Abstract
Phenological shifts, changes in the seasonal timing of life cycle events, are among the best documented responses of species to climate change. However, the consequences of these phenological shifts for population dynamics remain unclear. Population growth could be enhanced if species that advance their phenology benefit from longer growing seasons and gain a pre-emptive advantage in resource competition. However, it might also be reduced if phenological advances increase exposure to stresses, such as herbivores and, in colder climates, harsh abiotic conditions early in the growing season. We exposed subalpine grasslands to ~ 3 K of warming by transplanting intact turfs from 2000 m to 1400 m elevation in the eastern Swiss Alps, with turfs transplanted within the 2000 m site acting as a control. In the first growing season after transplantation, we recorded species’ flowering phenology at both elevations. We also measured species’ cover change for three consecutive years as a measure of plant performance. We used models to estimate species’ phenological plasticity (the response of flowering time to the change in climate) and analysed its relationship with cover changes following climate change. The phenological plasticity of the 18 species in our study varied widely but was unrelated to their changes in cover. Moreover, early- and late-flowering species did not differ in their cover response to warming, nor in the relationship between cover changes and phenological plasticity. These results were replicated in a similar transplant experiment within the same subalpine community, established one year earlier and using larger turfs. We discuss the various ecological processes that can be affected by phenological shifts, and argue why the population-level consequences of these shifts are likely to be species- and context-specific. Our results highlight the importance of testing assumptions about how warming-induced changes in phenotypic traits, like phenology, impact population dynamics.
Keywords: climate change, demography, global warming, phenological shifts, phenology, phenotypic plasticity, population dynamics, transplant experiment
Introduction
Warming-induced advances in phenology are one of the best documented biological responses to recent climate change (Fitter and Fitter 2002). Plants, fungi, insects, and birds in a variety of temperate ecosystems have shifted the timing of their activity to match an earlier start to the growing season (Parmesan 2006), and a later season end (Jeong et al. 2011). However, species vary widely in the magnitude and direction of their phenological shifts (CaraDonna et al. 2014). While most species advance their phenology in response to warming, some do so more than others, and a few species even delay phenology (Fitter and Fitter 2002). Some of these shifts reflect adaptive evolution, but most result from phenotypic plasticity (Anderson et al. 2012). Phenotypic plasticity, the range of phenotypes that a single genotype can express depending on the environment, could help species persist in the face of climate change by allowing them to rapidly adjust to new environmental conditions (Nicotra et al. 2010).
It is often expected that the flexibility imparted by a plastic phenology is beneficial to individual performance (i.e., that plasticity is adaptive), and that less plastic species are therefore more likely to experience negative impacts of climate change (Cleland et al. 2012, Socolar et al. 2017). However, this assumed link between phenological plasticity and population dynamics is rarely tested (McLean et al. 2016). A myriad of ecological processes can be affected by phenological shifts and their net impact on population dynamics is unclear (Elzinga et al. 2007). For example, while advancing phenology can help plants exploit longer growing seasons, it can also result in mismatches with the timing of activity of mutualists or impose a higher risk of suffering frost damage, particularly for early-flowering species (Inouye 2008, Hegland et al. 2009, Rafferty et al. 2015). Also, counter to the expected advantages of phenological plasticity, some species advance their phenology more than required to track changing climates (Visser and Both 2005, Tansey et al. 2017). That is, depending on the circumstances, phenological plasticity can be adaptive or maladaptive.
Despite mixed expectations for the impact of phenological plasticity on plant performance, both long-term observational studies (Willis et al. 2008, Hulme 2011) and a meta-analysis of terrestrial warming experiments (Cleland et al. 2012) have found a positive, albeit weak, relationship between species’ phenological advance and their population performance under climate change. It remains unclear, however, whether the large amount of unexplained variation in plant population performance is intrinsic to its relationship with phenological plasticity or whether it stems from environmental factors difficult to control in these types of analyses. In long-term observational studies, many aspects of the environment could change simultaneously with climate and affect plant population dynamics. Meanwhile, in meta-analyses, the diversity of experimental designs, methodologies, and ecosystems included could contribute to the variety of responses of species with similar phenological plasticity. Hence, within a given community, how well phenological plasticity predicts species’ population-level responses to climate change deserves further attention. Our understanding of the demographic consequences of phenological plasticity might therefore benefit from experiments that analyse the effects of a common climate change treatment on multiple species’ performance and phenology within a single system, thereby eliminating methodological and study-system variation. However, the data required for such an analysis were simply unavailable to the most comprehensive meta-analysis to date (Cleland et al. 2012) because most warming experiments at the time only measured phenological shifts and performance for one or several (and always < 10) species.
In this context, whole-community transplant experiments in mountains provide a powerful way to study the population-level consequences of phenological plasticity under climate change. First, mountain ecosystems in the future will experience similar temperatures to those already occurring at lower elevation along the same mountain slope (Alexander et al. 2015). Second, by transplanting entire turfs, we can study the consequences of phenological plasticity in a community context, where neighbouring species are also shifting their phenology and other traits in response to climate change. In particular, temperate mountain grasslands are diverse at small spatial scales (Wilson et al. 2012), providing numerous species to evaluate how phenological plasticity relates to population performance. Moreover, the diversity of mountain grassland species provides opportunities to test whether the effects of plasticity differ between functional groups, such as species active early or late during the growing season.
