Abstract
Desmoid tumors are benign but locally aggressive soft tissue tumors that arise from fibroblast cells. Magnetic resonance-guided focused ultrasound (MRgFUS) has emerged as an alternative to conventional therapies, showing promising results in reduction of tumor volume without significant side effects. The gold-standard assessment of the reduction of viable tumor volume post-treatment is non-perfused volume (NPV) and evaluation of NPV is typically performed with post-treatment gadolinium enhanced MR imaging. However, as gadolinium cannot be repeatedly administered during sonications, there is a need for alternative non-contrast monitoring of the tissue to prevent over and under treatment. In this work, we perform five MRgFUS treatments of lower extremity desmoid tumors with concurrent T2 mapping. We show that in all five sessions, T2 mapping showed good qualitative agreement with the post-contrast NPV. Thus, T2 mapping may be used to visualize the extent of ablation with focused ultrasound and can be used as a predictor of NPV prior to the administration of contrast during the post-treatment assessment.
Keywords: Desmoid tumors, T2 mapping, MRgFUS, HIFU, thermal ablation
Introduction
Magnetic resonance-guided focused ultrasound (MRgFUS), also known as high-intensity focused ultrasound (HIFU), is a noninvasive ablation technique that has been successfully used for the treatment of uterine fibroids, bone metastases, and essential tremor.[1, 2] More recently, focused ultrasound ablation has been investigated for indications such as treatment of focal breast lesions, osteoid osteomas, and desmoid tumors.[3, 4]
Desmoid tumors are benign but locally aggressive monoclonal soft tissue tumors that arise from fibroblast cells. Conventional therapies include surgical resection as a first line treatment, followed by radiation and chemotherapy. Despite these treatments, the tumors can have a recurrence rate of up to 50% in 5 years.[5] As an alternative treatment, focused ultrasound has shown promising results in reduction of tumor volume without significant side effects.[6–9] Specifically, a prior retrospective study of 15 patients with lower extremity desmoid tumors showed 63% reduction in mean tumor volume and pain improvement to 2.7 from 7.5, on a 1 to 10 Numerical Rating Scale.[8] MRgFUS is especially useful for desmoid tumors that are located in the lower extremity—this requires delicate technique as the tumors can grow to be quite large and often invade or grow proximally to neurovascular bundles.
However, visualization of the intra-procedural ablated volume remains a large problem in focused ultrasound therapy. Thermal dose maps from MR thermometry are used during the treatment to visualize the treated volume, but fail to reliably predict the extent of ablation.[10–13] Post-treatment contrast enhanced MR imaging allows assessment of the non-perfused volume (NPV), the gold standard assessment of the degree of tumor ablation. However, safety concerns regarding heating of tissue after gadolinium injection prevent further treatment following the NPV assessment.[14] Firstly, free gadolinium may disassociate and result in local tissue toxicity as lanthanides are known to contribute to pro-fibrotic and pro-inflammatory pathways and lead to apoptosis and necrosis.[15] Secondly, as a paramagnetic contrast agent, gadolinium can induce a phase shift, rendering MRgFUS PRF temperature monitoring unreliable—particularly problematic when using PRF thermometry in water-based tissues (muscle, myometrium, fibroids).[16]
Prior work demonstrated that changes in tissue T2 values can be used to measure the temperature change in fatty tissue for 1.5T.[17] Specifically, the T2 values of tissues such as muscle and fat have been shown to increase with temperature.[18] Further work built upon those results showed that T2-based thermometry can be used to monitor tissue temperature in both red and yellow bone marrow and abdominal fat, optimized for 3T.[19, 20]
In this study, we build upon these prior studies to investigate rapid T2 mapping as a way to visualize tissue changes during MRgFUS treatment. We report here our findings performing this technique during MRgFUS treatment of five patients with desmoid tumors.
Materials and Methods
The local Institutional Review Board (IRB) approved this study. Furthermore, informed consent was waived by the IRB for this retrospective study. MR-guided focused ultrasound (MRgFUS) ablation was performed on patients with desmoid tumors in the lower extremities using an Exablate 2100 system (INSIGHTEC, Haifa, Israel) integrated with a 3.0 Tesla MR scanner (GE Healthcare, Waukesha, WI, USA). Five separate MRgFUS sessions were performed.
