Abstract
Context
Diabetes insipidus (DI) is rare in the neonatal period but of great importance due to increased renal risk and mental retardation despite treatment.
Objective
This report describes the case of a patient with congenital nephrogenic diabetes insipidus (NDI). Detection of this pathology during the neonatal period, especially in premature newborns, is difficult because of the electrolyte variations that occur as a result of the immature kidney function.
Subjects and methods
The subject was a preterm infant with very low birth weight (VLBW) and persistent hypernatremic hyperosmolarity that developed polyuria and polydipsia in the first weeks of life.
Results
Taking into account blood and urine laboratory tests, vasopressin levels, as well as family history, the infant was diagnosed with congenital NDI. Early treatment allowed a good development, proving that the prevention of long-term complications is possible through multidisciplinary care and frequent monitoring. The particularity of this case was the presence of persistently elevated presepsin levels. This association prompted the investigation into underlying renal hypernatremia.
Conclusions
NDI is a rare condition and the onset in the neonatal period is a sign of severity and hereditary causality. Early diagnosis, symptomatic treatment and multidisciplinary monitoring may decrease the risk of long-term complications.
Keywords: preterm infant, presepsin, nephrogenic diabetes insipidus
INTRODUCTION
Congenital nephrogenic diabetes insipidus (NDI) is a rare condition that associates polyuria and polydipsia as a result of the inability to concentrate urine despite normal or increased levels of antidiuretic hormone (ADH). Undiagnosed and thus untreated, NDI results in growth delay, while seizures caused by hypernatremic dehydration may lead to irreversible brain damage and cognitive deficit (1). However, some studies have shown that early diagnosis and treatment decrease the incidence of neurological damage (1-3). Hypernatremia presented by the preterm infants is most probably caused by dehydration, and it requires prompt and gradual treatment in order to prevent secondary brain damage (4). In our case, a male premature infant born to a hypertensive mother gradually developed renal failure. Following adequate treatment that improved the patient’s clinical status, desmopressin testing confirmed the diagnosis of NDI. Further treatment for the congenital disease assured normal weight and normal neurological examination for corrected age at 10 months of age.
CASE REPORT
A male premature infant of 30 weeks gestational age (GA), weighing 1460 g, appropriate for GA, was delivered by emergency cesarean section because of an abnormal umbilical blood flow, having an Apgar score of 6/7.
Maternal history: 28 years old, primigravida, primipara, smoker and undergoing pre-pregnancy hypertension treatment, developed worsening high blood pressure during pregnancy despite correct treatment. The mother’s grandfather had suffered from an untreated polyuro-polydipsic syndrome during childhood and associated mild intellectual disability and mental instability.
Evolution during hospitalization: at birth the newborn presented cyanosis, secondary apnea, hypotonia and required positive pressure ventilation (PPV) with a fraction of inspired oxygen (FiO2) of 0.4. He was admitted in the neonatal intensive care unit (NICU) and placed in an incubator where he received oxygen by hood with an FiO2 of 40%, total parenteral nutrition (TPN) and prophylactic antibiotics.
From the second day of life (DOL), the infant developed mild abdominal distension and feeding intolerance associating bilious gastric residual volume (GRV). Based on the clinical status, abdominal X-ray appearance and maternal risk factors, intrauterine intestinal damage as an early start of necrotizing enterocolitis was suspected, which is often associated with renal decline. Treatment with low doses of dopamine (3 mcg/kg/min) in order to increase the mesenteric and renal perfusion was administered for eight days, with a favorable evolution. The newborn had lost about 8% of his initial birth weight (BW), regained by the 10th DOL.
During the first three postnatal weeks, laboratory tests revealed persistent hypernatremia (148-161.6 mEq/L), increased plasma osmolality (303.5-328.8 mOsm/kg), hypercalcemia (11.9-14 mg/dL), hyperuremia (81-85 mg/dL) and increased creatinine levels (1.04-1.32 mg/dL) (Fig. 1).
