ABSTRACT
Background
Fasting and timed feeding strategies normalize obesity parameters even under high-fat dietary intake. Although previous work demonstrated that these dietary strategies reduce adiposity and improve metabolic health, limited work has examined intestinal microbial communities.
Objectives
We determined whether timed feeding modifies the composition of the intestinal microbiome and mycobiome (yeast and fungi).
Methods
Male C57BL/6 mice were fed a high-fat diet (HF) for 6 wk. Animals were then randomly assigned to the following groups (n = 8–10/group): 1) HF ad libitum; 2) purified high-fiber diet (Daniel Fast, DF); 3) HF–time-restricted feeding (TRF) (6 h); 4) HF–alternate-day fasting (ADF); or 5) HF at 80% total caloric restriction (CR). After 8 wk, obesity and gut parameters were characterized. We also examined changes to the gut microbiome and mycobiome before, during, and following dietary interventions.
Results
Body mass gain was reduced with all restricted dietary groups. HF-fed microbiota displayed lower α-diversity along with reduced phylum levels of Bacteroidetes and increased Firmicutes. Animals switched from HF to DF demonstrated a rapid transition in bacterial taxonomic composition, α-, and β-diversity that initially resembled HF, but was distinct after 4 and 8 wk of DF feeding. Time-or calorie-restricted HF-fed groups did not show changes at the phylum level, but α-diversity was increased, with specific genera altered. Six weeks of HF feeding reduced various fungal populations, particularly Alternaria, Aspergillus, Cladosporium, and Talaromyces, and increased Candida, Hanseniaspora, and Kurtzmaniella. However, 8 wk of intervention did not change the fungal populations, with the most abundant genera being Candida, Penicillium, and Hanseniaspora.
Conclusions
These data suggest that timed-feeding protocols and diet composition do not significantly affect the gut fungal community, despite inducing measurable shifts in the bacterial population that coincide with improvements in metabolism.
Keywords: C57BL/6 mice, obesity, diet, time-restricted feeding, microbiome, mycobiome
Introduction
Obesity is an emerging epidemic in westernized societies that contributes to comorbidities and substantially elevates national medical care costs (1). Efforts aimed at preventing obesity have had limited impact on overall incidence, underscoring the complex nature of this condition in the context of environmental, lifestyle, and societal drivers. Regulation of energy expenditure and storage is influenced by physiological and endocrine signals, especially signals coming from the intestine, which are inseparably associated with the intestinal microorganisms (2). Microbial production of small molecules and fermentative by-products, including SCFAs and lactate, has been shown to stimulate host receptors within the intestinal epithelium and peripheral tissues that influence immune priming and tolerance (3) and host energy balance (4, 5). The assemblage of the microbiota community is orchestrated by environmental and host factors, including the use of antibiotics (6), host lifestyle and sanitation practices (7), and dietary intake (8). Of these factors, numerous animal and human studies demonstrate that dietary macronutrients serve as the major determinant of microbial composition and function (9). To date, the vast majority of microbiome research has focused on the bacterial communities. However, fungi also colonize the gut in considerable numbers but have been overlooked due to delays in next-generation sequencing specifically targeting fungi and because these organisms make up only 0.1–2% of total microbial DNA. Notably, fungi are 100 times larger in size than bacteria and therefore their contributions to microbial communities could be significantly underestimated. Indeed, emerging research demonstrates the strong effects these less abundant members of the microbiome have on host immune and metabolic functions (10, 11).
In addition to dietary macronutrient composition, emerging data show timing of dietary intake is fundamentally important for host metabolic outcomes (12, 13). Common dietary fasting protocols include caloric restriction, time-restricted feeding, and alternate-day fasting. Each approach has certain metabolic benefits independent of the macronutrient content. Most classically, caloric restriction has been shown to decrease body fat percentage and total LDL cholesterol, improve insulin sensitivity, and increase lifespan in multiple species (14, 15). More recent work reveals that time-restricted feeding influences body weight, cholesterol concentration, and insulin sensitivity, even when animals otherwise consume traditionally obesogenic diets (16). These findings build on the hypothesis that discordant oscillations in host metabolic activity with macronutrient intake has deleterious effects on metabolism. In line with this, our previous work demonstrated that time-restricted feeding has beneficial health outcomes, with early time-restricted feeding protocols showing the greatest improvement in glucose tolerance (17). Beyond restricted feeding within a 24-h period, another strategy is alternate-day fasting, where animals have ad libitum access to food for 24 h followed by a 24-h fasting cycle (18). This approach is gaining popularity and is reported to be as effective in animal studies as caloric restriction in lowering body weight, cholesterol concentrations, and fasting insulin, although its utility in human populations remains unclear.
Considering that changes in actual dietary composition (i.e., food type consumed) are difficult to adhere to, we now also focus on the impact that altered timing of dietary intake has on host metabolic outcomes. In the current study, we investigated the influence of various dietary interventions aimed at improving body weight and adiposity and focused upon effects on gut bacterial and fungal populations in our murine model. We hypothesized that both diet restriction and timed feeding interventions would normalize body weight with parallel alterations in gut microbial and fungal populations. Our findings demonstrate that diet restriction in the form of a plant-based, high-fiber diet or time-restricted feeding of a high-fat diet can improve weight and adiposity, and alter the composition of gut bacterial communities, but does not have a major effect on fungal populations.
Methods
Experimental animals and dietary interventions
Four-week-old C57BL/6 male mice were purchased from Envigo. Upon arrival at the University of Memphis campus, mice were housed (2 animals per cage) in a USDA-approved animal facility. All experiments were approved by the Institutional Care and Use of Animal Committee. Animals were entrained to a reverse light–dark schedule (12-h dark, 12-h light) with lights off between 07:00 and 19:00 for 2 wk with ad libitum access to an unpurified standard rodent diet (Teklad global 2018; Envigo Laboratories Inc; 18% fat, 58% carbohydrates, 24% protein; 3.1 kcal/g). After entrainment, all mice (n = 43) were fed a 45% high-fat diet (HF) (D12451; Research Diets, Inc; 45% fat, 35% carbohydrate, and 20% protein; 4.73 kcal/g; diet composition presented in Table 1) for 6 wk. This diet is commonly used to induce obesity in C57BL/6 mice (19). After 6 wk, mice were divided into 5 groups; group 1 (DF, n = 9) received a plant-based diet high in complex carbohydrates, including amylose, amylopectin, and inulin. This diet resembles a dietary restriction model known as the Daniel Fast (DF), where individuals are restricted to a plant-based diet with no additives, preservatives, and so forth. This diet was custom made by Research Diets, Inc, with 25% fat, 59% carbohydrate, 15% protein, and 3.9 kcal/g (Table 1) (20). The remaining 4 groups continued the HF diet, either ad libitum (HF, n = 8) or in a time- or caloric-restricted manner. The caloric-restricted group (CR, n = 8) were fed 80% of ad libitum intake as determined during week 6 of high-fat feeding prior to the intervention. The time-restricted groups followed either an alternate-day fasting protocol (ADF, n = 9) where animals had ad libitum access to the HF diet for 24 h followed by 24 h of fasting, continued for the remainder of the study, or a time-restricted protocol (TRF, n = 9) where ad libitum food access was allowed for the first 6 h of the active phase (07:00–13:00), followed by 18 h of fasting. All groups had ad libitum access to water and remained on their respective diets for 8–9 wk. Food consumption was determined daily, and mouse weight measured every other day. Fecal samples were collected at the end of the initial 6 wk of HF feeding (T0), and again at 3 wk (T1) and 7 wk (T2) following transition to dietary intervention. ADF fecal samples were collected under both fasting and nonfasting conditions. Fecal samples were collected fresh at the end of the active phase. After collection, samples were immediately frozen at −80°C until DNA extraction. A run-time-to-exhaustion test was performed 1 wk prior to killing. Results are published elsewhere (13). To avoid changes induced by exercise, fecal samples were collected prior to exercise. After 8–9 wk on their respective diets, mice were killed using isoflurane and cervical dislocation after an overnight fast. The gastrointestinal tract extending from the stomach to rectum was immediately removed and the length measured. The cecum was isolated and weighed with contents. Cecal and small intestinal contents were collected, frozen in liquid nitrogen, and stored at −80°C until DNA extraction.
