Abstract
How the behavior of cells in living tissues is orchestrated according to tissue needs, size and developmental stage is still poorly understood. Advances in these directions are essential in order to understand morphogenesis, “self-organization” phenomena, to build new tissues for regenerative medicine or to reverse the changes in deranged organs, such as in cancer or in genetic disorders. This review oulines a new scenario by which the crosstalk between the YAP/TAZ transcription factors and Notch signaling influence cell self-renewal, stem cell differentiation, cell fate decisions, epithelial stromal interactions, inflammation, morphogenesis and large-scale gene oscillations.
Keywords: YAP/TAZ, Notch, Stem Cells, Morphogenesis, Differentiation, Mechanotransduction
From tissue architecture to individual cell decisions
In the last decades, the intricate working of signaling pathways relevant for the control of cell behavior has been described. However, knowledge on the molecular mechanism of how cells respond to a given signal is insufficient to explain where and when a specific cell might change its behavior, and how this may impact on the behavior of surrounding cells. In fact, we still have only a scant understanding of how nature builds tissues with specific forms and functions, and how disturbance of normal cellular ecosystems results in the dysfunction of diseases. In other words, understanding spatial control of cell behavior at all levels requires the incorporation of new conceptual and molecular frameworks by which information on structural and architectural complexity of living tissues are imbued to individual cells and transmitted to neighboring cells [1,2]. In this review, we focus on evidence suggesting that one such new framework is represented by the connections between the biology of YAP/TAZ, two transcription factors serving as central sensors of the physical and mechanical properties of the microenvironment (see Glossary), and Notch signaling, one of the most established mediators of short-range signaling interactions by which neighboring cells mutually control each other's fate.
Glossary.
Fate specification: the process by which a ‘stem/progenitor’ is induced to differentiate by intrinsic or extrinsic signals, typically turning into a cell carrying out a specialised, tissue- specific function.
Hamartoma: benign, tumor-like malformation
Hydrogels: Polymeric biomaterials forming a 3D network that hold water; hydrogels can be engineered by adding adhesive extracellular matrix proteins, and their rigidity controlled by modulating the number of cross-links of their mesh. They offer culture conditions that are more “defined” in term of chemical and physical attributes over traditional tissue culture procedures, as required for “in human” use of stem cells.
Juxtacrine signaling: a signalling modality involving close contact between cells. Typically, this involves a receptor in one cell and a ligand on a neighboring cell. The signal may also be transmitted through gap junctions.
Lineage tracing: series of methodologies, and particularly genetic tools, allowing irreversible labelling (with fluorescent or other reporter genes), and thus tracking in space and time, the cells of a specific tissue, or specific subtypes of cells expressing a certain marker in one or multiple tissues.
Mechanotransduction: the mechanisms by which cells sense and translate physical forces into biochemical signals to adapt their behavior to the environment.
Microenvironment: indicates the set of physical, chemical and cellular elements that surrounds a cell or a portion of a tissue, influencing cell behaviour.
Multi-omics: the integrated analysis of a biological data set from different high-throughput ‘Omic’ technologies such as genomics, transcriptomics, proteomics, epigenomics or metabolomics.
Organoid: is an outgrowth of ‘stem/progenitor’ cells embedded within a three dimensional ECM, in which cells spontaneously self-organize into structures that recapitulate at least some histological features of the natural organs from which they derive. Organoids can be expanded through serial passaging.
RNA-seq: methods based on next-generation sequencing which reveal the presence and relative quantity of RNA species in a biological sample.
YAP/TAZ: sensors of the microenvironment
Since their identification more than 20 years ago [3] [4], the two highly related transcriptional co-factors YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif, also known as WWTR1) have been proved to operate as sensors of cell polarity, density, shape and cytoskeletal organization [1]. These features are connected to the 3D adhesive and spatial relationships of the cell with other cells or the surrounding extracellular matrix (ECM) (Figure 1A). For example, YAP/TAZ are activated in response to increasing mechanical stresses, such as when cells adopt a spread cell morphology, experience adhesion to a hard extracellular matrix, or undergo deformation due to substrate topology; all these conditions impact on the structural organization of the F-actin cytoskeleton thereby favouring focal adhesion and actin stress fibre formation [1]. Conversely, YAP/TAZ are inactivated when cells experience a soft ECM, adhesion to small substrate areas and formation of polarized epithelial sheets, that are conditions induced by high-cell density and typical of normal tissues [1].
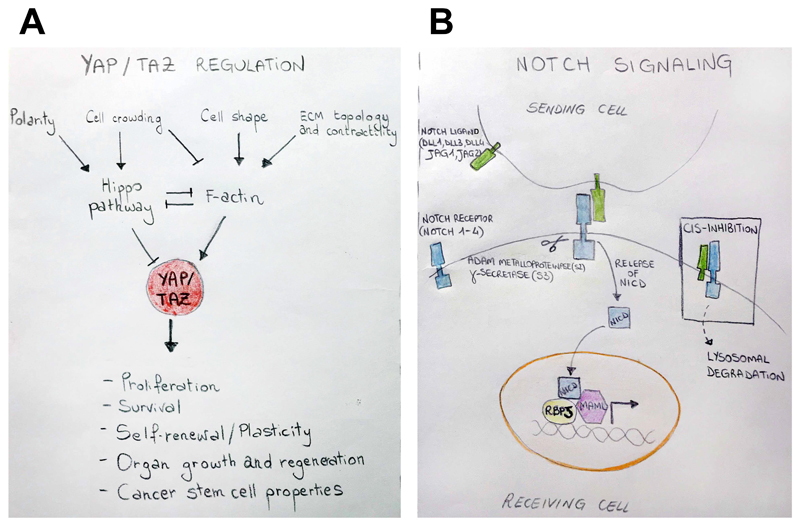
Figure 1. Overview of YAP/TAZ and Notch pathways.
