Abstract
Objective
A partial or complete deficiency of hormone secretion by pituitary gland (hypopituitarism) is commonly seen after a pituitary apoplexy caused by an infarction of a pituitary adenoma or pituitary hyperplasia (as in Sheehan’s syndrome). Hypopituitarism may also follow surgery, when hypovolemia, anticoagulation, fat/air/bone marrow microemboli can provoke a pituitary infarction/hemorrhage. Other causes of abrupt hypophyseal hypoperfusion, as hypovolemia during a septic shock, could also contribute. In the last mentioned situation, due to the complex endocrine-immune interrelation, sepsis could be masked and improperly managed.
Case report
We report a case of a 72 years-old Caucasian woman, previously healthy, who underwent an orthopedic surgery for a femoral fracture. This event apparently triggered a central-origin hypothyroidism, misinterpreted as “post-surgical psychosis”, which, in turn, masked a symptomatology of a subsequent severe sepsis. The patient was admitted in the infectious diseases department with a severe gut-origin sepsis, needing surgery and long course antibiotics. The pituitary insufficiency was reversed.
Conclusion
Pituitary apoplexy is an uncommon but potentially life-threatening disease, and could be precipitated by successive events – in our case an orthopedic surgery and a subsequent severe sepsis. It needs recognizing (has intrinsic severity and could mask other serious conditions), treat and monitor (could progress and/or reverse).
Keywords: surgery, sepsis, central hypothyroidism
INTRODUCTION
Hypopituitarism is defined as a partial or complete deficiency of hormones produced by the anterior or posterior hypophysis cerebri. The reported incidence is about 4/100000/year among the general population. About 50% of patients have three to five pituitary hormones deficiency (1). Although the most common cause of hypopituitarism in adult population is pituitary adenoma, which causes hormone deficits either by direct compression and subsequent destruction of the normal surrounding tissue or by adenoma’s infarction, a number of hypopituitarism cases appear after a pituitary apoplexy of a normal hypophysis, as usually seen in Sheehan’s syndrome (2, 3), but not exclusively. Any event that produces hemorrhage or infarction of the pineal gland can cause a pituitary apoplexy (4).
A pituitary apoplexy may rarely be caused by surgery. There have been postulated several pathophysiological mechanisms for pituitary apoplexy after surgery: a sudden drop of arterial blood pressure that induces ischemia, followed by infarction of the pituitary gland, which can further be followed by a second hemorrhage. There are patients that present only ischemic infarction without hemorrhage, while others only primary hemorrhage. The general consensus is that the pituitary apoplexy after surgery is caused by a multitude of factors that include perioperative hypotension related to anesthesia or hypovolemia, anticoagulation, and microemboli (fat, air, bone marrow or cement) (5-8).
Despite the fact that pituitary apoplexy classically appears as an overt clinical syndrome at the moment of its occurrence (intense and sudden headache, associated with visual impairment and a rapid and dramatic evolution towards an altered state of consciousness in severe cases) (9-11), and only in around 25% of cases can be relatively silent as a “subclinical pituitary apoplexy”(4), in most patients it remains undiagnosed and the endocrine insufficiencies, which develop gradually, commonly in months or even years (9,12,13), reveal the diagnosis. It could also appear as a cascade of events: the first one can have minimal or no clinical consequences; but the next event could unmask the insufficiencies.
The most frequent findings are thyrotropin (TSH) and gonadotropins (LH and FSH) deficiencies in about 50% and 75% of patients, respectively (9,12,13). Hormonal deficiencies are usually persistent, but there are few studies that suggest a reversibility which depends on the duration between the symptoms onset and the moment of diagnosis (9,10,14).
We illustrate with a case how a series of two subsequent events – an orthopedic surgery and a severe sepsis three months later – could cause and aggravate a central-origin hypothyroidism.
