Abstract
Context
Underweight, overweight and obesity are important global public health issues and risk factors for adverse perinatal outcomes.
Objective
To assess the distribution of the body mass index (BMI) in the Romanian obstetric population in the first trimester of pregnancy and its correlation with pregnancy outcomes. We also report the distribution of blood pressure (BP) parameters and their correlation with BMI.
Design
This retrospective study includes 9,064 women attending routine first trimester visit and ultrasound scan at 12.8(±0.6) gestational weeks. Characteristics, parity, method of conception, blood pressure (from 3,650 women), maternal weight and height, BMI and foetal ultrasound were recorded. Pregnancy outcomes were available for 1,607 deliveries. The Pearson correlation coefficient was assessed for each BMI group vs. blood pressure parameters, gestational age and birth weight. ANOVA analysis and post hoc tests were used to determine group differences. Linear regression was applied to estimate the contribution of BMI and gestational age to birth weight variance.
Results
In our population, 66.37% pregnant women had a normal BMI, 19.29% were overweight, and 7.56% were obese. There was a weak-to-medium positive correlation between BMI and blood pressure parameters, for all weight categories. The correlation between maternal BMI and birth weight was positive for normal and overweight.
Conclusions
Our findings highlight the need for more effective health strategies targeting reduction of weight-related problems in women of childbearing age.
Keywords: pregnancy, BMI, obesity, overweight, underweight, maternal age, blood pressure, mean arterial pressure
INTRODUCTION
Maternal underweight, overweight and obesity are being recognised globally as an important public health issue (1, 2). In Europe, 44.7% of the adult women (18 years or older) are either overweight or obese (3) and this has important implications during the reproductive years for their obstetric care. Maternal overweight and obesity are recognised risk factors for pregnancy complications like hypertensive disorders, gestational diabetes, stillbirth, infections and thromboembolic disease (4-7). Obesity is associated with an increased rate of instrumental deliveries and caesarean sections (8). Babies delivered by obese mothers are at risk for macrosomia, birth defects, stillbirth, intensive care admission and have a higher rate of perinatal death as reported in both clinical (4, 9, 10) and fundamental research studies (11-13). On the other hand, being underweight before pregnancy also increases the risk of adverse perinatal outcomes such as preterm birth and low birth weight (2).
There are great differences between studies reporting on the distribution of body mass index (BMI) categories during pregnancy. These differences are not only attributable to the socio-demographic difference of the studied populations, but also to the method of recording maternal BMI. This varies between studies from self-reporting to accurate measurement of weight and height before or during different gestation periods in pregnancy.
The distribution of BMI categories has not been reported in the Romanian obstetric population until now. The aim of this study was to assess the prevalence of underweight, overweight and obesity in the Romanian obstetric population in the first trimester of pregnancy using the BMI calculated from accurate measurement of weight and height at 11–13 weeks of gestation as proposed by Syngelaki et al. (4). We also report the distribution of blood pressure (BP) parameters at 11-13 weeks of pregnancy and the correlations between BMI & pregnancy outcomes and BMI & BP parameters.
MATERIAL AND METHODS
Study population
This was a retrospective study including 9,239 pregnant women attending for their routine first trimester visit and ultrasound scan between October 2009 and May 2018 at Filantropia Clinical Hospital, Bucharest, Romania. The study population was comprised mostly of young, white Caucasian women that conceived pregnancy spontaneously or assisted (in vitro fertilization (IVF) and intracellular sperm injection (ICSI)). The first trimester routine visit, which was held between 11+0 to 13+6 weeks of gestation, included recording of demographic characteristics, previous medical and obstetric history, parity, method of conception, measurement of maternal weight and height and calculation of BMI, measurement of the systolic blood pressure (SBP) and diastolic blood pressure (DBP) and an ultrasound examination for the measurement of the foetal crown-rump length to determine gestational age, measurement of the foetal nuchal translucency thickness as part of the screening for aneuploidies (14) and examination of the foetal anatomy for the diagnosis of major foetal defects (15) according to local protocols (16, 17). Data on pregnancy outcomes (gestational age at birth, birth weight, mode of delivery and Apgar scores) was collected from birth registries. This study was approved by the Filantropia Clinical Hospital Ethics Committee and all women were informed and gave written consent for their collected data to be used for research purpose.
Weight was recorded using a validated electronic scale and blood pressure was measured with a validated machine with the women in a sitting position with the arms supported, using a normal (22-32 cm) or large (33-42 cm) adult cuff depending on the mid-arm circumference of the patient. After rest for five minutes, two measurements of the SBP and DBP were taken from each arm simultaneously. We followed this protocol for blood pressure measurement as part of the first and third trimester screening for preeclampsia (18, 19) offered to pregnant women presenting to our hospital and to assess for chronic hypertension, which has important implications for the pregnancy outcome (20).
