Abstract
Background
Leptospirosis, caused by pathogenic Leptospira spp., is a zoonotic infection that affects humans, dogs and many other mammalian species. Virtually any mammalian species can act as asymptomatic reservoir, characterized by chronic renal carriage and shedding of a host-adapted leptospiral serovar. Environmental contamination by chronic shedders results in acquisition of infection by humans and susceptible animals.
Methods
In this study, we investigated if clinically normal shelter dogs and cats harbor leptospires in their kidneys by screening urine samples for the presence of leptospiral DNA by a TaqMan based-quantitative PCR (qPCR) that targets pathogen-associated lipl32 gene. To identify the infecting leptospiral species, a fragment of leptospiral rpoB gene was PCR amplified and sequenced. Additionally, we measured Leptospira-specific serum antibodies using the microscopic agglutination test (MAT), a gold standard in leptospiral serology.
Results
A total of 269 shelter animals (219 dogs and 50 cats) from seven shelters located in the tri-state area of western Virginia, eastern Tennessee, and southeastern Kentucky were included in this study. All cats tested negative by both qPCR and MAT. Of the 219 dogs tested in the study, 26/198 (13.1%, 95% CI: 8.4–17.8%) were positive for leptospiral DNA in urine by qPCR and 38/211 (18.0%, 95% CI: 12.8–23.2%) were seropositive by MAT. Twelve dogs were positive for both qPCR and MAT. Fourteen dogs were positive by qPCR but not by MAT. Additionally, leptospiral rpoB gene sequencing from a sub-set of qPCR-positive urine samples (n = 21) revealed L. interrogans to be the leptospiral species shed by dogs.
Conclusions
These findings have significant implications regarding animal and public health in the Cumberland Gap Region and possibly outside where these animals may be adopted.
Introduction
Leptospirosis, caused by pathogenic Leptospira spp., is a waterborne zoonotic infection that affects dogs and many other mammalian species [1,2,3]. Leptospires live in the proximal renal tubules of reservoir animals and are shed in the urine. The infection is contracted either through direct contact to urine of an infected animal or indirectly by exposure to Leptospira-contaminated water [4,5]. Leptospiral infection in dogs can result in a serious clinical outcome, such as acute hepatorenal failure, or it can also lead to asymptomatic chronic carrier state [6]. Chronic carriers may act as a source of infection and, for this reason, are of public health concern.
The prevalence of canine leptospirosis is increasing throughout the US, but some regions are considered hotspots of leptospirosis due to disproportionately large clusters of cases [7,8,9,10,11,12]. The Cumberland Gap Region (CGR), located close to the intersection of the state boundaries of Kentucky, Tennessee, and Virginia, is primarily a rural area that has all climatic, topographical, and socioeconomic factors that have been described as risk predictors for the occurrence of leptospirosis [13]. In a previous study, canine leptospirosis testing data collected over a period of 14 years in the United States was analyzed to develop predictive models for identifying regions of increased risk for leptospirosis. In that study, several counties in Appalachia had predictive probabilities for dogs testing seropositive. But the CGR was underrepresented in the testing data, likely due to the poor socio-economic status of the communities and a lack of veterinary care for pets in this region [13].
Since no information was available regarding prevalence of canine or feline leptospirosis in the region, we tested dogs and cats from seven shelters across three states in the CGR for the presence of leptospiruria and leptospiral antibodies. Leptospiral shedding was tested by screening urine samples for the presence of leptospiral DNA using a highly sensitive and specific TaqMan-based qPCR. In addition, dogs and cats were screened for the presence of leptospiral antibodies using microscopic agglutination test, a serodiagnostic gold standard.
Shelter dogs and cats are sentinels for many zoonotic diseases, likely due to unsanitary living conditions, high population density, stress, and exposure to rodents and other disease vectors. Here, we present our findings of leptospiral shedding and seropositivity among shelter animal populations in the CGR.
Materials and methods
Sample collection and DNA extraction
Dogs and cats from seven shelters located in three states (Tennessee, Kentucky and Virginia) were sampled in this study from April 2017 to Mar 2018 (Fig 1). Blood and urine samples were collected shortly after their arrival at the Lincoln Memorial University—College of Veterinary Medicine’s Small Animal Medical Center under the Shelter Outreach for the Appalachian Region (SOAR) program. The SOAR program provides spay/neuter services and veterinary care to unowned animals. These animals were clinically healthy and the number of animals included in the study from each shelter depended solely on the number of animals that were brought to the LMU-CVM Small Animal Medical Center for spay/neuter and basic veterinary care.