Figure 1 shows axial, sagittal, and coronal orientations of the standard setting of treatment in which the patient is positioned on the MRgFUS system, above the ultrasound transducer embedded within the MRI table. The gel pad is positioned between patient and the ultrasound window for acoustic coupling to the ultrasound transducer and to reduce heat to the near-field skin. A cooling water bag reduces heat to the far-field skin and serves as a conduit for acoustic energy to propagate beyond the air-skin interface.
Figure 1.
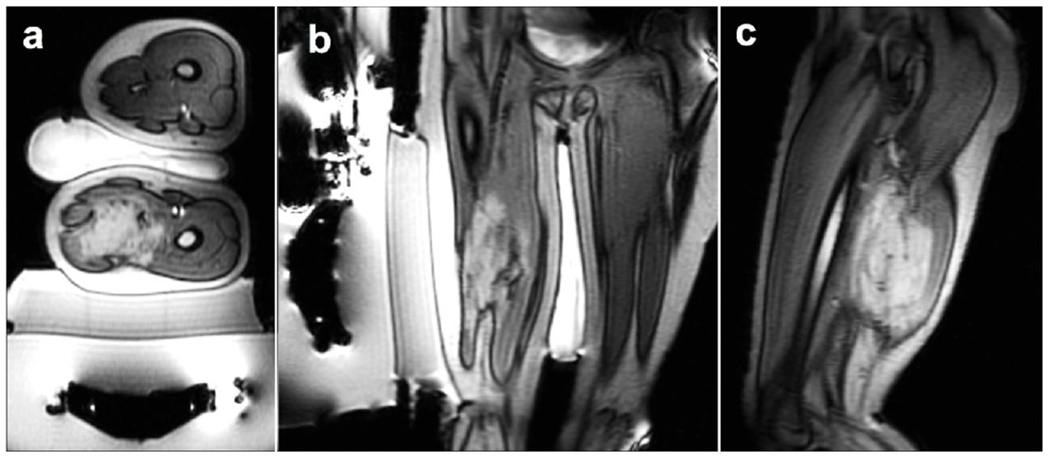
Positioning of patient 1 is shown via axial (a), coronal (b), and sagittal (c) view. These localizer images additionally show the transducer, gel pad, and cooling water bag.
For each patient, pre-procedural MRI sequences were performed to assess the position of the desmoid tumor in relation to other nearby nerves and vessels. Subsequently, sonications were performed with a duration of 20-30 sec and acoustic power between 49 and 107 W. The patient-specific acquisition parameters are summarized in Table 1.
Table 1.
Overview of sonication and acquisition data sorted by patient.
| # of MRgFUS treatment sonications | Energy range of sonications (J) | Temperature range of sonications (°C) | # of T2 map acquisitions | T2 map type | |
|---|---|---|---|---|---|
| Patient 1 | 69 | 1745-2268 | 49-77 | 6 | Double Echo FSE |
| Patient 2 | 70 | 1284-1556 | 50-96 | 4 | Double Echo FSE |
| Patient 3 | 79 | 1699-7897 | 38-83 | 6 | Multi-Echo FSE |
| Patient 4 | 78 | 1536-6591 | 37-80 | 4 | Multi-Echo FSE |
| Patient 5 | 76 | 1751-6882 | 40-94 | 4 | Multi-Echo FSE |
Patients received 2-10 verification sonications followed by treatment sonications over a course of 3-4 hours. MR thermometry was performed during each sonication with multiphase multislice echo planar imaging (FOV/slice thickness/TR/TE/flip angle/echo train length= 28 × 28 cm /4mm /210ms /18.3ms /35 /12).
