Abstract
Background
Venous thromboembolism (VTE) is the most common preventable cause of 30-day post-operative mortality, with many events occurring after hospital discharge. High-level evidence supports post-discharge VTE chemoprophylaxis following abdominal/pelvic cancer resection; however, some studies support a more tailored approach. Our objectives were to (1) identify risk factors associated with post-discharge VTE in a large cohort of patients undergoing colorectal cancer resection and (2) develop a post-discharge VTE risk calculator.
Methods
Patients who underwent colorectal cancer resection from 2012 to 2016 were identified from ACS NSQIP colectomy and proctectomy procedure–targeted modules. Multivariable logistic regression was used to identify factors associated with post-discharge VTE. Incorporating pre-operative, intra-operative, and post-operative variables, a post-discharge VTE risk calculator was constructed and validated.
Results
Of 51,139 patients, 387 (0.76%) developed post-discharge VTE. Pre-operative factors associated with post-discharge VTE included BMI (e.g., morbidly obese OR 2.27, 95% CI 1.65–3.12 vs. normal BMI), and thrombocytosis (OR 1.41, 95% CI 1.03–1.92). Intra-operative factors included operative time (4–6 h OR 1.56, 95% CI 1.12–2.17; > 6 h, OR 1.85, 95% CI 1.21–2.84, vs. < 2 h), and type of operation (e.g., open partial colectomy OR 1.67, 95% CI 1.30–2.16 vs. laparoscopic partial colectomy). Post-operative factors included anastomotic leak (OR 2.05, 95% CI 1.31–3.21) and post-operative ileus (OR 1.39, 95% CI 1.07–1.79). Using the risk calculator, the predicted probability of post-discharge VTE ranged from 0.04 to 10.29%. On a 10-fold cross validation, the calculator’s mean C-Statistic was 0.65.
Conclusions
Patient-specific factors are associated with varying rates of post-discharge VTE. We present the first post-discharge VTE risk calculator designed for use at the time of discharge following colorectal cancer resection.
Keywords: Colorectal cancer, Venous thromboembolism, Post-discharge VTE, ACS NSQIP, VTE risk calculator
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolus (PE), is the number one preventable cause of post-operative mortality, for which patients with intraabdominal malignancies have increased risk.1–3 Following abdominal and pelvic cancer surgery, patients are at risk both immediately after resection during the inpatient recovery period, and after they are discharged from the hospital.4 In colorectal cancer specifically, previous studies have revealed that at least 30% of VTE events following cancer resection occur after discharge from the hospital.5–7 Evidence from randomized controlled trials supports the benefit of 28 days of chemoprophylaxis following major abdominal or pelvic resection for cancer to mitigate this risk of VTE.8–10 As such, clinical practice guidelines, including those put forward by the American College of Chest Physicians, the American Society of Colon and Rectal Surgeons as well as the American Society of Clinical Oncology, recommend post-discharge VTE chemoprophylaxis with low molecular weight heparin.11–13 However, uptake of these recommendations is relatively low, with only 1.5% of Medicare beneficiaries undergoing colorectal cancer resection receiving post-discharge chemoprophylaxis.14
A potential explanation for low post-discharge VTE chemoprophylaxis adherence rates could be based upon prior work that has suggested that not all patients may benefit from post-discharge chemoprophylaxis, and targeted chemoprophylaxis prescribing may be warranted.15, 16 Individual patient factors are associated with increased VTE risk after undergoing surgical resection of intraabdominal malignancies, including race, body mass index (BMI), and comorbidities, for example.17–20 In addition, surgical approach (e.g., minimally invasive vs. open), operative time, and post-operative complications have been shown to be independently associated with VTEs.16, 21 Previous studies of patients undergoing colorectal resection utilizing the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database identified risk factors associated with post-operative VTE, and even post-discharge VTE.5, 6, 16, 22 However, these analyses were not specific to post-discharge VTE events in patients with malignancy, and they did not provide an accessible means of evaluating an individual patient’s risk of post-discharge events.
Therefore, an important knowledge gap remains, as providers are aware that patient-specific factors can impact an individual patient’s risk of developing a post-discharge VTE, but no tools specific to the post-operative period exist to quantify post-discharge VTE risk following colorectal cancer resection. Thus, the objectives of this study were (1) to identify pre-operative, intra-operative, and post-operative factors associated with post-discharge VTE in a large cohort of patients following colorectal cancer resection and (2) to develop and validate a post-discharge VTE risk calculator to be used at the time of discharge to quantify post-discharge VTE risk.
Materials and Methods
Data Source and Patient Population
All patients who underwent colorectal resection between January 1, 2012, and December 31, 2016, were identified from the ACS NSQIP colectomy and proctectomy procedure-targeted participant use datasets. ACS NSQIP is a validated, prospectively maintained surgical registry that captures patients undergoing surgical resection at participating hospitals. Over 150 patient variables including patient demographics, comorbidities, operative information, and 30-day post-operative complications are collected by trained nurse abstractors, as previously described.23 All patients are followed for 30 days after surgery or to death, whichever occurs first, and high-quality data collection is ensured via audits that have shown excellent inter-rater reliability.24, 25 As of 2012, hospitals have been able to voluntarily participate in procedure-targeted modules, including colectomy and proctectomy. These procedure-specific data contain additional variables such as operative indication, chemotherapy use, and post-operative complications such as anastomotic leak and post-operative ileus.
