Abstract
Background:
Depression is a heterogeneous collection of symptoms. Prior meta-analyses using symptom sum scores have shown the Internet intervention, Deprexis, to be an efficacious treatment for depression. However, no prior research has investigated how Deprexis (or any other Internet intervention for depression) impacts specific symptoms of depression. The current study utilizes symptom-level analyses to examine which symptoms are directly, indirectly, or minimally influenced by treatment.
Methods:
Network analysis and mean-level approaches examined which symptoms, assessed by the Quick Inventory of Depression Symptoms (QIDS-SR), were affected by an 8-week course of Deprexis compared to a waitlist in a nationally recruited sample from the United States (N = 295).
Results:
Deprexis directly improved the symptoms of sadness and indecision. Change in these symptoms, in turn, were associated with change in early insomnia, middle insomnia, self-dislike, fatigue, anhedonia, suicidality, slowness, and agitation. All of these symptoms (except for agitation and early insomnia) show decreases with Deprexis compared to a waitlist after correcting for multiple comparisons. Six additional symptoms, particularly the somatic symptoms, were not impacted by Deprexis compared to waitlist.
Conclusions:
In this sample, the efficacy of Deprexis was due to its direct impact on sadness and indecision. Examining treatment-related change in specific symptoms may facilitate a more nuanced understanding of how a treatment works compared to examining symptom sum scores. Symptom-level approaches may also identify symptoms that do not improve and provide important direction for future treatment development.
Keywords: Depression, Internet, Treatment Outcome
Introduction
Several psychotherapies are considered a well-established and effective treatments for depression (Cuijpers, van Straten, Andersson, & van Oppen, 2008); however, limited access remains a major barrier to receiving treatment (Bower & Gilbody, 2005). Internet delivered treatment offers a cost-effective option for disseminating evidence-based treatment (Andersson & Titov, 2014; Bower & Gilbody, 2005). Some internet treatments have demonstrated acceptability and efficacy for depression (Andrews et al., 2018; Karyotaki et al., 2017), with meta-analytic results supporting effect sizes equivalent to those of in-person delivered CBT (Carlbring, Andersson, Cuijpers, Riper, & Hedman-Lagerlöf, 2018).
A number of Internet-based treatments for depression have been developed (Karyotaki et al., 2017). One promising intervention, called Deprexis, is an individually tailored, web-based treatment for depression that integrates evidence-based approaches including cognitive restructuring, problem solving, behavioral activation, social skills training, as well as mindfulness and acceptance based exercises (Meyer et al., 2009). A recent meta-analysis of randomized clinical trials supports the effectiveness of Deprexis for reducing depression compared to control conditions, with a medium effect size of g = 0.54 (Twomey, O’Reilly, & Meyer, 2017).
In the vast majority of clinical trials, including Deprexis trials, depression severity is assessed by creating a depression symptom sum score, using clinician-administered or self-report measures. This method assumes that all symptoms are equally representative of the syndrome. Thus, symptoms can be added together. However, depression, by definition, is a heterogeneous mix of symptoms. Indeed, an MDE diagnosis can be met with 227 different symptom combinations--all yielding the same diagnosis (Zimmerman, Ellison, Young, Chelminski, & Dalrymple, 2015). Furthermore, if one considers that several symptoms include multiple components (e.g., psychomotor retardation or agitation), then it is possible to meet the criteria for major depression in over 1,000 different ways (Fried & Nesse, 2015a, 2015b). The individual symptoms assessed can differ substantially across depression measures (Fried, 2017) and there is little agreement over which depression symptoms are most important to assess (Fried, Epskamp, Nesse, Tuerlinckx, & Borsboom, 2016)
Symptom sum scores may also be problematic for measuring change in depression severity over time--a central aim for most clinical trials. Analysis of change over time using sum scores rests on two important statistical assumptions: unidimensionality and measurement invariance (Fried, van Borkulo, et al., 2016). Unidimensionality refers to the underlying factor structure of the measure. Specifically, in order to use sum scores, all of the individual items within the measure should load onto a single factor. This is generally not the case with depression measures, many of which are multifactorial. Measurement invariance refers to measuring the same construct across timepoints. In the case of depression scales, this requires confirmation that the distributions of the observed sum scores are constant across timepoints. This assumption is also frequently violated with the most widely used depression measures (Fried, Epskamp, et al., 2016).