Over the course of the treatment either double-echo (Patient 1,2) or multi-echo images (Patient 3,4,5) were acquired before, during and after the treatment. We performed either double or multi-echo FSEs with the goal of testing both techniques to evaluate the benefits of each. Double-echo Fast Spin Echo (FSE) images were acquired with the following parameters: TE= 35/186 ms (2 echoes), TR = 1500 ms, echo train length = 40, FOV = 24-28 cm, 128 x 128 matrix size, BW = 15.6 kHz, 10mm slice thickness, 1-2 slices, 15 sec acquisition time. Multi-echo Fast Spin Echo (FSE) images were acquired with the following parameters: TE = 8.8-141 ms (16 echoes), TR = 600-1377 ms, echo train length = 1, FOV = 30 cm, 128 x 128 matrix size, BW= 15.6 kHz, 6-8 mm slice thickness, 4-10 slices, 1:20-3:12 min acquisition time.
T2 maps were generated with an exponential fit. The multi-echo fitting was performed on all samples using software developed at our institution in IDL (Harris Geospatial Solutions, Broomfield, CO). Mean R2 value was calculated by placing circle ROIs on multiple slices within the tumor. Double-echo fitting was performed in Matlab. Processing took on the order of 1-10 seconds per image series. Mean T2 values were calculated in HOROS by drawing ROIs around untreated and treated regions of the tumor. ROIs were summed and a standard deviation was calculated.
At the end of the treatment, pre- and post-contrast 3D FSPGR (FOV/slice thickness/TR/TE/flip angle = 44×44cm/3.8mm/4.3msec/2msec/15) images were acquired. T2 maps were qualitatively compared to post-contrast MR images.
Results
Five MRgFUS treatments of desmoid tumors were performed for this study with concurrent T2 acquisitions as outlined in the Materials and Methods. For each patient, axial, sagittal, and coronal T2 images were acquired either via double or multi-echo sequences. Figure 2 shows one slice of the desmoid tumor of the upper thigh of patient 1. Fig. 2a is an image of the tumor prior to sonications. As the tumor treatment proceeded over 4 hours, T2 images were acquired at various timepoints (Figs. 2b–2f). This patient underwent sonication treatments between 10:55-11:50 (first set), 12:03 – 13:24 (second), 13:34-14:04 (third), and 14:08-14:12 (fourth). The dotted line in Fig. 2 (right) represents the final sonication. These T2 maps show a steady increase in the T2 value at the site of ablation over the course of the treatment, quantified in Fig. 2 (right). The last treatment sonication occurred at 14:12, 1 minute before point e. Point f occurs approximately 20 minutes after the final sonication and shows a slight decline in the T2 value. At this point the temperature of the tissue has returned closer to baseline, and so the decline is likely a result of tissue cooling due to artificially elevated values from the effect of temperature of prior sonications.
Figure 2.
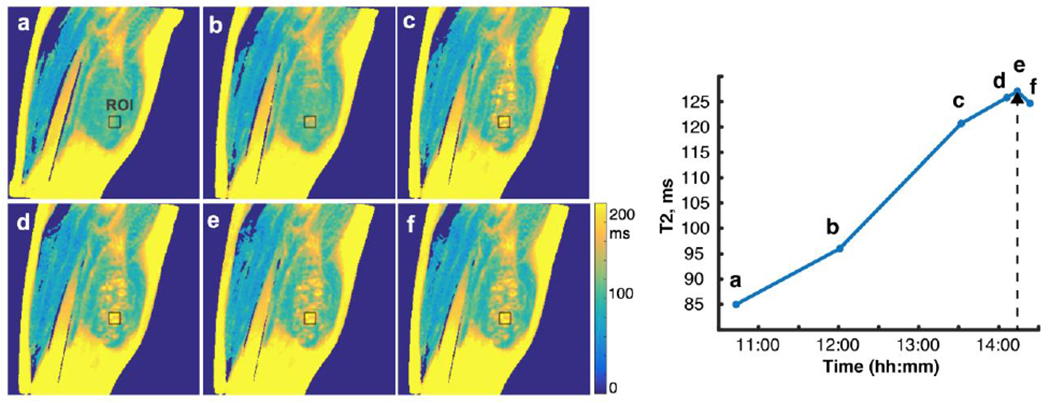
Sagittal T2 maps of one slice of the treatment region of patient 1. Fig. 2a demonstrates the tissue before sonication treatment. Figs. 2b through 2e demonstrate increased T2 value over the course of the treatment duration—four hours. 2f shows the T2 value approximately 20 minutes after treatment has ended. 2(right) is a quantification of this signal at the given ROI over time where the dotted arrowhead represents the final sonication treatment, just before point ‘e’. T2 maps were generated in MatLab.
