Abstract
Objectives
Polycystic ovarian syndrome (PCOS) occurs in 6-10% of all women in their reproductive age. In women with PCOS, controlled ovarian hyperstimulation (COH) often results in an increased risk of ovarian hyperstimulation syndrome (OHSS). In vitro maturation (IVM) of human oocyte is an alternative technique for in vitro fertilization (IVF). The aim of this study was to compare the morphometric analysis and morphology of oocytes after in- vitro maturation (IVM) between normal women and those suffering from polycystic ovary syndrome (PCOS).
Material and Methods
Thirty two women of 20 to 35 years of age that were undergoing controlled ovarian stimulation by the ICSI/IVF protocol were chosen for the study. The immature oocytes (n=108) were divided into two groups: the first oocyte group was comprised of 16 normal women (n=54); and the second group included 16 women with PCOS (n=54); then the oocytes were matured in vitro. After 24-48h of incubation, the oocyte maturation rate and morphometric and morphological characteristics were assessed using an inverted microscope, and then the images were compared.
Results
There were significant differences in the maturity of oocytes between normal women and those with PCOS after IVM (P<0.05). Moreover, morphometric assessments revealed that there were no significant difference in the total diameter (μm) (zona thickness (ZPT) + perivitelline space width (PVS) + cytoplasm (CD) of oocytes between normal women and those with PCOS (156.3±6.8 and 137.7±9.9), respectively (P>0.05). Evaluation of morphological oocytes showed that morphological abnormalities, including ooplasmic vacuolization and granulation were higher in PCOS women compared to normal women (P<0.05).
Conclusion
The increased quality of oocytes after IVM reflected a positive impact of IVM oocytes on normal women as compared to women with PCOS.
Keywords: Polycystic Ovarian Syndrome (PCOS), In vitro maturation (IVM), Human oocytes
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is the most common reproductive disorder in women who are in their reproductive age and it has a prevalence of 6-10%)(1). This syndrome is characterized by clinical or biochemical hyperandrogenism, oligo-anovulation and polycystic ovaries, with concurrent hormonal and metabolic alterations that alter the follicle maturation and reduce oocyte quality (2).
Gonadotropin treatment for women with PCOS can increase the risk of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies (3-4). Therefore, in-vitro maturation (IVM) of immature oocytes is used as an effective treatment for women’s infertility that is caused by PCOS (5). Moreover, IVM could potentially decrease the stimulating hormones before IVF or intracytoplasmic sperm injection (ICSI) (6-7). A previous study has shown that the IVM rate of collected GV oocytes differs from stimulated cycles, which can probably occur due to several factors, including IVM medium, the source of oocytes (non-stimulated or stimulated cycles) and cumulus cells that are associated with the oocyte (8). The IVM rate for collected GV oocytes is approximately 60%, while normal pregnancy and embryo implantation are still at rates of 30-35% and 10-15%, respectively (9). Cha and colleagues reported the first human live birth from IVM in 1991 (10). Moreover, Trounson and colleagues reported the first birth from an untreated polycystic ovarian (PCO) patient in 1994 (11).It has been revealed that a suitable oocyte diameter at the time of retrieval has an effect on IVM during oocyte maturation (12).
In addition, Mikkelsen and Lindenbery demonstrated that there was no difference in the morphology of in vitro matured oocytes of normal ovaries and polycystic ovaries (13).
Quality of oocytes plays a pivotal role in determining ART outcomes. Good quality oocyte plays an extremely important role in determining ART outcomes. Currently, the use of the non - invasive microscopic evaluation is one of the most reliable methods of oocyte quality evaluation. Moreover, there are mainly several cytoplasmic morphological criteria including the perivitelline space (normal/large), perivitelline debris (present/ not present), zona pellucida morphology (normal/ abnormal), cytoplasmic granularity (normal/ excessive), and cytoplasmic vacuoles (present/ not present) that are currently used to evaluate oocyte quality (8).
Therefore, the main aim of the current prospective study was to compare the morphology and morphometric assessment of IVM oocytes of normal women and of those with PCOS.
MATERIALS AND METHODS
The study was ethically approved by the Ethical Committee of Kurdistan University of Medical Sciences and no potential conflict of interests relevant to this article was reported. All patients were informed about the study protocol and signed their informed, written consent.
