Abstract
Introduction
There are no currently approved treatments for choroideremia, an X-linked progressive inherited retinal degeneration that leads to blindness by middle age. Several treatment options are being explored, but with major advances in adeno-associated vector (AAV) gene replacement therapy that has reached phase III clinical trials.
Areas covered
In this review we discuss new insights into the clinical phenotyping and genetic testing of choroideremia patients, that aid disease characterisation, progression and patient inclusion into clinical trials. Recent advances in in-vitro studies have resulted in the development of functional assays that can be used to confirm the diagnosis in challenging cases and to quantify vector potency for use in clinical trials. We review the progress in current gene therapy trials and some considerations towards gene therapy approval for the treatment of choroideremia. Lastly, we discuss developments in alternative therapies including optogenetics.
Expert Commentary
AAV gene replacement therapy is the most promising treatment strategy for choroideremia, that has developed exponentially over the last few years with a phase III clinical trial now underway. Optogenetics is a promising alternative strategy that might be applicable in late stages of degeneration.
Keywords: choroideremia, CHM gene, REP1, AAV gene therapy, clinical trials
1. Introduction
Choroideremia is an X-linked recessive inherited retinal degeneration caused by the loss of function or absence of Rab escort protein 1 (RPE1) which is encoded by the CHM gene (1,2). There are currently no approved treatments for choroideremia. Gene therapy is a very attractive treatment option for this monogenic eye disorder using an adeno-associated viral vector (AAV), which can easily contain the CHM cDNA cargo of 1.9kB. Proof-of-concept animal (3,4) and in-vitro studies (5–8) have demonstrated efficacy and restoration of REP1 expression, leading to multiple clinical trials (9–16). This review discusses the progress in pre-clinical research and the development of an in-vitro functional assay that can be used to confirm the diagnosis in challenging cases and to test the vector potency currently used in gene therapy clinical trials (17). The advances in ongoing clinical trials are also reviewed in view of the recently published reports from the leading centres in choroideremia research (11–14). Finally, we offer expert opinion on considerations necessary for regulatory approval of gene therapy for choroideremia and discuss development of alternative therapies including optogenetics for more advanced stages of the disease.
2. Diagnosis and genetic testing in choroideremia
Affected male patients with choroideremia typically experience nyctalopia in early childhood, followed by progressive loss of peripheral visual field in 20s and 30s. Most patients are registered legally blind by the fifth to sixth decade of life (18). Female carriers typically maintain good visual function throughout life, although some report nyctalopia and a few present with a severe phenotype similar to the male pattern of degeneration (19). Although REP1 deficiency is ubiquitous in patients with choroideremia, there are no known systemic associations to date. X chromosome disruptions in males (20, 21) and translocations in females (22) have nonetheless resulted in syndromic phenotypes. In males, chromosomal defects causing choroideremia has been associated with obesity, congenital deafness and developmental delay (21) and cleft lip and palate and severe developmental delay (20) whereas in female carriers, translocation between chromosomes X, 1 and 3 has led to choroideremia with ectodermal dysplasia (22). There are two reports in the literature regarding the presence (23) or absence (24) of intracellular crystals in lymphocytes and fatty acid abnormalities in serum and red blood cells in choroideremia patients compared to controls throwing conflicting evidence towards choroideremia being part of widespread systemic disease.
A male child with choroideremia manifests with early signs of peripheral pigment clumping similar to what is typically seen in adult female carriers (Figure 1). Progressive retino-choroidal degeneration soon leads to a characteristic barren appearance of sclera and prominent large choroidal vasculature. This pattern of degeneration is ideally captured by funds autofluorescence, which signals lipofuscin distribution in retinal pigment epithelium and to a lesser extent in photoreceptors, both of which are affected in choroideremia. Thus, loss of autofluorescence in periphery is contrasted by sharply demarcated stellar borders of increased signal from central degenerating tissue (15,16). This central degeneration has two phases, smooth para-foveal zone and a more mottled appearance towards the edges, both of which shrink progressively with a complete loss of the smooth area in later stages.
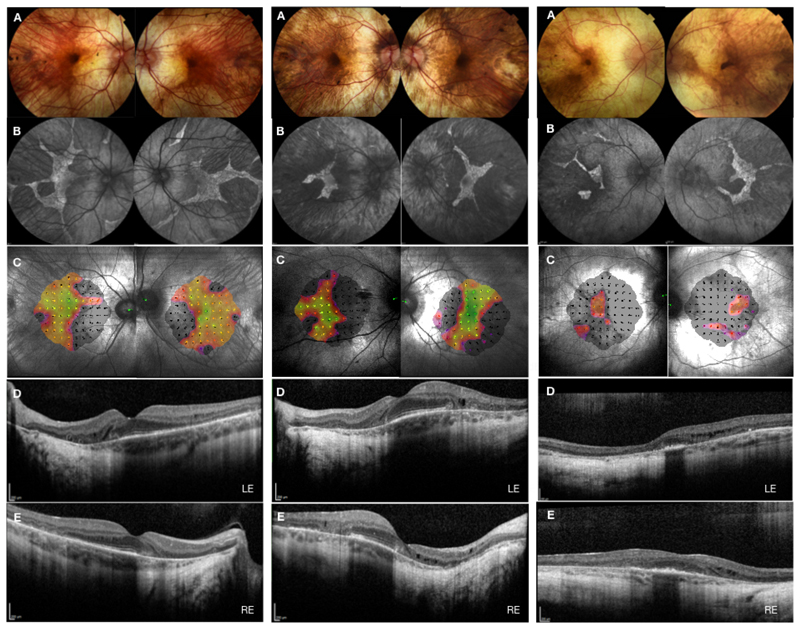
Figure 1. Multimodal retinal imaging of typical participants recruited in choroideremia gene therapy trials.
