ACE‐I vs Alfa1‐Antagonist, Outcome 8 Mean change in heart rate during treatment.
Comparison 10. Diazoxide vs Hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total of serious adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All cause mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non fatal cardiovascular events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acute Myocardial Infarction | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.06, 12.98] |
| 5 Witdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean change in systolic blood pressure during treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 13.56 [3.06, 24.06] |
| 7 Mean change in diastolic blood pressure during treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 14.67 [8.01, 21.33] |
| 8 Mean change in heart rate during treatment | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.4. Analysis.
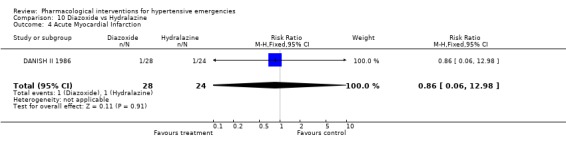
Comparison 10 Diazoxide vs Hydralazine, Outcome 4 Acute Myocardial Infarction.
10.6. Analysis.
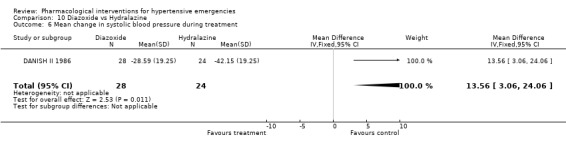
Comparison 10 Diazoxide vs Hydralazine, Outcome 6 Mean change in systolic blood pressure during treatment.
10.7. Analysis.
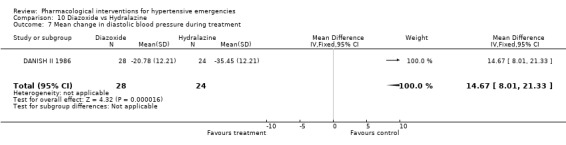
Comparison 10 Diazoxide vs Hydralazine, Outcome 7 Mean change in diastolic blood pressure during treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angeli 1991.
| Methods | Single‐site study (Italy). Single‐blind Method of randomization: reported as 1 to 1. No further details Concealment of allocation : NR Duration of treatment: single dose Follow‐up:24 hrs | |
| Participants | 22 patients with high blood pressure associated with symptoms and signs of end organ damage Note: There were two dropouts; one in each group * Inclusion criteria: Diastolic blood pressure of 140 mm Hg or greater after 20 minutes of bed rest associated with symptoms and signs of end‐organ damage ( angina, transient ischemic attack, hypertensive encephalopathy, and acute heart failure‐based on gallop rhythm, tachypnea, orthopnea and fine basal rales) * Exclusion criteria: An overt pulmonary edema, valvular heart disease, serious disturbance of consciousness and history of myocardial infarction or stroke. * Baseline characteristics for the two randomized groups: Nifedipine (N): n=10 Captopril (C): n=10 Unless otherwise indicated, values are expressed as mean ± SD age ( years) C: 61±12 N: 53 ± 12 Race: NR BP: (mm Hg) C:245/145 N:247/158 Patients previously receiving antihypertensive C:7/10 N:7/10 Secondary hypertension C:1/10 N:4/10 Diabetes C:1/10 N:0/10 |
|
| Interventions | Nifedipine (N): n=10 Captopril (C): n=10 Dose regimen: C: Single sublingual tablet of 25 mg under the patients' tongue and swallowed the saliva. N: Single sublingual perforated capsule of 10 mg under the patients' tongue and swallowed the saliva. | |
| Outcomes | Obtained from this trial for the two randomized groups:
Nifedipine (N): n=10
Captopril (C): n=10
Total SAE: NR
Mortality: nil during 24 hours of follow‐up.
Total Non fatal CVE: NR
Withdrawals: N/A as is a single dose regimen
BP: reported as magnitude of lowering effect during the first 60 minutes (text on page 680 last paragraph):
Captopril= SBP‐55 ± 24; DBP ‐29 ± 10
Nifedipine= ‐44 ± 20; DBP ‐39 ± 11
SD of change was reported on text, page 680, last paragraph. Note: there is also report of BP ± SE over 60 minutes in graph (we did not use this graph for entering BP into Revman) Heart rate: Captopril= ‐5.25±15 Nifedipine= 1.17±14 Note: We used HR data reported in a graph, p.681. |
|
| Notes | Author successfully contacted. Funding: Ministero dell Universita e della Ricerca Scientifica e tecnologica, progettto nazionale "Fisiopatologia del circolo" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Beltrame 1998.
| Methods | Single‐site study (Australia). Open‐label Method of randomization: NR Concealment of allocation : NR Follow‐up: until discharge from hospital Duration of treatment 24 hours | |
| Participants | 69 patients with elevated blood pressure and cardiogenic pulmonary edema (within 6 hours of onset) * Inclusion criteria: Acute onset of dyspnea within the preceding 6 hours, clinical findings consistent with pulmonary edema (increased respiratory work, gallop rhythm,widespread crepitations in the absence of chest infection or aspiration); radiological evidence of pulmonary edma * Exclusion criteria: Non cardiogenic pulmonary edema, cardiogenic shock ( SBP < 90). An overt AMI, valvular heart disease, obstructive airways disease, requiring immediate intubation, or cardioversion, or known in chronic renal failure * Baseline characteristics for the two randomized groups Furosemide/ morphine (F) (n=32) Nitroglycerin/ N‐acetylcysteine (N) (n=37) Note: Screened 87, (18 excluded‐ 10 ami, 3 chronic renal failure, 4 required immediate intubation, 1 unable to provide consent) Of 69 randomized, 4 were subsequently shown not to have acute pulmonary edema, all were included ITT analysis Unless otherwise indicated, values are expressed as mean ± SD age ( years) F:77± 6.6 N: 76± 9 Race: NR SBP: (mm Hg) F:164 ± 34 N:161 ± 32 HR (bpm) F:111± 21 N:115±21 Patients previously receiving antihypertensive F:nitrates 11(34%), diuretics 18(56%), CCB 9(28%),BB 4(13%),digoxin 10(31%), ACEi10(31%) N:nitrates 12(32%), diuretics 21(57%), CCB 8(22%),BB 3(8%),digoxin 3(8%*), ACEi11(30%) Past history F: ischaemic heart disease 11(34%), Chronic heart failure 17(53%),diabetes 12(38%) hypertension18(56%) N: ischaemic heart disease 15(41%), Chronic heart failure 20(54%),diabetes 14(38%) hypertension13(35%) |
|
| Interventions | Furosemide/ morphine (F) (n=32) Nitroglycerin/ N‐acetylcysteine (N) (n=37) Dose regimen: F: iv furosemide bolus 40 mg, second dose at 60 min, 3 and 24 hours. Morphine 1‐2 mg/5 min) to a maximum dose of 10 mg. (median dose received 80mg of furosemide, and 3 mg of morphine) N: intravenous nigroglycerin 2.5 mcg/min,( to max 10 mcg/min) at the same time patients receive N‐acetylcysteine at 6.6 ?g/min over 24 hours (median dose received 2.5 mcg /min during first hour) Assessment were performed at 30, 60, 3 hours, and 24 hours. Cointerventions: On arrival, patients were given 50 % oxygen , | |
| Outcomes | Obtained from this trial for the two randomized groups: Furosemide/ morphine (F) (n=32) Nitroglycerin/ N‐acetylcysteine (N) (n=37) Total SAE: NR Mortality : 3 patients died, but they were not reported according to group of allocation. Neither are reported the causes of death Total Non‐fatal CVE: NR AMI: Furosemide=4 /32 Nitroglycerin=6/37 Witdrawals due to adverse events: NR Blood Pressure: obtained from a table, p275, over 24 hours Calculated weighted mean BP change Furosemide: SBP ‐21± 23; DBP ‐13.25±15 Nitroglycerin: SBP‐23.75±22; DBP ‐16.25 ±19 Standard Deviation of change was not reported but Imputed from end point Heart Rate: also obtained from table: Calculated weighted mean HR change Furosemide: ‐13.25± 15 Nitroglycerin ‐16.25±19 Standard Deviation of change was not reported but Imputed from end point | |
| Notes | Funding: National Health and Medical Research Council of Autralia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
DANISH II 1986.
