ABSTRACT
Background
In Peru, tuberculosis (TB) is perceived as a nutritional disease. This perception, alongside factors including household food insecurity, may drive the food choices of people with TB and influence treatment outcomes.
Objectives
The objective of this qualitative study was to explore drivers of food choice among adults recently diagnosed with TB.
Methods
The study was conducted between April and December 2016 in the Huaycán district of Lima, Peru. Structured questionnaires were administered to 39 adults with TB at the time of diagnosis and after 1 mo of treatment to characterize food security and socioeconomic status. At 1 mo of treatment, 24-h dietary recalls, enhanced by recipes obtained from local street vendors, were administered to examine patterns of food consumption and determine mean daily intake of macro- and micronutrients. Among a subset of 9 participants, in-depth interviews were used to explore dietary beliefs and food choices associated with TB.
Results
Overall, 13.2% of participants were underweight at baseline, and 10.5% were overweight. At 1 mo of treatment, the mean caloric intake was 600 kcal/d over what was needed to maintain their current weight. Most of these additional kilocalories came from carbohydrates. Patients made active efforts to improve their diets during treatment, and were both receptive to, and actively sought out, nutritional advice. However, many patients reported significant unnecessary spending on questionable commercial products, such as expensive natural remedies and nutritional supplements.
Conclusions
The perceived connection between TB and diet creates both opportunities and challenges for treatment providers. Nutritional counseling provided through the national TB program should promote dietary quality through foods that are locally available, inexpensive, and aligned with cultural perceptions of health and wellness.
Keywords: tuberculosis, Peru, food security, qualitative, food choice, chronic disease
Introduction
Each year, 10 million new cases of tuberculosis (TB) are reported and 1.4 million people die of TB worldwide (1), mostly in low- and middle-income countries (LMICs). In 2015, Peru had the second highest TB incidence in the Americas, and was among the 30 countries with the highest burden of multidrug-resistant TB (MDR-TB).
TB is caused by the bacteria Mycobacterium tuberculosis. The disease most often affects the lungs, and is spread through coughing, sneezing, or speaking (2). Although ≤30% of the world's population may have a latent TB infection, only a small percentage progress into active disease (3). Recent infections (in the past 2 y), or infections of immunocompromised individuals, are most likely to result in active TB (4). Once diagnosed, the treatment requires 6 mo of antibiotic therapy for drug-sensitive illness and ≥20 months in the case of MDR-TB (5).
TB infection is biomedically linked to nutritional status (6). Some micronutrient deficiencies, particularly those of vitamin A and vitamin D, have been linked to an increased risk of disease (7, 8). Active TB is associated with cachexia (catabolic metabolism), associated with protein dysmetabolism, which leads to wasting (9). As a result, people with TB are often underweight at diagnosis (6). Weight gain after the initiation of chemotherapy is considered an early clinical indication of treatment response (10); however, the antibiotics used to treat TB can also cause nausea, vomiting, or a lack of appetite (11).
The Peruvian national TB program has received international recognition (12), with an estimated coverage of 80% of patients nationwide (). The program follows WHO guidelines by covering the full cost of treatment and offering psychological and nutritional counseling and monthly food baskets to all patients (13). The provision of food baskets is based on evidence that macronutrient supplementation increases treatment adherence, improves weight gain during treatment, and may decrease mortality (14, 15). However, in Peru, the urban population that bears the brunt of the TB burden is increasingly at risk of overweight and obesity (16). Furthermore, the prevalence of mild-to-moderate food insecurity, which may interfere with dietary quality more than dietary quantity, is high (17). This raises the question of whether nutritional recommendations originally developed for severely malnourished, severely food-insecure patients are fully meeting the needs of the current population.
Within Peru, the association between TB and weight loss, combined with a general cultural perception linking health and nourishment to body weight in general (18), has fueled the public perception of TB as a nutritional disease (19). This perception has implications for disease control, because Peruvians may be more likely to seek screening for TB because of weight loss than they are because of persistent cough (20). However, relatively little is known about how perceptions of the relation between TB and nutrition influence food choices after diagnosis.
Understanding how perceptions of nutrition and disease influence the food choices of TB patients during recovery can inform the development of appropriately tailored nutritional counseling messages and contextualize research evaluating the impact of macro- or micronutrient supplementation on TB recovery. Therefore, the aims of this study were to describe dietary patterns among people diagnosed with TB, both at diagnosis and after 1 mo of treatment, and to explore how knowledge and beliefs about TB affected food choices.
Methods
Setting
The study was conducted between April and December of 2016 at Huaycán Hospital. Huaycán is a district (21) in eastern Lima. As is also true of many other districts in the city, it was established in the mid-1980s by Andean migrants escaping terrorism (22). Huaycán was chosen because it is socioeconomically and culturally comparable with other impoverished neighboring districts.