One solution to the sum-score problem is examining individual symptoms with network analysis (Fried & Nesse, 2015a). Symptom networks can be represented visually as a series of nodes and edges (McNally et al., 2015). Nodes represent individual symptoms. Edges are partial correlations between symptoms, meaning that they represent the unique relationship between two symptoms while controlling for all of the other symptoms in the network.
Although it is possible to examine each symptom in isolation via traditional regressions, there are advantages to using a network approach (Borsboom & Cramer, 2013). A symptom-level approach can identify which symptoms directly change as a result of treatment. Network analysis also allows for examination of indirect effects (i.e., whether change in the symptom is mediated by change in another symptom), providing a better sense of how treatment impacts symptoms and the interplay among symptoms. .
To our knowledge, few depression studies to date have examined the effect of treatment using a symptom level network analysis approach (i.e., included treatment condition as a node in the network). In onestudy (Bekhuis et al., 2018), treatment condition (short-term psychodynamic supportive psychotherapy either alone or combined with pharmacotherapy) and symptom change scores were entered into a network analysis (N = 186). Adjunctive pharmacotherapy directly changed the symptoms of feeling entrapped and emotional lability. Notably, adjunctive pharmacotherapy may have also changed obsessive thoughts, blue mood, worry, low energy, and hopelessness indirectly, via changes in feeling entrapped and emotional lability. These interesting symptom level changes associated with treatment modality could not have been identified with traditional analyses relying on depression symptom sum scores. However, the complete a priori power, or being powered to detect all outcomes rather than just a single outcome (MDRC, 2016), for this sample was only 46% to detect halfway between a small and medium effect size (d = 0.325) for each symptom. Another study comparing an online CBT treatment for insomnia had 102 participants and 15 outcomes, with an a priori total power of 14% to detect d = 0.325 across 15 outcomes (Blanken et al., 2019). Therefore, the previous symptom level investigations into treatment for clinically elevated individuals appear to have been underpowered, though a better powered study (N = 325, total power = 87%) indicated attention bias modification training affected only the depression symptom of low interest among people with remitted depression (Kraft et al. 2019).
Building on this prior work, the present study examined the symptom change network for individuals receiving Deprexis compared to waitlist control in a larger sample (N = 295) than many prior studies. We sought to identify which DSM defined depression symptoms are influenced directly and indirectly by Deprexis compared to waitlist control.
Methods
Participants
Participants (N = 295) were referred to the study by others (4%) or self-referred through university mailing lists (27%), and advertisements placed on Reddit.com (22%), Google AdWords (5%), Craigslist.com (23%), Researchmatch.org (16%), or other sites (3%). Advertisements described the study as a self-guided Internet intervention for depression. The advertisements provided a link to a study website that provided additional information and screening questionnaires for those interested in determining whether they were eligible.
Inclusion criteria were: (a) age between 18 and 55; (b) English fluency; (c) reliable access to the Internet (i.e., dialup or broadband access); (d) willingness to provide saliva for DNA research; (e) presence of moderate levels of depression or greater (QIDS score > = 10) at time of eligibility screening; (f) treatment stability (no changes in psychotropic medication or psychosocial treatment in the 30 days before study entry); and (g) living in the United States of America. Exclusion criteria for these analyses were: (a) presence of psychotic or substance use symptoms via self-report on the Psychiatric Diagnosis Screening Questionnaire (Zimmerman & Mattia, 2001); (b) a diagnosis of bipolar disorder via semi-structured interview; or (c) suicidal risk (defined as having suicidal ideation with intent with/or without a plan in the last 90 days or attempting suicide in the past year). Participant characteristics are presented in Table 1.
Table 1.
