Abstract
Ideally, radiomics features and radiomics signatures can be used as imaging biomarkers for diagnosis, staging, prognosis, and prediction of tumor response. Thus, the number of published radiomics studies is increasing exponentially, leading to a myriad of new radiomics-based evidence for lung cancer. Consequently, it is challenging for radiologists to keep up with the development of radiomics features and their clinical applications. In this article, we review the basics to advanced radiomics in lung cancer to guide young researchers who are eager to start exploring radiomics investigations. In addition, we also include technical issues of radiomics, because knowledge of the technical aspects of radiomics supports a well-informed interpretation of the use of radiomics in lung cancer.
Keywords: Lung cancer, Biomarkers, Image processing
INTRODUCTION
What Is Radiomics?
The development of imaging techniques has led to the rapid expansion of medical imaging data for the diagnosis, staging, treatment planning, and response evaluation in patients with lung cancer. Although conventional interpretations provide information on lung cancer phenotypes, researchers have suggested that a great deal of biologic or prognostic information remains embedded within images. Radiomics is a field of study in which high-throughput imaging data is analyzed, and a vast amount of advanced quantitative features are extracted. By employing radiomics, we can capture immensely valuable cancer information that might have been overlooked or cannot be identified with the naked eye.
In the era of precision medicine, the demand for radio-phenotyping for accurate patient stratification is greater than ever. Ideally, radiomics features and signatures can be used as imaging biomarkers. Hence, not only the number but also the quality and sophistication of published radiomics studies is accelerating, leading to a myriad of new radiomics-based evidence in the field of lung cancer. Consequently, it is challenging for radiologists to keep up with the rapid development of radiomics features and clinical applications.
Therefore, the purpose of this review is to elaborate from the basics to advanced radiomics for lung cancer guide young researchers who are eager to start exploring radiomics investigations. In addition, we also include technical issues of radiomics, because knowledge of the technical aspects of radiomics supports a well-informed interpretation of the use of radiomics in lung cancer.
Steps of Radiomics Studies
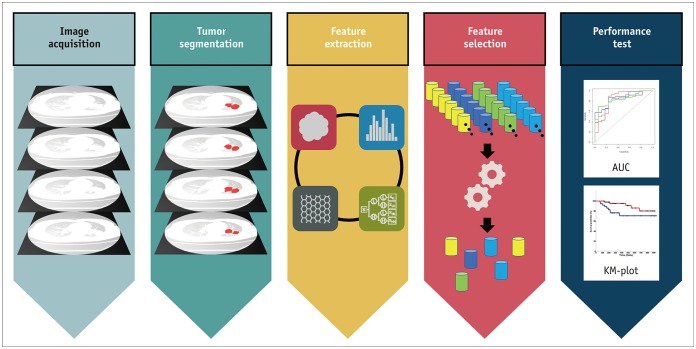
The radiomics process starts with image acquisition (Fig. 1). In general, there is no need to use sophisticated techniques for obtaining medical imaging scans. Most radiomics studies are retrospective and use conventional medical images obtained from routine clinical protocols. Nevertheless, different acquisition parameters may affect the extracted radiomics features, which might lead to questions regarding the stability of radiomics.
Fig. 1. Steps of radiomics: radiomics process starts with image acquisition and tumor segmentation, followed by feature extraction and selection, and ends with performance testing.
AUC = area under curve, KM = Kaplan-Meier
Following image acquisition, the tumor region of interest (ROI) is defined for analysis (i.e., tumor segmentation), and generally includes the entire tumor area. Next, from the defined ROI, a variety of radiomics features are extracted via software programs. Currently available radiomics features can be classified into multiple categories. Representative categories include morphological, histogram-based, texture and airway-related radiomics features (Table 1). Due to the continual development and refinement of radiomics features, the number of extractable features is rapidly growing. Finally, feature selection, model development, and performance testing are some of the most critical and demanding steps of a radiomics study. Feature selection may either be performed simultaneously with model development as a built-in procedure or may be done separately. Although numerous features can be extracted from a single tumor, the numerous available features need to be reduced to a practical number. Thus, through feature selection and model development, the most useful and prognostic radiomics features are summarized for clinical application. Several classifiers can be used for the model development depending on the number of cases, features, and characteristics. Commonly used methods are the random forest, principle component analysis, and least absolute shrinkage and selection operator. Lastly, testing the performance of the selected radiomics features is crucial to popularize radiomics in clinical research. Both internal and external validation is essential to ensure reliable radiomics results. Another prominent issue for radiomics analysis is overfitting and lack of generalizability, hence proper validation is imperative.
Table 1. Category of typical radiomics features in thorax.
| Category | Implications |
|---|---|
| Morphological features | Features reflecting shape and physical characteristics of ROI |
| Histogram-based features | Features based on intensity histogram of ROI. These features do not retain spatial information |
| Texture features | Features based on pixel neighborhood information. These features consider pixel and its neighbors. GLCM features belong to this category |
| Airway features | Feature that model airway property based on skeletonization |
GLCM = gray level co-occurrence matrix, ROI = region of interest
Clinical Applications of Radiomics
Employing radiomics features has been found to be useful in differentiating solitary pulmonary nodules, assessing tumor prognosis, correlating with genomics, and much more. In this section, we briefly review the currently available radiomics studies and applications in the thorax.
Pulmonary Nodule Evaluation
Several studies have shown that radiomics approaches may help lung nodule characterization when distinguishing between benign and malignant lung nodules. For example, a radiomics signature developed by He et al. (1) facilitates the differential diagnosis of solitary pulmonary nodules. Interestingly, the radiomics signature based on non-contrast computed tomography (CT) images showed better performance compared to the signatures using contrast-enhanced CT images. He et al. (1) suggested that the biologic heterogeneity within the tumor, depicted by radiomics features, may be confounded by intravenous contrast agents, leading to inferior discrimination between benign and malignant tumors.
Similarly, several studies used radiomics features in efforts to distinguish adenocarcinomas from granulomas. A prior study using Haralick texture features showed a sensitivity of 88% for distinguishing adenocarcinomas from granulomas but lacked external validation (2). In another study, three-dimensional (3D) shape-based radiomics features were used to discriminate adenocarcinomas from granulomas with an area under the curve (AUC) of 0.72 in an independent validation cohort (3). In a recent article, radiomics features from the intranodular and perinodular regions of nodules were used to distinguish adenocarcinomas from benign granulomas in non-contrast CT (4). Figure 2 demonstrates an example of generating the intratumor and peritumoral contours based on segmented tumor ROI.