MATERIALS AND METHODS
There is a case report of a central hypothyroidism, probably secondary to a pituitary infarction provoked by an orthopedic surgery and diagnosed during a septic shock that occurred three months later. The hormones were measured by CLIA method, the normal values being 0.35-5.50 μIU/mL for serum TSH, 0.89-1.76 ng/dL for free thyroxine (fT4) and 0.60-1.81 ng/mL for triiodothyronine (T3). The measurement of troponin – we measured troponin I, with normal values of 0-0.16 ng/mL. C-reactive protein (CRP) is measured in mg/L, with normal values < 3. The proBNP normal values: 0-125ng/L. The other measurements were performed using universally routine laboratory tests and the results were discussed in comparison with normal values: as normal, as slightly elevated/decreasing (when there is an elevation, decreasing, respectively, smaller than 1.5 times of normal values) or, when elevation/decreasing is higher than 1.5 times, we specify either the value itself or how many times that value raised above normal. Normal values for routine lab tests used in the case: hemogram - leukocytes: 3.9-9.6^103/μL, haemoglobin 12.1-17.2g/dL, platelets 200-400^103/μl; ESR women: upper limit = (age+10)/2; fibrinogen 200-400mg/dl; prothrombin concentration 80-115%; D-dimers 50-230ng/mL; Na 137- 145 mmol/L, K 3.6-5 mmol/L; LDH 313- 618 U/L; Creatine Kinase (CK) 30-135 U/L, CK-MB 30-135 U/L; Gamma Glutamyl Transferase (GGT) 12-43 U/L; direct bilirubin 0.0-0.4 mg/dL, indirect bilirubin 0.0- 1.10 mg/dL.
CASE REPORT
August-November
A 72 years-old active woman, with unremarkable medical history and in a good general health, fell down from a chair in August, breaking her left femur and underwent an orthopedic surgery in a regional hospital, with open reduction and internal fixation (femoral nails). After surgery, during the next three months, she had developed dyspnea, anxiety, depression and asthenia - interpreted as “post-traumatic psychosis”. She had also developed abdominal pain in both epigastric and hypogastric regions, nausea, vomiting and several episodes of urinary tract infections with E coli and Enterobacter spp, for which she received antibiotics – ciprofloxacin and cephalosporins.
November-December
Three months after surgery, at the beginning of November, she was sent to an infectious diseases (ID) clinic (National Institute for Infectious Diseases) with a diagnosis of septic shock, presenting fever >39°C, 60mmHg systolic blood pressure (BP) and being confused. A surgical consult and an abdominal ultrasound exam were performed before departure and they ruled out a surgical abdomen, although the imagist indicated a possible cholecystitis.
At admission in ID clinic, after 5-6 hours ride to the hospital by ambulance, with oxygen supplementation and vasoactive support (dopamine), she had an altered general state, was confused, extremely asthenic, with pale, dry, cold skin, no fever, complaining of chills, abdominal pain, nausea and vomiting. However, BP at admission was 120/80 mmHg, therefore vasoactive support was stopped. She had tachycardia (100bpm) with regular beats. Abdominal exam found tenderness in the superior quadrants and hypogastric area but no localized or generalized guarding. Lab tests showed mild leukocytosis (13^103/mm3) with neutrophilia (90%), anemia (Hb=9.8g/dL), a platelet count of 154^103/mm3, ESR=60mm/h, fibrinogen=410mg/dL (with 85% prothrombin concentration and D-dimers 10-fold higher), severe hypokalemia (2 mmol/L), with a normal sodium level (137 mmol/L), cholestasis (GGT=221U/L, direct bilirubin=1.8 mg/dL), elevated AST (=78U/L, ×2 normal value), normal ALT=32U/L, elevated LDH=1117U/L and CK=190U/L, with normal CK-MB=4U/L, important elevation of pro-BNP (=9003 ng/L) and a very high level of troponin I=3.5 ng/mL. Two sets of blood cultures were taken at admission.