Study sample characteristics.
| Variable | Treatment (n = 219) |
Waitlist (n = 76) |
Total (n = 295) |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender | Female | 162 | 74.0 | 60 | 79.0 | 222 | 75.2 |
| Male | 56 | 25.6 | 15 | 19.7 | 71 | 24.1 | |
| Transgender | 1 | 0.5 | 1 | 1.3 | 2 | 0.7 | |
| Mean age (SD) | 30.8 (10.4) | 33.7 (11.9) | 31.6 (10.9) | ||||
| Marital Status | Single | 141 | 65.3 | 53 | 69.7 | 194 | 66.4 |
| Married | 41 | 19.0 | 13 | 17.1 | 54 | 18.5 | |
| Divorced | 28 | 13.0 | 6 | 7.9 | 34 | 11.6 | |
| Separated | 0 | 0.0 | 1 | 1.3 | 1 | 0.3 | |
| Common Law Marriage | 5 | 2.3 | 3 | 4.0 | 8 | 2.7 | |
| Widowed | 1 | 0.5 | 0 | 0.0 | 1 | 0.3 | |
| Race | American Indian or Alaska Native | 2 | 0.9 | 0 | 0.0 | 2 | 0.7 |
| Asian | 15 | 6.9 | 6 | 7.9 | 21 | 7.1 | |
| Black or African American | 9 | 4.1 | 1 | 1.3 | 10 | 3.4 | |
| White | 170 | 77.6 | 63 | 82.9 | 233 | 79.0 | |
| Multiple Races | 15 | 6.9 | 4 | 5.3 | 19 | 6.4 | |
| None of the above | 4 | 1.8 | 0 | 0.0 | 4 | 1.4 | |
| Decline to answer | 4 | 1.8 | 2 | 2.6 | 6 | 2.0 | |
| Ethnicity | Hispanic or Latino | 29 | 13.2 | 4 | 5.3 | 33 | 11.2 |
| Not Hispanic or Latino | 186 | 84.9 | 70 | 92.1 | 256 | 86.8 | |
| Unknown / Decline to answer | 4 | 1.8 | 2 | 2.6 | 6 | 2.0 | |
| Education | High school graduate or less | 24 | 11.3 | 11 | 14.5 | 35 | 12.1 |
| 1 year of college or technical school | 13 | 6.1 | 8 | 10.5 | 21 | 7.2 | |
| 2 or more years of college | 42 | 19.6 | 12 | 15.8 | 54 | 18.6 | |
| Associates degree or technical degree | 6 | 2.8 | 5 | 6.6 | 11 | 3.8 | |
| College degree | 78 | 36.4 | 27 | 35.5 | 105 | 36.2 | |
| Postgraduate degree | 51 | 23.8 | 13 | 17.1 | 64 | 22.1 | |
| Income (SD) | 60,772 (54,489) | 56,522 (56,903) | 59,598 (55,088) | ||||
| Antidepressant | No | 134 | 61.2 | 38 | 50.0 | 172 | 58.3 |
| Yes | 85 | 38.8 | 38 | 50.0 | 123 | 41.7 | |
| Therapy | No | 156 | 71.2 | 53 | 69.7 | 209 | 76.6 |
| Yes | 63 | 28.8 | 23 | 30.3 | 86 | 23.4 | |
Procedure and Study Design
This was a parallel-group pragmatic randomized controlled trial. Participants who met inclusion/exclusion criteria were randomized to one of two groups: (a) immediate access to Deprexis (treatment, N = 219) or (b) access to Deprexis after an 8-week delay (waitlist, N = 76). Subjects provided written informed consent after receiving a complete description of the study. The overall procedure and trial design are described in more detail in the original publication (Beevers et al., 2017; Also see NCT01818453). This current study only involves people for whom we have complete pre and post treatment data on all depression symptoms (N = 295, see the CONSORT Diagram in the Supplementary Material Section 1), as network analyses require complete data and can be distorted by assumptions about missing values (Borsboom et al., 2017). This complete case approach could somewhat bias results if treatment condition or baseline symptom level predicted subsequent attrition (original N = 376). A two-sample test for the equality of proportions with continuity correction indicated that retention did not significantly differ across treatment conditions, X2(df = 1) = 0.04, p = 0.85. Neither individual symptom scores at baseline nor the depression sum score at baseline predicted attrition (See Supplemental Materials, Section 2). Using complete power, which accounts for being powered to test all outcomes in a study instead of just one (MDRC, 2016), this study had 82% a priori power to detect an effect size halfway between small and medium (d = 0.325) for 16 outcomes.