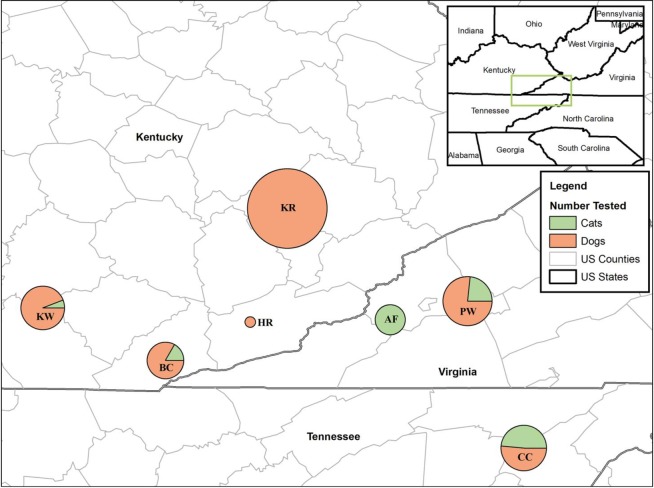
Fig 1. Map depicting the proportion of cats and dogs from seven shelters in Kentucky, Tennessee, and Virginia that were tested in this study.
Samples were collected from 113 animals (all dogs) from Shelter KR, 34 animals (32 dogs and 2 cats) from Shelter KW, 24 animals (20 dogs and 4 cats) from Shelter BC, 2 animals (both dogs) from Shelter HR, 43 animals (33 dogs and 10 cats) from Shelter PW, 16 animals (all cats) from Shelter AF, and 37 animals (19 dogs and 18 cats) from Shelter CC. Map created with ArcMap 10.6 (Esri, Redlands, CA).
Blood samples (1.5mL) were drawn by venipuncture and collected in IDEXX Vacuette SST-Serum Separator Tube. Tubes were then centrifuged (2,000 g for 10 min) and serum pipetted off, aliquoted and stored at -20°C. Urine was collected by free-catch method. Demographic data including sex, age, breed, and shelter were recorded in Excel (Microsoft, Redmond, WA). Additional information regarding blood urea nitrogen (BUN; Azostick®) results and month of sample collection were also recorded. Up to 1.5 mL of urine sample was centrifuged at 17000 g for 5 min, supernatant discarded and DNA from pellet extracted using the PureLink Genomic DNA Mini Kit (Invitrogen). Extracted DNA was stored at -20°C. Leptospira interrogans serovar Pomona was grown in Polysorbate-80 bovine serum albumin medium (NVSL) at 30°C, and genomic DNA was isolated and quantified as previously described [14] for use as a control in qPCR.
Quantitative Polymerase Chain Reaction (qPCR)
We used a TaqMan based quantitative PCR (qPCR) to target a 242 bp region of leptospiral lipl32 gene, as previously described [15]. The assay was performed in a MicroAmp Fast Optical 96-well reaction plate (Applied Biosystems, Foster City, CA, USA). Standard curve was created using DNA equivalent to 107, 106, 105, 104, 103, 102, 10, 1 leptospiral genome units. Each column, except positive control columns, had a no-template control. Each reaction was performed in a 25 μL final volume, using 5 μL of extracted DNA, 500 nM of LipL32-45F (forward primer; 5ꞌ-AAGCATTACCGCTTGTGGTG-3ꞌ), 500 nM of LipL32-286R (reverse primer; 5ꞌ-GAACTCCCATTTCAGCGATT-3ꞌ) and 100 nM of LipL32-189P (probe; FAM-5ꞌ-AAAGCCAGGACAAGCGCCG-3ꞌ-BHQ1) [15]. The assay was performed on a QuantStudio 3 using Platinum Quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA) and thermal conditions of a holding stage of 95°C for 20 s, and 40 cycles of 95°C for 3 s and 60°C for 30 s. Each sample was tested in duplicate and repeated at least twice.
Microscopic agglutination test
Microscopic agglutination test was performed following OIE protocol (https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.12_LEPTO.pdf). Two-fold serum dilutions from 1:100 to 1:6400 were tested against serovars Pomona, Hardjo, Grippotyphosa, Icterohaemorrhagiae, Canicola, Bratislava and Autumnalis. The titer was defined as the reciprocal of the highest dilution of a serum sample that agglutinated more than half of leptospires. Titers of more than or equal to 1:100 were considered positive for the presence of leptospiral antibodies.
Leptospiral rpoB gene sequencing
PCR amplification and sequencing of a fragment of leptospiral rpoB gene was performed for all positive urine samples as described previously [16]. Briefly, DNA from all qPCR-positive samples were subjected to PCR amplification of a 600bp fragment of rpoB gene using a Phusion High Fidelity polymerase (Thermofisher, Waltham, MA), primers Lept 1900f (5’-CCTCATGGGTTCCAACATGCA-3’) and Lept 2500r (5’-CGCATCCTCRAAGTTGTAWCCTT-3’), and thermal conditions as described previously [16]. PCR amplicons were sequenced at a commercial sequencing facility (Davis sequencing, Davis, CA), and compared to available sequences by BLAST search using the National Center for Biotechnology Information server (http://www.ncbi.nlm.nih.gov/BLAST/). Phylogenetic analyses were performed by the Neighbor-Joining method [17] using Geneious 9.0.5 software and phylogenetic distances measured by Tamura-Nei model.
