Abstract
Background
To evaluate the protective effect of Nigella sativa oil (NSO) on the myocardium in streptozotocin-induced diabetic rats.
Materials and methods
Thirty-two 7–8-week-old female Wistar albino rats (300–350 g) were equally divided into 4 groups: nondiabetic untreated animals (control), diabetes mellitus (DM), NSO, and DM+NSO groups. For the induction of diabetes, 45 mg/kg streptozotocin was applied to the rats in the DM and DM+NSO groups as a single intraperitoneal dose. NSO (400 mg/kg) was orally administered through an intragastric catheter once a day over 21 days. Formalin-fixed, paraffin-embedded tissue sections of the myocardium were evaluated histopathologically and immunohistochemically.
Results
Compared to the control, NSO, and DM+NSO groups, the myocardial tissue samples from the rats in the DM group had significantly higher myositis, hyaline degeneration, and Zenker’s necrosis. Moreover, the Bcl-2 expressions were significantly higher in the control, NSO, and DM+NSO groups than in the DM group.
Conclusion
NSO has a protective effect on the myocardium of streptozotocin-induced diabetic rats, most likely via suppressing apoptosis.
Keywords: Nigella sativa oil, diabetes mellitus, Bcl-2, apoptosis, myocardium
INTRODUCTION
Diabetes mellitus (DM) is one of the most common chronic diseases affecting the general population, with an increasing prevalence worldwide (1). The end-organ damage, including retinopathy, nephropathy, neuropathy, ischemic heart disease, stroke, and peripheral vascular disease, is the main cause of morbidity and mortality in uncontrolled diabetes. Hyperglycemia-induced oxidative stress plays a central role in the pathogenesis of organ damage in diabetes (1). The pathogenesis of organ damage involves many factors that cause reactive oxygen species accumulation in tissues, which in turn promotes cellular dysfunction and ultimately apoptosis (1, 2). It has been shown that apoptotic changes caused by hyperglycemia-induced oxidative stress are responsible for tubular kidney damage and pancreatic beta-cell loss in diabetes (3, 4). Therefore, in addition to controlling blood glucose level, protective measures against oxidative stress and apoptosis would delay the progression of diabetic tissue complications.
Nigella sativa is a flowering plant from the Ranunculaceae family mostly found in southwest Asia and the Middle East. Its seeds and oil have been commonly used in traditional herbal medicine to treat many diseases. In experimental and clinical studies, antimicrobial, antioxidant, anti-inflammatory, antitumor, antihypertensive, antihyperlipidemic, and antidiabetic effects have been shown (5–7). The pharmacological properties of Nigella sativa are attributed to thymoquinone, the major bioactive component of the essential oil, and its antioxidant effects (8, 9).
Bcl-2 is a member of the B-cell lymphoma (Bcl) protein family, which regulates apoptosis in cells. The overexpression of Bcl-2 enhances cell survival by suppressing apoptosis (10). It has been suggested that oxidative stress induces tissue damage in diabetic models by stimulating apoptosis (11, 12). Since oxidative stress and reactive oxygen species (which result in apoptosis) are known as the major causes of tissue damage induced by DM, we suggest that Nigella sativa oil (NSO) may play a protective role against tissue damage in diabetes by its antioxidant and antiapoptotic action.
Therefore, in this study, we aim to evaluate the protective effect of NSO on the myocardium in streptozotocin-induced diabetic rats. We also investigate the role of cell survival on the myocardial tissue.
MATERIALS AND METHODS
Study design and animals
Thirty-two 7–8-week-old female Wistar albino rats (300–350 g) (obtained from Van Yuzuncu Yil University Experimental Animal Unit) were used for the study. The rats were randomly divided into the following 4 groups, each containing 8 rats: nondiabetic untreated animals (control group), a DM group, a NSO group, and a DM+NSO group. Before the experiment, all of the rats were acclimatized to the environment for 3 weeks, during which time they were kept in cages at room temperature of 22±2ºC with a 12/12 h light/dark cycle and fed ad libitum.
Ethics Statement
This study was performed in strict accordance with the recommendations of the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs). The experimental protocol was approved by the Committee on the Ethics of Animal Experiments at Ataturk University (Permit Number: 46-02.03/2014).
Experimental procedure
The blood glucose levels of all rats were measured before the experiment with biosensor glucose measurement device and strips (Clever Chek-TD-4222). For induction of diabetes, 45 mg/kg streptozotocin (Sigma Chemical Co., St. Louis Missouri, USA) was dissolved in a cold citrate buffer at pH 4.5 and applied to the rats in the DM and DM+NSO groups as a single intraperitoneal dose on day 1 of the study.