Here, we examine whether phenological plasticity can predict population level-responses in subalpine plant communities transplanted to a site 600 m lower in elevation, with a ca. 3 K warmer climate (Barry 2008). We used species’ shifts in flowering time as a measure of their phenological plasticity and changes in cover as an indicator of species population performance. Specifically, we addressed three questions: (1) do subalpine plants advance their flowering phenology when transplanted to a lower, warmer site?, (2) are these phenological advancements correlated with cover changes in response to climate warming?, (3) does the strength of this correlation differ between early- and late-flowering species?. To further contextualize our findings, we end this paper with a synthesis of hypotheses for when we would expect phenological advances to be beneficial for species’ demography and population dynamics.
Materials and Methods
Field experiment
We selected two perennial grassland sites, one at 2000 m and one at 1400 m along the Calanda massif in the eastern Swiss Alps (46.88º N, 9.49º E). The climate at the lower site resembles that expected at the higher site over the next 50-100 years (CH2018, 2018). Namely, sites differ in mean annual temperature by ca. 3 K (based on adiabatic lapse rate) and in mean annual precipitation (1169 and 1355 mm at the lower and higher site, respectively, based on interpolations of Swiss climate from ca. 1961 to 1990 at 50 m; Alexander et al. 2015). Otherwise, the sites are similar in slope, aspect, and parent material (calcareous bedrock), and are only 2.1 km apart. This similarity is important for isolating the effects of climate differences from other environmental differences between sites. Thus, while the higher site was chosen for its high species diversity and compact turfs suitable for transplantation, the lower site was chosen to resemble the higher site as closely as possible (except the climate).
In September 2013, we extracted 20 turfs (0.5 × 0.5 m) from the 2000 m site to a depth of ca. 20 cm, which included most roots and the organic soil layer. We selected turfs haphazardly, avoiding rocky patches unsuitable for transplantation and areas heavily dominated by a single species (with > 50 % cover). We transplanted 10 of these turfs into the 1400 m site (climate change treatment), and 10 into different locations within the 2000 m site (transplant control). Given that temperatures experienced by low-statured subalpine plants tend to be decoupled from air temperatures (Körner 2007), we placed three temperature loggers (Onset HOBO Pendant Temperature/Light 64K Data Loggers) at ground level close to the turfs at each site to get a more accurate measure of the conditions actually experienced by the plants during the growing season. We also used these loggers to record light intensity and hence they were exposed to direct sunlight, which likely resulted in sensor overheating and some unreliably high temperature measurements. Therefore, we restricted temperature values to the maximum temperature recorded under low light conditions (< 50 Lux) at each site (29.7 and 33.4 ºC at the high and low sites, respectively).
We assumed that flowering phenology is a good proxy for the overall phenological development of subalpine species (Sola and Ehrlén 2007). Flowering is additionally relatively easy to monitor, and hence the phenological stage that has been most commonly studied. Therefore, in 2014 we monitored species’ flowering phenology once a week during the growing season (late-April to early-October) in every turf. Specifically, we recorded the date on which a species was first observed to flower in each turf (i.e., at least one individual had an open flower with reproductive organs exposed). Many subalpine species start forming flower buds one or several seasons before flowering (Körner 2003), and hence environmental conditions experienced at the high-elevation site could have influenced the probability of species flowering in 2014. Nonetheless, the influence of previous high-elevation conditions on flowering probability was the same for turfs in the control and climate change treatments. Moreover, the timing of flowering within a season is largely driven by environmental conditions during the current season (namely temperature, snowmelt time, and photoperiod), and sometimes during the preceding winter (Thórhallsdóttir 1998, Körner 2003, Hülber et al. 2010). Therefore, differences in flowering time between the control and climate change treatments in the year immediately following turf transplantation are expected to reflect species’ phenological plasticity.
We further assumed that the phenological responses of most species could be observed during this first year after transplantation. While acclimation after multiple years at lower elevation could accentuate some species’ phenological response to warming (Hoffmann et al. 2010), we assumed that this would have small effects on the ranking and relative magnitude of species’ phenological shifts. Indeed, phenological shifts in response to experimental warming tend to be relatively constant across years, despite large differences in the actual timing of flowering (Dunne et al. 2003). We examine the implications of violating this assumption in the ‘Study Limitations’ section of the Discussion.
Additionally, to evaluate species’ demographic responses to a warmer climate, we visually estimated species’ percentage cover within turfs in early June and late July for three consecutive years after transplantation (2014–2016). Since some species had started senescing by late July, we used the June cover data for the analyses except when a species was not recorded during that first survey. To complement these analyses and assess the generality of our results, we also analysed species’ cover responses in an earlier transplant experiment along the same elevation gradient, of almost identical design but set up one year earlier and using 0.56-m2 turfs (details in Alexander et al. 2015), and in which species’ cover, but not phenology, was recorded every summer from 2013 to 2016. We will hereafter refer to this latter experiment as the “four-year” experiment.