Statistical analysis
Statistical analysis was performed on data using IBM SPSS Statistics 24 (IBM, New York) and Epi Info 7.2.2.6 (CDC, Atlanta, GA). Briefly, Chi-square tests or Fisher’s exact tests were performed for the variables: sex, breed, season, and shelter with test results from the urine qPCR and serum MAT. Odds ratios with 95% confidence levels were also calculated for each variable. In addition, Chi-square tests were used to determine individual shelter differences. A kappa test was performed to determine agreement between the qPCR and MAT tests.
Ethics statement
All animal experiments were carried out in strict accordance with the recommendations in the Animal Welfare Act of 1966, its amendments and associated Regulations (https://www.nal.usda.gov/awic/animal-welfare-act). All protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Lincoln Memorial University (protocol number: 1703-RES-04).
Results
A total of 269 animals (219 dogs and 50 cats) from seven shelters located in the Cumberland Gap region of KY, TN and VA were included in this study. Blood and urine samples (blood and urine from 229 animals, only blood from 25 animals and only urine from 15 animals) were collected from shelter animals shortly after their arrival at the LMU-CVM Small Animal Medical Center. Blood and/or urine samples were collected from 113 animals (all dogs) from Shelter KR, 34 animals (32 dogs and 2 cats) from Shelter KW, 37 animals (19 dogs and 18 cats) from Shelter CC, 43 animals (33 dogs and 10 cats) from Shelter PW, 2 animals (both dogs) from Shelter HR, 16 animals (all cats) from Shelter AF, and 24 animals (20 dogs and 4 cats) from Shelter BC (Fig 1).
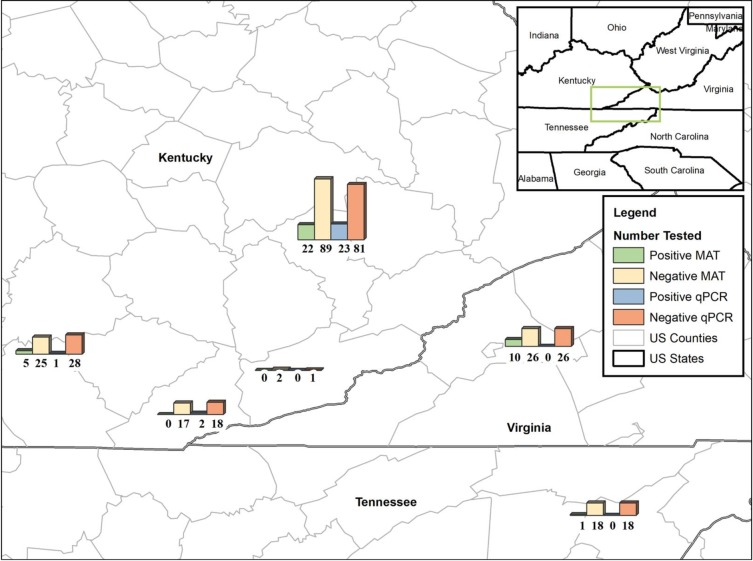
Out of 269 dogs and cats included in this study, we were able to collect urine samples from 244 animals (198 dogs and 46 cats). A TaqMan based qPCR that targets lipl32 gene of pathogenic Leptospira was used to screen DNA extracted from urine samples. Twenty six of 198 tested dogs (13.1%, 95% CI: 8.4–17.8%) were positive by qPCR (Table 1). Positive urine samples contained between 0.72 x 103–0.24 x 104 leptospiral genomic units/1.5ml of urine. Of 26 qPCR-positive dogs, 23 came from Shelter KR, 2 were from Shelter BC and 1 dog came from Shelter KW (Fig 2). All urine samples from cats tested negative for the presence of leptospiral DNA by qPCR.
Table 1. Prevalence of Leptospira spp. in shelter dogs and cats in the Cumberland Gap Region of Southeastern Appalachia.
| Sample Type | Dog Sera tested | Positive dog sera (percent; 95% CI) | Cat sera tested | Positive cat sera (percent; 95% CI) | Test |
|---|---|---|---|---|---|
| Urine | 198 | 26 (13.1%; 8.4–17.8%) | 46 | 0 | qPCR |
| Blood | 211 | 38 (18.0%; 12.8–23.2%) | 43 | 0 | MAT |
Fig 2. Map depicting MAT and qPCR test results for dogs from six shelters in Kentucky, Tennessee, and Virginia that were tested in this study.
A seventh shelter, which only housed cats, is not included in the figure. Map created with ArcMap 10.6 (Esri, Redlands, CA).