Measure
Participants self-reported their depression symptoms immediately pre- and post-treatment or waitlist using the Quick Inventory of Depression Symptoms (QIDS-SR; (Rush, Trivedi, et al., 2003). The QIDS-SR is sensitive to change with medications, psychotherapy, or somatic treatments (Rush et al., 2006), assesses all nine DSM 5 symptoms of depression (American Psychiatric Association, 2013; Rush et al., 2006), and is highly correlated (r = 0.84) with clinician rated depression assessed by the Hamilton Depression Rating Scale (Rush, Trivedi, et al., 2003). The measure contains 16 items, as the items assess both parts of compound symptoms and all stages of insomnia, rated on a scale from 0–3 (e.g. for sadness: 0 = “I do not feel sad”; 3 = “I feel sad nearly all the time”).
Treatment
Deprexis is an Internet-based intervention designed for adults with symptoms of unipolar depression (Meyer et al., 2009). The intervention consists of 10 content modules representing different psychotherapeutic approaches, plus one summary module, each of which can be completed in 10 to 60 min, depending on the user’s reading speed, interest, motivation, and individual path through the program. Modules are organized as simulated dialogues in which the program explains and illustrates concepts and techniques, engages the user in exercises, and continuously asks users to respond by selecting from response options. The modules cover a variety of therapeutic content that is broadly consistent with a cognitive–behavioral perspective, although the program is not restricted to one CBT manual. Instead, the program provides a variety of relevant therapeutic approaches and fits within the broad array of contemporary CBT. For more detail, see prior publications (Meyer et al., 2009).
Control Condition
Participants in the control condition were not influenced or advised to change their existing treatment plan (should one be in place). They were informed that they could receive access to the Deprexis program after an 8-week waiting period. Therefore, with respect to gaining access to Deprexis, this is a waitlist control condition, albeit with the caveat that participants were permitted to use any other treatments available to them (i.e., care as usual). This comparison condition was chosen in line with the logic governing pragmatic randomized control trials: to maximize external validity and test whether the intervention improves outcome compared with the heterogeneous care realities characterizing most health care systems. Participation in antidepressant and psychotherapy treatment was assessed and examined.
Analytic Plan
We first created residualized change scores for each symptom by predicting the post-treatment/waitlist score of each individual symptom with the baseline score of the symptom in a linear regression and extracting the residuals for each individual. We then used these continuous residuals for each symptom and a binarized treatment variable (0 = waitlist, 1 = immediate treatment) to estimate a network using a Mixed Graphical Model. A Mixed Graphical Model allows us to estimate binary and continuous variables in the same network (Haslbeck & Fried, 2017).
These network models include each variable as a node that is connected to other variables, or nodes, in the network via edges. Each network edge represents a unique partial association between two variables that accounts for all other nodes included in the network. Therefore, the network will allow us to identify which symptoms were directly changed by the treatment versus waitlist above and beyond their associations with changes in other symptoms.
To avoid topographical overlap, which can bias the interpretations of the network, we empirically assessed if any nodes were overlapping using the networktools package (Jones, 2018) in R (Version 3.6, R Core Team, 2019). Specifically, we followed standard practice and assessed whether 25% or fewer of the correlations with other symptoms significantly differed across each pair of nodes. In other words, we assessed whether the associations with other symptoms were so strongly overlapping between two nodes that estimating them separately could bias the network’s conclusions.
To avoid the inclusion of spurious relationships in our model, we utilized the LASSO regularization technique (Tibshirani, 2011) to shrink all edge-weights based on a set parameter. We used the extended bayesian information criterion as its estimates converge to known true networks in simulations as sample size increases (Ravikumar, Wainwright, & Lafferty, 2010). We also set the hyperparameter within this criterion to 0.00 to allow for regularization while still allowing for discovery of true unique associations (Epskamp, Borsboom, & Fried, 2018). Under these penalties, smaller, potentially spurious edge-weights shrink to a value of 0. The present network was fitted using the R-package mgm version 1.2–4 and was visualized using the qgraph version 1.5 R-package.
