Abstract
Background.
Carfilzomib improves survival in patients with relapsed myeloma. Given the strict eligibility criteria in the clinical trials, the actual frequency of cardiac (CAEs) and pulmonary adverse events (PAEs) and risk factors associated with these AEs in the general population needs to be established.
Methods.
We extracted myeloma cases in the SEER-Medicare linked database from 2000-2013 and corresponding claims through 2014. We then identified patients who received carfilzomib during their disease course. Subsequently, we used the International Classification of Disease, ninth revision (ICD-9) to identify all the codes for CAEs, PAEs and respiratory infections associated with carfilzomib use. Pre-existing diagnoses corresponding to the CAEs and PAEs of interest were excluded to distinguish toxicity from comorbidity. Multivariate Cox regression was performed to determine the variables independently associated with developing CAEs and PAEs.
Results.
Among 635 patients analyzed, the median age was 72 (range 36-94); 55% of the patients were male and 79% Caucasian. The median duration of carfilzomib treatment was 58 days (range 1-716). Overall, 66% had codes for either CAEs or PAEs. In terms of CAEs, 22% developed hypertension, 15% peripheral edema and 14% heart failure. In terms of PAEs, 28% developed dyspnea, 15% cough, and 15% pneumonia. Only COPD was independently associated with developing CAEs. Patients with pre-existing COPD had a 40% increase in hazard for developing CAEs (aHR 1.40, 95% CI 1.03-1.90).
Conclusions.
In older adults with myeloma receiving carfilzomib, new cardiac and pulmonary diagnoses were common. Patients with pre-existing COPD were at increased risk for developing CAEs.
Keywords: Multiple Myeloma, Carfilzomib, Older Adults, Cardiopulmonary Complications, SEER-Medicare Linked Database
Precis:
In older adults with myeloma receiving carfilzomib outside the clinical trials, new cardiac and pulmonary adverse events were common.
Older myeloma patients with pre-existing COPD who received carfilzomib had a 40% increase in hazard for developing cardiac adverse events.
Background
The growing armamentarium of treatment options in patients with multiple myeloma has resulted in significant improvement in depth of remission and survival outcomes. The next-generation proteasome inhibitor carfilzomib increases the frequency and depth of responses, and improves survival in heavily pretreated patients with multiple myeloma.1,2
The accelerated approval of carfilzomib in July 2012 was based on the promising results of a single-arm multicenter trial enrolling 266 patients with relapsed/refractory multiple myeloma. The overall response rate in this heavily pretreated population was 23% with a median duration of response of 7.8 months.3 Other landmark trials have further supported the efficacy of carfilzomib in patients with relapsed/refractory multiple myeloma as evidenced by significant improvements in the progression-free survival and overall survival of patients randomized to carfilzomib-based treatment arms.4,5
In addition to improvements in clinical outcomes, carfilzomib has not been associated with the debilitating and life-altering side effect of neuropathy, which has been frequently reported with bortezomib.6 Instead, its use has been associated mainly with cardiac and pulmonary adverse events.3–5 The most common cardiopulmonary adverse events (AEs) in all patients with relapsed/refractory multiple myeloma who received carfilzomib as monotherapy in phase II studies include dyspnea (35%), upper respiratory tract infection (28%), cough (26%), peripheral edema (24%), hypertension (14%), pneumonia (13%), and chest wall pain (11%).3
However, due to stringent eligibility criteria applied in clinical trials, healthier patients tend to be enrolled, and thus the real-world frequency of these events in the overall population is unknown. Furthermore, the risk factors associated with developing these adverse events remain to be established. The primary objective of this study was to estimate the incidence of cardiac and pulmonary adverse events occurring secondary to carfilzomib through reviewing administrative claims data in the SEER-Medicare linked database. In addition, we aimed to identify the risk factors associated with developing these adverse events.
Methods
We extracted all plasma cell myeloma cases (ICD-O-3 histology codes 9731, 9732, and 9734) in the SEER-Medicare linked database from 2000-2013 and corresponding claims data through 2014. We then used the Healthcare Common Procedure Coding System (HCPCS) codes for carfilzomib (C9295 and J9047) to identify patients who had received treatment with carfilzomib at any time point during their disease course. Due to the period under study, all patients received carfilzomib in 2013-2014, as carfilzomib became commercially available in the US in early 2013. Carfilzomib provided via a clinical trial, and not billed to Medicare, was not available in the dataset. Patients who had received additional anti-myeloma therapy in combination with carfilzomib, except for corticosteroids, were excluded since toxicity attribution could not be determined. Interruptions in carfilzomib-based treatment of more than 60 days were considered a change in treatment strategy/therapeutic option and only data from the first carfilzomib regimen was included in the analysis.
