Introduction
Lupus nephritis (LN) is a severe manifestation of systemic lupus erythematosus (SLE), leading to end-stage renal disease (ESRD) in 30% of patients, and is the most important predictor of SLE mortality. Although survival of patients with LN has improved significantly over the last few decades, the incidence of ESRD from LN which stabilized in the 1990s may now be rising, reflecting limits in effective management (1). One unmet need in LN management is an accurate, noninvasive method to assess renal disease activity and response to therapeutic interventions. While renal biopsy remains the gold standard for initial LN diagnosis, its invasiveness and inherent risks make it impractical to perform frequently and serially. Thus, there is a need to develop an accurate and noninvasive method to rapidly evaluate renal disease activity to inform immunosuppressive therapy.
The choroid and the kidney are “parallel” organs with similar vascular design and systemic exposures. Both the choroid and the glomerulus have fenestrated capillaries containing α3–5 type IV collagen, and are organized in lobules (2). Clinical choroidopathy is thought to be due to immune complex deposition, inflammatory cell infiltration, vascular leakage, and subsequent choroidal thickening (3). Histopathologically, similar findings are observed in proliferative LN with immunoglobulin/complement deposition, active inflammation, and subsequent glomerular injury (4). While clinical choroidopathy causing visual symptoms, is infrequent, patients with LN have high rates of subclinical choroidopathy compared to lupus patients without nephritis (5). Hyperfluorescence patterns similar to those seen in other macular diseases on indocyanine green angiography were observed in 100% of patients with LN but in none of the SLE patients without nephritis in one study (5).
Recently, it was shown that LN in remission was associated with increased choroidal thickness compared with non-LN SLE patients in remission (6). However, the degree of renal remission (partial vs. complete) was not characterized. Partial renal remission (i.e. proteinuria > 500 mg/day or creatinine above baseline) in the chronic setting could represent a heterogeneous group of patients with active renal disease or chronic renal damage, which could manifest differently in the choroid compared to the group of patients with complete renal remission. Thus, we specifically examined only those LN patients with complete renal remission. We hypothesized that choroidal thickness would not differ between patients in complete LN remission compared to non-LN SLE patients in remission.
Materials and Methods
Study population
This was a retrospective analysis of patients followed at the Washington University School of Medicine (WUSM) Lupus Clinic or identified through the WUSM Nephrology Clinic’s biopsy database. All patients met SLE classification using either the American College of Rheumatology (7) and/or Systemic Lupus International Collaborating Clinics (8). Patients with LN had biopsy-proven Renal Pathology Society/International Society of Nephrology class III, IV, or V lupus nephritis (9). This study was approved by the Washington University School of Medicine Institutional Review Board.
Chart review was conducted to identify SLE patients who received a comprehensive eye exam including OCT in the WUSM ophthalmology clinics between January 1, 2012 and December 31, 2018. Patients were excluded if they had conditions that could impact choroidal thickness: spherical equivalent > 6 or < −6 diopters, retinal disease, uveitis, pregnancy, or chronic renal disease stage 3 or greater (10). Spherical equivalent is an estimate of the refractive error of the eye, and was calculated from medical refraction as sphere + ½ cyl in diopter power.
Medical records pertaining to rheumatology and nephrology visits were reviewed before and after OCT imaging for evidence of clinical lupus nephritis activity and extra-renal flare. Complete renal remission was defined as urine protein < 0.5 g/day and return of serum creatinine to previous baseline. Flare was defined by Fortin criteria: 1) new or increased prednisone dose > 20 mg/day, 2) new or increased immunosuppression treatment, or 3) hospitalization or death from SLE (11).