Figure 1.
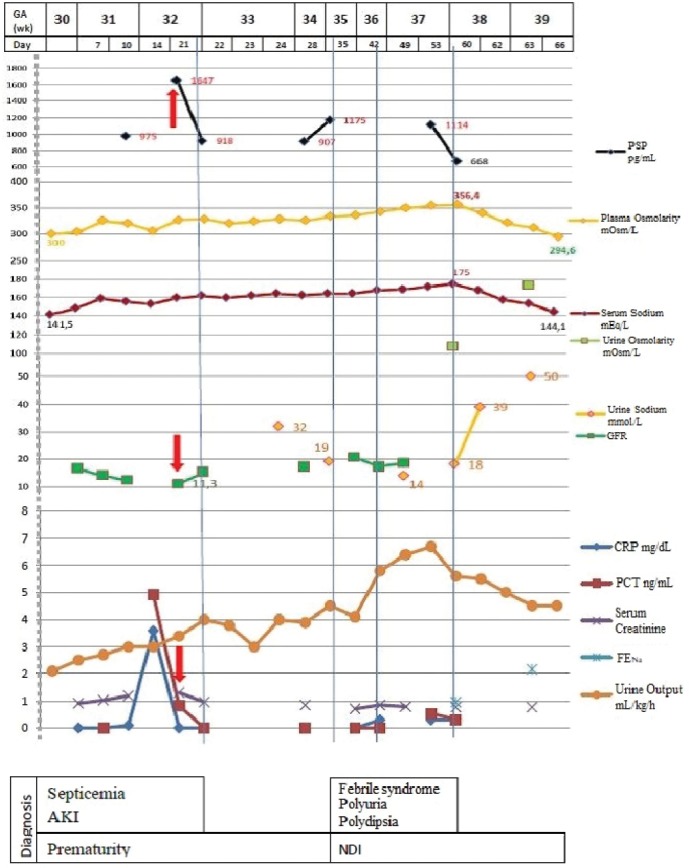
Laboratory tests and possible diagnosis. DI - diabetes insipidus; AKI - acute kidney injury (red arrow); PSP - presepsin; CRP - C reactive protein; PCT - procalcitonin; GFR - glomerular filtration rate; FENa - fractional excretion of sodium.
On the 10th DOL, the patient presented a febrile episode with a temperature of 38.5ºC and no other clinical findings. Laboratory tests revealed an inflammatory status most likely with an infectious origin, with C reactive protein (CRP) levels measuring 3.9 mg/dL, procalcitonin (PCT) 4.9 ng/mL and presepsin (PSP) 975 pg/mL, while creatinine levels rose to 1.2 mg/dL. Considering the clinical context as well as the associated risk factors (NICU patient having a central venous catheter of over one week), sepsis was suspected. Therefore, antibiotic and antipyretic treatment consisting of intravenous Vancomycin and Fluconazole and rectal Paracetamol was initiated.
In the second week of life, acute renal injury was confirmed based on elevated creatinine levels (1.32 mg/dL) and decreased glomerular filtration rate (GFR, 11.3 mL/min/1.73 m2), while PSP values increased to 1647 ng/mL.
Serum creatinine levels had a remarkable evolution, starting from an initial value of 0.92 mg/dL in the first DOL, reaching 1.04 mg/dL and 1.2 mg/dL in the 7th and 10th DOL respectively and attaining the peak value of 1.32 mg/dL in the 14th DOL. Following treatment, creatinine values started to improve, dropping to 0.97 mg/dL and 0.86 mg/dL in the 21st and 25th DOL respectively (Fig. 1).