CPT codes considered for analysis are listed in Supplemental Table 1. Patients were excluded from analysis if they underwent colorectal resection for any indication other than malignancy (n = 50,713), suffered an inpatient VTE prior to discharge from the index hospitalization (n = 634), remained in the hospital for 30 days or longer following surgical resection (n = 608), or suffered inpatient death (n = 637). These exclusion criteria were chosen so the study cohort only included patients who would be considered at risk for a post-discharge VTE event. Based on these exclusion criteria, 1879 patients with colorectal cancer were excluded from analysis.
Primary Outcome and Predictors
The primary outcome was clinically significant post-discharge VTE, as defined by PE or DVT diagnosed after discharge from the index hospitalization, but within 30 days of surgical resection. Possible predictors of post-discharge VTE were identified a priori based on a literature review or clinical face validity, and were categorized as pre-operative, intra-operative, or post-operative in nature.
Key pre-operative variables included age, sex, race, BMI, functional status, American Society of Anesthesiologists (ASA) classification, pre-operative albumin, pre-operative platelet count, a number of patient comorbidities including bleeding disorder, dyspnea, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), diabetes, steroid use, weight loss, and disseminated cancer, primary tumor location, and neoadjuvant therapy. Patient were considered to have received neoadjuvant therapy if they received either chemotherapy or radiation therapy within 90 days of surgical resection. Intra-operative variables included emergency case classification, operative time, operative approach, and operation. Post-operative variables included inpatient diagnosis of anastomotic leak, post-operative ileus, skin/soft tissue infection, pneumonia, reintubation, urinary tract infection (UTI), transfusion, and reoperation, as well as hospital length of stay. Pathologic features were not included for analysis, as this information may not be available to providers at the time of discharge from the index hospitalization following colorectal cancer resection.
Only complications diagnosed during the inpatient hospitalization (e.g., occurring on or prior to the day of discharge) were included for analysis. For anastomotic leak and post-operative ileus, day to event data was not available. These complications were considered inpatient events if there were no documented readmissions for a corresponding diagnosis based on ICD-9 or ICD-10 codes. For example, if a patient experienced an anastomotic leak and was also readmitted for an anastomotic leak, this complication was not considered to have occurred during the index hospitalization, and thus was not included as a risk factor for analysis.
Statistical Analysis
Bivariate analyses of individual pre-operative, intra-operative, and post-operative variables were assessed for association with post-discharge VTE events using separate chi-squared tests for categorical variables and t tests for continuous variables. Statistically significant predictors with a pre-determined P < 0.05 were entered into a multivariable logistic regression model. Collinear predictors were removed from the model. The Wald test was used to determine if categorical variable constructs significantly contributed to the estimated regression model. Those constructs with P > 0.05 on the Wald test were progressively removed from the regression model, until a final model was estimated. Model fit was evaluated for discrimination using the C-statistic, with a value closer to 1.0 indicating better discrimination.26 Model calibration was assessed using the Hosmer and Lemeshow (HL) chi-square, with any chi-square with P < 0.05 indicating poor calibration.27 Internal validation of the calculator was performed using 10-fold cross validation over 20 iterations, with the resultant C-statistics averaged over each iteration.28 In this technique, internal validation is evaluated by splitting the dataset into 10 equally sized cohorts. The model is estimated on 9 of the cohorts, and validated on one cohort, and then repeated such that each cohort is used for validation one time. The entire process is repeated 20 times, and the resultant model diagnostics averaged.
The same regression model was estimated on the logit scale, with resultant beta coefficients used to generate a risk calculator. By indicating which of the predictors an individual patient has, the corresponding beta coefficients are summed with the model intercept to generate the log odds of the probability (LP) of the outcome for that patient. Predicted probabilities can be calculated by exponentiating the LP using the following equation: probability of event = exp.(LP) / [1 + exp.(LP)], as previously described.29–33 Predicted probabilities were calculated for the entire cohort and plotted to evaluate for variation in the cohort. All statistical analyses were completed using Stata version 14.2 (Stata Corp). This study utilized deidentified data and thus was determined to be exempt from review by the Northwestern University Institutional Review Board.
Results
Characteristics of the Cohort
During the study period, 53,018 patients underwent colorectal resection for cancer, of which 1021 (1.93%) developed a VTE overall. 51,139 patients met the study inclusion criteria, of which 282 (0.55%) developed a post-discharge DVT, 151 (0.30%) developed a post-discharge PE, with 387 (0.76%) of patients having either a post-discharge DVT, PE, or both. Overall, 37.90% of VTEs following colorectal cancer resection occurred in the post-discharge period. The mean age was 65.9 years in the study cohort, which was comprised of 51.4% male and 71.3% White patients. The median time to post-discharge VTE was 11 days following hospital discharge (IQR 5–16 days). Additional patient demographics can be found in Table 1.
Table 1.
