Abstract
Background.
Endometrial cancer is the sixth most frequent type of cancer among women worldwide. Type I adenocarcinomas account for 80–85% of endometrial cancer cases and sometimes require more aggressive treatment than the remaining part of this group. Therefore, molecular markers to stratify adenocarcinomas are needed.
Materials and methods.
In this study, we analysed Notch and Wnt signalling in human endometrial cancer cases to evaluate these pathway elements as potential biomarkers for type I endometrial cancer. Endometrial samples were obtained from 47 women undergoing surgery for stage I–IV endometrial cancer in the National Cancer Institute (Vilnius, Lithuania) in 2015–2016. The expression at the mRNA level of signalling molecules genes (NOTCH1, NOTCH2, NOTCH3, NOTCH4, JAG1, JAG2, DLL1, HES1, AXIN2 and CTNNB1) was analysed by the quantitative real-time polymerase chain reaction. Relative expression of NOTCH1, NOTCH4, HES1 and β-catenin proteins in endometrioid adenocarcinoma was evaluated by the Western blot method.
Results.
The expression level of Notch receptors, ligands, and the target gene, as well as CTNNB1 and AXIN2, was reduced in stage I endometrioid adenocarcinoma if compared to the adjacent non-tumour tissue. The expression of all receptors, ligands, and target molecules was reduced in adenocarcinomas of later stages. The statistically significant correlations between transcript amounts of Notch receptors and ligands were found. There was a statistically significant difference in the gene expression of Notch signalling pathway components between different tumour differentiation grade samples. A positive correlation between mRNA and protein the expression level of NOTCH1, NOTCH4, HES1 was determined in stage I samples.
Conclusions.
Analysis of 47 human endometrial cancer samples revealeda reduction in the transcript levels of Notch and Wnt signalling molecule compared to the adjacent non-tumour tissue. These results suggest tumour suppressor function of Notch and Wnt signalling in human endometrial cancer. More detailed research on these signalling pathways should reveal their importance as potential biomarkers.
Keywords: Notch, WNT signalling pathway, endometrial cancer
Abstract
NOTCH IR WNT SIGNALINIŲ KELIŲ KOMPONENTŲ RAIŠKOS POKYČIAI SERGANT ENDOMETRIUMO VĖŽIU
Santrauka
Įvadas. Endometriumo vėžys yra šeštas pagal dažnį moterų piktybinis navikas pasaulyje. I tipo adenokarcinomos sudaro 80–85 % endometriumo vėžio atvejų. Siekiant parinkti adekvatų endometriumo adenokarcinomos gydymą gali padėti molekuliniai žymenys.
Medžiaga ir metodika. Atliekant šį tyrimą išanalizuoti Notch ir Wnt signalinių kelių komponentų ypatumai žmogaus endometriumo vėžio atveju siekiant įvertinti šių kelių komponentų, kaip potencialių I tipo endometriumo vėžio biožymenų, galimybes. Gimdos kūno audinio mėginiai gauti iš 2015–2016 m. Nacionaliniame vėžio institute (Vilnius, Lietuva) operuotų I–IV stadijos gimdos kūno vėžiu sergančių pacienčių (n = 47) operacinės medžiagos. Notch (NOTCH1, NOTCH2, NOTCH3, NOTCH4, JAG1, JAG2, DLL1, HES1) ir Wnt (AXIN2, CTNNB1) signalinių kelių genų raiška analizuota naudojant kiekybinį polimerazės grandininės reakcijos metodą. Santykinis NOTCH1, NOTCH4, HES1 ir β-katenino baltymų kiekis endometrioidinės adenokarcinomos mėginiuose buvo įvertintas Western blot metodu.
Rezultatai. Notch receptorių, ligandų ir taikinio, taip pat CTNNB1 ir AXIN2 genų raiška buvo sumažėjusi I stadijos endometrioidinės adenokarcinomos audiniuose, palyginti su aplinkiniu sveiku audiniu. Tirtų receptorių, ligandų ir taikinio genų raiška buvo sumažėjusi vėlesnių stadijų adenokarcinomų audiniuose. Nustatyta statistiškai reikšminga koreliacija tarp Notch receptorių ir ligandų transkriptų kiekio. Taip pat nustatyti statistiškai reikšmingi Notch signalinio kelio komponentų genų raiškos skirtumai esant skirtingai naviko diferenciacijai. Nustatyta teigiama koreliacija tarp NOTCH1, NOTCH4, HES1 genų raiškos ir baltymų kiekio ligos I stadijos atveju.