A typical participant recruited to phase II REGENERATE trial – cohort 1 with symmetrical disease in both eyes (left panel) and cohort 2 with asymmetrical disease between the two eyes (middle panel). A typical participant recruited to phase III STAR trial (right panel) at the Oxford study center, UK. A – fundus color images; B – fundus autofluorescence; C – microperimetry; D – optical coherence tomography of the left eye; E – optical coherence tomography of the right eye.
In addition to a characteristic phenotype and X-linked family history, genetic diagnosis of choroideremia is essential in the current era of gene therapy. There are 346 unique DNA variants in CHM reported to date (Leiden Open Variation Database, LOVD3, www.lovd.nl/CHM), most of which are functionally null either through deletions or nonsense sequence variations. Deletion vary in size from a few kilo bases to the entire gene deletion, leading to loss of function or complete absence of REP1. Nonsense variations result in premature stop codons and termination of protein translation. Unlike most retinal degenerations, the missense variants that result in pathological protein misfolding are rarely found in choroideremia (25). Any missense change therefore needs to be interpreted with caution to ensure a true pathogenic effect, rather than a simple mistake in the sequence readout. In cases of strong clinical suspicion and no identified genetic variant or in the case of a variant of uncertain pathogenicity, it is important to follow several additional steps to confirm the diagnosis. Further genetic testing can be requested to include sequencing of a recently reported novel deep intronic variant (26) and a variant in the promoter region of CHM (27) that are not routinely included in the panel. Several rare phenotypes that can mimic choroideremia such as, gyrate atrophy of the choroid (28), progressive bifocal chorioretinal atrophy (28), Oliver-McFarlane syndrome (29) and a phenotype associated with-dominant RPE65 variants (30, 31) need to be excluded. Although choroideremia is by far the most prevalent (1:50,000) of those masquerading conditions, it is helpful to examine a female relative to confirm the specific carrier phenotype. Lastly, functional in-vitro immunoblast assay to check for the absence, reduced levels or absent/reduced prenylation activity of REP1 in peripheral blood mononuclear cells can aid the clinical diagnosis (17).
2.1. In vitro functional assays
REP1 is a 653 amino acid long protein that is expressed ubiquitously and it plays an essential role in the intracellular trafficking pathways by prenylation of Rab GTPases (1,2). In choroideremia, there is deficiency of REP1 expression and reduction of the prenylation activity in all cells of the body, yet the phenotype only manifests in the eye, and mainly to the outer retina including retinal pigment epithelium and choroid. For reasons that remain unclear, proteins related to REP1, such as REP2, can compensate for REP1 deficiency in most cells of the body, but not the eye.
The levels of REP1 in peripheral blood mononuclear cells can be measured in vitro by immunoblot assays. The absence or reduced levels of REP1 can be used to support the diagnosis of choroideremia. Moreover, the detection of reduced prenylation activity can further aid the diagnosis. These premises led to the investigation of a method to test the biological activity of AAV vector carrying CHM gene currently under trial for choroideremia. We recently developed a robust in vitro prenylation assay using a biotinylated lipid donor to test the REP1 activity following transduction of HEK293 cells with AAV2-REP1 (17). Specifically, two Rab proteins, RAB27A and RAB6A, were used as substrates in a prenylation reaction in which a biotinylated lipid donor was incorporated. The amount of biotinylated lipid donor detected was proportional to the amount of AAV2-REP1 present in the reaction. We also found that the prenylation of RAB6A is more sensitive to REP1 protein expression compared to RAB27A. This method was found to be robust and reproducible in several cell lines. This assay can be used to test the biological activity of AAV vectors in choroideremia gene therapy clinical trials.
3. Gene therapy for choroideremia
Given the relative ease of clinical and genetic diagnosis of choroideremia compared to other retinal degenerations, gene replacement therapy is a very attractive treatment option for this monogenic blinding condition. Relative to other organs, the eye is easy to access and immune privileged, making it an ideal target for gene therapy. In addition, CHM cDNA is small enough (1.9kB) to fit into the AAV vector and given that most CHM sequence variants are functionally null, with no known dominant negative effects, expression of even a small amount of healthy functional protein is predicted to be therapeutic.
In order to achieve the maximum benefit and avoid potential complications, gene delivery to the target cells in choroideremia has been through several stages of development and improvement. Thus, a surgical approach that delivers the vector under the retina is now widely accepted as the method of necessity in choroideremia clinical trials and is likely to be one of the most important determinants of the successful treatment outcome (32). The surgical procedure involves standardised three port vitrectomy and detachment of posterior hyoid face. The vector is then delivered in a two-step process. During step 1, the balanced salt solution is used to initiate a subretinal bleb and create a space for the vector delivery during the step 2. The site of retinotomy is guided by the residual island of the retina as seen on autofluorescence imaging and the pattern of retinal vasculature. Ideally the same retinotomy site is used in both steps and it is possible to stain the BSS solution with membrane dual blue to visualise the site of retinotomy and any potential reflux into the vitreous. The real-time intraoperative OCT is used to guide the retinotomy into the correct tissue plane and monitor the retinal stretch throughout the injection. The retina is lifted in slow pulses using a foot pedal control of the viscous fluid injection system with special attention during foveal detachment to avoid excessive stretch and potential iatrogenic breaks. This is particularly important in advanced cases of choroideremia, where the degenerated retina is extremely thin and adherent to underlying tissue posing resistance to subretinal bleb formation. In such cases, it is possible to use perfluorocarbon liquid to protect the fovea from overstretching, whilst treating the proximal target area. In addition, a pneumatic displacement can be used to carefully extend the initial bleb in a controlled manner in order to cover a larger target area but at the same time minimise the potential injury to the retina from the injection related pressure.