We used the International Classification of Disease, ninth revision (ICD-9) to identify all the codes for cardiac and pulmonary adverse events associated with carfilzomib use that have previously been reported in the peer-reviewed literature (Supplementary Table 1). To define comorbidities, patients who were not enrolled in Medicare for 12 months preceding carfilzomib initiation were excluded. Adverse events were collected from the first carfilzomib dose through 28 days following the last dose or the start of additional anti-myeloma therapeutic agents, whichever was sooner. Codes present within the 90 days prior to initiation of carfilzomib were considered pre-existing conditions and were not considered an adverse events unless the patient had an admission for the condition during the follow-up period. A flow diagram for study methods is provided in the Figure 1.
Figure 1.
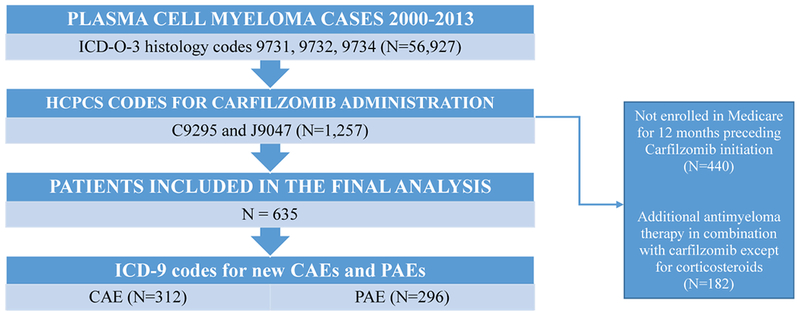
A flow diagram of methods utilized for patient selection and registration of adverse events of interest.
The frequency of cardiac adverse events (CAEs) and pulmonary adverse events (PAEs) were reported using descriptive statistics. Multivariate Cox regression analysis was performed to determine the variables that were independently associated with development of composite outcomes of specific events of interest including: cardiac adverse events (hypertension, ischemic heart disease, or congestive heart failure), pulmonary adverse events (deep venous thrombosis, pulmonary embolism, or pulmonary hypertension), and respiratory infections (cough, upper respiratory infection, or pneumonia). All statistical analyses were performed using SAS Enterprise Guide 5.1. A p value of < 0.05 was considered statistically significant. This study was performed under the supervision of Washington University’s Human Research Protection Office.
Results
The final analysis included 635 patients. The median age was 72 (range 36-94). Fifty-five percent of the patients were male, 79% Caucasian, 17% African-American, and 4% belonged to other races. The median duration of carfilzomib treatment was 58 days, and the median duration from the diagnosis of multiple myeloma to carfilzomib treatment was 54 months. The median overall survival of the population was 13.1 months (95% CI 11.9-14.3 months) from time of carfilzomib initiation (Table 1).
Table 1.
Baseline characteristics of patients receiving carfilzomib (N=635)
| Variables | (%) | |
|---|---|---|
| Age [median (range)] | 72 (36-94) | |
| Male | 350 (55%) | |
| Race | Caucasian | 502 (79%) |
| African-American | 108 (17%) | |
| Other | 25 (4%) | |
| Median duration of carfilzomib treatment (mean ± SD, range) | 58 days (95.7 ± 105.5, 1-716) |
|
| Median time from diagnosis of MM to carfilzomib treatment (mean ± SD, range ) | 54 months (59.7 ± 36.7, 3-170) |
|
| Median overall survival | 13.1 (95% CI 11.9-14.3) | |
SD: standard deviation; MM: multiple myeloma.
In total 66% of patients treated had ICD-9 codes indicative of a new cardiac or pulmonary adverse event and 10% had one or more admissions for these events. Nearly half (49%) of patients had ICD-9 codes indicative of new cardiac adverse events. In terms of specific cardiac adverse events, 22% had diagnostic codes for hypertension; 15% and 14% had diagnostic codes for peripheral edema and systolic/diastolic heart failure, respectively. New chest pain and ischemic heart disease was registered in 10% and 6% of the patients, respectively. The incidence of new cardiac dysrhythmias was noted in 7%.
Forty-seven percent of patients had ICD-9 codes indicative of new pulmonary adverse events. In terms of specific pulmonary adverse events, 28% of patients were noted to have diagnostic codes suggestive of developing new dyspnea and 15% had a code for new cough. Pneumonia and respiratory tract infections were each listed in 15% and 14% of patients, respectively. New venous thromboembolic events were documented in 6% of patients and 1% of the patients were noted to develop new pulmonary hypertension. The frequency of toxicities is presented in Figure 2.