(A) Schematic representation of YAP and TAZ (YAP/TAZ) regulatory inputs and biological functions. The Hippo pathway and mechanical cues are the two main pillars conveying a heterogeneous set of signals for YAP/TAZ regulation. Polarity and cell crowding can inhibit YAP/TAZ through the activation of the Hippo pathway, while mechanical features promoting a proficient F-actin cytoskeleton sustain YAP/TAZ activity. Additionally, Hippo signaling and F-actin cytoskeleton can negatively affect each other [1]. Activated YAP/TAZ enter the nucleus, where they regulate genetic programs required for fundamental cellular behavior (proliferation, survival, stem cell’s fate and plasticity) and biological processes (organ growth, tissue regeneration, tumorigenesis). (B) Notch signaling cascade. The Notch pathway is activated by the engagement of a receptor (blue) on the signal-receiving cell with the ligand (green) on the neighboring signal-sending cell. This transinteraction triggers two sequential proteolytic cleavages of the Notch receptor mediated by ADAM proteases (S2 cleavage) and gamma-secretase (γ-secretase) (S3 cleavage), leading to the release of the Notch intracellular domain (NICD) fragment. NICD then translocates to the nucleus, where it activates the transcription of Notch target genes together with the transcription factor RBPJ (yellow) and the nuclear effector Mastermind-like (MAML, purple). On the contrary, the interaction between ligands and receptors on the surface of the same cell results in cis inhibition of Notch signals.
As extensively reviewed elsewhere [1,5,6] (see Figure 1A), YAP/TAZ are regulated by the Hippo pathway (Box1) and by Hippo-independent signalling cascades. Biologically, YAP/TAZ are not required for normal physiology of most adult organs, but play essential roles in: i) organ growth during embryonic development; ii) promoting tissue repair after injury in adult tissues; iii) for ex-vivo expansion of organoids (GLOSSARY) from explanted primary epithelial cells; iv) for the emergence of multiple solid tumors in mouse and human experimental models [1,5].
Box 1. The Hippo pathway.
The early understandings of the biology and the biochemical regulation of YAP/TAZ came from seminal studies in Drosophila where, through genetic screens for mutations that control tissue overgrowth, the YAP/TAZ ortholog Yorkie (Yki) was first recognized as the downstream effector of the Hippo pathway. Pioneering studies using the fly model system also led to the identification of Hippo signaling as a potent tumor suppressor pathway, in which the hierarchical and evolutionary conserved kinases, Hpo/MST1/2 and Warts/LATS1/2, negatively regulate YAP/TAZ by phosphorylation, promote their cytoplasmic retention or ubiquitination and proteasomal degradation. Loss of Hippo fosters YAP/TAZ/Yki nuclear localization, where they regulate transcription mainly interacting with enhancer elements through the TEAD/Scalopped DNA-binding factors [6,81].
Of particular relevance for this review, is the regulation of YAP/TAZ by the structural features of the microenvironment that make these transcription factors a molecular bridge to understand how tissues control the behavior of their constituent cells with great spatial specificity. The interconnections between YAP/TAZ and the Notch pathway here outlined, represent an emerging example of this control
The basics of Notch signaling
Notch signaling promotes cell-cell communication through juxtacrine signaling (GLOSSARY), which originates by the interaction between single-pass transmembrane ligands and receptors of the Delta–Serrate–Lag (DSL) and Notch protein families, respectively [7,8]. In vertebrates, four Notch receptors (NOTCH1-4) and five Delta-Serrate-Lag (Dsl) ligands [Jagged 1 (JAG1), JAG2, Delta-like 1 (DLL1), DLL3, DLL4] have been identified (Figure 1B). Notch signaling is repeatedly used in development and homeostasis of multiple tissues, generating a diverse array of cellular responses; Notch activity regulates growth, differentiation, survival and stem cell behavior in a highly context-dependent manner [7,8].
The mechanisms of Notch signaling have been covered by several excellent reviews [7,9,10]. Here we would like to reprise a few aspects of Notch signaling. The first is the classic modality of "in trans" Notch signaling, occurring when Delta/Jagged ligands and Notch receptors are expressed on the membranes of adjacent cells: binding initiates a series of proteolytic cleavages of the Notch receptor, leading to the release of the Notch intracellular domain (NICD) fragment from the membrane. NICD then translocates to the nucleus, where it binds the transcription factor RBPJ [(recombining binding protein suppressor of hairless), also known as CBF1 or CSL in mammals] and the nuclear effector Mastermind-like (MAML), thereby activating transcription of Notch target genes, such as Hey- and Hes-like genes [9,10] (Figure 1B).
A second aspect of Notch signaling is "lateral" inhibition, by which small differences in Notch activity existing between neighboring cells are amplified [10]. Lateral inhibition is mediated by transcriptional mechanisms, by which, for example, activation of the Notch receptor leads to cell-autonomous transcriptional repression of Notch ligands, as such making the cell receiving Notch signaling progressively less and less capable of sending the same signal. This progressively turns off Notch signaling in the adjacent cells, thereby generating opposite ON-OFF Notch cell states [10].
A third feature of Notch signaling is "cis inhibition", which occurs when Notch ligands and receptors are co-expressed in the same cell. It is thought that this interaction makes Notch receptor refractory to in trans associations. In other words, cells experiencing cis inhibition are protected from receiving Notch signaling from ligands expressed by surrounding cells [10,11]. cis inhibition serves as a defense-system against ligand-independent Notch receptor activation, to reinforce (or initiate) lateral inhibition and as a threshold-setting system that reduces errors in cell decisions [12] (Figure 1B). Together, the spatial tissue distribution of cis and trans interactions defines clear-cut switches between mutually alternative signaling states and creates cell fate boundaries.