Data analysis
Due to the high variability among turfs in species’ phenological and cover responses to warming, our analyses only included species that were present in at least three turfs in both the climate change and control treatments. Although small, this minimum sample size allowed us to include 18 species in the analysis, and our results were similar when we only included the 10 species present in at least half of the turfs (see Appendix S2).
For analysis, we assumed that flowering actually occurred at the midpoint between the date it was first observed and the date of the prior survey (generally one week before). For consistency, if a species was observed flowering at the time of the first survey, we assumed that flowering occurred half a week before. We categorized species into early- and late-flowering groups depending on whether their median first flowering time in the control turfs was before or after June 1st, a date which lead to roughly equal numbers in each group and corresponds with the start of the meteorological summer. If, at the time of the first phenological survey (late April 2014), a species had already flowered in more than half of the turfs where it was present, we excluded it from the analyses because it was no longer possible to estimate the magnitude of its true phenological shift.
For each species, we fitted a multiple regression model of first flowering time as a function of treatment (i.e., climate change or transplantation control). We used species’ initial cover as a covariate in the models, since earlier flowering is probabilistically more likely in plots where a species is more abundant (Miller-Rushing et al. 2008). We used the regression coefficient of the treatment factor as an indicator of a species’ climate-related phenological plasticity. This coefficient, being the slope of the regression, can be interpreted as a reaction norm. Note that, throughout this paper, we refer to ‘phenological plasticity’ as the change in the calendar date of a phenological event. This change can result from actual developmental plasticity (e.g., faster developmental rates under warmer temperatures) but also from differences in the timing of environmental cues (e.g., snowmelt) at the lower and higher sites.
As a measure of each species’ population trend in each turf, we took the logarithm of the ratio of the final cover (year 3 or year 4 depending on the experiment) to the initial cover (each on a scale from 0 to 100% cover). We added 0.25 to the recorded covers to handle cases in which a species’ final cover was 0 (our results are robust to variation in the value of this constant, Appendix S2.1). Then, we fitted a linear model of the log cover ratio as a function of treatment and used the regression coefficient as an estimate of a species’ demographic response to climate change. Since the possible cover values for a species in a turf were bounded between 0 and 100, we included species’ initial cover as covariate in the model to account for the fact that when a species was initially abundant in a turf, its cover was less likely to show large proportional increases, and vice versa.
Finally, we analysed the relationship between species’ demographic and phenological responses to climate change with a multiple linear regression, including flowering time group (early or late) and its interaction with phenological plasticity as covariates (including species’ average initial cover across all plots as a covariate gave similar results). Since the interaction term was not significant, we tested the significance of the regression coefficients with an analysis of variance with type II sum of squares. Error in the estimates of phenological plasticity, the ‘independent’ variable in the regression model, biases the fitted slope towards zero, thereby reducing the power of the analysis (McArdle 2003). To account for the uncertainty in both the dependent and independent variables in our model, we conducted a resampling analysis. Sampling data from normal distributions based on the mean and standard error of cover responses and phenological shifts estimated for each species, we fitted models to 10,000 different datasets to characterize a distribution of slope estimates and P values. We prepared and analysed the data in R version 3.3.0 (R Core Development Team 2016). Code for data processing and analyses are available in Appendix S1 in Supporting Information.
Results
The mean daily temperatures at ground level during the growing season (March to August 2014) were 2.1 K higher at the low-elevation site than at the high-elevation site. During the preceding winter snow cover was intermittent at the low-elevation site. However, the timing of first snowmelt in spring and the start of the growing season (defined as the first date with mean daily temperature ≥ 5 ºC) happened almost simultaneously at both elevations in early March 2014, despite higher temperatures at the lower site (Fig. 1).
Figure 1.
Mean daily temperatures during the growing season of 2014 and the preceding winter, recorded with temperature loggers placed at ground level on the high-elevation (blue line) and low-elevation (red line) sites.
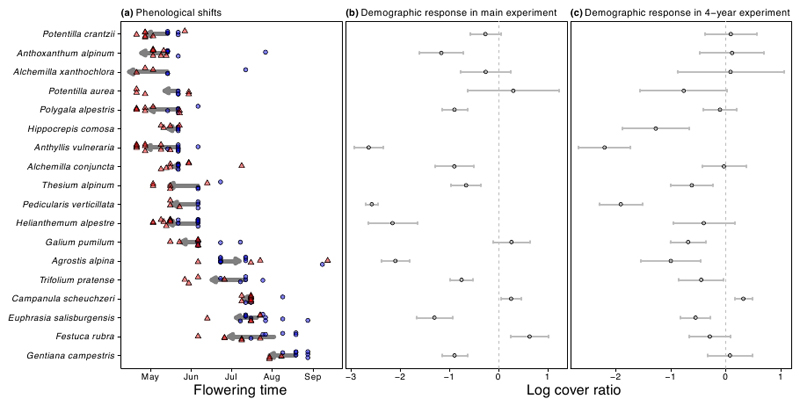
Despite the synchronous onset of the growing season, most species advanced their first flowering date with simulated climate change. However, the magnitude of the estimated shift varied widely across species, from −34.3 to +14.4 days (Fig. 2a). Species also differed in their among-turf variability in first flowering times; some species, such as Campanula scheuchzeri, flowered synchronously in almost all turfs, whilst others, such as Agrostis alpina, had a first flowering date that ranged over three months among turfs. Species also differed in their cover response to simulated climate change in both experiments (Fig.2b and c). On average, the cover of most species decreased in the climate change treatment. However, the responses of some species were highly variable across different turfs (e.g., Potentilla aurea) and across the two experiments (e.g., Gentiana campestris).