Using DNA extracted from qPCR positive urine samples, a 600 bp fragment of leptospiral rpoB gene was PCR amplified and sequenced. Twenty-one urine samples yielded good quality rpoB gene sequences. rpoB nucleotide sequences deposited in the GenBank have accession numbers MN731621-MN731641 (S1 Table). Analysis of these sequences revealed >99% homology with rpoB gene fragments of L. interrogans homologous gene fragments. By phylogenetic analysis, the rpoB genes of leptospiral strains MarleyKR, MarbleKR, DozerKR, LewisKR, BarneyKR, DarlaKR, HossKR, MirandaKR, BrandiKR, MaryAnnKR, ArielKR, JupiterKR, MandyKR, CindiKR, DaisyKR, GraceKR, HankKR, MeekoKR, CaliKR, HoldenKR and LandonKR (S1 Table) clustered very closely with the cognate gene of L. interrogans strains (S1 Fig). L. interrogans serovar Lai strain 56601 appeared to be the nearest neighbor of all leptospiral strains identified in this study, except Landon KR, which was the nearest neighbor to L. interrogans serovar Bataviae (S1 Fig).
Blood samples were drawn from 254 animals (211 dogs and 43 cats). Sera were tested for leptospiral antibodies using microscopic agglutination test (MAT). Of 211 dog sera tested, 38 contained Leptospira-specific antibodies (18.0%, 95% CI: 12.8–23.2%) (Table 1). Fifty per cent of the positive sera (19/38) reacted with only one of the seven tested serovars, with the majority (n = 11) being reactive to serovar Icterohaemorrhagiae, followed by Autumnalis (n = 4), Bratislava (n = 4), and Hardjo (n = 1) (Table 2). The remaining positive sera (n = 19) reacted to 2 (n = 7), 3 (n = 6), 4 (n = 4) or 5 (n = 2) serovars. Shelter KR had the highest number of MAT-positive dogs (n = 22), followed by Shelter PW (n = 10), Shelter KW (n = 5) and Shelter CC (n = 1) (Fig 1). None of the cats were positive by MAT and were excluded from further analyses.
Table 2. Seroreactivity of microscopic agglutination test (MAT)-positive dogs.
| Sample ID | Pomona | Hardjo | Grippotyphosa | Ictero.a | Canicola | Bratislava | Autumnalis |
|---|---|---|---|---|---|---|---|
| 18 | 100 | ||||||
| 23 | 100 | ||||||
| 27 P | 1600 | 100 | 400 | 200 | |||
| 28 | 100 | ||||||
| 36 | 200 | 400 | |||||
| 39 P | 200 | ||||||
| 42 P | 400 | 200 | 200 | ||||
| 44 P | 400 | 400 | 200 | ||||
| 45 P | 1600 | 400 | 200 | ||||
| 55 | 100 | ||||||
| 57 P | 800 | 400 | 400 | ||||
| 58 | 100 | ||||||
| 59 P | 200 | ||||||
| 60 P | 800 | 100 | |||||
| 66 P | 800 | 100 | |||||
| 67 P | 800 | ||||||
| 70 P | 100 | ||||||
| 76 | 400 | ||||||
| 89 | 100 | ||||||
| 102 | 100 | ||||||
| 104 | 200 | ||||||
| 109 | 100 | ||||||
| 115 | 100 | 100 | 400 | 400 | 400 | ||
| 116 | 100 | 400 | 200 | 400 | |||
| 130 | 200 | 400 | 200 | 400 | |||
| 139 | 100 | 100 | |||||
| 140 P | 100 | ||||||
| 149 | 100 | ||||||
| 166 | 400 | 100 | 100 | 100 | |||
| 172 | 100 | 200 | |||||
| 173 | 400 | ||||||
| 176 | 100 | 100 | |||||
| 180 | 100 | 100 | |||||
| 181 | 100 | 100 | 100 | ||||
| 182 | 200 | 100 | 200 | ||||
| 188 | 200 | ||||||
| 189 | 200 | ||||||
| 194 | 100 | 400 | 200 | 800 | 400 |
a, Icterohaemorrhagiae
P, positive for both MAT and urine qPCR
Twelve dogs were positive by both qPCR and MAT. Ten of these 12 dogs exhibited highest antibody titers for serovar Icterohaemorrhagiae, eight of which had a titer of ≥ 400. Fourteen dogs were positive by qPCR but negative by MAT.
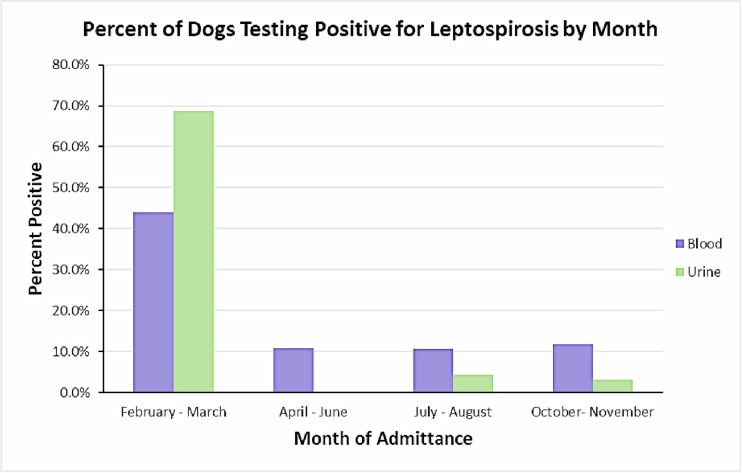
No analysis was performed on BUN results as all dogs in the study were within the normal range. Age was also dropped from analysis due to some validity concerns. The 95% CI for the variables sex and breed crossed the 1.0 threshold indicating there were no observed differences between the groups (Table 3). However, dogs admitted between February and June were 9.23 (95% CI: 3.04–28.02) times more likely to be qPCR positive and 2.66 (95% CI: 1.22–5.55) times more likely to be MAT positive than dogs admitted between July and November (Table 3). Further analysis of the variable season demonstrated statistically significant differences in the proportion of dogs admitted in Feb-March, April-June, July-August, and Oct-November (p<0.01) testing positive. In general, dogs were more likely to be positive earlier in the year than from summer through winter (p<0.01; Fig 3).