| Methods | Multi‐centre study (Denmark). Method of allocation / randomization: closed envelopes numbered consecutively, and statistical tables of randomized numbers were used. Duration of treatment: 4 h Follow‐up:24 hrs | |
| Participants | 52 patients with hypertensive encephalopathy * Inclusion criteria: Patients with diastolic blood pressure of 135 mm Hg or greater associated with cerebral symptoms (headache, consciousness disturbances, paresis, paresthesia, dizziness, blurred vision, nausea and vomits). The distribution of patients with those symptoms was not reported according to randomized group * Exclusion criteria: Aged over 70 years, cerebral apoplexy with hemiparesis, subarachnoidal haemorrhage, ischaemic heart disease, pulmonary oedema, uremia , creatinine > 500mcmol/l, pregnancy) * Base‐line characteristics for the two randomized groups: Diazoxide (D) group: n= 28 Dihydralazine (H) group: n=24 Mean age in years (range ) D:54 (33‐69) H: 52 (27‐69) Gender F/M D: 6/22 H:10/14 Hx of HTN D: 14/28 (50%) H: 9/24 (38%) Note: Twelve out of 64 patients achieved DBP levels of <125 mmHg within one hour after 40 mg of IV furosemide. These patients were not randomized but followed. We did not include these patients in our review. As such: 64‐12= 52 |
|
| Interventions | Diazoxide (D): n=28 Dihydralazine (H): n=24 Dose regimen: Diazoxide (D): two subgroups: A‐initial dose 75 mg IV then 150 mg IV every 15 min to reach DBP 110 mm Hg or max dose of 600 mg (12 patients). B‐ initial dose 75 mg then 75 mg every 30 min to reach DBP 110 mm Hg or max dose of 375 mg (16 patients) Dihydralazine (H): initial dose 6.25 mg I.M., then 12.5 mg I.M., every 30 min to reach DBP 110 mm Hg or max dose of 56.3 mg (24 patients) | |
| Outcomes | Outcomes obtained from this trial for the two randomized groups:
Diazoxide (D): n=28
Dihydralazine (H): n=24
Total SAE: NR
Mortality
Two deaths: However, the group to which the dead patients were originally allocated was not reported. One died from stroke at day 12, the other died from rupture of aortic aneurism at day 10.
Total Non‐fatal CVE:NR
Individual CVE:
AMI: Diazoxide =1/28 Hydralaline=1 /24
Withdrawals due to adverse events: NR
Blood pressure:
Except for the end point SBP/DBP values given in text (page 15 & 18; for dihydralazine, diazoxide groups, respectively), data was obtained from graphs, fig2, reported in page 18.The calculated weighted mean BP change was:
Diazoxide: SBP ‐29.63± NR; DBP ‐21.63±NR
Dihydralazine: SBP ‐43.40±NR; DBP ‐36.09±NR
Standard deviation of the change was not reported. In this case we imputed (according to our hierarchy) from other trials (any drug any dose) as there was no report whatsoever regarding SBP or DBP variability in this trial (including all publications).
However, on page 19 fig.3 there is a plot for MAP change according to groups. The calculated MAP variability (SD) for diazoxide group 19.46, and 22.25 for the dihydralazine group. Heart rate: There is no report on heart rate in the original or duplicate publications |
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Elliott 1990.
| Methods | Single‐site study (US). Open Label Method of randomization: not reported, Concealment of allocation: not reported Duration of treatment: 1 hour Follow‐up: 10 days. | |
| Participants | 28 patients with high blood pressure and acute end organ damage * Inclusion criteria: All patients had supine diastolic blood pressure > 120 mm Hg in association with acute end organ damage. (decrease in creatinine, cardiomegaly, left ventricular hypertrophy on ECG, > grade II fundoscopy abnormality. * Exclusion criteria: congestive heart failure * Baseline characteristics for the two randomized groups: Nitroprusside (N): n= 15 Fenoldopam (F): n = 13 Unless otherwise indicated, values are expressed as mean ± SD age ( years) N:42 ± 8 F: 51 ± 5 Race: black N:14/15 F:12/13, BP: (mm Hg) N:222/137 F:214/136 Presence previous of accelerated/ malignant HTN N:11/15 F:11/13 |
|
| Interventions | Nitroprusside (N): n= 15
Fenoldopam (F): n = 13 Dose regimen: IV Fenoldopam (dopamine1 receptor agonist) * Initial dose 0.1 mcg/kg/min and then increments of 0.05 ‐0.1 mcg/kg/min every 20 minutes to DBP 100‐110 mm Hg and stable for 1 hour. Then an oral treatment ( atenolol 100 mg and Furosemide 20 mg) was added. The IV drug was then taper down until stopping it. IV Nitroprusside Initial dose 0.5 mcg/kg/min and then increments of 0.25 ‐0.5 mcg/kg/min every 20 minutes to DBP 100‐110 mm Hg and stable for 1 hour. Then an oral treatment ( atenolol 100 mg and Furosemide 20 mg) was added. The IV drug was then taper down until stopping it. |
|
| Outcomes | Obtained from this trial for the two randomized groups: Nitroprusside (N): n= 15 Fenoldopam (F): n = 13 Total SAE: NR Mortality: NR Total non‐fatal CVE: NR Any CVE: NR Withdrawals due to adverse events: NR Blood Pressure: obtained from text, p.972, during treatment. Calculated weighted mean BP change: Fenoldopam: SBP‐34±18, DBP ‐30 ± 14 Nitroprusside: SBP‐48±19,DBP ‐32±12 Standard deviation of change was not reported but imputed from end point Heart rate: obtained from text, p.972, during treatment. Calculated weighted mean HR change: Fenoldopam: 4 ± 19 Nitroprusside: 6±11 Standard deviation of change was not reported but imputed from end point | |
| Notes | Funding: Not reported Although it said that creatinine would be monitored for 48‐72 hours and BP and clinical assessment would be done at day 7 to 10, no BP or clinical data was reported for 48‐72 hrs or day 7‐10 . |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hamilton 1996.
| Methods | Single‐site study (US). Double‐blind placebo‐controlled trial Method of Randomization: NR Method of Concealment of allocation : The investigators were given a numbered data collection instrument with a pre‐packaged set of four unmarked capsules that had previously been randomized. Duration of treatment: single dose ( readministrated at minute 60) Follow‐up: 2 h | |
| Participants | 48 patients with high blood pressure* and acute pulmonary edema * Based on values at baseline Note: of the 110 patients screen for ape 57 were enrolled; 3 patients were disqualified because they were intubated upon arrival. Five patients were eliminated due to incomplete data collection. One was mistakenly enrolled in the study and later disqualified. The etiology of acute pulmonary edema was due to acute myocardial infarction (31%) or exacerbation of chronic CHF (69%) Inclusion Criteria: Clinical appearance of acute pulmonary edema ( acute onset of dyspnea diaphoresis and rales> 50% of posterior lung fields). Exclusion Criteria: systolic BP < 90 mmHg, pregnancy, known ace inhibitor allergy or age < 18 years . By a prioriy design , patients who wer intubated within 15 minutes of arrival were disqualified from the study. * Baseline characteristics of the two randomized groups: Captopril (C ): n= 23 Placebo (P ): n= 25 age ( years) C:71 P: 66 Gender‐male C:11(47%) P: 15 (60%) MAP: (mm Hg) C: 132 P:120 Assuming a standard difference of 60 mm Hg, the calculated SBP/DBP (mm Hg) would be: C: 172/112 P: 160/100 |
|
| Interventions | Captopril (C ): n= 23
Placebo (P ): n= 25
Dose Regimen:
2 capsules of (lactose plus 12.5 mg captopril) or 2 capsules of (lactose powder)
Were emptied sublingually for patients who had a systolic BP > 110 mmHg
Or
1 capsule (Captopril) or 1 capsule (Placebo) for those who had systolic BP 90‐110 mmHg The dose was re‐administrated at minute 60 Cointerventions: standard treatment for all patients with oxygen, furosemide iv bolus ( 40 mg minimum , and nitroglycerin ( 0.4 mg ‐sublingually every 5 minutes for a total of three doses , morphine iv in 2 mg incrementes titrated against symptoms and BP . Treatment was repeated at investigator discretion. Treatment received at admission C: furosemide 23 (100%), sl. nitroglycerin 23(100%), morphine 16 (69%), iv nitroglycerin 13(57%) P: furosemide 25 (100%), sl. nitroglycerin 25(100%), morphine 18 (72%), iv nitroglycerin 18(72%) |
|
| Outcomes | Outcomes obtained from this trial for the two randomized groups:
Captopril (C ): n= 23
Placebo (P ): n= 25 Total SAE: NR Mortality :NR Total Non‐fatal CVE: NR Need for intubation: C: 2/23 (9%) P: 5/25(20%) Blood pressure change in (mm Hg) SBP: NR DBP: NR MAP: (obtained from table 1, page 207) C: ‐43 mmHg, P: ‐39 mm Hg Standard deviation of the change was not reported Heart Rate: NR |
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hirschl 1997.
