Abstract
Background
Thermotherapy is often used as adjunct in the treatment of rheumatoid arthritis (RA) by rehabilitation specialists.
Objectives
To evaluate the effectiveness of different thermotherapy applications on objective and subjective measures of disease activity in patients with RA.
Search methods
We searched MEDLINE, EMBASE, Pedro, Current Contents, Sports Discus and CINAHL up to and including September 2001. The Cochrane Field of Rehabilitation and Related Therapies and the Cochrane Musculoskeletal Review Group were also contacted for a search of their specialized registers. Hand searching was conducted on all retrieved articles for additional articles.
Selection criteria
Comparative controlled studies, such as randomized controlled trials, controlled clinical trials, cohort studies or case/control studies, of thermotherapy compared to control or active interventions in patients with RA were eligible. No language restrictions were applied. Abstracts were accepted.
Data collection and analysis
Two independent reviewers identified potential articles from the literature search (VR, LB). These reviewers extracted data using pre‐defined extraction forms. Consensus was reached on all data extraction. Quality was assessed by two reviewers using a 5 point scale that measured the quality of randomization, double‐blinding and description of withdrawals.
Main results
Seven studies (n=328 participants) met the inclusion criteria. The results of this systematic review of thermotherapy for RA found that there was no significant effect of hot and ice packs applications (Ivey 1994), cryotherapy (Rembe 1970) and faradic baths (Hawkes 1986) on objective measures of disease activity including joint swelling, pain, medication intake, range of motion (ROM), grip strength, hand function compared to a control (no treatment) or active therapy.
There is no significant difference between wax and therapeutic ultrasound as well as between wax and faradic bath combined to ultrasound for all the outcomes measured after one, two or three week(s) of treatment (Hawkes 1986). There was no difference in patient preference for all types of thermotherapy. No harmful effects of thermotherapy were reported.
Authors' conclusions
Superficial moist heat and cryotherapy can be used as palliative therapy. Paraffin wax baths combined with exercises can be recommended for beneficial short‐term effects for arthritic hands. These conclusions are limited by methodological considerations such as the poor quality of trials.
Plain language summary
Thermotherapy (heat treatment) for treating rheumatoid arthritis
Thermotherapy is a commonly used modality in treating rheumatoid arthritis (RA). Thermotherapy modalities include superficial moist heat fomentations (hot packs) at different temperatures, cryotherapy (ice packs), paraffin wax baths and faradic baths. All studies included in this review (n=7) are randomized controlled trials (RCT).
This review found there were no significant effects for hot and ice packs applications and faradic baths on objective measures of disease activity including joint swelling, pain, medication intake, range of motion (ROM), grip strength, hand function or patient preference compared to control (no treatment) or active therapy. However, there were positive results for paraffin wax baths alone for arthritic hands on objective measures of ROM, pinch function, grip strength, pain on non‐resisted motion, stiffness compared to control (no treatment) after four consecutive weeks of treatment.
There is no significant difference between wax and therapeutic ultrasound or between wax and faradic bath combined with ultrasound for any of the outcomes measures. The reviewers concluded that thermotherapy can be used as a palliative therapy or as an adjunct therapy combined with exercises for RA patients. Wax baths appear especially helpful in the treatment of arthritic hands. These conclusions are limited by methodological considerations such as the poor quality of trials.
Background
Superficial moist heat and cryotherapy are commonly used in physical rehabilitation for patients with rheumatoid arthritis (RA) to relieve pain (Oosterveld 1992c). Both can be easily applied at home by the patient but may also be combined with other rehabilitation interventions.
Thermotherapy is suggested as a potential intervention for the treatment of musculoskeletal conditions in the American Physical Therapy Association guidelines (APTA 2001). These guidelines are not based on evidence from comparative controlled trials. The Philadelphia Panel (Philadelphia 2001) developed Evidence Based Clinical Practice Guidelines for several musculoskeletal conditions (Philadelphia 2001). However, rheumatoid arthritis was not included in these guidelines. Clinicians require good evidence in order to make an informed decision regarding effective and appropriate treatment options.
There are several potentially beneficial physiological and clinical effects of thermotherapy for RA patients. Thermotherapy has effects on pain, muscle spasms, circulation and inflammation (Knight 1995). Furthermore, it can be applied by patients in their own home as needed. Despite the widespread use of heat and cold by patients with RA for the control of pain, this clinical application is solely based on empiric evidence. In fact, the effectiveness of heat or cold (i.e. cryotherapy) application relative to a placebo, to alternate therapies or even its role as an adjunct remains unclear.
Objectives
To evaluate the effectiveness of thermotherapy compared to placebo and to other alternate interventions on pain relief for treating patients with RA.
Methods
Criteria for considering studies for this review
Types of studies
According to an a priori protocol, all comparative controlled trials, including randomized controlled trials (RCT), controlled clinical trials(CCT), and case‐control and cohort studies were included. Trials which used the same patients as their own control were not accepted. The results were graded according to the strength of the study design.
No language limitations were imposed. Abstracts were accepted.
Types of participants
Adult patients with classic or definite rheumatoid arthritis (Arnett 1988) treated with heat or/and cryotherapy or other alternate thermotherapy modalities. All peripheral joints were considered while axial joints of the spine were excluded from the results of this review.
Types of interventions
Acceptable interventions included any form of heat (e.g. hot packs, paraffin wax bath) or cryotherapy (e.g. ice packs, cold gel packs). Balneotherapy was excluded as it has been evaluated in another Cochrane review (Verhagen 2000). Acceptable control groups were placebo, untreated, or alternate interventions such as paraffin wax baths, faradic baths and other forms of rehabilitation interventions. Concurrent interventions (e.g. NSAIDs, exercises) were accepted if they were given to both comparative groups.
Types of outcome measures
The primary outcome considered was pain. Secondary outcomes were selected from the potential core set identified by the Outcome Measures in Rheumatology Clinical Trials (OMERACT) conference on RA outcomes (OMERACT 1993). Our final set of secondary outcomes included:
Tender joint count Swollen joint count Physician global assessment Patient global assessment Functional status
In addition, two outcomes often used as measures of clinical effects in the practice of rehabilitation were included: Range of Motion (ROM) Strength
Physiological outcomes such as skin and joint temperature were not included in the analysis.
Search methods for identification of studies
The literature search was conducted up to September 2001 according to the sensitive search strategy for RCTs designed for the Cochrane Collaboration (Dickersin 1994), with modifications proposed by Haynes et al (Haynes 1994). Additional terms for study design were used to identify observational studies including: case‐control, cohort, comparative study and clinical trial. MEDLINE, EMBASE, HealthSTAR, Sports Discus, CINAHL, the Cochrane Controlled Trials Register, Pedro, the specialized registry of the Cochrane Musculoskeletal Group and the Cochrane Field of Rehabilitation and Related Therapies were searched using a keyword and text word search strategy (shown in Appendix 1). In addition, the reference lists of included trials were searched and content experts were contacted for additional studies.
Data collection and analysis
Two independent reviewers (VR, LB) examined the titles and abstracts of the trials identified by the search strategy to select trials that met the inclusion criteria. All trials classified as relevant by at least one of the reviewers were retrieved. The retrieved articles were re‐examined to ensure they met the inclusion criteria.
The results of the individual trials were extracted from each of the included trials using pre‐determined extraction forms by two independent reviewers (LB, VR). The data was cross‐checked by a third reviewer (BS). The extraction forms were developed and pilot‐tested, based on other forms used by the Cochrane Musculoskeletal Review Group. The extraction form documented specific information about the heat or cold therapy including 1) method (hot pack, paraffin wax, cold water); 2) therapeutic application (duration, frequency, temperature, total number of sessions and any specific skin preparation). The final data values were based on consensus of the two reviewers.
Statistical Analysis Most outcomes were continuous in nature (functional status, pain and strength). Where pooling of data from different trials was possible, these outcomes were analyzed using a weighted mean difference (WMD) a fixed effects model. For dichotomous data, relative risks were used. The effect measured in an individual trial is weighted by the amount of variability about the mean (measured by the standard deviation) in that study for that outcome. Graphical data was used in cases where table data was not available.
When applicable, heterogeneity was assessed with a Chi square test on N degrees of freedom where N is the number of studies. Where statistically significant heterogeneity existed, the results were analyzed by a random effects model. Furthermore, the contributions of pre‐determined hypotheses regarding different populations and interventions were examined as possible sources of heterogeneity.
Results
Description of studies
The literature search and handsearching identified 306 potential articles. Of these, seven RCTs were included involving 328 RA patients (Bulstrode 1986, Dellhag 1992, Hawkes 1986, Ivey 1994, Kirk 1968, Rembe 1970, Williams 1986).
Fifteen trials were excluded for several reasons: 1) Abramson 1964: no clinical outcomes; 2) Amundson 1979: not a clinical trial; 3) Bromley 1994: sample of healthy people; 3) Curkovic 1993: no sufficient statistical data; 4) Devereaux 1985: no control group; 5) DonTigny 1962: no participants with RA; 6) Feibel 1976: not a clinical trial; 7) Haines 1970: no RA patients; 8) Halliday Pegg 1969: no control group; 9) Harris 1955: No description of the statistical procedure used, no p values or standard deviations available; 10) Hoyrup 1986: sample of participants with trauma; 11) Mainardi 1979: no control group, patients were their own controls; 12) Oosterveld 1992c: sample of healthy people; 13) Oosterveld 1994a): sample of healthy people; 14) Oosterveld 1994b: mixed population with RA in minority; 15) Weinberger 1989: no clinical outcome; 16) Whipple 1992: sample of healthy people.
Two RCTs examined the effects of ice therapy versus control for the reduction of oedema and inflammation (Bulstrode 1986, Rembe 1970). One RCT compared the combination of parafin bath and exercise, to exercise only and control for pain relief on non‐resisted motion, morning stiffness and joint ROM , pinch function and grip strength (Dellhag 1992). Another RCT compared the relative efficacy of three different thermotherapy modalities: paraffin wax bath, faradic bath and ultrasound for their effects on hand strength, ROM, joint circumference and functional status (Hawkes 1986). One RCT investigated the effect of different temperatures of heat on medication intake (Ivey 1994). One RCT compared heat versus cryotherapy for the pain relief in patients with RA (Kirk 1968). Lastly, another RCT compared heat therapy to cryotherapy for pain relief and ROM (Williams 1986).
Strengthening exercises (Dellhag 1992, Williams 1986), ultrasound (Hawkes 1986) or medication (Rembe 1970) was prescribed concurrently in combination with various applications of thermotherapy.
Risk of bias in included studies
Methodological quality was assessed using a validated checklist (Jadad 1996). The components of quality focus on randomization, double‐blinding and description of withdrawals. Two independent reviewers (LB, VR) assessed quality and differences were resolved by consensus with a third reviewer (BS). Quality was used in subgroup analyses to test the hypothesis that poorly conducted trials demonstrate greater efficacy of the intervention under evaluation. Each item (i.e. randomization, blinding and withdrawals) was examined separately for its effect.