Table 3. Variable analysis of data collected from dogs admitted to LMU SOAR program.
Results stratified by diagnostic test performed.
| Test | Variable | Positive | Total | Percent positive | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
| qPCR | Sex | Female | 12 | 90 | 13.3% | 1.03 | 0.45–2.36 |
| Male | 14 | 108 | 13.0% | - | |||
| Breed* | Mixed | 23 | 159 | 14.5% | 0.49–9.64 | ||
| Other | 3 | 34 | 8.8% | - | |||
| Season | February-June | 22 | 84 | 26.2% | 9.23 | 3.04–28.02 | |
| July-November | 4 | 104 | 3.8% | - | |||
| MAT | Sex | Female | 16 | 99 | 16.2% | 0.78 | 0.38–1.59 |
| Male | 22 | 111 | 19.8% | - | |||
| Breed | Mixed Other |
33 4 |
172 28 |
19.2% 14.3% |
1.66 - |
0.55–5.06 |
|
| Season | February-June | 24 | 97 | 24.7% | 2.6 | 1.22–5.55 | |
| July-November | 12 | 106 | 11.3% | - |
* Odds Ratio not calculated due to < 5 as an expected value in a cell.
Fig 3. The percent of dog samples that tested positive for leptospiruria (urine) or leptospiral antibodies (blood) by month of admittance to the Lincoln Memorial University’s SOAR program.
Of note, animals were not admitted in January, September, and December.
A Chi-square test was performed to identify differences among shelters in relation to test results. A statistically significant difference was noted among the shelters in relation to the qPCR results (p<0.01). Shelter KR had 23 of 26 qPCR-positive dogs. For MAT results, no differences were found among shelters (p-value = 0.07). Kappa test statistic was performed to find if there was an agreement between qPCR and MAT results. Our data from qPCR and MAT produced an agreement of 0.33 (p<0.01), indicating a fair agreement.
Discussion
Pet overpopulation is an emerging issue in the Appalachian region relative to the rest of the United States, and this problem is likely attributed to various cultural and socio-economic factors unique to the region [18,19]. Stray animals have a higher exposure to rodents and contaminated standing water, which may be the only survival strategy available to many stray animals prior to adoption or arrival at an animal shelter. Once at a shelter, crowding, unsanitary housing conditions, and a lack of proper veterinary care further put these animals at a risk of getting infected, becoming carriers and transmitting diseases to both animals and humans.
In the present study, 13.1% of 219 tested apparently healthy dogs had leptospiral DNA in their urine indicating shedder status of these animals. Previous studies have shown that clinically normal dogs can chronically shed leptospires in urine, contaminating the surroundings and potentially exposing other animals and people in that environment to infection [20,21,22,23]. Identification of urinary shedders is thus important in preventing spread of leptospirosis. Culturing leptospires from clinical samples is extremely difficult, but molecular techniques, such as qPCR, provide a useful alternative tool for detecting leptospiral shedding. Several studies on renal carriage of leptospires show a wide range of prevalence among asymptomatic dogs. For example, a recent study from Switzerland reported urinary shedding in 0.2% of tested dogs [24], while a group from Brazil reported a prevalence of 19.8% [25]. Other studies have shown prevalence to be 8.2% (USA) [26], 7.05% (Ireland) [27], 14.2% (Brazil) [28], 3.7% (Columbia) [29], 4.8% (Algeria) [30], and 7.6% (New Caledonia) [31].
Classically, L. interrogans serovars, especially Canicola have been associated with asymptomatic renal carriage in dogs [32]. However, multiple studies have recovered other leptospiral species from asymptomatic dogs, including L. borgpetersenii, L. kirschneri, L. wolfii, and L. santarosai [33,34,22,35]. In the present study, sequence analysis of PCR-positive dog urine samples revealed that L. interrogans was the species involved in infections. L. interrogans has been associated with infection in humans, rodents and other animals [36–39]. Considering the animal and public health significance of these leptospiral species, the role of shelter dogs in transmission cycle in this region should be further investigated.