Imaging
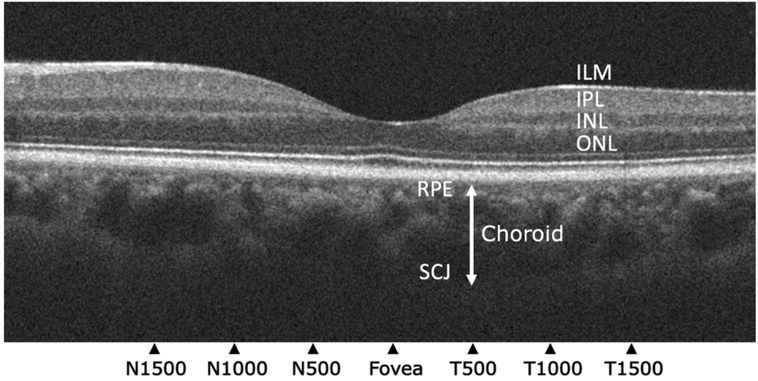
OCT images were collected using a Cirrus SD-OCT platform (Zeiss, Germany). Choroidal thickness was measured on high definition images through the fovea using Photoshop CS6 (Adobe Inc., San Jose, CA) in a method modified from that of Gupta et al (9). Image quality and measurements were assessed by two trained reviewers masked to group assignment. Choroidal thickness, defined as the linear distance between the retinal pigment epithelial band and the posterior sclerochoroidal junction (12), was measured at seven locations: 1500 μm, 1000 μm, and 500 μm nasal and temporal to the fovea, and underneath the foveal center [insert Figure 1.]. Only OCT images that clearly delineated the interface between the retinal pigment epithelium and posterior sclerochoroidal junction were included in analyses. Choroidal thickness measurement had an intra-rater reliability = 0.982 and interrater reliability = 0.96 after adjudication of measurements with a > 15% difference.
Figure 1.
Optical coherence tomography of the retina and choroid. Choroidal thickness was measured from the retinal pigment epithelium (RPE) to the sclerochoroidal junction (SCJ; white double-headed arrow). These locations were 1500 μm, 1000 μm, and 500 μm nasal and temporal to the fovea, and underneath the foveal center. Representative layers of the retina: ILM = Internal Limiting Membrane; IPL = Inner Plexiform Layer; INL = Inner Nuclear Layer; ONL = Outer Nuclear Layer (12).
Statistical analysis
SPSS 19 (IBM Corporation, 1989, 2010) was used for statistical analyses. For unadjusted comparisons, statistical significance was determined by independent Student’s t-test for continuous and normally distributed outcome variables, and Mann-Whitney U test for continuous and nonparametric outcome variables. Normality was tested using the Kolmogorov-Smirnov Z test. A linear mixed model was used to adjust for paired measurements with choroidal thickness at the above defined points as the outcome of interest. Known predictors of outcome including age, time of scan (dichotomized into morning and afternoon scans) (13), and spherical equivalent were entered into models using forced entry. p-value < 0.05 was considered statistically significant.
Results
The study cohort consisted of 23 SLE patients. There were 11 non-LN SLE patients and 12 LN patients. Of the LN patients, 10 (71%) had class III or IV nephritis, or mixed subtype with class III or IV and class V nephritis, and four (29%) had pure class V nephritis.