The mean methodological quality of the seven RCTs was two, with a range from one to three. Only one trial scored full points for randomization, none scored full points for double blinding, and five did not report withdrawals and dropouts. None of the included studies were double‐blind.
Effects of interventions
The results of this systematic review on thermotherapy in the treatment of RA found that there was no significant effect of hot or ice pack applications (Bulstrode 1986, Ivey 1994, Kirk 1968, Rembe 1970, Williams 1986) or faradic baths (Hawkes 1986) on objective measures of disease activity including joint swelling, pain, medication intake, ROM, grip strength or hand function when compared to a control (no active treatment) or alternate treatment.
There was an exception with the case of faradic baths when compared to control. For one outcome, an invalidated checklist of ability to perform daily activities, the results were conflicting since we observed significant borderline values favouring faradic baths at two weeks (0.30, 95%CI: 0.02 to 0.58) then favouring control group at three weeks (‐1.30, 95%CI: ‐2.51 to ‐0.09) (Hawkes 1986). There was also a tendency favouring cryotherapy versus no active treatment in the reduction of hand swelling after two days (‐5.80 % from baseline, 95%CI: ‐11.78 to 0.18) and three days (‐9.80 % from baseline, 95%CI: ‐19.91 to ‐0.31) post‐surgery of the hand (Rembe 1970).
One trial of paraffin wax alone or combined with exercise (Dellhag 1992) did not report sufficient detail to analyze the results using review manager: standard deviation was not reported. However, this trial reported statistically significant results for some outcomes: the wax + exercise group showed significantly greater improvement than the control group for flexion deficit (21% relative to control), pain on non‐resisted motion (44% relative to control), grip function (8% relative to control) and pinch function (5% relative to control) of the hand (p<.05). There was no significant effect of wax+exercise on stiffness or grip strength. This trial found no significant improvement from baseline with paraffin wax only versus control for any outcomes (pinch and grip function, pain, stiffness and range of motion deficits).
There was no statistically significant difference when comparing wax and therapeutic ultrasound or wax and faradic bath to ultrasound for outcomes measured after one, two or three week(s) of treatment (Hawkes 1986). In addition, there was no patient preference for the various thermotherapy modalities offered (Kirk 1968).
No subgroup analysis on high versus low quality applications were undertaken as none of the studies examined the same type of thermotherapy, used similar outcomes or studied the same joints. Due to the small number of trials, the remaining pre‐planned subgroup analyses (treatment duration, type of thermotherapy application, patient characteristics, disease characteristics, specific joint and design considerations) were not conducted. Publication bias was not assessed due to the small number of trials.
Discussion
Statistically significant results favouring paraffin wax combined with exercise to a control group were found in patients with RA of the hand after four weeks of treatment. Improvements were reported in objective measures of ROM in fingers flexion, pinch function, grip strength. These improvements ranged from 5 to 44% relative to the control group (Dellhag 1992).
No significant effect of hot pack or ice pack applications (Ivey 1994), cryotherapy (Rembe 1970) and faradic baths (Hawkes 1986) were reported for objective measures of disease activity including joint swelling, pain, medication intake, range of motion (ROM), grip strength, hand function when compared to a control group (no active treatment) or alternate therapy. No significant differences were detected between wax and therapeutic ultrasound or between wax and faradic bath combined to ultrasound for all the outcomes measured after one, two or three week(s) of treatment. There was no statistically significant difference in patient preference for any type of thermotherapy (Kirk 1968). These interventions can be used by the patient interchangeably.
Physiological studies have shown significant effects of cryotherapy on circulatory and temperature responses, muscle spasm and inflammation (Chapman 1991, Knight 1995), however, the mechanism of action is not yet fully understood (Knight 1995). Despite these identified physiologic effects, there appears to be no effect in human patients on objective measures of disease activity in controlled trials. Perhaps, the clinical outcome measures used in the included studies are not sensitive enough to capture the expected physiological effects.
Recent guidelines by the Philadelphia Panel (Philadelphia Panel 2001) did not include rheumatoid arthritis. Another systematic review (Puett 1994) for OA of the knee was unable to draw conclusions regarding thermotherapy, due to a lack of evidence. The American College of Rheumatology (ACR) (ACR 1996), The British Medical Journal (BMJ) (BMJ 1999) and the Manal & Snyder‐Mackler (Manal 1996) guidelines do not make recommendations regarding thermotherapy for RA.
Confounding variables, such as characteristics of the therapeutic application, characteristics of the population, characteristics of the disease and methodological considerations may have contributed to the lack of effect of thermotherapy (Morin 1996) in the studies reviewed. Some of the characteristics of the thermotherapy application that can affect efficacy are: type of thermotherapy, temperature of the application, duration of the application (Behnke 1973) and schedule of treatment (e.g. only four consecutive treatment sessions). Population characteristics of importance are: age, gender, disease duration (acute or chronic) and post‐surgery. It is crucial that details on these previously mentioned characteristics should be addressed in studies of thermotherapy and need to be reported consistently in published studies.
Methodological considerations that may have contributed to the lack of effect are the randomization method, quality of double‐blinding, sample size, study duration and selection of outcome measures. A good number of seven RCTs were retrieved from the literature. However, the low quality of the included RCTs may have caused an overestimation of effect. In particular, patients could not be blinded to thermotherapy. This is a common problem in trials of rehabilitation interventions (Deyo 1990). There are also issues in including crossover design (Kirk 1968) into systematic reviews (Altman 2001). Some outcome measures used in studies included in this review such as activity score (Hawkes 1986) or not recognized by OMERACT (OMERACT, 1993) such as stiffness (Dellhag 1992). Standardized outcomes measures (OMERACT 1993) and measurement periods should be used to facilitate the pooling of data of several studies.
Reporting data should also be standardized among the included RCTs. Mean and standard deviation of every outcome should be provided systematically. The use of statistical approximation derived from the p‐value to estimate the standard deviation and the borderline significant values of the upper and lower limits of the confidence interval could affect the conclusion on efficacy of wax therapy (Dellhag 1992), cryotherapy (Rembe 1970) and faradic bath (Hawkes 1986). Furthermore, some significant borderline results were also contradictory for the activity score at two different measurement periods (Hawkes 1986). Indeed, the results favoured the treatment group at two weeks and then the control group one week later (Hawkes 1986). Some studies expressed their results using the difference between baseline values and end of treatment values (Dellhag 1992). We had to recalculate the difference between groups at end of treatment.
Another confounding variable was concurrent therapy, such as combined exercises (Dellhag 1992, Williams 1986), ultrasound (Hawkes 1986) or medication (Rembe 1970). These could have influenced the efficacy of the thermotherapy. Even though it reflects common clinical practice in physiotherapy, these combined interventions introduce biases and the specific efficacy of an intervention for RA cannot be isolated. For example, endorphin and enkephalin production is stimulated by exercises (Coutts 1994) and may reduce arthritic pain in a combined thermotherapy modality. Combined thermotherapy modality, such as wax, may enhance the action of exercises in the treatment of RA for certain outcomes such as ROM and pain during non‐resisted movement.
The reviewers conclude that thermotherapy, especially wax baths, can be used as a palliative therapy and an adjunct therapy combined with exercises for the treatment of RA patients arthritic hands.
Authors' conclusions
Implications for practice.
This review has shown neither positive nor detrimental effects of heat therapy on important outcomes or on joint destruction in RA patients. The reviewers conclude that thermotherapy can be used as needed by patients with RA, as a palliative therapy and an adjunct therapy combined with exercises. However, these conclusions are limited by the poor methodological quality of the trials available and the large number of borderline values. This review has shown that thermotherapy can be used as an adjunct and palliative therapy. No harmful side effects were reported.
Implications for research.
More sensitive and valid clinical outcomes should be used in studies on thermotherapy to reflect the physiological effects found in the scientific literature. Detailed information regarding the temperature, duration of application and mode of application is needed in order to determine the optimal characteristics of the therapeutic application of thermotherapy for RA at different human joints.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. CMSG ID: C090‐R |
Acknowledgements
The authors thank Sarah Milne, Michael Saginur, Jessie McGowan, Shannon Rees, Guillaume Léonard, Marie‐Andrée Ouimet, and Catherine Lamothe for their work on this project and comments in earlier drafts.
Appendices
Appendix 1. Search strategy
1 exp osteoarthritis/ 2 osteoarthritis.tw. 3 osteoarthrosis.tw. 4 degenerative arthritis.tw. 5 exp arthritis, rheumatoid/ 6 rheumatoid arthritis.tw. 7 rheumatism.tw. 8 arthritis, juvenile rheumatoid/ 9 caplan's syndrome.tw. 10 felty's syndrome.tw. 11 rheumatoid.tw. 12 ankylosing spondylitis.tw. 13 arthrosis.tw. 14 sjogren$.tw. 15 or/1‐14 16 heat/tu 17 (heat or hot or ice).tw. 18 cryotherapy.sh,tw. 19 (vapocoolant or phonophoresis).tw. 20 exp hyperthermia, induced/ 21 (hypertherm$ or thermotherapy).tw. 22 (fluidotherapy or compression).tw. 23 15 and 22 24 clinical trial.pt. 25 randomized controlled trial.pt. 26 tu.fs. 27 dt.fs. 28 random$.tw. 29 placebo$.tw. 30 ((sing$ or doubl$ or tripl$) adj (masked or blind$)).tw 31 sham.tw. 32 or/24‐31 33 23 and 32
Data and analyses
Comparison 1. 50°F Thermal‐pad vs 60°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain measurement (amount of morphine injected ‐ mg/h) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.19, 0.59] |
| 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.14, 1.54] |
1.1. Analysis.
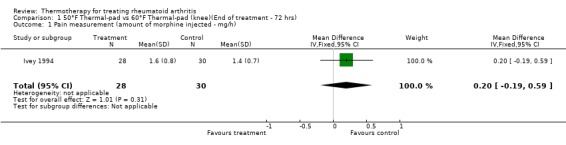
Comparison 1 50°F Thermal‐pad vs 60°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 1 Pain measurement (amount of morphine injected ‐ mg/h).
1.2. Analysis.
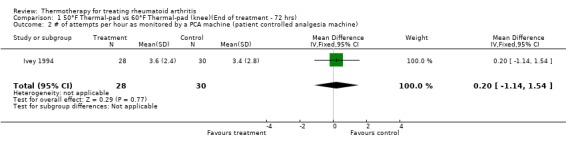
Comparison 1 50°F Thermal‐pad vs 60°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine).
Comparison 2. 50°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain measurement (amount of morphine injected ‐ mg/h) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.69, 1.09] |
| 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.07, 0.67] |
2.1. Analysis.
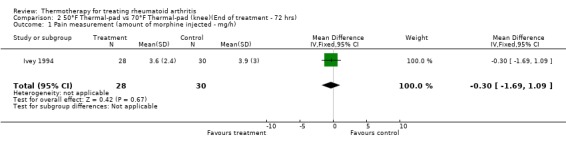
Comparison 2 50°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 1 Pain measurement (amount of morphine injected ‐ mg/h).