First-in-human gene therapy clinical trial in choroideremia started in Oxford, UK in 2011 (clinicaltrials.gov, NCT01461213). The vector construct contained human CHM transgene regulated by a ubiquitous cytomegalovirus-enhanced chicken β-actin (CAG) promoter, a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and a modified bovine polyadenylation (polyA) signal and was packaged in adeno-associated viral vector serotype 2, AAV2. The results of the first 6 patients from this safety trial who were injected subretinally with a low dose (1x1010 gp in 0.1ml) vector (AAV2-REP1) were initially reported in 2014 (9) and subsequently in 2016 (10) and 2018 (11). Two patients who had advanced disease and therefore reduced baseline visual acuity, improved by 21 and 11 Early Treatment Diabetic Retinopathy Study (ETDRS) letters respectively in the treated eye at 6 months post treatment (10). This gain in visual acuity was maintained at 5 years post treatment (patient 1: +25 letters in the treated eye and -58 letters in the control eye; patient 2: +20 letters in the treated eye and -3 letters in the control eye) (11). The other 4 patients had good baseline visual acuity which was maintained at 6 months in all 4 patients and in 3 out of 4 patients at 5 years post treatment. In the patient who had a decline in visual acuity (45 letters in the treated eye and 15 letters in the control eye at 5 years) with evidence of foveal degeneration, an intra-operative surgical complication of increased retinal stretch during retinal detachment precluded from injecting of the total vector dose (60µl injected instead of a total of 100µl). The full report of all 14 cases from this Phase I/II trial, including 7 patients who received high vector dose (1x1011 gp in 0.1ml), shows median gain of 4.5 letters in the treated group, compared to 1.5 letter loss in the control group at 2 years post-surgery, despite complications in two patients. Overall, 6 treated eyes gained more than 1 line of vision (5 letters). In addition to the surgical complication in the patient described above, one other patient had a post-operative course complicated by intra-ocular inflammation that resulted in -7 letter loss in the treated eye compared to -1 letter loss in the control eye. It is worth mentioning that the first 6 patients were treated without the aid of the intraoperative OCT or the use of foot pedal control of the injection system. The surgical technique was subsequently refined considerably to incorporate these systems to facilitate gene therapy surgery and this new technique is now widely used in gene therapy treatment centres.
Three other clinical trials (in Tubingen, Alberta and Miami) using the same vector as the Oxford group at the higher dose (1x1011 gp in 0.1ml) recruited 6 patients per trial and recently reported their Phase I/II results. The Tubingen group (13) reported a gain of 17 ETDRS letters in one treated eye, a loss of 14 letters in another treated eye and minor changes in visual acuity (-4 to +1 letters) in 4 treated eyes at 12 months post treatment. In addition, there was a mean improvement in retinal sensitivity in 5 out of 6 treated eyes (by 2.3dB) with no changes in anatomical endpoints. The Alberta group (12) showed a gain of 15 letters in one treated eye, loss of 8 letters in one treated eye (secondary to an intra-operative complication) and a maintenance of visual acuity in 4 treated eyes at 2 years post treatment. They also reported a gain of 15 letters in one control eye. There were no overall changes in retinal sensitivity and the authors observed a steady decline in the area of preserved retina as seen on the fundus autofluorescence in treated and untreated eyes. The Miami group (14) reported a gain of 10 and 5 letters respectively in two treated eyes, a gain in 4 letters in one untreated eye and maintenance of visual acuity within 2 letters of baseline in all other eyes at 2 years after treatment. There were no changes in retinal sensitivity at the end of 2-year study period. The excellent safety profile in the Miami group, with no surgically related complications, was attributed to the improved surgical technique with the use of intra-operative OCT and the foot pedal injection system from the outset of this study to facilitate subretinal gene delivery.
A phase II clinical trial, the REGENERATE trial, has recently completed recruitment of 30 patients at Oxford and Moorfields Eye Hospitals, UK This trial was designed to include patients with earlier stage choroideremia, with normal visual acuity and large areas of preserved central retina. The trial will test the long-term efficacy of AAV2-REP1 vector (1x1011 gp in 0.1ml) in slowing down the degeneration and preserving the healthy retina and visual function compared to the untreated control eye. Two cohorts of patients were included, cohort 1 with symmetrical areas of preserved central retina (Figure 1, left panel) and cohort 2 with non-symetrical (Figure 1, middle panel) areas. The results are anticipated in the next few years. Another Phase II trial, the GEMINI trial is now underway in Tubingen, Germany, where both eyes in a total of 15 subjects will be treated with the AAV2-REP1 vector (1x1011 gp in 0.1ml).
Based on the above safety data, a phase III international multi-centre gene therapy trial (NCT03496012) was initiated to evaluate safety and efficiency of the AAV2-REP1 vector through single-dose subretinal injection in 140 patients with choroideremia. Subjects were recruited at a more advanced stage of choroideremia with visual acuity already in decline to between 34 and 73 letters (Figure 1, right panel). Participants were randomised to high dose, low dose and no treatment groups with primary endpoint of best-corrected visual acuity at 12 months post treatment. The secondary efficacy outcomes include low level luminance vision, area of fundus autofluorescence, retinal structure on optical coherence tomography, retinal sensitivity as measured by the microperimetry, contrast sensitivity and colour vision. The read out and results are expected in late 2020.
The SOLSTICE study is an observational, long-term follow up study of 100 participants that evaluates the long-term safety and efficacy of the AAV2-REP1 used in the above-mentioned interventional choroideremia trials. In addition to the above interventional trials a large observational natural history study of the progression of choroideremia, the NIGHT study, is ongoing and the interim analysis have been reported (15, 16). Independent from the trials above, all of which are sponsored by the Nightstar Therapeutics (recently taken over by the Biogen, UK), Spark Therapeutics Inc are leading a phase I/II trial in Philadelphia, USA, using an AAV2-REP1 vector that is similar to the vector used in the Nightstar studies, but without the WPRE enhancer. There are no published reports to date.
Choroideremia gene therapy clinical trials are summarised in Table 1.
Table 1. Choroideremia gene therapy trials.