Out of the 9,239 viable pregnancies attending the first-trimester routine visit for an ultrasound scan in our hospital, we excluded 175 multiple pregnancies and the final study population consisted of 9,064 singleton pregnancies. Blood pressure measurements were available in only 3,650 patients. Six women reported to have been diagnosed with chronic hypertension before pregnancy. A flowchart with the algorithm for the selection of the study population is presented in Figure 1.
Figure 1.
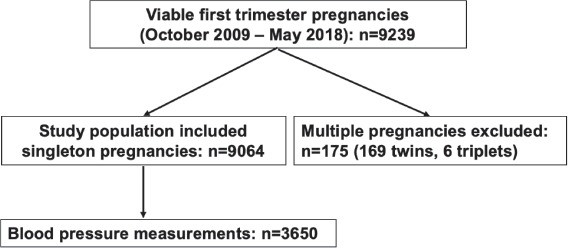
Algorithm of patient selection for the study.
Statistical analysis
The statistical analysis was performed using SPSS version 25.0 statistical software package and Jamovi software, version 1 (39, 40). Descriptive results are presented as mean and standard deviation or as percentage. Body mass index was calculated as weight in kilograms divided by height in meters squared and was classified in the following categories: normal BMI (NW, 18.5-24.9), underweight (UW, <18.5), overweight (OW, 25.0-29.9), and obesity (O) class I (30.0-34.9), class II (35.0-39.9), and class III (≥40) according to the definition of the World Health Organisation (WHO). In the underweight category, those with a BMI <16 were considered of severe thinness, between 16 and 16.99 of moderate thinness and between 17 and 18.49 of mild thinness. Mean arterial pressure (MAP) was calculated using the following formula: MAP = (2 * DBP + SBP)/ 3.
Correlation analysis was used to assess the strength and direction of the relationship between BMI and SBP, DBP & MAP. BMI association with pregnancy outcomes (such as gestational age at delivery and birth weight) was also tested.
Considering the normal distribution of our data, Pearson product-moment correlation coefficient, r, was calculated as a measure of the linear relationship between BMI and each of the parameters aforementioned. ANOVA analysis and post-hoc tests were run using the same software packages, SPSS and Jamovi (39-42), in order to determine significant differences between data groups. Additionally, linear regression analysis was applied using BMI and gestational age at birth to estimate the contribution of these two factors as birth weight predictors (39-41).
RESULTS
Maternal characteristics
The maternal characteristics for our study population consisting in 9,064 singleton pregnancies are presented as mean(±SD), with sample size as percentage, and include the following: mean maternal age - 30.1(±4.6) years, gestational age at study entry - 12.8(±0.6) weeks. A more detailed description of all maternal characteristics we considered is presented in Table 1, where additional information is provided on BMI values (four main subgroups: underweight, normal weight, overweight and obese, the first and the latter subgroups being subdivided into three classes each), blood pressure parameters (SBP 115.9 (9.7), DBP 72.7 (58.16) and MAP 87.1 (7.95)), parity (nulliparous (48.60%), primipara (40.37%), para-two (9.33%), para-three or more (1.67%)) and conception methods (spontaneous (97.88%), in vitro fertilization (IVF) or intracellular sperm injection (1.35%), ovulation induction without IVF (0.76%)).
Table 1.
Descriptive statistics of the study population. Sample size is expressed in % and all other values as mean (±SD)
| Included singleton viable pregnancies (Oct. 2009 – May 2018) n= 9064 | |
|---|---|
| Maternal age, years | 30.1 (±4.6) |
| Gestational age at inclusion, weeks | 12.8 (±0.6) |
| Mean crown-rump length, mm | 68.29 (±8.08) |
| Mean nuchal translucency, mm | 1.89 (±0.49) |
| Maternal weight, kg | 63.26 (±12.09) |
| Maternal height, cm | 164.77 (±6.08) |
| Maternal BMI, kg/m2 | 23.3 (±4.16) |
| BMI categories, kg/m2 (n, %) | |
| < 18.5 (underweight) | 613 (6.76 %) |
| Severe thinness < 16 | 16 (0.17 %) |
| Moderate thinness 16 – 16.99 | 100 (1.10 %) |
| Mild thinness 17 – 18.49 | 497 (5.48 %) |
| 18.5 – 24.99 (normal weight) | 6016 (66.37 %) |
| 25 – 29.99 (overweight) | 1749 (19.26 %) |
| > 30 (obese) | 686 (7.56 %) |
| Obese class I 30.00 - 34.99 | 540 (5.95 %) |
| Obese class II 35.00 - 39.99 | 117 (1.26 %) |
| Obese class III ≥ 40 | 29 (0.31 %) |
| Blood pressure in mmHg n = 3650 | |
| Systolic | 115.9 (±9.7) |
| Diastolic | 72.7 (±8.16) |
| Mean arterial pressure | 87.1 (±7.95) |
| Parity (n, %) | |
| Nulliparous | 4406 (48.60 %) |
| Primipara | 3660 (40.37 %) |
| Para-two | 846 (9.33 %) |
| Para three or more | 152 (1.67 %) |
| Conception (n, %) | |
| Spontaneous | 8872 (97.88 %) |
| IVF/ICSI | 123 (1.35 %) |
| Ovulation induction w/o IVF | 69 (0.76 %) |
SD – standard deviation; BMI – body mass index; kg – kilograms; cm – centimetres; m2 – squared meters; IVF – in vitro fertilization; ICSI – intracellular sperm injection.