Blood glucose levels were measured in blood samples obtained from the tail veins of the rats at 72 hours after the injection of streptozotocin. Rats with blood glucose levels over 250 mg/dL were diagnosed as type 1 DM.
For the rats in the NSO and DM+NSO groups, 400 mg/kg NSO was orally administered once a day over 21 days (13). Then, the myocardium of all the rats was formalin-fixed and paraffin-embedded for histopathological and immunohistochemical investigations.
Histopathological assessment
Tissue sections of 4 μm thickness were taken from each block and prepared on the slides, which were then stained with hematoxylin and eosin (H & E) and evaluated under a light microscope (Nikon Eclipse CI, Amsterdam, Netherlands). The presence and severity of histopathological changes such as myositis, hyaline degeneration, and Zenker’s necrosis were evaluated as none (-), mild (+), moderate (++), and severe (+++).
Immunohistochemistry
Immunohistochemical staining was done on 4 μm tissue sections of formalin-fixed, paraffin-embedded tumor samples. Bcl-2 alpha ab-1 (Richard Allan Scientific, Kalamazoo/USA) was used as the primary antibody. Staining was performed on a Ventana Benchmark XT autostainer with the XT ultraView DAB Kit (Ventana Medical Systems). All slides were counterstained with hematoxylin. To exclude unspecific staining, system controls were included. The immunopositivity for Bcl-2 expression was graded as none (-), mild (+), moderate (++), and severe (+++).
Statistical analysis
Statistical analyses were carried out using SPSS software (Statistical Package for Social Sciences, ver. 13.0, SPSS Inc., Chicago, Illinois, USA). The medians of histopathology and immunohistochemistry scores were reported. The differences in semi-quantitative data between multiple groups were evaluated by a non-parametric Kruskal-Wallis test followed by the Mann-Whitney U test for comparison of the two groups. The level of statistical significance was set to p<0.05.
RESULTS
Histopathology
The median severity grade for histologic lesions on the myocardium of the control, NSO, DM+NSO, and DM groups were 0 (0–1), 0 (0–1), 1 (1–3), and 2 (1–3), respectively (Kruskal-Wallis test, p<0.05). All the pairwise comparisons of the myocardium scores of the groups revealed statistical significance between the DM and DM+NSO groups (Mann-Whitney U test, p<0.05). However, there were no statistically significant differences between the control, NSO, and DM+NSO groups (Mann-Whitney U test, p>0.05).
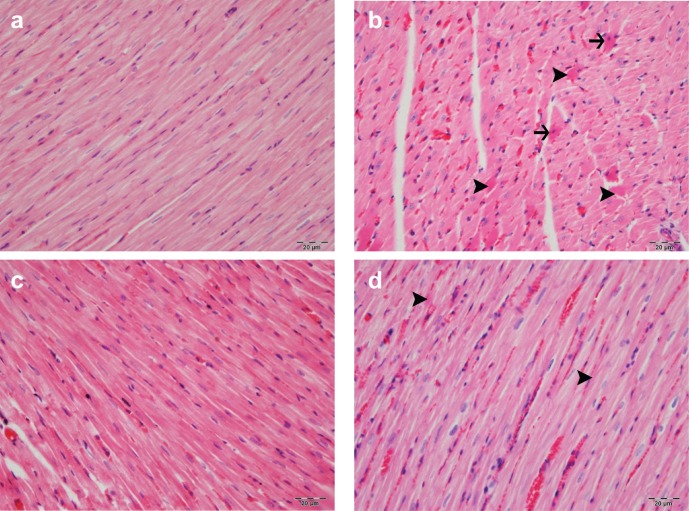
The heart tissue obtained from rats in the control and NSO groups revealed normal histologic structure. In the DM group, however, mild myositis, hyaline degeneration, and extensive Zenker’s necrosis were detected among the myocardial muscle fibers. In the DM+NSO group, hyperemia and hyaline degeneration were observed in a few muscle cells (Fig.1, Table 1).
Figure 1.
(a) Heart tissue of control group had normal histologic structure. (b) In DM, a mild myositis, severely hyaline degeneration (arrow head), and Zenker’s necrosis (arrow) were detected among the myocardial muscle fibers. (c) The heart tissue of NSO group had a normal histologic structure. (d) In DM+NSO group, hyperemia and slight hyaline degeneration were observed in a few muscle cells. (H&E; bar: 20 µm).
Table 1.