TABLE 1.
Composition of purified diets1
| HF | DF | |
|---|---|---|
| Macronutrients | ||
| Protein, % kcal | 20 | 15 |
| Carbohydrate, % kcal | 35 | 59 |
| Fat, % kcal | 45 | 25 |
| Energy, kcal/g | 4.73 | 3.9 |
| Ingredients, g | ||
| Casein, 80 mesh | 200 | 0 |
| Soy protein | 0 | 170 |
| dl-Methionine | 0 | 3 |
| l-Cysteine | 0 | 3 |
| Sucrose | 172.8 | 0 |
| Corn starch | 0 | 72.8 |
| Maltodextrin 10 | 100 | 150 |
| Corn-Starch-Hi Maize 260 (70%/30% ratio of Amylose/Amylopectin) | 0 | 533.5 |
| Cellulose, BW200 | 50 | 100 |
| Inulin | 0 | 50 |
| Soybean oil | 25 | 0 |
| Lard | 177.5 | 0 |
| Flaxseed oil | 0 | 71 |
| Safflower oil, high oleic | 0 | 59 |
| Mineral mix S10026 | 10 | 35 |
| Calcium carbonate | 5.5 | 4 |
| Potassium citrate | 16.5 | 0 |
| Dicalcium phosphate | 13 | 0 |
| Vitamin mix V10001 | 10 | 10 |
| Choline bitartrate | 2 | 0 |
Diets were formulated by Research Diets, Inc (www.researchdiets.com). DF, Daniel Fast; HF, high-fat diet.
SCFA analysis, ion chromatography
Analysis of SCFAs followed a previous protocol (21). The cecal samples were thawed, weight recorded, diluted in 0.18 M H2SO4 (0.5–1 mL/10 mg), and homogenized by sonication while kept on ice. The resulting suspension was diluted to 10% with additional H2SO4 (0.18 M) and centrifuged at 12,000 x g for 10 min at room temperature. The supernatant was filtered using a 0.2-µm filter into glass vials, and SCFAs were resolved with an increasing gradient of KOH using an AS15 analytical column preceded by a AG15 guard column incorporated in a Dionex ion chromatography system consisting of an AS50 autosampler, GP50 gradient pump, and ED50 electrochemical detector. Concentrations were calculated from peak heights based on standards containing acetate, propionate, formate, and butyrate.
DNA isolation for 16S and internal transcribed spacer amplification and next-generation sequencing
Stool, ileal, and cecal contents were mixed in 1 mL extraction buffer [50 mM Tris (pH 7.4), 100 mM EDTA (pH 8.0), 400 mM NaCl, 0.5% SDS] containing 20 μL proteinase K (20 mg/mL). Approximately 150 μL of 0.1-mm-diameter zirconia/silica beads (BioSpec Products) was added to extraction tubes and mechanically perturbed using a Mini-Beadbeater-8 cell disrupter (BioSpec Products) for 2 × 1 min. Tubes were incubated at 55°C overnight with agitation. DNA was extracted with the phenol:chloroform:isoamyl alcohol method, precipitated with ethanol, and dissolved in nuclease-free water. Purified DNA samples underwent 16S and internal transcribed spacer (ITS) library preparation and NextGen Illumina MiSeq sequencing at Argonne National Laboratory. Blank samples were passed through an identical collection, extraction, and amplification process but remained free of DNA amplification.
Statistical methods
Statistical analysis of datasets for body mass, food consumption, and tissue characteristic was performed with GraphPad Prism 8. Data are presented as means ± SEM and statistical significance was established at P < 0.05. Datasets were tested for homogeneity of variance by the D'Agostino–Pearson test. If a dataset passed normality, an ordinary 1-factor ANOVA with a Tukey multiple comparison test was performed, otherwise Kruskal–Wallis with a Dunn multiple comparison test was used. Changes in body mass over time were determined using a 2-factor ANOVA with Tukey multiple test.
Sequencing data were processed and analyzed using QIIME (Quantitative Insights Into Microbial Ecology) 1.9.1. Sequences were first demultiplexed, then denoised and clustered into sequence variants. For bacteria we rarefied to a depth of 2400 sequences. Representative bacterial sequences were aligned via PyNAST, and taxonomy assigned using the Ribosomal Database Project Classifier. Processed data were imported into Calypso 8.84 for further analysis and data visualization (22). The Shannon index was used to quantify α-diversity (intersample). Bray–Curtis analysis was used to quantify β-diversity (intrasample). We used LEfSe (Linear discriminant analysis Effects Size) to test for significance and perform high-dimensional biomarker identification (23). For fungi, sequences were aligned, and taxonomy was assigned using the UNITE (dynamic setting) database (24). Fungal operational taxonomic units were rarefied at a depth of 300 sequences for α-diversity using the Shannon index and β-diversity using the Jaccard abundance index (25). To quantify relative abundance of taxa between groups, we utilized ANOVA adjusted using the Bonferroni correction and false discovery rate for multiple comparisons.
Results
Body weight, adiposity, and food consumption
Two animals died during the study. An unassigned animal died during the initial 6-wk HF feeding from an injury to the hindlimb that was infected. An animal from the DF group was killed during the run-time-to-exhaustion test as result of getting trapped on the shock grid. All remaining animals completed the study.
Body weight and food consumption of all animals were monitored throughout the 8-wk intervention. Animals in the HF group gained more weight than all other groups (Figure 1A, B; P < 0.0001). At the end of the intervention, the average body mass of each group was as follows: HF: 39.56 ± 4.9 g; DF: 28.9 ± 1.4 g; CR: 33.85 ± 2.8 g; ADF: 28.65 ± 1.2 g; and TRF: 28.54 ± 1.7 g. The change in body weight was also reflected in the epididymal white adipose tissue (eWAT) accumulation, where ad libitum access to the HF diet resulted in significantly elevated eWAT compared with DF, TRF, and ADF groups (Figure 1C; P < 0.0001). Unexpectedly, however, the eWAT for the CR group was slightly reduced, but did not significantly differ from the HF control group (Figure 1C; P = 0.2). Fresh food was given to animals daily and consumption determined over a 24-h time period (Figure 1D). The animals that had access to food ad libitum (HF and DF) had a similar daily kilocalorie intake (P = 1), whereas the time-restricted feeding groups both consumed fewer calories than the HF and DF groups (Figure 1D; ADF, P = 0.0005; TRF, P < 0.01).
FIGURE 1.