| Methods | Single‐site study (Austria). Method of randomization/ allocation: not reported Duration of treatment : until response or maximum allowed dose Follow‐up:4 hrs | |
| Participants | 81 patients with elevated blood pressure and evidence of acute end organ damage * Inclusion criteria: Patients with systolic blood pressure > 200 mmHg ,and diastolic blood pressure > 110 mm Hg in association with clinical evidence of acute end organ damage ( encephalopathy, stroke, acute heart failure, angina, aortic dissection) * Exclusion Criteria: > 80 years old Acute or chronic renal failure Pheochromocytoma Organ transplant Pregnancy, Lactation * Baseline characteristics for the two randomized groups: Nitroprusside (N): n= 35 Urapidil (U): n= 46 Unless otherwise indicated, values are expressed as mean ± SD Age ( years) N:58 ±14.9 U: 62±12.9 Race: NR BP: (mm Hg) N:211/109 U:215/107 Type of acute end organ damage on admission Angina N:15 U:11 Neurological emergencies N:15 U:11 Acute heart failure N:2 U:7 Aortic dissection N:3 U:6 |
|
| Interventions | Nitroprusside (N): n= 35
Urapidil (U): n= 46 Dose regimen: IV Urapidil (peripheral alpha1 receptor blocker and central 5‐HT1A ‐receptor agonist). Initial dose 12.5 mg and then 12.5 mg every 15 minutes to a maximum of 75 mg or response. IV nitroprussiate . Initial dose of 0.5 mcg /kg/ min and then 0.5 mcg /kg/ min every 15 minutes to a maximum of 3 mcg /kg/ min or response. |
|
| Outcomes | Obtained from this trial
Total SAE: NR
Mortality: NR
Total non‐fatal CVE: NR
Individual CVE: NR
Withdrawals due to adverse events: not reported
Blood pressure:
Except for baseline values data was obtained from the graph in page 887 (fig.2). Weighted mean BP change was calculated as follow:
Nitroprusside: SBP ‐58.4 ± 17; DBP ‐28.4 ± 12
Urapidil: SBP ‐37.6 ± 17; DBP ‐17.6 ± 13
Standard deviation of the change was not reported but imputed from end point.
Heart rate:
Weighted mean HR change (at minute 90) was provided in the text (p.886) as follow:
Nitroprusside: ‐8.2 ± 14
Urapidil: ‐9.2 ± 21
Standard error of the change was provided. We converted it to SD. Primary outcome stated by authors: Percentage of responders within 90 min after start of therapy, the number of primary responders with a re‐elevation of BP and the percentage of major adverse events in each group (Hypotension greater than 50% and heart rate >120 bpm and aggravation of clinical symptoms requiring immediate intervention) and minor adverse events ( subjective symptoms). Secondary outcome: Extent of BP reduction, time to achieve BP control and the cumulative dose of each drug. |
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hirschl 1999.
| Methods | Single‐site study (Austria) Open label. Method of randomization / allocation: Not reported Duration of treatment: until response or maximum dose allowed Follow‐up:24 hrs | |
| Participants | 46 patients with high blood pressure and evidence of pulmonary oedema Inclusion Criteria: Patients found with Pulmonary edema (rales over both lungs) plus SBP > 200 mmHg or DBP > 100 mm Hg. Exclusion Criteria: If the patient required intubation or had cardiopulmonary arrest before initiating therapy. Baseline characteristics for the two randomized groups: Nitroglycerine (NTG) n=23 Enalaprilat(ENA) n=23 age ( years) NTG=74 ENA= 74 Male/female NTG=12/11 vs. ENA=9/14 race: NR BP (mm Hg): NTG=206/116 ENA=211/115 Patients previously receiving antihypertensive NTG=9/23 vs. ENA= 8/23 Diabetes NTG=6/23 vs. ENA= 4/23 Previous myocardial infarction NTG=4/23 vs. ENA= 6/23 |
|
| Interventions | Nitroglycerine (NTG) n=23
Enalaprilat(ENA) n=23
Dose regimen:
NTG = Sublingual, initial dose 0.8 mg as: repetitive application of 0.8 mg every 10 min. until a cumulative dose of 3.2 mg.
ENA = Initial dose: 2.5 mg IV; repetitive application of 2.5 mg every 30 min until a cumulative dose of 10 mg. The mean dose of drug given until admission was 1.6 ± 0.6mg of nitroglycerine and 3.7±1.5 mg of enalaprilat . Withdrawals due to adverse events were not reported. The number of patients requiring a second drug to reduce blood pressure was not reported. The time to achieve the target blood pressure was not reported. The mean time of drug infusion is not reported. |
|
| Outcomes | Obtained from this trial
Total SAE: NR
Mortality: nil at 24 hours of follow‐up.
Total non‐fatal CVE: NR.
Individual CVE
Nitroglycerine (N)= 0/23
Enalapril= 2/23; (1 asystole, 1 intubation).
Withdrawals due to adverse events: NR
Blood pressure:
Data was obtained from text (p.211). Weighted mean BP change was calculated as follow:
Nitroglycerine: SBP ‐52.3 ± 18; DBP ‐34.6 ± 12
Enalapril : SBP ‐55.6 ± 19; DBP ‐34.3 ± 11
Standard deviation of the change was not reported but imputed from end point.