AAV2 – adeno-associated viral vector serotype 2, CAG – ubiquitous cytomegalovirus-enhanced chicken beta-actin promoter, CHM – choroideremia transgene, WPRE – woodchuck hepatitis virus posttranscriptional regulatory element, polyA – a modified bovine polyadenylation signal, REP1 – Rab escort protein 1.
| Clinical trial registration (clinicaltrials.gov) | Location/Start Date | Phase/Design | Reference |
|---|---|---|---|
| NCT01461213 | University of Oxford, UK October 2011 |
Phase I/II Low and high dose, open label 14 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
Lancet, 2014 doi: 10.1016/S0140-6736(13)62117-0 NEJM, 2015 doi: 10.1056/NEJMc1509501 Nat Med, 2018 doi: 10.1038/s41591-018-0185-5 |
| NCT02341807 | Philadelphia, USA Spark Therapeutics January 2015 |
Phase I/II Low and high dose, open label 15 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-polyA) |
No reports to date |
| NCT02077361 | University of Alberta, Canada April 2015 |
Phase I/II Single dose, open label 6 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
Am J Ophthalmol, 2018 doi: 10.1016/j.ajo.2018.06.011 |
| NCT02553135 | University of Miami, USA September 2015 |
Phase II Single dose, open label 6 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
Am J Ophthalmol, 2019 doi: 10.1016/j.ajo.2018.09.012 |
| NCT02671539 | University of Tubingen, Germany THOR TRIAL January 2016 |
Phase II Single dose, open label 6 male participants, ubretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
Retina, 2018 doi: 10.1097/IAE.0000000000002360 |
| NCT02407678 | University of Oxford and Moorfields Eye Hospital, UK REGENERATE TRIAL August 2016 |
Phase II Randomised, single dose, open label 30 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
No reports to date |
| NCT03507686 | Nightstar Therapeutics, International, Multi-centre GEMINI TRIAL November 2017 |
Phase II Single dose, open label, two-period 15 male participants, Bilateral subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
No reports to date |
| NCT03496012 | Nightstar Therapeutics, International, Multi-centre STAR TRIAL December 2017 |
Phase III Control, low, high doseA Randomised, Open Label, Outcomes-Assessor Masked, Prospective, Parallel Controlled Group Study 140 male participants, Subretinal injection AAV2-REP1 (AAV2-CAG-CHM-WPRE-polyA) |
No reports to date |
| NCT03584165 | Nightstar Therapeutics, International, Multi-centre SOLSTICE TRIAL June 2018 |
Observational, Long-term follow up evaluating the safety and efficacy of AAV2-REP1 used in antecedent choroideremia studies, 100 participants |
No reports to date |
| NCT03359551 | Nightstar Therapeutics, International, Multi-centre NIGHT STUDY June 2015 |
Observational, Prospective, Natural History of the Progression of Choroideremia, 300 participants |
Am J Ophthalmol, 2017 doi: 10.1016/j.ajo.2017.05.002 Br J Ophthalmol, 2019 doi: 10.1136/bjophthalmol-2018-312620. |
4. Towards regulatory approval
The encouraging safety and early efficacy data from phase I/II choroideremia trials has led to the initiation of an international, multi-centre, phase III pivotal trial in December 2017 - the STAR trial.
The STAR trial was carefully planned and closely follows the vector design used in voretigene neparvovec-rzyl (Luxturna®, Spark Therapeutics Inc., USA), the US Food and Drug Administration (FDA) approved gene therapy for RPE65-related retinal dystrophy (33, 34). The vector construct used, AAV2-CAG-CHM-WPRE-polyA, is identical to the vector used in Luxturna®, except for the CHM transgene. In particular, the vector has been extensively optimised to include a strong ubiquitous promote, CAG, that targets multiple cell types including the retinal pigment epithelium and the photoreceptors, an optimised Kozak sequence, a WPRE sequence to enhance target gene expression and a modified polyA signal for stability. The final vector formulation included a surfactant (0.001% Pluronic F68) to prevent non-specific loss of vector to surfaces of vials used for storage and administration devices. The solution was adjusted to pH 7.3 and subjected to removal of empty capsids. The final vector preparation is stored at -80°C until clinical administration (35). It is subjected to an annual potency assay to check REP1 expression and prenylation activity against Rab substrates using a standardized protocol. The vector was granted the FDA and European Medicines Agency (EMA) Orphan Drug Designation in 2015.
In addition, the study protocol closely follows a perioperative immunomodulatory regimen established in the Philadelphia phase III AAV gene therapy clinical trial that lead to the approval of Luxturna® (34). Thus, in order to reduce risks related to any adverse immune responses, all trial subjects are given a 21-day course of oral prednisolone following closely the 17-day protocol established in the Philadelphia trial (5), except allowing an extra 4 days for tapering the dose at the end of the course. Subject are prescribed 1mg/kg/day of prednisolone for 10 days (beginning 2 days prior to gene therapy, on the day of surgery and for 7 days afterwards) followed by 0.5mg/kg/day for 7 days, 0.25mg/kg/day for 2 days, and 0.125 mg/kg/day for 2 days.
Finally, with the approval of Luxturna® the FDA has accepted a novel primary endpoint, the multi-luminance mobility test, for evaluation of patients with complex visual impairment (34). This is encouraging as it recognises that standard tests, such as visual acuity, may not be best suited to demonstrate the full efficacy of novel therapies in this patient population. It further opens the possibility for other clinical trial endpoints, such as low-level luminance visual acuity, fundus autofluorescence and microperimetry to become approved efficacy endpoints in retinal gene therapy trials.
The rigorous pathways undertaken for the FDA approval of Luxturna® gene therapy have required pioneering regulatory development for the use of novel investigational product that included adult and paediatric population. This was the first ever retinal gene therapy to receive the FDA approval and the first approved pharmacologic treatment for an inherited retinal degeneration. Given that the choroideremia gene therapy studies have closely followed the Luxturna® trials, including the vector design and manufacture, together with the encouraging safety and early efficacy data from phase I/II trials, the process for the regulatory approval of choroideremia gene therapy should follow a now established course. Phase III pivotal trial data is awaited in great anticipation.