2.2. Analysis.
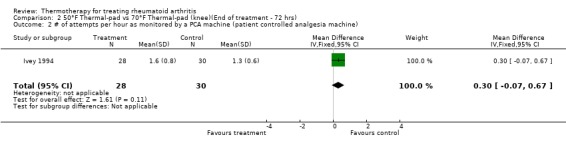
Comparison 2 50°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine).
Comparison 3. 60°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain measurement (amount of morphine injected ‐ mg/h) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.97, 0.97] |
3.1. Analysis.
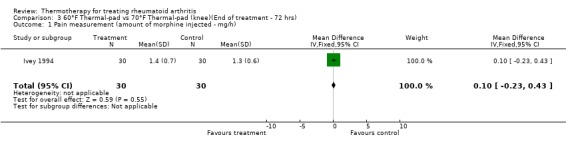
Comparison 3 60°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 1 Pain measurement (amount of morphine injected ‐ mg/h).
3.2. Analysis.
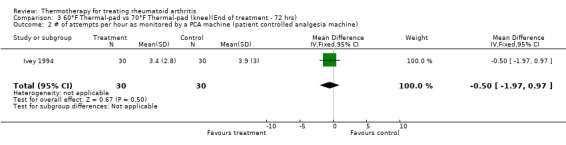
Comparison 3 60°F Thermal‐pad vs 70°F Thermal‐pad (knee)(End of treatment ‐ 72 hrs), Outcome 2 # of attempts per hour as monitored by a PCA machine (patient controlled analgesia machine).
Comparison 4. Ice packs vs Control (knee)(End of therapy ‐ 5 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Thermographic Index (derived from mean temperature, a decrease is good)) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| 2 Joint circumference (cm) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐1.46, 2.86] |
4.1. Analysis.
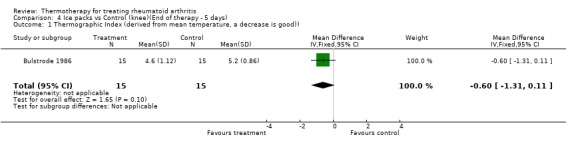
Comparison 4 Ice packs vs Control (knee)(End of therapy ‐ 5 days), Outcome 1 Thermographic Index (derived from mean temperature, a decrease is good)).
4.2. Analysis.
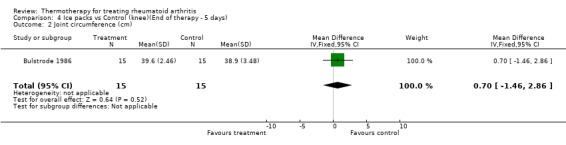
Comparison 4 Ice packs vs Control (knee)(End of therapy ‐ 5 days), Outcome 2 Joint circumference (cm).
Comparison 5. Ice packs vs Hot packs (knee)(End of treatment ‐ 5 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 # of patients prefering ice | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.40, 7.63] |
| 2 # of patients with improved pain grading | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.31, 5.67] |
| 3 # of patients with improved stiffness grading | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [0.77, 19.30] |
5.1. Analysis.
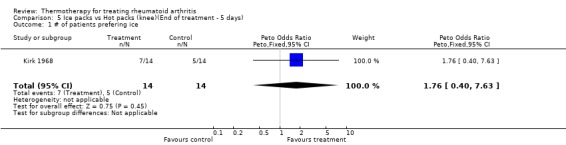
Comparison 5 Ice packs vs Hot packs (knee)(End of treatment ‐ 5 days), Outcome 1 # of patients prefering ice.
5.2. Analysis.
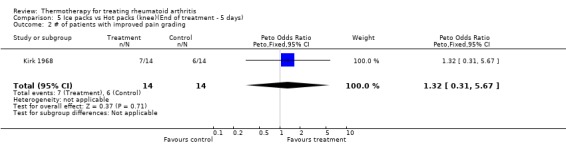
Comparison 5 Ice packs vs Hot packs (knee)(End of treatment ‐ 5 days), Outcome 2 # of patients with improved pain grading.
5.3. Analysis.
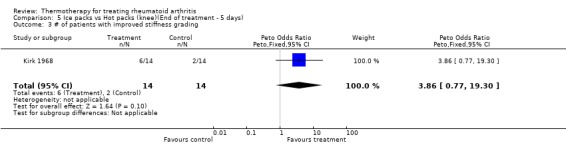
Comparison 5 Ice packs vs Hot packs (knee)(End of treatment ‐ 5 days), Outcome 3 # of patients with improved stiffness grading.
Comparison 6. Hot packs vs Ice packs (shoulder)(End of treatment ‐ 3 wks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (McGill Pain Questionnaire) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐7.59, 5.19] |
| 2 Flexion (degrees) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 7.20 [‐15.32, 29.72] |
| 3 Abduction ROM (degrees) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐24.65, 31.25] |
6.1. Analysis.
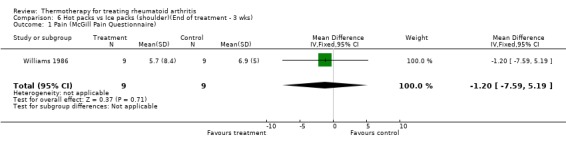
Comparison 6 Hot packs vs Ice packs (shoulder)(End of treatment ‐ 3 wks), Outcome 1 Pain (McGill Pain Questionnaire).
6.2. Analysis.
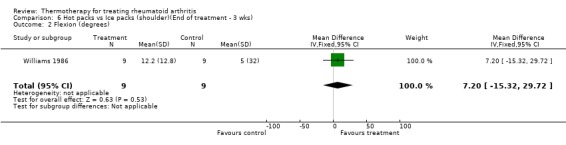
Comparison 6 Hot packs vs Ice packs (shoulder)(End of treatment ‐ 3 wks), Outcome 2 Flexion (degrees).
6.3. Analysis.
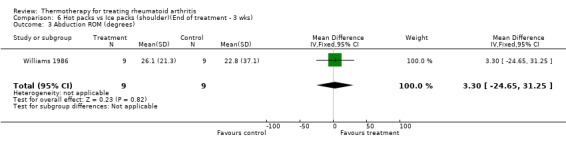
Comparison 6 Hot packs vs Ice packs (shoulder)(End of treatment ‐ 3 wks), Outcome 3 Abduction ROM (degrees).
Comparison 7. Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐19.1 [‐37.36, ‐0.84] |
| 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐11.90 [‐23.50, ‐0.30] |
| 3 Grip Function Test (0‐80 pts, 0=worst)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Pinch Function Test (0‐32 pts, 0=worst)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.78, ‐0.02] |
| 5 Grip strength (N) (average of dominant hand)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐18.76, ‐0.24] |
| 6 Pain on resisted motion (0‐9 pts, 0=no pain)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.00, 0.20] |
| 7 Pain, non‐resisted motion with both hands (VAS: 0‐100mm)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.20 [‐14.08, ‐0.32] |
| 8 Stiffness, both hands (VAS: 0‐100mm)* | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐6.32, ‐0.08] |
7.1. Analysis.
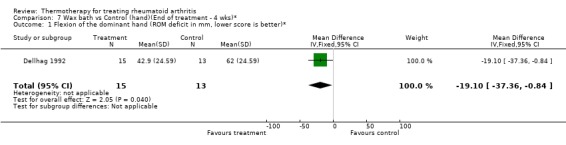
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better)*.
7.2. Analysis.
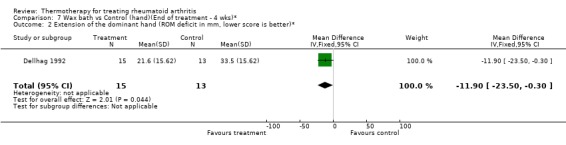
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)*.
7.3. Analysis.
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 3 Grip Function Test (0‐80 pts, 0=worst)*.
7.4. Analysis.
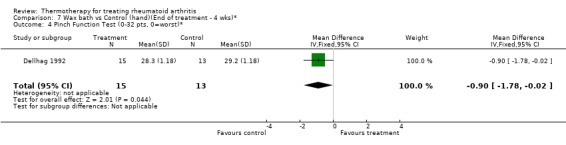
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 4 Pinch Function Test (0‐32 pts, 0=worst)*.
7.5. Analysis.
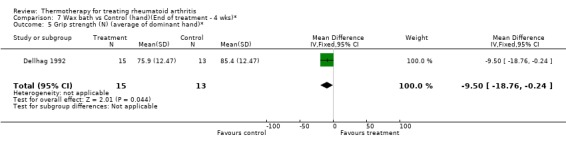
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 5 Grip strength (N) (average of dominant hand)*.
7.6. Analysis.
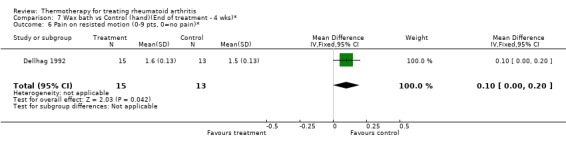
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 6 Pain on resisted motion (0‐9 pts, 0=no pain)*.
7.7. Analysis.
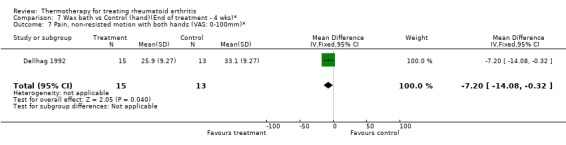
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 7 Pain, non‐resisted motion with both hands (VAS: 0‐100mm)*.
7.8. Analysis.
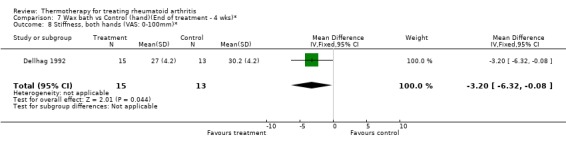
Comparison 7 Wax bath vs Control (hand)(End of treatment ‐ 4 wks)*, Outcome 8 Stiffness, both hands (VAS: 0‐100mm)*.
Comparison 8. Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better) * | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 8.30 [0.44, 16.16] |
| 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.18, ‐0.02] |
| 3 Grip Function Test (0‐80pts, 0=worst)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.55, ‐0.05] |
| 4 Pinch Function Test (0‐32 pts, 0=worst)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Grip strength (N) (average dominant hand)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐47.0 [‐92.38, ‐1.62] |
| 6 Pain on resisted motion (0‐9pts, 0=no pain)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.98, ‐0.02] |
| 7 Pain on non‐resisted motion with both hands (VAS:0‐100mm) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.10 [0.27, 9.93] |
| 8 Stiffness both hands (VAS: 0‐100mm)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐12.19, ‐0.21] |
8.1. Analysis.
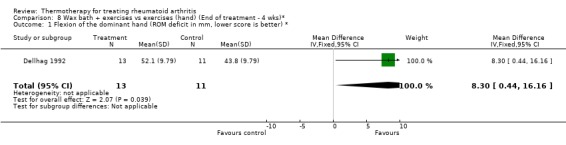
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better) *.