Figure 2.
(a) Species’ first flowering time in turfs transplanted to lower elevation (red triangles: climate change treatment) or within the same elevation (blue circles: control treatment). The arrows indicate the magnitude and direction of the phenological shift due to simulated climate change, estimated with a multiple regression of flowering time as a function of treatment, controlling for differences in species’ initial cover. Estimated effects (± 1 standard error) of climate change on species’ demographic trends (measured by the log ratio of final to initial percent cover) from a multiple regression with species’ initial cover as a covariate, for the main (b) and 4-year (c) experiments.
Species’ phenological plasticity was unrelated to their cover response to warming (F1,14 = 0.083, P = 0.78; Fig 3a). Moreover, early- and late-flowering species did not differ in their cover response to warming (F1,14 = 0.011, P = 0.92), nor in the association between cover response to warming and phenological plasticity (interaction term: F1,14 = 0.017, P = 0.90). Phenological plasticity also failed to predict mean cover response to warming in the four-year experiment (F1,13 = 0.243, P = 0.63; Fig. 3b), for both early- and late-flowering species (interaction term: F1,13 = 0.935, P = 0.35). We obtained similar results when we included species’ Landolt elevational scores (Landolt et al. 2010) as a covariate in the models to account for differences in species’ thermal affinity.
Figure 3.
Relationship between phenological shifts and cover responses to climate change for early- and late-flowering species (black and grey dots, respectively) in the main (a) and 4-year (b) experiments. Climate change was simulated by transplanting turfs to a site 600 m lower in elevation (i.e., ca. 3 K warmer) than the site of origin. Phenological shift is the effect of climate change on species first flowering time estimated with a multiple regression including species initial cover as a covariate. Cover response to climate change is the effect of downslope transplantation on the logarithm of the ratio of final to initial cover of a species in a turf. This effect was estimated with a multiple regression including species’ initial cover as covariate. Mean estimates (± 1 standard error) are shown.
We did not have enough data to test whether the effects of phenological plasticity varied among different functional groups. For example, we could only include three graminoid species in such an analysis (Agrostis alpina, Anthoxanthum alpinum, and Festuca rubra). However, a linear regression model testing the effects of phenological plasticity on the cover responses of these three grasses, averaged across the two experiments, reveals a marginally significant (F1,1 = 65.04, P = 0.08) negative relationship (i.e., positive effects of advancing phenology) that fits the data remarkably well (adjusted R2 = 0.97, Fig. S1).
While the resampling analysis revealed substantial variation in the estimated effects of phenological plasticity on cover trends under simulated climate change (Fig. S2), these were clearly centred around zero in both experiments (95 % CI = –0.020 to 0.019 in main experiment; –0.023 to 0.020 in 4-year experiment). Moreover, only a small minority of the slopes fitted to resampled data were significantly different from zero; 1.3 % and 0.3 % for the main and 4-year experiments, respectively.
Discussion
We found a large and consistent phenological advance of an entire subalpine community transplanted to a lower elevation, even though we monitored phenology in only one season and all species are perennials. Within this single subalpine plant community, demographic responses to simulated climate change varied substantially both between and within species (Fig. 2b and c), but this variability was not associated with phenological plasticity in flowering (Fig 3). Moreover, the effects of phenological plasticity on cover changes were not statistically different between early- and late- flowering species in our experiments. Though not a statistically significant effect, late-flowering species tended to benefit slightly more from advanced phenology, especially in the 4-year experiment (Fig. 3b). Earlier taxa may not derive similar benefits of advancing phenology due to a higher risk of frost damage during spring at the low-elevation site (Appendix S2). In fact, the potentially positive effects of advanced phenology for the three grasses included in the analysis may relate to the fact that grasses are likely to have a higher frost tolerance than forbs in this subalpine grassland (Taschler and Neuner 2004).
Our results suggest that the previously identified noise in the relationship between phenological advances and demographic responses to climate may be intrinsic to the relationship, and not simply due to uncontrolled environmental drivers (Willis et al. 2008, Hulme 2011) nor to the heterogeneous nature of studies pooled for analysis (Cleland et al. 2012). Here we show that, even when looking at a single plant community and using consistent methodology, the noise persists. We therefore conclude that plasticity in flowering phenology is a poor predictor of species performance following simulated climate change, at least in this subalpine grassland community. Indeed, Cleland et al. (2012)’s analysis of seven subalpine meadow species in Colorado found no significant relationship between phenological shifts and performance, and species with almost identical phenological plasticities had the highest and lowest performance under warming. Therefore, while there might be a general benefit of phenological plasticity in changing environments, the net demographic consequences are likely to depend strongly on species’ physiology and ecology (Pardee et al. 2019), and the degree to which the cues regulating species’ phenology maintain their temporal association with optimal environmental conditions. In what follows, after discussing the limitations of our approach, we further develop this hypothesis based on a brief literature review of the complex links between phenology and demography under climate change.