Gradually, the newborn’s weight became appropriate for age. The systemic inflammatory syndrome subsided and GFR values progressively increased to 20.6 mL/min/1.73 m2. Since elevated PSP levels (900 pg/dL) and hypernatremia (153.5-173.5 mEq/L) persisted, further investigations were conducted. Results revealed increased plasma osmolality (333-356.4 mOsm/kg), hypercalcemia (11.4-14 mg/dL), low urine sodium (Na) levels (14-19 mmol/L), low fractional excretion of Na (FENa, 0.96%) and urine osmolality below that of plasma, measuring 107.8 mOsm/kg (Fig. 1). Renal and cranial ultrasounds showed no changes, and an X-ray examination of the sella turcica showed no abnormality.
In the sixth week of life, the patient developed a febrile syndrome that lasted for 18 days, associating irritability and an increased appetite. The urine output exceeded 5 mL/kg/h or 2 L/m2/24h, associating persistent hypernatremia and serum hyperosmolality (Fig. 1).
Taking into account the onset of the polyuro-polydipsic syndrome, the associated hypernatremia, hyperosmolar plasma and hypoosmolar urine, as well as family history, NDI was suspected (Fig. 2). Therefore, treatment with thiazide diuretics was commenced, consisting of Hydrochlorothiazide 3 mg/kg/day divided into two doses with progressive decrease until normal plasma sodium and osmolality were reached and remission of polyuria and polydipsia were observed. After 10 days of treatment, Na levels dropped to 144 mEq/L, plasma osmolality reached 294 mOsm/kg, urine output decreased to 4.5 mL/kg/h and nutritional intake was estimated at 150 mL/kg/day.
Figure 2.
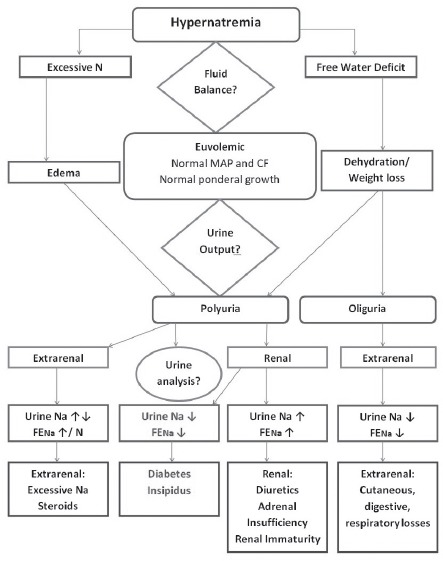
Hypernatremia differential diagnosis in the preterm infant. MAP - mean arterial pressure; CF - cardiac frequency.
At two months of life the patient was transferred in the pediatric ward for further investigations. Desmopressin testing was performed, proving that the kidney was insensitive to the action of vasopressin and thus confirming the diagnosis of NDI. Treatment with Indomethacin and Hydrochlorothiazide was administered along with enteral nutrition consisting of a low-calcium and vitamin D-free formula. Considerable improvement of the polyuro-polydipsic syndrome was observed, as well as normal levels of Na (143 mEq/L).
At 10 months of age, the patient had normal weight and normal neurological examination for corrected age.
DISCUSSION
Three possible causes could have stood at the root of the renal symptoms that the preterm infant was suffering from: immaturity, nephrologic pathology and sepsis (Fig. 3).
Figure 3.
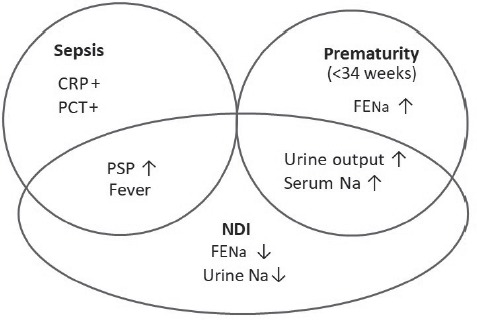
Renal dysfunction in prematurity.
