Abstract
Background:
Comparative studies of characteristics of optic neuritis (ON) associated with myelin oligodendrocyte glycoprotein-IgG (MOG-ON) and aquaporin-4-IgG (AQP4-ON) seropositivity are limited.
Objective:
To compare visual and optical coherence tomography (OCT) measures following AQP4-ON, MOG-ON, and multiple sclerosis-associated ON (MS-ON).
Methods:
In this cross-sectional study, 48 AQP4-ON, 16 MOG-ON, 40 MS-ON and 31 healthy control participants underwent monocular letter-acuity assessment and spectral-domain OCT. Eyes with a history of ON >3 months prior to evaluation were analyzed.
Results:
AQP4-ON eyes exhibited worse high-contrast letter-acuity (HCLA) compared to MOG-ON (−22.3±3.9 letters; p<0.001) and MS-ON eyes (−21.7±4.0 letters; p<0.001). Macular ganglion cell+inner plexiform layer (GCIPL) thickness was lower, as compared to MS-ON, in AQP4-ON (−9.1±2.0 μm; p<0.001) and MOG-ON (−7.6±2.2 μm; p=0.001) eyes. Lower GCIPL thickness was associated with worse HCLA in AQP4-ON (−16.5±1.5 letters per 10μm decrease; p<0.001) and MS-ON eyes (−8.5±2.3 letters per 10μm decrease; p<0.001), but not in MOG-ON eyes (−5.2±3.8 letters per 10μm decrease; p=0.17), and these relationships differed between the AQP4-ON and other ON groups (p<0.01 for interaction).
Conclusions:
AQP4-IgG seropositivity is associated with worse visual outcomes after ON compared with MOG-ON and MS-ON, even with similar severity of macular GCIPL thinning.
Keywords: optic neuritis, neuromyelitis optica, myelin oligodendrocyte glycoprotein, optical coherence tomography, multiple sclerosis
INTRODUCTION
Optic neuritis (ON) is a frequent manifestation of multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). In comparison to idiopathic or MS-associated ON, NMOSD-ON is characterized by worse visual outcomes, often resulting in blindness.1,2 The majority of cases of NMOSD are associated with antibodies directed against aquaporin-4 (AQP4-IgG).3 However, antibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) have been identified in a subset of NMOSD patients who are seronegative for AQP4-IgG, and appear to be associated with more favorable outcomes.4-6
Optical coherence tomography (OCT) studies have identified profound retinal nerve fiber layer (RNFL) and ganglion cell+inner plexiform layer (GCIPL) thinning in NMOSD-ON eyes.7 Furthermore, macular microcystoid pathology (MMP) of the inner nuclear layer (INL) occurs in ~20% of NMOSD-ON eyes (as compared to ~5% of MS eyes) and is associated with worse visual disability and increased severity of RNFL/GCIPL thinning.8-12 Notably, AQP4 is expressed in the retina by astrocytes and Müller cells and loss of AQP4 immunoreactivity has been demonstrated in a pathologic study of AQP4-IgG+ NMOSD retinas.13 Thus, it has been postulated that direct retinal damage may account for poor visual outcomes in AQP4-IgG associated ON. However, studies comparing visual function and retinal pathology (as assessed by OCT) following AQP4-IgG and MOG-IgG associated ON are limited and have shown conflicting findings.14-18 In this cross-sectional study, we sought to compare visual outcomes and retinal OCT measures following ON associated with AQP4-IgG, MOG-IgG, and MS.
METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
Johns Hopkins University Institutional Review Board approval was obtained for the study protocol, and written, informed consent was obtained from all participants prior to study enrolment.
Study Participants, MOG-IgG, and AQP4-IgG testing
In this cross-sectional study, subjects with a history of ON (monophasic or recurrent) and MOG-IgG (MOG-ON) or AQP4-IgG seropositivity (AQP4-ON) were recruited from the Johns Hopkins Neuromyelitis Optica, Transverse Myelitis, and Multiple Sclerosis Centers, and invited to undergo OCT and visual function testing during routine clinic visits.
AQP4-IgG antibody testing was performed by commercially available assays (Mayo Laboratories [cell-based assay; CBA] or Athena Diagnostics [ELISA]). The MOG-IgG antibody CBA was performed at Johns Hopkins University as previously described.19 MOG-IgG seropositivity was defined as a positive assay at a dilution of ≥1:20.
All MOG-IgG seropositive patients were seronegative for AQP4-IgG. Conversely, AQP4-IgG seropositive participants were not systemically tested for MOG-IgG. Additionally, healthy controls (HC) and subjects with a history of ON and a clinical diagnosis of relapsing-remitting MS (MS-ON), approximately age and sex-matched to the AQP4-ON and MOG-ON cohorts, were included from an ongoing prospective observational OCT study at our center. MS participants fulfilled 2010 McDonald criteria, and had a clinical course and imaging typical for MS.21 HC were recruited from Johns Hopkins University staff and patients’ spouses. OCT evaluations and visual function testing were performed for all participants at a timepoint between September 2008 and March 2018.
Only eyes with a history of ON were included in analyses. For participants with a bilateral history of ON (synchronous or asynchronous), both eyes were included in the analyses. Eyes that had experienced ON <3 months prior to evaluation were excluded from the analysis. Individuals with uncontrolled diabetes or hypertension, history of ocular surgery/trauma, glaucoma, and/or other ophthalmologic disorders were excluded from the study.
Optical coherence tomography
Retinal imaging was performed with spectral domain OCT (Cirrus HD-OCT, Model 5000; Carl Zeiss Meditec, Dublin, CA), as previously described.22 Briefly, peri-papillary and macular data were obtained with the Optic Disc Cube 200×200 protocol and Macular Cube 512×128 protocol, respectively. For eyes with poor visual function (unable to fixate), OCT scans were acquired with external fixation of the fellow eye. OCT scans underwent rigorous quality control, in accordance with OSCAR-IB criteria, and only scans passing the quality control process were included in the analyses.23
Global and quadrantal (i.e. superior, nasal, inferior and temporal) peri-papillary RNFL (pRNFL) thicknesses were estimated by use of the software incorporated in the Cirrus HD-OCT device. Automated macular segmentation was performed, as described in detail elsewhere.24 Average retinal layer thickness values were obtained within an annulus, centered on the fovea, with an internal diameter of 1mm and an external diameter of 5mm. Segmentations were reviewed for accuracy by a rater masked to clinical status (AF).