Body mass index
In our population 6,016 (66.37%) pregnant women had a normal BMI, however, 2,435 (26.86%) were either overweight or obese (Table 1). Obesity class I according to the WHO classification was recorded in 5.95% of the population and class II in 1.26%, while morbid obesity (class III) was found in only 0.31%. On the other hand, 613 (6.76%) women were found to be underweight, with 0.17% of extreme thinness, 1.10% of moderate thinness and 5.48% of mild thinness (Table 1). That is, in total 33.63% of all pregnancies examined were either underweight, overweight or obese. The mean maternal age of women with normal weight was significantly lower than that of obese and overweight women (29.9 ± 4.45 vs. 30.8 ± 4.72, years, p<0.001).
Blood pressure parameters
Basic features such as means and standard deviations for BMI, SBP, DBP and MAP of our data set (divided into underweight, normal weight, overweight and obese groups) are provided in Table 2. The ANOVA comparison and Tukey post-hoc tests of the blood pressure parameters between weight categories showed that mean SBP, DBP and MAP were significantly different (p<0.001) between all blood pressure measurements for all BMI groups. All three parameters follow the same ascending trend from underweight to obese categories.
Table 2.
Descriptive statistics of all BMI groups. p-values of the ANOVA comparison of blood pressure parameters between BMI groups and subclasses are shown BMI - body mass index; SBP - systolic blood pressure; DBP- diastolic blood pressure; MAP - mean arterial pressure.
| Severe thinness | Moderate thinness | Mild thinness | Underweight (all subgroups) | Normal weight | Overweight | Obese (all subgroups) | Obese class I | Obese class II | Obese class III | p-value between BMI categories | |
| n = 6 | n = 40 | n = 203 | n = 249 | n = 2339 | n = 747 | n = 315 | n = 245 | n = 57 | n = 13 | ||
| BMI | |||||||||||
| Mean (±SD) | 15.4 (0.778) | 16.6 (0.275) | 17.9 (0.402) | 17.6 (0.716) | 21.7 (1.72) | 26.9 (1.37) | 33.3 (3.01) | 32 (1.42) | 37 (1.43) | 42.1 (1.99) | <0.001 |
| SBP | |||||||||||
| Mean (±SD) | 105 (9.39) | 109 (8.27) | 111 (8.52) | 110 (8.54) | 114 (8.82) | 119 (9.59) | 123 (10.8) | 123 (10.8) | 123 (9.62) | 131 (14) | <0.001 |
| DBP | |||||||||||
| Mean (±SD) | 67.3 (6.36) | 70.3 (5.19) | 69.7 (7.77) | 69.8 (7.38) | 71.7 (7.72) | 74.8 (8.06) | 77.1 (9.41) | 77.1 (9.11) | 76 (10.2) | 82.1 (10.4) | <0.001 |
| MAP | |||||||||||
| Mean (±SD) | 79.8 (7.18) | 83.2 (5.54) | 83.4 (7.14) | 83.3 (6.91) | 86 (7.33) | 89.7 (7.92) | 92.6 (0.08) | 92.5 (8.87) | 91.6 (9.34) | 98.3 (10.6) | <0.001 |
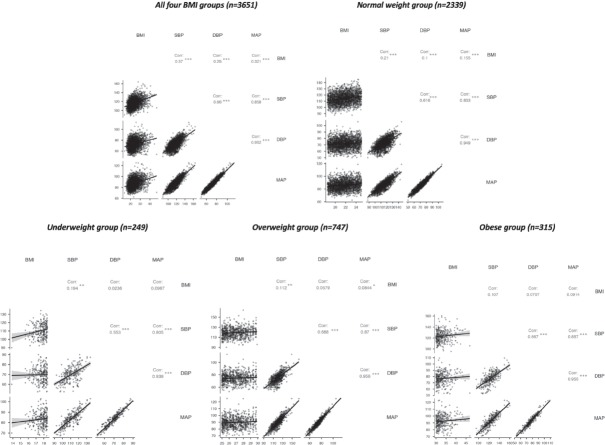
Pearson product-moment correlation coefficient, r, was estimated between blood pressure parameters and BMI for the whole pregnant population of 3,651 participants and for each BMI category. For the entire group of pregnant women, we found r values for BMI and each blood pressure parameter (BMI-BPP) between 0.2 and 0.4 (p < 0.001 for all correlations) suggesting a weak-to-medium positive correlation (Fig. 2). All correlations between blood parameters were significant, positive and medium to strong (r = 0.66 for SBP-DBP; r = 0.952 for DBP-MAP, p < 0.001). Yet, as this is the result for the entire cohort of 3,651 participants, further tests were conducted on each individual BMI category. Outcomes for all four subgroups revealed a rather weak positive correlation between BMI and any blood parameter, while all correlations between SBP, DBP and MAP remained significant (p < 0.001) and fairly stable. The normal weight group had BMI-BPP correlation coefficients