Median severity grade for histologic lesions and immunopositivity for Bcl-2 expression in myocardium of rats in study groups
| Control (n=8) | DM (n=8) | NSO (n=8) | DM+NSO (n=8) | ||
|---|---|---|---|---|---|
| Myocardium | Myositis | - | ++ | - | - |
| Hyaline degeneration | - | +++ | - | ++ | |
| Zenker’s necrosis | - | ++ | - | - | |
| Bcl-2 expression | + | + | + | +++ |
Severity for histologic lesions and immunopositivity for Bcl-2 expression were graded as none (-), mild (+), moderate (++), and severe (+++).
Immunohistochemistry
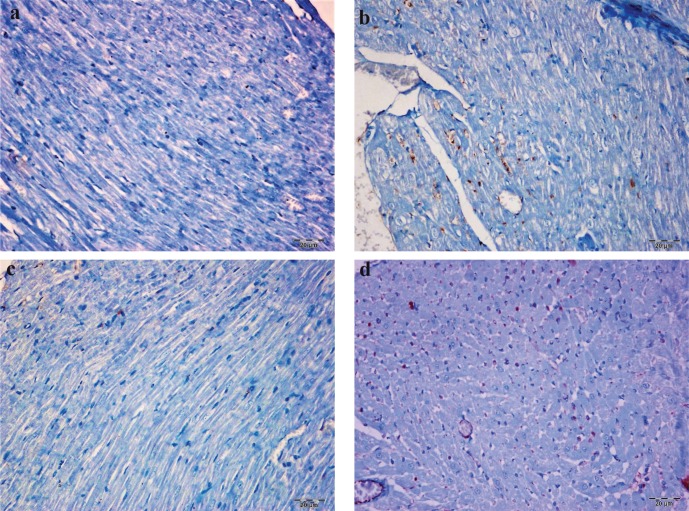
In the immunohistochemical evaluation, the Bcl-2 showed a moderate nuclear staining in all slides. The degree of Bcl-2 expression among myocardial cells was mild in the control and NSO groups, moderate in the DM group, and very high in the DM+NSO group (Fig. 2, Table 1).
Figure 2.
The cytoplasmic Bcl-2 positivity was observed in very few myocardial cells of control group (a) and Nigella sative oil group (c). Cytoplasmic Bcl-2 positivity was at moderate and highest degree in myocardial cells of DM group (b) and DM+NSO group (d), respectively. (IP; bar: 20 µm).
The median Bcl-2 expression in the myocardium of the control, NSO, DM+NSO, and DM groups was 1 (0–2), 1 (0–2), 3 (1–3), and 1 (0–3), respectively (Kruskal-Wallis test, p<0.05). The Bcl-2 expression was significantly higher in the DM+NSO group than in the other groups (Mann-Whitney U test, p<0.05). However, there were no statistically significant differences between the control, NSO, and DM groups (Mann-Whitney U test, p>0.05).
DISCUSSION
In this experimental study, we evaluated the protective effect of NSO against histologic damage to the myocardium caused by DM in a rat model of diabetes and the mechanism by which it exerts this effect. We found that NSO has a protective effect against diabetes-induced damage in the myocardium in streptozotocin-induced diabetic rats. To the best of our knowledge, this study is the first which evaluates the protective effect of NSO on the myocardium.
Since end-organ damage is the main cause of mortality and morbidity in both types 1 and 2 diabetes, it is important to develop effective strategies to protect the organs from damage caused by high blood glucose levels. Although controlling blood glucose level is the most effective strategy for this, other adjuvant therapeutics are needed to further protect the organs.
Several studies suggest a beneficial effect of Nigella sativa on a wide range of medical conditions such as hypertension, chemotherapy-induced nephrotoxicity, liver diseases, neurodegenerative conditions, and brain tumors (8, 14, 15). Although Nigella sativa and its active ingredient thymoquinone can improve the glycemic status and lipid profile in diabetes models (16, 17), studies on its effects on diabetes-induced tissue damage are scarce. Furthermore, the potential molecular mechanism by which NSO or thymoquinone exerts this action in diabetes has been still poorly characterized.
In the present study, there was remarkable histopathological damage, including myositis, hyaline degeneration, and Zenker’s necrosis in the myocardium in DM group. Atta et al. (18) suggest that thymoquinone prevents diabetes-induced testicular damage in rats due to its antioxidant and anti-inflammatory effects and upregulation of aromatase expression. Hamdy and Taha (19) show that NSO and thymoquinone correct neuropathy in streptozotocin-induced diabetic rats through their antioxidant effects. Kanter (20) reported that, in comparison to untreated streptozotocin-induced diabetic rats, thymoquinone treatment reduced the glomerular size, thickening of the capsular, glomerular, and tubular basement membranes, and increased levels of mesangial matrix and tubular dilatation and renal function. Therefore, our findings are in line with recent studies showing the protective effect of NSO and thymoquinone on the tissue damage induced by diabetes.