There were no dynamic changes on ECG. The troponin level increased in the next hours (4.52ng/mL, then 5.67ng/mL), so we decided to transfer her to the cardiology department, with the presumed diagnosis of acute arterial ischemia (coronary? mesenteric?).
Cardiology ward (Emergency Hospital “Floreasca”): Cardiac evaluation consisting of ECG, heart ultrasound and serum analyses failed to find a major acute cardiac pathology; however, the transthoracic echocardiography showed a small immobile mass on the mitral valve, with a preserved cardiac function and normal myocardial contractility; CRP was elevated = 192 mg/dL. Chest CT scan showed bilateral alveolar infiltrate, minimum pleural effusion, multiple splenic infarctions and important vesicular sludge. The case was interpreted as sepsis with respiratory involvement - severe bronchopneumonia and intravenous antibiotics were started - imipenem/cilastatin and vancomycin.
In the next 72 hours she developed arrhythmias and dyspnea and was transferred in ICU; AST, and in a lesser extent ALT, rapidly increased up to 30-fold higher (AST=1665U/L and ALT=814U/L) and became quasi-normal during the next days. The treatment in ICU was not specified in the retransfer notes, but we assumed that antibiotics were maintained, along with vasoactive and respiratory support; we do not know if she received a short course of corticotherapy or not. She was discharged from the ICU after three days. Because of persistent abdominal pain along with the imagery findings, a new surgery consult was performed which again ruled out an acute surgical abdomen. Antibiotics were maintained and after a total of nine days, she was considered stable and was transferred back to our ID clinic. In the meantime, the results of both blood cultures taken in the first day were positive for Enterobacter cloacae ESBL-positive (ESBL: extended spectrum beta-lactamases).
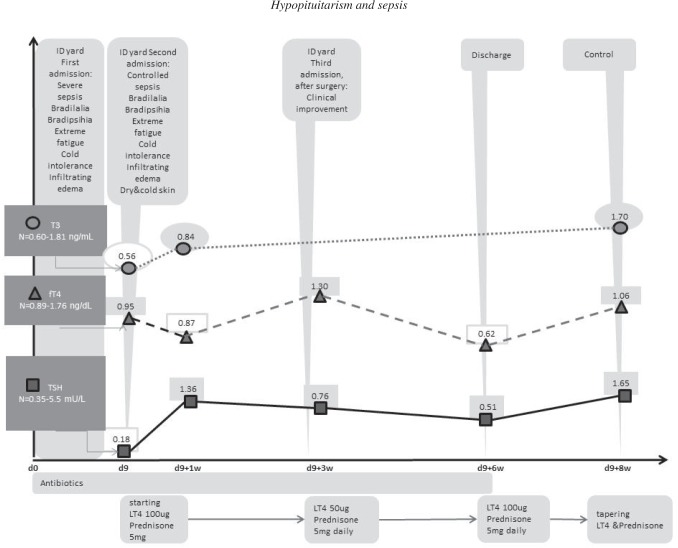
In the ID admission room - she presented bradylalia, intense fatigue, infiltrating edema, cold and dry skin and she complained of intense cold – reminding us of the clinical picture from her first admission, when these symptoms had been considered to be part of the exhaustion (the long ride with the ambulance) and sickness itself. These, in the light of the new findings, made us think about a severe thyroid insufficiency and thyroid hormones were subsequently tested. We found a low TSH level of 0.18 uIU/mL, with normal-low fT4=0.95 ng/dL and low T3=0.56 ng/mL. Facing a central hypothyroidism, a cranial CT scan targeting pituitary region was performed and showed partially