The polyuro-polydipsic syndrome was late-onset, associating increased plasma sodium levels and high PSP values. Although clinical signs of hypernatremia are rare, early diagnosis and treatment are pivotal due to the increased risk of developing an intracranial hemorrhage (5, 6). The main etiopathogenic mechanisms of hypernatremia include an increased intake of Na, free water deficit or simultaneous loss of water and sodium (7). In our case, the patient did not receive intravenous electrolytes during the first 7 DOL in order to limit the risk of iatrogenic hypernatraemia in a preterm VLBW infant.
Differential diagnosis of hypernatremia in preterm infants in the immediate postnatal period is difficult (8), and infants with very low birth weight (VLBW) are more prone to hypernatremic dehydration due to limited fluid intake, immature renal function and increased insensible losses caused by immaturity (4, 9, 10). Our patient received parenterally adjusted fluid intake depending on weight gain, which limited the physiological decrease in the first weeks of life to less than 10% of the birth weight. As a result, we could exclude dehydration caused by decreased fluid intake. Also, until the feeding tolerance was improved, the patient received TPN with 12 g/kg/day carbohydrates, 4 g/kg/day amino acids and 3 g/kg/day lipids, ensuring an adequate caloric intake.
Electrolyte imbalances and renal disorders that develop in this period can be attributed to sepsis-associated acute kidney injury (AKI), intrauterine renal dysfunction caused by maternal risk factors including maternal high blood pressure and the use of nephrotoxic drugs (certain antibiotics, nonsteroidal anti-inflammatory drugs), birth hypoxia, neonatal sepsis as well as renal immaturity (11). In NDI, the kidney’s ability to concentrate urine is affected as a result of resistance to antidiuretic hormone (ADH) associated with a decreased aquaporin-2 (AQP2) water channel expression in the collecting ducts (12-14). Preterm infants usually associate difficulties in retaining electrolytes and proteins due to the inability to concentrate urine, leading to the polyuric phase of AKI. Hypernatremia can cause mild fever and may be associated with moderate hypercalcemia and hyperglycemia, although the mechanism is not fully understood (6). Hypernatremia must be corrected as it increases the risk of brain damage and neurological sequelae especially in preterms (5, 6).
In the absence of dehydration and increased sodium intake, loss of free water is usually suspected (15). In the case of isolated free water deficit, clinical signs are not always obvious since fluid losses are seen at a cellular level while the intravascular volume status is normal. In order to differentiate between free water deficit mechanisms, urine output, urine characteristics and FENa must be analyzed. Oliguria and hypersthenuria are present in extrarenal free water losses, while renal free water losses are associating polyuria and hyposthenuria. FENa is increased in the cases of sodium poisoning and renal losses caused by prematurity, while dehydration and DI associate decreased FENa values (4). As the free water deficit in the present case associated an increased urine output and low FENa levels, DI was suspected.
DI may be central, caused by a defective arginine-vasopressin (AVP) secretion, or nephrogenic, caused by failure of the kidney to appropriately respond to AVP (16). Differential diagnosis between these two entities is crucial for a correct treatment. Measuring ADH levels, water deprivation testing as well as evaluating the response to desmopressin acetate facilitate the differential diagnosis (6, 16, 17). The water deprivation test is not necessary if concomitant hypernatremia and hyposthenuria are present (6).
NDI can be either congenital or acquired, and recognizing the etiology is necessary for appropriate treatment and prognosis. Congenital NDI is less common in children but cases are more severe and treatment is rather difficult (18). Most cases of inherited NDI develop as a result of a null mutation in the V2 vasopressin receptor (V2R) gene (16). Acquired NDI has a later onset and various risk factors that include metabolic imbalances such as hypercalcemia, hypokalemia (19), the use of certain medications like lithium, antibiotics, antineoplastic drugs (20) and sickle cell anemia (21). In our case, the mother underwent antihypertensive treatment with methyldopa during pregnancy. However, methyldopa has not been shown to have a negative impact on renal function of newborns.
