Abstract
In eukaryotic cells, ubiquitination and proteasomal degradation is an essential mechanism for regulating protein functions. For example, critical signaling proteins play their roles by controlling different cellular functions. Once a signaling protein has been activated, its activity needs to be quickly downregulated by different mechanisms, including ubiquitination/proteasome regulation. Failure to regulate the activity or expression levels of these proteins may cause human diseases. Protein ubiquitination involves a cascade of biochemical processes and requires three types of ubiquitin enzymes: E1 activating enzyme, E2 conjugating enzyme, and E3 ligase. Among these enzymes, E3 ubiquitin ligases play a specific role in recognizing specific protein substrates. There are several structurally diverse groups of E3 ubiquitin ligases in eukaryotic cells, and one type of these E3 ligases is the U-box ubiquitin ligases. Carboxyl terminus of Hsp70-interacting protein (CHIP) is a member of a family of U-box E3 ligases. It plays critical roles in multiple organs and tissues in the body. In this review article, we provide an update on some of the most recent discoveries about CHIP in normal physiological function and in disease.
Keywords: carboxyl terminus of Hsp70-interacting protein (CHIP), immunity, neurodegenerative diseases, inflammation, bone remodeling
Graphical abstract
Carboxyl terminus of Hsp70-interacting protein (CHIP) is a member of a family of U-box E3 ligases, which are involved in protein ubiquitination. It plays critical roles in multiple organs and tissues in the body. In this review article, the authors provide an update on some of the most recent discoveries about CHIP in normal physiological function and in disease.
Introduction
Protein ubiquitination is a multistep process. First, ubiquitin is activated by being attached to an E1 ubiquitin-activating enzyme. The ubiquitin is then transferred to an E2 ubiquitin-conjugating enzyme. The final transfer of ubiquitin to the substrate protein is mediated by an E3 ubiquitin ligase. In some cases, the ubiquitin is first transferred from E2 to E3 and then to the substrate protein. In other cases, the ubiquitin could be transferred directly from E2 to the targeted protein in a complex with E3. Most cells contain a single E1, but have many E2s and multiple families of E3 enzymes. Different members of the E2 and E3 families recognize different substrate proteins and mediate different biological functions. C terminus of Hsp70-interacting protein (CHIP) is a chaperone-dependent and U-box containing E3 ligase.1 It targets the degradation of proteins critical for multiple cellular functions and signaling pathways. The roles of CHIP in physiological functions and disease initiation and progression have been extensively investigated in recent years. In this review article, we will describe the roles of CHIP function as an E3 ligase in the development and progression of multiple diseases, such as neurodegenerative diseases, inflammation, and metabolic bone diseases.
CHIP functions as an E3 ligase
E3 ubiquitin ligases, which are required for recognizing substrate proteins, comprise several structurally diverse groups in eukaryotic cells.2 One type of E3 ligases are the U-box ubiquitin ligases (UULs). UULs contain a U-box domain, which is structurally related to the RING finger.3,4 Seven UUL-encoding genes have been identified in humans.5,6 The CHIP protein (encoded by the STUB1 gene) is a chaperone-dependent E3 ubiquitin ligase1 that interacts with Hsc70, Hsp70, and Hsp90 chaperone proteins through its N-terminal tetratricopeptide repeat (TPR) domain7,8 and mediates substrate protein ubiquitination through its C-terminal U-box.1 These chaperone proteins are important for CHIP functions. CHIP-K30A, which has a mutation in its chaperone-binding domain, fails to recognize substrate proteins, suggesting that CHIP interacts with its substrates through the participation of chaperone protein.9 Although CHIP usually interacts with its substrates through a chaperone protein, it occasionally interacts with substrate proteins in a chaperone-independent manner (Table 1).10-12
Table 1.