However, two fundamental questions remain. The first is, what establishes the initial asymmetries in Notch activity prior to their amplification through lateral inhibition and cis-trans interactions? Several studies have uncovered extensive signaling crosstalk between Notch and other cascades initiated by growth factors and morphogens, such as RTK ligands, Wnt, TGF-β or other signals [8,13–15]. However, soluble factors alone cannot explain sharp cell fate decisions that Notch signaling orchestrates on the micrometre scale between adjacent cells. For example, asymmetries in Notch signaling emerge in vitro, and without any morphogen gradient, within self-organizing organoids developing from single stem cells embedded in saturating amounts of growth factors [16]. The second question relates to the nature of the context-dependency of Notch-responses [8,10]. What is "context"? Distinct tissue-specific transcriptional partners of the Notch cascade have been proposed to operate as elements of tissue-specific responsiveness [17–19]. However, the heterogeneity of the Notch outcome is not only evident when comparing different tissues but also within the same tissue. Clearly, the effects of Notch must be understood in the context of an overarching molecular framework operating at the tissue level, which is somehow embedded into tissue architectures and microenvironments. In other words, mechanisms must exist to wire cell mechanics and cell-to-cell communication systems.
A YAP/TAZ-Notch signaling crosstalk
There are two main modalities by which YAP/TAZ and Notch signaling cascades have been reported to interact: YAP/TAZ-mediated transcriptional regulation of Notch ligands or receptors; and joined transcriptional co-regulation of common direct targets genes by YAP/TAZ and NICD (Figure 2). Below, we review different examples of these interactions in various cellular contexts. The reader should be aware that, in spite of the functional redundancy between YAP and TAZ, some studies investigated the sole loss of function of YAP in mouse models. The reason is, mostly, practical, as the availability of YAP conditional alleles predates that of TAZ alleles; alternatively, YAP may be, in some tissue types, more expressed than TAZ, and its inactivation offers a sufficient proxy to investigate YAP/TAZ functions.
Figure 2. Interaction between YAP/TAZ and Notch pathways.
(A) When YAP/TAZ (red) are activated, they translocate to the nucleus, where they can induce the gene expression of Notch receptors (blue, left panel) and/or Notch ligands (green, right panel) in order to regulate Notch signaling. (B) Concomitant activation of YAP/TAZ and Notch signaling induces nuclear translocation of YAP/TAZ and the transcriptionally active NICD peptide, thus co-operatively regulating the expression of common target genes. The different biological contexts are indicated on the figure. NICD, Notch intracellular domain; SCs, stem cells.
Early Embryonic Development
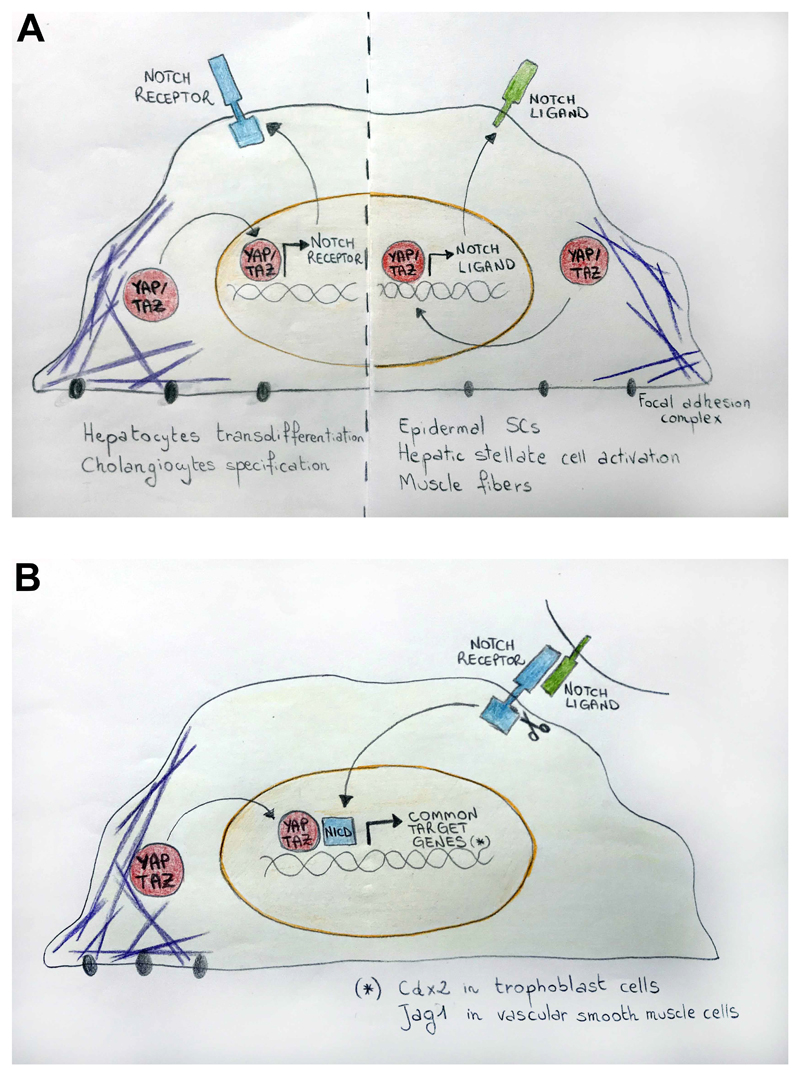
During the very early phases of embryonic development, YAP/TAZ are involved in the first lineage specification at the transition between the morula stage and blastocyst, being required for trophoectoderm (TE) specification in outer cells. The unpolarized cells inside the developing blastocyst retain inactive YAP/TAZ and acquire inner cell mass (ICM) identity [20]. Both Hippo pathway and Hippo-independent mechanical inputs, such as cytoskeletal contractility and pulling forces between different cells, are involved in the regulation of YAP/TAZ nuclear localization and transcriptional activity required to induce the expression of the TE master gene Cdx2 [20,21]. Notch activity is restricted to the outer cells of the blastocyst where it acts in cooperation with YAP/TAZ to regulate Cdx2 expression [22]. Mechanistically, YAP/TEAD4 and Notch/RBPJ transcriptional complexes bind to distinct sites in a trophectoderm-specific enhancer of Cdx2 [22] and synergistically regulate the expression of Cdx2 [23] (Figure 3A). Moreover, the observation that the YAP/TAZ DNA-binding partner TEAD4 is highly enriched in the promoter of Notch2 in mouse trophoblast cells, further suggests that YAP/TAZ could also regulate the transcription of this receptor, adding an additional level of feedback [24].