All macular cube scans were also assessed in a blinded fashion by experienced reviewers (AF and ESS) for abnormalities including macular microcystoid pathology (MMP; also referred to in the literature as microcystic macular edema [MME]).9,10
OCT methods and results are reported in accordance with consensus APOSTEL recommendations.25
Visual Function
Monocular, habitual-corrected visual acuity was assessed using standardized retro-illuminated eye charts (Precision Vision, La Salle, IL). High-contrast (100%) Early Treatment Diabetic Retinopathy Study (ETDRS) charts (at 4m) and low-contrast (2.5%) Sloan Letter charts (at 2m) were used. The total number of letters correctly identified on each chart was recorded to determine high-contrast (HCLA) and low-contrast (LCLA) letter-acuity scores for each contrast level (maximum score of 70 letters, corresponding to a Snellen visual acuity of 20/10). The presented letter-acuity scores may be converted to LogMAR (logarithm of the minimum angle of resolution) as follows: LogMAR = (−0.02) * Letters + 1.1
Statistical methods
Statistical analyses were performed with Stata 15 (StataCorp, College Station, TX). Statistical significance was defined as p<0.05. Analyses were not adjusted for multiple comparisons, given the exploratory nature of the study.26
Comparisons between groups were performed with one-way ANOVA (age), Kruskal-Wallis test (number of ON episodes, time elapsed from initial and last ON episode), and Fisher’s exact test (sex, race and presence of MMP). OCT measures were compared between groups with linear generalized estimating equations (GEE) models, accounting for within-subject inter-eye correlations (given inclusion of both eyes from participants with bilateral history of ON). Comparisons of OCT measures were performed in univariate models, as well as in models including age, sex, and race. Letter-acuity scores were similarly compared between groups with GEE. Analyses of HCLA were also performed in models including GCIPL or pRNFL thickness and their interactions with ON group, as well as in models further including the presence of MMP. Marginal effects were estimated from these models and compared between groups using the delta-method.
RESULTS
Study population and Clinical Characteristics
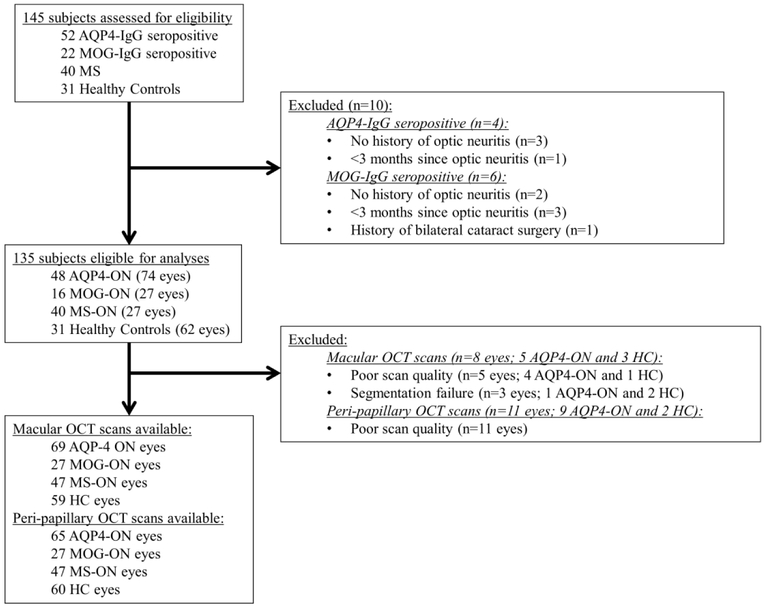
Eyes with a history of ON fulfilling study eligibility criteria from 16 MOG-ON (27 eyes), 48 AQP4-ON (74 eyes) and 40 MS-ON (47 eyes) participants were studied. In addition, 31 HC (62 eyes) participants were also assessed (Figure 1, Table 1). Age and sex did not differ between groups. Race differed between groups (p=0.04), with higher proportions of African-Americans in the AQP4-ON (48%) and MS-ON (38%) groups, compared to MOG-ON (19%) and HC (16%).
Figure 1. Study flowchart.
Abbreviations: ON: optic neuritis; AQP4: aquaporin-4; MOG: myelin oligodendrocyte glycoprotein; MS: multiple sclerosis
Table 1.
Demographics and optic neuritis history characteristics.
| AQP4-ON | MOG-ON | MS-ON | HC | P-value | |
|---|---|---|---|---|---|
| Subjects (eyes) | 48 (74) | 16 (27) | 40 (47) | 31 (62) | |
| Age, years, mean (SD) | 43.7 (12.7) | 43.8 (13.3) | 41.5 (12.6) | 41.5 (14.1) | 0.81 |
| Female sex, n (%) | 43 (90%) | 12 (75%) | 28 (70%) | 22 (71%) | 0.08 |
| Race, n (%)a | |||||
| Caucasian American | 23 (48%) | 11 (69%) | 24 (60%) | 24 (77%) | |
| African American | 23 (48%) | 3 (19%) | 15 (38%) | 5 (16%) | 0.04 |
| Other | 2 (4%) | 2 (13%) | 1 (3%) | 2 (6%) | |
| Microcystoid macular pathology, eyes, n (%) | 14 (19%) | 3 (11%) | 3 (6%) | - | 0.12 |
| Microcystoid macular pathology, patients, n (%) | 12 (26%) | 2 (13%) | 3 (8%) | - | 0.08 |
| Episodes of ON per eye, median (range) | 1 (1-5) | 2 (1-16) | 1 (1-2) | - | <0.001 |
| Time since initial ON (by eye), years, median (IQR)b | 5.9 (2.1-10.4) | 2.1 (1.0-3.1) | 4.6 (2.1-7.7) | - | <0.001 |
| Time since last ON (by eye), years, median (IQR)b | 4.8 (1.6-7.7) | 0.8 (0.4-3.1) | 4.1 (2.1-7.1) | - | <0.001 |
The sum of percentages is not 100% due to rounding
Time since initial/last ON refers to time elapsed from ON to OCT and visual function testing.