empty sella (Figs 1-3). We continued antibiotics with imipenem/cilastatin and provided hormonal substitution of thyroidal and also adrenal axis: levothyroxine (LT4), 100 μg/day and prednisone, 5 mg/day, respectively. We did not certify adrenal or gonadal insufficiency (FSH/LH). In the next 10 days her condition improved but abdominal pain persisted; the lab tests showed a very elevated GGT (=1248 U/L), with a slight elevation of pancreatic and liver enzymes; a new abdominal ultrasound was done and the vesicular sludge and also the splenic infarctions (described on CT scan) were reinterpreted in the context of positive blood-culture with an intestinal Gram-negative rod, as probable pus or abscesses – in the gallbladder and in the spleen, respectively. We asked for a third surgery consult and the abdomen was finally opened, revealing important amount of pus in the gallbladder and multiple splenic abscesses. Cholecystectomy and splenectomy were performed. A second transthoracic echocardiography was done – and revealed a rupture of chordae tendineae of anterior mitral valve, with severe mitral insufficiency and normal-sized cardiac cavities. After readmission in the ID clinic, the TSH and fT4 measurements registered improvements: TSH=0.76 uIU/mL, fT4=1.30 ng/dL, so we lowered the LT4 dose to 50 μg/day, but, at discharge, after another three weeks, when TSH was 0.51 uIU/mL and fT4=0.62 ng/dL, we increased the dosage of LT4 again to 100 μg/day; at the follow-up (2 weeks later), the levels of TSH (=1.65 uIU/mL), fT4 (=1.06 ng/dL) and T3 (1.70 ng/mL) were normal, so the hormonal replacements (LT4 and Prednisone) were gradually stopped, as seen in the graph (Fig. 3).
Figure 1.
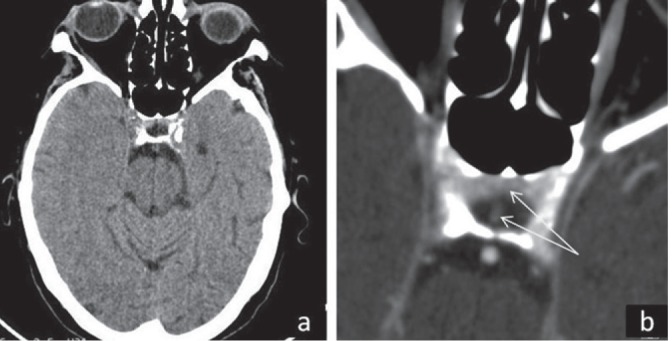
Cranial CT scan with contrast: partial empty sella: (a) transverse section – overview; (b) detail of sella turcica with white arrows showing a pituitary rest in anterior part of sella and pituitary stalk.
Figure 3.
The evolution after first admission (day zero = d0) in the ID (infectious diseases) clinic, three months after the orthopedic surgery; the values of T3 (circles), fT4 (triangles) and TSH (squares) were figured at the moments of day 9 (d9) when they were first checked, then after one week (d9+1w), three weeks (d9+3W), six weeks (d9+6w) and eight weeks (d9+8w), respectively – time is figured on the abscissa and the hormonal values on the ordinate.
Figure 2.
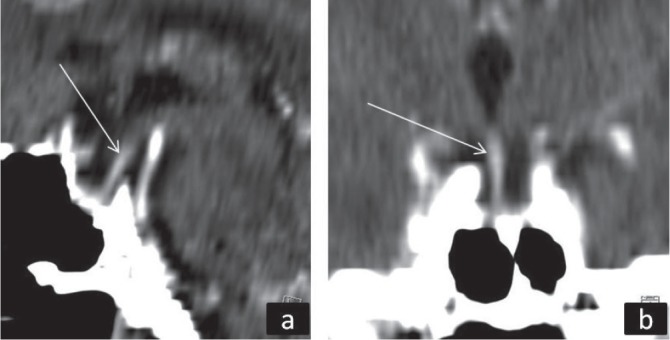
Cranial CT scan with contrast: (a) sagittal section, (b), coronal section of sella turcica with pituitary stalk which extends to the bottom of sella (arrows).