After treatment, post-contrast MRI sequences were obtained which demonstrated qualitative similarities between the T2 mapping of the non-perfused volume (NPV) and the post-contrast NPV (Figure 3). The same slice of the post-treatment desmoid area in patient 1 is shown for both the T2 mapping and post-contrast gadolinium sequences. The non-enhancing NPV corresponds directly to the areas of hyperintensity (highest T2 value (ms)) on T2 mapping images. Conversely, the gaps in the NPV highlighted by the red arrows in 3b, demonstrate agreement with the regions of lower T2 values on T2 mapping sequences. This reveals areas within the desmoid tumor that may yet require further treatment.
Figure 3.
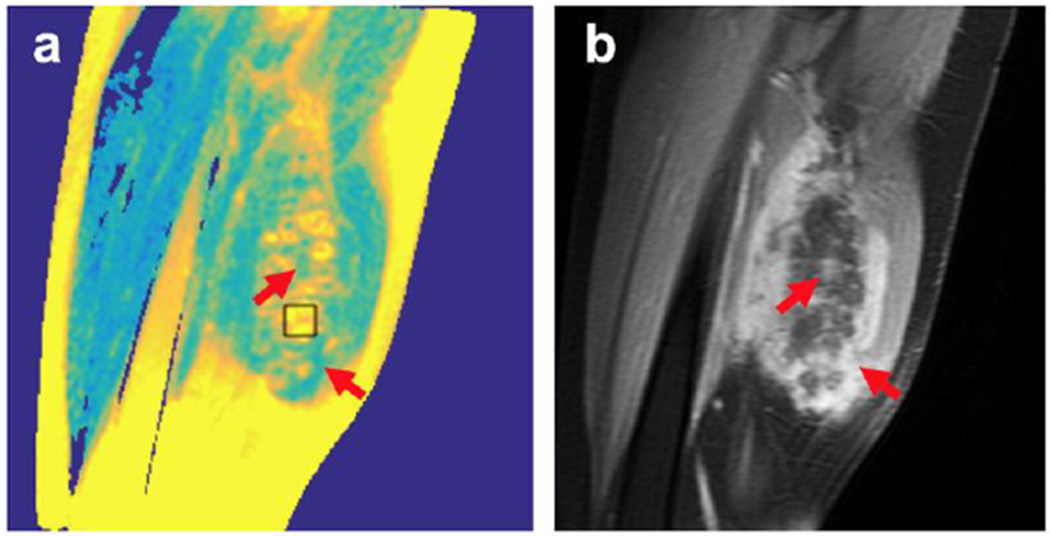
Comparison of a sagittal T2 map (a) with post-treatment post-contrast image (b) of same location for patient 1. The non-perfused volume (NPV) appears hyperintense on T2 maps (example given by ROI box). Consequently, the red arrows identify gaps in the NPV, which correspond to high enhancement in the post-contrast images.
Data for patient 2 is shown in Figure 4. Fig. 4a demonstrates the tissue before sonication treatment. Figs. 4b through 4c demonstrate increased T2 value over the course of the treatment duration. These axial T2 maps demonstrate that T2 mapping not only correlates well with post-contrast MR, but can provide more detailed information than post-contrast MR by more clearly delineating regions of partial treatment. This is seen in Figure 4—the post-contrast MR NPV is more homogeneous and less detailed than the T2 maps. Conversely, Fig. 4c shows distinct regions of high and low T2 elevation (red arrows) that more clearly show gradations of partial treatment.
Figure 4.

Axial T2 maps (a-c) of one slice of the treatment region of patient 2. Fig. 4a demonstrates the tissue before sonication treatment. Figs. 4b through 4c demonstrate increased T2 value over the course of the treatment duration. Fig. 4d is a post-contrast image of same location. Patient was repositioned, but the arrows indicate the ROI. T2 maps were generated in MatLab.