slightly modest, between 0.1 and 0.2, but still significant (p < 0.001) (Fig. 2). The BMI-SBP correlation coefficient was roughly the same for the underweight category as the normal weight group (r = 0.194, p < 0.01), but the BMI-DBP and BMI-MAP coefficients were insignificant and smaller. The BMI-SBP and BMI-MAP correlation coefficients obtained for the OW group were even smaller than those of NW & UW groups but significant (r = 0.11, p < 0.01 and r = 0.0844, p < 0.05). Statistical significance was completely lost for all BMI correlations in the O group. This aspect suggests that the relationship between BMI and blood parameters could depend on perturbating factors linked to weight increase (5). Overall, the strongest association was BMI-SBP for all groups.
Figure 2.
Correlation graphs for BMI, SBP, DBP and MAP. Top: correlation matrix for the entire pregnant population (left) and normal weight subgroup (right); Bottom: correlation matrix for the underweight (left), overweight (centre) and obese (right) groups; Note * p<0.05, ** p<0.01, *** p<0.001. BMI - body mass index; SBP - systolic blood pressure; DBP- diastolic blood pressure; MAP - mean arterial pressure.
Pregnancy outcomes
Pregnancy outcomes (gestational age at delivery, birth weight, Apgar scores and mode of delivery) were available for 1,607 singleton live births. Data characteristics are presented in Table 3. Mean gestational age, birth weight and both Apgar scores were not statistically different between BMI groups.
Table 3.
Pregnancy outcomes according to BMI category. Gestational age at birth (years), birth weight (grams) are expressed as mean (±SD) or sample size (%)
| Underweight | Normal weight | Overweight | Obese | |
| n = 98 | n = 1061 | n = 331 | n = 117 | |
| BMI | 17.5 (±0.855) | 21.7 (±1.72) | 26.9 (±1.36) | 33 (±2.64) |
| Gestational age (years) | 39 (±1.7) | 39 (±1.6) | 39 (±2) | 39 (±2.2) |
| Birth weight (grams) | 3177 (±522) | 3277 (±492) | 3296 (±591) | 3355 (±488) |
| Apgar score at 1’ | 8.43 (±1.1) | 8.54 (±0.9) | 8.47 (±0.86) | 8.33 (1±.11) |
| Apgar score at 5’ | 9.09 (±0.774) | 9.07 (±0.965) | 8.98 (±1.25) | 9.01 (±0.704) |
| Delivery mode (n, %) | ||||
| -Vaginal | 59 (59.00 %) | 520 (49.10 %) | 152 (45.92 %) | 62 (52.99 %) |
| -Caesarean | 37 (37.00 %) | 507 (47.87 %) | 168 (50.75 %) | 52 (44.44 %) |
| -Instrumental | 4 (4.00 %) | 32 (3.02 %) | 11 (3.32 %) | 3 (2.56 %) |
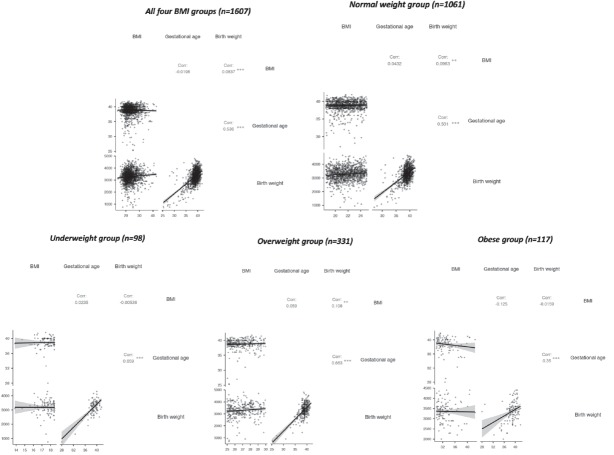
The same correlation analysis was applied to test the linearity of the relationship between BMI and pregnancy outcomes (gestational age and birth weight). The correlation coefficients obtained were fairly weak (r < 0.11) and with varying directions depending on the BMI category. The pooled groups results (Fig. 3) show a significant positive correlation between BMI and birth weight, but quite weak (r = 0.0837, p < 0.001). On the other hand, the strength and direction of the correlation between gestational age and BMI proved to be weaker, negative and not statistically significant (r = -0.01). Individual group tests were applied again. No negative correlations were discovered for the NW and OW groups (Fig. 3). Both weight categories had similar correlation strengths, though only BMI-BW was significant (NW: r = 0.0963, p < 0.01; OW: r = 0.108, p < 0.01). The UW and O groups had no statistically significant BMI related correlations. Moreover, BMI-BW coefficient was negative for both weight groups, while BMI-GA was negative only for the O group. Gestational age at delivery and birth weight correlations were always positive, moderately strong and significant (p < 0.001).