General cohort characteristics of patients undergoing colorectal cancer resection
| Patient characteristic (n = 51,139) | N(%) |
|---|---|
| Pre-operative factors | |
| Age, years, mean (SD) | 65.90 (13.60) |
| Sex | |
| Male | 26,291 (51.41%) |
| Female | 24,848 (48.59%) |
| Race | |
| White | 36,453 (71.28%) |
| Black | 4944 (9.67%) |
| Asian | 2185 (4.27%) |
| Other/not reported | 7557 (14.78%) |
| Body mass index (BMI) | |
| < 18.5 | 1277 (2.53%) |
| 18.5–24.9 | 15,032 (29.75%) |
| 25–29.9 | 17,188 (34.01%) |
| 30–34.9 | 10,050 (19.89%) |
| ≥ 35.0 | 6989 (13.83%) |
| Comorbidities | |
| Bleeding disorder | 1856 (3.63%) |
| Dyspnea (moderate exertion/rest) | 4127 (8.07%) |
| COPD | 2656 (5.19%) |
| CHF | 613 (1.20%) |
| Diabetes | 9323 (18.23%) |
| Steroid use | 1583 (3.10%) |
| Weight loss > 10% | 2992 (5.85%) |
| Disseminated cancer | 6007 (11.75%) |
| Functional status | |
| Independent | 49,633 (97.40%) |
| Dependent | 1324 (2.60%) |
| ASA class | |
| I/II | 19,631 (38.46%) |
| III/IV/V | 31,415 (61.54%) |
| Pre-operative albumin < 3 g/dL | 18,086 (35.37%) |
| Pre-operative platelet count | |
| < 150,000 | 3165 (6.43%) |
| 150,000–400,000 | 41,612 (84.50%) |
| > 400,000 | 4469 (9.07%) |
| Primary tumor location | |
| Colon | 35,299 (73.99%) |
| Rectum | 12,410 (26.01%) |
| Neoadjuvant therapy | 6345 (12.41%) |
| Intra-operative factors | |
| Emergency case classification | 2833 (5.54%) |
| Operative time, min, mean (SD) | 186.53 (101.93) |
| Operative approach | |
| Open | 16,692 (32.64%) |
| Laparoscopic | 26,953 (52.71%) |
| Laparoscopic converted to open | 3559 (6.96%) |
| Robotic | 3703 (7.24%) |
| Robotic converted to open | 232 (0.45%) |
| Operation | |
| Open partial colectomy | 14,454 (28.26%) |
| Laparoscopic partial colectomy | 19,626 (38.38%) |
| Open total colectomy | 720 (1.41%) |
| Laparoscopic total colectomy | 452 (0.88%) |
| Open proctectomy | 5475 (10.71%) |
| Laparoscopic proctectomy | 9100 (17.79%) |
| Open total proctocolectomy | 117 (0.23%) |
| Laparoscopic total proctocolectomy | 123 (0.24%) |
| APR/exoneration | 1072(2.10%) |
| Post-operative factors | |
| Inpatient post-operative complications | |
| Anastomotic leak | 1269 (2.48%) |
| Ileus | 7209 (14.10%) |
| SSI | 2006 (3.92%) |
| Pneumonia | 711 (1.39%) |
| Reintubation | 419 (0.82%) |
| UTI | 593 (1.16%) |
| Transfusion | 5304 (10.37%) |
| Reoperation | 1153 (2.25%) |
| Length of stay, days, mean, (SD) | 6.93 (5.68) |
| Outcomes | |
| Post-discharge DVT | 282 (0.55%) |
| Post-discharge PE | 151 (0.30%) |
| Any post-discharge VTE | 387 (0.76%) |
VTE, venous thromboembolism; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; ASA, American Society of Anesthesiologists; SSI, skin/soft tissue infection; UTI, urinary tract infection; DVT, deep vein thrombosis; PE, pulmonary embolism
Factors Associated with Post-Discharge VTE
On bivariate analysis, Asian patients as well as those patients with a race other than White, Black, or Asian developed post-discharge VTE less frequently than White and Black patients (0.41% of Asians and 0.44% of patients of other race categories vs. 0.83% of Whites and 0.89% of Blacks; p < 0.01). As pre-operative BMI category increased, so did rates of post-discharge VTEs, which reached 1.27% in morbidly obese patients (vs. 0.51% in normal BMI patients, p < 0.01). The only comorbid condition with which patients more frequently developed post-discharge VTE was disseminated cancer, with 1.02% of patients with this condition developing post-discharge VTE (p = 0.01). Pre-operative hypoalbuminemia was not statistically associated with post-discharge VTE, but 1.10% of patients with pre-operative thrombocytosis developed a post-discharge VTE, vs. 0.73% of patients with normal pre-operative platelet counts (p = 0.03). No differences in post-discharge VTE rates were found based upon primary tumor location (colon vs. rectal), nor the use of neoadjuvant therapy. Additional demographic information can be found in Table 2.
Table 2.