Study limitations and general caveats
Several limitations of our study and others like it must be kept in mind when interpreting our results. First, flowering phenology does not capture the full gamut of plastic phenological responses that influence species’ demography (Post et al. 2008, Yang and Rudolf 2010). For example, species in subarctic meadow communities advance their flowering in response to earlier snowmelt, but not so their root growth (Blume-Werry et al. 2017), which is presumably important to take advantage of an early start to the growing season. Thus, more holistic phenological measures might show a clearer association with species’ cover responses to climate change. Nonetheless, it is still relevant to study the potential of plasticity in flowering phenology as a predictor of population responses to climate change for two reasons. First, flowering time is among the easiest to detect and most often measured phenological events. Second, flowering is a crucial event in a plant’s life cycle and can only be successful within a narrow time window in subalpine environments (Körner 2003), which likely results in strong selection for adaptive plasticity in flowering phenology (Iwasa and Levin 1995).
Second, we only monitored phenology during one growing season, assuming that phenological plasticity is a species’ trait that will remain consistent in rank and relative magnitude when measured across species in different years. Some studies have reported consistent phenological shifts in response to warming over several years (Dunne et al. 2003), but others have found that the magnitude of shifts can change with inter-annual climatic variation (Walther et al. 2002, Jentsch et al. 2009). If the ranking of species’ phenological plasticity varied widely from year to year, any link between plasticity and demography would be blurred when correlating plasticity measured during one year with cover changes measured over several years. The ranking of species’ phenological shifts is especially likely to change when species rely on different environmental cues to regulate their phenology. For example, the phenology of a species relying exclusively on snowmelt as a flowering cue would have varied widely between 2014 (when snowmelt was simultaneous at the lower and higher sites) and 2015 (when snow melted one month earlier at the lower site). This suggests that the phenological shifts we observed may not be representative of phenological responses in other years. Nonetheless, the phenological shifts we observed can be interpreted as responses to warming per se, which we expect to be consistent across years (Dunne et al. 2003).
Third, some species’ phenological shifts lag climate change (Hoffmann et al. 2010). However, given the large shifts displayed already in the first season, we do not expect any lagged responses to substantially alter the relative plasticity observed across species, and thus to qualitatively change our results.
Fourth, short-term changes in species cover are likely an imperfect proxy for long-term population dynamics (but see Tredennick, Hooten, & Adler, 2017). On the short timescales of our experiments, cover changes in these perennial species are mostly driven by vegetative growth (Körner 2003). The demographic advantages of phenological plasticity might be better reflected by longer-term cover changes. In any case, the close association between cover change and growth makes it a relevant proxy for species performance and short-term dynamics in this subalpine community.
Finally, there is also a more general limitation of our study and others analysing correlations between phenological shifts and species’ performance. Advances in species’ flowering time are correlated with other functional traits that influence growth rate and competitive ability (König et al. 2017, Bucher et al. 2018). It is therefore possible that the observed correlations between phenological plasticity and species performance are mediated by other correlated traits and not by phenology per se.
A synthesis of hypotheses for the demographic consequences of phenological plasticity
Based on our finding that phenological plasticity is a poor predictor of species’ demographic responses to climate change, even within a single community, we end with a discussion of the complex links between phenology and demography during climate change, and synthesize hypotheses for when to expect phenological plasticity to be advantageous, and when not.
Theory suggests that, in temperate ecosystems, the optimal timing of life history events is a balance between avoiding harsh environmental conditions early in the season and avoiding intense competition later in the season (Iwasa and Levin 1995). Therefore, phenology should be under strong selective pressure to rely on environmental cues that signal the onset of optimal conditions for growth (Körner and Basler 2010, Forrest and Miller-Rushing 2010). Since seasonal dynamics in temperate ecosystems are largely driven by seasonal temperature fluctuations, species’ ability to track shifts in the timing of thermal cues must, in principle, be advantageous (Willis et al. 2008, Cleland et al. 2012). However, climate change involves simultaneous changes in many environmental variables (Garcia et al. 2014). It is not only the pattern of seasonal variation in temperature that changes, but also that of multiple elements of the abiotic and biotic environments (Fig. 4). Moreover, changes in the timing of different environmental variables differ in magnitude and even direction (Ovaskainen et al. 2013), and the degree to which specific variables remain temporally coupled differs strongly among regions (Liu et al. 2018). Therefore, the net effect of phenological plasticity on species’ performance should depend on the degree to which the environmental cues regulating phenology (e.g., temperature) maintain their temporal correlation with optimal environmental conditions (Fig. 4b; Post, 2013). What defines these optimal environmental conditions varies among species, depending on the relative impact of different variables on species’ demography and population growth (Pardee et al. 2019). Therefore, it is natural to expect a variety of demographic consequences of phenological plasticity within any community. For example, in the scenario depicted in Fig. 4c, phenological plasticity would be detrimental for a species using temperature as cue for frost risk, but beneficial for a species using it as a cue for herbivore density.