Figure 2.
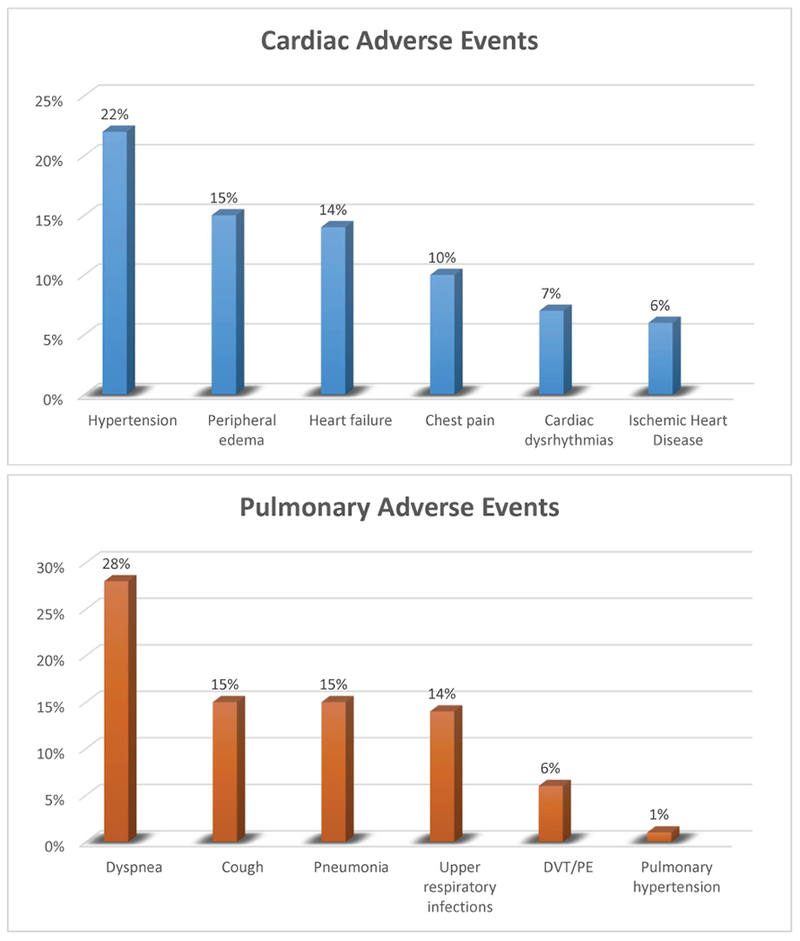
The incidence of cardiac and pulmonary adverse events in patients with multiple myeloma who have received carfilzomib.
Based on the literature review, to identify the risk factors associated with developing toxicities, we examined age, gender, and common comorbidities, including diabetes, dyslipidemia and chronic obstructive pulmonary disease (COPD) as independent variables.7–11 Of note, pre-existing comorbidities with the same codes as the examined CAEs and PAEs could not be examined as risk factors as patients with these were excluded. Among all the examined variables, COPD was found to be independently associated with developing adverse events. Patients with pre-existing COPD had a 40% increased hazard for developing HTN, ischemic heart disease, or congestive heart failure (adjusted hazard ratio 1.40, 95% confidence intervals 1.03-1.90) and a 40% increased risk in developing cough, upper respiratory infection, or pneumonia (adjusted hazard ratio 1.40, 95% confidence intervals 1.02-1.93) There was no other statistically significant association between the examined risk factors and other composite outcomes including pulmonary adverse events, and respiratory infections. The multivariate analysis is summarized in Table 2.
Table 2.
Multivariate analysis of variables associated with development of cardiac and pulmonary adverse events in patients with multiple myeloma who have received carfilzomib treatment.
| Variables | Cardiac Adverse Event of Interest aHR (95% CI) |
Pulmonary Adverse Events of
Interest aHR (95% CI) |
Respiratory Infections aHR (95% CI) |
|---|---|---|---|
| Age (per year) | 1.01 (0.99-1.02) | 1.00 (0.97-1.04) | 1.01 (0.99-1.03) |
| Female Gender | 0.89 (0.68-1.16) | 1.82 (0.96-3.44) | 0.82 (0.62-1.09) |
| Diabetes Mellitus | 1.25 (0.93-1.66) | 1.11 (0.57-2.16) | 0.92 (0.67-1.26) |
| Dyslipidemia | 1.19 (0.90-1.57) | 1.75 (0.88-3.48) | 1.05 (0.78-1.40) |
| COPD | 1.40 (1.03-1.90) | 1.58 (0.78-3.18) | 1.40 (1.02-1.93) |
COPD: chronic obstructive pulmonary disease; aHR: adjusted hazard ratio; CI: confidence interval. aHR adjusted for age, gender, diabetes mellitus, dyslipidemia and COPD.