8.2. Analysis.
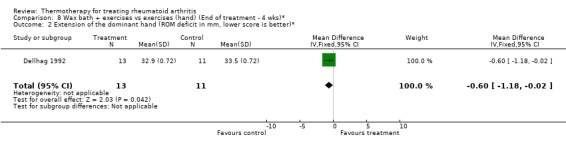
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)*.
8.3. Analysis.
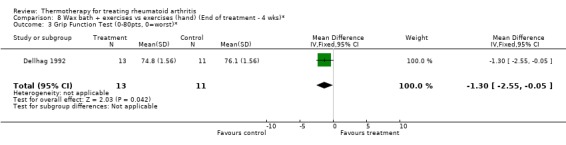
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 3 Grip Function Test (0‐80pts, 0=worst)*.
8.4. Analysis.
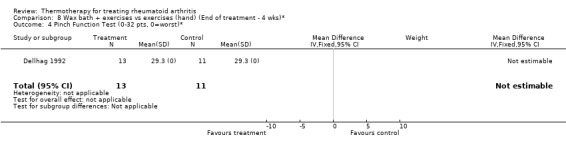
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 4 Pinch Function Test (0‐32 pts, 0=worst)*.
8.5. Analysis.
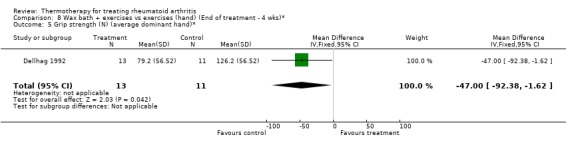
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 5 Grip strength (N) (average dominant hand)*.
8.6. Analysis.
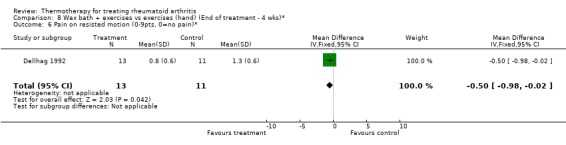
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 6 Pain on resisted motion (0‐9pts, 0=no pain)*.
8.7. Analysis.
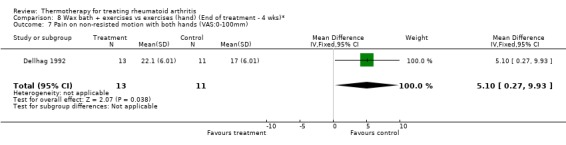
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 7 Pain on non‐resisted motion with both hands (VAS:0‐100mm).
8.8. Analysis.
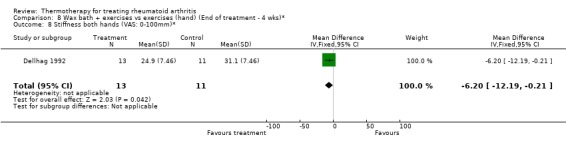
Comparison 8 Wax bath + exercises vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 8 Stiffness both hands (VAS: 0‐100mm)*.
Comparison 9. Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.76, ‐0.04] |
| 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐11.90 [‐23.45, ‐0.35] |
| 3 Grip Function Test (0‐80 pts, 0=worst)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.17, ‐0.03] |
| 4 Pinch Function Test (0‐32 pts, 0=worst)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.97, ‐0.03] |
| 5 Grip strength (N) (average dominant hand)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐50.3 [‐87.53, ‐13.07] |
| 6 Pain on resisted motion (0‐9pts, 0=no pain)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.01, 0.59] |
| 7 Pain on non‐resisted motion with both hands (VAS: 0‐100mm)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 8.90 [0.44, 17.36] |
| 8 Stiffness, both hands (VAS: 0‐100mm)* | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐4.10 [‐8.08, ‐0.12] |
9.1. Analysis.
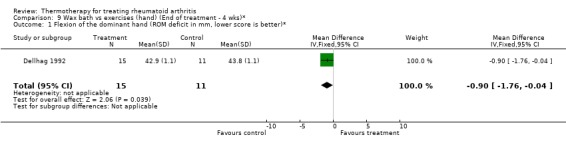
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 1 Flexion of the dominant hand (ROM deficit in mm, lower score is better)*.
9.2. Analysis.
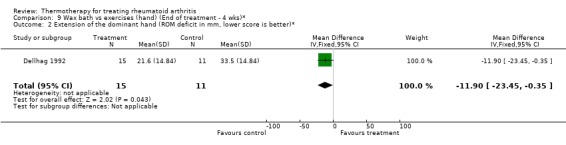
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 2 Extension of the dominant hand (ROM deficit in mm, lower score is better)*.
9.3. Analysis.
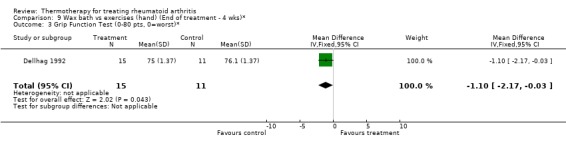
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 3 Grip Function Test (0‐80 pts, 0=worst)*.
9.4. Analysis.
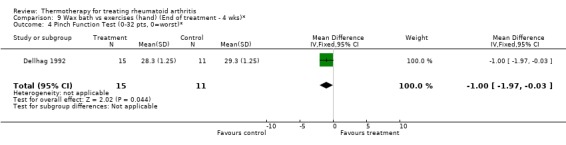
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 4 Pinch Function Test (0‐32 pts, 0=worst)*.
9.5. Analysis.
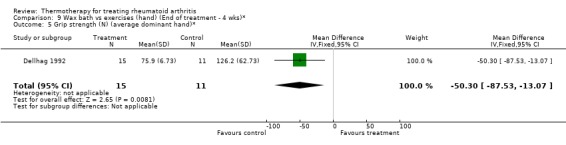
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 5 Grip strength (N) (average dominant hand)*.
9.6. Analysis.
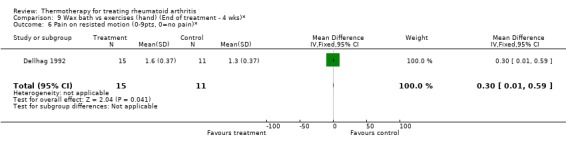
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 6 Pain on resisted motion (0‐9pts, 0=no pain)*.
9.7. Analysis.
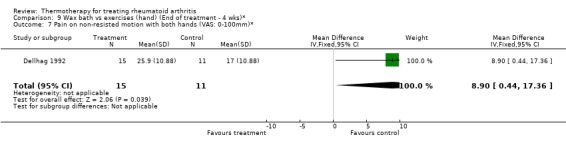
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 7 Pain on non‐resisted motion with both hands (VAS: 0‐100mm)*.
9.8. Analysis.
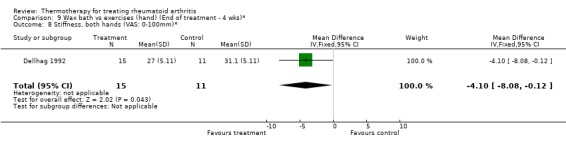
Comparison 9 Wax bath vs exercises (hand) (End of treatment ‐ 4 wks)*, Outcome 8 Stiffness, both hands (VAS: 0‐100mm)*.
Comparison 10. Exercises vs control (Hand) (End of treatment ‐ 4 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexion of dominant hand (ROM deficit in mm, lower score is better)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐18.20 [‐28.20, ‐8.20] |
| 2 Extension of dominant hand (ROM deficit in mm, lower score is better)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐18.47, ‐0.33] |
| 3 Grip Function Test (0‐80 pts, 0=worst)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.04, 2.16] |
| 4 Pinch Function Test (0‐32 pts, 0=worst)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.00, 0.20] |
| 5 Grip strength (N) (average of dominant hand)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 24.30 [0.84, 47.76] |
| 6 Pain on resisted motion (0‐9 pts, 0=no pain)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 7 Pain on non resisted motion with both hands (VAS: 0‐100mm)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐16.1 [‐31.35, ‐0.85] |
| 8 Stiffness, both hands (VAS: 0‐100mm)* | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.03, 1.77] |
10.1. Analysis.
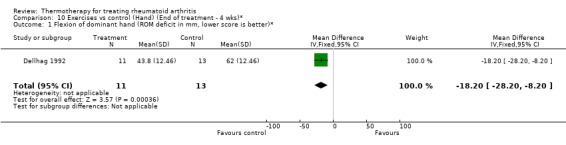
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 1 Flexion of dominant hand (ROM deficit in mm, lower score is better)*.
10.2. Analysis.
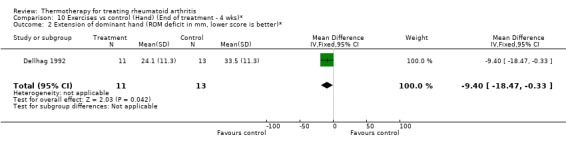
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 2 Extension of dominant hand (ROM deficit in mm, lower score is better)*.
10.3. Analysis.
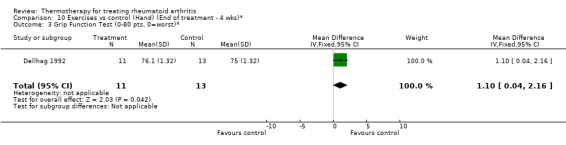
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 3 Grip Function Test (0‐80 pts, 0=worst)*.
10.4. Analysis.
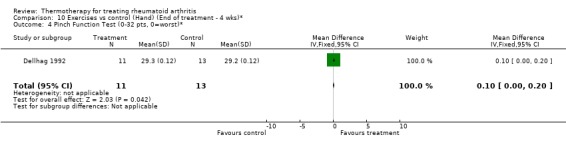
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 4 Pinch Function Test (0‐32 pts, 0=worst)*.
10.5. Analysis.
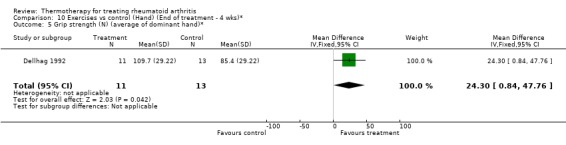
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 5 Grip strength (N) (average of dominant hand)*.
10.6. Analysis.
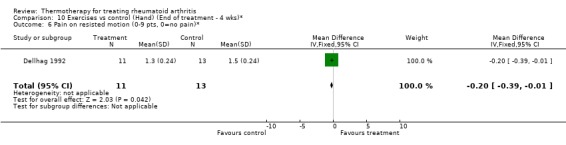
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 6 Pain on resisted motion (0‐9 pts, 0=no pain)*.
10.7. Analysis.
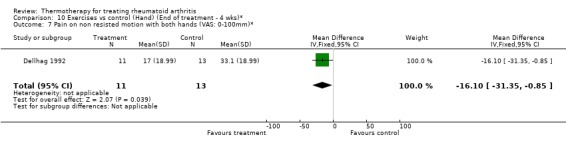
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 7 Pain on non resisted motion with both hands (VAS: 0‐100mm)*.