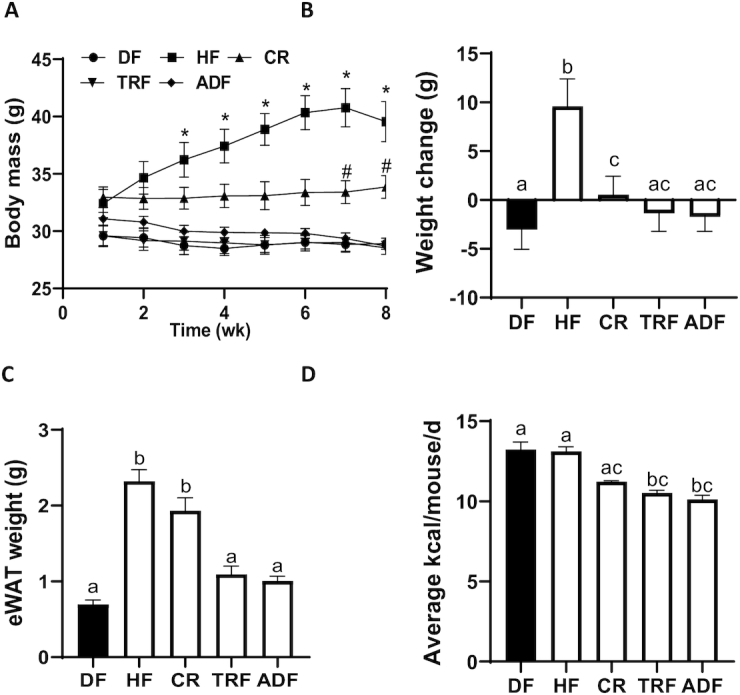
Body weight changes, adiposity, and caloric consumption. C57BL/6 male mice were fed a 45% fat diet for 6 wk and then switched to 1 of 4 dietary restrictions for an additional 8 wk. Body weight was monitored during the 8-wk intervention (A). Values are mean ± SEM, n = 8–9 per group. *P < 0.03, HF vs. DF, TRF, ADF; #P < 0.05, CR vs. DF, TRF, ADF (2-factor ANOVA). Weight change (B) and weight of epididymal white adipose tissue (eWAT) at the end of the 8-wk intervention (C). The average daily energy consumption was calculated for the 8-wk intervention time (D). Values are mean ± SEM, n = 8–9 per group. Means without a common letter differ, P < 0.05 (ANOVA). ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; TRF, time-restricted feeding.
Intestinal parameters and SCFAs
There was a significant difference in the intestinal length, with the DF group having an extended intestinal tract compared with all HF-fed animals (Figure 2A; P < 0.03). This trend in length was sustained for both small intestine and colon. The cecum was removed and immediately weighed with contents after harvest. The cecum from the DF group weighed significantly more than all the HF-fed groups (Figure 2B; P < 0.0001).
FIGURE 2.
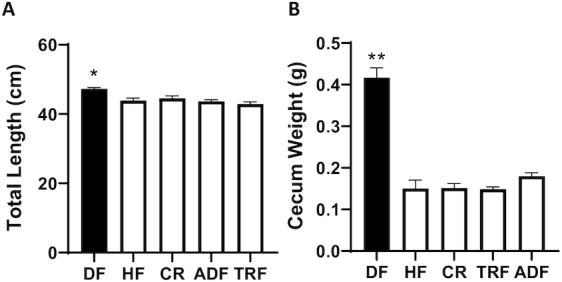
Gastrointestinal parameters after intervention. Intestinal length (A) and cecum weight (B) were measured immediately after killing. DF differs significantly from all other groups. Values are mean ± SEM, n = 8–10 per group. *P < 0.03; **P < 0.0001 (ANOVA). ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; TRF, time-restricted feeding.
SCFAs, including acetate, butyrate, formate, and propionate, were measured from the cecal content. As demonstrated in Table 2, there was significantly more acetate detected in the HF ad libitum group (77.1 ± 19.91 nmol/mg) compared with DF (42.6 ± 5.81 nmol/mg; P = 0.002). Although nonsignificant, this was also true for the restricted groups consuming the HF diet (CR, 65.0 ± 32.96 nmol/mg; TRF, 65.4 ± 20.0 nmol/mg; ADF, 63.7 ± 13.16 nmol/mg). Whereas butyrate and formate tended to be more abundant on average (nonsignificant) in the animals consuming the HF diet, the ADF group had concentrations more similar to the DF group. Propionate was significantly increased in the DF group (9.6 ± 2.69 nmol/mg) compared with the HF (5.31 ± 2.15 nmol/mg; P = 0.006) and TRF (5.48 ± 1.86 nmol/mg; P = 0.02) groups.
TABLE 2.
Cecal SCFA concentrations1
| Diet | Acetate | Butyrate | Formate | Propionate |
|---|---|---|---|---|
| DF | 42.6 ± 5.81* | 36.46 ± 27.61 | 24.21 ± 4.57 | 9.6 ± 2.69** |
| HF | 77.1 ± 19.91 | 65.58 ± 53.29 | 43.92 ± 27.55 | 5.31 ± 2.15 |
| CR | 65.0 ± 32.96 | 47.39 ± 35.17 | 54.28 ± 24.56 | 5.77 ± 2.26 |
| TRF | 65.4 ± 20.0 | 56.05 ± 57.87 | 53.93 ± 29.42 | 5.48 ± 1.86 |
| ADF | 63.7 ± 13.16 | 25.73 ± 13.02 | 29.48 ± 10.71 | 6.98 ± 2.91 |
Acetate, butyrate, formate, and propionate concentrations were measured in nanomoles per milligram and determined from cecal contents collected from C57BL/6 male mice after 8 wk on dietary interventions. Values are mean ± SD, n = 8–9 per group. Kruskal–Wallis with Dunn multiple comparison were used for analysis. *Acetate differed, DF vs. HF (P = 0.002); **propionate differed, DF vs. HF (P = 0.006), TRF (P = 0.02). ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; TRF, time-restricted feeding.
Fecal microbiome following high-fiber diet
After the 6 wk of high-fat feeding, animals started their intervention protocols. Animals that were switched to the DF diet had a rapid transition in taxonomic composition (Figure 3A), and change in α- and β-diversity (Figure 3B, C) as evident at 4 and 8 wk after the start of intervention. The altered microbiome induced by the DF diet resembled that of mice fed the standard rodent chow suggesting the high fiber content in these diets could contribute to the changes.
FIGURE 3.
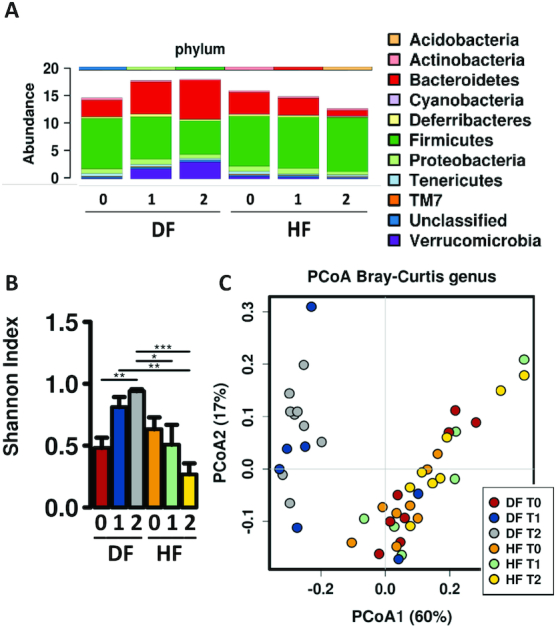
Shifts in the microbiome following changes in diet. (A) Fecal microbiota taxonomic abundance (phylum level) of animals on HF and DF diets prior to (0) and following 3 wk (1) and 7 wk (2) of dietary intervention. Animals switched from HF to DF initially resembled HF-fed microbiota and then shifted to more closely resemble the microbiome of the chow-fed animals. (B) Bar chart showing differences in α-diversity determined by Shannon index of the fecal microbiome across time points for HF and DF groups, *P< 0.05, **P< 0.005, ***P < 0.0001 (ANOVA). (C) Predicted principal components analysis (PCoA) plot of Bray–Curtis dissimilarity demonstrating β-diversity of the fecal microbiome across experimental time points, showing DF and HF feeding clustered distinctly along principal component 1, which explained 60% of composition dissimilarity. DF, Daniel Fast; HF, high-fat diet.