Discussion
Although the introduction of carfilzomib has significantly advanced the field of myeloma, the real-world incidence of adverse events associated with its use and factors associated with developing cardiac and pulmonary adverse events are not yet well understood. In our study the median duration of carfilzomib treatment was 58 days. This is significantly short and it’s noteworthy that in the real world outside of clinical trials, patients stayed on carfilzomib for a shorter time than we would anticipate based on the PFS in clinical trials. That may have been because the drug was new at the time of data collection and physicians did not yet know how to support patients and manage toxicities, or it may have been due to early disease progression.
Cardiopulmonary adverse events associated with carfilzomib in patients with multiple myeloma can be potentially life-threatening. In our study, hypertension, peripheral edema, systolic/diastolic heart failure, new chest pain, ischemic heart disease and cardiac dysrhythmias were the most common cardiac adverse events developed proximal to carfilzomib therapy. These incidence rates were similar to the incidence rates reported in the current literature. A large meta-analysis of 2594 patients in phase 1-3 clinical trials with multiple myeloma with a focus on the prevalence of cardiac adverse events (defined as heart failure, hypertension, ischemia, and arrhythmia), revealed all-grade and grades 3 and higher cardiac adverse events in 18% and 8% of patients, respectively. The cumulative relative risk of all-grade cardiac adverse events was 1.8 compared to patients who were not exposed to carfilzomib.10 Another analysis of a cohort of more than 2000 patients with relapsed/refractory multiple myeloma enrolled in phase 1-3 trials showed grades 3 and higher cardiac adverse events including hypertension (6%), dyspnea (5%), and cardiac failure (4%). However, it was noted that these adverse events rarely led to treatment discontinuation or death.7 In non-trial based studies, the rate of heart failure has been estimated to be 8-20%.8,9
As with cardiac adverse events, the incidence of the pulmonary adverse events collected through the SEER-Medicare linked database was similar to the rates of clinical adverse events gathered from clinical studies. In an analysis reviewing four phase II clinical studies, the most commonly reported pulmonary adverse events were dyspnea (42%) and cough (26%), which resulted in dose reduction in 2% and treatment discontinuation in an additional 2% of patients. The majority of dyspnea events were low grade, and resolved without dose reduction or discontinuation. Respiratory tract infections and pneumonia were reported in 19% and 13%, respectively. Other less common pulmonary adverse events included pleural effusion (4%), pulmonary hypertension (2%), pulmonary embolism (1%), hemoptysis (1%), and pneumonitis (<1%). No interstitial lung disease or pulmonary fibrosis was reported.11 There have been sporadic cases of more serious pulmonary adverse events. Fatal pulmonary toxicity manifested as acute respiratory distress syndrome (ARDS) and diffuse alveolar hemorrhage within one week of receiving the first dose of carfilzomib12, and carfilzomib-related severe pneumonitis have been reported in literature.13
In addition to measuring the real-world incidence of cardiac and pulmonary adverse events, it is also important to identify the underlying factors associated with developing adverse events. In our study, after examining age, gender, COPD, diabetes and dyslipidemia, COPD was the only statistically significant independent risk factor for developing cardiac adverse events. In the meta-analysis described earlier, carfilzomib doses of 45 mg/m2 or higher were associated with high-grade cardiac adverse events. Older age, prior lines of therapy, and concurrent myeloma therapies were not associated with developing cardiac adverse events.10 Similarly, age was not associated with increased risk of cardiac adverse events in our population. Given the growing enthusiasm in the field to move carfilzomib to earlier lines of therapy,14–16 and the heterogeneous pattern of dosing in various clinical trials, an in-depth understanding of patient-related and dose-related risk factors is essential to guide future treatment strategies in selecting the right patient population and modifying the dose based on patients’ characteristics.
The present study has several limitations. Although the SEER–Medicare linked database provides a large sample size with diverse demographic data, using claims data and ICD-9 codes to identify adverse events and define risk factors associated with adverse events has unavoidable deficiencies. It is possible that the ICD-9 codes are under- or over-reported due to miscoding of the claims data. In addition, the ICD-9 code does not differentiate the severity of the adverse events. Due to the limitations of ICD-9 codes, we were unable to tell which patients had baseline adverse events that worsened after initiation. Baseline adverse events were regarded as pre-existing conditions and were not categorized as an adverse event unless there was an admission for that reason. Because of this, it is likely that adverse events were under-estimated by the current study. Similarly, in our study, COPD was shown to increase the hazard of developing cardiac adverse events, but not pulmonary adverse events. Since patients with COPD may have shortness of breath and cough as pre-existing conditions, our methodology could not capture worsening of these pre-existing conditions as an adverse event. Future studies should include these patients to evaluate whether underlying comorbidities can affect the severity of toxicities.