All presenting patients who were ≥18 y old and had received a diagnosis of acute pulmonary TB in the past week were eligible to participate. All participants received directly observed therapy with appropriate chemotherapy from the Ministry of Health while enrolled in the study. All study procedures were conducted at the hospital with some exceptions that are described below. Our study included quantitative food security and 24-h dietary recall questionnaires, and qualitative semistructured interviews.
The study protocol was approved by the ethics review board of the Universidad Peruana Cayetano Heredia (UPCH). The Tulane University Human Research Protection Office formally deferred ethical oversight of the project to UPCH, and the Huaycán Hospital research board also reviewed and approved the study. Written informed consent was obtained from all study subjects. All participants were given a small food basket as a thank you for their participation in the study, and those who participated in semistructured interviews were given an additional small food gift.
Questionnaires
Baseline questionnaires were administered to collect clinical information, including prior TB, anthropometry, and the presence of any comorbidities, as well as age, sex, and socioeconomic status (SES). Information on drug sensitivity was not collected. Baseline dietary and food security information was collected via questions about the economic burden of TB, including costs specifically related to food and nutritional products (23); dietary diversity (defined as consumption of 12 possible food groups in the past 24 hours) (24); and coping strategies related to food insecurity in the past week (25), such as decisions to buy food on credit, to buy lower-cost less-preferred foods, or to reduce portion sizes. The questionnaire ended with 15 questions related to chronic food insecurity experienced over the past year, adapted to the Peruvian context by the Young Lives Study from the USDA's food insecurity and hunger module (26).
Dietary recalls
Between days 23 and 37 of treatment, questions about coping strategies and dietary diversity were repeated, and three 24-h dietary recalls were administered on nonconsecutive days, describing 2 weekdays and 1 weekend, by trained personnel. Wherever possible, ≥1 recall was conducted in the participant's home. A photobook of common foods and a selection of locally purchased containers were used as aids. When the patient cooked for themselves, their recipes were collected and analyzed. In addition, recipes of commonly consumed out-of-home foods were collected, from vendors who sold from stalls or restaurants near the hospital. Common food items, such as a portion of rice from a restaurant, were purchased 3 times, weighed, and the mean weight was taken.
In-depth interviews
The 9 participants enrolled between June and August of 2016 were invited to participate in in-depth interviews (IDIs). Every invited participant agreed to participate. These were conducted in the participant's home, or at the hospital if the patient preferred. IDIs were audio-recorded unless the participant declined, in which case detailed interview notes were taken. The interview guide is provided in Supplemental Table 1.
Analysis of questionnaire data
Food insecurity over the past year was calculated by rescaling the 15-item Young Lives questionnaire from 0 to 10, and using previously defined cutoffs to define “food secure,” and food insecure “without hunger,” “with moderate hunger,” and “with severe hunger” (Table 1) (26). Dietary diversity was split at the median, according to the recommendation (24). Data related to household costs were converted to US dollars at the rate of exchange at the midpoint of the study ($1 = 3.21 PEN) (27).
TABLE 1.
Baseline characteristics of study participants1
| Characteristic | |
|---|---|
| Women, % | 35.9 |
| Age | 28.8 ± 11.0 |
| Household size | 5 [3–7] |
| Baseline BMI | 21.9 ± 3.1 |
| Socioeconomic status score | 54.1 ± 9.4 |
| Prior reported tuberculosis, % | 20.5 |
| Baseline food insecure with moderate or severe hunger, % | 64.1 |
| Baseline reporting any coping strategies (past week), % | 74.4 |
| Baseline dietary diversity | 6 [4–7] |
Means ± SDs are reported for continuous variables that were distributed approximately normally, otherwise medians [IQRs] are reported, unless otherwise indicated.
The Progress out of Poverty index, developed and validated for use in Peru (28), was used to characterize SES. This score ranges between 0 and 100, with 0 representing the lowest and 100 the highest possible SES. Based on data availability, the education of the female head of the household was replaced with the education of the participant.
Baseline data from patients with incomplete and complete follow-up were compared using 2-sided t tests and chi-squared tests, as appropriate (Supplemental Table 2). Throughout all analysis, P values ≤0.05 were taken to indicate statistical significance.
Using the dietary data, energy, macronutrient, and micronutrient intakes were calculated using a previously developed Peruvian food composition table (FCT) (29). The nutrient content of recipes was calculated using retention factors from the USDA (30). For processed food items not found in the FCT, nutrient information was obtained from package labels. From this, energy (kcal/d), carbohydrate (grams/d), fat (grams/d), protein (grams/d), and specific micronutrient and mineral intakes (Supplemental Table 3) were estimated.
The resting basal metabolic rate (BMR) was calculated using the Schofield formula, accounting for the age, sex, and baseline weight of the participant, and adjusted by 14% to reflect energy expenditures resulting from illness-related catabolic metabolism (31). A ratio of total daily energy expenditures (TDEEs) over BMR of 1.5 was assumed (31). For minerals and micronutrients, the percentage of participants eating below the recommended nutrient intake (RNI) was estimated using age- and sex-specific cutoffs as defined by the WHO and FAO of the UN (32) and assuming moderate bioavailability of zinc and 10% bioavailability of iron. The 24-h recalls were also analyzed qualitatively by reviewing each recall and identifying specific dietary patterns also reported by patients during IDIs. All quantitative analysis was completed in Stata version 14 (StataCorp).