Figure 3.
Correlation graphs for BMI, gestational age and birth weight. Top: correlation matrix for the entire pregnant population (left) and normal weight subgroup (right); Bottom: correlation matrix for the underweight (left), overweight (centre) and obese (right) groups; Note * p<0.05, ** p<0.01, *** p<0.001. BMI - body mass index.
Linear regression results presented in Table 4 showed that BMI and gestational age can explain approximately 30% of the variance in birth weight (p< 0.001). There were two models used to estimate the variance in birth weight: 1) gestational age only and 2) BMI & gestational age (p< 0.001). Surprisingly, BMI adds only 1.8% towards the overall percentage of explained variance in birth weight, compared to gestational age which accounts for 28.7% (p< 0.001).
Table 4.
Linear regression results. Model 1: gestational age vs. birth weight. Model 2: BMI & gestational age vs. birth weight
| Model Fit Measures | |||||||
| Overall Model Test | |||||||
| Model | R | R² | Adjusted R² | F | df1 | df2 | p |
| 1 | 0.536 | 0.287 | 0.287 | 647 | 1 | 1605 | < .001 |
| 2 | 0.555 | 0.308 | 0.305 | 102 | 7 | 1599 | < .001 |
| Model Comparisons | ||||||
| Comparison | ||||||
| Model | Model | ΔR² | F | df1 | df2 | p |
| 1 | 2 | 0.0205 | 7.91 | 6 | 1599 | < .001 |
Model 1.
Gestational age vs. birth weight
| Omnibus ANOVA Test | |||||
| Sum of Squares | df | Mean Square | F | p | |
| Gestational age | 1.23e+8 | 1 | 1.23e+8 | 647 | < .001 |
| Residuals | 3.05e+8 | 1605 | 189754 | ||
| Model Coefficients | ||||
| Predictor | Estimate | SE | t | p |
| Intercept | -2923 | 244.08 | -12.0 | < .001 |
| Gestational age | 160 | 6.29 | 25.4 | < .001 |
Model 2.
BMI & gestational age vs. birth weight
| Omnibus ANOVA Test | |||||
| Sum of Squares | df | Mean Square | F | p | |
| Gestational age | 6.14e+7 | 1 | 6.14e+7 | 331.7 | < .001 |
| BMI category | 6.20e+6 | 3 | 2.07e+6 | 11.2 | < .001 |
| Gestational age * BMI category | 6.02e+6 | 3 | 2.01e+6 | 10.9 | < .001 |
| Residuals | 2.96e+8 | 1599 | 184977 |
| Model Coefficients | ||||
| Predictor | Estimate | SE | t | p |
| Intercept | -2882.5 | 338.81 | -8.51 | < .001 |
| Gestational age | 158.7 | 8.71 | 18.21 | < .001 |
| BMI category: | ||||
| overweight – normal w. | -1694.1 | 566.37 | -2.99 | 0.003 |
| obese – normal w. | 2991.5 | 787.72 | 3.80 | < .001 |
| underweight – normal w. | -1974.7 | 1049.86 | -1.88 | 0.060 |
| Gestational age * BMI category: | ||||
| Gestational age * (overweight – normal w.) | 44.7 | 14.60 | 3.06 | 0.002 |
| Gestational age * (obese – normal w.) | -74.9 | 20.31 | -3.69 | < .001 |
| Gestational age * (underweight – normal w.) | 47.7 | 26.95 | 1.77 | 0.077 |
DISCUSSION
This was a retrospective study on a large Romanian obstetric population in which the BMI was derived from accurate measurement of weight and height at 11-13 weeks of pregnancy. The study reports that 66.37% of the obstetric population in the first trimester had a normal weight, while 19.29%, 7.56% and 6.76% were overweight, obese and underweight, respectively. An abnormal BMI was frequent in the Romanian obstetric population in the first trimester of pregnancy with one in three women presenting for obstetric care being either underweight, overweight or obese. Our results are consistent with other studies that report data from our European region and reflect the socio-economic conditions of this area. In a study from Bulgaria on pregnant population within the first trimester, the prevalence of underweight was 12.5% and the one for overweight or obese was 23.3% (24). In Turkey, 27.2% of pregnant women at their first antenatal visit were reported overweight or obese and 7.9% were underweight (25). These figures are different from data reported from highly industrialized countries like UK and USA, where obesity rates are higher and the general percentage of underweight is much lower. For example, in a retrospective study conducted between 2004–2011 with 30,298 participants in the obstetric population in Great Britain, only 2.8% of women entering pregnancy were found to be underweight, 52.5% were normal, 27.8% were overweight and 17% were obese (26). In the USA, a study from the Washington state on 743,630 pregnant women in their first trimester reported also that only 3.2% were underweight, 47.5% had a normal weight, while 49% were either overweight or obese (overweight, 25.8%; obesity class I, 13.1%; obesity class II, 6.2%; and obesity class III, 4.2%) (27).