Figure 3. Biological responses controlled by YAP/TAZ and Notch crosstalk.
(A) The differentiation of the outer cells of the blastocyst to trophectoderm lineage is promoted by co-operative transcriptional regulation between YAP/TAZ (red) and Notch signaling (blue). Nuclear YAP/TAZ and NICD (red and blue stripes) jointly control the Cdx2 enhancer to activate its expression in prospective throphoblast cells. (B) In the basal keratinocytes of epidermis, mechanical signals from the basement membrane sustain YAP/TAZ nuclear localization and Notch cis-inhibition. YAP/TAZ and Notch signaling are active respectively in the basal (red) and suprabasal layers (blue). (C) Loss of Notch in corneal epithelial cells triggers chronic inflammation with consequent ECM remodeling in the underlying stromal tissue. (D) YAP/TAZ activation in mature hepatocytes directly regulates gene transcription of the Notch2 receptor, contributing to hepatocyte transdifferentiation into oval cells/HPCs. (E) Upon tissue damage, interleukin-mediated activation of YAP/TAZ upregulates Notch signaling in ISC promoting regeneration. (F) YAP/TAZ cooperate with Notch signaling to regulate the formation of a multilayered artery wall. Jag1 ligand expressed on the surface of endothelial cells activates Notch signaling in the neural crest-derived muscle progenitors, triggering their differentiation in VSMCs. In the newly forming VSMCs the Notch transcriptional complex recruits YAP to regulate gene expression of Jag1, leading to iterative sequences of muscle progenitors recruitment and VSMCs formation. (G) In the postmitotic muscle fibres of chick embryo, mechano-dependent activation of YAP by muscle contraction triggers gene expression of Jag2, which activates Notch signaling in satellite cells (muscle progenitors) to prevent their differentiation. ECM, extracellular matrix; ISC, intestinal stem cell; VSMCs: vascular smooth muscle cells.
Epidermis
The epidermis is a pluristratified epithelium with key protective and elastic properties [25,26]. Rapid epidermal turnover and tissue homeostasis are ensured by the keratinocyte basal layer, which contains the tissue progenitors. During epidermal stratification, as cells lose contact with the basement membrane they start to differentiate and undertake a complex process of morphological and structural changes that ends with the formation of the outermost cornified layer [26].
Nuclear YAP/TAZ localization is readily detected in the basal layer of the skin of newborn mice, while only very few cells retain nuclear YAP/TAZ in the adult epidermis of both mice and humans [27–29] (Totaro et al., unpublished). In transgenic mice, overexpression of an activated form of YAP in the basal layer of the epidermis induces expansion of the epidermal progenitor compartments at the expense of the differentiated suprabasal layers [27,28,30]. These phenotypes are recapitulated by genetic inactivation of the Hippo pathway [31] or of α-catenin, a component of the adherent junctions (AJ) proposed to favor YAP/TAZ cytoplasmic sequestration [32]. YAP/TAZ are also required for skin development, as YAP/TAZ knockout in the basal layer of the embryonic epidermis reduces keratinocyte proliferation with concomitant precocious terminal differentiation leading to skin thinning [30,32]. In adult mice, YAP/TAZ are required for epidermal wound-healing [29].
The mechanosensitive properties of the epidermis have been recently recapitulated in vitro by culturing epidermal progenitor cells into engineered surfaces or hydrogels (GLOSSARY) of defined elasticity, turning YAP/TAZ ON or OFF in order to inhibit or induce keratinocyte differentiation, respectively [30]. Notch signaling is a key determinant of keratinocyte differentiation and is required for the transition of keratinocytes from the basal to the suprabasal layers [7]. The activity of the Notch pathway is spatially regulated by the preferential distribution of Notch ligands and receptors throughout the different epidermal layers. The basal layer mainly expresses the Notch ligands Dll1 and Jag2, whereas the Notch receptors are enriched in the overlying spinous and granular layers, where the Notch signaling is most active [26,33].
The function of Notch in epidermal cells appears to recapitulate, at least in vitro, the effects of weak mechanical forces, and is antithetic to the activity of YAP/TAZ. Indeed, mechanical activation of YAP/TAZ preserves the epidermal SC fate by turning on the transcription of DLL1, DLL3 and JAG2 [30,34]. Mechanistically, YAP/TAZ-driven expression of Delta ligands is instrumental for cell-autonomous cis inhibition of Notch signaling, rendering basal progenitors under mechanical stress resistant to differentiation [30] (Figure 3B). In vivo, YAP overexpression in the skin of newborn mice turns on expression of Dll1 and is accompanied by a reduction of Notch transcriptional responses. Furthermore, conditional YAP/TAZ knockout turns off Notch ligand expression, unleashing Notch signaling and thus differentiation of epidermal progenitors [30]. In so doing, YAP/TAZ translate informational cues emanating from the geometry and physicality of their ECM attachment into a cell-to-cell signaling cascade for mutual and accurate regulation of cell fate decisions.
A mechanical interplay between Notch and YAP/TAZ has been also described in the corneal epithelium, a tissue serving as a protective barrier on the anterior ocular surface [35]. Chronic inflammation is a driver of epithelial aberrations and, in the corneal epithelium, it triggers corneal squamous cell metaplasia (CSCM), whereby differentiated corneal cells adopt a keratinized, skin-like fate. Notch and YAP/TAZ mechanically control CSCM, but in this case Notch operates upstream of YAP/TAZ, and the most relevant pool of YAP/TAZ is not the transcriptionally active nuclear pool, but the cytoplasmic one [35]. In fact, cytoplasmic YAP/TAZ are integrated into the Axin/APC destruction complex, where they participate in controlling β-catenin degradation [36,37]. In the corneal epithelium, loss of Notch triggers secretion of inflammatory cytokines, and continuous cycles of damage and repair [35,38,39]. Such persistent inflammation provokes ECM deposition and fibrosis leading to activation of YAP/TAZ mechanotransduction (GLOSSARY) [1]. Under these conditions, YAP/TAZ nuclear accumulation deprives cells of the anti-β-catenin cytoplasmic YAP/TAZ pool, thus favoring β-catenin nuclear accumulation and ultimately triggering CSCM [36] (Figure 3C).