Figure 4.
The demographic consequences of phenological plasticity depend on whether environmental cues regulating phenology maintain their association with the timing of optimal conditions for life history events. (a) Phenology has evolved to rely on cues that correlate with the timing of optimal conditions, such as low frost risk and low density of antagonist species. (b) If climate change alters the timing of these conditions, but not their association with phenological cues, then phenological plasticity should be advantageous. (c) However, when climate change alters the temporal association between phenological cues and optimal conditions, plasticity can be detrimental.
A decoupling of the seasonal association between different abiotic factors can lead to negative fitness consequences of phenological plasticity (Fig 4c). For example, increasing mean temperatures and advancing time of snowmelt in spring are often decoupled from the risk of experiencing freezing temperatures (Klein et al. 2018), with the degree of coupling varying substantially among regions (Liu et al. 2018). Indeed, frost damage due to advanced phenology has been linked to lower performance in observational studies (Inouye 2008, Wheeler et al. 2015), in experiments manipulating temperature (Rixen et al. 2012) and snowmelt dates (Wipf et al. 2009), and in plants transplanted to lower elevations (Scheepens and Stöcklin 2013). Therefore, phenological advances should be less advantageous (1) in areas where the risk of frost damage during spring has remained constant or increased despite rising temperatures (Liu et al. 2018), and (2) for species with low frost tolerance (Ladinig et al. 2013) active early in the growing season (Wheeler et al. 2015, Pardee et al. 2019).
Of course, the temporal distribution of biotic variables can also become decoupled under climate change. The wide variety of phenological responses both within (CaraDonna et al. 2014) and between (Ovaskainen et al. 2013) trophic levels reshapes species interaction networks, with complex consequences for community dynamics. Within trophic levels, time can be an important niche axis and interspecific competition may be weakened when species differ in their phenology (Wolkovich and Cleland 2014, Alexander and Levine 2019). Thus, understanding how phenological shifts affect competitive interactions requires analyzing shifts relative to other members in the same trophic guild (Yang and Rudolf 2010). Relative phenological advances can be advantageous if they allow for priority effects (Körner et al. 2008).
Finally, phenological sensitivity differs across trophic levels. Specifically, herbivores tend to advance their phenology to a greater extent than autotrophs and carnivores (Thackeray et al. 2016). Hence, advanced plant phenology will, generally, increase the intensity of interactions with both herbivores and pollinators (Elzinga et al. 2007, Ehrlén and Münzbergová 2009). But there are counterexamples to this general pattern. For example, advanced flowering during warm springs in Japan led to mismatches with pollinators in bumblebee-pollinated species, but not in fly-pollinated species (Kudo et al. 2004, Kudo and Ida 2013). Moreover, the net consequences of altered mutualistic and antagonistic interactions will vary across species and ecosystems. Indeed, whereas phenotypic selection for earlier flowering seems to be widespread in nature (Munguía-Rosas et al. 2011, Anderson et al. 2012), the opposite trend has been observed in systems and years with high abundance of insect herbivores (Pilson 2000).
In summary, the relationship between phenological plasticity and demographic responses to climate change depends on (1) the correlation between changes in the pattern of temporal variation of different environmental and biotic variables, which varies across ecosystems, and (2) the relative importance of those environmental variables for demography, which varies across species.
Future directions
Our results highlight the importance of testing the frequently assumed consequences of climate change driven phenological shifts on species’ performance and population dynamics (McLean et al. 2016). In highly seasonal environments, phenological shifts alter both the abiotic conditions under which organisms develop and their interactions with other species in the community, which may have complex, and sometimes opposing, effects on performance. Achieving mechanistic understanding of these processes requires studying how the plastic timing of different phenological stages affects species’ demographic rates, and how these in turn affect long-term population dynamics in environments that are under constant biotic and abiotic change (McLean et al. 2016).
Therefore, we suggest that future studies (1) distinguish the effects of shifting phenology on the abiotic environment under which a species develops from the effects mediated by the altered biotic interactions resulting from species differences in their phenological shifts (Pardee et al. 2019), (2) identify how these altered interactions affect the various demographic components of plant fitness (Ehrlén and Münzbergová 2009, Iler et al. 2019), and (3) assess the consequences of phenological shifts in the novel communities that result from asynchronous species range shifts (Alexander et al. 2015).
Supplementary Material
Additional supporting information may be found in the online version of this article.
Acknowledgments
We thank Marc-Jacques Mächler, Renato Guidon, Jonas Brännhage, Patrick Stettler, Saskia Minneboo, and Amarante Vitra for their contribution to field data collection, and the community of Haldenstein for providing field sites. We also thank the Plant Ecology Group at ETHZ for their feedback on the manuscript and Sabine Güsewell for her advice on the statistical analyses.
Funding – This research was funded by ETH Zurich and the Swiss National Science Foundation grant 31003A_173210. JMA received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 678841.
Footnotes
Author contributions – JMA and JML conceived and set up the field experiments; SB, JML, and JMA conceived the idea for the analysis; SB analysed the data and led the writing of the manuscript; all authors contributed critically to the revisions and gave final approval for publication.