Chaperone involvement in CHIP-mediated protein degradation
| Substrate protein | Chaperone involvement? | References |
|---|---|---|
| Tau | Yes | 37 |
| APP | Yes | 17 |
| Malin | Yes | 18 |
| Ataxin-1, ataxin-3 | Yes | 19 |
| ERBB2 | Yes | 22 |
| NIK | No | 29 |
| PKC-zeta/SRC | Yes | 21 |
| LRRK2 | Yes | 39 |
| IL-4R | Yes | 46 |
| Osterix | Yes | 47 |
| SMAD1, SMAD5 | Yes | 50 |
| RUNX2 | Yes | 10 |
| TRAF2 | Unknown | 54 |
| TRAF3 | Yes | 27 |
| TRAF5 | Unknown | 55 |
| TRAF6 | Yes | 54 |
| SIRT6 | No | 11-13 |
CHIP regulation in immunity
CHIP expression in innate and adaptive immune cells
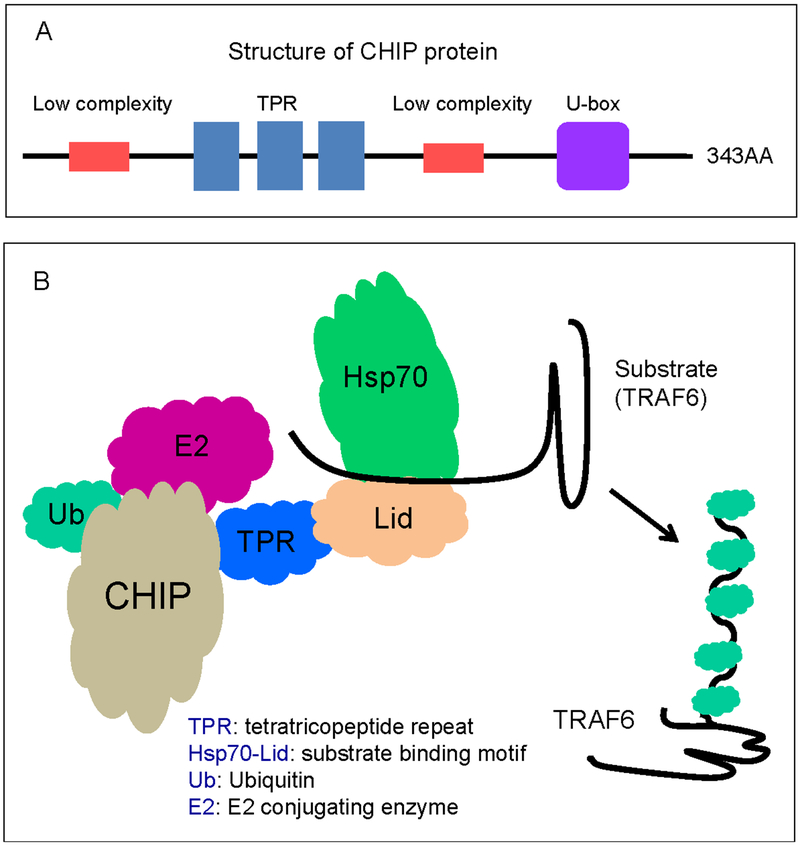
CHIP is expressed in innate and adaptive immune cells, including macrophages, CD11c+ dendritic cells (DCs), DX5+ natural killer (NK) cells, CD4+/CD8+ T cells, CD20+ B cells, etc.12 However, the exact function of CHIP in these cells is not clear. CHIP could interact directly or indirectly with its substrates (Table 1).13 The fates of CHIP substrates are partly reliant on different ubiquitination modifications mediated by different E2 enzymes.14 CHIP-mediated K63- or K27-linked polyubiquitination is mostly involved in signal transduction regulation, while CHIP-mediated K48-linked polyubiquitination usually targets proteins for proteasomal degradation.15 A model of the structure of the CHIP protein is shown in Figure 1.
Figure 1.
Structural model of carboxyl terminus of Hsp70-interacting protein (CHIP). (A) The domains of the CHIP protein structure are illustrated. (B) Illustrations of the modeling of CHIP protein interactions with other molecules involved in the ubiquitination process and substrate proteins.
CHIP regulation of NF-κB-inducing kinase
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-inducing kinase (NIK) is a central signaling molecule of the noncanonical NF-κB pathway and is an essential serine/threonine kinase for various functions of the immune system.16,17 Immunodeficiency disorders were observed in mice with an NIK loss-of-function mutation 18. NIK binds to TNF receptor-associated factor 3 (TRAF3).19 CHIP functions as a scaffolding protein to interact with Hsp70–NIK, as well as with TRAF3 through its TPR domain. The formation of the Hsp70/NIK/CHIP dimer/TRAF3 complex enhances the ubiquitination and degradation of NIK. The degradation of NIK is independent from the E3 ligase activity of CHIP and suppresses NIK-mediated non-canonical NF-κB signaling.20 It has been shown that hepatocyte-specific overexpression of CHIP in mice could inhibit NF-κB signaling and largely reverse NIK-induced liver inflammation and injury.20 These findings suggest that CHIP forms a complex with multiple proteins to induce the degradation of the substrate protein and that the degradation of this protein substrate is independent from the E3 ligase activity of CHIP.