Distal gut microbiome composition following dietary interventions and fasting protocols
Analysis of fecal microbiota at the experimental end point—after 8 wk of dietary intervention—demonstrated that the DF diet led to the largest increase in Bacteroidetes (DF 39% compared with HF 15%) and largest decrease in Firmicutes (DF 34% compared with HF 70%; Figure 4A). The DF diet also resulted in a relative increase in Verrucomicrobia (DF 15% compared with HF 1%; Figure 4A). The dietary restriction protocols, CR, TRF, and ADF, while consuming the HF diet, did not result in changes at phylum level between the various groups. LEfSe analysis identified Firmicutes and the genera Lactococcus and Enterococcus to be enriched in the HF group, whereas Actinobacteria and the genus Bifidobacterium were enriched in ADF, and Ruminococcus and Christensenellaceae in the TRF samples. The plant-based high-fiber DF group had several genera that were enriched: S247, Akkermansia, Clostridiales, Prevotella, Allobaculum, Bacteroides, Coprococcus RF32, and Dehalobacterium (Figure 4B). α-Diversity (Shannon index, Figure 4C) was increased in the DF, CR, and TRF groups compared with the HF group. However, the CR and TRF groups had much more variability than the DF group. The Bray–Curtis dissimilarity measure revealed that β-diversity of the DF-induced microbiome was distinct from all HF-fed animals, whereas all the animals fed the HF diet—irrespective of meal timing—clustered (Figure 4D). An analysis of abundance demonstrated that the following were significantly increased at genus level, specifically in the DF microbiota: S247, RF32, Clostridiales, Coprococcus, Bacteroides, Allobaculum, and Akkermansia, whereas Lactococcus, AF12, and Adlercreutzia were decreased (Figure 4E). Compared with HF ad libitum controls, fasting protocols affected the following: Clostridiales, Mucispirillum, Desulfovibrio, and Coprococcus increased with TRF, and Enterococcus decreased with ADF protocol. The taxonomic composition and α-diversity of ADF on fed days resembled HF under fed conditions, whereas ADF under fasting conditions more closely resembled CR and TRF. These findings suggest that the bacterial populations respond rapidly to food availability.
FIGURE 4.
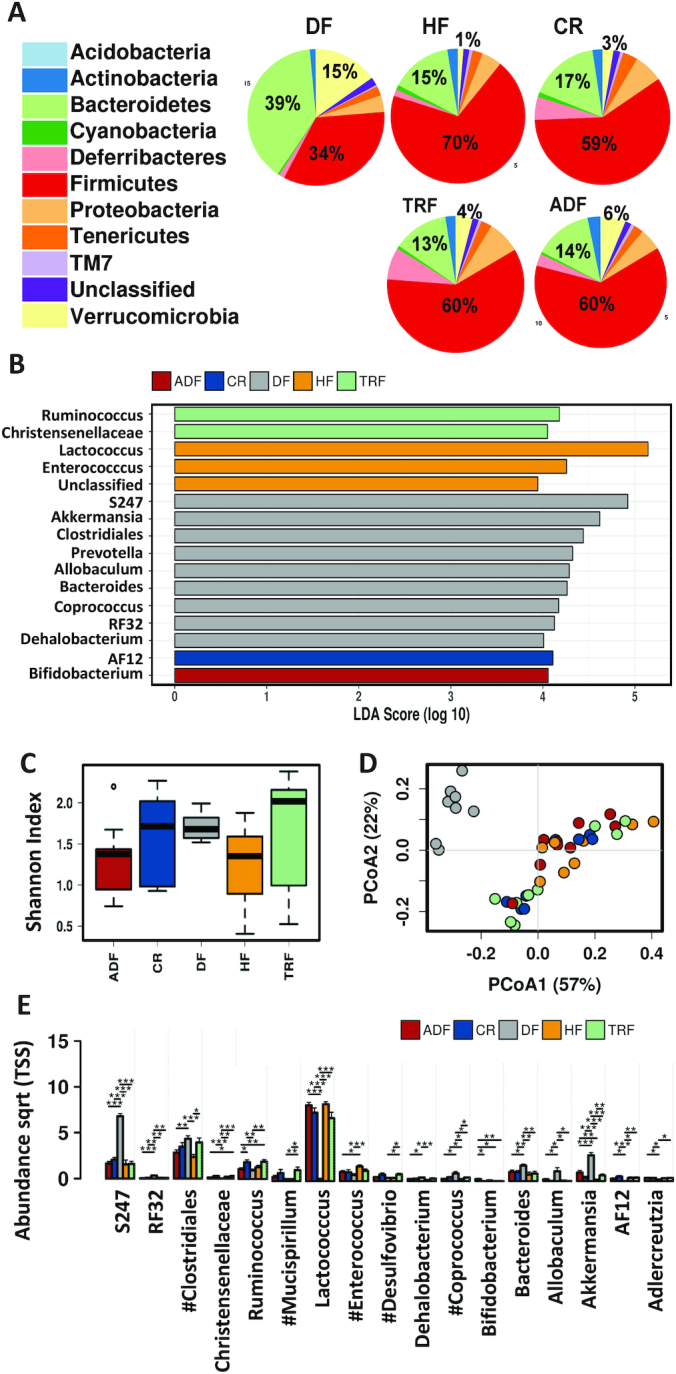
Fecal microbiome composition following intervention. (A) Pie charts showing relative abundance of fecal microbiota taxonomic composition for animals on various dietary modifications. (B) Linear discriminant analysis (LDA) effects size (LEfSe) analysis shows differentially abundant genera with an LDA score >4. (C) Box plots indicating α-diversity of the fecal microbiome for each group based on the Shannon index at the genus level. Whisker plots show mean surrounded by the upper and lower quartiles. Lines show variability outside of the quartiles. (D) Predicted principal components analysis (PCoA) plots using Bray–Curtis dissimilarity to demonstrate β-diversity at the genus level for all groups. HF-fed groups, irrespective of fasting time, are similar and distinct from the DF group. (E) Bar chart demonstrating abundance of indicated populations (genus level) that are altered by diet and fasting protocols. Significance (*P < 0.05; **P< 0.01; ***P< 0.005) determined by ANOVA. #Indicates significant differences induced by fasting. ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; Sqrt, square root; TRF, time-restricted feeding; TSS, total sum scaling.
Ileal and cecal microbiota composition following dietary timing interventions
To assess regional changes in microbiota along the intestinal axis, ileal and cecal contents were collected at the time of killing. Firmicutes were the dominant phylum of the ileal microbiota, with no significant differences observed at phylum level among any of the intervention groups. Genus-level analysis of the ileal content by principal components analysis (PCoA) (Figure 5A) demonstrated clustering of fasting groups (TRF, ADF) away from the ad libitum HF consumption group. This result coincided with a significant increase in abundance of Lactococcus and a decrease in Bilophila for the TRF and ADF groups. The high-fiber DF group also had significantly increased levels of Leuconostoc (Figure 5B).