Conflict of interest – All authors declare that they have no conflict of interest.
Data accessibility
All data analysed in this paper is be available in the Dryad Digital Repository <http://doi.org/10.5061/dryad.vt4b8gtn4>
References
- Alexander JM, Levine JM. Earlier phenology of a nonnative plant increases impacts on native competitors. Proc Natl Acad Sci. 2019 doi: 10.1073/pnas.1820569116. 201820569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, et al. Novel competitors shape species’ responses to climate change. Nature. 2015;525:515–518. doi: 10.1038/nature14952. [DOI] [PubMed] [Google Scholar]
- Anderson JT, et al. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc R Soc Lond B Biol Sci. 2012 doi: 10.1098/rspb.2012.1051. rspb20121051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RG. Mountain Weather and Climate. Cambridge Univ. Press; 2008. [Google Scholar]
- Blume-Werry G, et al. Root phenology unresponsive to earlier snowmelt despite advanced above-ground phenology in two subarctic plant communities. Funct Ecol. 2017;31:1493–1502. [Google Scholar]
- Bucher SF, et al. Traits and climate are associated with first flowering day in herbaceous species along elevational gradients. Ecol Evol. 2018;8:1147–1158. doi: 10.1002/ece3.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaraDonna PJ, et al. Shifts in flowering phenology reshape a subalpine plant community. Proc Natl Acad Sci. 2014;111:4916–4921. doi: 10.1073/pnas.1323073111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CH2018. CH2018 – Climate Scenarios for Switzerland, Technical Report. National Centre for Climate Services. 2018 [Google Scholar]
- Cleland EE, et al. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- Dunne JA, et al. Subalpine Meadow Flowering Phenology Responses to Climate Change: Integrating Experimental and Gradient Methods. Ecol Monogr. 2003;73:69–86. [Google Scholar]
- Ehrlén J, Münzbergová Z. Timing of Flowering: Opposed Selection on Different Fitness Components and Trait Covariation. Am Nat. 2009;173:819–830. doi: 10.1086/598492. [DOI] [PubMed] [Google Scholar]
- Elzinga JA, et al. Time after time: flowering phenology and biotic interactions. Trends Ecol Evol. 2007;22:432–439. doi: 10.1016/j.tree.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fitter AH, Fitter RSR. Rapid Changes in Flowering Time in British Plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc B Biol Sci. 2010;365:3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, et al. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science. 2014;344 doi: 10.1126/science.1247579. 1247579. [DOI] [PubMed] [Google Scholar]
- Hegland SJ, et al. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, et al. Phenological changes in six Australian subalpine plants in response to experimental warming and year-to-year variation. J Ecol. 2010;98:927–937. [Google Scholar]
- Hülber K, et al. Intraseasonal climate and habitat-specific variability controls the flowering phenology of high alpine plant species. Funct Ecol. 2010;24:245–252. [Google Scholar]
- Hulme PE. Contrasting impacts of climate-driven flowering phenology on changes in alien and native plant species distributions. New Phytol. 2011;189:272–281. doi: 10.1111/j.1469-8137.2010.03446.x. [DOI] [PubMed] [Google Scholar]
- Iler AM, et al. Reproductive losses due to climate change-induced earlier flowering are not the primary threat to plant population viability in a perennial herb. J Ecol. 2019;107:1931–1943. [Google Scholar]
- Inouye DW. Effects of Climate Change on Phenology, Frost Damage, and Floral Abundance of Montane Wildflowers. Ecology. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Levin SA. The timing of life history events. J Theor Biol. 1995;172:33–42. [Google Scholar]
- Jentsch A, et al. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Glob Change Biol. 2009;15:837–849. [Google Scholar]
- Jeong S-J, et al. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob Change Biol. 2011;17:2385–2399. [Google Scholar]
- Klein G, et al. Unchanged risk of frost exposure for subalpine and alpine plants after snowmelt in Switzerland despite climate warming. Int J Biometeorol. 2018;62:1755–1762. doi: 10.1007/s00484-018-1578-3. [DOI] [PubMed] [Google Scholar]
- König P, et al. Advances in flowering phenology across the Northern Hemisphere are explained by functional traits. Glob Ecol Biogeogr. 2017;27:310–321. [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer; 2003. [Google Scholar]
- Körner C. The use of ‘altitude’ in ecological research. Trends Ecol Evol. 2007;22:569–574. doi: 10.1016/j.tree.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Körner C, Basler D. Phenology Under Global Warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- Körner C, et al. Small differences in arrival time influence composition and productivity of plant communities. New Phytol. 2008;177:698–705. doi: 10.1111/j.1469-8137.2007.02287.x. [DOI] [PubMed] [Google Scholar]
- Kudo G, Ida TY. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology. 2013;94:2311–2320. doi: 10.1890/12-2003.1. [DOI] [PubMed] [Google Scholar]
- Kudo G, et al. Does seed production of spring ephemerals decrease when spring comes early? Ecol Res. 2004;19:255–259. [Google Scholar]