Pre-operative, intraoperative, and post-post-discharge venous thromboembolism following colorectal cancer resection
| No post-discharge VTE N (%) | Post-discharge VTE | p value* | |
|---|---|---|---|
| Total patients | 50,752 (99.24%) | 387 (0.76%) | |
| Pre-operative factors | |||
| Age, years, mean (SD) | 65.69 (13.61) | 65.56 (12.89) | 0.73** |
| Sex | |||
| Male | 26,087 (99.22%) | 204 (0.78%) | 0.61 |
| Female | 24,665 (99.26%) | 183 (0.74%) | |
| Race | |||
| White | 36,152 (99.17%) | 301 (0.83%) | <0.01 |
| Black | 4900 (99.11%) | 44 (0.89%) | |
| Asian | 2176 (99.59%) | 9 (0.41%) | |
| Other/not reported | 7524 (99.56%) | 33 (0.44%) | |
| Body mass index (BMI) | |||
| < 18.5 | 1274 (99.77%) | 3 (0.23%) | < 0.01 |
| 18.5–24.9 | 14,956 (99.49%) | 76 (0.51%) | |
| 25–29.9 | 17,064 (99.28%) | 124 (0.72%) | |
| 30–34.9 | 9957 (99.07%) | 93 (0.93%) | |
| ≥ 35.0 | 6900 (98.73%) | 89 (1.27%) | |
| Comorbidities | |||
| Bleeding disorder | 1839 (99.08%) | 17 (0.92%) | 0.42 |
| No bleeding disorder | 48,913 (99.25%) | 370 (0.75%) | |
| Dyspnea (moderate exertion/rest) | 4093 (99.28%) | 34 (0.82%) | 0.60 |
| No dyspnea | 46,659 (99.25%) | 353 (0.75%) | |
| COPD | 2638 (99.32%) | 18 (0.68%) | 0.63 |
| No COPD | 48,114 (99.24%) | 369 (0.76%) | |
| CHF | 610 (99.51%) | 3 (0.49%) | 0.44 |
| No CHF | 50,142 (99.24%) | 384 (0.76%) | |
| Diabetes | 9263 (99.36%) | 60 (0.64%) | 0.16 |
| No diabetes | 41,489 (99.22%) | 327 (0.78%) | |
| Steroid use | 1568 (99.05%) | 15 (0.95%) | 0.37 |
| No steroid use | 49,184 (99.25%) | 372 (0.75%) | |
| Weight loss > 10% | 2969 (99.23%) | 23 (0.77%) | 0.94 |
| Weight loss < 10% or no weight loss | 47,783 (99.24%) | 364 (0.76%) | |
| Disseminated cancer | 5946 (98.98%) | 61 (1.02%) | 0.01 |
| Non-disseminated cancer | 44,806 (99.28%) | 326 (0.72%) | |
| Functional status | |||
| Independent | 49,259 (99.25%) | 374 (0.75%) | 0.35 |
| Dependent | 1311 (99.02%) | 13 (0.98%) | |
| ASA Class | |||
| I/II | 19,499 (99.33%) | 132 (0.67%) | 0.09 |
| III/IV/V | 31,162 (99.19%) | 253 (0.81%) | |
| Pre-operative albumin < 3 g/dL | 17,940 (99.19%) | 146 (0.81%) | 0.33 |
| Pre-operative albumin ≥ 3 g/dL | 32,812 (99.27%) | 241 (0.73%) | |
| Pre-operative platelet count | |||
| < 150,000 | 3142 (99.27%) | 23 (0.73%) | 0.03 |
| 150,000–400,000 | 41,307 (99.27%) | 305 (0.73%) | |
| > 400,000 | 4420 (98.90%) | 49 (1.10%) | |
| Primary tumor location | |||
| Colon | 35,025 (99.22%) | 274 (0.78%) | 0.31 |
| Rectum | 12,325 (99.32%) | 85 (0.68%) | |
| Neoadjuvant therapy | 6298 (99.26%) | 47 (0.74%) | 0.88 |
| No neoadjuvant therapy | 44,454 (99.24%) | 340 (0.76%) | |
| Intra-operative factors | |||
| Emergency case classification | 2796 (98.69%) | 37 (1.31%) | < 0.01 |
| Non-emergent case classification | 47,956 (99.28%) | 350 (0.72%) | |
| Operative time, min, mean (SD) | 186.39 (101.76) | 205.16 (121.46) | < 0.01** |
| Operative approach | |||
| Open | 16,538 (99.02%) | 164 (0.98%) | < 0.01 |
| Laparoscopic | 26,793 (99.41%) | 160 (0.59%) | |
| Laparoscopic converted to open | 3522 (98.96%) | 37 (1.04%) | |
| Robotic | 3682 (99.43%) | 21 (0.57%) | |
| Robotic converted to open | 227 (97.84%) | 5 (2.16%) | |
| Operation | |||
| Open partial colectomy | 14,294 (98.89%) | 160 (1.11%) | < 0.01 |
| Laparoscopic partial colectomy | 19,505 (99.38%) | 121 (0.62%) | |
| Open total colectomy | 710 (98.61%) | 10 (1.39%) | |
| Laparoscopic total colectomy | 446 (98.67%) | 6 (1.33%) | |
| Open proctectomy | 5441 (99.38%) | 34 (0.62%) | |
| Laparoscopic proctectomy | 9057 (99.53%) | 43 (0.47%) | |
| Open total proctocolectomy | 113 (96.58%) | 4 (3.42%) | |
| Laparoscopic total proctocolectomy | 121 (98.37%) | 2 (1.63%) | |
| APR/exoneration | 1065 (99.35%) | 7 (0.65%) | |
| Post-operative factors | |||
| Inpatient post-operative complications | |||
| Anastomotic leak | 1245 (98.11%) | 24 (1.89%) | < 0.01 |
| No anastomotic leak | 49,507 (99.27%) | 363 (0.73%) | |
| Ileus | 7117 (98.72%) | 92 (1.28%) | < 0.01 |
| No Ileus | 43,635 (99.33%) | 295 (0.67%) | |
| SSI | 1994 (99.40%) | 12 (0.60) | 0.40 |