FIGURE 5.
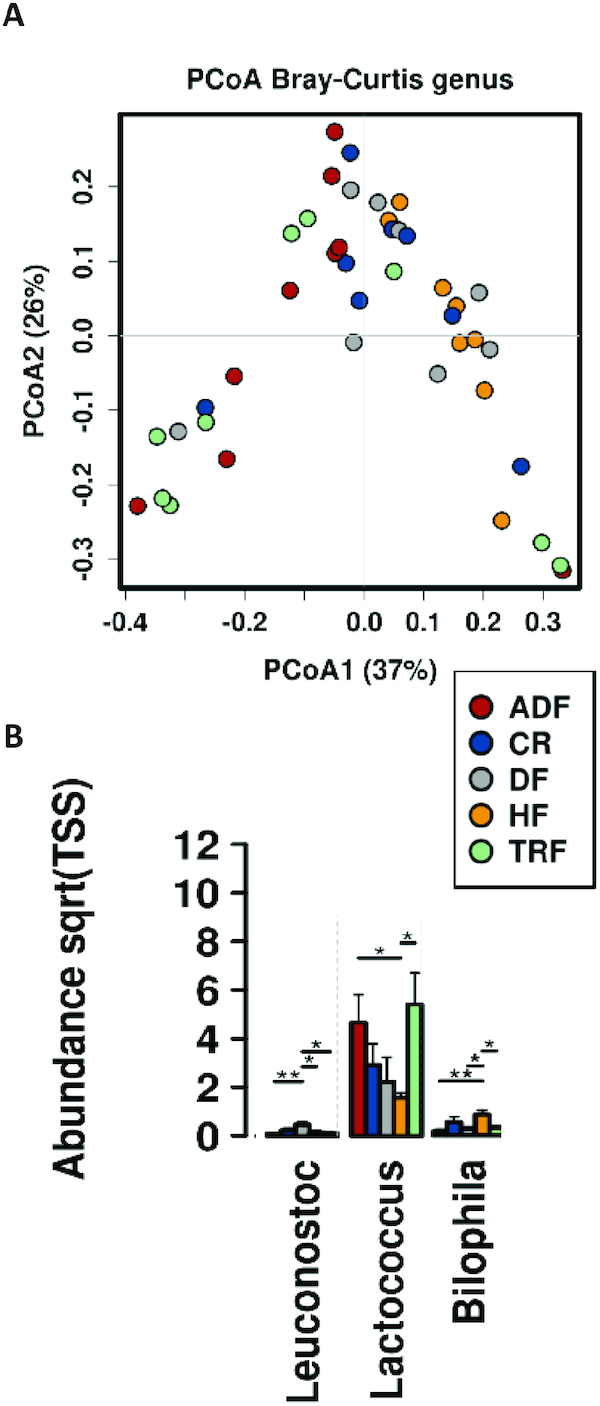
Ileal microbiome population following intervention. (A) Evaluation of the β-diversity (Bray–Curtis) of ileal samples. Predicted principal components analysis (PCoA) plots with Bray–Curtis dissimilarity metric demonstrating a change in community induced by fasting protocols. (B) Bar plot reports changes in abundance of bacterial population induced by either diet or fasting protocols. Significance (*P < 0.05; **P< 0.01) determined by ANOVA. ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; Sqrt, square root; TRF, time-restricted feeding; TSS, total sum scaling.
Compared with the ileum, where only fasting protocols had a significant effect on the bacterial population, diet composition induced dramatic changes in the cecal microbiota. Greater levels of the phylum Bacteroidetes were observed with DF relative to all HF groups (DF 29% compared with HF 17%, CR 16%, TRF 20%, and ADF 14%; Figure 6A). A striking increase was also observed in Verrucomicrobia with DF and to a lesser extent in all fasting groups (DF, 17%; HF, 2%; CR, 7%; TRF, 8%; and ADF, 8%). No significant differences were observed in α-diversity among the groups (Figure 6B), but interestingly, the DF group did not have the same expansion of diversity seen in the stool samples, despite having a very different taxonomic composition. β-Diversity in the cecal content showed clear clustering of the DF group in contrast to the tight clustering of all HF diet groups (Figure 6C), suggesting that timed feeding strategies do not effectively alter the cecal microbiota composition. At genus level, significant differences were observed between HF feeding and the DF group for the following: DF increased S274, Allobaculum, and Akkermansia, and decreased Rikenellaceae, Desulfovibrionaceae, Christensenellaceae, Ruminococcaceae, Oscillospira, Mucispirillum, Lactococcus, Desulfovibrio, Bilophila, and AF12 (Figure 6D). Lachnospiraceae and Peptococcaceae were both reduced by timed feeding protocols (Figure 6E).
FIGURE 6.
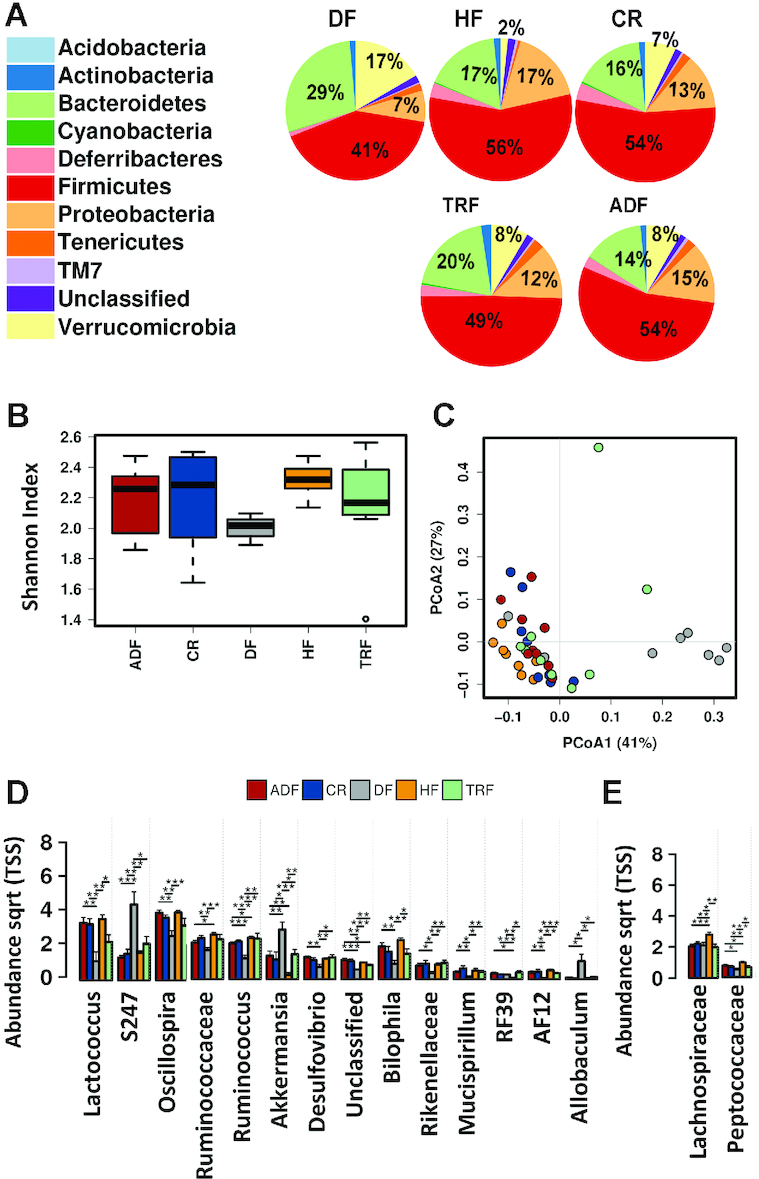
Cecal microbiome composition following intervention. (A) Pie chart showing relative abundance of cecal microbiota taxonomic composition for animals on various dietary modifications. (B) α-Diversity as measured by Shannon index of the cecal microbiome (genus level) for all dietary modifications. Whisker plots show mean surrounded by the upper and lower quartiles. Lines show variability outside of the quartiles. (C) Predicted β-diversity as shown by a PCoA plot (Bray–Curtis, genus level) of the cecal microbiome demonstrates that all HF-fed groups, irrespective of fasting time, clustered and are distinct from the DF group. (D) Bar chart showing abundance of specific populations (genus level) that are altered by dietary composition specifically. (E) Bar chart showing abundance of indicated populations that is altered by fasting protocols. Significance (*P < 0.05;**P <0.01;***P< 0.05) determined by ANOVA. ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; PCoA, principal components analysis; Sqtr, square toot; TRF, time-restricted feeding; TSS, total sum scaling.