5. Alternative therapies
In addition to gene therapy, there are several alternative strategies under development with a potential to treat choroideremia. In-frame nonsense mutations, resulting in premature termination codons and nonsense mediated decay could potentially be amenable to nonsense suppression therapy (36). Small molecule drugs, based on aminoglycosides, could promote ribosomal read-through and thus potentially treat phenotypes caused by nonsense-mediated disease (37). This nonsense suppression therapy has been investigated in other genetic disorders, including cystic fibrosis and Duchenne muscular dystrophy, with recent NICE approval of one of the oral compounds (PTC124, ataluren or Translarna™) for the treatment of Duchenne muscular dystrophy caused by a nonsense change in the dystrophin gene (37, 38). In-vitro and in-vivo pre-clinical testing of ataluren (and another optimised compound) in models of choroideremia, led to some promising results with improved REP1 expression (39). One of the challenges in future development of this potential treatment is the lack of specificity for the gene of interest with potential to override normal stop codons and express unwanted nonsense mutations in other genes.
In cases of advanced choroideremia, where gene replacement therapy may no longer be able to rescue photoreceptor degeneration, optogenetic therapy has potential to restore vision. Optogenetic therapy uses light sensing molecules to convert non-photoreceptor cells in the inner retina into directly light sensitive cells and thus restore vision (40). Many optogenetic tools for vision restoration are under development in pre-clinical trials (41–45) and more recently phase I clinical trials using microbial-based opsins for the treatment of advanced retinal degeneration have begun (www.clinicaltrials.gov, NCT02556736 and NCT03326336). One of the major challenges with this therapy is the amount of light the microbial opsins require for activation. Thus, therapy based on human opsins (42–45) has a major advantage where opsins are functional under physiological light conditions. Optogenetic therapy would be applicable to any retinal degeneration, irrespective to aetiology or genetic mutation. However, the inner retina needs to be preserved even at late stages of degeneration for targeting of optogenes to non-photoreceptor cells. Unlike in classical retinitis pigmentosa, narrowing of the retinal vessels and waxy pallor of the optic disc are not features of choroideremia phenotype, suggesting that the inner retinal cells are generally well preserved (46). This makes choroideremia one of the ideal retinal degeneration phenotypes amenable to optogenetic therapy. Future developments will focus on determining the optimal method of gene delivery, intravitreal versus subretinal, depending on the cellular target of interest.
An alternative way to restore vision after complete loss of photoreceptors is via electronic retinal implants. (47). Choroideremia patients with no light perception have been enrolled in clinical trials testing a 44-channel suprachoriodal Bionic Eye Device (NCT03406416) Melbourne, Australia and Intelligent Retinal Implant System, IRIS V1 (NCT01864486) and V2 (NCT02670980) Pixium Vision SA. Clinical trial outcomes are expected to give further insight into the safety of surgical procedures, longevity of the devices and potential benefits for vision restoration.
6. Conclusion
In conclusion, increasing knowledge from clinical phenotyping and genetic testing of choroideremia patients together with natural history studies on disease progression are providing insights into optimal timing of gene therapy treatment and establishment and interpretation of clinical trial endpoints. Results from recent Phase I/II gene therapy clinical trial for choroideremia have demonstrated good safety profile and signs of sustained improvements in visual acuity. Phase II and III randomised control trials are now underway and the results are anticipated within the next few years. Alternative therapies including small molecule translational read-through drugs, used for nonsense suppression therapy, are at pre-clinical stages of development and may be applicable for early intervention or in combination with gene therapy. Optogenetic strategies are showing very promising results with early clinical trials underway for the treatment of advanced retinal degeneration.
7. Expert Commentary
In the current climate of rapidly expanding gene therapy for the treatment of inherited retinal degenerations, many patients with choroideremia are now participating in one of the ongoing international gene therapy trials and their condition is closely monitored as per trial protocols. For those patients who are not part of a clinical trial, the standard ophthalmic care needs to include regular monitoring for signs of treatable conditions such as cataracts and cystoid macular oedema. In addition, patients should be offered supportive measures and low vision rehabilitation to improve quality of life and performance in daily activities. Patients are very keen to understand any new developments in the treatment of their condition and should be regularly updated on the progress of clinical trials and what the future might hold for them. The effect that this has on their overall wellbeing cannot be underestimated.
AAV gene replacement therapy is the most promising candidate to be a successful treatment strategy for most patients with choroideremia. The majority of identified mutations in choroideremia are null (compared to a small proportion that are nonsense), making gene augmentation therapy the most logical approach irrespective of the mechanism of causative mutations. This is in contrast to nonsense suppression therapy that would at best treat only a small proportion of affected individuals. In addition, no mutations in REP1 are known to exist to date that have a dominant-negative effect. The production of a relatively modest amount of normal protein from gene augmentation therapy for REP1 may thus have a large effect clinically, similar to the case for approved Luxturna® gene therapy that replaces even a modest amount of function of a missing RPE65 enzyme (34).
Experience from early clinical trials have led to advances in pre-operative clinical assessments and developments in surgical techniques that have significantly reduced the risks associated with subretinal vector administration resulting in successful surgeries and low complication rates. Thus, careful pre-operative assessments that confirm retinal structural integrity and improved surgical techniques that minimize excessive retinal stretch, air bubbles within the injection system and reflux of viral vector into the vitreous space, have reduced risks of surgical complications seen in early phase clinical trials that included patients with atrophic retinas associated with advanced disease. In majority of treated cases however, iatrogenic macular detachment was safely performed with the retinal structural and functional recovery to baseline seen within one month of subretinal surgery (11, 32).