| No SSI | 48,758 (99.24%) | 375 (0.76%) | |
| Pneumonia | 708 (99.58%) | 3 (0.42%) | 0.30 |
| No pneumonia | 50,044 (99.24%) | 384 (0.76%) | |
| Reintubation | 417 (99.52%) | 2 (0.48%) | 0.51 |
| No reintubation | 50,335 (99.24%) | 385 (0.76%) | |
| UTI | 589 (99.33%) | 4 (0.67%) | 0.82 |
| No UTI | 50,163 (99.24%) | 383 (0.76%) | |
| Transfusion | 5254 (99.06%) | 50 (0.94%) | 0.10 |
| No transfusion | 45,498 (99.26%) | 337 (0.74%) | |
| Reoperation | 1143 (99.13%) | 10 (0.87%) | 0.66 |
| No reoperation | 49,609 (99.25%) | 377 (0.75%) | |
| Length of stay, days, mean, (SD) | 6.93 (5.69) | 6.79 (4.02) | 0.62** |
Chi-Square test
t test VTE, venous thromboembolism; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; ASA, American Society of Anesthesiologists; SSI, skin/soft tissue infection; UTI, urinary tract infection
Mean operative time was greater in patients who developed post-discharge VTE compared with those who did not (205.16 min vs. 186.39 min; p < 0.01). Additionally, post-discharge VTE rates were lower in patients who underwent minimally invasive resection compared with open resection, or a minimally invasive converted to open approach (e.g., 0.59% of patients undergoing laparoscopic resection developed post-discharge VTE, vs. 2.16% of patients undergoing robotic converted to open operations; p < 0.01). Post-discharge VTE rates varied by operation performed from 0.47% to 3.42%; p < 0.01 (Table 2).
Considering only complications diagnosed during the index hospitalization, 1.89% of patients with anastomotic leak and 1.28% of patients with post-operative ileus developed post-discharge VTE. Patients with other post-operative complications diagnosed during the index hospitalization were not more likely to develop post-discharge VTE, based on bivariate analysis. Post-operative length of stay was not different between patients who developed post-discharge VTE (mean 6.79 days) and those who did not develop post-discharge VTE (mean 6.93 days; p = 0.62).
On multivariable logistic regression, after excluding factors with collinearity and those not significantly contributing to the model, 8 factors were significantly associated with post-discharge VTE after controlling for other variables in the model (Table 3). Pre-operative factors associated with post-discharge VTE included race other than White, Black, or Asian (OR 0.52, 95% CI 0.36–0.75), BMI (overweight OR 1.43, 95% CI 1.07–1.91; obese OR 1.84, 95% CI 1.35–2.50; morbidly obese OR 2.27, 95% CI 1.65–3.12; vs. normal BMI), and thrombocytosis (OR 1.41, 95% CI 1.03–1.92). Intra-operative factors associated with post-discharge VTE included emergency case classification (OR 1.61, 95% CI 1.11–2.33), operative time (4–6 h OR 1.56, 95% CI 1.12–2.17; > 6 h OR 1.85, 95% CI 1.21–2.84; vs. < 2 h), and operative type (open partial colectomy OR 1.67, 95% CI 1.30–2.16; open total proctocolectomy OR 4.41, 95% CI 1.57–12.38; vs. laparoscopic partial colectomy). Inpatient diagnosis of anastomotic leak (OR 2.05, 95% CI 1.31–3.21) and post-operative ileus (OR 1.39, 95% CI 1.07–1.79) were associated with increased risk of post-discharge VTE.
Table 3.
Association of pre-operative, intra-operative, and post-operative characteristics with post-discharge VTE following colorectal cancer resection
| Adjusted odds ratio (95% confidence interval) | p value | |
|---|---|---|
| Race | ||
| White | 1.00 | REF |
| Black | 0.93 (0.67–1.30) | 0.69 |
| Asian | 0.68 (0.35–1.33) | 0.26 |
| Other/not reported | 0.52 (0.36–0.75) | < 0.01 |
| BMI | ||
| < 18.5 | 0.28 (0.07–1.13) | 0.07 |
| 18.5–24.9 | 1.00 | REF |
| 25–29.9 | 1.43 (1.07–1.91) | 0.02 |
| 30–34.9 | 1.84 (1.35–2.50) | < 0.01 |
| ≥ 35.0 | 2.27 (1.65–3.12) | < 0.01 |
| Pre-operative platelet count | ||
| < 150,000 | 0.93 (0.61–1.43) | 0.75 |
| 150,000–400,000 | 1.00 | REF |
| > 400,000 | 1.41 (1.03–1.92) | 0.03 |
| Emergency case | ||
| No | 1.00 | REF |
| Yes | 1.61 (1.11–2.33) | 0.01 |
| Operative time | ||
| < 2 h | 1.00 | REF |
| 2–4 h | 1.18 (0.91–1.54) | 0.21 |
| 4–6 h | 1.56 (1.12–2.17) | 0.01 |
| > 6 h | 1.85 (1.21–2.84) | 0.01 |