Išvados. Išanalizavus 47-ių pacienčių endometriumo vėžio mėginius nustatytas Notch ir Wnt signalinių molekulių raiškos sumažėjimas, palyginti su jų raiška aplinkiniame sveikajame audinyje. Tyrimo rezultatai leidžia teigti, kad Notch ir Wnt signalai turi naviką slopinantį potencialą endometriumo vėžio atveju. Išsamesni atskirų šių signalinių kelių komponentų tyrimai turėtų atskleisti jų, kaip potencialių biožymenų, svarbą.
Raktažodžiai: Notch, WNT signalinis kelias, endometriumo vėžys
INTRODUCTION
Endometrial cancer (EC) is the sixth most frequent type of cancer among women and the most common gynaecological cancer worldwide (1). The incidence of uterine cancer is increasing. It is thought to be related to the current obesity epidemic, increased life expectancy, reduced fertility, and hormone replacement therapy (2). Based on histopathological and molecular criteria, two types of endometrial cancer are distinguished: oestrogen dependent type I endometrioid adenocarcinoma, usually associated with atypical endometrial hyperplasia; and type II non-endometrioid cancer, which is oestrogen independent and mostly is associated with endometrial atrophy. Type I adenocarcinomas, accounting for 80–85% of cases, are of endometrioid histology, more often well-differentiated and associate with a favourable prognosis. In contrast, type II cancers, including serous papillary and clear cell carcinomas as well as carcinosarcomas, are poorly differentiated, more aggressive, and associated with poorer survival (3, 4). Despite type I cancer cases being often less aggressive, some type I cases require more aggressive treatment. Therefore, there is a need to individualise therapy of type I cases in order to avoid overtreatment and its side effects. A better understanding of type I endometrial cancer-associated molecular mechanisms would allow the identification of the potential predictive and prognostic molecular markers of this cancer, which could be important for optimising treatment and follow-up of endometrial cancer patients.
The molecular mechanism underlying endometrial carcinoma has not yet been fully explored. It has been found that some abnormalities in signalling pathways are related to the development of endometrial carcinoma. Cell fate is regulated by a number of signalling pathways such as Notch and Wnt.
The Notch signalling pathway is highly conservative and starts in the form of ligand-receptor interactions between neighbouring cells. Notch signalling is mediated by four Notch receptors (NOTCH1-4) and five transmembrane ligands (Jagged 1 and Jagged 2 (JAG1, 2), Delta-like 1 (DLL1), DLL3, and DLL4). Upon the activation of the Notch receptor, the receptor is cleaved, and the intracellular fragment translocates to the nucleus and acts as a regulator, in order to improve the expression of target genes, such as the Hairy enhancer of split (Hes) and Hes-related (Hey) transcription factors (5). A unique characteristic of the Notch signalling pathway is the lack of an amplification step during signal transduction. During signalling, each activated Notch receptor is consumed yielding only one intracellular domain (NICD) (6); therefore, to generate an appropriate cellular response, signal strength is an important factor. Notch signalling can be extremely sensitive to deviations in gene expression. Malfunctioning of Notch signalling can lead to various pathological conditions such as cancer. In cancerous tissues, Notch signalling is tissue-specific and displays tumour suppressive or oncogenic properties (5, 7).
Like Notch signalling, the Wnt pathway is highly conservative and is known for regulating genes critical to normal embryonic development and tissue homeostasis. In the absence of Wnt signal activation, the large protein complex composed of APC, Axin, GSK3β, phosphorylates, and β-catenin induces its degradation through the ubiquitin-proteasome pathway. The Wnt ligand binds to the Frizzled (Fzd) family receptor and lipoprotein LRP5/6 to initiate signalling. In the canonical Wnt signalling – Wnt/β-catenin pathway, the Fzd receptor inhibits phosphorylation leading to stabilisation and accumulation of cytosolic β-catenin, which enters the nucleus and activates the Wnt transcriptional output (8). Aberrant Wnt signalling underlies a wide range of pathologies in humans. Mutations in β-catenin at its GSK-3 binding consensus site within exon 3 lead to aberrant activation of the Wnt pathway, and the activation is frequently associated with tumour growth (9). It was shown that Wnt is an oncogenic pathway, frequently activated in many cancers including gynaecological malignancies (10, 11). Deregulation of the Wnt signalling pathway in EC occurs by inactivating β-catenin mutations in approximately 10–45% of ECs. It has been found that the Wnt signalling pathway is involved in cross-talk with other signalling pathways (12).