Fig. 2. Generation of intratumor and peritumoral regions of lung adenocarcinoma in 57-year-old woman.
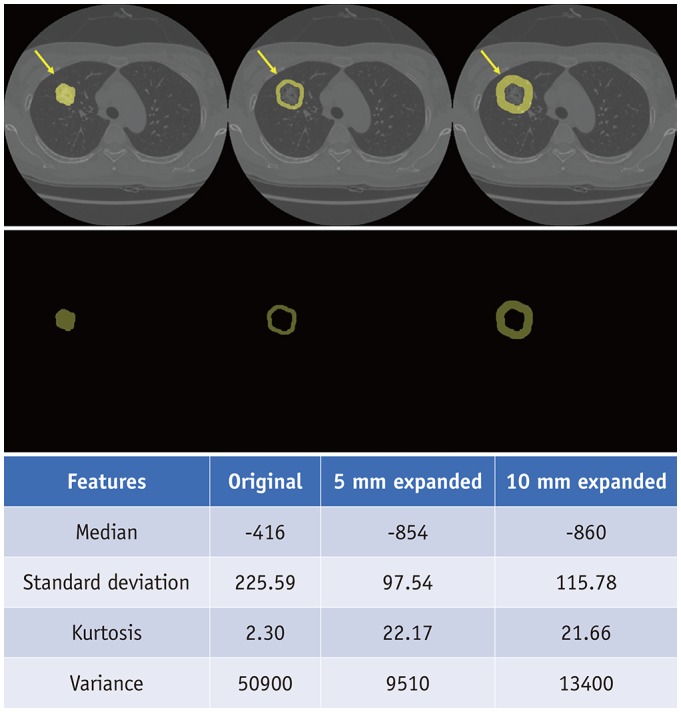
Yellow arrows indicate region of interests. Lower table demonstrates decrease of median Hounsfield units and increase in standard deviation from intratumor to 5 mm peritumoral and 10 mm peritumoral regions, suggesting reflection of tumor microenvironment.
Lung Cancer Prognostic Stratification and Treatment Response Evaluation
Aerts et al. (5), in their pioneering study, used a large number of radiomics features to demonstrate prognostic power in independent data sets of lung and head-and-neck cancer patients and suggested that there is prognostic and biologic information enclosed in routinely acquired CT scans. Hence, after allowing that tumor heterogeneity demonstrates the prognostic significance and may influence response to treatment, a large body of radiomics literature has focused on this subject (6). Evidence shows that radiomics features and signatures reflect the tumor microenvironment in terms of behavior and progression, thus emphasizing the role of radiomics for prognostication and assessing treatment response. Ganeshan et al. (7) found that tumor heterogeneity can be assessed by texture analysis of non-contrast CT scans and has the potential to provide an independent predictor of survival for patients with non-small cell lung cancer (NSCLC). In another study by the same authors, texture parameters identified relevant associations and demonstrated the potential for those to act as imaging correlates for tumor hypoxia and angiogenesis (8).
Meanwhile, Win et al. (9) evaluated tumor heterogeneity and permeability in pretreatment CT scans of positron-emission tomography (PET)/CT and found that in the radical treatment group, CT-derived textural heterogeneity was the only factor associated with survival, and in the palliative treatment group, CT-derived textural heterogeneity along with tumor stage and permeability was associated with survival. In the same context, Fried et al. (10) extracted texture features from pretreatment CT scans before undergoing definitive chemoradiation therapy, and found that radiomics features may provide prognostic information beyond what is obtained from conventional prognostic factors in NSCLC patients. Finally, Cherezov et al. (11) proposed a method for revealing tumor habitats using texture features based on the well-known concept that tumors are heterogeneous, and the level of heterogeneity may help to identify the malignancy and aggressiveness of tumors. Those results demonstrated an AUC of 0.9 and an accuracy of 85% to discriminate long-term and short-term survival rates among patients with lung cancer (11).
In addition, numerous studies have presented evidence that radiomics features extracted from pretreatment fluorodeoxyglucose (18F-FDG) PET scans are associated with prognosis and treatment response. For example, in an earlier study, texture features of PET scans were associated with nonresponse to chemoradiotherapy by Response Evaluation Criteria in Solid Tumors and with poorer prognoses (12). In another study, Cook et al. (13) reported that reduced heterogeneity on PET was associated with response to erlotinib and that changes in first-order entropy were independently associated with overall survival and treatment response in patients with NSCLC. Figure 3 demonstrates a case of clustering approach by combining FDG-PET and CT to identify intratumor heterogeneity in pretreatment and posttreatment lung adenocarcinoma.
Fig. 3. Clustering approach achieved by combining FDG-PET and contrast-enhanced CT to identify intratumor heterogeneity in 63-year-old woman with lung adenocarcinoma.