Qualitative interviews were transcribed (n = 8) or interviewer notes were used as the primary source material (n = 1). Using Dedoose software (33), codes were developed based on key themes that emerged from the transcripts. Transcripts were reread and coded blindly by 2 coders, any disagreements were discussed, and transcripts were blindly recoded in an iterative process until agreement was reached.
Data completeness
Five participants dropped out of treatment in the first 30 d and had no follow-up data available, 1 was referred to a health center elsewhere, and 4 did not drop out but had incomplete data (3 participants with ≥1 recall but no 30-d food security questionnaire, and 1 with a 30-d questionnaire but no dietary recalls). Of 32 participants with ≥1 recall, 3 recalls were successfully completed for 25 (78.1%), 2 were completed for 4 (12.5%), and 1 was completed for 3 participants (9.4%). Men (61% of the sample) had more incomplete study visits and were marginally more likely to drop out of treatment in the first month. Patients with prior self-reported TB were also more likely to drop out (Supplemental Table 1).
Results
A total of 39 patients were enrolled in the study (age range: 18–62 years old). One was HIV-positive and one had type 2 diabetes (both previously diagnosed). Thirty-one had TB for the first time, and 8 participants had had a previous episode of TB.
At enrollment, participants had spent a median $44 on their illness (IQR: $19–140). By 30 d of treatment, this rose to $161 (IQR: $57–242). This does not account for losses resulting from unemployment, although 66.7% of participants said they stopped working because of illness (reduced to 48.3% by day 30). More than one-third (39.4%) spent extra money on food in the first week of treatment (median spent: $10.8; IQR: $4–23), and 58.6% spent extra money on food after 1 mo (median: $23; IQR: $9–62). Just under half (43.6%) had spent money on natural remedies at day 0 (median: $7; IQR: $6–16), and 48.3% had done so at day 30 (median: $13; IQR: $9–44).
The most commonly reported natural remedy purchased was “frog extract” (“extracto de rana”) (8 participants; median: $47/mo), an Andean recipe where a frog is pureed (boiled or live) into a juice with fruit or herbs. This extract cost $2.50–3.00/serving. The second most common remedy reported was “alfalfa extract” (5 participants; median spent: $8/mo). One participant spent $44 on HerbaLife® because “they were told it was good for the lungs” (interview note, 19-y-old woman) and 1 purchased a “complete packet” of HerbaLife® for $234 (18-y-old man). A third spent $218 on “vitamins” but could not describe the brand. Other common, lower-cost remedies reported included various herbs, “banana syrup” (“jarabe de platano”), and “tokosh,” an Andean fermented potato porridge.
Food insecurity and costs related to TB
Overall, 64.1% of participants reported baseline food insecurity with moderate or severe hunger, and 74.4% reported ≥1 food insecurity–related coping strategy in the past week (Tables 1, 2). The frequency of reported coping strategies was similar at baseline and 30 d of treatment, but dietary diversity was significantly higher at day 30 (P = 0.0453, paired t test among participants with complete data at both visits).
TABLE 2.
Characteristics of participants at day 0 and day 301
| Day 0 (n = 39) | Day 30 (n = 29) | |
|---|---|---|
| Food insecure with moderate or severe hunger | 64.1 | N/A |
| Coping strategies | 3 [0–7] | 1 [0–6] |
| Dietary diversity | 6 [4–7] | 7 [6–8] |
| Costs related to extra food | 0 [0–14] | 25 [0–90] |
| Costs related to nutritional supplements | 0 [0–20] | 0 [0–32] |
| Changed diet because of illness | ||
| No change | 20.5 | 41.4 |
| Same foods as usual but more | 5.1 | 10.3 |
| Same foods as usual but less | 5.1 | 0.0 |
| Fewer kinds of foods than before | 5.1 | 3.5 |
| More kinds of foods than before2 | 59.0 | 41.4 |
| Other | 5.1 | 3.5 |
| Sources of nutritional advice (categories are nonexclusive) | ||
| Family | 82.1 | 82.8 |
| Neighbors | 7.7 | 20.7 |
| Health professional | 10.3 | 31.0 |
| TV/Internet | 7.7 | 17.2 |
| Nobody/didn't look for advice | 15.4 | 20.7 |
| Received a MINSA packet (PANTBC) | 10.3 | 48.3 |
| Among those who received packet, felt packet was important to household | 90.0 | 100 |
| Received ≥1 visit with nutritionist | 18.0 | 55.2 |
| Among those who received visit, felt time with nutritionist was useful | 75.0 | 100 |
| Among those who received visit, wanted more time with nutritionist | 87.5 | 43.8 |
Ministry of Health (MINSA); Program for Nutrition of People with Tuberculosis and Family (PANTBC); Values are medians [IQRs] for nonnormally distributed continuous variables, or percentages; N/A, not available.