Leptospiruria or renal carriage of leptospires is not necessarily associated with the seropositivity [40]. The kappa statistics performed on our data showed a fair agreement between qPCR and MAT results, indicating a low correlation. Although it is not surprising, a low correlation between qPCR and MAT results implies that serological tests are not suitable for identification of asymptomatic infected dogs, and these leptospiruric but MAT-negative dogs perhaps pose a greater risk to animal and public health as they are less likely to be detected by routinely recommended serological tests, such as MAT.
A significant number of dogs tested in this study had leptospiral antibodies as detected by MAT. Most of the MAT positive sera were reactive to serovar Icterohaemorrhagiae, followed by serovars Bratislava and Autumnalis. These three serovars have previously been isolated from dogs [39,41]. Association between Icterohaemorrhagiae and rodents is well documented [42]. Since these shelters have limited resources, rodent infestation of the premises is very common in this region. A recent study from our group has shown that 62.3% of rodents in the Cumberland Gap region carry leptospires in their kidneys, potentially contaminating the environment and infecting animals and humans at risk [43].
Earlier studies from different parts of the world have shown urinary shedding and/or seropositivity in cats, suggesting that cats may have a role as a reservoir or accidental host [44–48]. However, in our study, all tested cats were negative for the presence of leptospiral antibodies and leptospiruria. How shelter cats fit in the leptospiral transmission conundrum in this region needs to be reevaluated.
Shelter animals may be exposed to rodents, other wild reservoirs, and stagnant water prior to arriving at shelters or even after entering shelters, if vermin control and disinfection protocols are inadequate. Exposure could also happen when animals come in contact with infected urine of another shelter animal. In a shelter environment, asymptomatic urinary shedders have the potential to infect other animals as well as workers and adopters. Shelter workers are occupationally exposed to dog and cat urine on a daily basis as they provide basic husbandry to these animals. Our study provides evidence-based information to educate shelters on the risk of leptospiral exposure to shelter workers and the importance of vermin control, vaccinating animals, and implementing proper disinfection and hygiene protocols.
Leptospirosis should be on the differential diagnosis list for any animal that has clinical signs such as vomiting, diarrhea, fever, lethargy and anorexia, especially if it has been adopted from a shelter, and prior vaccination status is unknown. Also, prevalence data can help a veterinarian determine an animal’s risk of exposure and the need for vaccination for leptospirosis in their region, since it is a non-core vaccine as per American Animal Health Association Canine Vaccination Guidelines [49]. This study provides veterinarians in this region with supporting evidence to make a case for the need and importance of yearly vaccination of dogs for leptospirosis when discussing preventive care with pet-owners.
Supporting information
The tree was generated using Geneious 9.0.5.
(PPTX)
(PPTX)
Acknowledgments
We thank Ellen Cho, Samantha Runser, Kayla Tubbs, Hanna Barnhart, Kenneth Gallatin, Emma Pawlowski, Rae Strobel, Susanna Kitts-Morgan and John Dascanio for technical assistance or helpful comments on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Please note the funding for this study was provided by Lincoln Memorial University- College of Veterinary Medicine’s Intramural Grant Program to Dawn Spangler and Ashutosh Verma (Grant#17DS001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Faine S, Adler B, Bolin C, Perolat P (1999) Leptospira and Leptospirosis, 2nd edition, MediSci. [Google Scholar]
- 2.Levett PN (2001) Leptospirosis. Clin Microbiol Rev 14: 296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757–771. 10.1016/s1473-3099(03)00830-2 [DOI] [PubMed] [Google Scholar]
- 4.Ko AI, Goarant C, Picardeau M (2009) Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7: 736–747. 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler B, de la Pena Moctezuma A (2010) Leptospira and leptospirosis. Vet Microbiol 140: 287–296. 10.1016/j.vetmic.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Nelson RW, Couto CG (2003) Small Animal Medicine. 3rd Ed Mosby, St. Louis, MO. [Google Scholar]
- 7.Ward MP, Glickman LT, Guptill LE (2002) Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). J Am Vet Med Assoc. 220(1):53–58. 10.2460/javma.2002.220.53 [DOI] [PubMed] [Google Scholar]
- 8.Glickman LT, Moore GE, Glickman NW, Caldanaro RJ, Aucoin D, Lewis HB (2006) Purdue University-Banfield National Companion Animal Surveillance Program for emerging and zoonotic diseases. Vector Borne Zoonotic Dis. 6(1):14–23. 10.1089/vbz.2006.6.14 [DOI] [PubMed] [Google Scholar]