Heart rate:
Weighted mean HR change was also calculated from data provided in the text (p.211) as follow:
Nitroglycerine: ‐29 ± 7
Enalapril: ‐33.5 ± 12
Standard deviation of the change was not reported but imputed from end point. Primary outcome of trial: The aim of the antihypertensive treatment was Reduction of systolic blood pressure below 160 mm Hg and diastolic BP below 90 mm Hg at admission to the emergency department. Secondary outcome: Chest x‐ray congestion, adverse events, metabolic and respiratory parameters. |
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marigliano 1988.
| Methods | Single‐site study (Italy). Open‐label Method of randomization/allocation: NR Duration of treatment : single dose Follow‐up:2 hrs | |
| Participants | 44 patients with high blood pressure and acute symptoms * Inclusion Criteria: Elderly patients with systolic blood pressure of 210 mm Hg or greater associated with acute symptoms (dyspnoea, cephalgia, angina, mental aberration) * Exclusion Criteria: Not stated Except for BP and HR, baseline characteristics were not reported according to randomization group |
|
| Interventions | Captopril (C): n=22 Nifedipine (N): n=22 Dose regimen: C: Single sublingual tablet of 50 mg. N: Single sublingual capsule of 10 mg. | |
| Outcomes | Outcomes obtained from this trial Total SAE: NR Mortality: NR Total non‐fatal CVE: NR Individual Cardiovascular events: NR Withdraw due to adverse events= N/A (as single dose was given) Blood Pressure: Except for baseline BP values and standard deviation of the change provided on text of page S92, data was obtained from the graph fig.1&2 (p. S92). Weighted mean BP change calculated was: Captopril: SBP‐60.33 ±18; DBP‐21±12 Nifedipine: SBP‐60.6 ±18; DBP‐37±14 Heart rate: Except for baseline HR values and standard deviation of the change provided on text of page S92, data was obtained from the graph fig.1&2 (page. S92). Weighted mean HR change calculated was: Captopril: ‐10.5±5 Nifedipine: +20.8±7 | |
| Notes | Funding : Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nelson 1983.
| Methods | Single‐centre (US), randomized, single‐blind, controlled trial Method of randomization/ allocation: NR Duration of treatment : 1.5 h Follow‐up: 48 h | |
| Participants | 28 patients with acute heart‐failure blood pressure levels that met our threshold for this category of patients. Base‐line characteristics for the two randomized groups: Isosorbide (N): n=14 Furosemide (F): n=14 Mean age in years N: 56 F: 56 Mean SBP ± SE N: 130±7 F: 119±4 Mean DBP ± SE N: 75±3 F: 72±2 |
|
| Interventions | Isosorbide (N): n=14
Furosemide (F): n=14 Dose regimen: N: Intravenous infusion of isosorbide dinitrate at initial dose of 50 mcg /kg and max 200 mcg/kg/h F: IV infusion of furosemide 1 mg/kg Study treatment lasted 1.5 hours after randomization and the target was to reduce systemic BP by 10 mm Hg. Treatment started between 5‐14 h of AMI Mean dose administrated : Isosorbide dinitrate: mean cumulative dose 13.2 mg (146 mcg/min ‐considering 90 min of infusion). Furosemide: mean dose 80 mg Co‐interventions: NR |
|
| Outcomes | Obtained from this trial for the two randomized groups: Isosorbide (N) group: n=14 Furosemide (F) group: n=14 Total SAE: NR Mortality : nil during 48 hours of follow‐up Total Non‐fatal CVE: NR Individual CVE: NR Withdrawals due to adverse events: NR Blood pressure: Data was obtained from table II page 731. The calculated BP weighted mean change was: Isosorbide: SBP: ‐6.6±22.4; DBP‐1.6±18.7 Furosemide: SBP: 1.6±18.7; DBP 1±11.2 The standard deviation of the change was not reported but it was imputed from the end point. Heart Rate: Data was obtained from table II page 731. The calculated HR weighted mean change was: Isosorbide: 3±18.7 Furosemide: 2±14.96 The standard deviation of the change was not reported but it was imputed from the end point. | |
| Notes | Funding: Yorkshire Regional Hospital: West Riding Medical Research trust. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pastorelli 1991.
| Methods | Single‐site study (Italy). Method of randomization/ allocation: NR Duration of treatment: single dose Follow‐up: 1 h | |
| Participants | 96 patients with hypertensive emergencies* Inclusion Criteria: Patients with observed hypertensive emergencies (*defined as acute target organ damage and high blood pressure). The different types of hypertensive emergencies were uniformly present in each group. No further details Exclusion Criteria: Not stated Not reported |
|
| Interventions | Nifedipine sublingual (N): n=16, Captopril sublingual (Cs): n=27, Captopril oral (Co): n=14, Ketanserine sublingual (K): n=15, Placebo (P):20 Dose Regimen: single dose. | |
| Outcomes | Obtained from this trial: Total SAE: NR Mortality: NR Total non‐fatal CVE: NR Individual Cardiovascular events: NR Withdraw due to adverse events: N/A (single dose) Blood Pressure; Data was obtained from graphs in page 861 and 862. The calculated weighted mean BP change was: Nifedipine: SBP‐26.66±12.45; DBP‐18.16±9.13, Placebo: SBP‐7.2±13.5; DBP‐7.8±9 Ketanserine: SBP‐13.6±7; DBP‐14.6±9 Captopril: SBP‐22.56±9.32; DBP‐14.74±9 It was not specified if SD or SE was reported on the text or graphs. It was assumed to be SD. Standard deviation of change was not reported but imputed from end point | |
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rubio‐G 1999.
| Methods | Single‐site study (Mexico) Open label trial Method of randomization /allocation: not reported Duration of treatment: single dose Follow‐up: 6 hrs | |
| Participants | 60 patients with high blood pressure and evidence of end organ organ damage Inclusion Criteria: Mean arterial pressure > 130 mmHg and evidence of end organ damage. Exclusion Criteria: Liver failure, chronic renal failure, drug or ethanol abuse, pregnancy. * Baseline characteristics for the two randomized groups: Isosorbide dinitrate aerosol (I): n= 30 Nifedipine (N): n= 30 mean age ± SD (years) I:51 13 N: 51±11 Race: NR BP: (mm Hg) NR Data was extracted from graphs and text based on ITT analysis Distribution of patients according to the type of end organ damage at admission Encephalopathy I:18 N:18 Intracraneal Haemorrage I:2 N:4 Stroke I:5 N:4 Myocardial Ischaemia I:2 N:0 Acute pulmonary edema I:2 N:1 Retinal haemorrhage I:0 N:2 Pulmonary congestion by CXR I:0 N:1 Papilledema I:1 N:0 |
|
| Interventions | Isosorbide dinitrate aerosol (I): n= 30
Nifedipine (N): n= 30 I: Initial dose 1.25 mg through oral mucosa when admitted and a second dose given 15 min later when MAP decreased less than 15%. N: 10 mg sublingually as a single dose. |
|
| Outcomes | Outcomes obtained from this trial Total SAE: NR Mortality: NR Total non‐cardiovascular events: NR Individual Cardiovascular events: Nifedipine = 2/30 (subepicardial ischemia) Isosorbide= 0/30 Withdraw due to adverse events= NR Blood pressure Data was obtained from text (page 474). The calculated weighted mean BP change was: Isosorbide: SBP ‐34±15, DBP‐29±7 Nifedipine: SBP ‐37±26, DBP‐29±6 Standard deviation of change was not reported but imputed from end point Heart rate Data was obtained from text (page 474) The calculated weighted mean HR change was: Isosorbide: ‐13±14 Nifedipine: 10±23 Standard deviation of change was not reported but imputed from end point | |
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schreiber 1998.