Multi-Echo T2 mapping was performed in three patients whose T2 maps are shown in Figure 5. In each, red arrows identify the region of T2 elevation over time that corresponds well to the NPV on post-contrast ROI. The mean T2 values for patients 3, 4, 5 is between 87.4 ± 10 ms (in untreated desmoid) and 147.1 ms ± 18 ms (treated desmoid). The multi-echo fitting was performed on all samples and mean R2 values for patients 3, 4, 5 were found to be 0.93, 0.98, and 0.96, respectively. Patient 5 is a patient that was treated once before, thus the red arrow is pointing to the site of most current ablation.
Figure 5.
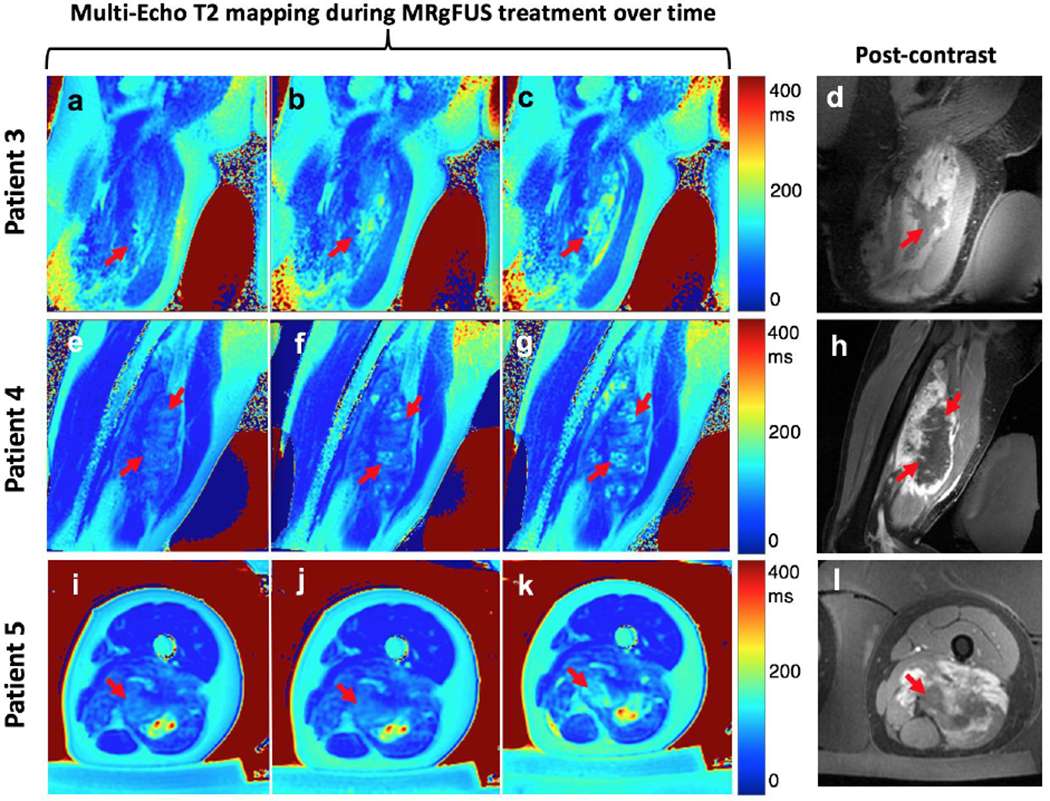
Multi-slice T2 maps were generated (one slice shown). T2 maps for three additional patients are shown pre-sonication (first column: Fig. 5a, 5e, 5i), mid-sonication (second column: 5b, 5f, 5j) and end-sonication (final column: 5c, 5g, 5k). This set of final T2 maps is intended for comparison to Figs. 5d, 5h, 5l which demonstrate the corresponding post-contrast images.
Discussion
Our work presents, to the best of our knowledge, the first application of T2 mapping for treatment assessment of soft tissue tumors during MRgFUS ablation. The results in Figs. 2–5 show that T2 mapping could be used to visualize the changes in tissue during focused ultrasound treatments of desmoid tumors. The areas of T2 elevation showed good agreement with the non-perfused volumes in the post-contrast MR images, both for double-echo and multi-echo FSE T2 map acquisitions. Thus, T2 mapping can be used as a non-contrast indicator for ablated area mid-procedure. To this end, we determined mean T2 values that correspond with NPV as reported in the results (147.1 ms ± 18 ms for treated desmoid). While we believe that qualitative comparisons between T2 maps and post-contrast images can be most useful to clinicians mid-procedure, this T2 value can additionally inform regions that do not require further treatment. We note, however, that this value is based on our small sample size and to more accurately determine a true threshold, a larger statistical sample of patients is needed.