Most common foods mentioned: pulses, 22.1%; fruit, 22.1%; and fish, 13.3%.
Dietary intake
The median number of kilocalories reported per participant per day was 2594 (IQR: 1877–3401), an estimated 617 kilocalories (SD: 1213) or 22.1% over TDEE (IQR: −3.0% to 53.1%) (Table 3). The mean percentages of kilocalories derived from carbohydrate, protein, and fat were 69.9%, 8.3%, and 21.8%, respectively. The most common micronutrient and mineral deficiencies were calcium (75.0%), vitamin D (62.5%), and iron (56.3%) (Supplemental Table 2). The only difference in micronutrient adequacy by sex was for iron (92.8% of women below the RNI compared with 27.8% of men, P < 0.001), although the difference in zinc adequacy was marginally significant (0.0% of women below the RNI compared with 22.2% of men, P = 0.0623).
TABLE 3.
Estimated macronutrient intake of participants1
| Intake | Recommendation (13, 34) | TDEEs | Recommendation to gain weight at 10% TDEE/wk | |
|---|---|---|---|---|
| Kcal/d | 2797 ± 1172 | N/A | 2215 ± 293 | 2437 ± 322 |
| % Energy from carbohydrates | 69.9 | 45–65 | N/A | N/A |
| % Energy from protein | 8.3 | 15–30 | N/A | N/A |
| % Energy from fat | 21.8 | 25–35 | N/A | N/A |
n = 32. Values are means ± SDs or percentages. N/A, not applicable; TDEE, total daily energy expenditure.
Weight gain was described as a primary motivator of food choice. These were confirmed in the dietary recalls with participants eating 2 full meals in a row (2 participants); supplementing their diet with drinkable yogurt, whole milk, or Ensure® (4 participants); or eating 750- to 2500-g quantities of fruit per day, most often bananas (5 participants).
IDIs
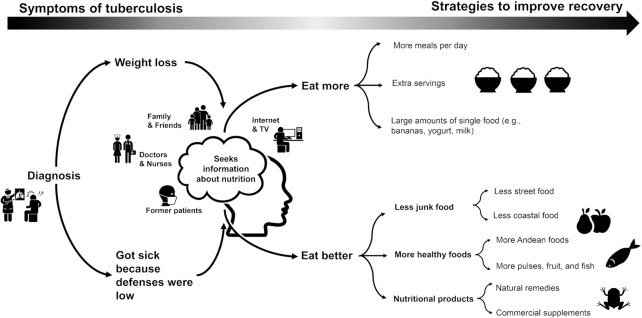
Nine participants were recruited for a semistructured IDI. From these, 3 key themes were examined: causes of and recovery from TB, key beliefs and changes in diet (Figure 1, Table 4), and health messaging and support.
FIGURE 1.
Mind map of key themes. Icons are taken from the Noun Project (https://thenounproject.com/) with permission.
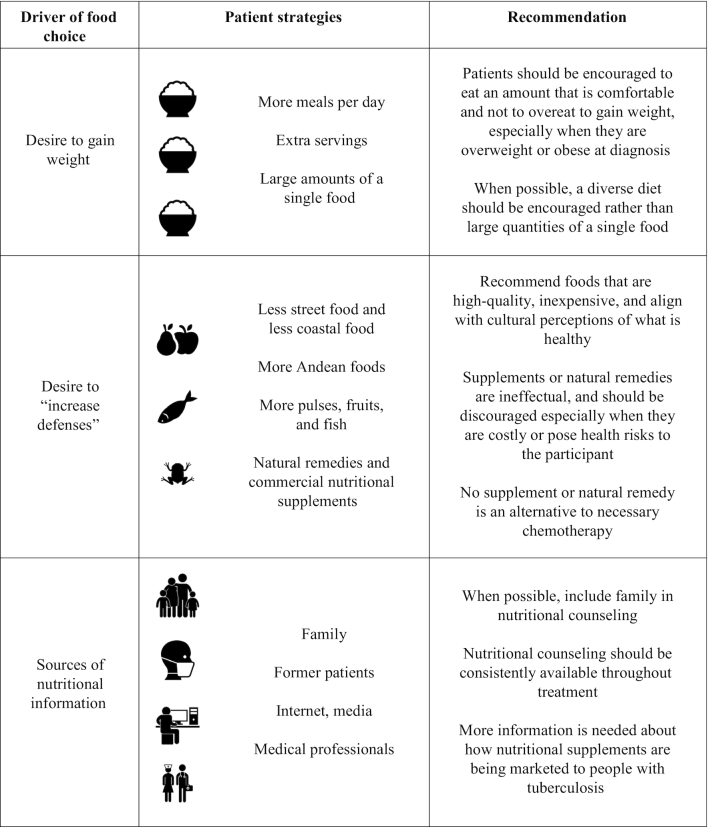
TABLE 4.