| Methods | Single‐site study (Austria). Open label Method of randomization: NR Concealment of allocation: NR Duration of treatment: 6 hours Follow‐up:6 hours | |
| Participants | 133 patients* with pulmonary oedema and high blood pressure. * Note: not all randomized patients were included in the analysis of the original publication (please see below discussion) The total number of patients included in the original analysis was: n=112 45 patients from the urapidil group 67 patients from the nitroglycerin group This is because 20 (15%) patients withdrew or dropped out from the trial. However there is a discrepancy in the numbers as follows: Of those 20, 13 were reported according to the randomization group and 7 not according to the randomization group. "Withdrawals/dropouts reported according to randomization group: 13 Urapidil : 11 (3 due to AMI, 9 due to dose violation) Nitroglycerin : 2 ( due to AMI) "Withdrawals/dropouts reported NOT according to randomization group: 7 So, 112 + 20 = 132 patients. Thus, there is a discrepancy between the number of patients described in the text (total 132) with those described as total randomized patients (133). Therefore, total randomized (133) minus those included in the analysis (112) equals 21(16%) not included in the original analysis We tried to contact the authors to explain this discrepancy but they did not replied to our request. Inclusion Criteria: Patients with systolic blood pressure > 200 mmHg ,and diastolic blood pressure > 100 mm Hg in association with clinical evidence of pulmonary edema ( rales over both lungs) Exclusion Criteria: Allergic reactions Pregnancy, Myocardial infarction Respiratory insufficiency requiring intubation or coma at the time of the emergency physician arrived. * Baseline characteristics for the two randomized groups: Nitroglycerine (N): n= 67 Urapidil (U): n= 45 Mean age ± SD ( years) N:74 9 U: 73±11 BP: (mm Hg) N:216/116 U:218/118 |
|
| Interventions | Nitroglycerine (N): n= 67
Urapidil (U): n= 45
Dose regimen:
Sublingual Nitroglycerine : Initial dose of 0.8 mg and then 0.8 mg every 10 minutes. If after hospital admission SBP was > 180 mm Hg and/or DBP 100 mm Hg the drug was still continued but changed to IV infusion on a rate of 0.5‐ 5mg/ h to reach SBP < 160 mm Hg and DBP below 90 mmHg within 30 min after admission and no re‐elevation of BP for 6 h. IV Urapidil (peripheral alpha1 receptor blocker and central 5‐HT1A ‐receptor agonist): Initial dose 12.5 mg and then 12.5 mg every 15 minutes. If after hospital admission SBP was > 180 mm Hg and/or DBP 100 mm Hg the drug was still continued but changed to IV infusion on a rate of 5‐25mg/ h to reach SBP < 160 mm Hg and DBP below 90 mmHg within 30 min after admission and no re‐elevation of BP for 6 h. |
|
| Outcomes | Obtained from this trial for the two randomized groups:
Nitroglycerine (N): n= 67
Urapidil (U): n= 45 Total SAE: not reported (NR) Mortality = nil Total non‐fatal cardiovascular events: NR Individual CVE: Left ventricular failure requiring intubation: Urapidil: 0 Nitroglycerin: 2 Blood pressure: Data was obtained from table 1 page 560 ( base‐line values) and from text on page 559 & 560. It was not possible to follow full ITT principles due to inconsistencies in the report. (see notes above) The calculated weigthed mean BP change was: Nitroglycerin: SBP ‐59.5 ±20; DBP‐33.5±11 Urapidil: SBP ‐73.5 ±21; DBP‐42±13 Standard deviation of change was not reported but imputed from end point. Heart rate: Data was obtained from table 1 page 560 ( base‐line values) and from text on page 559 & 560. It was not possible to follow full ITT principles due to inconsistencies in the report. (see notes above) The calculated weigthed mean HR change was: Nitroglycerin: ‐17.5±9 Urapidil: SBP ‐15±7 Standard deviation of change was not reported but imputed from end point. |
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Verma 1987.
| Methods | Single‐site study (UK) Single‐blind Method of randomization: NR Concealment of allocation: NR Duration of treatment: 90 minutes Follow‐up: Until hospitalization discharge | |
| Participants | 48 patients with acute left ventricular failure and blood pressure levels that met our threshold for this category of patients. Inclusion criteria ECG for transmural myocardial infarction Radiographic changes consistent with diagnosis of left ventricular failure Left ventricular filling pressure > 20 Systolic P >100 mmHg Exclusion criteria Sustained arrhythmias Valvular disease requiring surgery Base‐line characteristics for the 4 randomized groups Furosemide (F), n=12 Isosorbide dinitrate (I) n =12 Hydralazine (H), n =12 Prenalterol n=12 (this group is not considered any further, as this drug is not an anti‐hypertensive drug. It is a beta‐adrenergic agonist. Mean age in years ± sd . F= 57 I = 58 H = 56 Mean SBP ± sd F=117±4 I =131±8 H =134±6 Mean DBP ± sd F =73±4 I =75±3 H =77±3 |
|
| Interventions | Furosemide 1 mg/kg IV bolus (N=12)
Isosorbide dinitrate 50‐200 mcg/kg/h IV infusion (N=12)
Hydralazine 0.15 mg/kg IV over 5 minutes (N=12) Target: to reduce mean arterial pressure 10 mm hg but not to reduce SBP < 100 mmHg. Mean dose administrated: Furosemide 84 mg Isosorbide dinitrate 11.8 mg [8.3‐15.6 ] Hydralazine= 12.8 mg [10.2‐16] Co‐interventions: all patients received 5 mg of intramuscular diamorphine |
|
| Outcomes | Outcomes obtained from this trial
Total serious adverse events: not reported (NR)
Mortality
Isosorbide = 0/12
Furosemide=0/12
Hydralazine =1/12
Prenalterol=1/12
Total Non‐fatal cardiovascular events: NR
Individual cardiovascular events: NR
Withdrawals due to adverse events: NR Blood pressure: All data was obtained from table 2 page 41. The calculated weighted mean BP change was: Isosorbide: SBP‐8±24, DBP‐1.6±10.4 Furosemide: SBP 1±14, DBP1.3±10 Hydralazine: SBP‐4.3±17.3, DBP‐5±10 The standard deviation of the change was not reported but imputed from end point. Heart rate: All data was obtained from table 2 page 41. The calculated weighted mean HR change was: Isosorbide: 2.6±17.3 Furosemide: 2±17.3 Hydralazine: 8±20.8 The standard deviation of the change was not reported but imputed from end point. |
|
| Notes | Funding: Yorkshire Regional Hospital: West Riding Medical Research trust. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wu 1993.
| Methods | Multi‐centre study (Taiwan). Open label Method of randomization: NR Concealment of allocation: NR Duration of treatment: single dose Follow‐up: 2 h | |
| Participants | 64 patients with high blood pressure and cerebral signs or symptoms symptoms (headache, dizziness, convulsion, coma) during haemodyalisis. Inclusion Criteria: Patients with SBP >190 or DBP >120 associated with symptoms (headache, dizziness, convulsion, coma) during haemodyalisis. Exclusion Criteria: Patients with increasing BP less than 20 min after initiating haemodyalisis. Patients with drop of BP to level less than 170/110 within 20 min were also excluded. * Except for BP and HR, baseline characteristics were not provided. The type of emergencies was not reported according to randomized group. |
|
| Interventions | Nifedipine (N):n=30, Captopril (C): n=35 Prazosin (P):n=27 Dose regimen: sublingual single dose Nifedipine 10 mg , Captopril 25 mg , and Prazosin 10 mg | |
| Outcomes | Outcomes obtained from this trial: Total SAE: Not reported (NR) Mortality: NR Total non‐fatal cardiovascular events: NR Individual CVE: NR Withdraw due to adverse events= N/A (single dose) Blood pressure: Data was obtained from tables 1,2 and 3 on page 285‐286. Standard deviation of change was not reported but imputed from end point. The calculated weighted mean BP change was: Captopril: SBP‐41±8, DBP‐27.71±10 Nifedipine: SBP‐42±10, DBP‐35.85±8 Prazosin: SBP‐14.6±6, DBP‐21.57±8 Heart rate: Data was obtained from tables 1,2 and 3 on page 285‐286. Standard deviation of change was not reported but imputed from end point. The calculated weighted mean HR change was: Captopril: ‐1.28±9 Nifedipine: ‐4.28±17 Prazosin: ‐0.85±9 | |
| Notes | Source of Funding : NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang 2004.