- 9.Moore GE, Guptill LF, Glickman NW, Caldanaro RJ, Aucoin D, Glickman LT (2006) Canine leptospirosis, United States, 2002–2004. Emerg Infect Dis. 12(3):501–503. 10.3201/eid1203.050809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward MP (2002) Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Prev Vet Med. 56(3):215–226. 10.1016/s0167-5877(02)00160-5 [DOI] [PubMed] [Google Scholar]
- 11.Gautam R, Guptill LF, Wu CC, Potter A, Moore GE (2010) Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. Prev Vet Med. 96(1–2):122–131. 10.1016/j.prevetmed.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 12.Hennebelle JH, Sykes JE, Carpenter TE, Foley J (2013) Spatial and temporal patterns of Leptospira infection in dogs from northern California: 67 cases (2001–2010). J Am Vet Med Assoc. 242(7):941–947. 10.2460/javma.242.7.941 [DOI] [PubMed] [Google Scholar]
- 13.White AM, Zambrana-Torrelio C, Allen T, Rostal MK, Wright AK, Ball EC, et al. (2017) Hotspots of canine leptospirosis in the United States of America. Vet J. 222:29–35. 10.1016/j.tvjl.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Levett PN, Morey RE, Galloway RL, Turner DE, Steigerwalt AG, Mayer LW (2005) Detection of pathogenic leptospires by real-time quantitative PCR. J Med Microbiol. 54 (Pt 1):45–49. 10.1099/jmm.0.45860-0 [DOI] [PubMed] [Google Scholar]
- 15.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR (2009) Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis. 64(3):247–255. 10.1016/j.diagmicrobio.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 16.La Scola B, Bui LT, Baranton G, Khamis A, Raoult D (2006) Partial rpoB gene sequencing for identification of Leptospira species. FEMS Microbiol Lett. 263(2):142–147. 10.1111/j.1574-6968.2006.00377.x [DOI] [PubMed] [Google Scholar]
- 17.Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425. 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 18.Clifton M. Record Low Shelter Killing Raises Both Hopes & Questions. Available online: http://www.animals24-7.org/2014/11/14/record-low-shelter-killing-raises-both-hopes-questions/
- 19.Rowan A, Kartal T (2018) Dog Population & Dog Sheltering Trends in the United States of America. Animals (Basel) 8(5): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harkin KR, Roshto YM, Sullivan JT, Purvis TJ, Chengappa MM (2003) Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J Am Vet Med Assoc. 222(9):1230–1233. 10.2460/javma.2003.222.1230 [DOI] [PubMed] [Google Scholar]
- 21.Brown CA, Roberts AW, Miller MA, Davis DA, Brown SA, Bolin CA, et al. (1996) Leptospira interrogans serovar grippotyphosa infection in dogs. J Am Vet Med Assoc. 209(7):1265–1267. [PubMed] [Google Scholar]
- 22.Zakeri S, Khorami N, Ganji ZF, Sepahian N, Malmasi AA, Gouya MM, et al. (2010) Leptospira wolffii, a potential new pathogenic Leptospira species detected in human, sheep and dog.Infect Genet Evol. 10(2):273–277. 10.1016/j.meegid.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 23.Miotto BA, Guilloux AGA, Tozzi BF, Moreno LZ, da Hora AS, Dias RA, et al. (2018) Prospective study of canine leptospirosis in shelter and stray dog populations: Identification of chronic carriers and different Leptospira species infecting dogs. PLoS One. 13(7):e0200384 10.1371/journal.pone.0200384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaude A, Rodriguez-Campos S, Dreyfus A, Counotte MJ, Francey T, Schweighauser A, et al. (2017) Canine leptospirosis in Switzerland-A prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Prev Vet Med. 141:48–60. 10.1016/j.prevetmed.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 25.Sant'anna R, Vieira AS, Grapiglia J, Lilenbaum W (2017) High number of asymptomatic dogs as leptospiral carriers in an endemic area indicates a serious public health concern. Epidemiol Infect. 145(9):1852–1854. 10.1017/S0950268817000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harkin KR, Roshto YM, Sullivan JT (2003) Clinical application of a polymerase chain reaction assay for diagnosis of leptospirosis in dogs. J Am Vet Med Assoc. 222(9):1224–1229. 10.2460/javma.2003.222.1224 [DOI] [PubMed] [Google Scholar]
- 27.Rojas P, Monahan AM, Schuller S, Miller IS, Markey BK, Nally JE (2010) Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis. 29(10):1305–1309. 10.1007/s10096-010-0991-2 [DOI] [PubMed] [Google Scholar]
- 28.Oliveira ST, Messick JB, Welker Biondo A, Pires A, Santos D, Stedile R, et al. (2012) Exposure to Leptospira spp. in Sick Dogs, Shelter Dogs and Dogs from an Endemic Area: Points to Consider. Acta Sci Vet. 40(403):1056–1056. [Google Scholar]
- 29.Calderón A, Rodríguez V, Máttar S, Arrieta G (2014) Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop Anim Health Prod. 46(2):427–32. 10.1007/s11250-013-0508-y [DOI] [PubMed] [Google Scholar]