Importantly, the research into molecular pathogenesis in endometrial cancer has lagged far behind breast, ovarian, and cervical cancer. Therefore, a study of signalling pathways could facilitate the understanding of the interactions of disease-causing aberrant signalling pathways.
Several investigations have revealed the crosstalk between Notch and Wnt signalling pathways. Further understanding of the crosstalk between Notch and Wnt may not only reveal the regulation of these signalling pathways but also assist in the identification of diagnostic or therapy targets.
This study aimed to determine the expression profile of Notch and Wnt signalling molecules and to explore the possible relationship between both pathways in endometrial cancer tissues.
MATERIALS AND METHODS
Patients and specimens
Human endometrial samples were obtained from 47 women undergoing surgery in 2015 and 2016. The tissue samples were obtained from the National Cancer Institute (Vilnius, Lithuania). All samples had a paired control sample – an adjacent non-tumour endometrial tissue (determined by histopathologists). The tissue samples were promptly snap frozen in liquid nitrogen and stored at –80°C until use.
The age of the patients ranged from 44 to 79 years. Thirty-five patients were with stage I according to the International Federation of Gynaecology and Obstetrics (FIGO) classification, 6 with stage II, 3 with stage III, and 3 with stage IV cancer. Clinicopathological characteristics of the patients are shown in the Table.
Table.
Clinicopathological characteristics of the patients
| Characteristics | Number of patients |
|---|---|
| FIGO stage | |
| IA | 18 |
| IB | 17 |
| II | 6 |
| III | 3 |
| IV | 3 |
| Histologic type | |
| Endometrioid adenocarcinoma | 47 |
| Grade (G) | |
| G1 | 22 |
| G2 | 18 |
| G3 | 7 |
| Menopausal status | |
| Premenopausal | 6 |
| Postmenopausal | 41 |
FIGO, International Federation of Gynaecology and Obstetrics
This study was approved by the Lithuanian Bioethics Committee (No. 158200-05-180-43, 2010-05-05). All samples were collected with patients’ written informed consent in accordance with ethics approval.
Sample preparation
Frozen tumour and paired normal tissues were homogenised and extracted using the Ambion® PARIS™ system (Life Technologies). Protein concentration in lysate was determined with bicinchoninic acid (BCA).
Quantitative real-time PCR
0.5–1 μg of total RNA was reverse transcribed using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo scientific), according to the manufacturer’s instructions. The expression at the mRNA level of signalling molecule genes was analysed by the quantitative real-time polymerase chain reaction (Q-PCR). HPRT1 was used as the endogenous control genes used for normalisation. The following TaqMan primer-probe sets were used:
NOTCH1 (Hs01062014_m1, NM_017617.3),
NOTCH2 (Hs01050702_m1, NM_001200001.1),
NOTCH3 (Hs01128541_m1, NM_000435.2),
NOTCH4 (Hs00965889_m1, NM_004557.3),
JAG1 (Hs01070032_m1, NM_000214.2),
JAG2 (Hs00171432_m1, NM_002226.3),
DLL1 (Hs00194509_m1, NM_005618.3),
HES1 (Hs00172878_m1, NM_005524.3),
AXIN2 (Hs00610344_m1, NM_004655.3),
CTNNB1 (Hs00355045_m1, NM_001904.3).
The expression of the genes was normalized to HPRT1 (Hs02800695_m1, NM_000194.2). All TaqMan primer-probe sets and TaqMan Gene Expression Master Mix were from Life Technologies. Quantitative real-time PCR was performed with StepOne™ 2.2.2. (Applied Biosystems, Life Technologies) using the default thermal cycling conditions (10 min at 95°C and 45 cycles of 15 s at 95°C plus 1 min at 60°C). Relative quantification was performed using the Comparative Ct (threshold cycle) method.