We do recognize that developments in gene therapy surgery must ultimately go hand-in-hand with development of novel vectors, including those that would penetrate outer retinal from the vitreous and potentially avoid the risks of subretinal delivery to the fragile tissues in certain advanced cases.
Unfortunately, the complexity associated with these biological tools has meant that there has been a significant lag in their advancements to clinical trials. The AAV vector used in current choroideremia trials, namely AAV2, is unable to undergo transport from the vitreous to the outer retina and would thus not be useful for an intravitreal administration. New generation vectors show improved transduction efficiencies in rodents, and to a much lower extent in primates, with additional caveat of higher necessary doses that are known to be pro-inflammatory with adverse ocular effects, thus limiting their use in clinical applications for choroideremia in foreseeable future (48).
Clinical research supports that choroideremia may be one of the more treatable forms of inherited retinal degenerations, especially if gene replacement therapy is given before major degeneration and loss of choroid and retina occur throughout the macula. However, the disease process in choroideremia is more complex and proving to be increasingly individual. The genotype-phenotype correlation remains at present poorly characterised. One of the main aspects of subretinal gene therapy that requires special consideration is the timing of intervention. Given that choroideremia progresses relatively slowly, and that most patients retain reasonable visual acuity until the very late stage of the disease process, the therapeutic window is long, with the potential to treat even at the very late stage. The latest evidence suggests that most choroideremia patients undergo similar rate of exponential decay, but that the most important determinant of the disease severity is the age of onset of degeneration (49). Hence, once the degeneration starts, it seems to progress at a steady rate. The gene therapy treatment needs to be administered soon after the visual acuity starts to decline, to capture the potential for rescue of visual function, but before the irreversible loss has occurred. It may equally be possible to treat patients, including affected female carriers and children, at early stages of the disease process when the peripheral fields start to become affected, but the central vision is still intact. The aim of this treatment would be to halt or slow down the central degeneration and preserve the visual acuity for a longer period of time. In addition, future therapy may involve treatment of the peripheral retina to preserve or reverse the loss of visual field. Better understanding of genotype-phenotype correlation may shed light on the triggers and timing of onset of retinal degeneration and hence the optimal timing for intervention with potential to maximise the therapeutic effect in as many patients as possible.
The precise reasons why REP2 does not prevent disease in the eye are poorly understood. The defect of REP1 is present in all cells of the body, yet the clinical feature of deficiency of REP1 in affected males is limited to the eye and mainly to the outer retina and choroid. The reason why this might be the case is commonly explained by the presence of a second gene involved in prenylation, REP2, located on the long arm of chromosome 1 at 1q43. The REP2 encodes Rab escort proteins throughout the body and they serve the needs of prenylation for all cells in the body except for those in the eye (the choroid and outer retina), which have a specific need for the proteins encoded by REP1. The specifics as to why REP1 is required for the eye is poorly understood but is likely related to the evolutionary origin of the REP2 gene.
REP2 is an X-linked retrogene of REP1, one of 36 known retrogenes in human genome. The insertion of X-linked retrogenes is known to be random and coding sequence of RNA templates have no introns, hence direct comparison of genes is not restricted to coding regions alone. Since promoter and splicing affect gene expression, it is logical to assume that expression of REP2 will be random and levels will be variable, which could explain why REP2 is unable to compensate for REP1 deficiency in retinal pigment epithelium. However, it should be noted that in transgenic mouse REP2 is unable to compensate for REP1 in placenta, which makes condition embryologically lethal in mice. This may explain why to date, no naturally occurring animal models of choroideremia have been identified. In addition, as a retrogene on chromosome 1 and now free of its normal regulation, REP2 coding sequence may have undergone changes whereby specific functions essential for the health of the choroid and retina may have been altered or lost. It is notable that the reading frame of REP2 is considerably longer than REP1. The REP2 gene may have also developed differences in protein specificity or regulation from local promoters that resulted in REP2 being less efficient compared to REP1, and hence unable to keep the choroid and retina healthy. Elucidating the exact mechanisms that allow the failure of protection of the choroid and retina from dystrophy or degeneration in the absence of REP1, could lead to additional targets for novel therapies of choroideremia.
8. Five-year view
Considerable progress has been made in the development of novel therapies for choroideremia. Results from phase I/II gene therapy clinical trials show good safety profile and promising early efficacy data. The approval of LUXTURNA® gene therapy, has made the regulatory bodies more aware of the potential risks and benefits of ocular gene therapy in the treatment of blinding retinal diseases, paving the way for approval of future gene therapies. Choroideremia is close to being the second ocular gene therapy to complete a pivotal phase III clinical trial and undergo consideration for regulatory approval.
Future innovations in the field of gene therapy are likely to include the use of robot assisted vector delivery. The precision and the accuracy of the robot assisted gene therapy is beyond to what is achievable with manual surgery allowing for the infusion of the vector over a long period of time. This would allow for the very controlled detachment of the retina, improving the balance between the rate of fluid infusion and the rate of fluid absorption. In addition, the switch between the balanced salt solution in step one and the vector solution in step two could be done externally, without the need to move the injection cannula from the initial injection site, thus minimising the size of the retinotomy and any intravitreal reflux. The first in human robotic eye surgery (50) has demonstrated safety and the proof of concept of robot assisted delivery of a therapeutic substance under the retina paving the way for future robot assisted gene therapy.
Future clinical trials for nonsense suppression therapy with small molecule drugs will shed light on the safety and efficacy of this alternative treatment option for a subset of choroideremia patients carrying nonsense mutations. Upcoming clinical trials in optogentics will determine the potential benefits in late stage choroideremia.
9. Article highlights.
Clinical phenotyping of choroideremia patients, genetic testing and increasing data from natural history studies on disease progression are providing insights into optimal timing of gene therapy treatment and establishment and interpretation of clinical trial endpoints.