| Operation | ||
| Open partial colectomy | 1.67 (1.30–2.16) | < 0.01 |
| Laparoscopic partial colectomy | 1.00 | REF |
| Open total colectomy | 1.59 (0.79–3.18) | 0.19 |
| Laparoscopic total colectomy | 1.75 (0.76–4.05) | 0.19 |
| Open proctectomy | 0.92 (0.62–1.36) | 0.67 |
| Laparoscopic proctectomy | 0.72 (0.50–1.03) | 0.07 |
| Open total proctocolectomy | 4.41 (1.57–12.38) | 0.01 |
| Laparoscopic total proctocolectomy | 2.18 (0.52–9.09) | 0.28 |
| Abdominoperineal resection/exoneration | 0.91 (0.42–2.01) | 0.82 |
| Anastomotic leak | ||
| No | 1.00 | REF |
| Yes | 2.05 (1.31–3.21) | < 0.01 |
| Post-operative Ileus | ||
| No | 1.00 | REF |
| Yes | 1.39 (1.07–1.79) | 0.01 |
BMI, body mass index
Post-Discharge VTE Risk Calculator
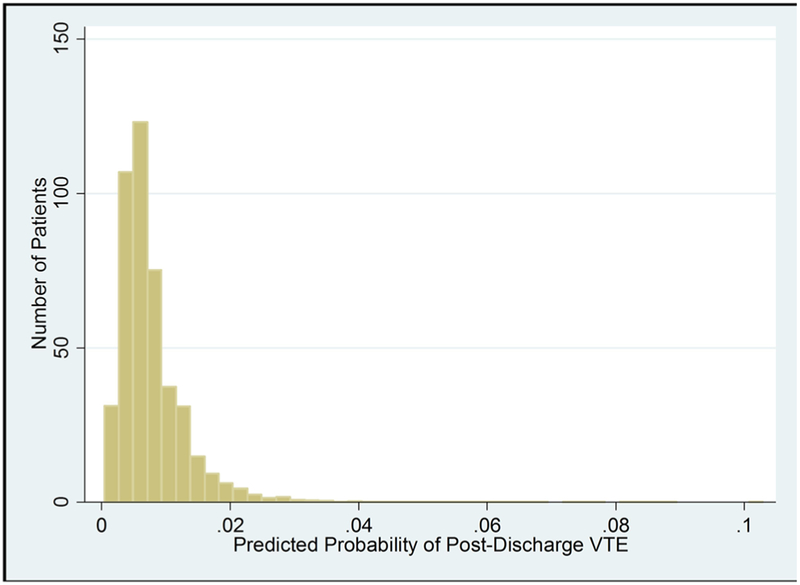
The beta coefficients from the multivariable logistic regression model were used to construct a post-discharge VTE risk calculator, which can be used to predict the probability of post-discharge VTE (Supplemental Table 2). In this cohort, the predicted probability of post-discharge VTE ranged from 0.04 to 10.29% (Fig. 1). The calculator’s C-statistic was 0.68 and the Hosmer-Lemeshow chi-square was 12.62 (p = 0.13), demonstrating good model discrimination and calibration. On 10-fold cross validation over 20 iterations, the mean test group C-Statistic was 0.65.
Fig. 1.
Predicted probability of post-discharge VTE following colorectal resection for malignancy
Discussion
Venous thromboembolism is the most common cause of preventable morbidity following major abdominal or pelvic resection for cancer, with risk extending beyond the index hospitalization.3, 4 Prior studies have indicated that at least 30% of VTEs occur after discharge following cancer resection.5–7 Several randomized clinical trials have shown the benefit of post-discharge chemoprophylaxis for 28 days following abdominal or pelvic cancer resection.8–10 However, more recent evidence demonstrates that patient-specific factors are associated with varying post-discharge VTE risk.34 Thus, in the era of precision medicine and targeted interventions, tools are needed to stratify individual patient risk for post-discharge VTE to allow for tailored decision-making surrounding post-discharge VTE prophylaxis prescribing.
In this retrospective cohort study of patients undergoing colorectal cancer resection, we identified a 0.76% incidence of post-discharge VTE, with pre-operative, intra-operative, and post-operative factors associated with post-discharge VTE risk. Subsequently, we developed a post-discharge VTE risk calculator including pre-operative, intra-operative, and post-operative factors diagnosed within the index hospitalization to generate patient-specific predicted probabilities of post-discharge events. This tool could be beneficial to clinicians deciding when to prescribe post-discharge prophylaxis and in counseling patients regarding the importance of this therapy.
Factors Associated with Post-Discharge VTE
Prior studies have evaluated individualized risk factors associated with post-operative VTE in patients undergoing colorectal resection for any reason, and have even identified factors associated with post-discharge VTE in the same population.5, 6, 16, 22 These studies identified factors associated with VTE including ASA score, BMI, hypoalbuminemia, race, steroid use, disseminated caner, open surgical approach, operative time, length of hospital stay, reoperation, and post-operative ileus. Similarly, in this study of post-discharge VTE following colorectal cancer resection, we identified race, BMI, operative time, and post-operative ileus as being associated with post-discharge VTEs after controlling for confounders. Additionally, we identified pre-operative platelet count, emergency class classification, operative type, and anastomotic leak as being associated with post-discharge VTE. No previous study to our knowledge has evaluated risk factors, including pre-operative, intra-operative, and post-operative factors, associated with post-discharge VTE in this population.