CHIP regulation of Toll-like receptors
Toll-like receptors (TLRs) play a major role in the innate immune system by sensing invading pathogens through recognition of conserved structures in pathogens.21 TLRs can be classified into two groups according to their cellular localization. The cell surface group, containing TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11, mainly recognize and target microbial membrane components such as lipids, lipoproteins, and proteins. The other group includes TLR3, TLR7, TLR8, and TLR9, which recognize microbial nucleic acids. This latter group of TLRs is only expressed in intracellular vesicles, such as the endoplasmic reticulum, endosomes, lysosomes, and endolysosomes.22 It has been demonstrated that CHIP directs the Hsp70-mediated assembly of TLR4/9 and possibly TLR2/7 complexes, but not that of TLR3.22 CHIP binds directly to Hsp70/protein kinase C (PKC)-ζ/SRC through its TPR domain in the TLR4/9 signaling pathway. This interaction causes ubiquitination of both SRC and PKC-ζ in a K63-linked manner, leading to the activation of NF-κB signaling.22 However, the mechanisms of NF-κB activation by PKC-ζ/SRC remain to be determined. In contrast, it has been shown that CHIP/Hsp70 mediates the ubiquitination and degradation of TLR4, resulting in reduced LPS-induced NF-κB activation, IL-6 expression, and apoptosis in intestinal epithelial cells.23 It has also been shown that peptidoglycan (PGN) induces the expression and activity of CHIP in RAW264.7 cells through the TLR2/c-Jun N-terminal kinase (JNK) signaling pathway. 24 These findings suggest that CHIP could affect NF-κB signaling through its influence on TLR protein degradation.
CHIP regulation of inflammation
Because CHIP regulates immune cell function, it is not surprising that CHIP also regulates inflammation in the body. Interleukin 4 receptor (IL-4R) and its signaling play important roles in inflammation and associated diseases, such as rheumatoid arthritis (RA).25 IL-4R is a key regulator of the Th2 immune response. IL-4Rα, as a common receptor, mediates the signaling of IL-4 and IL-13. IL-4Rα interacts with the cytokine receptor common gamma chain (γc) and forms a type I IL-4 receptor, which mediates IL-4 signaling. IL-4Rα also pairs with IL-13Rα1 to form a type II IL-4 receptor, which mediates both IL-4 and IL-13 signaling pathways.26 IL-4 or IL-13 binds to IL-4 receptors and activates Janus kinases, leading to the activation of several signaling cascades. This signaling activation finally results in the binding of phosphorylated signal transducer and activator of transcription 6 (STAT6) to the promoter regions of IL-4 and IL-13 target genes.27,28 CHIP interacts with IL-4Rα and induces its ubiquitin-dependent degradation.28 These findings suggest that CHIP may affect inflammation and RA development through regulation of IL-4Rα proteasomal degradation.
Tumor necrosis factor (TNF) α is overproduced in inflamed joints leading to local erosion of bone and cartilage in patients with inflammatory arthritis. The mechanisms by which TNF-α regulates osteoblast function have not been fully defined. A recent study suggests that TNF-α inhibits osteoblast differentiation through up-regulation of CHIP expression, which promotes Osterix degradation in osteoblasts.29 Osterix is a key transcription factor for osteoblast differentiation.30 Co-immunoprecipitation (co-IP) results revealed that CHIP interacts with Osterix and induces Osterix ubiquitination. The K55 and K386 residues have been shown to be the key ubiquitination sites for the Osterix protein.29 A recent study demonstrated that CHIP regulates NF-κB signaling in bone tissues.31,32 It is known that NF-κB signaling is closely related to inflammation33; thus it is conceivable that CHIP may also regulate inflammation mediated by NF-κB signaling.
CHIP regulation of neural function
Neurodegenerative diseases, which are characterized by a massive loss of specific neurons, are progressively disabling. The accumulation of aberrant proteins, probably leading to the formation of protein aggregates, is associated with the pathology of the most frequent neurodegenerative diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD).34 To remove and abrogate the toxic misfolded proteins in neural cells, chaperones, ubiquitin ligases and proteasomes are required.35-37 Chaperones not only help to appropriately fold newly synthesized polypeptides, but also play an essential role in removal of protein aggregates either by the ubiquitin–proteasome system or by autophagy.36 Cumulative evidence has indicated that the Hsc70, Hsp70, and Hsp90 complexes play a pivotal role in proteasomal degradation of damaged proteins.34 CHIP, serving as a co-chaperone and an E3 ubiquitin ligase, is a crucial component of the Hsc70, Hsp70, and Hsp90 protein complexes responsible for the ubiquitination and degradation of proteins that play important roles in neurodegenerative disorders.38,39 Therefore, the proper function of CHIP in protein folding and degradation in neural cells should be ensured.