The interplays reported for epidermal or corneal cells may also apply in other contexts. For example, in mammary gland and airway epithelia YAP/TAZ are also expressed in basal layers, where, by inducing Dsl-ligand expression [40], they may at once preserve stemness through cis inhibition and, through trans signaling, foster differentiation of overlying luminal cells (that lack YAP/TAZ activity). Future studies are required to validate these possibilities.
Liver
Inactivation of the Hippo pathway components in the liver induces organ overgrowth. As such, the liver has been used to facilitate study of YAP/TAZ biology in vivo[41]. The hepatocytes are the parenchymal cell of the liver and account for the majority of the cell population, while a small population of epithelial cells, the colangiocytes, form the bile ducts. Upon damage, hepatic progenitor cells (HPCs, also known as “oval cells”), show characteristics of expanded bile duct cells are found in the liver [42]. By lineage tracing (GLOSSARY), it is clear that at least a fraction of oval/HPCs is generated by dedifferentiation of mature hepatocytes [43]. In line, elegant work from Yimlamai et al. demonstrated that in transgenic mice overexpressing YAP the oval cell population expands through a cell-autonomous, Notch-dependent transdifferentiation of mature hepatocytes [44]. YAP/TAZ/TEAD-dependent transcriptional upregulation of Notch2 has been proposed as one mechanisms to explain these events. In line, hepatocyte-specific knockout of the Notch transcriptional coactivator RBPJ inhibits the YAP-mediated reprogramming of hepatocytes in cells that are morphologically similar to oval/HPCs but that in fact also display functional traits as bipotent liver progenitors [44] (Figure 3D).
Inactivation of the sole YAP in the fetal liver is sufficient to trigger bile duct paucity and to limit the appearance of oval/HPCs after cholestatic injury [41]. Interestingly these defects resemble those observed upon liver-specific deletion of Notch2 in mice or as found in Alagille syndrome (AGS), a multiorgan genetic disorder caused by mutation in JAG1 or NOTCH2 genes in humans [7]. Consistent with the effect of YAP gain of function, livers from embryonic NF2-knockout mice are characterized at birth by increased numbers of biliary precursors and primitive ducts, which ultimately cause hamartomas (GLOSSARY) in older mice [45]. This increased biliary fate specification (GLOSSARY) is phenocopied by the overexpression of the intracellular domain of the Notch2 receptor in the liver [7]. In agreement, liver specific inactivation of the Hippo pathway component NF2, with ensuing upregulation of endogenous YAP/TAZ activity, promotes Notch signaling in cholangiocytes and increases the expression of Notch2 and the Notch target genes Hes1 and Sox9. Conversely, loss of Notch2 in NF2-null mouse livers background prevents bile duct overgrowth [45]. However, a contrasting report has more recently placed YAP downstream of Notch signaling in the context of bile duct regeneration induced by partial hepatectomy, suggesting that the RBPJ/NICD complex works as a repressor of YAP transcription [46].
The YAP/Notch interplay may also occur non-cell autonomously, as in stromal-epithelial interactions. Hepatic stellate cells (HSCs) are liver-specific mesenchymal cells, playing an important role during liver development, homeostasis and regeneration after injury [47]. YAP is required for HSC activation into myofibroblast-like cells in response to liver damage [48,49]. Upon biliary injury, HSC-derived myofibroblasts upregulate the expression of the Notch ligand Jag1, promoting Notch signaling and biliary specification in oval/HPCs [50]. Interestingly Jag1 is a YAP target in hepatocytes [44] and human hepatocellular carcinoma [51]. This suggests a scenario in which, upon liver damage, YAP could direct fate specification of liver progenitors toward biliary cells by acting in HSCs and inducing Jag1 expression.
Intestinal epithelium
The epithelium of the small intestine is a tissue with rapid turnover that is composed of a single layer of cells organized in crypts and villi. While the villus constitutes the functional unit of the organ, the crypt structure contains distinct populations of intestinal stem cells (ISCs) [52]. The LGR5+ (Leu-rich repeat-containing G protein-coupled receptor 5-expressing) fast-cycling crypt base columnar (CBC) cells are a pool of ISCs located at the bottom of the crypt where they are intercalated with Paneth cells. CBC cells maintain normal tissue homeostasis as they can either self-renew or generate transit amplifying (TA) tissue progenitor cells. Differentiated cells lost from the surface of the villi are rapidly replaced from cells migrating from the TA region and differentiating into the two main cell lineages of absorptive enterocytes or secretory cells [52].
By immunohistochemistry, nuclear YAP and TAZ can be readily detected at the bottom of the crypt in CBC cells [36,53]. Increasing YAP/TAZ activity through overexpression [54], or after inactivation of the Hippo pathway [53,55,56], induces proliferation of ISCs.
There are intriguing overlaps between YAP/TAZ and Notch signaling in intestinal biology, but also substantial differences. The original idea of an interaction between YAP/TAZ and Notch in the regulation of ISCs came from the seminal work of Camargo and Brummelkamp who found that the expansion of the intestinal progenitors induced by YAP overexpression could be blunted by blocking Notch signaling with a γ-secretase inhibitor [54]. However, independent lines of evidence from various mouse models bearing YAP and/or TAZ knockout alleles ultimately indicated that YAP/TAZ are not required for intestinal homeostasis and maintenance of ISCs [36,55,57]. In contrast to YAP/TAZ, Notch signaling is essential for adult intestinal homeostasis. Signaling mediated by the Notch1 and Notch2 receptors is active in ISCs [58], and induced by the Dll1 and Dll4 ligands expressed in neighboring Paneth cells [59]. As such, intestine-specific deletion of the Notch transcriptional mediator Rbpj [60] results in the loss of intestine progenitors, a phenotype also recapitulated by the genetic inactivation of Notch1/2 [61], or of Dll1/Dll4 [59].