Genetic NDI is more severe than the acquired form, becomes apparent in the first weeks of life, with polyuria and polydipsia dominating the clinical picture (2). Other clinical aspects include fever, vomiting, dehydration and even failure to thrive caused by non-caloric fluid intake (18). Long-term complications include non-obstructive hydronephrosis, megacystis and hydroureter secondary to prolonged excessive fluid intake (22).
Severe early-onset symptoms associated with electrolyte imbalance and reduced kidney function together with positive family history, all suggested the diagnosis of NDI transmitted in an X-linked manner. X-linked NDI was later confirmed by genetic testing.
Treatment
Congenital NDI is difficult to treat. The goal of the treatment is to provide nutrition and caloric intake which ensure optimal growth. Dehydration and hypervolemia must be avoided while renal complications should be reduced to a minimum (18). Growth is improved while urine output is decreased when adopting a diet with an increased calorie to osmolality ratio associated with decreased Na intake (<1 mEq/kg/24 hours) (6). Current medication is symptomatic, involving thiazide diuretics which have a paradoxical effect on the renal function. Urine output is reduced by decreasing GFR and increasing Na excretion associated with proximal tubular reabsorption of Na and water. Indomethacin and amiloride amplify the thiazide’s effects in polyuria. Indomethacin decreases urine output and increases urine concentrating ability while amiloride maintains appropriate serum potassium levels (23, 24).
However, despite early diagnosis and treatment, incidence of growth failure and mental retardation is increased. Mental retardation may be associated with cerebral calcifications secondary to hypercalcemia and hypernatremia (25-27). The patient showed a normal appearance on the transfontanellar ultrasound since he experienced only a short period of hypernatremia and hypercalcemia as a result of early treatment that assured normal sodium levels.
Complications
Repeated episodes of dehydration can result in mental retardation. Early diagnosis and treatment may reduce the incidence of neurological damage (1-3). Psychological development can also be hindered by the persistent need to drink and urinate (1).
The particularity of the case consists of the simultaneous presence of elevated plasma PSP values, hypernatremia and polyuria that persisted until after treatment initiation. PSP is a biomarker of sepsis (28) that, together with the febrile syndrome, managed to conceal the renal pathology, thus hampering the diagnosis. Congenital NDI is usually caused by a genetic mutation affecting the vasopressin V2 receptor that is located in the collecting ducts, the thin segment of the ascending limb of the loop of Henle and the periglomerular tubules (29). Another cause of congenital NDI is a mutation of the AQP2 gene that leads to insufficient or absent aquaporin 2 water channels in the apical membrane of the collecting ducts (30).
The clearance mechanism of PSP is relevant since its plasma levels seem to be influenced by renal function and are inversely proportional to GFR (31). Although activity in vivo is unclear, observations in adult and older patients as well as presepsin’s low molecular weight (13 kDa) elicit the assumption that it undergoes glomerular filtration and reabsorption followed by catabolism in the proximal duct cells (32, 33). Since the patient had normal GFR for GA despite persistent symptoms and electrolyte imbalance, the increase in plasma PSP levels suggests reabsorption in the collecting ducts.