It is also possible that carfilzomib treatments received by patients were not fully captured by the HCPCS codes for carfilzomib (C9295 and J9047). Although the sensitivity and specificity of Medicare claims for MM treatment have not been reported, previous work indicates a sensitivity of 93% (95% CI, 88%-96%) for administration of parenteral chemotherapy.17 In addition, HCPCS codes do not specify the dose of carfilzomib. As we know, the incidence rates of adverse events reported in different phase 2-3 clinical trials differ based on weekly or twice-weekly dosing schedule.18
To our knowledge, this is the first SEER-Medicare linked study estimating the incidence of new cardiac and pulmonary adverse events and associated risk factors in patients with multiple myeloma who are treated with carfilzomib. With the expanding role of carfilzomib in the treatment of newly diagnosed and relapsed/refractory multiple myeloma, and the interest for weekly dosing rather than twice-weekly dosing for patients’ convenience, it is essential to identify the incidence of adverse events associated with its use in real-life practice settings. It is also critical to recognize risk factors that predispose patients to developing these adverse events, to modify treatment and mitigate toxicity.
Supplementary Material
Acknowledgements
This research was made possible by Grant Number K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest relevant to the manuscript.
REFERENCES
- 1.Muchtar E, Gertz MA, Magen H. A practical review on carfilzomib in multiple myeloma. European journal of haematology 2016;96:564–77. [DOI] [PubMed] [Google Scholar]
- 2.Tzogani K, Camarero Jimenez J, Garcia I, et al. The European Medicines Agency Review of Carfilzomib for the Treatment of Adult Patients with Multiple Myeloma Who Have Received at Least One Prior Therapy. The oncologist 2017;22:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herndon TM, Deisseroth A, Kaminskas E, et al. U.S. Food and Drug Administration Approval: Carfilzomib for the Treatment of Multiple Myeloma. Clinical Cancer Research 2013;19:4559–63. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. The Lancet Oncology 2017;18:1327–37. [DOI] [PubMed] [Google Scholar]
- 5.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. New England Journal of Medicine 2015;372:142–52. [DOI] [PubMed] [Google Scholar]
- 6.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112:1593–9. [DOI] [PubMed] [Google Scholar]
- 7.Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood advances 2018;2:1633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood advances 2017;1:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain T, Narayanasamy H, Mikhael J, et al. Systolic dysfunction associated with carfilzomib use in patients with multiple myeloma. Blood cancer journal 2017;7:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah C, Bishnoi R, Jain A, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leukemia & lymphoma 2018:1–13. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Cheng J. Overview and management of cardiac and pulmonary adverse events in patients with relapsed and/or refractory multiple myeloma treated with single-agent carfilzomib. Oncology (Williston Park, NY) 2013;27 Suppl 3:24–30. [PubMed] [Google Scholar]
- 12.Lataifeh AR, Nusair A. Fatal pulmonary toxicity due to carfilzomib (Kyprolis). Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 2016;22:720–4. [DOI] [PubMed] [Google Scholar]
- 13.D D, K N, G T, D J, E K Carfilzomib-Related Severe Pneumonitis. Am J Respir Crit Care Med 2018;197.29420904 [Google Scholar]
- 14.Bortezomib or Carfilzomib With Lenalidomide and Dexamethasone in Treating Patients With Newly Diagnosed Multiple Myeloma. . ClinicalTrialsgov identifier
- 15.Daratumumab in Combination With Lenalidomide and Dexamethasone in Relapsed and Relapsed-Refractory Multiple Myeloma. . ClinicalTrialsgov identifier
- 16.Jakubowiak AJCA, Lonial S, Weiss BM, Comenzo RL, Wu K, Khokhar NZ, Wang J, Doshi P, Usmani SZ. Daratumumab (DARA) in combination with carfilzomib, lenalidomide, and dexamethasone (KRd) in patients (pts) with newly diagnosed multiple myeloma (MMY1001): An open-label, phase 1b study. J Clin Oncol 35, 2017 (suppl; abstr 8000) 2017. [Google Scholar]
- 17.Lamont EB, Herndon JE 2nd, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. Journal of the National Cancer Institute 2005;97:1080–3. [DOI] [PubMed] [Google Scholar]
- 18.Moreau P, Mateos MV, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. The Lancet Oncology 2018;19:953–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