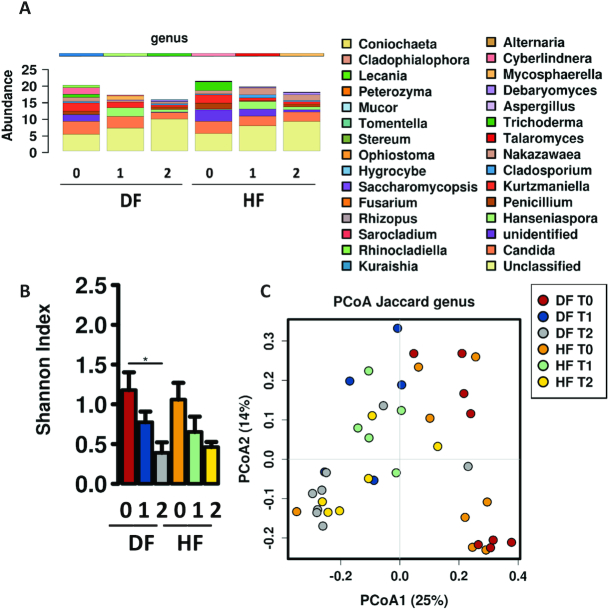
Fecal mycobiome following high-fiber diet
After 6 wk of high-fat feeding animals were switched to their respective intervention diets. The DF diet did not significantly alter fecal fungal populations compared with HF in relative composition for the duration of the experiment (Figure 7A). Compared with baseline, the DF and HF groups exhibited declines in α-diversity over time (Figure 7B) and shifted in β-diversity (Figure 7C). In contrast to differences found in bacterial community structure observed between DF and HF groups during the experiment, these results demonstrate that fungal communities responded similarly to both HF diets and were possibly less sensitive to the inclusion of fiber in dietary composition.
FIGURE 7.
Shifts in fungal composition following changes in diet. (A) Fecal fungal taxonomic abundance of animals on HF and DF diets prior to (0) and following 4 wk (1) and 8 wk (2) of dietary intervention. Animals switched from HF to DF initially remain close in composition to HF. (B) Bar chart showing differences in α-diversity determined by Shannon index (order level) of the fecal fungal community across time points for HF and DF groups, where DF and HF lead to decreased α-diversity (P = 0.012, ANOVA). (C) Predicted principal components analysis (PCoA) plot of Jaccard dissimilarity demonstrating β-diversity of the fecal microbiome across experimental time points demonstrating that the DF and HF groups move in a similar direction over time along PCoA1. DF, Daniel Fast; HF, high-fat diet.
Mycobiome composition following dietary interventions
We further examined content from the cecum and distal gut for the presence of fungi for all intervention groups. α-Diversity as indicated by the Shannon index and β-diversity as determined by PCoA using the Jaccard diversity index did not differ between any of the intervention groups in the cecal or fecal samples (Figure 8A, B). In contrast to dietary timing strategies, we also examined the fungal populations in a group that consumed a nonpurified chow diet as well as animals that were switched from HF diet onto chow. The groups demonstrated distinct fungal populations with increased abundances of Saccharomyces, Cladosporium, Talaromyces, and Aspergillus compared with the experimental groups (Figure 8C). However, within the timed feeding groups and DF, no significant differences were observed in taxonomic structure at the genus level (Figure 8C), despite dramatic changes in bacterial communities observed in the same samples (Figure 3C). Ascomycota was the dominant phylum (82% in the cecum and 81% in the fecal samples) and the most abundant fungi at the genus level measured in all intervention groups were Candida, Penicillium, and Hanseniaspora. (Figure 8D, Hanseniaspora not shown). These results again suggest that fungal populations, in contrast to bacterial populations, are less sensitive to variables such as timing of dietary intake, consistent with their longer replication times compared with the smaller bacterial counterparts.
FIGURE 8.
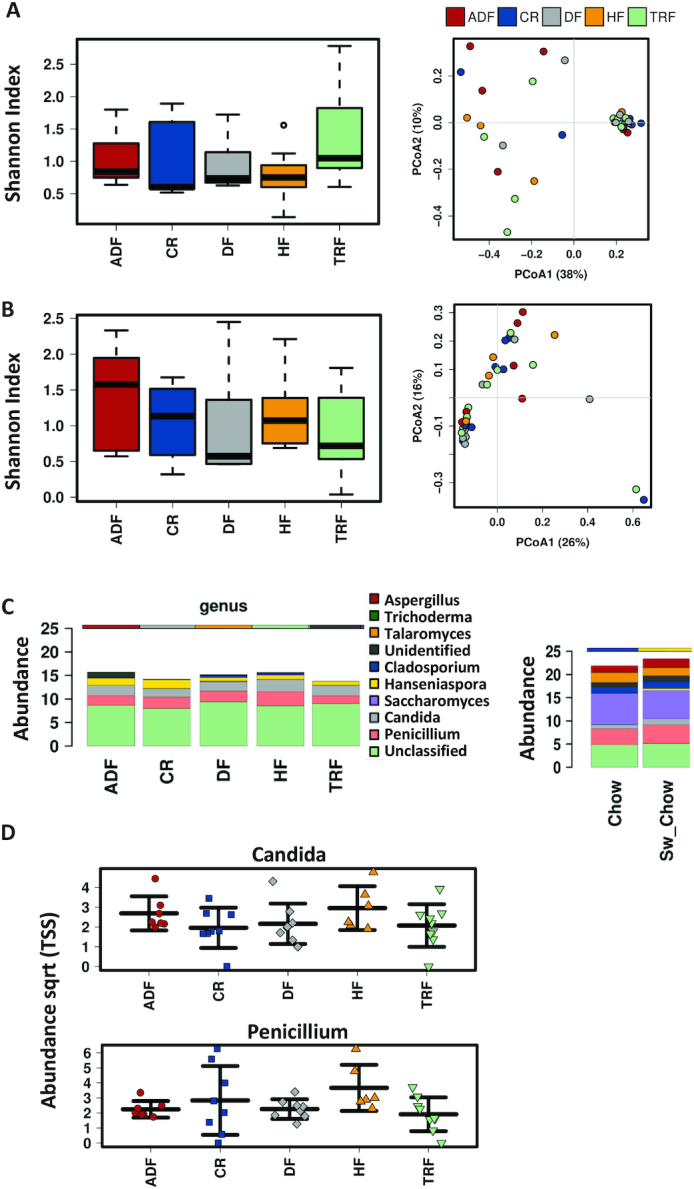
Fungal composition following dietary intervention in cecal and fecal samples. Diversity of fungal samples (operational taxonomic unit level); α-diversity as demonstrated by box plots and Shannon index (left panel). Predicted PCoA plots based on Jaccard analysis showing β-diversity (right panel) from (A) cecal and (B) fecal samples. (C) Bar chart of genus-level differences in fecal fungal populations for all experimental groups (left panel). Comparison of abundance of fecal fungal populations, indicating the 10 most abundant fungi. The right panel shows the abundance of fungal populations determined in groups that were included as age- and gender-matched controls fed chow or were reverted to the chow diet after the HF feeding period to demonstrate the influence of a nonpurified diet on the fungal population. (D) Scatterplots demonstrating the most abundant fungal populations (genus level) present in the fecal samples. No significant differences were observed in Candida or Penicillium as a consequence of diet or fasting protocols. Significance determined by ANOVA. ADF, alternate-day fasting; CR, caloric-restricted; DF, Daniel Fast; HF, high-fat diet; PCoA, principal components analysis; Sqrt, square root; TRF, time-restricted feeding; TSS, total sum scaling.