Collection of oocytes
The current study was experimental. A total of 108 immature oocytes were obtained from 32 consenting patients, which included women with PCOS (n=16) and normal women (n=16) who underwent the ICSI/IVF protocol. All the women were between 20-35 years of age and their body mass index (BMI) was between 18.2 and 22.3 (kg/m2). Women suffering from endometriosis, endocrinopathies such as Cushing’s syndrome, thyroid disorders, and diabetes were excluded from this study. Among the immature oocytes, 86 were observed in the germinal vesicle (GV) stage and 22 in the metaphase I (MI) oocytes. In the current study, decision for ovarian stimulation and the FSH starting dose according to the patients’ clinical outcome of previous cycles and baseline characteristics ovarian stimulation (women’s age and markers of ovarian reserve including antral follicle count (AFC) and anti-Müllerian hormone (AMH) level; in addition serum day-three FSH has been recommended (14).
Ovarian stimulation was performed using a combination of a gonadotrophin-releasing hormone (GnRH) antagonist and a follicle-stimulating hormone (FSH) analogue recombinant FSH (inj. Gonal-F, Merck Serono) was initiated on Day 2 of the cycle (75-200 IU daily) and GnRH antagonist daily injection of Cetrorelix acetate (Cetrotide - Merck-Serono, Switzerland) 0.25 mg. Treatment was started when the follicle reached a diameter of 14 mm and/or the estradiol levels were >400 pg/ml. When the ovarian follicles reached 18-20 mm in diameter, the recombinant hCG(r-HCG) was administered; then, ultrasound-guided cumulus-oocyte complex (COC) pick-up was conducted after 36 h using a 18-gauge single-lumen aspiration needle. The cumulus cells were then removed by 3 min exposures to HEPES buffered medium containing hyaluronidase (Irvine Scientific, CA, USA), and by pipetting with a pasteur pipette. Denuded oocytes were evaluated for nuclear maturity using a stereomicroscope (Olympus, Tokyo, Japan). Oocytes lacking the first polar body (PB) extrusion were considered as immature and thus a candidate for IVM (Fig. 1). In the IVM technique, immature oocytes were washed in 3 drops of washing medium (SAGE IVF,USA) and were placed in the maturation medium (SAGE IVF,USA) supplemented with 75 mUI/mL FSH and 75 mUI/mL LH (Ferring) at 37ºC and 6% CO2.
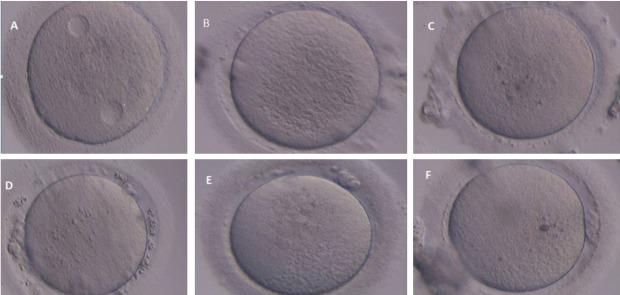
Figure 1.
Various morphological abnormalities of human oocytes were observed after IVM oocytes. A: Cytoplasmic vacuoles in human oocyte. B: Central granularity in human oocytes; C: Refractile bodies within ooplasm; D: Wide PVS with debris in human oocytes; E: Fragmented polar body in human oocytes; F: Bull’s eye within ooplasm.
In order to define maturity, the oocytes were again evaluated for the presence of the first PB after 24-48 h. In the next step, mature oocytes were individually placed into culture medium; then, the diameter and morphology of mature oocytes were measured using an Olympus IX71 inverted microscope (Olympus America Inc., Melville, NY) equipped with ZILOS laser and a software program (Hamilton-Thorne Research, Beverly, MA). Afterwards, the mean and standard deviations of each measurement were automatically calculated. The diameters of total oocytes (zona pellucida + perivitelline space width + cytoplasm) (total diameter) were obtained using the average of measurements of each oocyte.
The morphologies of the oocytes were classified according to the presence of abnormalities, which was comprised dark oocytes, cytoplasmic vacuoles, refractile bodies (RF), ooplasm granulation, bull’s eye (central aggregation of organelles and vesicles within ooplasm), wide ZP, wide perivitelline space (PVS), and fragmented polar body (Fig. 2) (8).
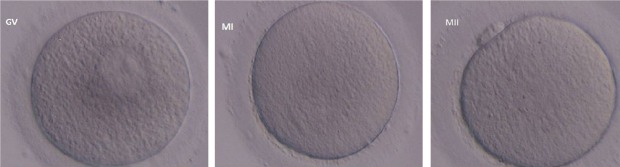
Figure 2.