Although the function of Notch and YAP/TAZ signaling is clearly distinct during homeostasis, they ostensibly intersect each other during intestinal regeneration, a response for which YAP/TAZ are essential [53,57,62,63]. The IL-6 co-receptor gp130 is activated during intestinal inflammation, and expression of a constitutively active form of gp130 activates and requires YAP to induce a hyperproliferative response of intestinal cells and for intestinal regeneration in a model of inflammatory colitis [63]. The activation of YAP in villin-gp130Act transgenic mice is associated with the upregulation of the mRNA level of both Notch receptors and ligands, and with increased expression of the Notch target Hes1 in intestinal crypts. The YAP-mediated restorative response is repressed by treatment with a γ-secretase inhibitor, while YAP deletion prevents upregulation of Notch signaling [63]. This suggests that, by undefined mechanisms, Notch signaling is downstream of YAP/TAZ activation during intestinal repair after inflammation (Figure 3E).
Vascular smooth muscle
Notch signaling plays an important role in the organization and thickening of vascular smooth muscle cells (VSMCs) along artery walls [64]. Vascular SMCs of the ascending aorta, the aortic arch and pulmonary trunk are derived from the neural crest. In these locations, vascular endothelial cells expressing the Notch ligand Jag1 activate Notch receptors on neural crest precursors, promoting their differentiation into VSMCs [64]. Interestingly, the activation of Notch signaling in smooth muscle precursors induces a feed-forward loop with upregulation of Jag1 ligand in newly differentiating SMCs. Throughout sequential activation of this positive feedback response, also known as lateral induction, the developing artery is able to recruit additional smooth muscle precursors and give rise to multiple concentric layers around the endothelial layer in aortic arch derivatives [65]. Neural crest-specific deletion of YAP and TAZ abrogates Notch signaling in SMCs, and impairs development of the aortic arch arteries [66]. This phenotype recapitulates that of mice in which RBPJ has been deleted in neural crest cells [64]. Mechanistically, YAP and NICD can physically interact and regulate the expression of Jag1 [66]. Mechano-regulation of YAP/TAZ activity have been involved in the regulation of VSMC fate in vessel development and homeostasis [1], as well as in the regulation of Notch signaling [30]. It is thus tempting to speculate that the physical forces produced by blood flow could impinge on YAP/TAZ as a mechano-sensor of arterial thickness, fostering Notch-driven layering of VSMCs during vascular development, or terminating it when the final size of the mature arterial wall has been reached (Figure 3F).
Somite development and skeletal muscle
Molecular oscillators are involved in a broad variety of biological functions [67]. The "segmentation clock" is a prominent example of such rhythmic gene activity, by which pairs of blocks of mesodermal cells called somites are progressively formed along the antero-posterior axis of vertebrate embryos. The somites generate the vertebral column, the ribs, all skeletal muscles and part of the dermis, and are responsible for the segmental organization of nerves and blood vessels. The segmentation clock is essentially a set of periodic waves of gene expression traveling through the presomitic mesoderm (PSM), which entails periodic activation of Notch signaling [68]. The nature of this oscillatory behavior remains elusive. A recent report intriguingly highlighted the convergence of YAP/TAZ mechanotransduction and Notch signaling as part of an excitable system that initiates oscillations [69]. Attenuating YAP, by lowering mechanotransduction, has been shown to be sufficient to initiate an oscillatory gene expression pattern even in a single PSM cell. Conversely, oscillations were blocked by artificially raising YAP levels. In part, this is mediated by YAP negatively impacting on Notch signaling, but also by YAP controlling classic NICD target genes, such as Hes7, in a Notch-independent manner [69].
In adult skeletal muscle, satellite cells represent a reservoir of quiescent muscle stem cells located in close proximity to mature myofibers. Upon tissue damage or physical exercise, satellite cells must activate, proliferate, commit to a myoblast lineage and ultimately differentiate to fuse with each other or to pre-existing myofibers, as such those regenerating the muscle or mediating hypertrophy [70]. Satellite cells express higher levels of Notch receptors, while differentiated myotubes and fibers constitute the source of Notch ligands [71]. Notch signaling in satellite cells inhibits their differentiation. Conversely, YAP activity promotes proliferation of satellite cells in vitro [72]. Recently, work in chick embryos provided an elegant demonstration of how YAP and its regulation by muscle mechanics determines the fate of satellite cells through Notch signaling [73]. It was found that YAP is mechanically activated by muscle contractility in differentiated, post-mitotic muscle fibers. Here, YAP-dependent transcriptional regulation of the Notch ligand Jag2 activates Notch signaling in neighboring satellite cells, preserving their potency and preventing their differentiation (Figure 3G). Upon muscle immobilization, loss of YAP mechanical activation causes a reduction of Jag2 expression in myofibers, with consequent differentiation of the satellite cells. In line, experimental overexpression of either a constitutive active YAP or of Dll1 in paralyzed muscle fibers prevented the decrease of muscle progenitors [73]. This mechanism might be potentially relevant to foster muscle regenerative responses; however further studies are required to validate its existence in mammals.
Recently, a bidirectional crosstalk between YAP/TAZ and Notch signaling has been described in human Embryonal Rhabdomyosarcomas (ERMS), a soft tissue sarcoma of mesenchymal origin characterized by the persistence of a satellite-like cell state at the expense of myoblast differentiation [74,75]. Both YAP/TAZ and Notch signalling are hyperactivated in human ERMS tumors and rhabdomyosarcoma-derived cell lines [76–78]. Based on in vitro studies, it has been proposed that Notch signalling transcriptionally upregulates YAP levels, promoting ERMS rhabdosphere proliferation. In turn, the increase of YAP/TAZ transcriptional activity can feed Notch signalling by upregulating the expression of Jag1 and Dll1 ligands and the Rbpj transcription factor [78].