Abbreviations: ON: optic neuritis; AQP4-ON: aquaporin-4-IgG seropositive ON; MOG-ON: myelin oligodendrocyte glycoprotein-IgG seropositive ON; MS-ON: multiple sclerosis ON; HC: healthy control; OCT: optical coherence tomography; SD: standard deviation; IQR: interquartile range
MMP was present in 3 MOG-ON eyes (11%), 14 AQP4-ON eyes (19%) and 3 MS-ON eyes (6%). MOG-ON eyes (median: 2 ON episodes) had experienced more prior episodes of ON compared to both AQP4-ON (median: 1 ON episode; p=0.004) and MS-ON eyes (median: 1 ON episode; p<0.001), and AQP4-ON eyes had experienced more episodes of ON compared to MS-ON eyes (p=0.02). As defined in our inclusion criteria, all eyes included in the analyses were >3 months from an ON episode. However, time elapsed between first or last ON and OCT/visual function testing was shorter in MOG-ON eyes as compared to both AQP4-ON (p<0.001) and MS-ON eyes (p<0.001), but did not differ between AQP4-ON and MS-ON eyes.
Of the MOG-ON and AQP4-ON participants, 7 (44%) and 10 (21%), respectively, had not experienced clinical neurological syndromes other than optic neuritis prior to evaluation. Other clinical manifestations in the MOG-ON group included transverse myelitis (n=7; 44%), encephalopathy (n=4; 25%) and a brainstem syndrome (n=1; 6%). In the AQP4-ON group other clinical manifestations included transverse myelitis (n=37; 77%) and brainstem syndromes (n=11; 23%). MOG-IgG testing had been performed >30 days from a clinical event in the majority of MOG-ON participants (n=13; 81%). All MOG-ON participants tested within 30 days of a clinical event (n=3; 19%) had experienced recurrent ON and at least one clinical attack involving a CNS location other than the optic nerve (transverse myelitis, brainstem syndrome and/or acute disseminated encephalomyelitis), consistent with a MOG-IgG related disease phenotype.
Optical coherence tomography and visual function measures by group
OCT and visual function measures are summarized and compared between groups in Table 2 and Table 3, respectively. Unadjusted analyses revealed that, as expected, GCIPL, global pRNFL, and quadrantal pRNFL thicknesses were lower in all ON groups compared to HC (p≤0.01 for all). MOG-ON and AQP4-ON eyes had lower GCIPL and global pRNFL thicknesses compared to MS-ON eyes (p≤0.001 for all), but these did not differ significantly between MOG-ON and AQP4-ON eyes. Similar to the global pRNFL, superior, inferior, and nasal pRNFL thicknesses were thinner in MOG-ON and AQP4-ON compared to MS-ON eyes (p≤0.001 for all), but did not differ between MOG-ON and AQP4-ON eyes. However, temporal pRNFL thickness was lower in MOG-ON eyes compared to AQP4-ON (p=0.003) and MS-ON (p=0.02) eyes, but did not differ between AQP4-ON and MS-ON eyes. ONL thickness was lower in AQP4-ON eyes compared to MOG-ON (p=0.04), MS-ON (p=0.004) and HC (p=0.02), but did not differ otherwise between groups. INL thickness did not differ between groups. Analyses including age, sex, and race did not alter any of the above findings. Comparisons amongst ON groups were additionally performed accounting for number of ON episodes (single vs. multiple) and findings were similarly unchanged, with the exception of the difference in ONL thickness between AQP4-ON and MOG-ON which did not retain statistical significance (p=0.052).
Table 2.
OCT measures and letter-acuity scores by group.
| AQP4-ON | MOG-ON | MS-ON | HC | |
|---|---|---|---|---|
| OCT measures, μm, mean (SD) | ||||
| Global pRNFLa | 67.5 (15.6) | 60.9 (11.2) | 78.9 (11.5) | 91.3 (10.0) |
| Superior quadranta | 80.4 (23.5) | 72.8 (16.3) | 100.6 (19.4) | 114.2 (15.3) |
| Nasal quadranta | 60.0 (10.3) | 56.9 (7.4) | 65.3 (8.8) | 71.5 (12.1) |
| Inferior quadranta | 80.8 (30.5) | 74.3 (18.6) | 102.2 (19.2) | 119.7 (17.7) |
| Temporal quadranta | 48.6 (12.3) | 39.8 (10.6) | 47.3 (10.6) | 60.1 (10.0) |
| GCIPLb | 54.9 (10.9) | 56.5 (6.5) | 64.3 (10.4) | 75.9 (4.7) |
| INLb | 43.9 (4.0) | 44.6 (3.7) | 44.7 (4.7) | 45 (2.5) |
| ONLb | 65.9 (5.6) | 69.6 (5.9) | 69.2 (6.0) | 69 (6.2) |
| Letter-acuity scoresc, median (IQR) | ||||
| 100% contrast (HCLA) | 43.5 (0-54.5) | 57 (53-60) | 59 (50-64) | 60 (58-65) |
| 2.5% contrast (LCLA) | 0 (0-19.5) | 11 (3-15) | 28 (14-34) | 33.5 (28-36) |
Available for 65 AQP4-ON, 27 MOG-ON, 47 MS-ON and 60 HC eyes
Available for 69 AQP4-ON, 27 MOG-ON, 47 MS-ON and 59 HC eyes
Available for 56 AQP4-ON, 21 MOG-ON, 47 MS-ON and 58 HC eyes
Abbreviations: ON: optic neuritis; AQP4-ON: aquaporin-4-IgG seropositive ON; MOG-ON: myelin oligodendrocyte glycoprotein-IgG seropositive ON; MS-ON: multiple sclerosis ON; HC: healthy control; SD: standard deviation; IQR: inter-quartile range; OCT: optical coherence tomography; pRNFL: peri-papillary retinal nerve fiber layer; GCIPL: ganglion cell+inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer; HCLA: high-contrast letter-acuity; LCLA: low-contrast letter acuity
Table 3.