Overall study findings and related recommendations
|
Causes of and recovery from TB
As reported elsewhere (20), most participants described TB as infectious, but also reported that poor eating habits increased susceptibility and were therefore a primary reason for contracting TB. This idea was reinforced by health professionals, with 1 participant reporting that “nurses at the health post told me that it is because of poor diet” (62-y-old man). Common responses around causes of TB included, “If you don't nourish yourself, you get ill” (19-y-old woman), “[I was] not eating like I should have been” (33-y-old woman), or “careless eating” (62-y-old man). Physical manifestations of malnutrition and perceived undernutrition were also perceived as a sign of weakness and susceptibility to TB: “I was very weak. I was very skinny as well” (21-y-old man).
Regaining an outwardly well-nourished appearance was a significant part of recovery. Describing recovery in terms of “gaining weight” (19-y-old woman) or “recuperating the weight that you lost” (19-y-old woman) was common. Being fatter was a good thing, with 1 participant describing another patient completing treatment as having “left fat and everything, and healthy” (26-y-old man).
Key beliefs and changes in diet related to TB
Most participants referred to specific dietary changes made after their diagnosis of TB. When asked if there were any differences related to a TB-specific diet, most participants reported that they should “eat well… I mean eat as I should” (62-y-old man). This included avoiding junk food and street food, avoiding condiments, and increasing the consumption of healthy foods, often including pulses, fruit, fish, and home-cooked meals.
Patients also reiterated that being and feeling full were essential for recovery and reported the desirability of increasing the quantity of food consumed through larger portion sizes and more meals throughout the day. One participant reported that “yes [the nurse] told me the same. To eat 5 times a day… but not junk food” (21-y-old-man) and another reported that they “had to eat a lot, I have to eat more… More than anything everyone told me that I have to eat, feed myself well, eat pulses… things like this” (19-y-old man). This was further supported by family members offering seconds and sometimes encouraging the participant to eat first.
Health messaging and support
Participants were positive about their experiences with the TB program nutritionist. Several noted that this was their first exposure to nutritional information and reported a desire to learn more, with 1 participant recounting Internet searches about TB and nutrition: “More than anything, [I used] the Internet, I went to the nutritionist once, and she said… more than anything [eat] pulses and fruit” (19-y-old man).
Friends and acquaintances who had previously undergone treatment of TB were also mentioned as helpful, solicited sources of information. Family members were the most commonly reported source of advice and support; however, some participants reported unsolicited, unhelpful advice with disagreement on what constituted “healthy” compared with “unhealthy” foods and the quantities that should be consumed. One participant described preferring to take advice from a nutritionist: “… my mother does not know much and she tells me this… The [nutritionist] knows more, she said to me already, you can eat this, this is good” (19-y-old woman).
Andean heritage foods were perceived as particularly healthy: “… from our childhood we ate quinoa, beans, corn, things from the mountains because we are from there. There we ate potato, olluco, oca, mashua, everything they said was for growth” (44-y-old woman). In contrast, ceviche, a common coastal food, was mentioned negatively: “yes sometimes I ate junk, sometimes ceviche, the ceviche does not have many vitamins” (19-y-old woman).
Discussion
A large body of literature describes the positive relation between food incentives, treatment adherence, and treatment outcomes among TB patients (35). The need for food incentives to be culturally appropriate and balanced in macro- and micronutrients has been emphasized. Dietary counseling is a WHO-recommended component of the TB control, complementing necessary chemotherapy (13). However, very little operational research, to our knowledge, has been conducted to identify how best to tailor nutritional messaging and support.
Our study found that people with TB in Huaycán, Peru, have positive dietary habits that should be supported, and some negative practices to discourage. The perception that TB was a nutritional disease played into these practices. On the one hand, it created a population of patients who, often together with their families, were receptive to nutritional advice and active in attempting to nutritionally manage their disease. However, it also put patients at risk of expenditures on unnecessary and ineffectual commercial supplements and expensive natural remedies, and caused them to overemphasize the need to eat for weight gain, and underemphasize the importance of dietary quality, during early treatment.
Our estimate that participants ate ∼600 kcal/d over their TDEEs is similar to those for TB patients in other countries and contexts (36, 37) and supported by other studies in urban Lima where TB patients gained an mean 0.7 kg in the first month of treatment. However, the same study found less underweight (15%) and more overweight (22%) than our population at baseline, increasing to 33% overweight by 2 months of treatment (JG Hernandez and G Comina, Tulane University, 2017, personal communication). Peru, like many other LMICs, is undergoing a rapid nutritional transition (38, 39), and Huaycán, a peri-urban district, may be relatively less affected than the urban center of Lima.
Nevertheless, the implication of these findings is that, whereas some patients may present with underweight, others may be overweight or obese. Similarly, some patients present with severe food insecurity, whereas others are mild or moderately food insecure. The macronutrient supplementation provided by the national TB program may be particularly critical to the former group. For the latter group, although weight gain remains a clinically meaningful indicator of recovery, recommendations should emphasize dietary quality, encourage protein consumption, and provide encouragement to eat a comfortable amount rather than to overeat with the intention of gaining weight. Although eating large quantities of refined carbohydrates for the purpose of gaining weight should generally be discouraged, for patients dealing with medication-induced nausea, a positive calorie balance should nevertheless be encouraged through whatever foods are palatable. These results reinforce the importance of the individualized nutritional counselling provided by the TB program.