Western blot
Samples containing 30 μg of total protein were subjected to 12% SDS-PAGE at 120 V. Proteins were transferred to the nitrocellulose membrane (BioRad) by semi-dry blotting. After blocking with 5% BSA, the membrane was incubated with rabbit monoclonal anti-Cleaved NOTCH1 (4147S, Cell Signalling) or anti-NOTCH4 (1:200; sc-5594, Santa Cruz Biotechnology), anti-HES1 antibody (PA5-28802, Thermo Scientific), or anti-non-phospho β-catenin antibody (8814S, Cell Signalling) and mouse monoclonal anti-β-actin antibody (MA5-15739, Thermo Scientific) for detection of β-actin as a loading control overnight at 4°C. Membrane-bound primary antibodies of NICD1, HES1, and β-catenin were detected by horseradish-peroxidase-conjugated secondary anti-rabbit antibody (31460, Thermo Scientific). The antibodies of β-actin were detected by horseradish-peroxidase-conjugated secondary anti-mouse antibody (31430, Thermo Scientific). The immunoreactive bands were developed using Pierce® ECL Western Blotting Substrate (Thermo Scientific). The amount of Notch signalling protein was normalised to the amount of β-actin. The relative amount of Notch signalling protein was calculated as the ratio of protein amount in carcinoma vs. normal tissue.
Statistical analysis
Sigma Plot software was used for statistical analysis. The data of the two groups were compared using the Mann-Whitney U Test. The correlation between expression levels was calculated using the Spearman Correlation. The significance of the difference between data was determined by P values < 0.05.
RESULTS
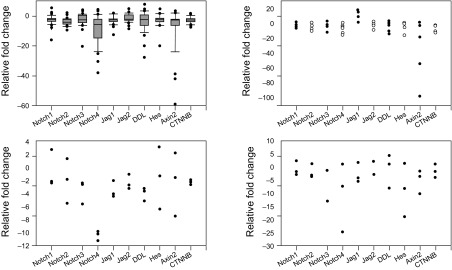
For the detection of Notch and Wnt signalling molecules, human endometrial samples obtained from 47 women undergoing surgery were analysed. All tested samples were endometrioid adenocarcinomas. According to the FIGO classification, most of the patients had stage I. To evaluate the expression of Notch and Wnt related genes, Q-PCR analysis was performed using paired samples of endometrial cancer and the adjacent non-tumour tissue. For comparison of the expression levels in accordance to the stage of cancer, the patients were divided into groups: stage I (35 patients; stage IA – 18 patients, stage IB – 17 patients), stage II (6 patients), stage III (3 patients) and stage IV (3 patients). The expression level of Notch receptors was reduced in stage I adenocarcinoma; the medians were 1.6-fold for NOTCH1, 3.3-fold for NOTCH2, 1.8-fold for NOTCH3, and 5-fold for NOTCH4 (Fig. 1A).
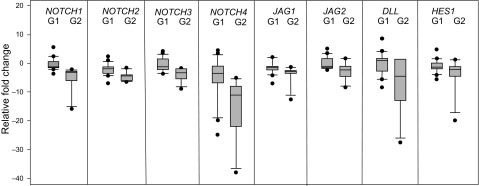
The expression level of Notch ligands coding JAG1, JAG2, and DLL was also reduced compared to the adjacent non-tumour tissue: 1.7-fold, 1.5-fold, and 1.6-fold as median in stage I (Fig. 1A). The mRNA level of CTNNB1 and AXIN2 was reduced 2-fold as the median in cancer cells if compared to the adjacent non-tumour tissue. The signalling pathway target gene HES1 expression in stage I endometrial adenocarcinoma was also reduced up to 1.4-fold. We also evaluated the mRNA levels of Notch and Wnt pathway molecules in stages II, III, and IV of endometrial adenocarcinoma cells. The expression of all receptors, ligands, and target molecules was reduced in most of the adenocarcinomas of later stages (Fig. 1B, C, D). According to the Spearman correlation test, we found significant correlations between transcript amounts of Notch receptors and ligands (r > 0.6, P < 0.05). We determined a positive correlation between the mRNA of all Notch receptors and target gene HES1 (for NOTCH1 r = 0.592; NOTCH2 r = 0.616; NOTCH3 r = 0.579; NOTCH4 r = 0.521, P < 0.05), as well as ligand DLL1 with HES1 (r = 0.613, P < 0.05) and between CTNNB1 and HES1 (r = 0.45, P < 0.05). Gene expression based on the tumour differentiation grade was analysed to evaluate activating Notch and Wnt signals depending on tumour differentiation. Stage 1 with G1 and G2 grade adenocarcinomas were chosen for analysis (Fig. 2).