DISCUSSION
The hypothyroidism of central origin in our patient was clinically and biologically diagnosed during the severe sepsis, three months after the orthopedic surgery. But she could have been retrospectively diagnosed as having hypothyroidism by clinical criteria sooner, after the surgical procedure, when she developed bradylalia, anxiety, depression, slowed mental function, cold-intolerance misinterpreted as “post-traumatic psychosis”. Her family reported that she was a very active person before the operation. We cannot rule out a previous pituitary impairment – like Sheehan syndrome or a silent pituitary adenoma clinically visible after surgery, but this seems improbable: she breastfed her daughter, she had a normal menopause onset and her sella turcica was not radiologically enlarged. On the contrary, the CT scan showed a partial empty sella, that could be preexistent or, most probably, a consequence of ischemia during the orthopedic surgery, three months prior.
Thus, we consider the orthopedic surgery as the primary event, being a procedure a priori known to be accompanied by a large blood loss that could lead to ischemia by hypoperfusion, and/or association of other factors, as intraoperative or postoperative hypotension related to anesthesia, anticoagulation and/or fat/air/bone marrow microemboli (3,5-7).
A second pituitary hypoperfusion probably took place during the septic shock, three months after the first event and further decompensated the hypophysis.
The main differential diagnosis in our case was between central hypothyroidism and euthyroid sick syndrome which can also appear during a severe septic shock.
The euthyroid sick syndrome (or nonthyroidal illness syndrome) does not have specific clinical findings and consists of a multitude of important cytokinic alterations in the hypothalamic-pituitary-thyroid axis which causes changes in the patient’s thyroid hormonal profile. This syndrome can appear in patients with sepsis and a history of trauma or major surgery. The most common type of hormonal imbalance syndrome consists of low total and free T3 levels with normal T4 and TSH, but it could go towards a low T3-T4, high T4 level or even low TSH levels. (15-17).
In opposition, central hypothyroidism is a clinical disease secondary to a functional or an anatomic disorder of the hypothalamus or pituitary gland – in our case pituitary gland is presumed to be involved. It is a consequence of low circulating TSH levels. The positive diagnosis of the acquired form of the central hypothyroidism consists of: suggestive history (risk factors, including head traumas, surgery, vascular accidents), clinical signs of a hypothyroid state, alterations in the hypothalamus/pituitary imaging and, in the absence of any interference, a low FT4 combined with an abnormally low TSH (18).
Thus, being a clinically manifested hypothyroidism which seemed to be preexistent to the severe sepsis, persisting despite of sepsis control, with a long period of TSH and thyroid hormones fluctuations between low or normal-low values, our diagnosis is strongly in favor of a central hypothyroidism, as a consequence of a subclinical pituitary apoplexy caused by orthopedic surgery, which gradually manifested in the next months as a mild thyroidal insufficiency and which was abruptly revealed during the sepsis. The severe sepsis might represent the second event that affected the pineal gland (by hypovolemia or by embolic septic foci during bacteremia) or might be the condition for a superposed euthyroid sick syndrome. In both situations, the sepsis aggravated the presumed preexistent central hypothyroidism.
Hormone deficiencies have been reported to become clinically relevant several months after the apoplexy. The most common findings are TSH and gonadotropins (LH and FSH) deficiencies in about 50% and 75% of patients, respectively (9,12,13). As it happened with our patient, the clinical evolution can be confusing, misinterpreted as neurological or psychiatric pathology, exposing the patients to complications due to delayed or wrong diagnosis.
Patients with combined pituitary deficits have a reduced life expectancy when compared to the general population. This is due to cardiovascular and cerebrovascular disease, but can also be caused by infectious diseases arising from gaps of normal host defenses (19-21). On the other hand, sepsis can lead to endocrine dysfunction or accentuate a pre-existing one –as probably was the case in our patient. This is usually a transient event, but it is associated with a poor prognosis (22).