As others have reported, participants were appreciative of the nutritional counseling they received (40). However, due to personnel turnover, consultations with the program nutritionist were limited during the first half of the study, which may have influenced the frequency with which participants sought outside information. Participants who received program counseling often reported that it was their first exposure to nutritional information. Notably, decisions about food were made at the household level, and participants often sought nutritional advice from their families. Household contacts of TB patients are at increased risk of developing TB owing to shared risk profiles and risks of secondary infection, and vitamin A deficiency in household contacts is associated with the risk of developing TB (8). Therefore, dietary advice targeted not only toward patients but also toward their households, when feasible, may increase adherence and reduce the risk of secondary infections.
To the best of our knowledge there is no evidence supporting the effectiveness of any of the natural remedies or commercial nutritional supplements reported by participants. Their use should therefore be discouraged, particularly when they are costly or present health risks. The most common nutritional product purchased by participants was “frog extract,” typically consumed for respiratory problems, which should be strongly discouraged because of the expense to the patient, the documented risk of Salmonella contamination (41), and because the frog in question—Telmatobius culeus—is classified as endangered by the Peruvian government and internationally (42).
This descriptive study investigated a small number of participants intensely and was not designed to compare between dietary patterns and treatment outcomes. We also did not collect control data on the dietary habits of healthy adults in the community, limiting our capacity to conclude that patients changed their diets because of illness. Nevertheless, several factors support this supposition. Participants directly reported making changes: they enumerated what changes were made and their reasons for this. Unusual dietary patterns were also observed that, according to the participants’ own report, were intended to facilitate weight gain.
A further limitation of this study is that it characterizes the food choices and nutritional status of TB patients soon after diagnosis, and therefore represents only a moment in the participant's TB care. TB requires 6 mo of treatment for drug-sensitive disease, and substantially longer for MDR-TB. Therefore, there is a need to understand food choices over the full course of treatment. Increasing attention has recently been payed to the role of TB as a potential risk factor for subsequent chronic disease, including cardiovascular disease (43) and diabetes (44). Nutritional support in late treatment may therefore serve not only to improve treatment outcomes, but also to reduce the risk of future morbidity.
Dietary recommendations that align with underlying perceptions of what is healthy and culturally valued may be more intuitive, and result in better adherence, than recommendations that misalign with underlying beliefs and values around food (45). Huaycán is a distinct community, comprised of first- and second-generation Andean migrants. The history of the community was evident, because Andean foods (particularly quinoa, a good source of dietary protein, and “Seven Seeds” as breakfast foods) were frequently consumed, and “extracto de rana” and other commonly mentioned natural remedies were also of Andean origin. Health-related TV shows such as “DoctorTV” (46), mentioned by several participants, also promote Andean foods as healthy. Because Andean foods are valued and familiar, recommendations that emphasize financially accessible and locally available Andean foods could be utilized in health communication messaging to increase uptake of nutritional recommendations.
Although not the emphasis of this report, many participants reported a sense of shame around their illness. Although many reported that a poor diet had left them susceptible to illness, they often qualified that this was a result of busy work schedules that left them with limited time to eat well, an assertion previously noted by others (20). In this way, participants may have attempted to push back against a narrative of stigma that equates malnutrition to poverty, ignorance, and a disordered lifestyle. After diagnosis, the sometimes-extreme efforts participants made to nutritionally manage their disease may also have helped them to reassert control over their own bodies, and to assume an active role in their own recovery. Medical professionals and nutritionists working with TB patients should avoid perpetuating stigma by implying that people with TB are to blame for their disease because of their “unhealthy” eating habits or other poor behaviors.
Our results suggest that the National Tuberculosis Control Program in Peru should provide early and ongoing access to a nutritionist, who should provide reassurance that expensive commercial nutritional products are not necessary, while simultaneously recommending low-cost locally available foods to promote dietary diversity and increase protein intake. Our findings also have implications for TB control at a global level, including emphasizing the need to tailor nutritional recommendations to account for local perceptions of illness and diet.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Juan Carlos Llontop and the Tuberculosis team at Hospital Huaycán for his support of the study, as well as Lic. Raul Chuquiyauri for his administrative support of the study. We also thank Mirella Villena for her helpful comments regarding Telmatobius coleus, Joanna Brown for her assistance with translations and other support throughout the study, and Dr. Laura E Caulfield and the MAL-ED study for allowing us the use of their food composition tables. The authors’ responsibilities were as follows—GOL, VAP-S, AG, CT-M, RA, CU-G, GC, GH, and RO: designed the research; GOL and CT-M: provided essential materials; AG, KVP, KO, and NN: conducted the research; GOL, VAP-S, ARR-P, and AG: analyzed the data; GOL and ARR-P: wrote the paper; GOL: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by NIH grant 5D43TW009349-03 “Inter-American Training for Innovations in Emerging Infectious Diseases” (to RO) and T37MD001424 "Tulane Xavier Minority Training in International Health" (to RO).