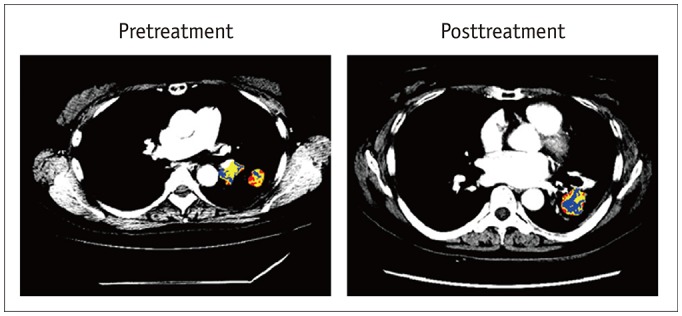
Pretreatment image exhibits heterogeneous tumor areas of various colors showing multiple patterns of tumor vascularity from contrast-enhanced CT and glucose metabolism from FDG-PET. One-year posttreatment with afatinib reveals tumor with central area of low vascularity and low metabolism (blue), suggesting effective treatment. Blue area represents low vascularity and low metabolism, yellow area represents low vascularity and high metabolism or high vascularity and low metabolism, and red area represents high vascularity and high metabolism. FDG = 18F-fluorodeoxyglucose, PET = positron-emission tomography
Quantification of Severity in Diffuse Lung Disease
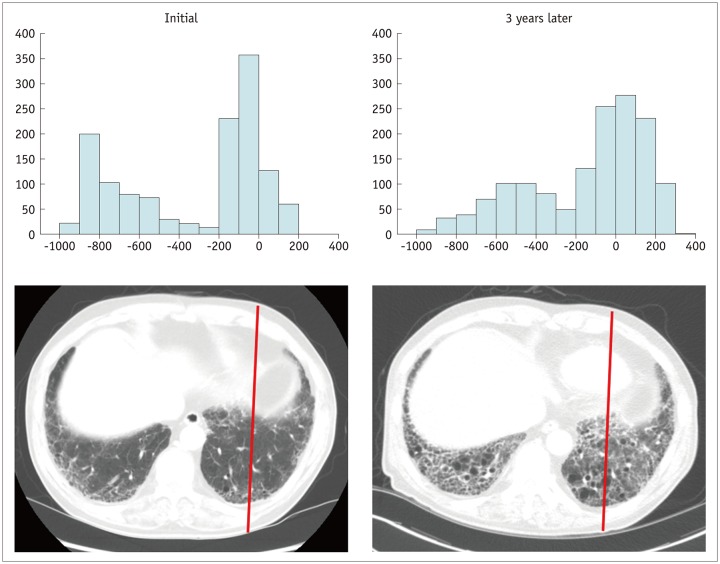
The prediction of postoperative lung function is mandatory in the preoperative evaluation of lung cancer patients, especially in those with reduced lung function (14). The current standard for predicting postoperative lung function uses spirometry, such as forced expiratory volume in one second, and diffusing capacity of the lung for carbon monoxide or radionuclide lung scanning (15). In much earlier research, Wu et al. (16) used quantitative CT and found that it correlated well with postoperative lung function in patients with lung cancer. Nowadays, in the emerging era of radiomics, the pattern and severity of lung fibrosis can be quantitatively analyzed from conventional CT images, by using histogram-based quantification, texture-based quantification, and deep learning (DL) (Fig. 4). Several studies have shown that automated quantification of radiological patterns of interstitial lung disease can predict lung function, disease severity, and progression, including normality, ground-glass opacity (GGO), reticular opacity, honeycombing, emphysema, and consolidation (17,18,19,20,21).
Fig. 4. Interval CT images of 70-year-old man with idiopathic pulmonary fibrosis.
Compared to histogram of initial CT scan, histogram of CT scan obtained three years later demonstrates right-side shifting of Hounsfield unit pixels due to microscopic interstitial fibrosis, suggesting progression of idiopathic pulmonary fibrosis.
Mediastinal Lymph Node Evaluation
Lymph node metastasis is a crucial factor related to survival and recurrence in lung cancer patients. Traditionally, the evaluation of lymph node status was dependent upon morphological changes such as the size and presence of necrosis in CT scans and increased metabolic uptake in PET scans.
Radiomics analysis of the primary tumor helps predict lymph node metastasis in lung cancer patients. Yang et al. (22) built a radiomics signature using 14 radiomics features selected from a set of 94 features. The model was able to significantly correlate the nature of these features with lymph node metastasis of lung cancer, with an AUC of 0.871 in the training group and an AUC of 0.856 in the validation cohort (22). Zhong et al. (23) extracted 300 radiomics features from a large study group of 492 lung adenocarcinoma patients. The accuracy of the radiomics signature for predicting lymph node metastasis was 91.1% in receiver operating characteristic curve analysis, suggesting that the radiomics signature of the primary tumor can be used for quantitative and noninvasive prediction of lymph node metastasis in patients with lung cancer (23).
However, few studies have extracted radiomics features from mediastinal lymph nodes itself. Bayanati et al. (24) assessed radiomics features of mediastinal lymph nodes and found that combined textural and shape features identified malignant lymph nodes with 81% sensitivity and 80% specificity (AUC of 0.87). Similarly, Andersen et al. (25) reported that texture analysis demonstrated a significant difference between malignant and benign lymph nodes with an AUC of 83.4% and excellent reproducibility. Interestingly, Coroller et al. (26) performed radiomics analyses on both the primary tumor and the lymph nodes and demonstrated that the lymph node phenotype could present essential information in addition to that offered by the primary tumor site alone. Regarding PET/CT scans, Li et al. (27) explored the use of standardized uptake value (SUV)-based radiomics features from lymph nodes in comparison to primary tumor features, and reported that lymph node features added value for predicting overall relapse.
Imaging Genomics
Imaging genomics or radio-genomics refers to the identification of genomic profiling within a tumor's deoxyribonucleic acid by using radiomics features. Both semantic features and quantitative features have been used to recognize the presence of specific mutations and alterations, thus leading to treatment decisions and outcomes in lung cancer patients. For example, Gevaert et al. (28) reported that a final decision tree (semantic features of emphysema, airway abnormality, percentage of GGO component, and the type of tumor margin) was predictive of epidermal growth factor receptor (EGFR) mutations. Rizzo et al. (29) reported that there were significant associations between qualitative CT features and EGFR, anaplastic lymphoma kinase (ALK), and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations in 285 NSCLC patients. Yoon et al. (30) showed that adenocarcinomas with ALK, c-ros oncogene 1 (ROS1), or rearranged during transfection (RET) fusion phenotypes could be identified from CT and PET images. Finally, a recent study presented a radio-genomics map of NSCLC, which linked image phenotypes with ribonucleic acid (RNA) signatures captured by metagenes, showing their association with molecular pathways (31). In that study, Zhou et al. (31) assessed 87 semantic features and RNA sequencing in 113 patients with NSCLC to find multiple associations between 35 semantic features and the top 10 metagenes. According to that study, a metagene representing the EGFR pathway was significantly associated with GGO and irregular nodules or nodules with poorly defined margins (31).
In terms of PET, Nair et al. (32) reported that among several prognostic metagene signatures, the most predictive one correlated with survival at both external validation sites. Thus, a crucial goal for imaging genomics research is to improve the knowledge of tumor biology and develop imaging surrogates for genetic testing. In the era of precision medicine and targeted therapy, we believe that the role of the radiologist will expand and incorporate genomic and phenotypic information along with the conventional interpretation of lung cancers.