Our patient was admitted in the ID department with severe gut-origin sepsis (pyocholecystitis) with ESBL-positive E cloacae with endocardial involvement (mitral endocarditis) and secondary splenic abscesses who required surgical intervention. Endocardial involvement (endocarditis)could appear anytime during the last three months, with alleviations when she was receiving antibiotics in short cures. Regarding sepsis - we could more clearly appreciate its appearance - as in less than two days before admission. The preexistent hypopituitarism masked the clinical features of sepsis, making it even more difficult to diagnose. Moreover, depression of thyroidal axis, is, in turn, exacerbated by the severe sepsis. At the readmission moment (in the ID clinic), when the infection was controled, her clinical status was obviously hypothiroidian (very bradilalic, extremely asthenic, with cold, dry and infiltrated skin, slightly edematous), she had a very low TSH level and also normal-low values of T3 and fT4, these findings being highly suggestive for a preexistent hypothyroidism. We speculate that if we had taken the hormonal values at the moment of her first admission, we would have found even lower values, because in 10 days of appropriate antibiotherapy, she had a partial recovery most probably due to sepsis correction.
When hypopituitarism occurs, hormone replacement treatment can be necessary. Even with correct replacement treatment, pituitary function should be reassessed periodically, because both remission of existing deficiencies or development of new ones has been reported (23-25). In our patient there was a restauration of TSH production that permitted us to cease the hormonal replacement therapy. Even so, the period between the beginning of hormonal replacement and the recovery was around two months (Fig 4). As reported in the literature, in her case it could be a connection between recovery and the short period from the symptoms onset (24,25), if we take into account that the first event that happened three months prior and the second trigger for her insufficiencies (endocarditis and sepsis with E cloacae) that happened more recently, were relatively rapid corrected.
In conclusion, in our severely ill patient with partial empty sella and potentially reduced thyrotrope reserve due to a post-surgical pituitary infarction, the superposed infectious-related events - pituitary new damage/euthyroid sick syndrome contributed to a clinical picture of overt secondary hypothyroidism, which masked the sepsis symptoms.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Prabhakar VK, Shalet SM. Aetiology, diagnosis, and management of hypopituitarism in adult life. Postgrad Med J. 2006;82:259–266. doi: 10.1136/pgmj.2005.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bills DC, Meyer FB, Laws ER, Jr., Davis DH, Ebersold MJ, Scheithauer BW, Ilstrup DM, Abboud CF. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33(4):602–609. doi: 10.1227/00006123-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZH, Tu PH, Pai PC, Chen NY, Lee ST, Chuang CC. Predisposing factors of pituitary hemorrhage. European Journal of Neurology. 2012;19(5):733–738. doi: 10.1111/j.1468-1331.2011.03619.x. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, Lanyon M, Markey A, Plant G, Powell M, Sinha S, Wass J. UK guidelines for the management of pituitary apoplexy. Pituitary Apoplexy Guidelines Development Group: May 2010. Clinical Endocrinology. 2011;74:9–20. doi: 10.1111/j.1365-2265.2010.03913.x. [DOI] [PubMed] [Google Scholar]
- 5.Khandelwal M, Chhabra A, Krishnan S. Pituitary apoplexy following bilateral total knee arthroplasty. J Postgrad Med. 2005;51:155–156. [PubMed] [Google Scholar]