Another important finding of our study is that the average maternal age at the beginning of a pregnancy was 30.1 years. Although our data relate to an urban tertiary referral centre, we believe that similar trends are likely to exist in other maternity health care settings in the country and reflect the generalised tendency to postpone pregnancy for later age (28).
Several studies have examined the effects of maternal weight on blood pressure levels during different periods of normal pregnancy. In our study increasing BMI was associated, as expected, with increasing MAP in the first trimester. The correlation coefficients we obtained are showing that there is a weak-to-medium positive correlation between BMI and any of the three blood pressure parameters considered. After testing these results for significance, p<0.001, we concluded that there is a moderate linear positive correlation between BMI and SBP, DBP, MAP for the 3,651 cohort of pregnant women. Overall, for this large population, it appears that the BMI can only explain around 10% (r²) of the variance of the blood pressure measurements. However, the association of the aforementioned parameters seems to be slightly modest for the normal weight group but still significant, while for the underweight and overweight groups, the correlation coefficient decreases but loses significance for the BMI-DBP & BMI-MAP cases (underweight) and BMI-DBP (overweight), respectively. The obese group presented no significant association between BMI and any blood pressure parameter. These results suggest that the abnormality of the BMI produces an imbalance in the body making any relationship between physiological parameters more difficult to model and estimate. Such a problem becomes even more difficult in the case of a weak linear relationship between physiological parameters, such as BMI and blood pressure parameters. Being overweight, obese or morbidly obese is associated with higher SBP and DBP during pregnancy and consequently there are increased risks of gestational hypertensive disorders (29-33). Obesity is a known independent risk factor for cardiovascular disease and hypertension is one of the most common obesity related complications (34).
As far as the association between BMI and pregnancy outcomes is concerned, there is no direct relationship discovered between BMI category and gestational age or birth weight, as pointed out by the weak correlation coefficients we reported. Our conclusion regarding this aspect is that birth weight is less likely to be highly affected by BMI only, but a combination between BMI and other parameters (e.g. gestational age) may play a more important role for the pregnancy outcomes as shown by the results of the linear regression analysis, where gestational age accounts for 28.7% of the variance in birth weight.
There are three main conclusions to draw: 1) pregnancy evolution is directly dependent on the socio-economic factors as shown by our study and other related studies undergone within the same geographical area, 2) BMI does influence blood pressure parameters and to a smaller extent the pregnancy outcomes, but more investigations are required to fully understand the degree of its influence, and 3) gestational age, as expected, is a predictor for birth weight. We are also aware that there might be other physiological parameters and confounders that require attention, this being one of our primary aims for future studies. However, the nature of the relationship between BMI and all the parameters mentioned above must be further investigated to better understand the factors that can influence the evolution of pregnancy.
Strengths and limitations of this study
The major strengths of our study are: (1) it includes a large cohort of Romanian obstetric population, (2) it provides a BMI calculation that was derived from accurate measurement of weight and height, rather than from patient-self reporting, which can be inaccurate (35), and (3) it provides measurements taken at a specific time interval during pregnancy, between 11 to 13 weeks, rather than at a larger span, which could make results difficult to interpret.
An important limitation of this study is that accurate measurements of weight and height could not be obtained from an earlier time in pregnancy as it could be arguable that at the end of the first trimester there could already be some pregnancy related weight gain. Other limitations are that we did not have a reliable recording of smoking behaviour and previous medical and obstetrical history for all the participating patients. Also, the related pregnancy outcomes recorded were only available for 1,607 women of our cohort and did not include adverse events.