There was a statistically significant difference between different grade samples: for NOTCH1 (median G1 (–1.25); G2 (–2.78); P < 0.001); NOTCH2 (median G1(–1.85); G2 (4.35); P < 0.001); NOTCH3 (median G1 (–1.16); G2 (–3.31); P < 0.001); NOTCH4 (median G1 (–3.47); G2 (–10.95); P < 0.001); JAG1 (median G1 (–1.46); G2 (–2.84); P = 0.002); JAG2 (median G1 (–1.08); G2 (–2.13); P < 0.001); DLL1 (median G1 (1.12); G2 (–4.56); P = 0.01): HES1 (median G1 (–1.27); G2 (–2.16); P = 0.04). The difference between the CTNNB1 and AXIN2 mRNA level in G1 and G2 adenocarcinomas was not significant (P = 0.22 and P = 0.13).
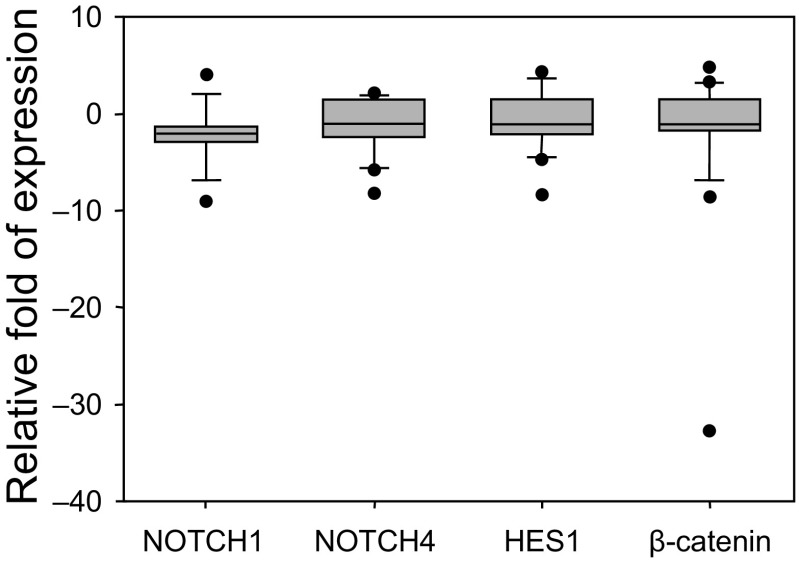
The amount of NOTCH1 (NICD domain), NOTCH4, HES1, and CTNNB1 proteins was determined by Western blot analysis (Fig. 3).
Fig. 1.
Expression of mRNA coding Notch and Wnt signalling pathway molecules in the endometrioid adenocarcinoma tissue compared to the normal endometrium of the same patient
(a) Changes in mRNA expression in stage I; (b) Changes in mRNA expression in stage II; (c) Changes in mRNA expression in stage III; (d) Changes in mRNA expression in stage IV. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers (error bars) above and below the box indicate the 90th and 10th percentiles
Fig. 2.
Expression of mRNA coding Notch signalling pathway molecules in stage I endometrioid adenocarcinoma tissue
Changes in mRNA expression in stage I, G1 and G2 grade adenocarcinomas compared to the normal endometrium of the same patient. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers (error bars) above and below the box indicate the 90th and 10th percentiles
Fig. 3.