Author disclosures: the authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: BMR, basal metabolic rate; FCT, food composition table; IDI, in-depth interview; LMIC, low- and middle-income country; MDR-TB, multidrug-resistant tuberculosis; RNI, recommended nutrient intake; SES, socioeconomic status; TB, tuberculosis; TDEE, total daily energy expenditure; UPCH, Universidad Peruana Cayetano Heredia.
References
- 1. World Health Organization. Global Tuberculosis Report. Geneva (Switzerland): WHO; 2016. [Google Scholar]
- 2. Turner RD, Bothamley GH. Cough and the transmission of tuberculosis. J Infect Dis. 2015;211:1367–72. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva (Switzerland): WHO; 2018. [PubMed] [Google Scholar]
- 4. Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31:e00021–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO (World Health Organization). Compendium of WHO guidelines and associated standards: ensuring optimum delivery of the cascade of care for patients with tuberculosis [Internet]. 2nd ed Geneva (Switzerland: ): WHO; 2018. [Accessed 2017 Jan 1]. Available from: https://www.who.int/tb/publications/Compendium_WHO_guidelines_TB_2017/en/. [Google Scholar]
- 6. MacAllan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34:153–7. [DOI] [PubMed] [Google Scholar]
- 7. Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–9. [DOI] [PubMed] [Google Scholar]
- 8. Aibana O, Franke M, Huang C, Galea J, Calderon R, Zhang Z, Becerra M, Smith E, Ronnenberg A, Contreras C et al.. The impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis. 2017;65:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwenk A, Hodgson L, Wright A, Ward LC, Rayner CFJ, Grubnic S, Griffin GE, Macallan DC. Nutrient partitioning during treatment of tuberculosis: gain in body fat mass but not in protein mass. Am J Clin Nutr. 2004;79:1006–12. [DOI] [PubMed] [Google Scholar]
- 10. Gler MT, Guilatco R, Caoili JC, Ershova J, Cegielski P, Johnson JL. Weight gain and response to treatment for multidrug-resistant tuberculosis. Am J Trop Med Hyg. 2013;89:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patil K, Bagade S, Bonde S, Sharma S, Saraogi G. Recent therapeutic approaches for the management of tuberculosis: challenges and opportunities. Biomed Pharmacother. 2018;99:735–45. [DOI] [PubMed] [Google Scholar]
- 12. WHO (World Health Organization). Health, a key to prosperity: successful stories in developing countries. Geneva (Switzerland): WHO; 2002. [Google Scholar]
- 13. World Health Organization. Guideline: nutritional care and support for patients with tuberculosis. [Internet] Geneva (Switzerland): WHO; 2013. [Accessed 2017 Jan 1]. Available from: https://extranet.who.int/iris/restricted/handle/10665/94836. [PubMed] [Google Scholar]
- 14. Grobler L, Nagpal S, Sudarsanam TD, Sinclair D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2016;(6):CD006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Pee S, Grede N, Mehra D, Bloem MW. The enabling effect of food assistance in improving adherence and/or treatment completion for antiretroviral therapy and tuberculosis treatment: a literature review. AIDS Behav. 2014;18(Suppl 5):S531–41. [DOI] [PubMed] [Google Scholar]
- 16. Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart. 2011;97:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Humphries DL, Dearden KA, Crookston BT, Fernald LC, Stein AD. Cross-sectional and longitudinal associations between household food security and child anthropometry at ages 5 and 8 years in Ethiopia, India, Peru, and Vietnam. J Nutr. 2015;145(8):1924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loret de Mola C, Pillay TD, Diez-Canseco F, Gilman RH, Smeeth L, Miranda JJ. Body mass index and self-perception of overweight and obesity in rural, urban and rural-to-urban migrants: PERU MIGRANT study. PLoS One. 2012;7:e50252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koethe JR, Von Reyn CF. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis. 2016;20:857–63. [DOI] [PubMed] [Google Scholar]
- 20. Baldwin MR, Yori PP, Ford C, Moore DAJ, Gilman RH, Vidal C, Ticona E, Evans CA. Tuberculosis and nutrition: disease perceptions and health seeking behavior of household contacts in the Peruvian Amazon. Int J Tuberc Lung Dis. 2004;8:1484–91. [PMC free article] [PubMed] [Google Scholar]
- 21. Peru21. Huaycán ya es distrito y esto es lo que debes saber [Internet]. Lima (Peru): Peru21; 2017. [Accessed 2018 Jan 1]. Available from: https://peru21.pe/lima/huaycan-distrito-esto-debes-67907. [Google Scholar]
- 22. Muñoz H, Meyers C, Vásquez MA, Williams P. Believers and neighbors: “Huaycán is one and no one shall divide it”. J Inter Am Stud World Aff. 1999;41:73–92. [Google Scholar]