CHIP may function as an E3 ligase alone or form a complex with other E3 ligases, such as with parkin (PRKN), a RING-in-between-RING (RBR) ubiquitin ligase.40-42 Mutations in PRKN could lead to familial Parkinson disease. It has been reported that CHIP promotes the ubiquitination of other factors that are important in neurodegenerative diseases, such as tau, APP, malin, ataxin-1, and ataxin-3. Tau and APP play important roles in AD,36,38,39 while malin is another E3 ligase involved in Lafora disease,43 and ataxin-1 and ataxin-3 are associated with spinocerebellar ataxia types 1 and 3.44,45 These results also indicate that CHIP may play significant roles in the nervous system and the development of neurodegenerative diseases.
It has been proposed that CHIP controls protein folding homeostasis that determines whether to refold or dephosphorylate pathologic aggregates in neural cells. If CHIP is not able to function properly, the degradation process will be severely compromised and accumulation of proteins will occur because of overburdened proteasomal and lysosomal systems.34 Thus, CHIP regulates the protein quality control process and has been involved in neurological disorders featured by protein misfolding and aggregation.46,47 In disease conditions, the mechanism of how CHIP protects against neural toxicity caused by protein aggregation remains unclear. However, it has been reported that insufficiency of CHIP generates oxidative toxicity, resulting in neural defects.37 CHIP-positive tau inclusions were detected in several neurodegenerative disorders, such as AD, progressive supranuclear palsy, and Pick’s disease. In addition, CHIP was also detected in Lewy body-like hyaline inclusions in the mouse model of ALS.46 Gordon Holmes syndrome (GHS), manifested by ataxia and hypogonadism, is a rare neurodegenerative disorder. The disruption of CHIP function is known to be one of the factors causing GHS because mice harboring a mutation in the CHIP gene (Stub1) could phenocopy certain symptoms of GHS, including ataxia and hypogonadism.48,49, 37 CHIP is a central convergence point for manifold degenerative neural processes. Hence, GHS is actually a part of the phenotypic cluster of STUB1 mutations.50
CHIP regulation of bone cell function
As an E3 ligase, CHIP also regulates the degradation of multiple signaling molecules that play important roles in bone remodeling. Early in vitro studies demonstrated that CHIP regulates the degradation of multiple SMAD proteins and is involved in transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) signaling.51,52 Other in vitro studies also showed that CHIP induces runt-related transcription factor 2 (RUNX2) degradation and inhibits osteoblast differentiation.10 RUNX2 is the master transcription factor controlling osteoblast differentiation53 and SMAD1/5 are key signaling molecules in BMP signaling.54,55 However, in vivo studies using Stub1 KO mice demonstrated that CHIP had no significant effects on the steady-state protein levels of RUNX2 and SMAD1/5. Instead, CHIP promotes the degradation of multiple TRAF family members, including TRAF2, TRAF5, and TRAF6.31,32 A mutant CHIP (H260Q) lacking ubiquitination activity could not induce TRAF6 degradation.31 The consequence of CHIP-induced multiple TRAF protein degradation is the inhibition of NF-κB signaling. We found that the nuclear translocation of NF-κB subunit p65 was significantly enhanced in Stub1 KO mice.31 K48-linked polyubiquitin chain (Lys48) and K63-linked polyubiquitin chain (Lys63) play important roles in regulating the activity of the NF-κB pathway.56 It has been shown that TRAF2 and TRAF5 activate NF-κB signaling and mediate TNF-α-induced osteoclast formation.57,58 The effect of TNF-α-induced osteoclast formation was severely impaired in Traf2 or Traf5 deficient cells.57,58 Moreover, TRAF6 interacts with receptor activator of nuclear factor κ B (RANK) to activate NF-κB signaling that further regulates osteoclast formation.59-62 Osteoclast numbers were largely increased in Stub1 KO mice.57In contrast, osteoblast activity was significantly inhibited in postnatal Stub1 KO mice.31 The addition of a NF-κB inhibitor significantly reversed the osteoblast inhibitory effect caused by the deletion of Stub1 in bone marrow cells.32 These findings indicate that the bone mass reduction found in Stub1 KO mice may be attributed to the combination of increased osteoclast formation and reduced osteoblast differentiation.