Our findings highlight that underweight, overweight and obesity are important public health issues for our population. One in three pregnant women was either underweight, overweight or obese. Regarding the relationship with blood pressure values, BMI does significantly affect these parameters and therefore it should be considered as a marker to monitor and detect abnormal weight and consequently to early prevent pregnant women about associated risk factors. A lower or higher BMI than normal increases the risk of adverse maternal and perinatal outcomes. High BMI is associated with increased rates of caesarean section, higher maternal morbidity, neonatal morbidity, neonatal intensive care utilisation and length of hospital stay, while lower BMI is associated with a high risk of preterm birth and a low birth weight (4-7). Moreover, gestational age showed its value in predicting birth weight, hence more efforts should be invested into identifying new predictors in order to better estimate birth weight. All these aspects have important implications for the cost of health care delivery. Thus, routine collection of data on maternal BMI and gestational age can provide valuable information for resource planning for obstetric and neonatal facilities.
In conclusion, there is a strong need for better implementation of strategies aimed at reducing the prevalence of underweight, overweight or obesity in women at reproductive age in the Romanian population. As they enter their reproductive age and plan pregnancy, women should be counselled by primary care physicians not only to avoid alcohol, smoking or teratogenic drugs (36), but also on the importance of weight management (37, 38) and the impact of BMI on pregnancy outcomes.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Gregg EW, Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377(1):80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 2.Han Z, Mulla S, Beyene J, Liao G, McDonald SD, Knowledge Synthesis Group Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40(1):65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 3.Overweight and obesity – BMI statistics Eurostat. Accessed 26th of May 2018. http://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_and_obesity_-_BMI_statistics.
- 4.Syngelaki A, Bredaki FE, Vaikousi E, Maiz N, Nicolaides KH. Body mass index at 11-13 weeks’ gestation and pregnancy complications. Fetal Diagn Ther. 2011;30(4):250–265. doi: 10.1159/000328083. [DOI] [PubMed] [Google Scholar]
- 5.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obesity. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 6.Panaitescu AM, Peltecu G, Gestational Diabetes Obstetrical Perspective. Acta Endo (Buc) 2016;12(3):331–334. doi: 10.4183/aeb.2016.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 9.ACOG Practice Bulletin No 156 Obesity in Pregnancy. Obstet Gynecol. 2015 Dec;126(6):e112–126. doi: 10.1097/AOG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 10.Mc Donald SD, Han Z, Mulla S, Beyene J. Knowledge Synthesis Group: Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isac S, Panaitescu AM, Spataru A, Iesanu M, Totan A, Udriste A, Cucu N, Peltecu G, Zagrean L, Zagrean AM. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neurosci Lett. 2017;653:308–313. doi: 10.1016/j.neulet.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Isac S, Panaitescu AM, Iesanu M, Grigoras IF, Totan A, Udriste A, Cucu N, Peltecu G, Zagrean L, Zagrean AM. Maternal high-fat diet modifies the immature hippocampus vulnerability to perinatal asphyxia in rats. Neonatology. 2018 doi: 10.1159/000491383. [DOI] [PubMed] [Google Scholar]
- 13.Panaitescu AM, Isac S, Pavel B, Ilie AS, Ceanga M, Totan A, Zagrean L, Peltecu G, Zagrean AM. Oxytocin reduces seizure burden and hippocampal injury in a rat model of perinatal asphyxia. Acta Endo (Buc) 2018;14(3):315–319. doi: 10.4183/aeb.2018.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijders RJ, Noble P, Sebire NJ, Souka AP, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10–14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998;352:343–346. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]
- 15.Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides KH. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11-13 weeks. Prenat Diagn. 2011;31(1):90–102. doi: 10.1002/pd.2642. [DOI] [PubMed] [Google Scholar]
- 16.Veduta A, Vayna AM, Duta S, Panaitescu A, Popescu F, Bari M, Peltecu G, Nedelea F. The first trimester combined test for aneuploidies - a single center experience. J Matern Fetal Neonatal Med. 2018;31(16):2091–2096. doi: 10.1080/14767058.2017.1336220. [DOI] [PubMed] [Google Scholar]
- 17.Vayna AM, Veduta A, Duta S, Panaitescu AM, Stoica S, Buinoiu N, Nedelea F, Peltecu G. Diagnosis of Fetal Structural Anomalies at 11 to 14 Weeks. J Ultrasound Med. 2018 doi: 10.1002/jum.14561. [DOI] [PubMed] [Google Scholar]
- 18.Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11-13 weeks’ gestation. Fetal Diagn Ther. 2012;31(1):42–48. doi: 10.1159/000335366. [DOI] [PubMed] [Google Scholar]
- 19.Panaitescu AM, Wright D, Militello A, Akolekar R, Nicolaides KH. Proposed clinical management of pregnancies after combined screening for pre-eclampsia at 35-37 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;50(3):383–387. doi: 10.1002/uog.17419. [DOI] [PubMed] [Google Scholar]
- 20.Panaitescu AM, Akolekar R, Kametas N, Syngelaki A, Nicolaides KH. Impaired placentation in women with chronic hypertension who develop pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50(4):496–500. doi: 10.1002/uog.17517. [DOI] [PubMed] [Google Scholar]
- 21. Table of Critical Values: Pearson Correlation http://www.statisticssolutions.com/table-of-critical-values-pearson-correlation/
- 22.Critical Values of the Spearman’s Ranked Correlation Coefficient. http://webspace.ship.edu/pgmarr/Geo441/Tables/Spearman%20Ranked%20Correlation%20Table.pdf.