In keeping with existing robustness techniques (Epskamp et al., 2018), we evaluated the accuracy of our edge-weights using bootstrapped 95% CIs. CIs were calculated using the range of 100 bootstrapped samples for each edge-weight, with larger edge-weight CIs indicating more variable and less precise estimates for those edges (Fried & Haslbeck, 2018).
We also conducted t-tests with residualized symptom scores as the outcome and treatment group as the individual variable. We also calculated effect sizes for treatment on each symptom. To correct for multiple comparisons (16 comparisons, one for each symptom), we used the Holm-Bonferroni approach. After this correction, any p-values less than .05 were taken to mean there was a significant treatment effect on the symptom. We then cross-validated, or tested the potential out-of-sample performance, all models where there was a significant effect of treatment (Yarkoni & Westfall, 2017).
Results
Descriptive statistics for all individual symptoms on the QIDS-SR are presented by time (baseline, post-treatment/waitlist) and treatment group (immediate, waitlist) in Table 2. As this sample differed slightly from the previously reported sample, we calculated the effect of treatment on QIDS-SR sum score (See Supplementary Section 3 for Cronbach’s alpha at both pre- and post-). The effect of treatment on sum score depression was significant and large (p < .001, d = 0.90). None of the individual symptoms differed by treatment group at baseline after correcting for multiple comparisons.
Table 2.
Descriptive Statistics of QIDS-SR Depression Symptoms
| Wait-List | Deprexis | ||||
|---|---|---|---|---|---|
| Symptom | Pre | Post | Pre | Post | p |
| Early Insomnia | 1.78 (0.92) | 1.43 (0.98) | 1.52 (1.01) | 1.08 (0.98) | 0.64 |
| Middle Insomnia | 1.79 (0.88) | 1.62 (1.01) | 1.55 (1.02) | 1.12 (0.97) | 0.73 |
| Late Insomnia | 0.96 (1.09) | 0.72 (0.95) | 0.68 (0.95) | 0.55 (0.89) | 0.73 |
| Hypersomnia | 0.86 (0.83) | 0.67 (0.81) | 0.91 (0.93) | 0.69 (0.84) | 1.00 |
| Sadness | 2.00 (0.73) | 1.84 (0.83) | 1.94 (0.73) | 1.20 (0.84) | 1.00 |
| Appetite Loss | 0.51 (0.76) | 0.41 (0.64) | 0.57 (0.79) | 0.31 (0.66) | 1.00 |
| Appetite Gain | 1.03 (1.17) | 0.61 (1.05) | 1.01 (1.17) | 0.46 (0.90) | 1.00 |
| Weight Loss | 0.46 (0.92) | 0.38 (0.73) | 0.49 (0.88) | 0.23 (0.55) | 1.00 |
| Weight Gain | 0.92 (1.25) | 0.75 (1.03) | 0.84 (1.05) | 0.46 (0.87) | 1.00 |
| Indecision | 1.66 (0.66) | 1.54 (0.74) | 1.72 (0.67) | 1.03 (0.86) | 1.00 |
| Self-Dislike | 1.89 (1.14) | 1.46 (1.10) | 1.83 (1.10) | 0.89 (1.05) | 1.00 |
| Suicidality | 0.88 (0.80) | 0.68 (0.82) | 0.89 (0.83) | 0.35 (0.62) | 1.00 |
| Anhedonia | 1.50 (0.82) | 1.30 (0.86) | 1.60 (0.84) | 0.87 (1.01) | 1.00 |
| Fatigue | 1.68 (0.72) | 1.54 (0.86) | 1.67 (0.78) | 1.00 (0.97) | 1.00 |
| Slowness | 0.88 (0.73) | 0.72 (0.76) | 0.86 (0.79) | 0.43 (0.68) | 1.00 |
| Agitation | 1.04 (0.97) | 0.72 (0.86) | 0.99 (0.91) | 0.61 (0.78) | 1.00 |
| Total | 15.20 (3.53) | 13.00 (3.90) | 15.04 (3.94) | 8.92 (5.43) | 1.00 |
Note. p indicates the p-values when comparing symptom severity across groups at baseline. To correct for multiple comparisons, p-values were Holm-Bonferonni corrected such that any p-values < 0.05 indicate a significant difference. Degrees of freedom = 117.26 – 149.79 following Satterthaite-Welch adjustment.