10.8. Analysis.
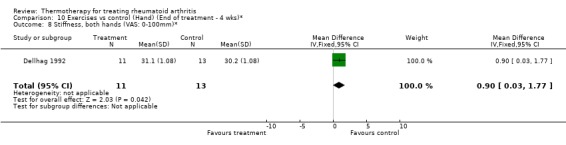
Comparison 10 Exercises vs control (Hand) (End of treatment ‐ 4 wks)*, Outcome 8 Stiffness, both hands (VAS: 0‐100mm)*.
Comparison 11. Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand Grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐253.38, 289.38] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐24.15, 36.15] |
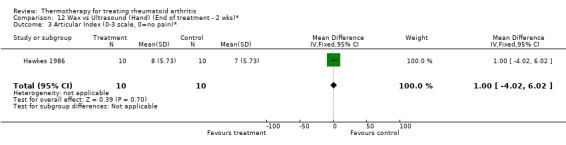
| 3 Articular Index (0‐3 scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.02, 6.02] |
| 4 Timed Task (sec.)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐51.23, 39.23] |
| 5 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.40, 0.60] |
11.1. Analysis.
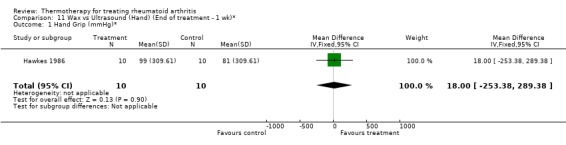
Comparison 11 Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*, Outcome 1 Hand Grip (mmHg)*.
11.2. Analysis.
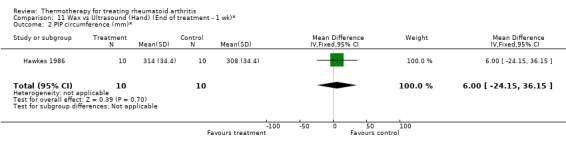
Comparison 11 Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*, Outcome 2 PIP circumference (mm)*.
11.3. Analysis.
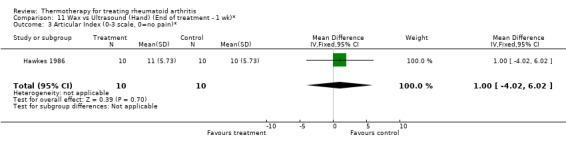
Comparison 11 Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*, Outcome 3 Articular Index (0‐3 scale, 0=no pain)*.
11.4. Analysis.
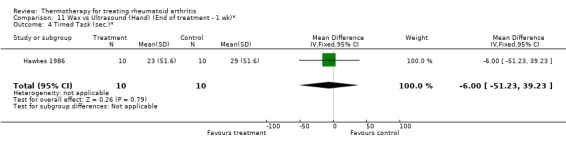
Comparison 11 Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*, Outcome 4 Timed Task (sec.)*.
11.5. Analysis.
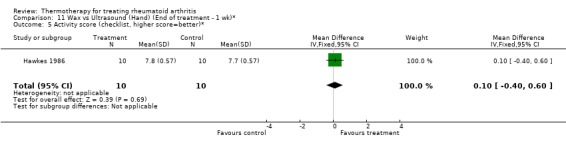
Comparison 11 Wax vs Ultrasound (Hand) (End of treatment ‐ 1 wk)*, Outcome 5 Activity score (checklist, higher score=better)*.
Comparison 12. Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand Grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 23.0 [‐323.23, 369.23] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐20.13, 30.13] |
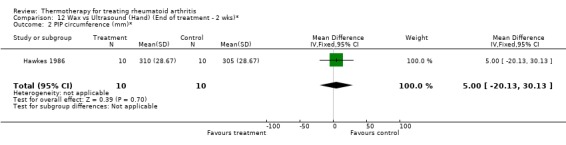
| 3 Articular Index (0‐3 scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.02, 6.02] |
| 4 Timed Task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐59.77, 45.77] |
| 5 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.21, 1.81] |
12.1. Analysis.
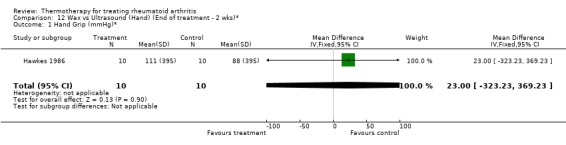
Comparison 12 Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*, Outcome 1 Hand Grip (mmHg)*.
12.2. Analysis.
Comparison 12 Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*, Outcome 2 PIP circumference (mm)*.
12.3. Analysis.
Comparison 12 Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*, Outcome 3 Articular Index (0‐3 scale, 0=no pain)*.
12.4. Analysis.
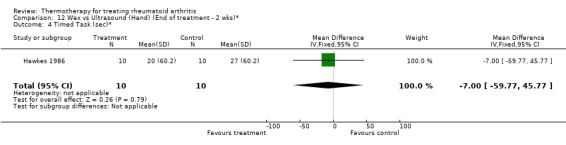
Comparison 12 Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*, Outcome 4 Timed Task (sec)*.
12.5. Analysis.
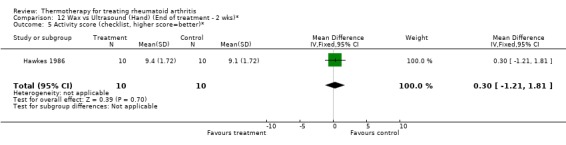
Comparison 12 Wax vs Ultrasound (Hand) (End of treatment ‐ 2 wks)*, Outcome 5 Activity score (checklist, higher score=better)*.
Comparison 13. Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand Grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 22.0 [‐309.68, 353.68] |
| 2 PIP circumference (mm) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐24.15, 36.15] |
| 3 Articular Index (0‐3 scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.02, 6.02] |
| 4 ROM (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 21.0 [‐2.79, 44.79] |
| 5 Timed Task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐59.77, 45.77] |
| 6 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.81, 1.21] |
13.1. Analysis.
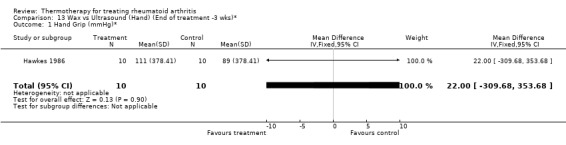
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 1 Hand Grip (mmHg)*.
13.2. Analysis.
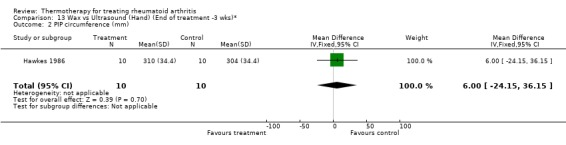
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 2 PIP circumference (mm).
13.3. Analysis.
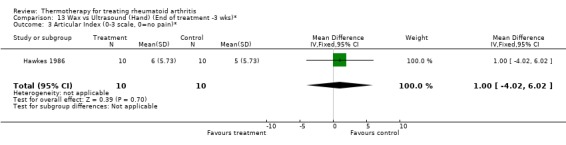
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 3 Articular Index (0‐3 scale, 0=no pain)*.
13.4. Analysis.
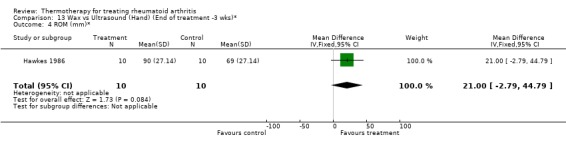
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 4 ROM (mm)*.
13.5. Analysis.
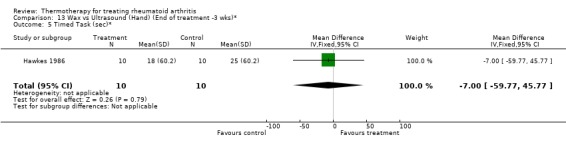
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 5 Timed Task (sec)*.
13.6. Analysis.
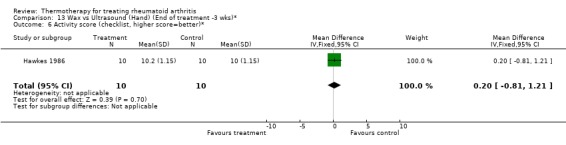
Comparison 13 Wax vs Ultrasound (Hand) (End of treatment ‐3 wks)*, Outcome 6 Activity score (checklist, higher score=better)*.
Comparison 14. Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐154.85, 176.85] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 27.0 [‐108.69, 162.69] |
| 3 Articular Index (0‐3 scale, 0=no pain)* | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐7.63, 11.63] |
| 4 Time Task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐68.30, 52.30] |
| 5 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐6.69, 7.69] |
14.1. Analysis.
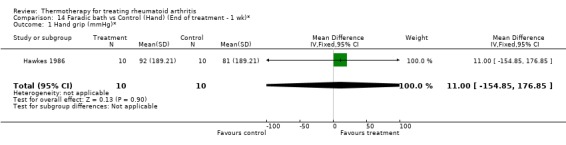
Comparison 14 Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*, Outcome 1 Hand grip (mmHg)*.
14.2. Analysis.
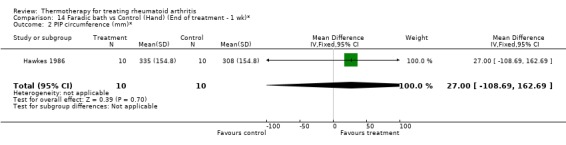
Comparison 14 Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*, Outcome 2 PIP circumference (mm)*.
14.3. Analysis.
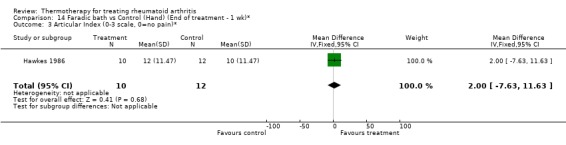
Comparison 14 Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*, Outcome 3 Articular Index (0‐3 scale, 0=no pain)*.
14.4. Analysis.
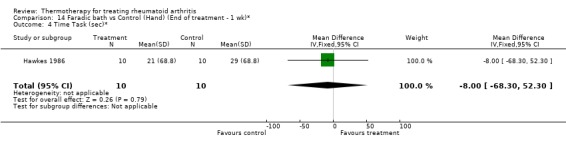
Comparison 14 Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*, Outcome 4 Time Task (sec)*.
14.5. Analysis.
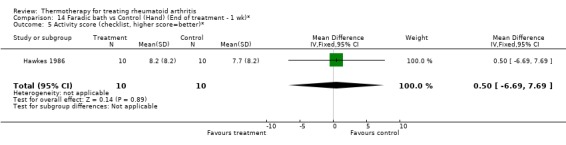
Comparison 14 Faradic bath vs Control (Hand) (End of treatment ‐ 1 wk)*, Outcome 5 Activity score (checklist, higher score=better)*.