Comparison of optical coherence tomography measures and letter-acuity scores between groups
| AQP4-ON vs. MOG-ON |
AQP4-ON vs. MS-ON |
MOG-ON vs. MS-ON |
AQP4-ON vs. HC |
MOG-ON vs. HC |
MS-ON vs. HC |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Betad (95% CI) |
P | Betad (95% CI) |
P | Betad (95% CI) |
P | Betad (95% CI) |
P | Betad (95% CI) |
P | Betad (95% CI) |
P | |
| OCT measures, μm | ||||||||||||
| Global pRNFLa | 6.0 (−0.5 to 12.5) |
0.07 |
−11.5 (−16.8 to −6.3) |
<0.001 |
−17.5 (−23.8 to −11.2) |
<0.001 |
−24.1 (−29.3 to −18.8) |
<0.001 |
−30.1 (−36.3 to −23.8) |
<0.001 |
−12.5 (−17.5 to −18.8) |
<0.001 |
| Superior quadranta | 6.8 (−2.7 to 16.3) |
0.16 |
−20.5 (−28.8 to −12.2) |
<0.001 |
−27.3 (−36.8 to −17.7) |
<0.001 |
−34.1 (−41.8 to −26.4) |
<0.001 |
−40.9 (−49.9 to −31.9) |
<0.001 |
−13.6 (−21.4 to −26.4) |
0.001 |
| Nasal quadranta | 2.4 (−1.7 to 6.6) |
0.25 |
−5.6 (−9.3 to −1.9) |
0.001 |
−8.0 (−12.2 to −3.9) |
<0.001 |
−11.9 (−16.7 to −7.0) |
<0.001 |
−14.3 (−19.5 to −9.1) |
<0.001 |
−6.3 (−11.1 to −7.0) |
0.01 |
| Inferior quadranta | 5.4 (−5.9 to 16.8) |
0.35 |
−21.9 (−31.2 to −12.6) |
<0.001 |
−27.3 (−37.9 to −16.7) |
<0.001 |
−39.5 (−49.0 to −30.0) |
<0.001 |
−44.9 (−55.7 to −34.2) |
<0.001 |
−17.6 (−26.1 to −30.0) |
<0.001 |
| Temporal quadranta |
9.1 (3.1 to 15.1) |
0.003 | 1.8 (−2.8 to 6.4) |
0.43 |
−7.2 (−13.2 to −1.3) |
0.02 |
−11.2 (−15.9 to −6.5) |
<0.001 |
−20.2 (−26.3 to −14.2) |
<0.001 |
−13 (−17.7 to −6.5) |
<0.001 |
| GCIPLb | −1.6 (−5.5 to 2.3) |
0.43 |
−9.1 (−13.1 to −5.1) |
<0.001 |
−7.6 (−11.9 to −3.2) |
0.001 |
−21.0 (−23.9 to −18) |
<0.001 |
−19.4 (−22.8 to −16.0) |
<0.001 |
−11.8 (−15.4 to −18.0) |
<0.001 |
| INLb | −0.5 (−2.7 to 1.6) |
0.62 | −0.6 (−2.5 to 1.2) |
0.50 | −0.1 (−2.4 to 2.2) |
0.93 | −1.1 (−2.5 to 0.3) |
0.14 | −0.5 (−2.5 to 1.5) |
0.60 | −0.4 (−2.2 to 0.3) |
0.62 |
| ONLb |
−3.4 (−6.6 to −0.2) |
0.04 |
−3.7 (−6.2 to −1.2) |
0.004 | 1.1 (−3.0 to 5.2) |
0.61 |
−3.2 (−5.9 to −0.6) |
0.02 | 0.1 (−3.4 to 3.6) |
0.95 | 0.5 (−2.4 to 3.4) |
0.75 |
| Letter-acuity scoresc | ||||||||||||
| 100% contrast (HCLA) |
−22.3 (−30.0 to −14.7) |
<0.001 |
−21.7 (−29.4 to −14.0) |
<0.001 | 0.6 (−5.5 to 6.7) |
0.85 |
−28.0 (−34.7 to −21.3) |
<0.001 |
−5.7 (−10.4 to −1.0) |
0.02 |
−6.3 (−11.1 to −1.4) |
0.01 |
| 2.5% contrast (LCLA) | −3.1 (−8.7 to 2.5) |
0.28 |
−14.7 (−19.4 to −9.9) |
<0.001 |
−11.6 (−17.7 to −5.5) |
<0.001 |
−23.0 (−27.0 to −19.1) |
<0.001 |
−20.0 (−25.4 to −14.5) |
<0.001 |
−8.4 (−13.0 to −3.7) |
0.001 |
Available for 65 AQP4-ON, 27 MOG-ON, 47 MS-ON and 60 HC eyes
Available for 69 AQP4-ON, 27 MOG-ON, 47 MS-ON and 59 HC eyes
Available for 56 AQP4-ON, 21 MOG-ON, 47 MS-ON and 58 HC eyes
Results are derived from unadjusted linear generalized estimating equations (GEE) models. For OCT measures, the beta coefficients correspond to difference in μm between groups. For letter-acuity scores, the beta coefficients correspond to difference in number of letters correct between groups.
Abbreviations: ON: optic neuritis; AQP4-ON: aquaporin-4-IgG seropositive ON; MOG-ON: myelin oligodendrocyte glycoprotein-IgG seropositive ON; MS-ON: multiple sclerosis ON; HC: healthy control; CI: confidence interval; pRNFL: peri-papillary retinal nerve fiber layer; GCIPL: ganglion cell+inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer; HCLA: high-contrast letter-acuity; LCLA: low-contrast letter acuity
HCLA and LCLA were worse in all ON eye groups compared to HC (HCLA: p≤0.02; LCLA p≤0.001). AQP4-ON eyes had worse HCLA compared to MOG-ON (−22.3±3.9 letters; p<0.001) and MS-ON eyes (−21.7±4.0 letters; p<0.001), and HCLA did not differ between MOG-ON and MS-ON. LCLA was better in MS-ON compared to MOG-ON (−11.6±3.1 letters; p=0.002) and AQP4-ON (−14.7±2.4 letters; p<0.001), and did not differ between MOG-ON and AQP4-ON.