Results from recent early phase clinical trials show promising results with good safety profile and signs of efficacy. Phase II and III randomised control trials are now underway and the results are expected within the next few years.
Alternative therapies are under pre-clinical testing with promising developments in nonsense suppression therapy and optogenetics.
Funding
The authors are funded by Oxford NIHR Biomedical Research Centre, Oxford, UK. J Cehajic-Kapetanovic is also funded by Global Ophthalmology Awards Fellowship, Bayer, Switzerland.
Footnotes
Declaration of interest
RE MacLaren is the scientific founder of Nightstar Therapeutics Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Cremers FP, van de Pol DJ, van Kerkhoff LP, et al. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- 2.Seabra MC, Brown MS, Goldstein JL. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993;259:377–381. doi: 10.1126/science.8380507. [* First study to identify the prenylation defect in choroideremia.] [DOI] [PubMed] [Google Scholar]
- 3.Tolmachova T, Tolmachov OE, Barnard AR, et al. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med (Berl) 2013;91:825–837. doi: 10.1007/s00109-013-1006-4. [*First proof-of-principle pre-clinical study for gene therapy in choroideremia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black A, Vasireddy V, Chung DC, et al. Adeno-associated virus 8-mediated gene therapy for choroideremia: preclinical studies in in vitro and in vivo models. J Gene Med. 2014;16:122–130. doi: 10.1002/jgm.2768. [DOI] [PubMed] [Google Scholar]
- 5.Anand V, Barral DC, Zeng Y, et al. Gene therapy for choroideremia: in vitro rescue mediated by recombinant adenovirus. Vision Res. 2003;43:919–926. doi: 10.1016/s0042-6989(02)00389-9. [DOI] [PubMed] [Google Scholar]
- 6.Vasireddy V, Mills JA, Gaddameedi R, et al. AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2013;8:e61396. doi: 10.1371/journal.pone.0061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cereso N, Pequignot MO, Robert L, et al. Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient. Mol Ther Methods Clin Dev. 2014;1:14011. doi: 10.1038/mtm.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong TT, Vasireddy V, Ramachandran P, et al. Use of induced pluripotent stem cell models to probe the pathogenesis of choroideremia and to develop a potential treatment. Stem Cell Res. 2018;27:140–150. doi: 10.1016/j.scr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 9.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014 Mar 29;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0. [** First in human study of gene therapy for choroideremia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards TL, Jolly JK, Groppe M, et al. Visual Acuity after Retinal Gene Therapy for Choroideremia. N Engl J Med. 2016 May 19;374(20):1996–8. doi: 10.1056/NEJMc1509501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue K, Jolly JK, Barnard AR, Rudenko A, Salvetti AP, Patrício MI, Edwards TL, Groppe M, Orlans HO, Tolmachova T, Black GC, et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med. 2018 Oct;24(10):1507–1512. doi: 10.1038/s41591-018-0185-5. [** Long-term follow up of phase 1/2 clinical trial for choroideremia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after aav2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol. 2018;193:130–142. doi: 10.1016/j.ajo.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MD, Ochakovski GA, Beier B, et al. Changes in Retinal Sensitivity After Gene Therapy in Choroideremia. Retina. 2018 Oct 9; doi: 10.1097/IAE.0000000000002360. [DOI] [PubMed] [Google Scholar]
- 14.Lam BL, Davis JL, Gregori NZ, MacLaren RE, et al. Choroideremia Gene Therapy Phase 2 Clinical Trial: 24-Month Results. Am J Ophthalmol. 2019 Jan;197:65–73. doi: 10.1016/j.ajo.2018.09.012. Epub 2018 Sep 19. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AH, Velaga SB, Girach A, et al. Measurement and reproducibility of preserved ellipsoid zone area and preserved retinal pigment epithelium area in eyes with choroideremia. Am J Ophthalmol. 2017;179:110–117. doi: 10.1016/j.ajo.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Hariri AH, Ip MS, Girach A, Lam BL, et al. Macular spatial distribution of preserved autofluorescence in patients with choroideremia. For Natural History of the Progression of Choroideremia (NIGHT) Study Group. Br J Ophthalmol. 2019 Jul;103(7):933–937. doi: 10.1136/bjophthalmol-2018-312620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrício MI, Barnard AR, Cox CI, Blue C, MacLaren RE. The Biological Activity of AAV Vectors for Choroideremia Gene Therapy Can Be Measured by In Vitro Prenylation of RAB6A. Mol Ther Methods Clin Dev. 2018 Mar 28;9:288–295. doi: 10.1016/j.omtm.2018.03.009. [** Development of first in-vitro prenylation assay to test vector potency in choroideremia gene therapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aleman TS, Han G, Serrano LW, et al. Natural history of the central structural abnormalities in choroideremia. Ophthalmology. 2017;124:359–373. doi: 10.1016/j.ophtha.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards TL, Groppe M, Jolly JK, et al. Correlation of retinal structure and function in choroideremia carriers. Ophthalmology. 2015;122:1274–1276. doi: 10.1016/j.ophtha.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 2012;33:57–65. doi: 10.3109/13816810.2011.620056. [DOI] [PubMed] [Google Scholar]
- 21.Ayazi S. Choroideremia, obesity, and congenital deafness. Am J Ophthalmol. 1981;92:63–69. doi: 10.1016/s0002-9394(14)75909-4. [DOI] [PubMed] [Google Scholar]