Several molecular mechanisms have been suggested in the literature by which NSO or thymoquinone exerts its action in diabetes. Karandrea et al. (21) suggest that thymoquinone ameliorates the diabetic phenotype in the diet-induced obesity mouse model of type 2 diabetes via activation of the SIRT1-dependent pathways. Thymoquinone has been suggested to have a protective effect against type 1 diabetes via the nitric oxide inhibitory pathway (22). El-Shemi et al. (23) report that thymoquinone regenerates pancreatic β-cells, ameliorates pancreatic inflammation and oxidative stress, suppresses apoptosis of β-cells, and enhances islet revascularization in streptozotocin-induced diabetic rats. Assaf et al. (24) have also recently suggested that thymoquinone has an inhibitory effect on apoptosis in leukemic cells. Dincel and Yildirim (11) suggest that overexpression of endothelial nitric oxide synthase and inducible nitric oxide synthase induces diabetic nephropathy by mediating apoptosis in streptozotocin-induced rats.
Apoptosis refers to the genetically-controlled morphological process of controlled cellular self-destruction by which an organism eliminates unnecessary cells (25). Bcl is a family of proteins with both proapoptotic and antiapoptotic properties that plays a pivotal role in the life and death of cells (26). The various Bcl proteins control the sensitivity of cells to apoptotic stimuli (27). Bcl-2 belongs to the antiapoptotic member of the Bcl family. It is localized to the outer mitochondrial and endoplasmic reticulum membranes and plays a role on the inhibition of apoptosis in a number of subjects to various apoptosis-inducing stimuli including hyperglycemia (28, 29). Recent studies indicate that de-activation of Bcl-2 along with activation of the proapoptotic Bcl protein family members initiates beta-cell apoptosis in the pancreas and a corresponding reduction in islet insulin content in diabetes (4). Based on the present literature on the antiapoptotic effect of thymoquinone, we hypothesized that NSO protects the myocardium from diabetes-induced damage via its inhibitor action on apoptosis. In order to test this hypothesis, we evaluated the expression of Bcl-2 in the myocardium and found that while the expression of Bcl-2 was low in the control, DM, and NSO groups, a large number of cells in the myocardial tissue of the DM+NSO group had Bcl-2 positivity. The overexpression of Bcl-2 only in the DM+NSO group suggests that NSO prevents myocardial cell damage induced by DM by inhibiting apoptosis.
The main limitation of the present study was the use of NSO itself, which is the phytochemical extract of the plant and contains various ingredients in addition to the active ingredient thymoquinone (5, 30). Although thymoquinone has been suggested to be responsible for the pharmacological activity of NSO, the dosage and safety profile of the active ingredient should be determined for the clinical management of diabetes and its complications. Additionally, we only assessed the expression of Bcl-2, but not other markers of apoptosis. In order to reach a more definitive conclusion on the mediatory action of NSO on apoptosis in diabetes, the expression of other apoptosis and antiapoptosis markers and the tissue levels of oxidative stress markers should be determined in further studies.