Post-operative complications have a large impact on patient recovery following colorectal cancer resection, with reported complication rates reaching as high as 35%.35, 36 Furthermore, complications are often compounded, with subsequent complications more likely to occur in patients who have already suffered one adverse event.35 Specifically, Kronberg et al. identified post-operative ileus as a high-risk complication in patients undergoing laparoscopic colon resection, with patients with post-operative ileus having a 7-fold greater incidence of DVT compared with patients without ileus.37 Based on these findings, we questioned whether complications during the index hospitalization were associated with post-discharge VTE events, and identified both anastomotic leak and post-operative ileus as risk factors associated with post-discharge VTE. These complications may result in decreased mobility, which could be a potential mechanism leading to increased VTE risk. Interestingly, there was no difference in average length of hospital stay between patients who did and did not develop post-discharge VTE. Although these complications may result in prolonged hospital stay, a prolonged hospital stay results in a narrower window within which post-discharge VTE events could be diagnosed in this study, as follow-up was limited to 30 days.
Post-Discharge VTE Risk Calculator
Prior studies using ACS NSQIP data have developed various risk calculators to predict post-operative complications in patients undergoing surgical resection.29–32 Since these tools are beneficial to providers, we sought to develop a patient-specific post-discharge VTE risk calculator to be used to discuss risk of post-discharge VTE following colorectal cancer resection. An individual patient’s predicted probability of developing a post-discharge VTE can be generating by summing the applicable beta coefficients as found in Supplemental Table 1. As an example, a 72-year-old White male with a BMI of 25.5 and a pre-operative platelet count of 176,000 underwent an emergent open partial colectomy with a 245-min operative time. His post-operative course was complicated by anastomotic leak requiring reoperation as well as post-operative ileus. Utilizing the beta coefficients listed in Supplemental Table 1, the summed LP for this patient is − 5.67 + 0.36 + 0.47 + 0.45 + 0.52 + 0.72 + 0.33 = − 2.82. Plugging this into predicted probability equation of probability of event = exp.(LP) / [1 + exp.(LP)] = 0.056 or 5.6% risk of post-discharge VTE. This patient has an over 5-fold higher rate of VTE than the average risk patient and should most certainly receive post-discharge VTE chemoprophylaxis.
Future work will focus on expanding this risk calculator to include patients undergoing surgical resection for other types of malignancy, as well as the development of an online tool with which providers can quickly calculate an individual patient’s predicted probability of post-discharge VTE. Ultimately, we would like to identify a risk threshold, above which the risk of post-discharge VTE outweighs the costs and inconvenience of post-discharge prophylaxis. This calculator would thus allow for targeted post-discharge prophylaxis prescribing in patients undergoing surgical resection for intraabdominal malignancy.
Limitations
This study has several limitations. First, the cohort of patients was derived from hospitals participating in ACS NSQIP’s procedure targeted programs for colectomy and proctectomy. Thus, there may be a selection bias as hospitals must be resource intensive enough to participate in this program in order to have patients represented in this cohort analysis. This may limit the generalizability of our findings. Second, this study is an observational cohort study, and therefore only associations can be drawn from this study, not causation. Third, a limitation of the ACS NSQIP database is that medication information is not provided. Therefore, we are unable to assess for either inpatient VTE chemoprophylaxis compliance or post-discharge VTE chemoprophylaxis. Presumably, some but not all patients in this cohort received post-discharge VTE chemoprophylaxis. Therefore, our study likely underestimates the post-discharge VTE rate that would occur in this population in the absence of any post-discharge chemoprophylaxis. Nevertheless, the risk factors identified as associated with post-discharge VTE are unlikely to change. Finally, VTE risk can extend beyond 30-days following colorectal cancer resection, but ACS NSQIP only follows patients for 30-days post-operatively. Again, this is a limitation of the dataset, and likely underestimates the true post-discharge VTE rate in this population.
Conclusion
In this multicenter, patient-centered evaluation of post-discharge VTEs following colorectal cancer resection, we identified pre-operative, intra-operative, and post-operative factors associated with post-discharge VTEs. We developed a risk calculator incorporating these patient specific factors to be used at the time of discharge following colorectal cancer resection to guide post-discharge prophylaxis prescribing and to communicate the importance of post-discharge chemoprophylaxis in this population. Considering these patient-specific factors, the predicted probability of post-discharge VTE ranged from 0.04 to 10.29% in this study.
Supplementary Material
Funding
This study was supported by the Northwestern Institute for Comparative Effectiveness Research in Oncology (NICER-Onc) of the Robert H. Lurie Comprehensive Cancer Center. ADY is supported by the National Heart, Lung and Blood Institute (K08HL145139), DJB is supported by the Veteran’s Administration Health Services Research and Development Program (I01HX002290), KYB is supported by the Agency for Healthcare Research and Quality (R01HS024516), and RPM is supported by the Agency for Healthcare Research and Quality (K12HS026385) and an Institutional Research Grant from the American Cancer Society (IRG-18-163-24).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11605-019-04354-2) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors declare that they have no conflict of interest.