Human oocytes in various maturation stages include Germinal Vesicle (GV), Metaphase I (MI), and Metaphase II (MII).
Statistical analysis
Data was compared using Student’s t-test or Chi-squared analysis. The P-value of <0.05 was considered significant.
RESULTS
A total of 74 (68.5 %) immature oocytes reached the MII stage following in vitro culture for 24-48 h, reached the MII stage, and GV(n= 39) and MI (n=7) oocytes were observed in normal women and GV (n=24) and MI (n=4) oocytes were observed in women with PCOS. There were significant differences in the maturity of oocytes between normal women and those with PCOS after IVM (P< 0.05). Moreover, in women with PCOS, the rates of degeneration and arrested oocytes were significantly increased after IVM in comparison with normal women (P<0.05) Table (1).
Table 1.
Comparison of in vitro maturation rates of oocytes between normal women and women with PCOS
| Variable | Normal Women Oocyte-IVM (n=54) | PCOS Women Oocyte-IVM (n=54) | Odd ratio(95%CI) | P-Value |
|---|---|---|---|---|
| Matured oocytes(MII) | 46 (85.2%) | 28 (51.8%) | 5.33 (1.97-15.38) | 0.0002 |
| Arrested oocytes | 6 (11.1%) | 18 (33.3%) | 0.25 (0.07-0.75) | 0.005 |
| Degenerated oocytes | 2 (3.7%) | 10 (18.51%) | 0.16 (0.1-0.86) | 0.01 |
The morphometric and morphological results of in vitro matured oocytes were compared between the two groups (Table 1). Comparison of the morphometric assessment revealed that there were no significant differences in the mean oocytes of total diameters (TD μm) (zona thickness (ZPT) + perivitelline space width (PVS) + cytoplasm diameter (CD) between normal women and those with PCOS (156.3±6.8 and 137.7±9.9), respectively (P=0.3) (Table 2). In addition, in the present study, morphological criteria showed that there were no significant differences between the two groups with regard to fragmented polar body, regularity of shape, retractile body, bull’s eye, PVS debris, and the width of PVS. However, the rates of abnormalities, including vacuolization and ooplasm granulation were higher in PCOS women compared with normal women (P<0.05) (Table 3).
Table 2.
Comparison of morphometric assessment of MII oocytes between normal women and women with PCOS after IVM
| Variable | Normal Women Oocyte-IVM (n=54) | PCOS Women Oocyte-IVM (n=54) | P-Value |
|---|---|---|---|
| Oocyte diameter (μm) | 116.3 ± 6.8 | 114.07 ± 5.9 | P >0.05 |
| Ooplasm diameter (μm) | 105.3 ± 4.6 | 100.02 ± 5.5 | P >0.05 |
| Zona pellucida width (μm) | 15.8 ± 2.5 | 15.3 ± 2.6 | P >0.05 |
Table 3.
Comparison of morphological MII oocytes between normal women and women with PCOS after IVM
| Variables % | MII Oocyte in Normal Women after IVM(n=54) | MII Oocyte in Women with PCOS after IVM(n=54) | Odd ratio(95%CI) | P-Value |
|---|---|---|---|---|
| Refractile body | 33(61.1%) | 37(68.5%) | 0.72(0.3-1.71) | 0.4 |
| Fragmented polar body | 20(37.04% ) | 32(59.2%) | 0.4(0.17-0.96) | 0.02 |
| Regular shape | 35(64.8%) | 38(70.3%) | 0.77(0.31-1.87) | 0.5 |
| Ooplasmic granulation | 14(25.9%) | 26(48.1%) | 0.37(0.15-0.9) | 0.01 |
| Vacuole | 12(22.2%) | 14(25.9%) | 0.81(0.3-2.16) | 0.6 |
| Bull’s eye | 5(9.2%) | 7(12.9%) | 0.68(0.16-2.71) | 0.5 |
| Debris PVS | 3(5.5%) | 4(7.4%) | 0.7(0.1-4.6) | 0.6 |
| Width PVS | 12(22.2%) | 17(31.5%) | 0.6(0.2-1.59) | 0.2 |
DISCUSSION
Many factors impact oocyte maturation which occurs in the follicular environment (15). Oocytes obtain various sizes during the stages of oocyte maturation (16). IVM is a safe technique that maintains the high maturation rate of oocytes. In this study, the results of maturity of oocytes after IVM in normal women were higher than in women with PCOS. Endocrine abnormalities in PCOS can influence oocyte maturity (17). Teissier and colleagues reported that low meiotically-competent oocytes in patients with PCOS existed due to the fact that testosterone and progesterone concentrations were high in their follicular fluid (18).