Because NF-κB signaling plays an important role in regulation of bone remodeling, CHIP may also serve as a key regulator in bone remodeling. To fully understand the function of CHIP in bone remodeling and diseases related to the defects of bone remodeling, such as osteoporosis, we need to generate Stub1flox/flox mice and Stub1 conditional KO mice. To achieve this goal, we have recently generated Stub1flox/flox mice and Stub1OsxER conditional KO mice (unpublished data). With these important tools, we will be able to analyze changes in bone remodeling longitudinally and in aged mice. In addition, it has been recently demonstrated that NF-κB also plays an important role in the development of osteoarthritis (OA), 63,64 thus we could also use the Stub1 conditional KO mice to determine if they develop the OA phenotype in aged mice. The Stub1 conditional KO mouse model is a useful tool for further studies of CHIP functions, especially at the adult stage.
CHIP regulation of aging
The mechanisms of aging, characterized by progressive decline in tissue and organ function and increased risk of mortality, remain to be defined. Common hallmarks of aging have been proposed, including systemic inflammation, macromolecular damage leading to genomic instability, telomere attrition, epigenetic alteration, mitochondrial dysfunction, cellular senescence, and stem cell exhaustion.65 The aging process is characterized by structural and functional changes affecting almost all tissues and organs. A defined feature of aging is the decline in regenerative capacity associated with reduced adult stem cell function. In the late stage of adult life, the aggregation of aberrant proteins become overwhelming as a result of the age-related decline of chaperone activity and the activity of the proteasomal system.35 From the results of early studies in C. elegans and D. melanogaster, it has been speculated that molecular chaperones, like Hsp70, are directly associated with aging.66,67 In addition, considering the crucial role of CHIP in maintaining protein homeostasis in the cell, the role of CHIP in the aging process cannot be ignored. Stub1-deficient mice exhibit overloaded denatured protein, the up-regulation of aging-related biomarkers, and significantly shortened life spans, demonstrating that CHIP may play a protective role in the prevention of aging process, possibly through maintaining the quality control of CHIP-regulated proteins.68
Given the central role of CHIP in neurodegenerative disorders, several in vivo studies have been conducted to elucidate the neuroprotective role of CHIP. An aging mouse model was established by the deletion of Stub1 and the removal of CHIP causes protein aggregation in neural cells and decreased neuronal survival. In contrast, the overexpression of Stub1 attenuated an early aging phenotype.34 The expression and toxicity of leucine-rich repeat kinase 2 (LRRK2), an aging-related molecule, were upregulated in the Stub1 KO mouse model of PD, while the overexpression of Stub1 offered protection from the toxicity of mutant LRRK2.69,34
Sirtuin 6 (SIRT6) is a stress responsive protein deacetylase and mono-ADP ribosyltransferase enzyme encoded by the SIRT6 gene.70 SIRT6 functions are involved in multiple molecular pathways related to aging, including DNA repair, telomere maintenance, glycolysis, and inflammation.70 CHIP stabilizes SIRT6 protein by preventing its proteasomal degradation.11 In Stub1-deficient cells, the half-life of SIRT6 protein is substantially reduced due to increased proteasomal degradation. SIRT6 interacts with a CHIP lacking the TPR domain (∆TPR CHIP) and with a CHIP mutant that has an inactivating point mutation in the U-box domain (H260Q CHIP), but not with a CHIP mutant lacking the entire U-box domain (∆U-box CHIP). These findings demonstrate that interaction of CHIP with SIRT6 is not chaperone-dependent, but requires the U-box domain of CHIP. SIRT6 K170 mediates susceptibility to protein degradation in the absence of CHIP. These results suggest that another E3 ligase may be involved in SIRT6 ubiquitination.11 One of the potential mechanisms for CHIP regulation of the aging process may be through maintaining SIRT6 protein stability, allowing SIRT6 to participate in histone deacetylation and DNA repair activities.