Special Considerations Regarding Multimodal Applications
As new diagnostic imaging methods continue to develop, each imaging technique provides unique information that reflects tumor biology or tumor behavior (33). For instance, PET/CT provides both metabolic and anatomic information about the tumor, diffusion-weighted magnetic resonance imaging (MRI) can demarcate tissue cellularity or tumor differentiation, and dynamic contrast-enhanced (DCE) CT and MRI captures tumor vascularity. A study showed that combining metabolic and functional imaging biomarkers using PET and diffusion-weighted imaging (DWI) leads to more useful stratification of patients in lung adenocarcinoma compared to the results of each image type alone (34). Likewise, a multimodal approach yields comprehensive information to better assess the biological status of the tumor compared to that from a single modality. Intratumoral heterogeneity affecting the prognosis could be expressed as imaging phenotypes. The partitioning-based approach to dividing the whole volume into smaller subregions provides quantitative information regarding intratumoral heterogeneity (35). Using a data-driven partitioning approach with PET and DWI, intratumor subregions with high SUVs and low apparent diffusion coefficients reflect high aggressiveness of a tumor and are significant predictors of survival in lung adenocarcinoma (35). Another study divided the subregions using imaging of metabolic activity (FDG-PET/CT), hypoxia (HX4 PET/CT), and tumor vasculature (DCE-CT) in NSCLC patients treated with definitive chemoradiation (36). According to that study, metabolically active subregions that were highly hypoxic and demonstrated intermediate tumor perfusion were related to high-risk tumor type and worse survival, and data-driven sub-regional analysis for multimodal imaging may be used as a biomarker to predict the prognosis of the NSCLC patients (36). Thus, although significant work remains to be done, the role of the radiologist is expanding to the combined application of medical images obtained from multiple modalities, leading to an informed decision for better diagnosis, staging, and treatment response.
Technical Issues
Segmentation Issues
Segmentation is defined as the process of separating the tumor from the surrounding lung tissue by hand (manual segmentation), machine (automated segmentation), or both (semiautomated segmentation). Usually, this process is performed without difficulty, but in some cases, it may be challenging due to indistinct tumor margins.
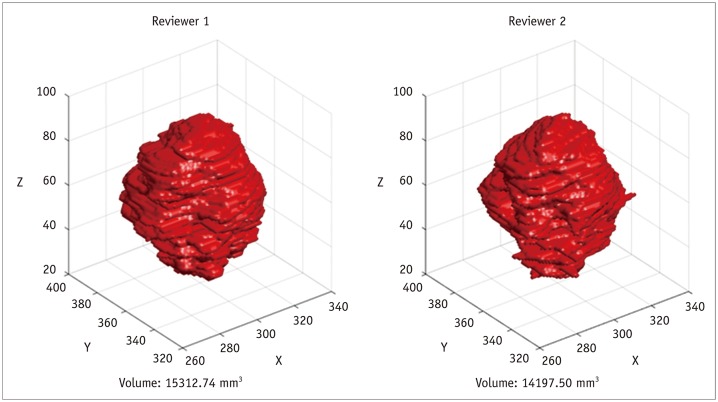
Largely, there are three methods of tumor segmentation before radiomics feature analysis. Although it is considered most accurate when drawn by a chest radiology expert, manual segmentation may not be appropriate for routine clinical usage because it is a time-consuming, labor-intensive task, and is prone to inter- and intrareader variability (Fig. 5). On the other hand, semiautomatic segmentation refers to tumor margin editing, by an experienced expert, of the automatically selected volume of interest. According to Parmar et al. (37), in a study comparing manual and semiautomatic segmentation, the radiomics features derived from the latter demonstrated significantly higher reproducibility (p = 0.0009; intraclass correlation coefficients 0.85 and 0.77 for semiautomatic segmentation and manual segmentation, respectively). They were more robust than those derived from manual contouring (37). Given the reduced contrast of hazy, increased attenuation in CT images (particularly GGO), semiautomatic segmentation may be of particular use for part-solid adenocarcinomas, which have GGO components (38).
Fig. 5. Example of tumor volume segmentation by two different reviewers.
Results show inter-reader variability.
Meanwhile, fully automatic segmentation can be considered a rapid and accurate method of tumor segmentation. However, it is generally accepted that there is variation among software packages and should not be used interchangeably (39,40). Recently, several investigators have incorporated DL technology in tumor segmentation by training convolutional neural networks and showing that DL is capable of performing accurate localization and segmentation of tumors in multiple organs (41,42). Though most of these studies were based on MRI scans, such as those of the brain, prostate, and rectum, DL shows the potential to improve the accuracy and robustness of tumor segmentation.
Measurement Variability Issues
By definition, radiomics features are objective, quantitative measurements, and ideally, extracted radiomics features may provide accurate anatomical and biological information about the tumor. However, the radiologist must keep in mind that variability exists, even in “objective” CT metrics, and any combination of these factors may affect extracted radiomics features, thus altering important tumor information.
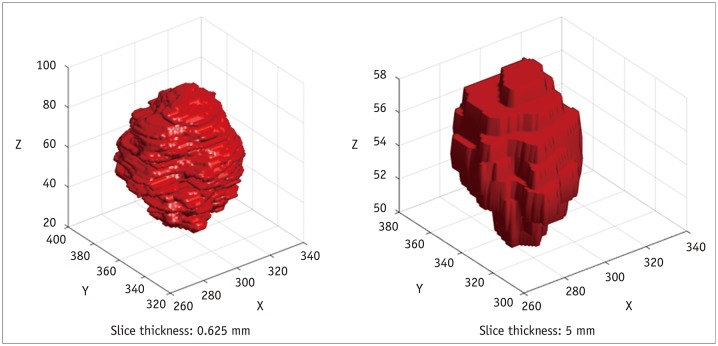
First, it is understood and accepted that variations in slice thickness may impact subsequent feature analyses to a great extent (Fig. 6). According to a phantom study calculating 14 radiomics features, all features were significantly different between 1.25 mm slice and 5 mm slice images (43). The authors suggested that thinner (1.25 mm and 2.5 mm) slices were better than thicker slices, and that thin and thick slice images should not be used interchangeably. Similarly, in a study of 240 lung cancer patients using 150 extracted radiomics features, a radiomics signature based on thin slices (1.25 mm) showed better diagnostic performance than that applied to thick slices (5 mm) (1). In addition, Zhao et al. (44) obtained repeated CT scans from 32 lung cancer patients, and the reproducibility of radiomics features was evaluated in terms of slice thickness and reconstruction algorithms. As a result, thinner slices (1.25 mm and 2.5 mm) and a smoother reconstruction algorithm were more favorable for reproducibly extracting radiomics features. Zhao et al. (44) suggested that the smoother reconstruction algorithm reduces more noise and may hold back useful texture details compared to the sharp reconstruction algorithm. Accordingly, agreement levels were worse when changing both the reconstruction kernel and the slice thickness (44).
Fig. 6. Effect of CT slice thickness on tumor visualization.