- 23. Wingfield T, Boccia D, Tovar M, Gavino A, Zevallos K, Montoya R, Lönnroth K, Evans CA. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med. 2014;11:e1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy G, Ballard T, Dop M. Guidelines for measuring household and individual dietary diversity. Rome (Italy): FAO; 2013. [Google Scholar]
- 25. Maxwell DG. Measuring food insecurity: the frequency and severity of “coping strategies.”. Food Policy. 1996;21:291–303. [Google Scholar]
- 26. Vargas S, Penny ME. Measuring food insecurity and hunger in Peru: a qualitative and quantitative analysis of an adapted version of the USDA's Food Insecurity and Hunger Module. Public Health Nutr. 2010;13:1488–97. [DOI] [PubMed] [Google Scholar]
- 27. Historical Rates for the USD/PEN Currency Conversion on 13 July 2016 (13/07/2016) [Internet]. Wokingham (UK): Pound Sterling Live; 2016; [cited 2017 July 16]. Available from: https://www.poundsterlinglive.com/best-exchange-rates/us-dollar-to-peruvian-nuevo-sol-exchange-rate-on-2016-07-13. [Google Scholar]
- 28. Schreiner M. Progress out of Poverty: A Simple Poverty Scorecard for Peru. New Haven, CT: Innovations for Poverty Action (IPA); 2012. [Google Scholar]
- 29. Caulfield LE, Bose A, Chandyo RK, Nesamvuni C, De Moraes ML, Turab A, Patil C, Mahfuz M, Ambikapathi R, Ahmed T et al.. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin Infect Dis. 2014;59:S248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, release 27. USDA; 2016. [Google Scholar]
- 31. Raj T, D'Souza G, Elia M, Kurpad AV. Measurement of 24 h energy expenditure in male tuberculosis patients. Indian J Med Res. 2006;124:665–76. [PubMed] [Google Scholar]
- 32. WHO (World Health Organization), FAO of the UN. Vitamin and mineral requirements in human nutrition. 2nd ed Geneva (Switzerland: ): WHO; 2004. [Google Scholar]
- 33. Dedoose Version 7.0.23, Web Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data [Internet]. Los Angeles (CA): SocioCultural Research Consultants, LLC; 2016. [Accessed 2018 Jan 1]. Available from: www.dedoose.com. [Google Scholar]
- 34. Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids [Internet]. Washington (DC): National Academies Press; 2005. [Accessed 2018 Jan 1]. Available from: http://www.nap.edu/openbook.php?isbn=0309085373. [Google Scholar]
- 35. Martins N, Morris P, Kelly PM. Food incentives to improve completion of tuberculosis treatment: randomised controlled trial in Dili, Timor-Leste. BMJ. 2009;339:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frediani JK, Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, Ramakrishnan U, Tangpricha V, Blumberg HM et al.. A culture-specific nutrient intake assessment instrument in patients with pulmonary tuberculosis. Clin Nutr. 2013;32:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frediani JK, Sanikidze E, Kipiani M, Tukvadze N, Hebbar G, Ramakrishnan U, Jones DP, Easley KA, Shenvi N, Kempker RR et al.. Macronutrient intake and body composition changes during anti-tuberculosis therapy in adults. Clin Nutr. 2016;35(1):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popkin B, Adair L, Ng S. Now and then: the global nutrition transition: the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaparro PM, Estrada L. Mapping the nutrition transition in Peru: evidence for decentralized nutrition policies. Rev Panam Salud Publica. 2012;32:241–4. [DOI] [PubMed] [Google Scholar]
- 40. Paz-Soldán VA, Alban RE, Jones CD, Oberhelman RA. The provision of and need for social support among adult and pediatric patients with tuberculosis in Lima, Peru: a qualitative study. BMC Health Serv Res. 2013;13:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villena M, Morales S, Enciso MA. Salmonella enterica na ra gigante do lago titicaca telmatobius culeus (Anura: Leptodactylide): Estudo Preliminar. XXXI Congreso Anual da Sociedade de Zoologicos do Brazil 2007. [Google Scholar]
- 42. Ramos L, Gallegos N, Quispe LS. Evaluación de la Información Disponible del Suri, Pisaca y Rana Gigante del Titica, Peru: Binational Authority of Lake Titicaca and the National University of the Antiplano-Puno; 2000. [Google Scholar]
- 43. Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines. 2015;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Magee M, Salindri A, Gujral U, Auld S, Bao J, Haw J, Lin H, Kornfeld H. Convergence of non-communicable diseases and tuberculosis: a two-way street?. Int J Tuberc Lung Dis. 2018;22:1258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yates-Doerr E. The weight of obesity: hunger and global health in postwar Guatemala. Berkeley (CA): University of California Press; 2015. [Google Scholar]
- 46. América Televisión. Dr TV. [Internet] Lima (Peru): América TV; [cited 2017 Aug 30]. Available from: http://www.americatv.com.pe/doctor-tv/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.