One feature of aging is a progressive decline in protein homeostasis (proteostasis), aggravating the risk for protein aggregation diseases. A recent study also demonstrated that CHIP targets insulin receptor and induces its degradation and controls insulin receptor turnover. The outcome of this regulatory mechanism is to inhibit insulin signaling, leading to an anti-aging effect.71,72 Since it is well known that insulin receptor levels are linked to insulin and IGF1 signaling and longevity, the study also identified CHIP acting as a potential molecular target for aging regulation. Proteotoxic stress promotes insulin receptor stability and inhibits insulin receptor turnover, and drives the aging process and shortens lifespan. CHIP may possess anti-aging activity by counteracting proteotoxic stress.
Summary
CHIP is an E3 ligase that regulates the stability and functions of multiple proteins in different cell types. It has been demonstrated that CHIP plays critical roles in neurodegeneration, immunity, inflammation, and bone remodeling and aging. However, the detailed molecular mechanisms of CHIP in multiple cell functions need to be further investigated. In addition, the tissue-specific effects of CHIP and the role of CHIP in aging also need to be further studied using tissue-specific and inducible Stub1 KO mice.
Acknowledgments
This work was supported by National Institutes of Health Grants R01AR054465 and R01AR070222 to D.C. This work was also partially supported by National Natural Science Foundation of China (grant no. 81874011, 81572104 and 81301531) to T.W.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.McDonough H, & Patterson C. 2003. CHIP: a link between the chaperone and proteasome systems. Cell. Stress. Chaperones. 8: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz AL & Ciechanover A. 2009. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Ann. Rev. Pharmacol. Toxicol. 49: 73–96. [DOI] [PubMed] [Google Scholar]
- 3.Aravind L & Koonin EV. 2000. The U box is a modified RING finger-a common domain in ubiquitination. Curr. Biol. 10: R132–134. [DOI] [PubMed] [Google Scholar]
- 4.Ohi MD, Vander KCW, Rosenberg JA, et al. 2003. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 10:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatakeyama S, Yada M, Matsumoto M, et al. 2001. U box proteins as a new family of ubiquitin-protein ligases. J. Biol. Chem. 276: 33111–33120. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Qian W, Qian Y, et al. 2009. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2: ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballinger CA, Connell P, Wu Y, et al. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19: 4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connell P, Ballinger CA, Jiang J, et al. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3: 93–96. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Huang M, Zheng H, et al. 2008. CHIP promotes Runx2 degradation and negatively regulates osteoblast differentiation. J. Cell Biol. 181: 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronnebaum SM, Wu Y, McDonough H, et al. 2013. The ubiquitin ligase CHIP prevents SirT6 degradation through noncanonical ubiquitination. Mol. Cell. Biol. 33: 4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp Y, Lang W-H, Schuster TB, et al. 2017. CHIP as a membrane-shuttling proteostasis sensor. eLife 6: e29388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SF, Deb S, Paul I, et al. 2012. The Chaperone-assisted E3 Ligase C Terminus of Hsc70-interacting Protein (CHIP) Targets PTEN for Proteasomal Degradation. J Biol Chem 287: 15996–16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Marcu M, Yuan X, et al. 2002. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci USA 99: 12847–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yau R & Rape M. 2016. The increasing complexity of the ubiquitin code. Nat. Cell. Biol. 18: 579–586. [DOI] [PubMed] [Google Scholar]
- 15.Edkins AL 2015. CHIP: a co-chaperone for degradation by the proteasome. Sub-Cell. Biochem. 78: 219–242. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Wu L, Wesche H, et al. 2001. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291: 2162–2165. [DOI] [PubMed] [Google Scholar]