Concluding Remarks
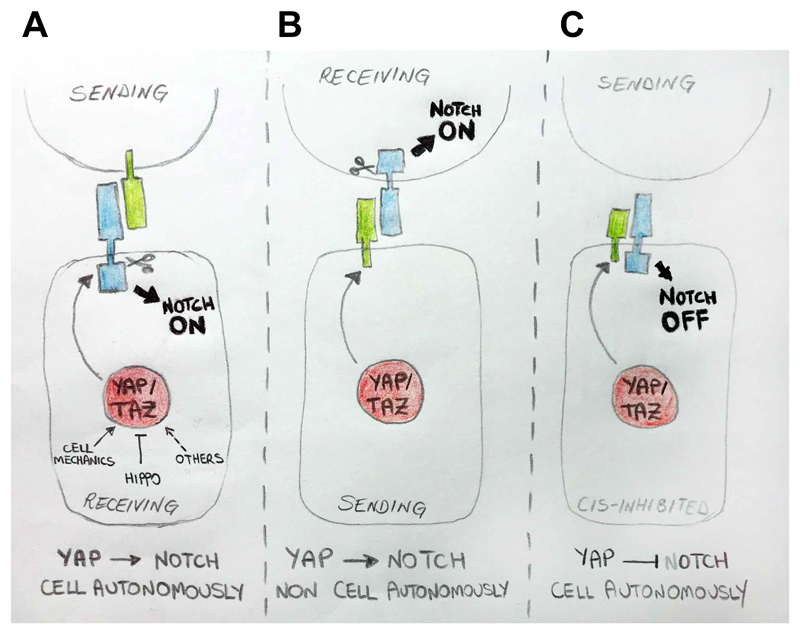
An intriguing scenario emerging is one in which structural features of the microenvironment control Notch signaling through cellular mechanotransduction and YAP/TAZ. The ability of YAP/TAZ to directly control the transcription of Dsl ligands raises the possibility of understanding how localized mechanical signals - such as attachment to the basement membrane, local deformations of tissues or the shape of individual cells - create complex spatial patterns of cell fate determination (See Outstanding Questions). For example, the Delta-like or Jagged ligands expressed by the YAP/TAZ ON cells may cause a NOTCH OFF state cell autonomously, through in cis inhibition, and a NOTCH ON state in the adjoining cells through in trans signaling (Figure 4). Notably, these basic modules can be complicated, but still rationalized, if we consider these interplays occurring between distinct cell types, for example between stromal and epithelial cells, or between stem cells and their niches. Clearly, much more work is required to validate these models.
Figure 4. YAP/TAZ dictate context-specific Notch signaling outcomes.
Regulation of YAP/TAZ by different inputs, can affect Notch signaling in a spatial and context-specific manner. (A) YAP/TAZ can promote cell-autonomously Notch signaling, by regulating the gene expression of Notch receptors, which are activated in trans by ligands expressed on the cell surface of neighbouring cells. (B) Regulation of Notch ligands' expression in signal-sending cells by YAP/TAZ, in turn inducing Notch signaling non-cell autonomously in signal-receiving cells. (C) The YAP/TAZ-dependent expression of Notch ligands can inhibit Notch signaling cell autonomously, by cis-interaction of ligands and receptors.
As we appreciate the potential relevance of these mechanical and juxtacrine interactions in generating fine-tuned tissue patterning, we should also acknowledge that most of our present tools have been quite inadequate to tackle the reality of this spatial, temporal and functional heterogeneity. A substantial part of our knowledge on YAP/TAZ and Notch signaling in vivo has been derived from analyses of bulk tissues, relaying information on "averaged" cells that may, in fact, not even exist. Clearly, new approaches such as in situ single-cell RNA-seq (GLOSSARY) or comparable advancements in single cell multi-omics (GLOSSARY) will be extremely valuable at dissecting the pattern of YAP/TAZ -Notch activities in living tissues [79]. This may also shed light into the otherwise nebulous concept of "context dependency" of the effects of Notch signaling, as depending on specific mechanical conditions, YAP may recruit varying proportions of cells into a Notch ON or OFF state, often with dramatically opposite outcomes (Figure 4). Tumor biology may be a case in point. In several tumors, YAP/TAZ and Notch are pro-oncogenic but are at odds with other effects of Notch such as lineage-committing, pro-differentiating functions. However, this paradox may be resolved, at least in some cases, by envisioning that YAP/TAZ may turn on Delta to inhibit differentiation of cancer stem cells while preventing their expansion, and concomitantly fostering Notch and amplification of their immediate neighboring descendants. In the absence of specific markers and single cell signatures it would be hard to dissect or rationalize the real complexity of these events from bulk tissue analyses. Similarly, functional investigations should be also directed at individual cells developing within tissues, for example by implementing optogenetic tools in cultured organoids or in vivo. At the same time, reconstructing ex-vivo the outcomes of Notch signaling may require the phenocopy of natural niches by sophisticated microfabrication tools for example 3D scaffolds of defined shapes with localized deposition of Notch ligands.
Finally, the widespread role of Notch in human cancer and genetic diseases makes Notch a potentially very effective route of therapeutic intervention. However, the appeal of this endeavor is clearly questioned by the broad generality of Notch signaling in several tissues, as any anti-Notch therapy would invariably be met by toxicity [80]. Yet, the YAP/TAZ mechanotransduction mechanisms supporting Notch in disease states may offer an alternative, as tackling YAP/TAZ has been shown to be effective at preventing tumor progression and other diseases while being dispensable for homeostasis of normal tissues [1,5].