Additionally, though NPV has historically been the gold standard to determine ablated area, it is worth noting that NPV may overestimate the actual ablated area immediately after treatment. Several studies have reported that NPV increases between 2-48 hours post ablation and can decrease in volume over time with reabsorption of necrotic cells.[21–23] As our treatments were on the scale of several hours, the post contrast NPV may yet be an overestimation. With this discussion in mind, we recognize that in Figure 5, for example, T2 mapping appears to underestimate NPV, however, the former may be a truer indication of ablated area. Further work is needed to compare T2 mapping to NPV on serial post-procedural MRI evaluation.
For clinicians, it is worth noting that we present both double-echo FSE and multi-echo FSE acquisitions to show the breadth of the T2 mapping technique, however, the double-echo technique is more feasible to integrate clinically. The multi-echo acquisition generates much better exponential fitting due to the acquisition of more data points (Fig. 5). However, longer acquisition times make it more challenging to run during a time-sensitive treatment session. Over the course of this work, we were able to optimize the double-echo acquisition time to 15 seconds and still generate high quality T2 maps (Fig. 2, Fig. 4). Thus, we believe the double-echo acquisition may be the superior technique to generate adequate T2 maps while simultaneously preventing prolongation of MRgFUS procedure time.
Our study was not without some limitations. Because this work was preliminary, we performed a primarily qualitative comparison of T2 maps for five treatment sessions. Quantitative volumetric analysis comparing T2 maps and post-contrast images was not pursued as we did not acquire T2 mapping sequences through the entirety of the tumor for every treatment (so as not to significantly delay the length of the treatment). However, we saw consistent agreement between T2 maps and post-contrast images. We intend the T2 mapping technique to be used by clinicians during procedure, and thus if there is good qualitative correlation between T2s and post-contrast images, then intra-procedural T2 maps can be used to inform the clinician which regions may require further ablation. Secondly, evaluating the T2 maps in Figs. 2–5, it is important to note that T2 values do have some temperature sensitivity. Although some of the observed T2 elevation could be caused by the heat from the previous sonications, the elevated T2 values were observed at every analyzed slice throughout the interior of the tumor. Fig. 2 (right) is also evidence that approximately 20 minutes after the final sonication, the temperature of the tissue has significantly decreased while the T2 value only decreases slightly (point f), further underscoring that the temperature effect is minimal. Additionally, Patient 5 (Fig. 5k) is evidence of a case where the NPV from an old (prior treatment) ablation generates a higher T2 value than the hotter, more currently ablated tissue (red arrows). Prior work has investigated this temperature contribution to T2 maps during ablation of trabecular bone.[19] Future work may evaluate changes in T2 values in the tumor after return to baseline temperature. Finally, all tissue has some inherent baseline T2 heterogeneity. In prior work, Ozhinsky et al showed T2 maps both with and without a baseline subtraction.[20] This is easiest to achieve when the tissue is immobilized, and due to some repositioning in the course of these desmoid treatments, we did not pursue the same subtraction here. Nevertheless, the post-subtraction T2 maps were still representative of the pre-subtraction T2 maps, and so we do not believe a subtraction is always necessary.
Conclusions
MRgFUS has been shown to be a safe, reliable and broadly applicable treatment option for patients with desmoid tumors in recent years. However, a limitation of MRgFUS has been a shortage of quick, quantifiable techniques to monitor the ablated tissue during treatment. Though the use of gadolinium contrast agents to evaluate the NPV of the tissue has been the gold standard, the gadolinium injection prevents and complicates further treatment within the same session.
In this work, we have shown that double-echo and multi-echo T2 mapping can be used to visualize the extent of ablation with focused ultrasound and be used as a predictor of NPV without the need for contrast injections. This could be a useful technique for physicians to ensure complete ablation of the tissue within the region of treatment.