In conclusion, renal complications are common in preterm infants during the first weeks of life and, although they are usually transient, in rare cases they can hinder the detection of a congenital kidney disease. NDI is a rare condition, and the onset in the neonatal period is a sign of severity and hereditary causality. Although there is no specific treatment, early diagnosis and symptomatic treatment along with multidisciplinary monitoring (neonatology, pediatrics, nephrology and endocrinology) can decrease the risk of long-term complications.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Milano S, Carmosino M, Gerbino A, Svelto M, Procino G. Hereditary Nephrogenic Diabetes Insipidus: Pathophysiology and Possible Treatment. An Update. 2017;18(11) doi: 10.3390/ijms18112385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocrine reviews. 2013;34(2):278–301. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivas-Crespo MF, Miñones-Suárez L, G-Gallarza SS. Rare neonatal diabetes insipidus and associated late risks: case report. BMC pediatrics. 2012;12:56. doi: 10.1186/1471-2431-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloherty John P., H AR, Stark Ann R., Eichenwald Eric C. Fluid and Electrolyte Management. 7th ed. Lippincott Williams & Wilkins; 2012. Cloherty and Stark’s Manual of Neonatal Care; pp. 269–283. [Google Scholar]
- 5.Mujawar NS, Jaiswal AN. Hypernatremia in the Neonate: Neonatal Hypernatremia and Hypernatremic Dehydration in Neonates Receiving Exclusive Breastfeeding. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2017;21(1):30–33. doi: 10.4103/0972-5229.198323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliegman Robert M., BMDS, Geme Joseph St., Schor Nina F. 20th ed. Elsevier; 2016. Nephrogenic Diabetes Insipidus. Nelson Textbook of Pediatrics; pp. 2532–2533. [Google Scholar]
- 7.Kliegman Robert M., BMDS, Geme Joseph St., Schor Nina F. Nelson Textbook of Pediatrics. 20th ed. Elsevier; 2016. Sodium; pp. 350–353. [Google Scholar]
- 8.Marcialis MA, Dessi A, Pintus MC, Marinelli V, Fanos V. Frontiers in bioscience. Elite edition. 2012. Hyponatremia and hypernatremia in the newborn: in medio stat virtus; pp. 132–140. 4. [DOI] [PubMed] [Google Scholar]
- 9.Hammarlund K, Sedin G. Water evaporation and heat exchange with the environment in newborn infants. Acta paediatrica Scandinavica Supplement. 1983;305:32–35. doi: 10.1111/j.1651-2227.1983.tb09856.x. [DOI] [PubMed] [Google Scholar]
- 10.Cardiello V, Zecca E, Corsello M, Pianini T, Serrao F, Costa S, Cota F. Semipermeable membranes and hypernatremic dehydration in preterms. A randomized-controlled trial. Early human development. 2018;119:45–50. doi: 10.1016/j.earlhumdev.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL. Neonatal Acute Kidney Injury. Pediatrics. 2015;136(2):e463–73. doi: 10.1542/peds.2014-3819. [DOI] [PubMed] [Google Scholar]
- 12.Sandra Lee Gardner BSC, Enzman-Hines Mary I., Jacinto A. Neonatal Nephrology Merenstein & Gardner’s Handbook of Neonatal Intensive Care. 20th ed. Mosby; 2015. Hernandez; pp. 689–726. [Google Scholar]
- 13.Tamarappoo BK, Yang B, Verkman AS. Misfolding of mutant aquaporin-2 water channels in nephrogenic diabetes insipidus. The Journal of biological chemistry. 1999;274(49):34825–34831. doi: 10.1074/jbc.274.49.34825. [DOI] [PubMed] [Google Scholar]
- 14.Deen PM, van Balkom BW, Kamsteeg EJ. Routing of the aquaporin-2 water channel in health and disease. European journal of cell biology. 2000;79(8):523–530. doi: 10.1078/0171-9335-00075. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW. Hypernatremia: successful treatment. Electrolyte & blood pressure: E & BP. 2006;4(2):66–71. doi: 10.5049/EBP.2006.4.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalra S, Zargar AH, Jain SM, Sethi B, Chowdhury S, Singh AK, Thomas N, Unnikrishnan AG, Thakkar PB, Malve H. Diabetes insipidus: The other diabetes. Indian journal of endocrinology and metabolism. 2016;20(1):9–21. doi: 10.4103/2230-8210.172273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein RE. Chapter 67 - Diabetes Insipidus. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. Second Edition. St. Louis: W.B. Saunders; 2015. pp. 357–362. [Google Scholar]