Relative expression of NOTCH1, NOTCH4, HES1, and β-catenin proteins in endometrioid adenocarcinoma
Relative expression of the protein in stage I endometrial adenocarcinoma tissue compared to the normal endometrium of the same patient. After densitometric analysis of the bands, which was performed with Image J software, the amount of Notch signalling protein was normalised to β-actin. The relative expression protein was calculated as the ratio of the protein amount in adenocarcinoma to that of the healthy tissue. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers (error bars) above and below the box indicate the 90th and 10th percentiles
The amount of NOTCH4 receptor and HES1 in stage I adenocarcinoma was similar to the level in the healthy tissue and decreased by 1.1 and 1.1-fold as median. The median of the relative NOTCH1 amount in cancer tissue decreased 2-fold compared to the amount in the non-tumour tissue (min/max, –9.03/4.06). There was no significant difference in the NOTCH4 (median, –1.06; min/max, –8.23/2.17), HES1 (median, –1.13; min/max, –8.33/4.67) and β-catenin (median, –1.02; min/max, 32.83/4.78) relative protein level in stage I cancer vs. healthy tissues. A correlation between the level of NOTCH1 and NOTCH4 proteins (r = 0.592) was detected. Also, according to the Spearman correlation test, a positive correlation between mRNA and protein the expression level of NOTCH1, NOTCH4, HES1 was determined in stage I samples (r = 0.69, r = 0.51, r = 0.68, P < 0.05).
DISCUSSION
Endometrioid adenocarcinomas represent a range of neoplasms, from well- to poorly-differentiated tumours. This type of carcinomas is associated with endometrial hyperplasia. Its progression is closely related to unopposed estrogenic stimulation and is often associated with obesity (13, 14). Obesity risk factors and hormones are likely to play an important role in increasing rates of endometrial cancer (15). Most of the women, whose tissues were used for investigation, were overweight or obese: the body mass index (BMI) of only four women was <25 and indicated normal weight; nine women were overweight with their BMI ranging from 25 to 29.9; the BMI of the rest of the women was ≥30, which is classified as obesity. Also, the majority of investigated women (41) were in the postmenopausal stage. The endometrium undergoes a controlled process of proliferation and differentiation in premenopausal women. Under oestrogen stimulation, during the proliferative phase, endometrial cells are in a state of intense proliferation. After the menopause, the depletion of the endometrium hormone causes changes in the endometrium: atrophy and shrinkage (16).
Several reviews discuss the involvement of signalling pathways in the homeostasis of the endometrium and various pathologies, but the relationship between endometrial carcinoma and signalling pathways is sophisticated. Some investigations show that NOTCH1 and JAG1 increase from the proliferative to the secretory phase, while, in contrast, NOTCH4 decreases; moreover, high expression levels of JAG1/NOTCH1 were associated with a poor prognosis (17). Our previous studies have shown that Notch receptors, ligands, and target HES1 had an overall decreased expression of mRNA in endometrial cancer when compared to the normal endometrium (18); also, Notch signalling receptors, ligands JAG2 and DLL4, as well as direct target genes HES5 and HEY1 decreased in the eutopic endometrium (19). The malignant transformation of endometriosis may be affected by the hormonal changes associated with the menopause. There are few reports of Wnt signalling in endometrial cancers. β-catenin, an essential player of Wnt signalling, was abnormally expressed in endometrial adenocarcinomas. Immunohistochemistry studies reported accumulation of β-catenin in some cases of endometrial cancers (20).
This study aimed to test the expression profile of Notch and Wnt pathways signalling molecules and determine the relationship between both systems in endometrial cancer tissues. We examined the mRNA coding Notch receptors NOTCH1-4, ligands JAG1 and 2, DLL1, Wnt signalling transductor CTNNB1, and regulator AXIN2, as well as target HES1 in 47 patients with endometrial adenocarcinoma. The expression was normalised to the corresponding non-tumour tissue sample of the same patient. We reported the reduced expression level of mRNA coding in all analysed genes. We found positive correlations between transcript amounts of Notch receptors, ligands and target gene HES1 and between CTNNB1 and HES1. The family of HES transcription factors are a target of the Notch in many tissues, and HES1 expression has been regarded as an indicator of Notch activation (21). Endometrial cancer is commonly categorised by grade: well-differentiated G1, moderately-differentiated G2, and poorly-differentiated G3. These categories are based on tumour differentiation and the percentage of solid growth and have prognostic significance in endometrial cancer (22). Notch signalling is involved in the differentiation of many types of tissues, including the human endometrium (23). We found significant differences in expression between G1- and G2-grade adenocarcinomas, indicating that Notch signalling pathways’ suppression is associated with cell differentiation. Our investigations showed that the level of NOTCH1 domain NICD in stage I endometrial adenocarcinoma was reduced compared to adjacent non-tumour endometrial tissue. NICD1 formation describes activation of the Notch canonical pathway; cleaved NICD1 goes to the nucleus and regulates transcription. Besides, we determined a positive correlation between the mRNA and the amount of NICD. This confirms that the Notch signalling pathway is less active in the endometrial adenocarcinoma tissue.