- 17.Sun SC 2011. Non-canonical NF-κB signaling pathway. Cell Res. 21: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willmann KL, Klaver S, Dogu F, et al. 2014. Biallelic loss-offunction mutation in NIK causes a primary immunodeficiency with multifaceted aberrant lymphoid immunity. Nat. Commun. 5: 5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarnegar BJ, Wang Y, Mahoney DJ, et al. 2008. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9: 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang B, Shen H, Chen Z, et al. 2015. Carboxyl terminus of HSC70-interacting protein (CHIP) down-regulates NF-κB-inducing kinase (NIK) and suppresses NIK induced liver injury. J. Biol. Chem. 290: 11704–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T & Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Wang C, Zhu X, et al. 2011. E3 ubiquitin ligase CHIP facilitates Toll-like receptor signaling by recruiting and polyubiquitinating Src and atypical PKCzeta. J. Experimen. Med. 208: 2099–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afrazi A, Sodhi CP, Good M, et al. 2012. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J. Immunol. 188: 4543–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Y, Chen C, Wang L, et al. 2013. Toll-like receptor-2 ligand peptidoglycan upregulates expression and ubiquitin ligase activity of CHIP through JNK pathway. Cell Physiol Biochem: Int. J. Experimen. Cell Physiol. Biochem. Pharmacol. 32: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 25.Burgos PI, Causey ZL, Tamhane A, et al. 2010. Association of IL4R single-nucleotide polymorphisms with rheumatoid nodules in African Americans with rheumatoid arthritis. Arthritis Res Ther 12:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuperman DA & Schleimer RP. 2008. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr. Mol. Med. 8: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh CK, Geba GP, & Molfino N. 2010. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur. Respir. Rev. 19: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadava K, & Marsland BJ. 2014. IL-4Ra, a STUB-strate for Proteasomal Degradation: Understanding the Termination of Cytokine Signaling in Asthma. Am J Respir Crit Care Med 189(1):4–6. [DOI] [PubMed] [Google Scholar]
- 29.Xie J, & Gu J. 2015. Identification of C-terminal Hsp70-interacting protein as a mediator of tumour necrosis factor action in osteoblast differentiation by targeting Osterix for degradation. J. Cell. Mol. Med. 19: 1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakashima K, Zhou X, Kunkel G, et al. 2002. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Shu B, Zhang Y, et al. 2014. CHIP regulates osteoclast formation through promoting TRAF6 protein degradation. Arthritis Rheumatol. 66: 1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Li S, Yi D, et al. 2018. CHIP regulates bone mass by targeting multiple TRAF family members in bone marrow stromal cells. Bone Res. 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Zhang L, Joo D, and Sun S-C. 2017. NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickey CA, Patterson C, Dickson D, et al. 2007. Brain CHIP: removing the culprits in neurodegenerative disease. Trends Mol. Med. 13: 32–38. [DOI] [PubMed] [Google Scholar]
- 35.Joshi V, Amanullah A, Upadhyay A, et al. 2016. A decade of boon or burden: what has the CHIP ever done for cellular protein quality control mechanism implicated in neurodegeneration and aging? Front. Mol. Neurosci. 9: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanack AJ, Newsom OJ, & Scaglione KM. 2018. Most mutations that cause spinocerebellar ataxia autosomal recessive type 16 (SCAR16) destabilize the protein quality-control E3 ligase CHIP. J. Biol. Chem. 293: 2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrucelli L, Dickson D, Kehoe K, et al. 2004. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 13: 703–714. [DOI] [PubMed] [Google Scholar]
- 38.Kalia LV, Kalia SK, Chau H, et al. 2011. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5). PLoS. One 6: e14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar P, Ambasta RK, Veereshwarayya V, et al. 2007. CHIP and HSPs interact with β-APP in a proteasome-dependent manner and influence Aβ metabolism. Hum. Mol. Genet. 16: 848–864. [DOI] [PubMed] [Google Scholar]
- 40.Jiang J, Ballinger CA, Wu Y, et al. 2001. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 276: 42938–42944. [DOI] [PubMed] [Google Scholar]
- 41.Murata S, Minami Y, Minami M, et al. 2001. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Soda M, Hatakeyama S, et al. 2002. CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Mol. Cell 10: 55–67. [DOI] [PubMed] [Google Scholar]
- 43.Shimura H, Schwartz D, Gygi SP, et al. 2004. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J. Biol. Chem. 279: 4869–4876. [DOI] [PubMed] [Google Scholar]
- 44.Rao SN, Sharma J, Maity R, et al. 2010. Co-chaperone CHIP stabilizes aggregate-prone malin, a ubiquitin ligase mutated in Lafora disease. J. Biol. Chem. 285: 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi JY, Ryu JH, Kim HS, et al. 2007. Co-chaperone CHIP promotes aggregation of ataxin-1. Mol. Cell. Neurosci. 34: 69–79. [DOI] [PubMed] [Google Scholar]