To date, it has been defined that hyperandrogenism, high LH level and insulin resistance play an important role in oocyte quality. So, alterations in these lead to the lower fertilization rate and clinical pregnancy. Myo-inositol (MI), used as an insulin-sensitizing drug, in improving ovulatory function and reducing androgen excess in PCOS (19). Moreover, it is suggested that melatonin and Myo-inositol significantly improved morphologically MII and the development of the maturing oocyte (20).
Yazdanpanah and colleagues showed that after IVM, the maturation rates in patients with male infertility were higher than in patients with ovarian infertility (21).
In accordance with the present study, Barnes and colleagues discovered that the maturation rate of immature oocytes obtained from women with normal ovaries (78%) was significantly higher than the maturation rate of immature oocytes that were collected from women with PCOS (26%) (22). In addition, Russell showed that the maturation rates in oocytes obtained from normal women (63%) and women with PCOS were similar to the current study (23). It has indicated that the process of IVM during the growth phase influences oocyte quality (24).
The present study’s results showed that after IVM, the arrest rates for GV and MI oocytes in women with PCOS were higher compared to normal women. Jonard and colleagues reported that amplification of intra-ovarian hyperandrogenism in PCOS patients influences the follicular fluid and increases the degeneration of oocytes (25). Moreover, this study demonstrated that after IVM, the rates of oocyte abnormalities such as vacuolization endoplasmic granularity were higher in women with PCOS compared to normal women. Balaban et al. showed that abnormalities in the morphology of oocytes consist of a distinct central cytoplasmic granularity, expanded perivitelline space, cytoplasmic vacuoles, RF, ooplasm granulation, bull’s eye, wide PVS, and fragmented polar body (8). It is widely accepted that many organelles in the oocytes contribute to their maturation (16); therefore, any dysfunction in the oocyte organelles, such as meiotic spindle, cortical granules, and mitochondria, can reduce oocyte competence (26). In patients with PCOS, denuded oocytes with FPB may have impaired cytoplasmic competence which is associated with abnormal hormones, perifollicular vascularity, granulose cell abnormality, cytogenetic maturity, and chromosomal aberrations (27). In the current study, morphometric comparison of the oocytes of normal women with women suffering from PCOS following IVM revealed that there were noticeable differences between the two groups.
The mean morphometric assessment of oocytes in terms of total diameters (TD) (zona pellucida + perivitelline space width + cytoplasm) in normal women and those with PCOS (116.3±6.8μm and 114.07±5.9μm, respectively, (P>0.05). In addition, all matured oocytes had ZP thicknesses of 17.2 and 18 μm respectively, which were related to normal women and women with PCOS. In agreement with the present study’s results, Cavilla and Ubaldi showed that the normal size of in vivo and in vitro matured oocytes is approximately 110-120 μm, excluding the ZP; in addition, the total size of an oocyte is approximately 150 μm, and during its growth, its diameter increases from 30 to 110 μm (28). Moreover, Bertrand and colleagues reported that the thickness of ZP differs from 10 to 30 μm with a mean of 17.5 μm (29), which is in conformity with the present study’s results.
Strength and limitations
Limitations of our study could be the small number of women included, therefore, more studies in large number of women are necessary to confirm clinical usefulness and cost-effectiveness of this technique. However, the strict inclusion and exclusion criteria could be considered strength of the study.
In conclusion, the current study showed that the morphological abnormalities of oocytes, such as ooplasm granularity and cytoplasmic vacuolation, are higher in patients suffering from PCOS. Therefore, IVM of oocyte with supplemented culture media by other investigational approaches may be useful in the treatment of patients with PCOS.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
The authors would like to appreciate Vice chancellor of research and technology of Kurdistan University of Medical Sciences for funding and support this research.