Previous studies have shown that abnormal activation of the Wnt signalling pathway occurs in about 40% of endometrial cancers, and in most cases, it was due to β-catenin, APC, and Axins carcinogenic mutations. In addition, nuclear accumulation of β-catenin, which promoted tumour growth, was reported (24, 25). Receptor component FZD5 was downregulated in endometrial adenocarcinoma, in comparison with normal endometrium, but there was no significant difference in Wnt1, FZD1, and Wnt5 (26). Two immunohistochemical studies investigated the expression of ligand WNT7A. The first study found that WNT7A was overexpressed (27), and the second study revealed the decrease in the expression in endometrial cancer (28). We detected a downregulated expression of CTNNB1 and AXIN2 genes in stage I adenocarcinoma samples, but no significant changes in the protein level in most of the patients. There was no correlation between mRNA and protein expression. In our research, in 26% of the patients, the β-catenin amount was increased. Various authors indicate a different percentage of β-catenin accumulation in endometrial cancers. It was proposed to examine the importance of Wnt/β-catenin across a range of sites in various countries, taking into account the role of obesity in the pathogenesis of endometrial cancer (15). We found positive correlations between transcript amounts of CTNNB1 and HES1. Several investigations demonstrate crosstalk between Notch and Wnt signalling pathways. These interactions occur at several steps. A direct interaction between the proteins of these two pathways occurs; for example, during Notch and β-catenin interaction in mammalian neural progenitor cells (29) and NICD binding to Axin (30). It has also been demonstrated that HES1 can be activated by the Wnt signalling pathway (31).
CONCLUSIONS
Analysis of 47 human endometrial cancer samples revealed a reduction in the transcript levels of Notch and Wnt signalling molecule compared to the adjacent non-tumour tissue. These results suggest tumour suppressor function of Notch and Wnt signalling in human endometrial cancer. More detailed research on these signalling pathways should reveal their importance as potential biomarkers.
Acknowledgments
This project received funding from the European Social Fund (project No. 09.3.3-ESFA-V-711-01-0001) under a grant agreement with the Research Council of Lithuania (LMTLT).
Nadežda Lachej, Violeta Jonušienė, Augustina Mažeikė, Aušra Sasnauskienė, Daiva Dabkevičienė, Julija Šimienė, Kęstutis Sužiedėlis, Janina Didžiapetrienė
References
- Ferlay J Soerjomataram I Dikshit R Eser S Mathers C Rebelo M et al. . Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136: E359–E86. [DOI] [PubMed] [Google Scholar]
- Kitchener HC, Trimble EL. Endometrial Cancer Working Group of the Gynecologic Cancer Intergroup: Endometrial cancer state of the science meeting. Int J Gynecol Cancer. 2009; 19: 134–40. [DOI] [PubMed] [Google Scholar]
- Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, Gargett CE. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009; 69: 8241–8. [DOI] [PubMed] [Google Scholar]
- Kalampokas E, Payne F, Gurumurthy M. An update on the use of immunohistochemistry and molecular pathology in the diagnosis of pre-invasive and malignant lesions in gynecological oncology. Gynecol Oncol. 2018; 150: 378–86. [DOI] [PubMed] [Google Scholar]
- Dunford RG, Mejia EB, Salbador GW, Gerth WA, Hampson NB. Diving methods and decompression sickness incidence of Miskito Indian underwater harvesters. Undersea Hyperb Med. 2002; 29: 74–85. [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011; 138: 3593–612. [DOI] [PubMed] [Google Scholar]
- Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003; 3: 203–5. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim M, Jho EH. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem J. 2013; 450: 9–21. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434: 843–50. [DOI] [PubMed] [Google Scholar]