Thick-section CT scan (right) demonstrates more partial volume artifacts compared to thin-section CT scan (left).
Second, in terms of respiratory variability, PET images are influenced more by respiration due to longer acquisition times compared to chest CT scans. Yip et al. (45) reported that texture features were significantly blurred out by respiratory motion during conventional 3D PET acquisition and that respiratory-gated four-dimensional (4D) PET texture features may have better prognostic value as they are less susceptible to motion. Similarly, Oliver et al. (46) extracted 56 radiomics features, including shape as well as first-order and second-order texture features, using conventional 3D PET protocol and respiratory-gated 4D PET protocol. In that study, only 26.6% of all features had a percent difference of less than 5% between the two PET protocols, while the majority of radiomics features seemed to be susceptible to respiration (46). In a recent study, Du et al. (47) applied the 4D CT technique to 20 NSCLC patients and studied the impact of respiration on 841 radiomics features.
Last, few studies have investigated the effects of iterative reconstruction algorithms on radiomics features, and most radiomics features were significantly affected. According to Kim et al. (48), the impact of reconstruction algorithms was significant for most first-order tumor intensity features and second-order gray level co-occurrence matrix (GLCM)-based features. Similarly, other research also reported that radiomics features were dependent upon the iterative reconstruction algorithm and radiation dose (49,50).
Recent Advances in Radiomics
Temporal Approach Using Delta Radiomics
In contrast to most radiomics studies, which are based on features extracted at a single time point (usually at the time of diagnosis), delta radiomics evaluates changes in radiomics features between interval studies. In a study by Fave et al. (51), the inclusion of delta radiomics features had a statistically significant impact on the model for overall survival compared to a model with only clinical and pretreatment radiomics features; however, the impact on the model's prognostic abilities was generally negligible. Another study, by Alahmari et al. (52), showed that delta radiomics features improved the performance of the models in a lung cancer screening setting.
Although published research using delta radiomics in lung cancer studies is scarce and does not yet show convincing evidence, delta radiomics demonstrates the fact that radiomics features change during treatment. Ideally, delta radiomics may be used as imaging biomarkers for evaluating treatment response between interval medical images. In turn, a point that needs to be addressed, for which future research is needed, is that the concept of delta radiomics requires high accuracy during the process of image registration.
Spatial Approach Using Multiple ROIs and Inter-Site Modeling
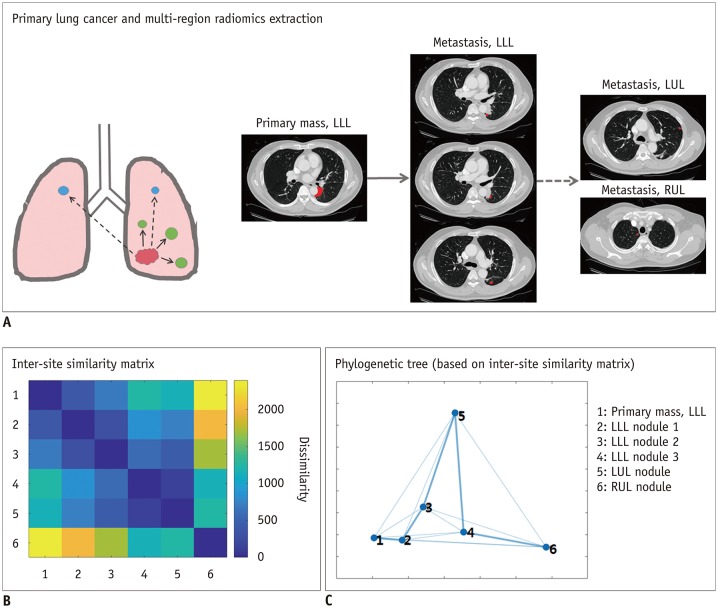
Radiomics studies have typically focused on a single primary ROI per subject. There are many cases where a given patient has multiple tumor sites, which could be primary or metastatic. Focusing on a single ROI makes the statistical analyses easier, but such an approach does not adequately model the potentially rich interactions between tumor sites. Vargas et al. (53) proposed an approach to capture inter-site variations in texture features using multidimensional scaling for ovarian cancers. They were able to identify imaging markers of overall survival and incomplete surgical resection. Similar efforts in lung cancer are scarce but are expected to emerge. There is a rich body of literature in computer vision for modeling inter-object (our case inter-tumor) relationships using graph theory, including stochastic neighbor embedding and spectral clustering (54). Using these methods will almost certainly help improve inter-site relationship models (Fig. 7).
Fig. 7. Tracking cancer evolution from primary tumor to metastases using multi-region radiomics and inter-site modeling.
A. Multi-region radiomics extraction from patient with primary lung cancer in LLL and multiple metastatic nodules in LLL, LUL, and RUL. (B) Inter-site similarity matrix and (C) phylogenetic tree (based on inter-site similarity matrix) demonstrate degrees of dissimilarity with each other, which indicates evolutional sequences through all lesions. Numbers indicate numeric codes for lesion locations. LLL = left lower lobe, LUL = left upper lobe, RUL = right upper lobe
Multidimensional Textural Approach Beyond the Intensity
Texture information plays a leading role in the radiomics field. Typically, the texture is computed from the intensity distributions from neighborhood voxels (compared to a single voxel) in the form of a GLCM. Besides intensity, the gradient of intensity can be defined for each voxel. Gradient by definition is a vector with magnitude and angle. The magnitude of the gradient is already captured with an edge detecting filter. Prasanna et al. (55) exploited the angle information similar to GLCM, where co-occurrences of gradient directions (intensities in GLCM) were used to compute texture. They named the approach the co-occurrence of local anisotropic gradient orientations and demonstrated its efficacy in radiation necrosis for brain tumor patients, different molecular subtypes of breast cancer, and NSCLC. Gradient direction captures distinct aspects of heterogeneity, and we expect many future radiomics studies to include this type of direction-based texture.