| Methods | Single‐site study (Korea) Open label Method of randomization: NR Concealment of allocation : NR Duration of treatment: 1 hour Follow‐up: 1 hour | |
| Participants | 40 patients with acute pulmonary edema and high blood pressure (SBP >160) Inclusion Criteria: Systolic pressure > 160 and a diastolic pressure > 100 mmHg accompanied by cardiovascular abnormalities and acute pulmonary edema Exclusion Criteria: Not stated * Baseline characteristics for the two randomized groups: Nitroprusside:(NTP) n = 20 Nicardipine (NIC) n= 20 Age (years) ± sd NTP:60 ± 14 NIC: 59±12 BP: (mm Hg) NTP:195/115; ± 27/20 NIC:196/114 ± 14/13 |
|
| Interventions | Nitroprusside infusion at a starting dose of 1 mcg /kg x min., for 1 hour
Nicardipine infusion at a starting dose of 3 mcg /kg per min for 1 hour
The dose regimen was titrated to maintain the BP at 80% of the initial mean arterial pressure. ‐mean dose given of NTP was 1.5‐mean dose given was 1.5±0.4 mcg/kg x min ‐mean dose given of NIC was 3.5±0.5mcg/kg per min Co‐interventions: NR |
|
| Outcomes | Outcomes obtained from this trial:
Total SAE: Not reported (NR)
Mortality: NR
Total Non‐fatal cardiovascular events: NR
Individual cardiovascular events: NR
Withdrawals due to adverse events: NR
Despite the author's statement that patients were to be remove from the study if they experience an excessive drop in BP , developed arrhythmia or respiratory difficulty , or became unresponsive or lost of consciousness, there is no report of these withdrawals. Blood pressure: Data was obtained from table 2 page 121. The calculated weighted mean BP change was: Nitroprusside: SBP‐41.25± 24; : DBP‐26.5±12 Nicardipine: SBP‐49± 23; : DBP‐30±12 Standard deviation of the change was not reported but imputed from end point Heart rate: Data was obtained from table 2 page 121. The calculated weighted mean HR change was: Nitroprusside: 2±16 Nicardipine: ‐1.5±20 Standard deviation of the change was not reported but imputed from end point |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Glossary: AEOD=acute end organ damage NTP=sodium Nitroprusside NR=Not reported LVEF=left ventricular ejection fraction SBP =systolic blood pressure MAP=mean arterial pressure DBP=diastolic blood pressure HR=heart rate CO=cardiac output
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Annane 1996 | Only responders to an immediate previously given antihypertensive treatment . Thus, the results of this trials cannot be generalize to another situations. |
| Bertel 1983 | It does not report any of the outcome of interest, actually it does not show results of the clonidine group. Baseline values are not separated according to the randomization groups . |
| Borghi 1999 | BP was not part of inclusion criteria; and BP at baseline was too low (143/88 mmHg) for our acute myocardial infarction threshold (180/110 mm Hg) |
| Bussmann 1992 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate beteween those. |
| Ceyhan 1990 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Conen 1988 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Cotter 1998 | It compares wrong comparators: two different doses of a combination regimen |
| Dadkar 1993 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Franklin 1986 | Results from patients non‐randomized were mixed with randomized patients |
| Guerrera 1990 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Lisk 1993 | Blood pressure inclusion criteria (>170 mmHg) and baseline bp values, 172‐178/98‐104 (calculated from MAP 122‐128 mm Hg) were lower than our blood pressure thershold, 180/110, for acute stroke. |
| Marghli 1997 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Moritz 1989 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Nelson 1984 | It is a cross over trial |
| Neutel 1994 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those |
| Nielsen 1980 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Panacek 1995 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Pascale 1992 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Perez 1991 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Pilmer 1993 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Pujadas 1987 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Reisin 1990 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
| Risler 1998 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Rohr 1994 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Spah 1988 | Hypertensive emergency and non‐emergency patients are mixed in the same trial. Results do not discriminate those. |
| Yoshida 1998 | It was excluded because it compared two drugs of the same class (calcium channel blocker). Note: this is the only trial found that studied exclusively patients with acute aortic dissection. |
| Zampaglione 1994 | It was not possible to determine whether the population studied included exclusively patients with acute end organ damage |
AEOD: Acute end organ damage SBP= systolic blood pressure DBP= diastolic blood pressure
Contributions of authors
Marco Perez and James Wright formulated the idea for the review, and developed the basis for the protocol.
Marco Perez took the lead roles in searching, identifying and assessing studies, in data extraction and in writing up the review.
Vijaya Musini is an independent reviewer. She helped with the methodology of the review and independently checked the data extraction.
Sources of support
Internal sources
Departments of Pharmacology & Therapeutics and Medicine, Faculty of Medicine, University of Britisish Columbia, Canada.
External sources
British Columbia Ministry of Health Grant to the Therapeutics Initiative, Canada.
Instituto Mexicano del Seguro Social, Mexico.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Angeli 1991 {published data only}
- (2) Angeli P, Chiesa M, Caregaro L, Merkel C, Sacerdoti D, Rondana M, et al. Comparison of sublingual captopril and nifedipine in immediate treatment of hypertensive emergencies. A randomized, single‐blind clinical trial. [see comments]. Archives of Internal Medicine 1991;151(4):678‐682. [PubMed] [Google Scholar]
Beltrame 1998 {published data only}
- Beltrame JF, Zeitz CJ, Unger SA, Brennan RJ, Hunt A, Moran JL, Horowitz JD. Nitrate therapy is an alternative to furosemide/morphine therapy in the management of acute cardiogenic pulmonary edema. Journal of Cardiac Failure 1998;4(4):271‐279. [DOI] [PubMed] [Google Scholar]
DANISH II 1986 {published data only}
- Krogsgaard AR, McNair A, Hilden T, Nielsen PE. Reversibility of cerebral symptoms in severe hypertension in relation to acute antihypertensive therapy.Danish Multicenter Study.. Acta Medica Scandinavica 1986;220(1):25‐31. [DOI] [PubMed] [Google Scholar]
- Krogsgaard, A.R, Hilden, T, McNair, A, Nielsen, P.E. Cerebral symptoms and blood pressure during parenteral administration of chlorpromazine, dihydralazine and diazoxide. Danish multicentre study on acute severe hypertension. Acta Medica Scandinavica 1983;Suppl 678:51‐60. [DOI] [PubMed] [Google Scholar]
- McNair A, Krogsgaard AR, Hilden T, Nielsen PE. Severe hypertension with cerebral symptoms treated with furosemide, fractionated diazoxide or dihydralazine. Danish Multicenter Study. Acta Medica Scandinavica 1986;220(1):15‐23. [DOI] [PubMed] [Google Scholar]
- McNair A, Krogsgaard AR, Hilden T, Nielsen PE. Reversibility of cerebral symptoms in severe hypertension in relation to acute antihypertensive therapy. Danish Multicenter study.. Acta medica Scandinavica.Supplementum. 1985;693:107‐110. [DOI] [PubMed] [Google Scholar]