Consistent with prior studies, comparisons of MMP and non-MMP ON eyes, accounting for ON group, revealed that presence of MMP was, independently of diagnosis, associated with reduced GCIPL (−8.1±1.7μm; p<0.001) and pRNFL thickness (−9.1±2.4μm; p<0.001), increased INL thickness (5.0±1.1μm; p<0.001), and worse HCLA (−17.9±5.1 letters; p<0.001) and LCLA (−12.2±2.5 letters; p<0.001). MMP was not associated with ONL thickness (1.3±1.0μm; p=0.17).
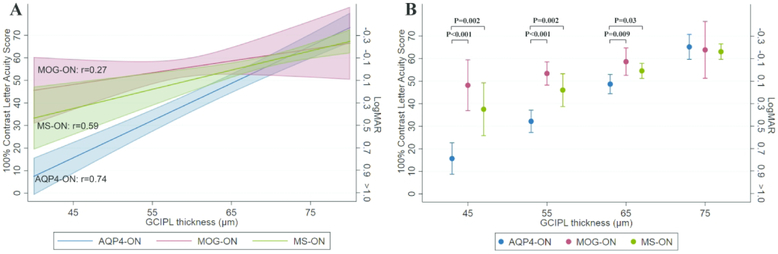
Given the relative preservation of HCLA in MOG-ON, despite severely reduced inner retinal layer thicknesses, further multivariate analyses were performed including additionally GCIPL or pRNFL thickness and their interactions with ON group. Lower GCIPL thickness was associated with worse HCLA in AQP4-ON (−16.5±1.5 letters per 10μm decrease; p<0.001) and MS-ON eyes (−8.5±2.3 letters per 10μm decrease; p<0.001), but not in MOG-ON eyes (−5.2±3.8 letters per 10μm decrease; p=0.17). Importantly, the relationship between GCIPL and HCLA differed significantly between AQP4-ON and the other ON groups (MOG-ON: p=0.006; MS-ON: p=0.004), but not between MOG-ON and MS-ON (p=0.46), supporting that a decrease in GCIPL thickness of the same amount is associated with more severe visual loss in AQP4-ON compared to MOG-ON and MS-ON (Figure 2a). Figure 2b shows results of comparisons between ON groups of point estimates of HCLA obtained from this model, at GCIPL thicknesses of 45 to 75μm in increments of 10μm (the range of GCIPL thickness in ON eyes was approximately 40 to 80μm).
Figure 2. Estimated HCLA by group and GCIPL thickness.
Figure 2a demonstrates estimates (solid line) and 95% confidence intervals (shaded area) of HCLA by GCIPL thickness, separately for AQP4-ON (blue), MOG-ON (red) and MS-ON (green), derived from generalized estimating equations (GEE) model including ON group, GCIPL thickness and their interaction. Pearson correlation coefficients are shown for each group.
Figure 2b shows, similarly derived, point estimates and 95% confidence intervals of HCLA by group at specific values of GCIPL thickness. P-values are shown for statistically significant comparisons between groups.
Abbreviations: ON: optic neuritis; AQP4-ON: aquaporin-4-IgG seropositive ON; MOG-ON: myelin oligodendrocyte glycoprotein-IgG seropositive ON; MS-ON: multiple sclerosis ON; CI: confidence interval; GCIPL: ganglion cell+inner plexiform layer; HCLA: high-contrast letter-acuity
Results of analyses including pRNFL similarly revealed that lower pRNFL thickness was associated with worse HCLA in AQP4-ON (−7.1±1.4 letters per 10μm decrease; p<0.001) and MS-ON eyes (−7.4±1.9 letters per 10μm decrease; p<0.001), but not in MOG-ON eyes (1.6±0.9 letters per 10μm decrease; p=0.072). The relationship between pRNFL and HCLA differed significantly between MOG-ON and the other ON groups (p<0.001), but not between AQP4-ON and MS-ON (p=0.91).
Importantly, addition of MMP to these models did not alter these results, and presence of MMP was not independently associated with HCLA when accounting for GCIPL thickness (−6.8±5.0 letters; p=0.17), but was significant when accounting for pRNFL thickness (−14.5±5.4 letters; p=0.01). Notably, models including GCIPL thickness (R2: 0.60 without MMP, 0.61 with MMP) had better fit than models including pRNFL thickness (R2: 0.39 without MMP, 0.45 with MMP).
Furthermore, despite the observation of lower ONL thickness in AQP4-ON eyes, there was no association between ONL thickness and HCLA in any of the groups (not shown). Given the basement effect observed with LCLA (most prominent in the AQP4-ON group in which 57% of eyes with available LCLA had a score of 0 letters), correlation analyses of LCLA with OCT measures were not performed.
Finally, given the wide-range of HCLA in the AQP4-ON relative to the MOG-ON eyes (Table 1), we performed a sensitivity analysis comparing AQP4-ON and MS-ON eyes matched 1:1 to MOG-ON eyes based on HCLA (Table 4). In the case that more than one eligible eye was available in the AQP4-ON or MS-ON groups, only one was randomly selected. Despite matching on HCLA, MOG-ON eyes had lower GCIPL and pRNFL thicknesses, as well as lower LCLA, compared to both AQP4-ON and MS-ON matched eyes.
Table 4.