Deep Learning Approach
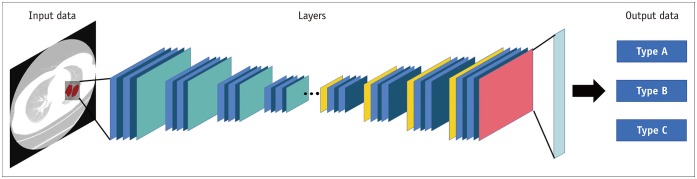
DL is a subset of machine learning that has shown remarkable performance gains in various domains (Fig. 8). The adoption of DL is occurring in all specialties in radiology, and the lung is no exception. Recent radiomics studies have used DL in novel features, feature selectors, classifiers, and predictors, as described in review articles (56,57). However, the DL approach in medical imaging has limitations that must be solved. DL is capable of learning relevant features from the data and thus could be applied to any data in theory. However, the learned features are challenging to interpret, in stark contrast to radiomics features, where each feature has an analytical formula with possible physical interpretations. Many efforts have been made to address this interpretability issue (58,59). Another issue is the scarcity of pre-trained DL networks for medical imaging. DL models are made from many layers of artificial neurons, and we need to solve for thousands of millions of parameters. Such effort requires a massive amount of training data; thus, a common approach is to transfer a model already trained from a similar domain to the target domain, so that optimization of the parameters becomes feasible. There are many well-established models for natural images (e.g., dogs, cats, flowers), but established pre-trained models in medical imaging are scarce, and in lung imaging even more so. Many efforts have been made to address the construction of pre-trained models specific to medical imaging (60).
Fig. 8. Example of deep learning architectures for lung cancer classification based on multi-layers.
Task of Data Sharing
Radiomics analysis is a high-dimensional analysis technique. The statistical power of radiomics studies improves with an increased sample size. With the expected adoption of DL, this becomes even more important. A team of researchers is likely to have limited resources, and accruing many samples could be difficult. Data sharing is the obvious and only solution, and we as a research community need to agree on a plan to do this. In the meantime, one short-term solution is not to share the anonymized raw data, but to share the derived radiomics feature data.
More recently, researchers have initiated collaborative efforts for radiomics-based biomarkers, and several imaging repositories with hundreds of thousands of medical images with correlating clinical information are available. The most extensive database is the National Lung Cancer Screening Trial American College of Radiology Imaging Network Biomarker Repository (61). This repository consists of lung cancer screening patient CT scans along with blood, urine, sputum, and tissue specimens. Other large databases or study cohorts are the Dutch-Belgian Randomized Lung Cancer Screening Trial (Dutch acronym: NELSON study) and the Pamplona International Early Lung Cancer Detection Program (62,63).
Limitations of Radiomics Analysis
Investigations employing radiomics approaches show promising results of radiomics features or signatures as sources of robust imaging biomarkers for assessing tumor prognosis and predicting treatment response by correlating with genomics. Nevertheless, a critical weakness of the radiomics approach is the lack of reproducible research. Radiomics analysis is quantitative; the features are often defined using mathematical formulae. However, the full details of the feature computation are not clearly disclosed, thus limiting model validation. Therefore, there is a clear need to make the radiomics models available to the researchers. In this regard, using an open-source software package and uploading the codes to GitHub (for preprocessing or modeling) would be the first practical steps for the development and validation of radiomics analysis across multiple institutions. Pyradiomics is an open-source package for extracting radiomics features. Similar to physical libraries, Pyradiomics provides a comprehensive radiomics library made up of a collection of radiomics in the Python language (https://www.python.org). Insight ToolKit (www.Itk.org) is another open-source software package developed in C++ which provides a subset of radiomics features, which may be used with other segmentation tools. Imaging biomarker Explorer is an open infrastructure software platform developed using the MATLAB (The MathWorks, Inc., Natick, MA, USA) and C/C++ that flexibly supports common radiomics workflow tasks.
CONCLUSION
Current clinical workflows require most lung cancer patients to undergo medical imaging such as CT and PET. Although these conventional modalities provide crucial information for lung cancer diagnosis and phenotypes, a great deal of genetic and prognostic information remains unrevealed. However, the subjective interpretation of medical images by humans is naturally biased, and it is impossible to decode the entire tumor biology from imaging data. In this regard, radiomics may help radiologists discover and unravel this important tumor information embedded within the medical images. We believe that radiomics will not replace the radiologists' role; instead, radiomics will become a powerful tool for radiologists and a strategy to improve the knowledge of tumor biology and develop imaging surrogates for genetic testing that will advance precision medicine.
Acknowledgments
We are thankful to Seung-Hak Lee, and Jonghoon Kim, PhD, from Department of Electronic Electrical and Computer Engineering, Sungkyunkwan University, Suwon, Korea, who devoted their time and knowledge in technical support to provide figures for this article.
Footnotes
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which was funded by the Ministry of Health & Welfare (HI17C0086), and by a National Research Foundation of Korea grant funded by the Korean government (Ministry of Science, ICT, & Future Planning) (Nos. NRF-2016R1A2B4013046 and NRF-2017M2A2A7A02018568).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z. Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Sci Rep. 2016;6:34921. doi: 10.1038/srep34921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennie C, Thornhill R, Sethi-Virmani V, Souza CA, Bayanati H, Gupta A, et al. Role of quantitative computed tomography texture analysis in the differentiation of primary lung cancer and granulomatous nodules. Quant Imaging Med Surg. 2016;6:6–15. doi: 10.3978/j.issn.2223-4292.2016.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alilou M, Beig N, Orooji M, Rajiah P, Velcheti V, Rakshit S, et al. An integrated segmentation and shape-based classification scheme for distinguishing adenocarcinomas from granulomas on lung CT. Med Phys. 2017;44:3556–3569. doi: 10.1002/mp.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, et al. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology. 2019;290:783–792. doi: 10.1148/radiol.2018180910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802. doi: 10.1007/s00330-011-2319-8. [DOI] [PubMed] [Google Scholar]