- 22.Mukkamala K, Gentile RC, Willner J, et al. Choroideremia in a woman with ectodermal dysplasia and complex translocations involving chromosomes X, 1, and 3. Ophthalmic Genet. 2010;31:178–182. doi: 10.3109/13816810.2010.497529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang AY, Mysore N, Vali H, et al. Choroideremia is a systemic disease with lymphocyte crystals and plasma lipid and RBC membrane abnormalities. Invest Ophthalmol Vis Sci. 2015;56:8158–8165. doi: 10.1167/iovs.14-15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radziwon A, Cho WJ, Szkotak A, Suh M, MacDonald IM. Crystals and Fatty Acid Abnormalities Are Not Present in Circulating Cells From Choroideremia Patients. Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4464–4470. doi: 10.1167/iovs.18-25112. [DOI] [PubMed] [Google Scholar]
- 25.Simunovic MP, Jolly JK, Xue K, Edwards TL, Groppe M, Downes SM, MacLaren RE. The Spectrum of CHM Gene Mutations in Choroideremia and Their Relationship to Clinical Phenotype. Invest Ophthalmol Vis Sci. 2016 Nov 1;57(14):6033–6039. doi: 10.1167/iovs.16-20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carss K, Arno G, Erwood M, et al. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am J Hum Genet. 2017;100:75–90. doi: 10.1016/j.ajhg.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radziwon A, Arno GK, Wheaton D, et al. Single-base substitutions in the CHM promoter as a cause of choroideremia. Hum Mutat. 2017;38:704–715. doi: 10.1002/humu.23212. [DOI] [PubMed] [Google Scholar]
- 28.Hayasaka S, Shoji K, Kanno C, et al. Differential diagnosis of diffuse choroidal atrophies. Diffuse choriocapillaris atrophy, choroideremia, and gyrate atrophy of the choroid and retina. Retina. 1985;5:30–37. [PubMed] [Google Scholar]
- 29.Sampson JR, Tolmie JL, Cant JS. Oliver McFarlane syndrome: a 25-year follow-up. Am J Med Genet. 1989;34:199–201. doi: 10.1002/ajmg.1320340213. [DOI] [PubMed] [Google Scholar]
- 30.Hull S, Mukherjee R, Holder GE, et al. The clinical features of retinal disease due to a dominant mutation in RPE65. Mol Vis. 2016;22:626–635. [PMC free article] [PubMed] [Google Scholar]
- 31.Bowne SJ, Humphries MM, Sullivan LS, et al. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur J Hum Genet. 2011;19:1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue K, Groppe M, Salvetti AP, et al. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond) 2017;31:1308–1316. doi: 10.1038/eye.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [**Phase 3 clinical trial for the first approved gene therapy for retinal degeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JF, Wellman J, High KA. Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr Gene Ther. 2010;10:341–349. doi: 10.2174/156652310793180715. [DOI] [PubMed] [Google Scholar]
- 36.Moosajee M, Ramsden SC, Black GC, et al. Clinical utility gene card for: choroideremia. Eur J Hum Genet. 2014 Apr;22(4) doi: 10.1038/ejhg.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 38.Richardson R, Smart M, Tracey-White D, et al. Mechanism and evidence of nonsense suppression therapy for genetic eye disorders. Exp Eye Res. 2017;155:24–37. doi: 10.1016/j.exer.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Moosajee M, Tracey-White D, Smart M, et al. Functional rescue of REP1 following treatment with PTC124 and novel derivative PTC- 414 in human choroideremia fibroblasts and the nonsense-mediated zebrafish model. Hum Mol Genet. 2015;25:3416–3431. doi: 10.1093/hmg/ddw184. [DOI] [PubMed] [Google Scholar]
- 40.Baker CK, Flannery JG. Innovative Optogenetic Strategies for Vision Restoration. Front Cell Neurosci. 2018 Sep 21;12:316. doi: 10.3389/fncel.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta A, et al. Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol Med. 2016;8:1248–1264. doi: 10.15252/emmm.201505699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R, Davis KE, Bishop PN, Lucas RJ. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr Biol. 2015 Aug 17;25(16):2111–22. doi: 10.1016/j.cub.2015.07.029. [**Proof-of-principle study for efficacy of optogenetics as an alternative therapy in advanced retinal degeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eleftheriou CG, Cehajic-Kapetanovic J, Martial FP, Milosavljevic N, Bedford RA, Lucas RJ. Meclofenamic acid improves the signal to noise ratio for visual responses produced by ectopic expression of human rod opsin. Mol Vis. 2017 Jun 16;23:334–345. [PMC free article] [PubMed] [Google Scholar]
- 44.De Silva SR, et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc Natl Acad Sci USA. 2017;114:11211–11216. doi: 10.1073/pnas.1701589114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry MH, Holt A, Salari A, Veit J, Visel M, Levitz J, Aghi K, Gaub BM, Sivyer B, Flannery JG, Isacoff EY. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat Commun. 2019 Mar 15;10(1):1221. doi: 10.1038/s41467-019-09124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu DJ, Xue K, Jolly JK, MacLaren RE. A detailed in vivo analysis of the retinal nerve fibre layer in choroideremia. Acta Ophthalmol. 2019 Jun;97(4):e589–e600. doi: 10.1111/aos.13973. [DOI] [PubMed] [Google Scholar]
- 47.Zrenner E. Fighting blindness with microelectronics. Sci Transl Med. 2013 Nov 6;5(210):210ps16. doi: 10.1126/scitranslmed.3007399. [DOI] [PubMed] [Google Scholar]
- 48.Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, Vijayasarathy C, Marangoni D, Ziccardi L, Kjellstrom S, Park TK, et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol Ther. 2018 Sep 5;26(9):2282–2294. doi: 10.1016/j.ymthe.2018.05.025. [*Clinical trial describing safety results from gene therapy via intravitreal delivery.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aylward JW, Xue K, Patrício MI, Jolly JK, Wood JC, Brett J, Jasani KM, MacLaren RE. Retinal Degeneration in Choroideremia follows an Exponential Decay Function. Ophthalmology. 2018 Jul;125(7):1122–1124. doi: 10.1016/j.ophtha.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Edwards TL, Xue K, Meenink HCM, Beelen MJ, Naus GJL, Simunovic MP, Latasiewicz M, Farmery AD, de Smet MD, MacLaren RE. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018 Jun 18;2:649–656. doi: 10.1038/s41551-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]