- Ladinig U, et al. How endangered is sexual reproduction of high-mountain plants by summer frosts? Frost resistance, frequency of frost events and risk assessment. Oecologia. 2013;171:743–760. doi: 10.1007/s00442-012-2581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt E, et al. Flora indicativa. Ecological indicator values and biological attributes of the flora of Switzerland and the Alps. Haupt. 2010 [Google Scholar]
- Liu Q, et al. Extension of the growing season increases vegetation exposure to frost. Nat Commun. 2018;9:426. doi: 10.1038/s41467-017-02690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle BH. Lines, models, and errors: Regression in the field. Limnol Oceanogr. 2003;48:1363–1366. [Google Scholar]
- McLean N, et al. Predicting when climate-driven phenotypic change affects population dynamics. Ecol Lett. 2016;19:595–608. doi: 10.1111/ele.12599. [DOI] [PubMed] [Google Scholar]
- Miller-Rushing AJ, et al. How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol. 2008;96:1289–1296. [Google Scholar]
- Munguía-Rosas MA, et al. Meta-analysis of phenotypic selection on flowering phenology suggests that early flowering plants are favoured. Ecol Lett. 2011;14:511–521. doi: 10.1111/j.1461-0248.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Ovaskainen O, et al. Community-level phenological response to climate change. Proc Natl Acad Sci. 2013;110:13434–13439. doi: 10.1073/pnas.1305533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee GL, et al. The individual and combined effects of snowmelt timing and frost exposure on the reproductive success of montane forbs. J Ecol. 2019;107:1970–1981. [Google Scholar]
- Parmesan C. Ecological and Evolutionary Responses to Recent Climate Change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- Pilson D. Herbivory and natural selection on flowering phenology in wild sunflower, Helianthus annuus. Oecologia. 2000;122:72–82. doi: 10.1007/PL00008838. [DOI] [PubMed] [Google Scholar]
- Post E. Ecology of Climate Change: The Importance of Biotic Interactions. Monographs in Population Biology. Princeton Univ. Press; 2013. Life History Variation and Phenology. [Google Scholar]
- Post ES, et al. Phenological sequences reveal aggregate life history response to climatic warming. Ecology. 2008;89:363–370. doi: 10.1890/06-2138.1. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- Rafferty NE, et al. Phenological shifts and the fate of mutualisms. Oikos. 2015;124:14–21. doi: 10.1111/oik.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixen C, et al. Evidence of enhanced freezing damage in treeline plants during six years of CO2 enrichment and soil warming. Oikos. 2012;121:1532–1543. [Google Scholar]
- Scheepens JF, Stöcklin J. Flowering phenology and reproductive fitness along a mountain slope: maladaptive responses to transplantation to a warmer climate in Campanula thyrsoides. Oecologia. 2013;171:679–691. doi: 10.1007/s00442-012-2582-7. [DOI] [PubMed] [Google Scholar]
- Socolar JB, et al. Phenological shifts conserve thermal niches in North American birds and reshape expectations for climate-driven range shifts. Proc Natl Acad Sci. 2017;114:12976–12981. doi: 10.1073/pnas.1705897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola AJ, Ehrlén J. Vegetative phenology constrains the onset of flowering in the perennial herb Lathyrus vernus. J Ecol. 2007;95:208–216. [Google Scholar]
- Tansey CJ, et al. Estimating the ability of plants to plastically track temperature mediated shifts in the spring phenological optimum. Glob Change Biol. 2017;23:3321–3334. doi: 10.1111/gcb.13624. [DOI] [PubMed] [Google Scholar]
- Taschler D, Neuner G. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ. 2004;27:737–746. [Google Scholar]
- Thackeray SJ, et al. Phenological sensitivity to climate across taxa and trophic levels. Nature. 2016;535:241–245. doi: 10.1038/nature18608. [DOI] [PubMed] [Google Scholar]
- Thórhallsdóttir TE. Flowering phenology in the central highland of Iceland and implications for climatic warming in the Arctic. Oecologia. 1998;114:43–49. doi: 10.1007/s004420050418. [DOI] [PubMed] [Google Scholar]
- Tredennick AT, et al. Do we need demographic data to forecast plant population dynamics? Methods Ecol Evol. 2017;8:541–551. [Google Scholar]
- Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc Lond B Biol Sci. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wheeler HC, et al. Phenological mismatch with abiotic conditions—implications for flowering in Arctic plants. Ecology. 2015;96:775–787. doi: 10.1890/14-0338.1. [DOI] [PubMed] [Google Scholar]
- Willis CG, et al. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc Natl Acad Sci. 2008;105:17029–17033. doi: 10.1073/pnas.0806446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JB, et al. Plant species richness: the world records. J Veg Sci. 2012;23:796–802. [Google Scholar]
- Wipf S, et al. Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Clim Change. 2009;94:105–121. [Google Scholar]
- Wolkovich EM, Cleland EE. Phenological niches and the future of invaded ecosystems with climate change. AoB PLANTS. 2014;6 doi: 10.1093/aobpla/plu013. plu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LH, Rudolf VHW. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett. 2010;13:1–10. doi: 10.1111/j.1461-0248.2009.01402.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.