Discussion
Obesity is an urgent threat to health in westernized countries due to its co-occurrence with comorbidities including cancer, type II diabetes, and chronic inflammatory diseases. Although many genetic and environmental factors contribute to obesity, diet is identified as a major risk factor for this condition. Dietary practices are also a major determinant of the composition of the intestinal microbiome, and the interaction of dysbiosis with both obesity and high-fat feeding is now well established (26, 27). Consumption of a high-fat diet by C57BL/6 mice mimics the metabolic dysfunction observed in obese humans, making this an effective model to test potential dietary interventions aimed at reducing obesity and improving metabolism. Consistent with previous work (28), we observed that in addition to the induction of metabolic dysfunction, feeding of an obesogenic diet results in dysbiosis with increased Firmicutes and decreased Bacteroidetes. Emerging data now support the hypothesis that, in addition to diet composition, time of feeding or fasting cycles also influence the incidence of obesity and metabolic syndrome (16, 28). We therefore further examined the role of various dietary restriction models on adiposity and its association with gut microbial and fungal populations.
To investigate the effect of dietary timing on obesity, 3 experimental control groups were employed: a traditional calorie restriction model where animals were fed 80% of their predetermined “habitual” diet, and 2 protocols that would be classified as intermittent fasting, namely time-restricted feeding (6 h ad libitum consumption per 24 h) and alternate-day fasting (24 h ad libitum consumption; 24 h fasting). All 3 of these groups were fed the same obesogenic diet as the controls that consumed ad libitum throughout the study. An additional group of animals were restricted to a plant-based high-fiber diet resembling the human dietary intervention known as the Daniel Fast. This dietary strategy has previously been shown to improve health parameters in both animal and human studies (29, 30) and was included in this work as an extension of previous work and as a reference of an alternative high-fat diet that contains other macronutrient sources.
Compared with the high-fat controls, all animals on restricted dietary protocols had a reduction in weight gain associated with lower adiposity and improved body composition and measures of insulin sensitivity—these results are published (13)—demonstrating that timed feeding while consuming a high-fat diet can be a successful strategy for weight management and the treatment of obesity and its associated parameters. These data are consistent with previous work that demonstrated improvement in body composition with both calorie restriction and timed feeding protocols (31). Compared with the DF group, all HF diet groups reduced the length of the bowel and cecal weight, consistent with decreased fermentative activity of the gut under high-fat nutrition. A study by Gabel et al. (32) demonstrated that alternate-day fasting produced greater reductions in fasting insulin and insulin resistance in insulin-resistant individuals. An alternate-day fasting protocol, while consuming a high-fat diet, also increased weight loss and improved glucose tolerance in obese mice (33), and protected the liver from high-fat-diet–induced inflammation (34) Many of the benefits seen with alternate-day fasting are also observed with time-restricted feeding (16, 35, 36). In addition to affecting metabolism, the present study corroborates a previous report that feeding times affect the gut microbiome (37).
We therefore further investigated the role of dietary timing strategies upon microbial community composition with 2 complementary approaches, dual amplicon sequencing of the bacterial and fungal community signatures. The gut microbiome encompasses 1014 microorganisms, including bacteria, viruses, protista, and fungi. Of these, bacteria are the most well studied due to their total abundance in biomass, contribution to total microbial DNA, and rapid advances in next generation sequencing tools available to curate them. However, emerging research demonstrates that fungi can also play a contributory role in modulating host signaling, but these organisms remain almost entirely undescribed under many dietary intervention settings (10, 11, 38).
Diet is considered as the primary driver of gut microbiome community structure. Consistent with this, the plant-based high-fiber diet caused a reduction in Firmicutes and an increase in Bacteroidetes in the cecum, and also in fecal samples collected temporally. The increase in Bacteroidetes was primarily driven by the increase in S274, a population that is specific to the mouse gut. Although α-diversity was not increased in the cecal sample, the plant-based diet group clustered distinct from all other groups consuming the high-fat diet. Lactococcus, Ruminococcaceae, and Bilophila were increased in the high-fat compared with the plant-based diet, with no significant effect of timed feedings. The mucin-degrading bacterium Akkermansia muciniphila was dramatically increased with the plant-based diet, possibly due to the presence of inulin, because previous work had shown Akkermansia to be increased by fructo-oligosaccharide and inulin treatment (39). Interestingly, calorie- and time-restricted HF-fed groups also increased this mucin-degrading population suggesting that food restriction reprograms bacterial communities to use host-derived carbon resources. This increase has previously been shown to be specific for animals consuming a high-fat diet (40). Akkermansia remains of great interest in dietary studies that modulate the gut microbiome, because Akkermansia is elevated in lean individuals compared with obese individuals and diabetics, weight loss elevates it, and experimental administration restores insulin sensitivity (39, 41–44). In contrast, Lachnospiraceae appeared to be sensitive to fasting. Compared with the plant-based diet, the HF diet increased this population, but all fasting protocols reduced the Lachnospiraceae to the level of the plant-based diet. Intermittent leucine deprivation had also previously been shown to reduce Lachnospiraceae, whereas colonization of germ-free ob/ob mice by Lachnospiraceae significantly increased fasting blood glucose and adiposity (45). The changes induced by the different dietary interventions in the cecum persisted in the fecal samples with additional changes observed: the plant-based diet increased RF32, Allobaculum, Bacteroides, Coprococcus, and Clostridiales, whereas extended fasting times as seen with the ADF group resulted in increased Bifidobacterium.
Although the fungal populations make up 0.1% of total DNA and 1% of the gut microbial biomass, these organisms are longer lived and have diverse metabolism profiles, and therefore are interesting targets to examine in the context of health and disease (38, 46). We monitored the gut fungal populations through sequencing of the ITS2 region of the fungal genome. Ascomycota was the dominant phylum detected in the cecal and stool samples, with Candida, Penicillium, and Hanseniaspora being the most abundant genera. The dietary interventions did not alter the diversity of fungal populations and the taxonomic composition of these populations. Although associations have been made between fungal and bacterial populations and between fungi and diet, these complex relations are not well understood (47). Others have demonstrated that dietary contamination can influence the intestinal mycobiome (8, 38). However, here we demonstrated that in a C57BL/6 male murine model, diet composition and timing of feeding did not have a significant effect on the fungal populations, despite inducing dramatic changes in the bacterial community. The relative stability of the fungal community and the relations with health and disease are poorly understood.