Comparison of optical coherence tomography measures and letter-acuity scores between eyes matched to the MOG-ON group on high-contrast letter acuity.
| AQP4-ON (n=21 eyes) |
MOG-ON (n=21 eyes) |
MS-ON (n=21 eyes) |
AQP4-ON vs. MOG-ON | AQP4-ON vs. MS-ON | MOG-ON vs. MS-ON | ||||
|---|---|---|---|---|---|---|---|---|---|
| Beta* (95% CI) | P-value | Beta* (95% CI) | P-value | Beta* (95% CI) | P-value | ||||
| OCT measures, um, mean(SD) | |||||||||
| Global pRNFL | 76.5 (17.9) | 61.2 (11.7) | 79.6 (11.8) | 17.2 (7.2 to 27.3) | 0.001 | −1.1 (−10.7 to 8.5) | 0.82 | −18.4 (−26.3 to −10.4) | <0.001 |
| Superior quadrant | 96.7 (25.0) | 73.1 (17.2) | 101.9 (20.3) | 25.9 (11.8 to 40.1) | <0.001 | −2.9 (−17.2 to 11.4) | 0.69 | −28.9 (−41.2 to −16.5) | <0.001 |
| Nasal quadrant | 62.6 (13.0) | 56.6 (7.2) | 64.6 (8.8) | 6.9 (0 to 13.8) | 0.05 | −0.8 (−7.9 to 6.3) | 0.82 | −7.7 (−12.8 to −2.6) | 0.003 |
| Inferior quadrant | 98.5 (35.9) | 74.5 (18.1) | 102.3 (21.5) | 26.2 (7.1 to 45.2) | 0.007 | −2.0 (−21.0 to 16.9) | 0.83 | −28.2 (−41.8 to −14.7) | <0.001 |
| Temporal quadrant | 47.8 (10.0) | 41.2 (11.3) | 49.4 (9.4) | 7.7 (0 to 15.3) | 0.049 | −0.3 (−6.4 to 5.8) | 0.92 | −7.9 (−15.6 to −0.3) | 0.04 |
| GCIP | 63.6 (9.3) | 56.5 (6.9) | 64.7 (11.1) | 7.6 (2.1 to 13.0) | 0.006 | −0.8 (−7.1 to 5.4) | 0.80 | −8.4 (−14.4 to −2.4) | 0.006 |
| INL | 44.2 (2.8) | 44.3 (4.0) | 44.4 (6.7) | −0.3 (−2.8 to 2.3) | 0.84 | −0.5 (−3.7 to 2.7) | 0.76 | −0.2 (−4.0 to 3.5) | 0.90 |
| ONL | 67.2 (4.5) | 68.4 (5.6) | 68.2 (12.7) | −1.2 (−4.9 to 2.6) | 0.54 | −1.0 (−7.1 to 5.0) | 0.74 | 0.1 (−6.3 to 6.6) | 0.97 |
| Letter-acuity scores, median (IQR) | |||||||||
| 100% contrast (HCLA) | 56 (53-59) | 57 (53-60) | 57 (53-60) | 0.0 (−5.9 to 5.8) | 0.99 | −0.5 (−6.2 to 5.3) | 0.87 | −0.4 (−6.6 to 5.7) | 0.89 |
| 2.5% contrast (LCLA) | 24 (8-30) | 11 (3-15) | 27 (18-30) | 8.4 (1.2 to 15.5) | 0.02 | −2.3 (−9.5 to 5.0) | 0.54 | −10.6 (3.7 to 17.5) | 0.002 |
For OCT measures corresponds to difference in μm between groups. For letter-acuity scores corresponds to difference in number of letters correct between group.
Abbreviations: ON: optic neuritis; AQP4-ON: aquaporin-4-IgG seropositive ON; MOG-ON: myelin oligodendrocyte glycoprotein-IgG seropositive ON; MS-ON: multiple sclerosis ON; CI: confidence interval; pRNFL: peri-papillary retinal nerve fiber layer; GCIPL: ganglion cell+inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer; HCLA: high-contrast letter-acuity; LCLA: low-contrast letter acuity
DISCUSSION
We have found that AQP4-IgG seropositivity is associated with worse visual outcomes after ON, as compared with MOG-IgG or MS associated ON, a disparity that increases with decreasing GCIPL thickness. This finding suggests that macular structure-function correlation, as defined by evaluation of macular GCIPL thickness (considered to represent macular retinal ganglion cell integrity) and visual acuity, may vary by ON etiology. Also, this lends support to the notion that GCIPL thickness in ON may be representative of pathologically heterogeneous processes and/or that additional pathologic factors, possibly not involving the retinal ganglion cells, may contribute to visual dysfunction following ON.
Interestingly, in analyses of structure-function correlation including pRNFL rather than GCIPL thickness, we found similar findings when comparing MOG-ON to AQP4-ON eyes, however the relationship of pRNFL thickness and HCLA did not differ between MS-ON eyes and AQP4-ON eyes. However, the fit was better for models including GCIPL thickness, which is expected given that pRNFL thickness is representative of global retinal axonal integrity, whereas GCIPL thickness is a macular measure, thus corresponding to injury of axons originating in areas serving central vision. Thus, it is plausible that the findings when pRNFL thickness was included in the analysis may be affected by differences in the topographical involvement of the optic nerve fibers between the ON groups, however given that the GCIPL thickness measure is specific to fibers serving central vision, this would not be expected to be a contributor to the differential relationships of GCIPL thickness and HCLA by ON group.
The pathoetiology of worse visual function in AQP4-ON compared to MOG-ON and MS-ON, even with similar degree of macular GCIPL thinning, is not clear. MMP has been proposed as a factor that may contribute to poor visual outcomes following ON.10,12 In line with prior studies, we found that MMP eyes, as compared to non-MMP eyes, had reduced GCIPL and pRNFL thickness, as well as worse visual function, and the prevalence of MMP was highest in AQP4-ON.8-12 However, when accounting for GCIPL thickness and ON etiology, MMP was not independently associated with HCLA. This finding suggests that MMP may represent a marker of optic neuropathy severity, rather than an independent process that directly contributes to visual dysfunction after ON.