Comparison 15. Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand Grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐98.53, 112.53] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 26.0 [‐104.66, 156.66] |
| 3 Articular Index (0‐3 scale, 0=pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐12.08, 18.08] |
| 4 Timed Task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐59.77, 45.77] |
| 5 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.02, 0.58] |
15.1. Analysis.
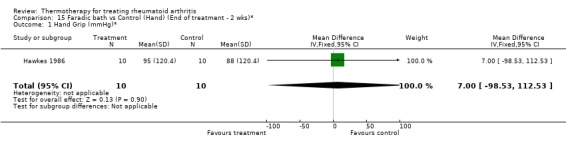
Comparison 15 Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*, Outcome 1 Hand Grip (mmHg)*.
15.2. Analysis.
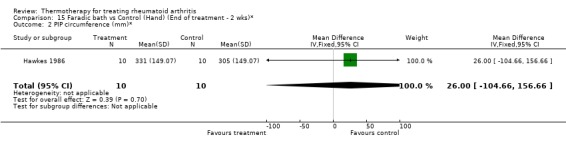
Comparison 15 Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*, Outcome 2 PIP circumference (mm)*.
15.3. Analysis.
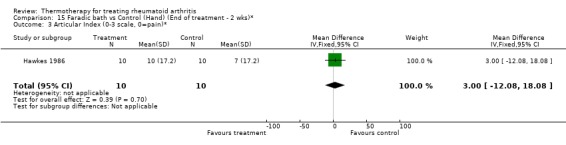
Comparison 15 Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*, Outcome 3 Articular Index (0‐3 scale, 0=pain)*.
15.4. Analysis.
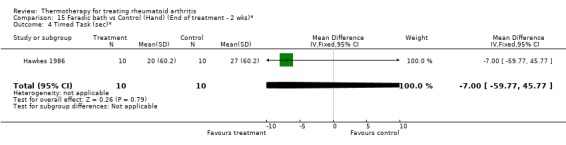
Comparison 15 Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*, Outcome 4 Timed Task (sec)*.
15.5. Analysis.
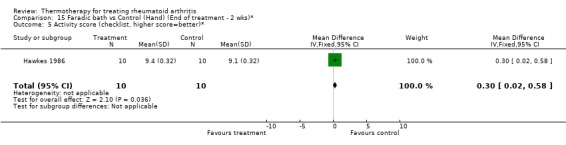
Comparison 15 Faradic bath vs Control (Hand) (End of treatment ‐ 2 wks)*, Outcome 5 Activity score (checklist, higher score=better)*.
Comparison 16. Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand Grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐140.77, 160.77] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [‐100.64, 150.64] |
| 3 Articular Index (0‐3) scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐20.13, 30.13] |
| 4 ROM (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐1.06, 17.06] |
| 5 Timed Task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐42.69, 32.69] |
| 6 Activity score (checklist, higher score=better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.51, ‐0.09] |
16.1. Analysis.
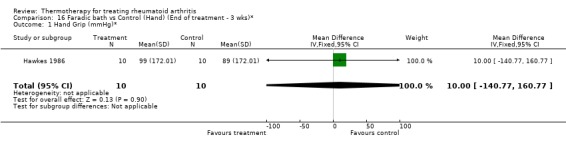
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 1 Hand Grip (mmHg)*.
16.2. Analysis.
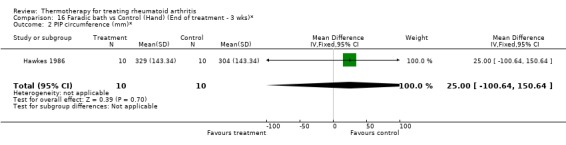
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 2 PIP circumference (mm)*.
16.3. Analysis.
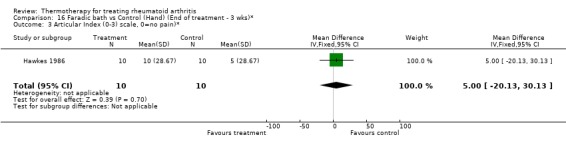
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 3 Articular Index (0‐3) scale, 0=no pain)*.
16.4. Analysis.
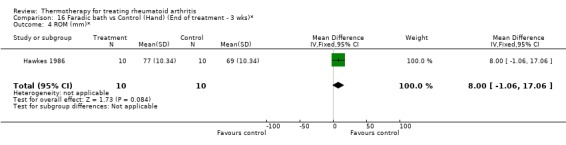
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 4 ROM (mm)*.
16.5. Analysis.
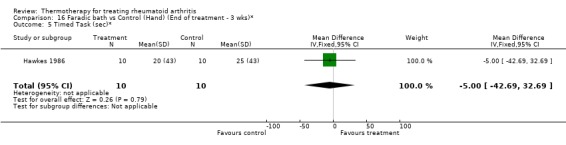
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 5 Timed Task (sec)*.
16.6. Analysis.
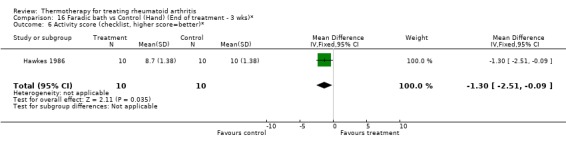
Comparison 16 Faradic bath vs Control (Hand) (End of treatment ‐ 3 wks)*, Outcome 6 Activity score (checklist, higher score=better)*.
Comparison 17. Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐98.53, 112.53] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐21.0 [‐126.53, 84.53] |
| 3 Articular index (0‐3 scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.02, 4.02] |
| 4 Timed task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐13.08, 17.08] |
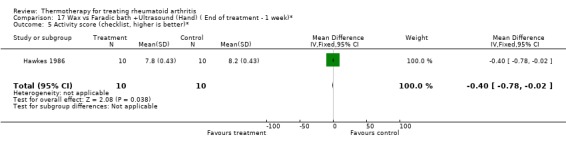
| 5 Activity score (checklist, higher is better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.78, ‐0.02] |
17.1. Analysis.
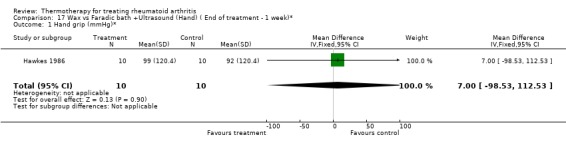
Comparison 17 Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*, Outcome 1 Hand grip (mmHg)*.
17.2. Analysis.
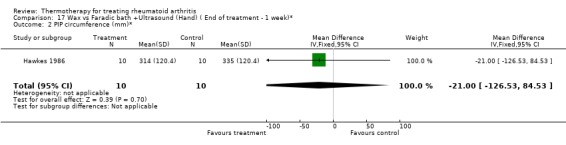
Comparison 17 Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*, Outcome 2 PIP circumference (mm)*.
17.3. Analysis.
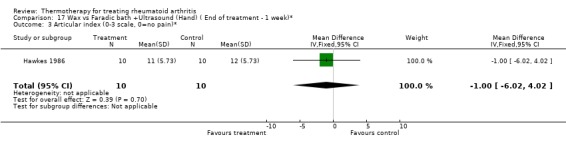
Comparison 17 Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*, Outcome 3 Articular index (0‐3 scale, 0=no pain)*.
17.4. Analysis.
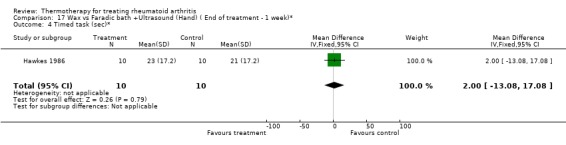
Comparison 17 Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*, Outcome 4 Timed task (sec)*.
17.5. Analysis.
Comparison 17 Wax vs Faradic bath +Ultrasound (Hand) ( End of treatment ‐ 1 week)*, Outcome 5 Activity score (checklist, higher is better)*.
Comparison 18. Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [‐225.23, 257.23] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐21.0 [‐126.53, 84.53] |
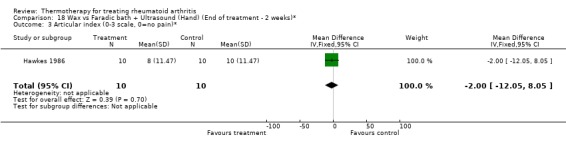
| 3 Articular index (0‐3 scale, 0=no pain)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐12.05, 8.05] |
| 4 Timed task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Activity score (checklist, higher is better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
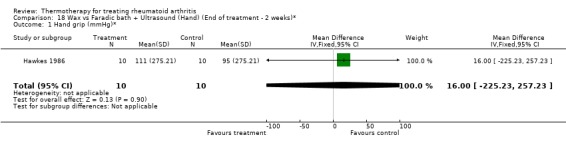
Comparison 18 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*, Outcome 1 Hand grip (mmHg)*.
18.2. Analysis.
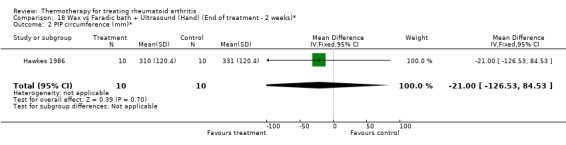
Comparison 18 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*, Outcome 2 PIP circumference (mm)*.
18.3. Analysis.
Comparison 18 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*, Outcome 3 Articular index (0‐3 scale, 0=no pain)*.
18.4. Analysis.
Comparison 18 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*, Outcome 4 Timed task (sec)*.
18.5. Analysis.
Comparison 18 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 2 weeks)*, Outcome 5 Activity score (checklist, higher is better)*.
Comparison 19. Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand grip (mmHg)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [‐168.56, 192.56] |
| 2 PIP circumference (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐19.0 [‐114.49, 76.49] |
| 3 Articular index (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐24.10, 16.10] |
| 4 Timed task (sec)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐17.08, 13.08] |
| 5 Activity score (checklist, higher is better)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.10, 2.90] |
| 6 ROM (mm)* | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐1.73, 27.73] |
19.1. Analysis.
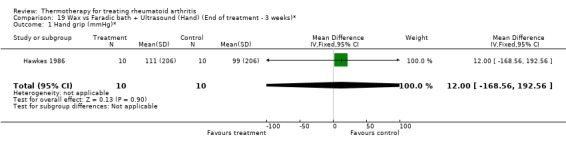
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 1 Hand grip (mmHg)*.
19.2. Analysis.
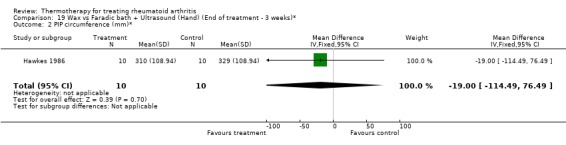
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 2 PIP circumference (mm)*.
19.3. Analysis.
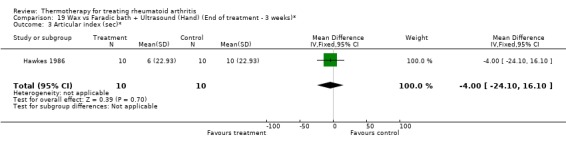
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 3 Articular index (sec)*.