Presentation Presented at the Society of Surgeons of the Alimentary Tract Small Bowel and Colorectal Plenary Session; San Diego, CA; May 21, 2019.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117(7):1334–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanden P, Svensson PJ, Sjalander A. Venous thromboembolism and cancer risk. J Thromb Thrombolysis. 2017;43(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnelli G, Bolis G, Capussotti L, et al. A Clinical Outcome-Based Prospetive Study on Venous Thromboembolism After Cancer Surgery The @Ristos Project. Ann Surg. 2006;243(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano PE, Parpia S, Linkins LA, et al. Venous Thromboembolism Events Following Major Pelvic and Abdominal Surgeries for Cancer: A Prospective Cohort Study. Ann Surg Oncol. 2018;25(11):3214–3221. [DOI] [PubMed] [Google Scholar]
- 5.Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg. 2014;18(22):2169–2177. [DOI] [PubMed] [Google Scholar]
- 6.Davenport DL, Vargas HD, Kasten MW, Xenos ES. Timing and perioperative risk factors for in-hospital and post-discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost. 2012;18(6):569–575. [DOI] [PubMed] [Google Scholar]
- 7.Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for etended prohylaxis. Ann Surg. 2011;2554(1):131–137. [DOI] [PubMed] [Google Scholar]
- 8.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of Prophylaxis Against Venous Thromboembolism with Enoxaparin after Surgery for Cancer. NEJM. 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4(11):2384–2390. [DOI] [PubMed] [Google Scholar]
- 10.Kakkar VV, Balibrea JL, Martinez-Gonzalez J, Prandoni P, Group CS. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8(6):1223–1229. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–2204. [DOI] [PubMed] [Google Scholar]
- 12.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the Prevention of Venous Thromboembolic Disease in Colorectal Surgery. Dis Colon Rectum. 2018;61(1):14–20. [DOI] [PubMed] [Google Scholar]
- 14.Merkow RP, Bilimoria KY, Sohn MW, et al. Adherence with postdischarge venous thromboemboism chemoprophylaxis recommendations after colorectal cancer surgery among elderly Medicare beneficiaries. Ann Surg. 2014;260(1):103–108. [DOI] [PubMed] [Google Scholar]
- 15.Sammour T, Chandra R, Moore JW. Extended venous thromboembolism prophylaxis after colorectal cancer surgery: the current state of the evidence. J Thromb Thrombolysis. 2016;42(1):27–32. [DOI] [PubMed] [Google Scholar]
- 16.Beal EW, Tumin D, Chakedis J, et al. Which Patients Reuire Extended Thromboprophylaxis After Colectomy? Modeling Risk a n d A s s e s s i n g I n d i c a t i o n s f o r P o s t - d i s c h a r g e Pharmacoprophylaxis. World J Surg. 2018;42(7):2242–2251. [DOI] [PubMed] [Google Scholar]
- 17.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219–230. [DOI] [PubMed] [Google Scholar]
- 18.Crous-Bou M, Harrington LB, Kabrhel C. Environmental and Genetic Risk Factors Associated with Venous Thromboembolism. Semin Thromb Hemost. 2016;42(8):808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Guo Q, Hu W. Incidence, risk factors, and outcomes of venous thromboembolism after oncologic surgery: A systemic review and meta-analysis. Thromb Res. 2019;173:48–56. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Haines K, Nelson LG, Gallagher SF, Murr MM. Predictive factors of thromboembolic events in patients undergoing Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(1):30–35. [DOI] [PubMed] [Google Scholar]
- 22.Moghadamyeghaneh Z, Alizadeh RF, Hanna MH, et al. Post-Hospital Discharge Venous Thromboembolism in Colorectal Surgery. World J Surg. 2016;40(5):1255–1263. [DOI] [PubMed] [Google Scholar]
- 23.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19–S27. [DOI] [PubMed] [Google Scholar]
- 24.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical factors. J Am Coll Surg. 2013;217(2):336–346. [DOI] [PubMed] [Google Scholar]
- 25.Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6–16. [DOI] [PubMed] [Google Scholar]
- 26.Merkow RP, Hall BL, Cohen ME, et al. Relevance of the c-statistic when evaluating risk-adjustment models in surgery. J Am Coll Surg. 2012;214(5):822–830. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung Y, Hu J. A K-fold Averaging Cross-vaidation Procedure. J Nonparametr Stat. 2015;27(2):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208(6):1009–1016. [DOI] [PubMed] [Google Scholar]
- 31.Hyder JA, Wakeam E, Habermann EB, Hess EP, Cima RR, Nguyen LL. Derivation and validation of a simple calculator to predict home discharge after surgery. J Am Coll Surg. 2014;218(2):226–236. [DOI] [PubMed] [Google Scholar]
- 32.Mansmann U, Rieger A, Strahwald B, Crispin A. Risk calculators methods, development, implementtion, and validation. Int J Colorectal Dis. 2016;31(6):1111–1116. [DOI] [PubMed] [Google Scholar]
- 33.Parikh P, Shiloach M, Cohen ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford). 2010;12(7):488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;21(1): CD004318. [DOI] [PubMed] [Google Scholar]
- 35.Tevis SE, Kennedy GD. Postoperative Complications: Looking Forward to a Safer Future. Clin Colon Rectal Surg. 2016;29(3): 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo WE, Virgo KS, Johnson FE, et al. Risk factors for mobidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43(1):83–91. [DOI] [PubMed] [Google Scholar]
- 37.Kronberg U, Kiran RP, Soliman MS, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg. 2011;253(1):78–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.