- 8.Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology. 2013;266:326–336. doi: 10.1148/radiol.12112428. [DOI] [PubMed] [Google Scholar]
- 9.Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:3591–3599. doi: 10.1158/1078-0432.CCR-12-1307. [DOI] [PubMed] [Google Scholar]
- 10.Fried DV, Tucker SL, Zhou S, Liao Z, Mawlawi O, Ibbott G, et al. Prognostic value and reproducibility of pretreatment CT texture features in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90:834–842. doi: 10.1016/j.ijrobp.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherezov D, Goldgof D, Hall L, Gillies R, Schabath M, Müller H, et al. Revealing tumor habitats from texture heterogeneity analysis for classification of lung cancer malignancy and aggressiveness. Sci Rep. 2019;9:4500. doi: 10.1038/s41598-019-38831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 13.Cook GJ, O'Brien ME, Siddique M, Chicklore S, Loi HY, Sharma B, et al. Non-small cell lung cancer treated with erlotinib: heterogeneity of 18F-FDG uptake at PET—Association with treatment response and prognosis. Radiology. 2015;276:883–893. doi: 10.1148/radiol.2015141309. [DOI] [PubMed] [Google Scholar]
- 14.Papageorgiou CV, Antoniou D, Kaltsakas G, Koulouris NG. Role of quantitative CT in predicting postoperative FEV1 and chronic dyspnea in patients undergoing lung resection. Multidiscip Respir Med. 2010;5:188–193. doi: 10.1186/2049-6958-5-3-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poonyagariyagorn H, Mazzone PJ. Lung cancer: preoperative pulmonary evaluation of the lung resection candidate. Semin Respir Crit Care Med. 2008;29:271–284. doi: 10.1055/s-2008-1076747. [DOI] [PubMed] [Google Scholar]
- 16.Wu MT, Chang JM, Chiang AA, Lu JY, Hsu HK, Hsu WH, et al. Use of quantitative CT to predict postoperative lung function in patients with lung cancer. Radiology. 1994;191:257–262. doi: 10.1148/radiology.191.1.8134584. [DOI] [PubMed] [Google Scholar]
- 17.Humphries SM, Yagihashi K, Huckleberry J, Rho BH, Schroeder JD, Strand M, et al. Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology. 2017;285:270–278. doi: 10.1148/radiol.2017161177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado F, Moua T, Rajagopalan S, Karwoski RA, Raghunath S, Decker PA, et al. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur Respir J. 2014;43:204–212. doi: 10.1183/09031936.00071812. [DOI] [PubMed] [Google Scholar]
- 19.Moon JW, Bae JP, Lee HY, Kim N, Chung MP, Park HY, et al. Perfusion- and pattern-based quantitative CT indexes using contrast-enhanced dual-energy computed tomography in diffuse interstitial lung disease: relationships with physiologic impairment and prediction of prognosis. Eur Radiol. 2016;26:1368–1377. doi: 10.1007/s00330-015-3946-2. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, Lee SM, Song JW, Lee SM, Oh SY, Kim N, et al. Texture-based automated quantitative assessment of regional patterns on initial CT in patients with idiopathic pulmonary fibrosis: relationship to decline in forced vital capacity. AJR Am J Roentgenol. 2016;207:976–983. doi: 10.2214/AJR.16.16054. [DOI] [PubMed] [Google Scholar]
- 21.Yoon RG, Seo JB, Kim N, Lee HJ, Lee SM, Lee YK, et al. Quantitative assessment of change in regional disease patterns on serial HRCT of fibrotic interstitial pneumonia with texture-based automated quantification system. Eur Radiol. 2013;23:692–701. doi: 10.1007/s00330-012-2634-8. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Pan X, Liu H, Gao D, He J, Liang W, et al. A new approach to predict lymph node metastasis in solid lung adenocarcinoma: a radiomics nomogram. J Thorac Dis. 2018;10(Suppl 7):S807–S819. doi: 10.21037/jtd.2018.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Y, Yuan M, Zhang T, Zhang YD, Li H, Yu TF. Radiomics approach to prediction of occult mediastinal lymph node metastasis of lung adenocarcinoma. AJR Am J Roentgenol. 2018;211:109–113. doi: 10.2214/AJR.17.19074. [DOI] [PubMed] [Google Scholar]
- 24.Bayanati H, Thornhill RE, Souza CA, Sethi-Virmani V, Gupta A, Maziak D, et al. Quantitative CT texture and shape analysis: can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur Radiol. 2015;25:480–487. doi: 10.1007/s00330-014-3420-6. [DOI] [PubMed] [Google Scholar]
- 25.Andersen MB, Harders SW, Ganeshan B, Thygesen J, Torp Madsen HH, Rasmussen F. CT texture analysis can help differentiate between malignant and benign lymph nodes in the mediastinum in patients suspected for lung cancer. Acta Radiol. 2016;57:669–676. doi: 10.1177/0284185115598808. [DOI] [PubMed] [Google Scholar]
- 26.Coroller TP, Agrawal V, Huynh E, Narayan V, Lee SW, Mak RH, et al. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J Thorac Oncol. 2017;12:467–476. doi: 10.1016/j.jtho.2016.11.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Becker N, Raman S, Chan TC, Bissonnette JP. The value of nodal information in predicting lung cancer relapse using 4DPET/4DCT. Med Phys. 2015;42:4727–4733. doi: 10.1118/1.4926755. [DOI] [PubMed] [Google Scholar]
- 28.Gevaert O, Echegaray S, Khuong A, Hoang CD, Shrager JB, Jensen KC, et al. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci Rep. 2017;7:41674. doi: 10.1038/srep41674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. 2016;26:32–42. doi: 10.1007/s00330-015-3814-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoon HJ, Sohn I, Cho JH, Lee HY, Kim JH, Choi YL, et al. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine (Baltimore) 2015;94:e1753. doi: 10.1097/MD.0000000000001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, Leung A, Echegaray S, Gentles A, Shrager JB, Jensen KC, et al. Non-small cell lung cancer radiogenomics map identifies relationships between molecular and imaging phenotypes with prognostic implications. Radiology. 2018;286:307–315. doi: 10.1148/radiol.2017161845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair VS, Gevaert O, Davidzon G, Napel S, Graves EE, Hoang CD, et al. Prognostic PET 18F-FDG uptake imaging features are associated with major oncogenomic alterations in patients with resected non-small cell lung cancer. Cancer Res. 2012;72:3725–3734. doi: 10.1158/0008-5472.CAN-11-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 34.Lee HY, Jeong JY, Lee KS, Yi CA, Kim BT, Kang H, et al. Histopathology of lung adenocarcinoma based on new IASLC/ATS/ERS classification: prognostic stratification with functional and metabolic imaging biomarkers. J Magn Reson Imaging. 2013;38:905–913. doi: 10.1002/jmri.24080. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Ryu SY, Lee SH, Lee HY, Park H. Clustering approach to identify intratumour heterogeneity combining FDG PET and diffusion-weighted MRI in lung adenocarcinoma. Eur Radiol. 2019;29:468–475. doi: 10.1007/s00330-018-5590-0. [DOI] [PubMed] [Google Scholar]