Elliott 1990 {published data only}
- Elliott WJ, Weber RR, Nelson KS, Oliner CM, Fumo MT, Gretler DD, McCray GR, Murphy MB. Renal and hemodynamic effects of intravenous fenoldopam versus nitroprusside in severe hypertension. Circulation 1990;81(3):970‐977. [DOI] [PubMed] [Google Scholar]
Hamilton 1996 {published data only}
- Hamilton RJ, Carter WA, Gallagher EJ. Rapid improvement of acute pulmonary edema with sublingual captopril.[see comment]. Academic Emergency Medicine 1996;3(3):205. [DOI] [PubMed] [Google Scholar]
Hirschl 1997 {published data only}
- Hirschl MM, Binder M, Bur A, Herkner H, Mullner M, Woisetschlager C, Laggner AN. Safety and efficacy of urapidil and sodium nitroprusside in the treatment of hypertensive emergencies. Intensive Care Med 1997;23(8):885‐888. [DOI] [PubMed] [Google Scholar]
Hirschl 1999 {published data only}
- Hirschl MM, Schreiber W, Woisetschlager C, Kaff A, Raab H. [Sublingual nitroglycerin or intravenous enalaprilat in preclinical treatment of hypertensive patients with pulmonary edema]. [German]. Zeitschrift fur Kardiologie 1999;88(3):208‐214. [DOI] [PubMed] [Google Scholar]
Marigliano 1988 {published data only}
- Marigliano V, Santilli D, Fiorani M, Ariani A, Cacciafesta M, Ferri C, Piccirillo G. Hypertensive emergencies in old age: effects of angiotensin converting enzyme inhibition. J Hypertens Suppl 1988;6(1):S91‐S93. [PubMed] [Google Scholar]
Nelson 1983 {published data only}
- Nelson GI, Silke B, Ahuja RC, Hussain M, Taylor SH. Haemodynamic advantages of isosorbide dinitrate over frusemide in acute heart‐failure following myocardial infarction. Lancet 1983;1(8327):730‐733. [DOI] [PubMed] [Google Scholar]
Pastorelli 1991 {published data only}
- Pastorelli R, Ferri C, Santucci A, Balsano F. New therapeutic possibilities in hypertensive emergencies. Current Therapeutic Research, Clinical & Experimental 1991;50(6):857‐868. [Google Scholar]
Rubio‐G 1999 {published data only}
- Rubio‐Guerra AF, Vargas‐Ayala G, Lozano‐Nuevo JJ, Narvaez‐Rivera JL, Rodriguez‐Lopez L. Comparison between isosorbide dinitrate aerosol and nifedipine in the treatment of hypertensive emergencies. J Hum Hypertens 1999;13(7):473‐476. [DOI] [PubMed] [Google Scholar]
Schreiber 1998 {published data only}
- Schreiber W, Woisetschlager C, Binder M, Kaff A, Raab H, Hirschl MM. The nitura study ‐ Effect of nitroglycerin or urapidil on hemodynamic, metabolic and respiratory parameters in hypertensive patients with pulmonary edema. Intensive Care Med 1998;24(6):557‐563. [DOI] [PubMed] [Google Scholar]
Verma 1987 {published data only}
- Verma SP, Silke B, Hussain M. First‐line treatment of left ventricular failure complicating acute myocardial infarction: A randomised evaluation of immediate effects of diuretic, venodilator, arteriodilator, and positive inotropic drugs on left ventricular function. Journal of cardiovascular pharmacology 1987;10(1):38‐46. [DOI] [PubMed] [Google Scholar]
Wu 1993 {published data only}
- Wu SG, Lin SL, Shiao WY, Huang HW, Lin CF, Yang YH. Comparison of sublingual captopril, nifedipine and prazosin in hypertensive emergencies during hemodialysis. Nephron 1993;65(2):284‐287. [DOI] [PubMed] [Google Scholar]
Yang 2004 {published data only}
- Yang HJ, Kim JG, Lim YS, Ryoo E, Hyun SY, Lee G. Nicardipine versus nitroprusside infusion as antihypertensive therapy in hypertensive emergencies. The Journal of international medical research 2004;32(2):118‐123. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Annane 1996 {published data only}
- Annane D, Bellissant E, Pussard E, Asmar R, Lacombe F, Lanata E, Madonna O, Safar M, Giudicelli JF, Gajdos P. Placebo‐controlled, randomized, double‐blind study of intravenous enalaprilat efficacy and safety in acute cardiogenic pulmonary edema. Circulation 1996;94(6):1316‐1324. [DOI] [PubMed] [Google Scholar]
Bertel 1983 {published data only}
- Bertel O, Conen D, Radu EW, Muller J, Lang C, Dubach UC. Nifedipine in hypertensive emergencies. British Medical Journal Clinical Research Ed 1983;. 286(6358):19‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borghi 1999 {published data only}
- Borghi C, Bacchelli S, Esposti DD, Bignamini A, Magnani B, Ambrosioni E. Effects of the administration of an angiotensin‐converting enzyme inhibitor during the acute phase of myocardial infarction in patients with arterial hypertension. SMILE Study Investigators. Survival of Myocardial Infarction Long‐term Evaluation. American journal of hypertension : journal of the American Society of Hypertension 1999;12(7):665‐672. [DOI] [PubMed] [Google Scholar]
Bussmann 1992 {published data only}
- Bussmann WD, Kenedi P, Mengden HJ, Nast HP, Rachor N. Comparison of nitroglycerin with nifedipine in patients with hypertensive crisis or severe hypertension. Clinical Investigator 1992;70(12):1085‐1088. [DOI] [PubMed] [Google Scholar]
Ceyhan 1990 {published data only}
- Ceyhan B, Karaaslan Y, Caymaz O, Oto A, Oram E, Oram A, Ugurlu S. Comparison of sublingual captopril and sublingual nifedipine in hypertensive emergencies. Japanese Journal of Pharmacology 1990;52(2):189‐193. [DOI] [PubMed] [Google Scholar]
Conen 1988 {published data only}
- Conen D, Ruttimann S, Noll G, Schneider K, Muller J. Short‐ and long‐term cerebrovascular effects of nitrendipine in hypertensive patients. Journal of cardiovascular pharmacology 1988;12 Suppl 4:S64‐S68. [DOI] [PubMed] [Google Scholar]
Cotter 1998 {published data only}
- Cotter G, Metzkor E, Kaluski E, Faigenberg Z, Miller R, Simovitz A, Shaham O, Marghitay D, Koren M, Blatt A, Moshkovitz Y, Zaidenstein R, Golik A. Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.[see comment]. Lancet 1998;351(9100):389. [DOI] [PubMed] [Google Scholar]
Dadkar 1993 {published data only}
- Dadkar VN, Karnik ND, Izar M, Sharma SR, Gandhi YP, Narawane NM, Vyawahare SS, Sudhakar S, Gore AG, Kapadia NM. Sublingual nifedipine and captopril in hypertensive urgencies and emergencies. Indian Heart Journal 1993;45(3):185‐187. [PubMed] [Google Scholar]
Franklin 1986 {published data only}
- Franklin C, Nightingale S, Mamdani B. A randomized comparison of nifedipine and sodium nitroprusside in severe hypertension. Chest 1986;90(4):500‐503. [DOI] [PubMed] [Google Scholar]
Guerrera 1990 {published data only}
- Guerrera G, Melina D, Capaldi L, Mauro R, Colivicchi F, Cardillo C, Guerrera G, Musumeci V, Savi L, Santoliquido A. [Sublingually administered captopril versus nifedipine in hypertension emergencies]. [Italian]. Minerva Cardioangiologica 1990;38(1‐2):37‐44. [PubMed] [Google Scholar]
Lisk 1993 {published data only}
- Lisk DR, Grotta JC, Lamki LM, Tran HD, Taylor JW, Molony DA, Barron BJ. Should hypertension be treated after acute stroke? A randomized controlled trial using single photon emission computed tomography. Archives of Neurology 1993;50(8):855. [DOI] [PubMed] [Google Scholar]
Marghli 1997 {published data only}
- Marghli S, Bouida W, Elatrous S, Boujdaria R, Nouira S, Abroug F. A prospective, randomized and controlled study of three intravenous antihypertensive drugs in severe hypertension. Reanimation Urgences 1997;6(3):289‐296. [Google Scholar]
Moritz 1989 {published data only}
- Moritz RD, Queiroz LP, Pereira MR, Scotinni MA. [Comparative study of the use of nifedipine and captopril in hypertensive emergencies]. [Portuguese]. Arquivos Brasileiros de Cardiologia 1989;52(6):323‐326. [PubMed] [Google Scholar]
Nelson 1984 {published data only}