- 6.Goel V, Debnath UK, Singh J, Brydon HL. Pituitary apoplexy after joint arthroplasty. J Arthroplasty. 2009;24:826–829. doi: 10.1016/j.arth.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Thomason K, Macleod K, Eyres KS. Hyponatraemia after orthopaedic surgery - a case of pituitary apoplexy. Ann R Coll Surg Engl. 2009;91:W3–5. doi: 10.1308/147870809X400912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennon M, Seigne P, Cunningham AJ. Pituitary apoplexy after spinal anaesthesia. Br J Anaesth. 1998;81:616–618. doi: 10.1093/bja/81.4.616. [DOI] [PubMed] [Google Scholar]
- 9.Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJL. Acute management of pituitary apoplexy—surgery or conservative management? Clinical Endocrinology. 2004;61(6):747–752. doi: 10.1111/j.1365-2265.2004.02162.x. [DOI] [PubMed] [Google Scholar]
- 10.Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy: presentation, surgical management, and outcome in 21 patients. Neurosurgery. 1990;26(6):980–986. [PubMed] [Google Scholar]
- 11.Johnston PC, Hamrahian AH, Weil RJ, Kennedy L. Pituitary tumor apoplexy. Journal of Clinical Neuroscience. 2015;n22(6):939–944. doi: 10.1016/j.jocn.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Sibal L, Ball SG, Connolly V, James RA, Kane P, Kelly WF, Kendall-Taylor P, Mathias D, Perros P, Quinton R, Vaidya B. Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary. 2004;7(3):157–163. doi: 10.1007/s11102-005-1050-3. [DOI] [PubMed] [Google Scholar]
- 13.Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CBT, Wass JAH. Classical pituitary apoplexy: clinical features, management and outcome. Clinical Endocrinology. 1999;51(2):181–188. doi: 10.1046/j.1365-2265.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 14.Karagiannis AKA, Dimitropoulou F, Papatheodorou A, Lyra S, Seretis A, Vryonidou A. Pituitary abscess: a case report and review of the literature. Endocrinol Diabetes Metab Case Rep. 2016;2016:160014. doi: 10.1530/EDM-16-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutch M, Kumar S, Gupta KK. Prognostic value of thyroid profile in critical care condition. Indian J Endocrinol Metab. 2018;22(3):387–391. doi: 10.4103/ijem.IJEM_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbas T, Sahin IE, Ozturk A. Alterations in thyroid hormones in brain-dead patients are related to non-thyroidal illness syndrome. Endokrynol Pol. 2018;69(5):545–549. doi: 10.5603/EP.a2018.0056. [DOI] [PubMed] [Google Scholar]
- 17.Cho EB, Min JH, Cho HJ, Seok JM, Lee HL, Shin HY, Lee KH, Kim BJ. Low T3 syndrome in neuromyelitis optica spectrum disorder: Associations with disease activity and disability. J. Neurol. Sci. 2016;370:214–218. doi: 10.1016/j.jns.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Persani L. Central Hypothyroidism: Pathogenic diagnostic, and Therapeutic Challenges. The Journal of Clinical Endocrinology & Metabolism. 2012;97(9):3068–3078. doi: 10.1210/jc.2012-1616. [DOI] [PubMed] [Google Scholar]
- 19.Bates AS, Van’t Hoff W, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81:1169–1172. doi: 10.1210/jcem.81.3.8772595. [DOI] [PubMed] [Google Scholar]
- 20.Rosen T, Bengtsson B. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 21.Sharma MD, Sagar B, Wang S, White AC, Jr, Balasubramanyam A. High frequency of serious infections in patients with panhypopituitarism: a case-control study. Clin Infect Dis. 2001;32(1):153–158. doi: 10.1086/317533. [DOI] [PubMed] [Google Scholar]
- 22.Gheorghiţă V, Barbu AE, Gheorghiu ML, Căruntu FA. Endocrine dysfunction in sepsis: a beneficial or deleterious host response? Germs. 2015;5(1):17–25. doi: 10.11599/germs.2015.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Archives of Endocrinology and Metabolism. 2015;59(3):259–264. doi: 10.1590/2359-3997000000047. [DOI] [PubMed] [Google Scholar]
- 24.Walia R, Bhansali A, Dutta P, Shanmugasundar G, Mukherjee KK, Upreti V, Das A. An uncommon cause of recurrent pyogenic meningitis: pituitary abscess. BMJ Case Reports. 2010 doi: 10.1136/bcr.06.2009.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin WS, Xu WG, Yin ZN, Li HM, Li J, Zhang XP, Wang GL. Endocrine dysfunction and follow-up outcomes in patients with pituitary abscess. Endocrine Practice. 2015;21:339–347. doi: 10.4158/EP14457.OR. [DOI] [PubMed] [Google Scholar]