- 30.Zaidi S, Bouam A, Bessas A, Hezil D, Ghaoui H, Ait-Oudhia K, et al. (2018) Urinary shedding of pathogenic Leptospira in stray dogs and cats, Algiers: A prospective study. PLoS ONE 13(5): e0197068 10.1371/journal.pone.0197068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gay N, Soupé-Gilbert M-E, Goarant C (2014) Though not Reservoirs, Dogs might Transmit Leptospira in New Caledonia. Int J Environ Res Public Health. 11(4):4316–4325. 10.3390/ijerph110404316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuller S, Francey T, Hartmann K, Hugonnard M, Kohn B, Nally JE, et al. (2015) European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 56(3):159–179. 10.1111/jsap.12328 [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn JR, Krupka-Dyachenko I, Rettinger AL, Dyachenko V, Stamm I, Kopp PA, et al. (2016) Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from Upper Bavaria. Berl Munch Tierarztl Wochenschr. 129(5–6):251–257. [PubMed] [Google Scholar]
- 34.da Cunha CEP, Felix SR, Neto ACPS, Campello-Felix A, Kremer FS, Monte LG, et al. (2016) Infection with Leptospira kirschneri Serovar Mozdok: First Report from the Southern Hemisphere. Am J Trop Med Hyg. 94: 519–521. 10.4269/ajtmh.15-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miotto BA, Moreno LZ, Guilloux AGA, Sousa GO, Loureiro AP, Moreno AM, et al. (2016) Molecular and serological characterization of the first Leptospira santarosai strain isolated from a dog. Acta Trop. 162:1–4. 10.1016/j.actatropica.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 36.Dietrich M, Wilkinson DA, Soarimalala V, Goodman SM, Dellagi K, Tortosa P (2014) Diversification of an emerging pathogen in a biodiversity hotspot: Leptospira in endemic small mammals of Madagascar. Mol Ecol. 23(11):2783–2796. 10.1111/mec.12777 [DOI] [PubMed] [Google Scholar]
- 37.Vinetz. JM, Wilcox. BA, Aguirre. A, Gollin. LX, Katz. AR, Fujioka. RS, et al. (2005) Beyond Disciplinary Boundaries: Leptospirosis as a Model of Incorporating Transdisciplinary Approaches to Understand Infectious Disease Emergence. EcoHealth. 2(4):291–306. [Google Scholar]
- 38.Thaipadungpanit J, Wuthiekanun V, Chierakul W, Smythe LD, Petkanchanapong W, Limpaiboon R, et al. (2007) A dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl Trop Dis. 1(1):e56 10.1371/journal.pntd.0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Xu J, Zhang T, Qiu H, Li Z, Zhang E, et al. (2019) Genetic characteristics of pathogenic Leptospira in wild small animals and livestock in Jiangxi Province, China, 2002–2015. PLoS Negl Trop Dis 13(6): e0007513 10.1371/journal.pntd.0007513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene CE, Sykes JE, Moore GE, Goldstein RE, Schultz RD. (2012) Leptospirosis In: Greene C.E. Infectious Diseases of the Dog and Cat, Fourth Edition, Elsevier, St Louis, 431–446. [Google Scholar]
- 41.López MC, Vila A, Rodón J, Roura X (2019) Leptospira seroprevalence in owned dogs from Spain. Heliyon. 5(8):e02373 10.1016/j.heliyon.2019.e02373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boey K, Shiokawa K, Rajeev S (2019) Leptospira infection in rats: A literature review of global prevalence and distribution. PLoS Negl Trop Dis 13(8): e0007499 10.1371/journal.pntd.0007499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma A, Beigel B, Smola C, Kitts-Morgan S, Kish D, Nader P, et al. Evidence of leptospiral presence in the Cumberland Gap region. PLoS Negl Trop Dis (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickeson D, Love DN (1993) A serological survey of dogs, cats and horses in south-eastern Australia for leptospiral antibodies. Aust Vet J. 70(10):389–390. 10.1111/j.1751-0813.1993.tb00823.x [DOI] [PubMed] [Google Scholar]
- 45.Lapointe C, Plamondon I, Dunn M (2013) Feline leptospirosis serosurvey from a Quebec referral hospital. La Rev Vet Can. 54(5):497–499. [PMC free article] [PubMed] [Google Scholar]
- 46.Dos Santos LF, Guimarães MF, de Souza GO, da Silva IWG, Santos JR, Azevedo SS, et al. (2017) Seroepidemiological survey on Leptospira spp. infection in wild and domestic mammals in two distinct areas of the semi-arid region of northeastern Brazil. Trop Anim Health Prod. 49(8):1715–1722. 10.1007/s11250-017-1382-9 [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez J, Blais M-C, Lapointe C, Arsenault J, Carioto L, Harel J (2014) Serologic and urinary PCR survey of leptospirosis in healthy cats and in cats with kidney disease. J Vet Intern Med. 28(2):284–293. 10.1111/jvim.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weis S, Rettinger A, Bergmann M, Llewellyn JR, Pantchev N, Straubinger RK, et al. (2017) Detection of Leptospira DNA in urine and presence of specific antibodies in outdoor cats in Germany. J Feline Med Surg. 19(4):470–476. 10.1177/1098612X16634389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford R, Larson L, McClure K, Schultz R, Welborn L (2017). 2017 American Animal Hospital Association Canine Vaccination Guidelines (www.aaha.org/aaha-guidelines/vaccination-canine-configuration/vaccination-canine/). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The tree was generated using Geneious 9.0.5.
(PPTX)
(PPTX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.