In conclusion, NSO has a protective effect on diabetes-induced damage to the myocardium in streptozotocin-induced diabetic rats, most likely via suppressing apoptosis, as indicated by the overexpression of Bcl-2. Therefore, NSO may be a potential biological adjuvant therapy to be used as a remedy to protect the myocardium from diabetes-induced damage, which needs to be confirmed with further clinical studies.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42:698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 3.Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT, Garibotto G, Deferrari G. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol. 2004;15:85–87. doi: 10.1097/01.asn.0000093370.20008.bc. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer S, Prehn JH. The esoteric roles of Bcl-2 family proteins in glucose homeostasis and cell survival. Cell Death Dis. 2015;6:e1968. doi: 10.1038/cddis.2015.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gholamnezhad Z, Havakhah S, Boskabady MH. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J Ethnopharmacol. 2016;190:372–386. doi: 10.1016/j.jep.2016.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin J Nat Med. 2016;14:732–745. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- 7.Sahebkar A, Soranna D, Liu X, Thomopoulos C, Simental-Mendia LE, Derosa G, Maffioli P, Parati G. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J Hypertens. 2016;34:2127–2135. doi: 10.1097/HJH.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 8.Mollazadeh H, Hosseinzadeh H. The protective effect of Nigella sativa against liver injury: a review. Iran J Basic Med Sci. 2014;17:958–966. [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 11.Dincel GC, Yildirim S. Increased expressions of eNOS and iNOS correlate with apoptosis of diabetic nephropathy in streptozotocin-induced type 1 diabetic rats. Kafkas Univ Vet Fak Derg. 2016;22:381–390. [Google Scholar]
- 12.Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017;49:837–844. doi: 10.1007/s11255-016-1488-4. [DOI] [PubMed] [Google Scholar]
- 13.Fararh KM, Atoji Y, Shimizu Y, Takewaki T. Isulinotropic properties of NSO in Streptozotocin plus Nicotinamide diabetic hamster. Res Vet Sci. 2002;73:279–282. doi: 10.1016/s0034-5288(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 14.Cascella M, Palma G, Barbieri A, Bimonte S, Amruthraj NJ, Muzio MR, Del Vecchio V, Rea D, Falco M, Luciano A, Arra C, Cuomo A. Role of Nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: evidences from experimental animal studies. Nutrients. 2017;9:E625. doi: 10.3390/nu9060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmaci I, Altinoz MA. Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomed Pharmacother. 2016;83:635–640. doi: 10.1016/j.biopha.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Complement Ther Med. 2015;23:275–282. doi: 10.1016/j.ctim.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Sangi SM, Sulaiman MI, El-Wahab MF, Ahmedani EI, Ali SS. Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. Pharmacogn Mag. 2015;11:S251–257. doi: 10.4103/0973-1296.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atta MS, Almadaly EA, El-Far AH, Saleh RM, Assar DH, Al Jaouni SK, Mousa SA. Thymoquinone defeats diabetes-ınduced testicular damage in rats targeting antioxidant, ınflammatory and aromatase expression. Int J Mol Sci. 2017;18:E919. doi: 10.3390/ijms18050919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamdy NM, Taha RA. Effects of NSO and thymoquinone on oxidative stress and neuropathy in streptozotocin-induced diabetic rats. Pharmacology. 2009;84:127–134. doi: 10.1159/000234466. [DOI] [PubMed] [Google Scholar]
- 20.Kanter M. Protective effects of thymoquinone on streptozotocin-induced diabetic nephropathy. J Mol Histol. 2009;40:107–115. doi: 10.1007/s10735-009-9220-7. [DOI] [PubMed] [Google Scholar]
- 21.Karandrea S, Yin H, Liang X, Slitt AL, Heart EA. Thymoquinone ameliorates diabetic phenotype in diet-induced obesity mice via activation of SIRT-1-dependent pathways. PLoS One. 2017;12:e0185374. doi: 10.1371/journal.pone.0185374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, El-Sayed M, Takewaki T. Successful abrogation by thymoquinone against induction of diabetes mellitus with streptozotocin via nitric oxide inhibitory mechanism. Int Immunopharmacol. 2005;5:195–207. doi: 10.1016/j.intimp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.El-Shemi AG, Kensara OA, Alsaegh A, Mukhtar MH. Pharmacotherapy with thymoquinone improved pancreatic β-cell integrity and functional activity, enhanced islets revascularization, and alleviated metabolic and hepato-renal disturbances in streptozotocin-induced diabetes in rats. Pharmacology. 2017;101:9–21. doi: 10.1159/000480018. [DOI] [PubMed] [Google Scholar]
- 24.Assaf MD, Semaan J, El-Sabban M, Al-Jaouni SK, Azar R, Kamal MA, Harakeh S. Inhibition of proliferation and induction of apoptosis by thymoquinone via modulation of TGF family, p53, p21 and Bcl-2α in leukemic cells. Anticancer Agents Med Chem. 2017 Sep 12; doi: 10.2174/1871520617666170912133054. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Mallat Z, Tedgui A. Apoptosis in the vasculature: mechanisms and functional importance. Br J Pharmacol. 2000;130:947–62. doi: 10.1038/sj.bjp.0703407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 27.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene. 2001;20:1939–1952. doi: 10.1038/sj.onc.1204288. [DOI] [PubMed] [Google Scholar]
- 29.Hasnan J, Yusof MI, Damitri TD, Faridah AR, Adenan AS, Norbaini TH. Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singapore Med J. 2010;51:50–55. [PubMed] [Google Scholar]
- 30.Akram Khan M, Afzal M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology. 2016;24:67–79. doi: 10.1007/s10787-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]