- 23.Hauke Jan, Kossowski Tomasz. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaestiones geographicae. 2011;30(2):87–93. [Google Scholar]
- 24.Kamburova M, Hristova P, Georgieva S, Khan A. Adverse effects of maternal age, weight and smoking during pregnancy in Pleven, Bulgaria. South Eastern European Journal of Public Health. 2015;4(1) doi: 10.4119/UNIBI/SEEJPH-2015-11. [DOI] [Google Scholar]
- 25.Daşıkan Z, Kavlak O. Maternal obesity: pregnancy complications and management of pregnant women: review. Turkiye Clin. J. Nurs. Sci. 2009;1:39–46. [Google Scholar]
- 26.Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG. 2013;120(8):932–939. doi: 10.1111/1471-0528.12193. [DOI] [PubMed] [Google Scholar]
- 27.Lisonkova S, Muraca GM, Potts J, Liauw J, Chan WS, Skoll A, Lim KI. Association Between Prepregnancy Body Mass Index and Severe Maternal Morbidity. JAMA. 2017;318(18):1777–1786. doi: 10.1001/jama.2017.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod. 2011;17:848–860. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo D, Poon LC, Fernandez M, Wright D, Nicolaides KH. Prediction of preeclampsia by mean arterial pressure at 11-13 and 20-24 weeks’ gestation. Fetal Diagn Ther. 2014;36(1):28–37. doi: 10.1159/000360287. [DOI] [PubMed] [Google Scholar]
- 30.Panaitescu AM, Baschat AA, Akolekar R, Syngelaki A, Nicolaides KH. Association of chronic hypertension with birth of small-for-gestational-age neonate. Ultrasound Obstet Gynecol. 2017;50(3):361–366. doi: 10.1002/uog.17553. [DOI] [PubMed] [Google Scholar]
- 31.Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol. 2007;21:487–494. doi: 10.1111/j.1365-3016.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 32.Helmreich RJ, Hundley V, Varvel P. The effect of obesity on heart rate (heart period) and physiologic parameters during pregnancy. Biol Res Nurs. 2008;10:63–78. doi: 10.1177/1099800408321077. [DOI] [PubMed] [Google Scholar]
- 33.Thompson ML, Williams MA, Miller RS. Modelling the association of blood pressure during pregnancy with gestational age and body mass index. Paediatr Perinat Epidemiol. 2009;23:254–263. doi: 10.1111/j.1365-3016.2009.01027.x. [DOI] [PubMed] [Google Scholar]
- 34.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:969–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 35.Fattah C, Farah N, O’Toole F, Barry S, Stuart B, Turner MJ. Body Mass Index (BMI) in women booking for antenatal care: comparison between self-reported and digital measurements. Eur J Obstet Gynecol Reprod Biol. 2009;144(1):32–34. doi: 10.1016/j.ejogrb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Le Duc D, Spataru A, Ceanga M, Zagrean L, Schöneberg T, Toescu EC, Zagrean AM. Developmental exposure to ethanol increases the neuronal vulnerability to oxygen-glucose deprivation in cerebellar granule cell cultures. Brain Res. 2015;1614:1–13. doi: 10.1016/j.brainres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Roman G, Bala C, Creteanu G, Graur M, Morosanu M, Amorin P, Pîrcalaboiu L, Radulian G, Timar R, Achimas Cadariu A. Obesity and Health-Related Lifestyle Factors in the General Population in Romania: a Cross Sectional Study. Acta Endo (Buc) 2015;11(1):64–72. [Google Scholar]
- 38.Mocanu V. Overweight, Obesity and Dieting Attitudes Among College Students in Romania. Acta Endo (Buc) 2013;9(2):241–248. [Google Scholar]
- 39. The Jamovi project (2019). Jamovi. (Version 0.9) [Computer Software] Retrieved from https://www.jamovi.org.
- 40. R Core Team (2018). R: A Language and environment for statistical computing. [Computer Software]. Retrieved from https://cran.r-project.org.
- 41.Fox J., Weisberg S. (2018). car: Companion to Applied Regression. [R package]. Retrieved from https://cran.r-project.org/package=car.
- 42.Lenth R. (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. [R package]. Retrieved from https://cran.r-project.org/package=emmeans.