Importantly, the underlying pathophysiology differs between these conditions. AQP4-IgG associated disease is recognized as an autoimmune astrocytopathy, MOG-IgG associated disease likely results directly from an autoimmune response directed against MOG on myelin sheaths with pathologic studies demonstrating prominent antibody and complement deposition (resembling type II MS lesions), and MS represents a complex and pathologically heterogeneous entity with neuropathologic changes including inflammatory demyelination, axonal transection/degeneration and gliosis.20,27 Direct retinal damage involving AQP4-IgG is an important consideration as an explanation for worse visual outcomes as AQP4 is highly expressed in the retina by Müller cells (the cell bodies of which are located in the INL) and astrocytes (mainly located in the RNFL), especially in end-feet membranes facing blood vessels. Notably, Müller cells are involved in multiple homeostatic functions in the retina and loss of AQP4 immunoreactivity on Müller cells has been demonstrated in a pathologic study of AQP4-IgG+ NMOSD retinas.13 AQP4 deletion results in a decreased capacity of Müller cells to withstand osmotic stress and induces retinal inflammation, and selective ablation of Müller cells has been shown to lead to photoreceptor apoptosis and vascular retinal abnormalities.28,29
Interestingly, thinning of the fovea has been observed in AQP4-IgG+ eyes without a history of ON and in the absence of GCIP or pNRFL thinning, and it has been postulated that this may represent a subclinical primary retinal pathology due to direct targeting of retinal Müller cells.30 Furthermore, retinal vascular alterations have been reported in vivo in NMO and pathologic studies have identified prominent vascular fibrosis and hyalinization in NMO lesions.31,32 This is a potential explanation for the decreased ONL thickness that was found in AQP4-ON eyes; however, the observed differences between groups were modest and ONL thickness was not associated with visual function. Another consideration is that differences in the ability for functional compensation of the visual system between these conditions may have influenced our results.
Additionally, we found that the quadrantal pattern of thinning differed in AQP4-ON compared to MOG-ON and MS-ON. Prior studies have demonstrated that in NMO, pRNFL thinning is more pronounced in the superior and inferior quadrants, compared to the typical temporal predominant pRNFL thinning pattern that is observed in MS.31,33 Our results confirm this finding in AQP4-ON, in which despite severely decreased global pRNFL thickness relative to MS-ON, there was no difference in the temporal quadrant pRNFL thickness. Interestingly however, in MOG-ON we observed thinning in all pRNFL quadrants compared to MS-ON eyes, as well as compared to AQP4-ON, despite the fact that AQP4-ON and MOG-ON eyes had similar global pRNFL thickness. This suggests that the pattern of pRNFL thinning may have diagnostic utility in distinguishing ON etiology. The pathophysiology underlying the observed differences in the quadrantal patterns is not clear; however, the pattern observed in AQP4-ON is consistent with vascular optic neuropathies in which the arcuate fibers (located in the superior and inferior quadrants) are predominantly affected, which further supports the possibility of a vascular contribution to optic neuropathy in AQP4-ON.31
Furthermore, our results demonstrate in MOG-ON an impressive discordance between the severity of inner retinal layer thinning and visual outcomes. Despite severely reduced inner retinal layer thicknesses in MOG-ON, to a similar degree to that observed in AQP4-ON, visual outcomes differed markedly, with relative preservation of visual acuity in MOG-ON eyes. Retinal pathologic studies in MOG-ON are lacking, but a small number of reported histopathologic studies of diagnostic brain biopsies in MOG-IgG associated disease have shown plaque-like myelin loss with relative axonal preservation.27 Importantly, the retina is normally an unmyelinated structure, and thus devoid of MOG.34 Consequently retinal changes observed in MOG-ON would be expected to be due to retrograde degenerative processes. The severity of inner retinal layer thinning detected with OCT in MOG-ON appears to support that retinal neuro-axonal integrity is severely compromised in MOG-ON. However, given the relatively preserved visual acuity in MOG-ON compared to AQP4-ON and the reported neuropathologic findings in MOG-IgG associated disease, an important consideration is that the relative contributions of the retinal ganglion cells to GCIPL and RNFL thickness may differ between these two conditions. Notably, a large proportion of RNFL thickness is accounted for by astrocytes and their processes, and microglia are distributed in a laminar pattern in the plexiform layers (including the inner plexiform layer, which is a major component of GCIPL thickness).35,36 Thus, it is conceivable that differences between AQP4-ON and MOG-ON in the dynamics of glial activation could lead to differing compositions and thicknesses of these layers.
Our study has a number of limitations that warrant discussion. Firstly, although this is one of the largest studies assessing MOG-ON and AQP4-ON eyes to date, our sample size was relatively low, which is expected given the rarity of these conditions. Thus, independent validation of these findings in other cohorts will be especially important. Another limitation is that MS-ON participants were not systemically evaluated for presence of AQP4-IgG or MOG-IgG antibodies. However, MS-ON participants were included only if they had a typical clinical course and imaging findings. In a large US study of a patient population carrying a diagnosis of MS, AQP4-IgG+ NMOSD was misdiagnosed as MS only in 0.2% of MS patients and all the misdiagnosed cases had a history of longitudinally extensive transverse myelitis.37 Furthermore, it has been estimated that across 25 studies employing the MOG-CBA with immunofluorescence (which was applied in the present study), only 1% of people with MS were MOG-IgG seropositive, of which half were pediatric cases, and predominantly included patients with borderline titers.20 Studies employing MOG-CBA with flow cytometry (mostly those published prior to 2016) found that 6% of people with MS were considered to be MOG-IgG seropositive, although it has been postulated that this observation may reflect use of sub-optimal assay cut-offs.20 Overall, this evidence supports that the likelihood of MOG-IgG or AQP4-IgG seropositivity in our adult MS-ON cohort is extremely low. Also in our study, AQP4-ON participants were not evaluated systematically for MOG-IgG, however existing data supports that “double-positive” cases are exceedingly rare, making it extremely unlikely that this would impact our findings.20 Finally, MOG-ON eyes at baseline had a shorter time since their initial and last ON episode, relative to AQP4-ON and MS-ON eyes. However, only eyes that were >3 months from an episode of acute ON were included in the analysis, and the literature supports that the vast majority of GCIPL and pRNFL thinning, as well as maximal visual recovery, has already occurred at 3 months following ON.38,39 It is conceivable that the time course of change in retinal layer thicknesses and visual recovery may differ in rarer causes of ON (including AQP4-ON and MOG-ON); however, one would expect that the differences in our groups would lead us to underestimate the severity of GCIPL and pRNFL thinning and overestimate the severity of visual dysfunction in MOG-ON (given the shorter time elapsed since ON). Contrary to this, we found marked GCIPL and pRNFL thinning in MOG-ON eyes with relatively preserved visual acuity.