- Bodnar L Stanczak A Cierniak S Smoter M Cichowicz M Kozlowski W et al. . Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J Ovarian Res. 2014; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JY, Norman AW, Lübbert M, Collins ED, Uskokovic MR, Koeffler HP. Novel vitamin D analogs that modulate leukemic cell growth and differentiation with little effect on either intestinal calcium absorption or bone mobilization. Blood. 1989; 74: 82–93. [PubMed] [Google Scholar]
- Dellinger TH, Planutis K, Tewari KS, Holcombe RF. Role of canonical Wnt signaling in endometrial carcinogenesis. Expert Rev Anticancer Ther. 2012; 12: 51–62. [DOI] [PubMed] [Google Scholar]
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983; 15: 10–7. [DOI] [PubMed] [Google Scholar]
- Suarez AA, Felix AS, Cohn DE. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol Oncol. 2017; 144: 243–9. [DOI] [PubMed] [Google Scholar]
- Coopes A, Henry CE, Llamosas E, Ford CE. An update of Wnt signalling in endometrial cancer and its potential as a therapeutic target. Endocr Relat Cancer. 2018; 10.1530/ERC-18-0112 [DOI] [PubMed] [Google Scholar]
- Winder SJ, Sutherland C, Walsh MP. Biochemical and functional characterization of smooth muscle calponin. Adv Exp Med Biol. 1991; 304: 37–51. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi Y, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology. 2012; 60: 826–37. [DOI] [PubMed] [Google Scholar]
- Jonusiene V Sasnauskiene A Lachej N Kanopiene D Dabkeviciene D Sasnauskiene S et al. . Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol. 2013; 30: 438. [DOI] [PubMed] [Google Scholar]
- Su RW Strug MR Joshi NR Jeong JW Miele L, Lessey BA et al. . Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015; 100: E433–E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten AN, Creutzberg CL, van den Broek LJ, Noordijk EM, Smit VT. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol. 2003; 201: 460–5. [DOI] [PubMed] [Google Scholar]
- Sun Y Gao X Liu J Kong QY Wang XW Chen XY et al. . Differential Notch1 and Notch2 expression and frequent activation of Notch signaling in gastric cancers. Arch Pathol Lab Med. 2011; 135: 451–8. [DOI] [PubMed] [Google Scholar]
- Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002; 9: 145–84. [DOI] [PubMed] [Google Scholar]
- Cobellis L Caprio F Trabucco E Mastrogiacomo A Coppola G Manente L et al. . The pattern of expression of Notch protein members in normal and pathological endometrium. J Anat. 2008; 213: 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi T Sakamoto M Tsuda H Maruyama K Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998; 58: 3526–8. [PubMed] [Google Scholar]
- Moreno-Bueno G Hardisson D Sánchez C Sarrió D Cassia R García-Rostán G, et al. . Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002; 21: 7981–90. [DOI] [PubMed] [Google Scholar]
- Menezes MP Oshima CT Filho LB Gomes TS Barrezueta LF Stávale JN et al. . Canonical and noncanonical Wnt pathways: A comparison between endometrial cancer type I and atrophic endometrium in Brazil. Sao Paulo Med J. 2011; 129: 320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y Meng F Xu Y Yang S Xiao M Chen X et al. . Overexpression of Wnt7a is associated with tumour progression and unfavorable prognosis in endometrial cancer. Int J Gynecol Cancer. 2013; 23: 304–11. [DOI] [PubMed] [Google Scholar]
- Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of wnt7a in human endometrial carcinoma. Obstet Gynecol Int. 2012; 10.1155/2012/134962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T Kagawa T Inoue T Nonaka A Takada S Aburatani H et al. . Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008; 28: 7427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Descalzo S, Tkocz K, Balayo T, Arias AM. Modulation of the ligand-independent traffic of Notch by Axin and Apc contributes to the activation of Armadillo in Drosophila. Development. 2011; 138: 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F, Giaimo BD. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia pathways. Biochim Biophys Acta. 2016; 1863: 303–13. [DOI] [PubMed] [Google Scholar]