References
- 1.Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132:321–336. doi: 10.1097/AOG.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 2.Reyes-Muñoz E, Sathyapalan T, Rossetti P, Shah M, Long M, Buscema M, Valenti G, La Rosa VL, Cianci S, Vitale SG. Polycystic Ovary Syndrome: Implication for Drug Metabolism on Assisted Reproductive Techniques -A Literature Review. Adv Ther. 2018;35(11):1805–1815. doi: 10.1007/s12325-018-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2006;61(2):234–248. doi: 10.1002/mrd.1153. [DOI] [PubMed] [Google Scholar]
- 4.Baumber J, Ball BA, Linfor JJ, Meyers SA. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl. 2003;24(4):621–628. doi: 10.1002/j.1939-4640.2003.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 5.Bucak MN, Ateşahin A, Yüce A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze–thawing process. Small Ruminant Research. 2008;75(2-3):128–134. [Google Scholar]
- 6.Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts Views Vis Obgyn. 2014;6(2):77–80. [PMC free article] [PubMed] [Google Scholar]
- 7.Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, Ricciardo-Lamonica G, Boscaro M, Lenzi A, Littarru G. Coenzyme Q 10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009;91(5):1785–1792. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 8.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12(5):608–615. doi: 10.1016/s1472-6483(10)61187-x. [DOI] [PubMed] [Google Scholar]
- 9.Combelles CM, Albertini DF, Albertini Racowsky C., Racowsky C. Distinct microtubule and chromatin characteristics of human oocytes after failed in vivo and in vitro meiotic maturation. Hum Reprod. 2003;18(10):2124–2130. doi: 10.1093/humrep/deg419. [DOI] [PubMed] [Google Scholar]
- 10.Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. FertilSteril. 1991;55(1):109–113. doi: 10.1016/s0015-0282(16)54068-0. [DOI] [PubMed] [Google Scholar]
- 11.Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62(2):353–362. doi: 10.1016/s0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil. 2010;13(4):217–225. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 13.Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum reprod. 2001;16(8):1714–1718. doi: 10.1093/humrep/16.8.1714. [DOI] [PubMed] [Google Scholar]
- 14.Di Paola R, Garzon S, Giuliani S, Laganà AS, Noventa M, Parissone F, Zorzi C, Raffaelli R, Ghezzi F, Franchi M, Zaffagnini S. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch Gynecol Obstet. 2018;298(5):1029–1035. doi: 10.1007/s00404-018-4906-2. [DOI] [PubMed] [Google Scholar]
- 15.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99(6):1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wani AR, Khan MZ, Sofi KA, Malik AA, Lone FA, Bhat FA. Effect of follicular size on in vitro maturation, fertilization and culture of sheep embryos. Iran J Vet Res. 2013;14(40):299–304. [Google Scholar]
- 17.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(1):38. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15(12):2471–2477. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- 19.Nestler JE, Unfer V. Reflections on inositol(s) for PCOS therapy: steps toward success. Gynecol Endocrinol. 2015;31(7):501–505. doi: 10.3109/09513590.2015.1054802. [DOI] [PubMed] [Google Scholar]
- 20.Vitale SG, Rossetti P, Corrado F, Rapisarda AM, La Vignera S, Condorelli RA, Valenti G, Sapia F, Laganà AS, Buscema M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int J Endocrinol. 2016:4987436. doi: 10.1155/2016/4987436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdanpanah F, Khalili MA, Eftekhar M, Karimi H. The effect of vitrification on maturation and viability capacities of immature human oocytes. Arch Gynecol Obstet. 2013;288(2):439–444. doi: 10.1007/s00404-013-2777-0. [DOI] [PubMed] [Google Scholar]
- 22.Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro-matured primary human oocytes. Fertil steril. 1996;65:1151–1156. doi: 10.1016/s0015-0282(16)58330-7. [DOI] [PubMed] [Google Scholar]
- 23.Russell JB. Immature oocyte retrieval combined with in-vitro oocyte maturation. Hum Reprod. 1998;13(3):63–70. doi: 10.1093/humrep/13.suppl_3.63. [DOI] [PubMed] [Google Scholar]
- 24.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reprod. 2001;121(1):51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 25.Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Human Reproduction Update. 2004;10(2):107–117. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 26.Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001;122(1):155–163. [PubMed] [Google Scholar]
- 27.Rose BI, Laky D. Polar body fragmentation in IVM oocytes is associated with impaired fertilization and embryo development. J Assist Reprod Genet. 2013;30(5):679–682. doi: 10.1007/s10815-013-9982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavilla JL, Kennedy CR, Byskov AG, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod. 2008;23(1):37–45. doi: 10.1093/humrep/dem178. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand E, Van den Bergh M, Englert Y. Fertilization and early embryology: Does zona pellucida thickness influence the fertilization rate? Hum reprod. 1995;10(5):1189–1193. doi: 10.1093/oxfordjournals.humrep.a136116. [DOI] [PubMed] [Google Scholar]