In conclusion, our study provides compelling evidence that AQP4-IgG seropositivity is associated with worse visual outcomes after ON, as compared with MOG-ON and MS-ON, even with similar severity of GCIPL thinning. The pathophysiological underpinnings of the diverging macular structure-function correlations in these conditions are not clear. Future studies are necessary to confirm and expand on these findings, and potentially identify novel therapeutic targets in ON.
ACKNOWLEDGEMENTS
We would like to acknowledge support for the statistical analysis from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Study Funding: This study was funded by the National Institutes of Health (5R01NS082347 to P.C.; 4K08NS078555 to M.L.) and National MS Society (FP-1607-24999 to E.S.; RG-1606-08768 to S.S).
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
Disclosures:
Elias Sotirchos is funded by a Sylvia Lawry physician fellowship award from the National Multiple Sclerosis Society (NMSS).
Angeliki Filippatou, Santiago Pardo, Jiangxia Wang, Esther Ogbuokiri, Norah Cowley, Nicole Pellegrini, Olwen Murphy, and Maureen Mealy report no disclosures.
Kathryn Fitzgerald is funded by postdoctoral fellowships from the NMSS and Consortium of MS Centers (CMSC).
Sara Salama receives funding from the Egyptian Ministry of Higher Education.
Jerry Prince is a founder of Sonovex, Inc. and serves on its Board of Directors. He has been a paid consultant for JuneBrain, Inc. within the last year and has currently funded research projects from Biogen, Inc. and 12Sigma Technologies.
Michael Levy currently receives research support from: National Institutes of Health, Maryland Technology Development Corporation, Sanofi, Genzyme, Alexion, Alnylam, Shire, Acorda and Apopharma. He also received personal compensation for consultation with Alexion, Acorda, and Genzyme and he serves on the scientific advisory boards for Alexion, Acorda and Quest Diagnostics.
Peter Calabresi has received personal honorariums for consulting from Disarm Therapeutics and Biogen. He is PI on research grants to Johns Hopkins from MedImmune, Annexon, Biogen, and Genzyme.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen-Idec, Genzyme, Genentech Corporation, EMD Serono & Novartis. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer. He is also the site investigator of a trial sponsored by MedDay Pharmaceuticals.
REFERENCES
- 1.Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53: 1107–14. [DOI] [PubMed] [Google Scholar]
- 2.Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007; 114: 810–5. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005; 202: 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 5.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014; 82: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol 2018; 195: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler 2015; 21: 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012; 135: 1786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013; 80: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS ONE 2013; 8: e71145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelfand JM, Cree BA, Nolan R, et al. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol 2013; 70: 629–633. [DOI] [PubMed] [Google Scholar]
- 13.Hokari M, Yokoseki A, Arakawa M, et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol 2016; 79: 605–624. [DOI] [PubMed] [Google Scholar]
- 14.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 2016; 13: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Lapiscina EH, Sepulveda M, Torres-Torres R, et al. Usefulness of optical coherence tomography to distinguish optic neuritis associated with AQP4 or MOG in neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2016; 9: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havla J, Kümpfel T, Schinner R, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol 2017; 264: 139–151. [DOI] [PubMed] [Google Scholar]
- 17.Stiebel-Kalish H, Lotan I, Brody J, et al. Retinal Nerve Fiber Layer May Be Better Preserved in MOG-IgG versus AQP4-IgG Optic Neuritis: A Cohort Study. PLoS ONE 2017; 12: e0170847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao G, Chen Q, Huang Y, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol 2018; 265: 33–40. [DOI] [PubMed] [Google Scholar]
- 19.Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015; 2: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reindl M and Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 2018. [DOI] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler 2010; 16: 829–839. [DOI] [PubMed] [Google Scholar]
- 23.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB Consensus Criteria for Retinal OCT Quality Assessment. PLOS ONE 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang A, Carass A, Hauser M, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express 2013; 4: 1133–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016; 86: 2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender R and Lange S. Adjusting for multiple testing--when and how?. J Clin Epidemiol 2001; 54: 343–9. [DOI] [PubMed] [Google Scholar]
- 27.Weber MS, Derfuss T, Metz I, et al. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord 2018; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannicke T, Wurm A, Iandiev I, et al. Deletion of aquaporin-4 renders retinal glial cells more susceptible to osmotic stress. J Neurosci Res 2010; 88: 2877–88. [DOI] [PubMed] [Google Scholar]
- 29.Shen W, Fruttiger M, Zhu L, et al. Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci 2012; 32: 15715–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oertel FC, Kuchling J, Zimmermann H, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm 2017; 4: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green AJ and Cree BA. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatr 2009; 80: 1002–5. [DOI] [PubMed] [Google Scholar]
- 32.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 2002; 125: 1450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology 2009; 72: 1077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FitzGibbon T and Nestorovski Z. Morphological consequences of myelination in the human retina. Exp Eye Res 1997; 65: 809–819. [DOI] [PubMed] [Google Scholar]
- 35.Ogden TE. Nerve fiber layer of the primate retina: thickness and glial content. Vision Res 1983; 23: 581–587. [DOI] [PubMed] [Google Scholar]
- 36.Silverman SM and Wong WT. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu Rev Vis Sci 2018; 4: 45–77. [DOI] [PubMed] [Google Scholar]
- 37.Pittock SJ, Lennon VA, Bakshi N, et al. Seroprevalence of Aquaporin-4–IgG in a Northern California Population Representative Cohort of Multiple Sclerosis. JAMA Neurol 2014; 71: 1433–1436. [DOI] [PubMed] [Google Scholar]
- 38.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 2012; 135: 521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck RW, Cleary PA and Backlund JC. The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology 1994; 101: 1771–1778. [DOI] [PubMed] [Google Scholar]