- Nelson GI, Verma SP, Silke B, Abdulali S, Taylor SH. Management of hypertension complicating acute myocardial infarction ( MI): comparison of sympathetic (labetalol) and calcium channel ( nifedipine) blockade. [abstract]. Aust NZ J Med Suppl 1984;14(4):577. [Google Scholar]
Neutel 1994 {published data only}
- Neutel JM, Smith DH, Wallin D, Cook E, Ram CV, Fletcher E, Maher KE, Turlepaty P, Grandy S, Lee R. A comparison of intravenous nicardipine and sodium nitroprusside in the immediate treatment of severe hypertension. American Journal of Hypertension 1994;7(7 Pt 1):623‐628. [DOI] [PubMed] [Google Scholar]
Nielsen 1980 {published data only}
- Nielsen PE, Krogsgaard A, McNair A, Hilden T. Emergency Treatment of Severe Hypertension Evaluated in a Randomized Study. Acta Medica Scandinavica 1980;208:473‐480. [PubMed] [Google Scholar]
Panacek 1995 {published data only}
- Panacek EA, Bednarczyk EM, Dunbar LM, Foulke GE, Holcslaw TL. Randomized, prospective trial of fenoldopam vs sodium nitroprusside in the treatment of acute severe hypertension. Fenoldopam Study Group. [see comments]. Academic Emergency Medicine 1995;2(11):959‐965. [DOI] [PubMed] [Google Scholar]
Pascale 1992 {published data only}
- Pascale C, Zampaglione B, Marchisio M. Management of hypertensive crisis: Nifedipine in comparison with captopril, clonidine and furosemide. Current Therapeutic Research, Clinical & Experimental 1992;51(1):9‐18. [Google Scholar]
Perez 1991 {published data only}
- Perez C, Dougnac A, Alvarez M, Andresen M, Diaz O, Geni R, Prat G, Vasquez M. [Sublingual captopril versus nifedipine in the treatment of hypertensive crisis]. [Spanish]. Revista Medica de Chile 1991;119(4):402‐405. [PubMed] [Google Scholar]
Pilmer 1993 {published data only}
- Pilmer BL, Green JA, Panacek EA, Elliot WJ, Murphy MB, Rutherford W, Nara AR. Fenoldopam mesylate versus sodium nitroprusside in the acute management of severe systemic hypertension. Journal of Clinical Pharmacology 1993;33(6):549‐553. [DOI] [PubMed] [Google Scholar]
Pujadas 1987 {published data only}
- Pujadas R, Jane J, Fornos C, Gago MJ, CN. Comparison of sublingual captopril and nifedipine in hypertensive crises. Archives of Internal Medicine 1987;147(1):175‐176. [PubMed] [Google Scholar]
Reisin 1990 {published data only}
- Reisin E, Huth MM, Nguyen BP, Weed SG, Gonzalez FM. Intravenous fenoldopam versus sodium nitroprusside in patients with severe hypertension. Hypertension 1990;15(2 Suppl):I59‐I62. [DOI] [PubMed] [Google Scholar]
Risler 1998 {published data only}
- Risler T, Bohm R, Wetzchewald D, Nast HP, Koch HH, Stein G, Erley CM. A comparison of the antihypertensive efficacy and safety of felodipine IV and nifedipine IV in patients with hypertensive crisis or emergency not responding to oral nifedipine. European Journal of Clinical Pharmacology 1998;54(4):295‐298. [DOI] [PubMed] [Google Scholar]
Rohr 1994 {published data only}
- Rohr G, Reimnitz P, Blanke P. Treatment of hypertensive emergency. Comparison of a new dosage form of the calcium antagonist nitrendipine with nifedipine capsules. Intensive Care Medicine 1994;20(4):268‐271. [DOI] [PubMed] [Google Scholar]
Spah 1988 {published data only}
- Spah F, Grosser KD. Treatment of hypertensive urgencies and emergencies with nitrendipine, nifedipine, and clonidine: effect on blood pressure and heart rate. Journal of cardiovascular pharmacology 1988;12 Suppl 4:S154‐S156. [DOI] [PubMed] [Google Scholar]
Yoshida 1998 {published data only}
- Yoshida Noriko ea. Antihypertensive Effect of the Long‐Acting Ca Antagonist Amlodipine vs. Sustained‐Release Nifedipine in Patients with Acute Aortic Dissection. Rinsho to Kenkyu (The Japanese Journal of Clinical and Experimental Medicine) 1998;75(6):1419‐1422. [Google Scholar]
Zampaglione 1994 {published data only}
- Zampaglione B, Pascale C, Marchisio M, Santoro A. The use of lacidipine in the management of hypertensive crises: a comparative study with nifedipine. Journal of cardiovascular pharmacology 1994;23 Suppl 5:S116‐S118. [DOI] [PubMed] [Google Scholar]
Additional references
Antman 2004
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, American College of Cardiology, American Heart Association Task Force on Practice Guidelines, Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction).[erratum appears in Circulation. 2005 Apr 19;111(15):2013‐4]. Circulation 2004;110(9):e82‐292. [PubMed] [Google Scholar]
BASC 2001
- Blood pressure in Acute Stroke Collaboration (BASC). Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI] [PubMed] [Google Scholar]
Cherney 2002
- Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: A systematic review of the literature. Journal of General Internal Medicine 2002;17(12):937‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalen 1979
- Dalen JE, Howe JP, III. Dissection of the aorta. Current diagnostic and therapeutic approaches. JAMA 1979;242(14):1530‐2. [PubMed] [Google Scholar]
Duley 2006
- Duley L, Henderson‐Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy.[update of Cochrane Database Syst Rev. 2006]. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI] [PubMed] [Google Scholar]
Gifford 1959
- Gifford RW. Treatment of Hypertensive Emergencies, including use of Sodium Nitroprusside. Mayo Clin Proc 1959;34(16):387. [PubMed] [Google Scholar]
JNC‐7
- Chobanian, A.V, Bakris, G.L, Black, H.R, Cushman, W.C, Green, L.A, Izzo, J.L, Jr, Jones, D.W, Materson, B.J, Oparil, S, Wright, J.T, Jr, Roccella, E.J, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
Krogsgaard 1983
- Krogsgaard, A.R, Hilden, T, McNair, A, Nielsen, P.E. Cerebral symptoms and blood pressure during parenteral administration of chlorpromazine, dihydralazine and diazoxide. Danish multicentre study on acute severe hypertension. Acta Medica Scandinavica 1983;Suppl 678:51‐60. [DOI] [PubMed] [Google Scholar]
Krogsgaard 1986‐D
- Krogsgaard AR, McNair A, Hilden T, Nielsen PE. Reversibility of cerebral symptoms in severe hypertension in relation to acute antihypertensive therapy.Danish Multicenter Study.. Acta Medica Scandinavica 1986;220(1):25‐31. [DOI] [PubMed] [Google Scholar]
Mattu 2005
- Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emergency Medicine Clinics of North America 2005;23(4):1105‐25. [DOI] [PubMed] [Google Scholar]
McNair 1985‐D
- McNair A, Krogsgaard AR, Hilden T, Nielsen PE. Reversibility of cerebral symptoms in severe hypertension in relation to acute antihypertensive therapy. Danish Multicenter study.. Acta medica Scandinavica.Supplementum. 1985;693:107‐110. [DOI] [PubMed] [Google Scholar]
McNair 1986
- McNair A, Krogsgaard AR, Hilden T, Nielsen PE. Severe hypertension with cerebral symptoms treated with furosemide, fractionated diazoxide or dihydralazine. Danish Multicenter Study. Acta Medica Scandinavica 1986;220(1):15‐23. [DOI] [PubMed] [Google Scholar]
Roberts 2004
- Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, Laloe V, Munoz‐Sanchez A, Arango M, Hartzenberg B, Khamis H, Yutthakasemsunt S, Komolafe E, Olldashi F, Yadav Y, Murillo‐Cabezas F, Shakur H, Edwards P, CRASH tc. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo‐controlled trial.[see comment]. Lancet 2004;364(9442):1321‐1328. [DOI] [PubMed] [Google Scholar]
Wilson 2005
- Wilson SR, Hirsch NP, Appleby I. Management of subarachnoid haemorrhage in a non‐neurosurgical centre. Anaesthesia 2005;60(5):470‐85. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Perez 2008
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. Journal of Human Hypertension 17 Apr 2008;advance online publication. [DOI: 10.1038/jhh.2008.25] [DOI] [PubMed] [Google Scholar]