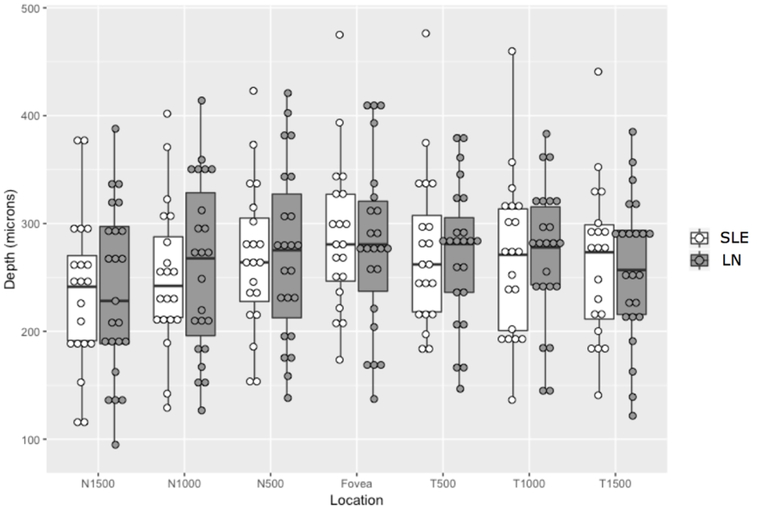
In the group of patients with LN (Table 1), all subjects were in complete renal remission (median spot protein/creatinine ratio of 0.2 [IQR 0.1–0.3], median serum creatinine 0.84 [IQR 0.71–1.04]). All non-LN SLE patients had urine spot protein/creatinine ratio of 0.1 and median serum creatinine was 0.83 (IQR 0.62–0.93). There was no difference in choroidal thickness between patients with LN in complete renal remission compared with SLE patients without LN at any of the seven measured locations through the fovea [insert Figure 2.] (Table 2). Thus, in the absence of extra-renal disease activity and renal activity, subjects with LN had equivalent CT choroidal measurements compared to those without LN.
Table 1.
Demographics and Characteristics of non-LN SLE and LN patients at the time of OCT scan
| Non-LN SLE (n=11) | LN (n=12)1 | |
|---|---|---|
| Clinical Characteristics | ||
| Female (%) | 11 (100) | 10 (83) |
| African-American (%) | 6 (54) | 9 (75) |
| Age at scan (yrs), mean (SD) | 47 (12) | 34 (8.8) |
| Lupus duration at scan (yrs), median (IQR) | 5.5 (1.7–21.8)2 | 9.0 (7.3–13.0) |
| Lupus nephritis duration at scan (yrs), median (IQR) | - | 8.5 (6.0–10.8) |
| Spot protein/creatinine ratio3 (median; IQR) | 0.1 | 0.2 (0.1–0.3) |
| Serum creatinine (median; IQR) | 0.83 (0.62–0.93) | 0.84 (0.71–1.04) |
| Any steroids at scan (%) | 4 (36) | 3 (25) |
| Scan Characteristics | ||
| Afternoon scan | 6 (55) | 7 (58) |
| Spherical equivalent (median; IQR) | −0.25 (−2.25–[−0.25]) | −1.6 (−4.75–[−1.6]) |
| # eyes scanned | 20 | 23 |
yrs, years; SD, standard deviation; IQR, interquartile range.
All patients with LN were in complete renal remission
Year of onset was not available for one patient
Figure 2.
Choroidal thickness of lupus (SLE) and lupus nephritis (LN) patients in complete clinical remission. There was no difference between choroidal thickness in SLE and LN patients in complete clinical remission.
Table 2.
Mean choroidal thickness (μm (SD)) of non-LN SLE and LN patients at seven locations
| Measurement Location | Non-LN SLE1 | LN | p-value |
|---|---|---|---|
| N1500 | 236 (72) | 237 (77) | 0.991 |
| N1000 | 251 (68) | 260 (79) | 0.687 |
| N500 | 268 (69) | 272 (79) | 0.862 |
| Fovea | 288 (70) | 281 (78) | 0.766 |
| T500 | 274 (72) | 271 (64) | 0.859 |
| T1000 | 268 (74) | 271 (63) | 0.889 |
| T1500 | 263 (71) | 255 (67) | 0.718 |
N, nasal to the fovea; T, temporal to the fovea.
mean (μm, SD)
Discussion
SLE is an autoimmune disease that may affect multiple organs, including the kidney. While lupus nephritis is the most important predictor of mortality, involvement of other organs during lupus flares also results in morbidity (14). In lupus nephritis, kidney biopsy has been the gold standard for diagnosis and prognosis of disease, but frequent repeat biopsies are not often done due to the invasiveness of the procedure. Clinical biomarkers including urine protein and serum creatinine are often used to follow LN activity, but clinical remission may be discordant with histologic remission in 30% of cases (15). Thus, major unmet needs in the clinical care of SLE patients are noninvasive and accurate methods for assessing LN. This study comprises preliminary work to assess the association between choroidal thickness and lupus nephritis.