19.4. Analysis.
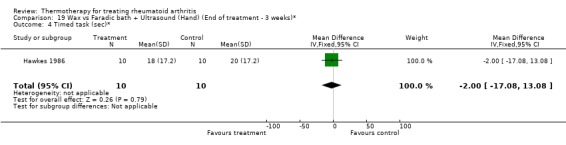
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 4 Timed task (sec)*.
19.5. Analysis.
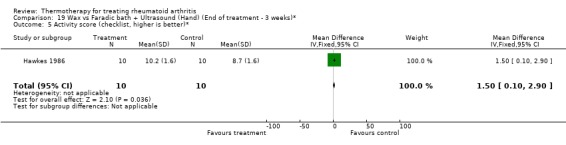
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 5 Activity score (checklist, higher is better)*.
19.6. Analysis.
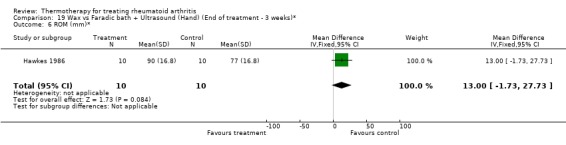
Comparison 19 Wax vs Faradic bath + Ultrasound (Hand) (End of treatment ‐ 3 weeks)*, Outcome 6 ROM (mm)*.
Comparison 20. Cryotherapy vs Control (Hand) (End of treatment‐2 days)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % of change in post‐surgery oedema increase (lower is better)* | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐5.80 [‐11.78, 0.18] |
20.1. Analysis.
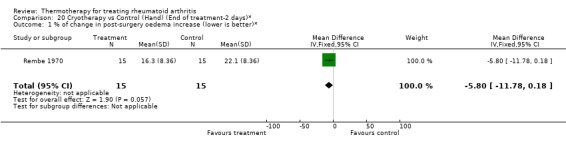
Comparison 20 Cryotherapy vs Control (Hand) (End of treatment‐2 days)*, Outcome 1 % of change in post‐surgery oedema increase (lower is better)*.
Comparison 21. Cryotherapy vs Control (Hand) (End of treatment‐3 days)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % of change in post‐surgery oedema increase (lower % is better)* | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐9.80 [‐19.91, 0.31] |
21.1. Analysis.
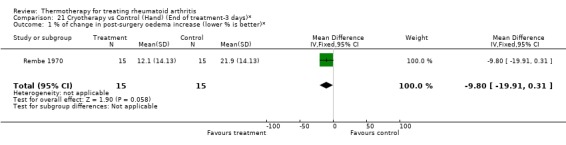
Comparison 21 Cryotherapy vs Control (Hand) (End of treatment‐3 days)*, Outcome 1 % of change in post‐surgery oedema increase (lower % is better)*.
Comparison 22. Cryotherapy vs Control (Hand) (End of treatment‐4 days)*.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % of change in post‐surgery oedema increase (lower % is better)* | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.62, 0.02] |
22.1. Analysis.
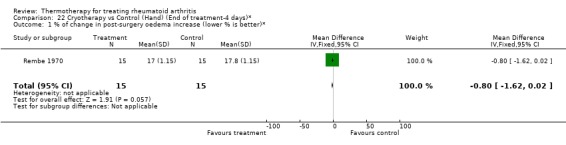
Comparison 22 Cryotherapy vs Control (Hand) (End of treatment‐4 days)*, Outcome 1 % of change in post‐surgery oedema increase (lower % is better)*.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bulstrode 1986.
| Methods | Randomized, single‐blind (assessor) controlled trial Sample size at entry: 24 | |
| Participants | Inpatients confined to bed rest with classical or definite RA according to ARA criteria and clinically obvious effusion of 1 or both knee joints Men: 17 Seropositive: 19 | |
| Interventions | 1‐Experimental group: Ice therapy (2 kg of crushed ice wrapped in damp towels applied to one of knee joints for 10 minutes daily for 5 days) 2‐Control group: No ice therapy Contralateral control: patients with both knees affected, only one knee was assigned to ice therapy and the other served as a contralateral control Concurrent therapy: no intra‐articular injections, joint aspiration, no changes in drug therapy, no other forms of physical therapy |
|
| Outcomes | 1‐Inflammation measured by infrared thermography (Thermographic Index), derived from mean temperature of anterior knee 2‐Circumference (cm) |
|
| Notes | R=1 B=0 W=1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dellhag 1992.
| Methods | Randomized, Controlled with matching, parallel group, Sample size at entry for group 1: 13 group 2: 11 group 3: 15 group 4: 13 | |
| Participants | Resident in the city of Gothenburg, age equal or younger than 70 yrs, disease duration:6‐10 yrs, functional class. I or II, hand problems defined as a decrease in the ROM and/or grip strength, Mean age for females: 51.8 yrs (n=33), Mean age for males: 56.3 yrs (n=19), Stage of disease: chronic. | |
| Interventions | Group 1: Wax bath & Exercises Group 2: Exercises only Group 3: Wax bath only Group 4: control (no treatment) |
|
| Outcomes | 1‐ROM deficit (mm) :
‐ flexion of dom. hand.
‐ flexion of non‐dom. hand.
‐ extension of dom. hand.
‐ extension of non‐dom. hand. 2‐Grip strength Test (0‐80 pts) 3‐Pinch function Test (0‐32 pts) 4‐ Grip strength (N) : ‐ max. dom. hand. ‐ max. non‐dom. hand. ‐ average of dom. hand ‐ average of non‐dom. hand. 5‐Pain: ‐ resisted motion of dom. hand (0‐9 pts) ‐ non‐resisted motion, both hands (0‐100mm) 6‐Stiffness both hands (0‐100mm) |
|
| Notes | R=1 B=0 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hawkes 1986.
| Methods | Randomized, placebo‐controlled study. Sample size at entry for group 1: 10; for group 2: 10 | |
| Participants | Patients with classical/definite RA disease involved in both hands, pain swelling and limitation of movement | |
| Interventions | 1‐ Wax + exercises;
2‐Ultrasound + exercises;
2‐Ultrasound + faradic exercises 3‐Ultrasound +Faradic bath |
|
| Outcomes | 1‐ Hand grip (mmHg) 2‐ PIP circumference (mm) 3‐ Articular Index (0‐3) 4‐ Pain score (mm) 5‐ROM (mm) 6‐ Timed task (sec) 7‐ Activity score (checklist) | |
| Notes | R=1 B=0 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Ivey 1994.
| Methods | Randomized, parallel group, Placebo controlled, Sample size at entry for Group 1: 30 Group 2: 30 Group 3: 30 | |
| Participants | Patients undergoing primary knee arthroplasty, Mean age or the following groups: Group 1: 64.5 yrs SD: 8.1 yrs Group 2: 64.2 yrs SD: 10.3 yrs Group 3: 66.9 yrs SD: 11.6 yrs | |
| Interventions | 1‐Thermal‐pad of 50°F 2‐Thermal‐pad of 60°F 3‐Thermal‐pad of 70°F | |
| Outcomes | 1‐Amount of morphine injected (pain measurement) (mg/h) 2‐No. of PCA (Patient controlled analgesia attempts) | |
| Notes | R=2 B=0 W=1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kirk 1968.
| Methods | Randomized, cross‐over trial Washout: 9 days with no therapy Sample size at entry: 14 patients, 20 knees Treatment duration: 5 days Trial duration: 19 days | |
| Participants | Chronic rheumatoid arthritis, admitted to hospital with "definite or classic" RA Mean age: not reported Mean symptom duration: 14 years SD=12.5 | |
| Interventions | 1‐Ice packs in damp towels for 20 minutes, 1/day for 5 days 2‐Hot packs: wrapped in Turkish towel and wrapped around the knee for 20 minutes, 1/day for 5 days |
|
| Outcomes | 1‐Preference for cold
2‐Preference for heat
3‐Improved by 1 pain grade (heat and ice)
4‐Stiffness improved (heat and ice) Pain, stiffness, range of movement, knee circumference, joint temperature, treatment preference |
|
| Notes | R=1 B=0 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rembe 1970.
| Methods | Randomized, parallel group, Placebo controlled, Sample size at entry for Group 1: 15 Group 2: 15 | |
| Participants | Patients hospitalized for surgical procedures to the hand | |
| Interventions | at 10 degrees 1‐cryotherapy, submerging involved hand, covered in glove in water at 10 degrees Celsius for 4 mins., 2/day, after 48 hrs post‐op until 72 hours post‐op. 2‐ no therapy 2‐Hot packs: wrapped in Turkish towel and wrapped around the knee for 20 minutes, 1/day for 5 days | |
| Outcomes | 1‐% of increase oedema over preoperative volume | |
| Notes | R=1 B=0 W=1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Williams 1986.
| Methods | Randomized, parallel group, Sample size at entry for group 1: 9, for group 2: 9 | |
| Participants | Onset of rheumatoid arthrtis within the proceeding 5 yrs, painful shoulder, normal sensations (hot&cold), stage of disease: early & advanced, Group 1 mean age: 59 yrs, Group 2 mean age: 55 yrs, Mean disease duration for group 1: 31 mths, Mean disease duration for group 2: 36 mths. | |
| Interventions | 1‐Heat (hot packs) for 20 min. + 20 min.of exercise program 2‐Ice (20 min.) + exercises program | |
| Outcomes | 1‐Pain (McGill Pain Questionnaire) 2‐Flexion (degrees) 3‐Abduction ROM (degrees) | |
| Notes | R=1 B=0 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramson 1964 | No clinical outcome |
| Amundson 1979 | Not a clinical trial |
| Bromley 1994 | Healthy subjects |
| Curkovic 1993 | No sufficient statistical data |
| Devereaux 1985 | No control group |
| DonTigny 1962 | No subjects with rheumatoid arthritis |
| Feibel 1976 | Not a clinical trial |
| Haines 1970 | No RA patients. Survey to estimate the number of hospitals who find it worth while to use cold therapy. |
| Halliday Pegg 1969 | No control group |
| Harris 1955 | No description of the statistical procedure used, no p values and no standard deviations available |
| Hoyrup 1986 | Subjects with traumas |
| Mainardi 1979 | No control group. Patient was his own control. |
| Oosterveld 1992c | Healthy subjects |
| Oosterveld 1994 | Mixed population with rheumatoid arthritis in minority |
| Oosterveld 1994b | Literature review |
| Weinberger 1989 | No clinical outcome |
| Whipple 1992 | Healthy subjects |
Contributions of authors
VR was responsible for writing the manuscript, extracting and analyzing data and selecting trials of the initial review.
LB contributed data extraction, update of the selection of the reference list, update of the analyses and update of the interpretation of results.
MJ developed the search strategy.
BS, GW and PT contributed methodological expertise and commented on early drafts.
MJ provided feedback and editorial support for final drafts.
LC contributed data extraction and update of the analyses.