Acknowledgements
We would like to thank Misung Han for the help with protocol optimization and David Newitt for his help with multi-echo fitting parameters. This study has been funded by NIH R00 HL097030 and by GE Healthcare.
Footnotes
Declaration of Interest Statement: No conflicts of interest to declare.
References
- 1.Boutet A, et al. , Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain, 2018. 141(12): p. 3405–3414. [DOI] [PubMed] [Google Scholar]
- 2.Catane R, et al. , MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience. Ann Oncol, 2007. 18(1): p. 163–7. [DOI] [PubMed] [Google Scholar]
- 3.Zippel DB and Papa MZ, The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer, 2005. 12(1): p. 32–8. [DOI] [PubMed] [Google Scholar]
- 4.Arrigoni F, et al. , Magnetic-resonance-guided focused ultrasound treatment of non-spinal osteoid osteoma in children: multicentre experience. Pediatr Radiol, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Peng PD, et al. , Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann Surg Oncol, 2012. 19(13): p. 4036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghanouni P, et al. , Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol, 2017. 27(2): p. 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avedian RS, et al. , Is MR-guided High-intensity Focused Ultrasound a Feasible Treatment Modality for Desmoid Tumors? Clin Orthop Relat Res, 2016. 474(3): p. 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucknor MD and Rieke V, MRgFUS for desmoid tumors within the thigh: early clinical experiences. J Ther Ultrasound, 2017. 5: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wang W, and Tang J, Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia, 2011. 27(7): p. 648–53. [DOI] [PubMed] [Google Scholar]
- 10.Winter L, et al. , Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int J Hyperthermia, 2016. 32(1): p. 63–75. [DOI] [PubMed] [Google Scholar]
- 11.Rieke V and Butts Pauly K, MR thermometry. J Magn Reson Imaging, 2008. 27(2): p. 376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odeen H and Parker DL, Magnetic resonance thermometry and its biological applications - Physical principles and practical considerations. Prog Nucl Magn Reson Spectrosc, 2019. 110: p. 34–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitton RR, et al. , Improving thermal dose accuracy in magnetic resonance-guided focused ultrasound surgery: Long-term thermometry using a prior baseline as a reference. J Magn Reson Imaging, 2016. 43(1): p. 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinger SC, Fridjhon P, and Rubin DM, Thermal Excitation of Gadolinium-Based Contrast Agents Using Spin Resonance. PLoS One, 2016. 11(6): p. e0158194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogosnitzky M and Branch S, Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals, 2016. 29(3): p. 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hijnen NM, et al. , The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. J Ther Ultrasound, 2013. 1: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron P, et al. , In vivo T2 -based MR thermometry in adipose tissue layers for high-intensity focused ultrasound near-field monitoring. Magn Reson Med, 2014. 72(4): p. 1057–64. [DOI] [PubMed] [Google Scholar]
- 18.Graham SJ, Bronskill MJ, and Henkelman RM, Time and temperature dependence of MR parameters during thermal coagulation of ex vivo rabbit muscle. Magn Reson Med, 1998. 39(2): p. 198–203. [DOI] [PubMed] [Google Scholar]
- 19.Ozhinsky E, et al. , T2-based temperature monitoring in bone marrow for MR-guided focused ultrasound. J Ther Ultrasound, 2016. 4: p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozhinsky E, et al. , T2-based temperature monitoring in abdominal fat during MR-guided focused ultrasound treatment of patients with uterine fibroids. J Ther Ultrasound, 2015. 3: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hectors SJ, et al. , MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: Current status and future needs. Magn Reson Med, 2016. 75(1): p. 302–17. [DOI] [PubMed] [Google Scholar]
- 22.Wijlemans JW, et al. , Evolution of the ablation region after magnetic resonance-guided high-intensity focused ultrasound ablation in a Vx2 tumor model. Invest Radiol, 2013. 48(6): p. 381–6. [DOI] [PubMed] [Google Scholar]
- 23.Fite BZ, et al. , Magnetic resonance imaging assessment of effective ablated volume following high intensity focused ultrasound. PLoS One, 2015. 10(3): p. e0120037. [DOI] [PMC free article] [PubMed] [Google Scholar]