- 36.Even AJG, Reymen B, La Fontaine MD, Das M, Mottaghy FM, Belderbos JSA, et al. Clustering of multi-parametric functional imaging to identify high-risk subvolumes in non-small cell lung cancer. Radiother Oncol. 2017;125:379–384. doi: 10.1016/j.radonc.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Parmar C, Rios Velazquez E, Leijenaar R, Jermoumi M, Carvalho S, Mak RH, et al. Robust radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One. 2014;9:e102107. doi: 10.1371/journal.pone.0102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lassen BC, Jacobs C, Kuhnigk JM, van Ginneken B, van Rikxoort EM. Robust semi-automatic segmentation of pulmonary subsolid nodules in chest computed tomography scans. Phys Med Biol. 2015;60:1307–1323. doi: 10.1088/0031-9155/60/3/1307. [DOI] [PubMed] [Google Scholar]
- 39.Ashraf H, de Hoop B, Shaker SB, Dirksen A, Bach KS, Hansen H, et al. Lung nodule volumetry: segmentation algorithms within the same software package cannot be used interchangeably. Eur Radiol. 2010;20:1878–1885. doi: 10.1007/s00330-010-1749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devaraj A, van Ginneken B, Nair A, Baldwin D. Use of volumetry for lung nodule management: theory and practice. Radiology. 2017;284:630–644. doi: 10.1148/radiol.2017151022. [DOI] [PubMed] [Google Scholar]
- 41.Havaei M, Davy A, Warde-Farley D, Biard A, Courville A, Bengio Y, et al. Brain tumor segmentation with deep neural networks. Med Image Anal. 2017;35:18–31. doi: 10.1016/j.media.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Trebeschi S, van Griethuysen JJM, Lambregts DMJ, Lahaye MJ, Parmar C, Bakers FCH, et al. Deep learning for fully-automated localization and segmentation of rectal cancer on multiparametric MR. Sci Rep. 2017;7:5301. doi: 10.1038/s41598-017-05728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B, Tan Y, Tsai WY, Schwartz LH, Lu L. Exploring variability in CT characterization of tumors: a preliminary phantom study. Transl Oncol. 2014;7:88–93. doi: 10.1593/tlo.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B, Tan Y, Tsai WY, Qi J, Xie C, Lu L, et al. Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep. 2016;6:23428. doi: 10.1038/srep23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yip S, McCall K, Aristophanous M, Chen AB, Aerts HJ, Berbeco R. Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One. 2014;9:e115510. doi: 10.1371/journal.pone.0115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliver JA, Budzevich M, Zhang GG, Dilling TJ, Latifi K, Moros EG. Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol. 2015;8:524–534. doi: 10.1016/j.tranon.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du Q, Baine M, Bavitz K, McAllister J, Liang X, Yu H, et al. Radiomic feature stability across 4D respiratory phases and its impact on lung tumor prognosis prediction. PLoS One. 2019;14:e0216480. doi: 10.1371/journal.pone.0216480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H, Park CM, Lee M, Park SJ, Song YS, Lee JH, et al. Impact of reconstruction algorithms on CT radiomic features of pulmonary tumors: analysis of intra- and inter-reader variability and inter-reconstruction algorithm variability. PLoS One. 2016;11:e0164924. doi: 10.1371/journal.pone.0164924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo P, Young S, Kim HJ, Brown MS, McNitt-Gray MF. Variability in CT lung-nodule quantification: effects of dose reduction and reconstruction methods on density and texture based features. Med Phys. 2016;43:4854. doi: 10.1118/1.4954845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon J, Mileto A, Nelson RC, Roy Choudhury K, Samei E. Quantitative features of liver lesions, lung nodules, and renal stones at multi-detector row CT examinations: dependency on radiation dose and reconstruction algorithm. Radiology. 2016;279:185–194. doi: 10.1148/radiol.2015150892. [DOI] [PubMed] [Google Scholar]
- 51.Fave X, Zhang L, Yang J, Mackin D, Balter P, Gomez D, et al. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer. Sci Rep. 2017;7:588. doi: 10.1038/s41598-017-00665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alahmari SS, Cherezov D, Goldgof D, Hall L, Gillies RJ, Schabath MB. Delta radiomics improves pulmonary nodule malignancy prediction in lung cancer screening. IEEE Access. 2018;6:77796–77806. doi: 10.1109/ACCESS.2018.2884126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vargas HA, Veeraraghavan H, Micco M, Nougaret S, Lakhman Y, Meier AA, et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol. 2017;27:3991–4001. doi: 10.1007/s00330-017-4779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Maaten L. Accelerating t-SNE using tree-based algorithms. J Mach Learn Res. 2014;15:3221–3245. [Google Scholar]
- 55.Prasanna P, Tiwari P, Madabhushi A. Co-occurrence of Local Anisotropic Gradient Orientations (CoLlAGe): a new radiomics descriptor. Sci Rep. 2016;6:37241. doi: 10.1038/srep37241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, et al. Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. 2017;18:E805. doi: 10.3390/ijms18040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, et al. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. 2017;28:1191–1206. doi: 10.1093/annonc/mdx034. [DOI] [PubMed] [Google Scholar]
- 58.Kim ST, Lee JH, Lee H, Ro YM. Visually interpretable deep network for diagnosis of breast masses on mammograms. Phys Med Biol. 2018;63:235025. doi: 10.1088/1361-6560/aaef0a. [DOI] [PubMed] [Google Scholar]
- 59.Tang Z, Chuang KV, DeCarli C, Jin LW, Beckett L, Keiser MJ, et al. Interpretable classification of Alzheimer's disease pathologies with a convolutional neural network pipeline. Nat Commun. 2019;10:2173. doi: 10.1038/s41467-019-10212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tajbakhsh N, Shin JY, Gurudu SR, Hurst RT, Kendall CB, Gotway MB, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35:1299–1312. doi: 10.1109/TMI.2016.2535302. [DOI] [PubMed] [Google Scholar]
- 61.Patz EF, Jr, Caporaso NE, Dubinett SM, Massion PP, Hirsch FR, Minna JD, et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network specimen biorepository originating from the contemporary screening for the detection of lung cancer trial (NLST, ACRIN 6654): design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol. 2010;5:1502–1506. doi: 10.1097/JTO.0b013e3181f1c634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11 Spec No A:S79–S84. doi: 10.1102/1470-7330.2011.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Salcedo P, Berto J, de-Torres JP, Campo A, Alcaide AB, Bastarrika G, et al. Lung cancer screening: fourteen year experience of the Pamplona early detection program (P-IELCAP) Arch Bronconeumol. 2015;51:169–176. doi: 10.1016/j.arbres.2014.09.019. [DOI] [PubMed] [Google Scholar]