Sources of support
Internal sources
Ottawa Health Research Institute, Canada.
Institute for Population Health, University of Ottawa, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Bulstrode 1986 {published data only}
- Bulstrode S, Clarke A, Harrison R. A controlled trial to study the effects of ice therapy on joint inflammation in chronic arthritis. Physiotherapy Practice 1986;2:104‐108. [Google Scholar]
Dellhag 1992 {published data only}
- Dellhag B, Wollersjö I, Bjelle A. Effect of active hand exercise and was bath treatment in rheumatoid arthritis patients. Arthritis Care and Research 1992;5(2):87‐92. [3‐TH] [DOI] [PubMed] [Google Scholar]
Hawkes 1986 {published data only}
- Hawkes J, Care G, Dixon JS, Bird HA, Wright VA. Comparison of three different treatments for rheumatoid arthritis of the hands. Physiotherapy Practice 1986;2:155‐160. [21‐TH] [Google Scholar]
Ivey 1994 {published data only}
- Ivey M, Johnston RV, Ulchida T. Cryotherapy for postoperative pain relief following knee arthroplasty. The Journal of Arthroplasty 1994;9(3):285‐290. [20‐TH] [DOI] [PubMed] [Google Scholar]
Kirk 1968 {published data only}
- Kirk JA, Kersley GD. Heat and Cold in the Physical Treatment of Rheumatoid Arthritis of the Knee. A Controlled Clinical Trial. Annals of Physical Medicine 1968;9(7):270‐274. [DOI] [PubMed] [Google Scholar]
Rembe 1970 {published data only}
- Rembe EC. Use of Cryotherapy on the Postsurgical Rheumatoid Hand. Physical Therapy 1970;50:19‐21. [DOI] [PubMed] [Google Scholar]
Williams 1986 {published data only}
- Williams J, Harvey J, Tannenbaum H. Use of superficial heat versus ice for the rheumatoid arthrtis shoulder: A pilot study. Physiotherapy Canada 1986;38(1):8‐13. [19‐TH] [Google Scholar]
References to studies excluded from this review
Abramson 1964 {published data only}
- Abramson DI, Tuck S, Chu LSW, Augustin C. Effect of paraffin bath and hot fomentations on local tissue temperatures. Archives of Physical Medicine and Rehabilitation 1964;45:87‐94. [PubMed] [Google Scholar]
Amundson 1979 {published data only}
- Amundson H. Thermography and cryotherapy : effects on joint degeneration in rheumatoid arthritis. Physiotherapy Canada 1979;31:258‐262. [22‐TH] [Google Scholar]
Bromley 1994 {published data only}
- Bromley J, Unsworth A, Haslock I. Changes in Stiffness Following Short‐ and Long‐term Application of Standard Physiotherapeutic Techniques. British Journal of Rheumatology 1994;33:555‐561. [16‐TH] [DOI] [PubMed] [Google Scholar]
Curkovic 1993 {published data only}
- Curkovic B, Vitulic V, Babic‐Naglic D, Durrigl T. The influence of heat and colde on pain arthritis. Zeitschrift fur Rheumatologie 1993;52(5):289‐91. [2‐TH] [PubMed] [Google Scholar]
Devereaux 1985 {published data only}
- Devereaux MD, Parr GR, Page Thomas DP, Hazleman BL. Disease activity indexes in rheumatoid arthritis; a prospective, comparative study with thermography. Annals of the Rheumatic Diseases 1985;44:434‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
DonTigny 1962 {published data only}
- DonTigny R, Sheldon K. Simultaneous use of heat and cold in treatment of muscle spasm. Archives of Physical Medicine and Rehabilitation 1962;43:235‐237. [PubMed] [Google Scholar]
Feibel 1976 {published data only}
- Feibel A, Fast A. Deep heating of joints : a reconsideration. Archives of Physical Medicine and Rehabilitation 1976;57(11):513‐4. [PubMed] [Google Scholar]
Haines 1970 {published data only}
- Haines J. A study into a report on cold therapy. Physiotherapy 1970;56(11):501‐502. [23‐TH] [PubMed] [Google Scholar]
Halliday Pegg 1969 {published data only}
- Halliday Pegg SM, Littler TR, Littler EN. A trial of ice therapy and exercise in chronic arthritis. Physiotherapy 1969;55:51‐6. [9‐TH] [PubMed] [Google Scholar]
Harris 1955 {published data only}
- Harris R, Millard JB. Paraffin wax baths in the treatment of Rheumatoid Arthritis.. Ann Rheum 1955;14:278‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoyrup 1986 {published data only}
- Hoyrup G, Kjorvel L. Comparison of whirpool and wax treatments for hand therapy. Physiotherapy Canada 1986;38:79‐82. [Google Scholar]
Mainardi 1979 {published data only}
- Mainardi CL, Walter JM, Spiegel PK, Goldkamp OG, Harris ED. Rheumatoid Arthritis: Failure of Daily Heat Therapy to Affect its Progression. Arch Phys Med Rehabil 1979;60:390‐393. [PubMed] [Google Scholar]
Oosterveld 1992c {published data only}
- Oosterveld FGJ, Rasker JJ, Jacobs JW, Overmars HJ. The effect of local heat and cold therapy on the intraarticular and skin surface temperature of the knee. Arthritis and Rheumatism 1992;35(2):146‐151. [17‐TH] [DOI] [PubMed] [Google Scholar]
Oosterveld 1994 {published data only}
- Oosterveld FGJ, Rasker JJ. Effects of local heat and cold treatment on surface and articular temperature of arthritic knees. Arthritis and Rheumatism 1994;37(11):1578‐1582. [18‐TH] [DOI] [PubMed] [Google Scholar]
Oosterveld 1994b {published data only}
- Oosterveld FGJ, Rasker JJ. Treating arthritis with locally applied heat or cold. Seminars in Arthritis and Rheumatism 1994;24(2):82‐90. [DOI] [PubMed] [Google Scholar]
Weinberger 1989 {published data only}
- Weinberger, Fadilah R, Lev A, Pinkhas J. Intra‐articular temperature measurements after superficial heating. Scandinavian Journal of Rehabilitation Medicine 1989;21(1):55‐57. [PubMed] [Google Scholar]
Whipple 1992 {published data only}
- Whipple‐Ellsworth A, Klebba M, Walden J, Kulig K. A comparison of the analgesic effects of ice massage and brief intense transcutaneous electrical nerve stimulation. Physical Therapy 1992;72(6):S69. [Google Scholar]
Additional references
ACR 1996
- American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Guidelines for the management of rheumatoid arthritis. Arthritis & Rheumatism 1996;39:713‐22. [PubMed] [Google Scholar]
Altman 2001
- Altman D, Elbourne D. Incorporating crossover trials into systematic reviews. Proceedings of the 9th International Cochrane Colloquium. 2001:131.
APTA 2001
- American Physical Therapy Association. Guide to Physical Therapist Practice: Part One: A Description of Patient/Client Management. Alexandria, Va: American Physical Therapy Association, 2001. [Google Scholar]
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neudstadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 Revised Criteria for Classification of Rheumatoid Arthritis. Arthritis Rheumatology 1988;31:315‐24. [DOI] [PubMed] [Google Scholar]
Behnke 1973
- Behnke RS. Cryotherapy and vasodilatation. Athl Train 1973;8(106):133‐7. [Google Scholar]
BMJ 1999
- BMJ. British Medicine Journal Clinical Evidence. A Compendium of the Best Available Evidence for Effective Health Care. British Medical Journal, 1999. [Google Scholar]
Chapman 1991
- Chapman CE. Can the use of physical modalities for pain control be rationalized by the research evidence?. Canadian Journal of Physiology and Pharmacology 1991;69:704‐12. [DOI] [PubMed] [Google Scholar]
Coutts 1994
- Coutts RD, Toth C, Kaita JH. The role of continuous passive motion in the rehabilitation of the total knee patient. In: Hungerford DS, Krackow K, Kenne RV editor(s). Total Knee Arthroplasty: A Comprehensive Approach. Baltimore: Williams and Wilkins, 1994:126‐32. [Google Scholar]
Deyo 1990
- Deyo RA, Walsh NE, Schoenfeld LS, Ramamurty S. Can trials of physical treatments be blinded: the example of transcutaneous electrical nerve stimulation for chronic pain. American Journal of Physical Medicine and Rehabilitation 1990;69:6‐10. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 1994
- Haynes R, Wilczynski N, McKibbon KA, et al. Developing optimal search strategies for detecting clinically sound strategies in MEDLINE. Journal of the American Medical Informatics Association 1994;1:447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Knight 1995
- Knight KL. Cryotherapy for sport injuries management. Windsor, Canada: Human Kinetics, 1995. [Google Scholar]
Manal 1996
- Manal R.J. Snyder‐Mackler, L. Practice guidelines for anterior cruciate ligament rehabilitation: criterion‐based rehabilitation progression.. Operative techniques in Orthopaedics 1996;6:190‐6. [Google Scholar]
Morin 1996
- Morin M, Brosseau L, Quirion‐DeGrardi C. A theoretical framework on low level laser therapy (classes I, II and III) application for the treatment of OA and RA. Proceedings of the Canadian Physiotherapy Association National Congress, Victoria (B.C.). 1996.
Mulrow 1997
- Mulrow CD, Oxman AD (eds). Cochrane Collaboration Handbook [updated September 1997]. In: The Cochrane Library. The Cochrane Collaboration Update Software; 1994, issue 4.
OMERACT 1993
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Journal of Rheumatology 1993;20:526‐91. [PubMed] [Google Scholar]
Philadelphia 2001
- The Philadelphia Panel. The Philadelphia Panel Evidence‐Based Clinical Practice Guidelines on Selected Rehabilitation Interventions. Physical Therapy 2001;81:1641‐74. [PubMed] [Google Scholar]
Primer 1997
- Arthritis Foundation. Primer on the Rheumatic Diseases. 11. Atlanta: Ed Klippel, J.H, 1997. [Google Scholar]
Puett 1994
- Puett DW, Griffin MR. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Annals of Internal Medicine 1994;121:133‐40. [DOI] [PubMed] [Google Scholar]
Semble 1990
- Semble EL, Loeser RF, Wise CM. Therapeutic exercise for rheumatoid arthritis and osteoarthritis. Seminars in Arthritis and Rheumatism 1990;20:32‐40. [DOI] [PubMed] [Google Scholar]
Verhagen 2000
- Verhagen AP, Vet HCW, Bie RA, Kessels AGH, Boers M, Knipschild PG. Balneotherapy for rheumatoid arthritis and osteoarthritis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000518] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Harris 1955
- Harris R, Millard JB. Paraffin wax baths in the treatment of Rheumatoid Arthritis. Annals of the Rheumatic Diseases 1955;14:278‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philadelphia Panel
- Philadelphia Panel, Ottawa Methods Group. Philadelphia Panel Guidelines on rehabilitation interventions for knee pain. Physical Therapy Submitted August 2000.