We found that LN in complete renal remission was not associated with an increase in choroidal thickness compared to lupus patients without a history of LN. While Braga et al observed that the choroid was thicker in LN in remission compared to non-LN SLE patients in remission (6), the clinical characteristics of the study cohorts are dissimilar. Braga et al did not define renal and extra-renal inactivity or remission. In addition, our LN group had a lower proportion of class III/IV and higher proportion of class V LN (71% and 29% vs 93% and 7%, respectively), and included only patients who were in complete renal remission with 24-hour urine protein < 500 mg and serum creatinine at baseline. Thus, the findings of these two studies may not be contradictory, but rather represent a spectrum of subclinical choroidal changes in different groups of LN patients. Supporting this theory is a case report of a patient with LN and clinical choroidopathy in whom achievement of complete remission led to resolution of both clinical choroidopathy and a reduction in choroidal thickness (16).
The strengths of our study include the biopsy documentation of lupus nephritis and the examination of only patients who were in complete renal remission and without extra-renal flare. Our main limitations are small sample size and retrospective design, which precluded using standardized clinical examinations performed synchronously with OCT scanning, and standardized validated instruments to measure lupus activity.
In summary, our findings add to existing data on the potential association between choroidal thickness and LN. Larger, prospective studies are required to better characterize this relationship and explore putative mechanisms underlying the variation in choroidal thickness in SLE patients, including the role of immune complex deposition, and endothelial damage.
References
- 1.Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971–2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol. 2016;68(6):1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan SJ. Retina. 5th ed. London: Saunders/Elsevier; 2013. [Google Scholar]
- 3.Aronson AJ, Ordonez NG, Diddie KR, Ernest JT. Immune-complex deposition in the eye in systemic lupus erythematosus. Arch Intern Med. 1979;139(11):1312–3. [PubMed] [Google Scholar]
- 4.Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med. 2009;133(2):233–48. [DOI] [PubMed] [Google Scholar]
- 5.Baglio V, Gharbiya M, Balacco-Gabrieli C, Mascaro T, Gangemi C, Di Franco M, et al. Choroidopathy in patients with systemic lupus erythematosus with or without nephropathy. J Nephrol. 2011;24(4):522–9. [DOI] [PubMed] [Google Scholar]
- 6.Braga J, Rothwell R, Oliveira M, Rodrigues D, Fonseca S, Varandas R, et al. Choroid thickness profile in patients with lupus nephritis. Lupus. 2019;28(4):475–82. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 8.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–50. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Sidhartha E, Girard MJ, Mari JM, Wong TY, Cheng CY. A simplified method to measure choroidal thickness using adaptive compensation in enhanced depth imaging optical coherence tomography. PLoS One. 2014;9(5):e96661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortin PR, Ferland D, Moore AD, Belisle P, Joseph L, Clarke AE. Rates and predictors of lupus flares. Arthritis Rheum. 1998;41(9):S218. [Google Scholar]
- 12.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography P. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121(8):1572–8. [DOI] [PubMed] [Google Scholar]
- 13.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–6. [DOI] [PubMed] [Google Scholar]
- 14.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health-related quality of life in patients with systemic lupus erythematosus. J Rheumatol. 2010;37(3):568–73. [DOI] [PubMed] [Google Scholar]
- 15.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. 2017;32(8):1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes M, Dias-Santos A, Gois M, Domingues I, Proença R, Francisca Moraes-Fontes M. Bilateral Choroidopathy, Nephritis and Hypertension in Systemic Lupus Erythematosus. J Clin Exp Opthamol. 2018;9:729. [Google Scholar]