References
- 1.Panciera T, et al. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller E, Fuchs E. Tissue patterning and cellular mechanics. J Cell Biol. 2015;211:219–231. doi: 10.1083/jcb.201506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. [PubMed] [Google Scholar]
- 4.Kanai F, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. The EMBO Journal. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanconato F, et al. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng Z, et al. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 8.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 9.Kovall RA, et al. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Developmental Cell. 2017;41:228–241. doi: 10.1016/j.devcel.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guruharsha KG, et al. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaron A, Sprinzak D. The cis side of juxtacrine signaling: a new role in the development of the nervous system. Trends Neurosci. 2012;35:230–239. doi: 10.1016/j.tins.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Palmer WH, Deng W-M. Ligand-Independent Mechanisms of Notch Activity. Trends in Cell Biology. 2015;25:697–707. doi: 10.1016/j.tcb.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson ER, et al. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 14.Collu GM, et al. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71:3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazio C, Ricciardiello L. Inflammation and Notch signaling: a crosstalk with opposite effects on tumorigenesis. Cell Death Dis. 2016;7:e2515–e2515. doi: 10.1038/cddis.2016.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci USA. 2011;108:14908–14913. doi: 10.1073/pnas.1109023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard F, et al. Specificity of Notch pathway activation: twist controls the transcriptional output in adult muscle progenitors. Development. 2010;137:2633–2642. doi: 10.1242/dev.053181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cave JW, et al. A DNA transcription code for cell-specific gene activation by notch signaling. Curr Biol. 2005;15:94–104. doi: 10.1016/j.cub.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 20.Chazaud C, Yamanaka Y. Lineage specification in the mouse preimplantation embryo. Development. 2016;143:1063–1074. doi: 10.1242/dev.128314. [DOI] [PubMed] [Google Scholar]
- 21.Plusa B, Hadjantonakis A-K. Mammalian development: Mechanics drives cell differentiation. Nature. 2016;536:281–282. doi: 10.1038/nature18920. [DOI] [PubMed] [Google Scholar]
- 22.Rayon T, et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental Cell. 2014;30:410–422. doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe Y, et al. Notch and Hippo signaling converge on Strawberry Notch 1 (Sbno1) to synergistically activate Cdx2 during specification of the trophectoderm. Sci Rep. 2017;7 doi: 10.1038/srep46135. 46135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Home P, et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA. 2012;109:7362–7367. doi: 10.1073/pnas.1201595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson CL, et al. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu Y-C, et al. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proceedings of the National Academy of Sciences. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbediwy A, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totaro A, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:1–13. doi: 10.1038/ncomms15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccolo S, et al. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 32.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowell C, Radtke F. Cutaneous Notch signaling in health and disease. Cold Spring Harb Perspect Med. 2013;3:a017772. doi: 10.1101/cshperspect.a017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowell S, et al. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 35.Nowell CS, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol. 2016;18:168–180. doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzolin L, et al. YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Azzolin L, et al. Role of TAZ as Mediator of Wnt Signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Demehri S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumortier A, et al. Atopic Dermatitis-Like Disease and Associated Lethal Myeloproliferative Disorder Arise from Loss of Notch Signaling in the Murine Skin. PLoS ONE. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panciera T, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SH, et al. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology. 2017;152:533–545. doi: 10.1053/j.gastro.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyajima A, et al. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Tarlow BD, et al. Bipotential Adult Liver Progenitors Are Derived from Chronically Injured Mature Hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yimlamai D, et al. Hippo Pathway Activity Influences Liver Cell Fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu N, et al. The Hippo signaling functions through the Notch signaling to regulate intrahepatic bile duct development in mammals. Lab Invest. 2017;97:843–853. doi: 10.1038/labinvest.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu J, et al. Notch Signaling Coordinates Progenitor Cell-Mediated Biliary Regeneration Following Partial Hepatectomy. Sci Rep. 2016;6 doi: 10.1038/srep22754. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin C, et al. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannaerts I, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Caliari SR, et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep. 2016;6 doi: 10.1038/srep21387. 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boulter L, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschaharganeh DF, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. 2013;144:1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 53.Gregorieff A, et al. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 54.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 55.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein(Yap) overabundance. Proceedings of the National Academy of Sciences. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imajo M, et al. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 57.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fre S, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS ONE. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pellegrinet L, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240.e1–7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 61.Riccio O, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto R, et al. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G23–35. doi: 10.1152/ajpgi.90225.2008. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mead TJ, Yutzey KE. Notch pathway regulation of neural crest cell development in vivo. Dev Dyn. 2012;241:376–389. doi: 10.1002/dvdy.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manderfield LJ, et al. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125:314–323. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manderfield LJ, et al. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015;142:2962–2971. doi: 10.1242/dev.125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonnen KF, Aulehla A. Dynamic signal encoding--from cells to organisms. Semin Cell Dev Biol. 2014;34:91–98. doi: 10.1016/j.semcdb.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Hubaud A, Pourquié O. Signalling dynamics in vertebrate segmentation. Nat Rev Mol Cell Biol. 2014;15:709–721. doi: 10.1038/nrm3891. [DOI] [PubMed] [Google Scholar]
- 69.Hubaud A, et al. Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell. 2017;171:668–682.e11. doi: 10.1016/j.cell.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tierney MT, Sacco A. Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis. Trends in Cell Biology. 2016;26:434–444. doi: 10.1016/j.tcb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mourikis P, Tajbakhsh S. Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev Biol. 2014;14:2. doi: 10.1186/1471-213X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Judson RN, et al. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J Cell Sci. 2012;125:6009–6019. doi: 10.1242/jcs.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esteves de Lima J, et al. Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis. Elife. 2016;5:3593. doi: 10.7554/eLife.15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia SJ, et al. Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther. 2002;1:97–104. doi: 10.4161/cbt.51. [DOI] [PubMed] [Google Scholar]
- 75.Conti B, et al. Recent Insights into Notch Signaling in Embryonal Rhabdomyosarcoma. Curr Drug Targets. 2016;17:1235–1244. doi: 10.2174/1389450116666150907105756. [DOI] [PubMed] [Google Scholar]
- 76.Ignatius MS, et al. The NOTCH1/SNAIL1/MEF2C Pathway Regulates Growth and Self-Renewal in Embryonal Rhabdomyosarcoma. Cell Rep. 2017;19:2304–2318. doi: 10.1016/j.celrep.2017.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tremblay AM, et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 2014;26:273–287. doi: 10.1016/j.ccr.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 78.Slemmons KK, et al. A Novel Notch-YAP Circuit Drives Stemness and Tumorigenesis in Embryonal Rhabdomyosarcoma. Mol Cancer Res. 2017;15:1777–1791. doi: 10.1158/1541-7786.MCR-17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Regev A, et al. The Human Cell Atlas. Elife. 2017;6:503. doi: 10.7554/eLife.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 81.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]