- 46.Williams AJ, Knutson TM, Colomer GVF, et al. 2009. In vivo suppression of polyglutamine neurotoxicity by C-terminus of Hsp70-interacting protein (CHIP) supports an aggregation model of pathogenesis. Neurobiol. Dis. 33: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahara N, Murayama M, Mizoroki T, et al. 2005. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 94: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 48.Al-Ramahi I, Lam YC, Chen HK, et al. 2006. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 281: 26714–26724. [DOI] [PubMed] [Google Scholar]
- 49.Schuster S, Schelling Y, Synofzik M, et al. 2018. Establishment of STUB1/CHIP mutant induced pluripotent stem cells (iPSCs) from a patient with Gordon Holmes syndrome/SCAR16. Stem Cell Res. 29: 166–169. [DOI] [PubMed] [Google Scholar]
- 50.Shi CH, Schisler JC, Rubel CE, et al. 2014. Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum. Mol. Genet. 23: 1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayer SN, Deconinck T, Bender B, et al. 2017. STUB1/CHIP mutations cause Gordon Holmes syndrome as part of a widespread multisystemic neurodegeneration: evidence from four novel mutations. Orphanet. J. Rare Dis. 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Xin H, Xu X, et al. 2004. CHIP mediates degradation of Smad proteins and potentially regulates Smad-induced transcription. Mol. Cell. Biol. 24: 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Liu Y, Hao R, et al. 2011. Molecular mechanism of the negative regulation of Smad1/5 protein by carboxyl terminus of Hsc70-interacting protein (CHIP). J. Biol. Chem. 286: 15883–15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroeder TM, Jensen ED, & Westendorf JJ. 2005. Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res (Part C) 75: 213–225. [DOI] [PubMed] [Google Scholar]
- 55.Chen D, Zhao M, and & Mundy GR. 2004. Bone morphogenetic proteins. Growth Factors 22: 233–241. [DOI] [PubMed] [Google Scholar]
- 56.Hill CS 2016. Transcriptional Control by the SMADs. Cold Spring Harb Perspect Biol 8: a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohtake F, Saeki Y, Ishido S, et al. 2016. The K48-K63 branched ubiquitin chain regulates NF-κB signaling. Mol Cell 64: 251–266. [DOI] [PubMed] [Google Scholar]
- 58.Kanazawa K & Kudo A. 2005. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J. Bone Miner. Res. 20: 840–847. [DOI] [PubMed] [Google Scholar]
- 59.Kanazawa K, Azuma Y, Nakano H, et al. 2003. TRAF5 functions in both RANKL- and TNFα-induced osteoclastogenesis. J. Bone Miner. Res. 18: 443–450. [DOI] [PubMed] [Google Scholar]
- 60.Teitelbaum SL & Ross FP. 2003. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4: 638–649. [DOI] [PubMed] [Google Scholar]
- 61.Ye H, Arron JR, Lamothe B, et al. 2002. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418: 443–447. [DOI] [PubMed] [Google Scholar]
- 62.Kadono Y, Okada F, Perchonock C, et al. 2005. Strength of TRAF6 signalling determines osteoclastogenesis. EMBO Rep. 6: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh MC & Choi Y. 2003. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 14: 251–263. [DOI] [PubMed] [Google Scholar]
- 64.Marcu KB, Otero M, Olivotto E, et al. 2010. NF-κB signaling: multiple angles to target OA. Curr. Drug Targets 11: 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang SH, Mori D, Kobayashi H, et al. 2019. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat Commun 10.1038/s41467-019-09491-5 [DOI] [PMC free article] [PubMed]
- 66.Lopez-Otın C, Blasco MA, Partridge L, et al. 2013. The hallmarks of aging. Cell 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esser C, Scheffner M, & Hohfeld J. 2005. The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J. Biol. Chem. 280: 27443–27448. [DOI] [PubMed] [Google Scholar]
- 68.Strahler JR, Kuick R, and Hanash SM. 1991. Diminished phosphorylation of a heat shock protein (HSP 27) in infant acute lymphoblastic leukemia. Biochem. Biophys. Res. Commun. 175: 134–142. [DOI] [PubMed] [Google Scholar]
- 69.Min JN, Whaley RA, Sharpless NE, et al. 2008. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol. Cell. Biol. 28: 4018–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ko HS, Baileyg R, Smithe WW, et al. 2009. CHIP regulates leucine-rich repeat kinase-2 ubiquitination, degradation, and toxicity. Proc. Natl. Acad. Sci. USA 106(8): 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frye RA 2000. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Co. 273: 793–98. [DOI] [PubMed] [Google Scholar]
- 72.Tawo R, Pokrzywa W, Kevei E, et al. 2017. The ubiquitin ligase CHIP integrates proteostasis and aging by regulation of insulin receptor turnover. Cell 169: 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Branicky R and Hekimi S. 2017. Proteostasis or aging: Let the CHIPs fall where they may. Developmental Cell 41: 126–127. [DOI] [PubMed] [Google Scholar]