In conclusion, the current study compares several dietary interventions, including high-fat diet and timed feeding interventions, that are used to improve health and weight management. From the results we aver that high fiber, plant-based diets and timed feeding protocols can be beneficial for weight management and metabolic health. These beneficial dietary protocols occur with changes in the gut bacterial populations, but do not induce dramatic alterations in gut fungal populations.
ACKNOWLEDGEMENTS
We thank Matt Butawan from the School of Health Studies at University of Memphis for help with animal experiments, and Kary Buddington for veterinary support.
The authors' contributions were as follows—MvdM, RJB, JFP: designed the research; MvdM, SS, JLC, NJS, CKG, RKB, JFP: conducted the research; MvdM, JFP: analyzed the data; MvdM, JFP: wrote the manuscript; MvdM, JFP: had primary responsibility for the final content; and all authors: edited the manuscript, and read and approved the final manuscript.
Notes
This study was supported by the University of Memphis and Children's Foundation Research Institute, Memphis.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ADF, alternate-day fasting; CR, caloric restriction; DF, Daniel Fast (purified high-fiber diet); eWAT, epididymal white adipose tissue; HF, high-fat diet; ITS, internal transcribed spacer; LDA, linear discriminant analysis; LEfSe, linear discriminant analysis effects size; PCoA, principal components analysis, rRNA, ribosomal RNA; SDS, sodium dodecyl sulfate; TRF, time-restricted feeding.
References
- 1. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–85. [PubMed] [Google Scholar]
- 2. Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39(4):509–21. [DOI] [PubMed] [Google Scholar]
- 3. Haase S, Haghikia A, Wilck N, Müller DN, Linker RA. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology. 2018;154(2):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74(3):227–34. [DOI] [PubMed] [Google Scholar]
- 5. Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S et al.. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1(11):16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung WSF, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N et al.. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–15. [DOI] [PubMed] [Google Scholar]
- 10. Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M et al.. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Limon JJ, Tang J, Li D, Wolf AJ, Michelsen KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI et al.. Malassezia is associated with Crohn's disease and exacerbates colitis in mouse models. Cell Host Microbe. 2019;25(3):377–88. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith NJ, Caldwell JL, van der Merwa M, Sharma S, Butawan M, Puppa M, Bloomer RJ. A comparison of dietary and caloric restriction models on body composition, physical performance, and metabolic health in young mice. Nutrients. 2019;11(2):E350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health benefits of fasting and caloric restriction. Curr Diab Rep. 2017;17(12):123. [DOI] [PubMed] [Google Scholar]
- 15. Das SK, Balasubramanian P, Weerasekara YK. Nutrition modulation of human aging: the calorie restriction paradigm. Mol Cell Endocrinol. 2017;455:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaiz A, Joens M, Fitzpatrick JA et al.. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delahaye LB, Bloomer RJ, Butawan MB, Wyman JM, Hill JL, Lee HW, Liu AC, McAllan L, Han JC, van der Merwe M. Time-restricted feeding of a high-fat diet in male C57BL/6 mice reduces adiposity but does not protect against increased systemic inflammation. Appl Physiol Nutr Metab. 2018;43(10):1033–42. [DOI] [PubMed] [Google Scholar]
- 18. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J et al.. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shing CM, Peake JM, Lim CL, Briskey D, Walsh NP, Fortes MB, Ahuja KD, Vitetta L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur J Appl Physiol. 2014;114(1):93–103. [DOI] [PubMed] [Google Scholar]
- 20. Daniels JL, Bloomer RJ, van der Merwe M, Davis SL, Buddington KK, Buddington RK. Intestinal adaptations to a combination of different diets with and without endurance exercise. J Int Soc Sports Nutr. 2016;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pourcyrous M, Nolan VG, Goodwin A, Davis SL, Buddington RK. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J Pediatr Gastroenterol Nutr. 2014;59(6):725–31. [DOI] [PubMed] [Google Scholar]
- 22. Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion MJ, Berger B, Krause L. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics. 2017;33(5):782–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glockner FO, Tedersoo L et al.. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47(D1):D259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Health AC, Leibel RL et al.. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulz MD, Atay Ç, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C et al.. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagpal R, Newman TM, Wang S, Jain S, Lovato JF, Yadav H. Obesity-linked gut microbiome dysbiosis associated with derangements in gut permeability and intestinal cellular homeostasis independent of diet. J Diabetes Res. 2018;2018:1–9., 3462092. Available from: 10.1155/2018/3462092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–21. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloomer RJ, Schriefer JHM, Gunnels TA, Lee SR, Sable HJ, van der Merwe M, Buddington RK, Buddington KK. Nutrient intake and physical exercise significantly impact physical performance, body composition, blood lipids, oxidative stress, and inflammation in male rats. Nutrients. 2018;10(8):E1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bloomer RJ, Kabir MM, Trepanowski JF, Canale RE, Farney TM. A 21 day Daniel Fast improves selected biomarkers of antioxidant status and oxidative stress in men and women. Nutr Metab. 2011;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, Varady KA. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. 2018;37(6 Pt A):1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Ceinfuegos S, Kalam F, Varady KA. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity (Silver Springs). 2019;27(9):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joslin PMN, Bell RK, Swoap SJ. Obese mice on a high-fat alternate-day fasting regimen lose weight and improve glucose tolerance. J Anim Physiol Anim Nutr (Berl). 2017;101(5):1036–45. [DOI] [PubMed] [Google Scholar]
- 34. Yang W, Cao M, Mao X, Wei X, Li X, Chen G, Zhang J, Wang Z, Shi J, Huang H et al.. Alternate-day fasting protects the livers of mice against high-fat diet-induced inflammation associated with the suppression of Toll-like receptor 4/nuclear factor κB signaling. Nutr Res. 2016;36(6):586–93. [DOI] [PubMed] [Google Scholar]
- 35. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):E1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation, but does not affect energy expenditure in humans. Obesity (Silver Springs). 2019;27(8):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20(6):1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8(6):e66019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu L, Qin S, Zhai S, Gao Y, Li L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol Lett. 2017;364(10):1–7. [DOI] [PubMed] [Google Scholar]
- 40. Zheng X, Zhou K, Zhang Y, Han X, Zhao A, Liu J, Qu C, Ge K, Huang F, Hernandez B et al.. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J. 2018;32(9):4878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med [Internet]. 2013;5(178):178ra41 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3652229&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borgo F, Verduci E, Riva A, Lassandro C, Riva E, Morace G, Borghi E. Relative abundance in bacterial and fungal gut microbes in obese children: a case control study. Child Obes. 2017;13(1):78–84. [DOI] [PubMed] [Google Scholar]
- 43. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L et al.. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–36. [DOI] [PubMed] [Google Scholar]
- 44. Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–35. [DOI] [PubMed] [Google Scholar]
- 45. Kameyama K, Itoh K.. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29(4):427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, Baldassano RN, Lewis DJ, Buschman FD, Wu GD. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li J, Chen D, Yu B, He J, Zheng P, Mao X, Yu J, Luo J, Tian G, Huang Z et al.. Fungi in gastrointestinal tracts of human and mice: from community to functions. Microb Ecol. 2018;75(4):821–9. [DOI] [PubMed] [Google Scholar]