- 18.Mishra G, Chandrashekhar SR. Management of diabetes insipidus in children. Indian journal of endocrinology and metabolism. 2011;15(Suppl 3):S180–S187. doi: 10.4103/2230-8210.84858. (Suppl3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna A. Acquired nephrogenic diabetes insipidus. Seminars in nephrology. 2006;26(3):244–248. doi: 10.1016/j.semnephrol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: A systematic review. American Journal of Kidney Diseases. 2005;45(4):626–637. doi: 10.1053/j.ajkd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Tharaux PL, Hagege I, Placier S, Vayssairat M, Kanfer A, Girot R, Dussaule JC. Urinary endothelin-1 as a marker of renal damage in sickle cell disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(11):2408–2413. doi: 10.1093/ndt/gfi111. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto M, Okada S-I, Kawashima Y, Nishimura R, Miyahara N, Kawaba Y, Hanaki K, Nanba E, Kondo Y, Igarashi T, Kanzaki S. Clinical overview of nephrogenic diabetes insipidus based on a nationwide survey in Japan. Yonago acta medica. 2014;57(2):85–91. [PMC free article] [PubMed] [Google Scholar]
- 23.Loffing J. Paradoxical antidiuretic effect of thiazides in diabetes insipidus: another piece in the puzzle. Journal of the American Society of Nephrology : JASN. 2004;15(11):2948–29450. doi: 10.1097/01.ASN.0000146568.82353.04. [DOI] [PubMed] [Google Scholar]
- 24.Cesar KR, Magaldi AJ. Thiazide induces water absorption in the inner medullary collecting duct of normal and Brattleboro rats. The American journal of physiology. 1999;277(5):F756–60. doi: 10.1152/ajprenal.1999.277.5.F756. [DOI] [PubMed] [Google Scholar]
- 25.Bindu PS, Kovoor JM. Nephrogenic diabetes insipidus: a rare cause of intracranial calcification in children. Journal of child neurology. 2007;22(11):1305–1307. doi: 10.1177/0883073807307087. [DOI] [PubMed] [Google Scholar]
- 26.Ray M, Dixit A, Singhi P. Nephrogenic diabetes insipidus with intracranial calcifications. Indian pediatrics. 2002;39(2):197–202. [PubMed] [Google Scholar]
- 27.Bajpai A, Kabra M, Thapliyal R, Gulati S, Kalra V. Nephrogenic diabetes insipidus presenting with developmental delay and intracranial calcification. Indian journal of pediatrics. 2005;72(6):527–528. doi: 10.1007/BF02724433. [DOI] [PubMed] [Google Scholar]
- 28.Zou Q, Wen W, Zhang X-C. Presepsin as a novel sepsis biomarker. World journal of emergency medicine. 2014;5(1):16–9. doi: 10.5847/wjem.j.issn.1920-8642.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bichet DG, Bockenhauer D. Genetic forms of nephrogenic diabetes insipidus (NDI): Vasopressin receptor defect (X-linked) and aquaporin defect (autosomal recessive and dominant) Best practice & research. Clinical endocrinology & metabolism. 2016;30(2):263–276. doi: 10.1016/j.beem.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Ando F, Uchida S. Activation of AQP2 water channels without vasopressin: therapeutic strategies for congenital nephrogenic diabetes insipidus. Clinical and experimental nephrology. 2018;22(3):501–507. doi: 10.1007/s10157-018-1544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE. Presepsin (sCD14-ST) in emergency department: the need for adapted threshold values? Clinica chimica acta; international journal of clinical chemistry. 2014;427:34–36. doi: 10.1016/j.cca.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Nagata T, Yasuda Y, Ando M, Abe T, Katsuno T, Kato S, Tsuboi N, Matsuo S, Maruyama S. Clinical impact of kidney function on presepsin levels. PloS one. 2015;10(6):e0129159–e. doi: 10.1371/journal.pone.0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotera A, Sagishima K, Tashiro T, Niimori D, Kamohara H, Kinoshita Y. A validation of presepsin levels in kidney dysfunction patients: four case reports. Journal of intensive care. 2014;2(1):63. doi: 10.1186/s40560-014-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