Utilization of concurrent treatment did not differ across randomized groups (see Supplementary Materials, section 4). Importantly, the symptom change network had adequate accuracy. The goldbricker function from the summarytools package indicated changes in middle insomnia and changes in early insomnia, changes in appetite gain and weight gain, and changes in anhedonia and changes in fatigue as pairs had overlapping enough associations with other symptoms (i.e., fewer than 25% of their associations with other nodes in the network significantly differed from one another) that estimating them together in the network could bias its conclusions. We therefore created composites of these pairs of symptoms to include in the network and standardized all symptom change variables to put all nodes on the same scale. No other nodes in the symptom change network were empirically overlapping. We assessed changes in all symptoms separately in non-network analyses. There were many edges with non-overlapping confidence intervals and edges not included in the network were all estimated as greater than 0 in less than 10% of the bootstrapped samples (see Supplementary Materials, section 5).
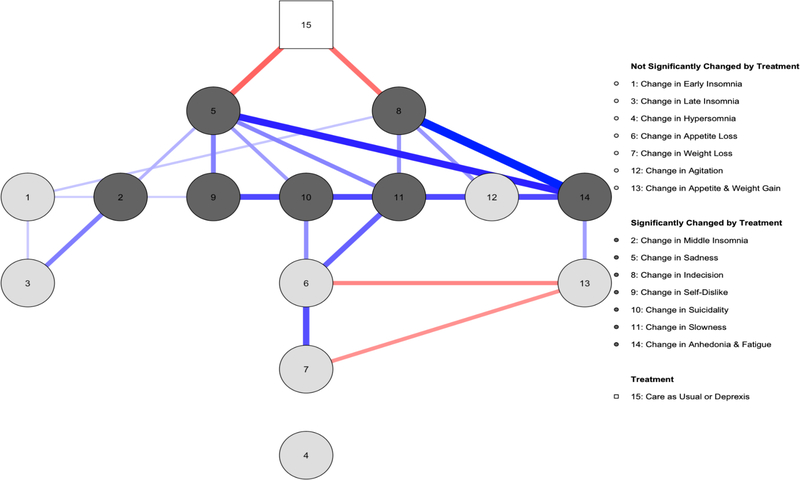
The symptom network revealed that treatment directly caused decreases in sadness and indecision (Figure 1). Changes in an additional eight symptoms/seven nodes (early insomnia, middle insomnia, self-dislike, fatigue, anhedonia, suicidality, slowness, and agitation) were one node removed from being directly associated with treatment (i.e., were indirectly associated with treatment via changes in sadness, changes in indecision, or both). Changes in four symptoms/three nodes (late insomnia, appetite loss, appetite gain, and weight gain) were two nodes removed from being directly associated with treatment (i.e., was indirectly associated with treatment via change in sadness, indecision, and then changes in either middle insomnia, slowness, suicidality or fatigue/anhedonia). Changes in weight loss were three nodes removed from being directly associated with treatment (i.e. was indirectly associated with treatment via changes in sadness, changes in indecision, or both, then changes in at least one of and then changes in either at least one of middle insomnia, slowness, suicidality or fatigue/anhedonia, and finally changes in at least one of appetite loss, appetite gain, and/or weight gain). Changes in one symptoms/one node was not associated, even indirectly, with treatment (hypersomnia). This general pattern was robust to whether we used residualized scores or raw change scores (see Supplementary Materials, section 6).
Figure 1.
A network analysis of unique associations between Deprexis versus Care As Usual and depression symptom change
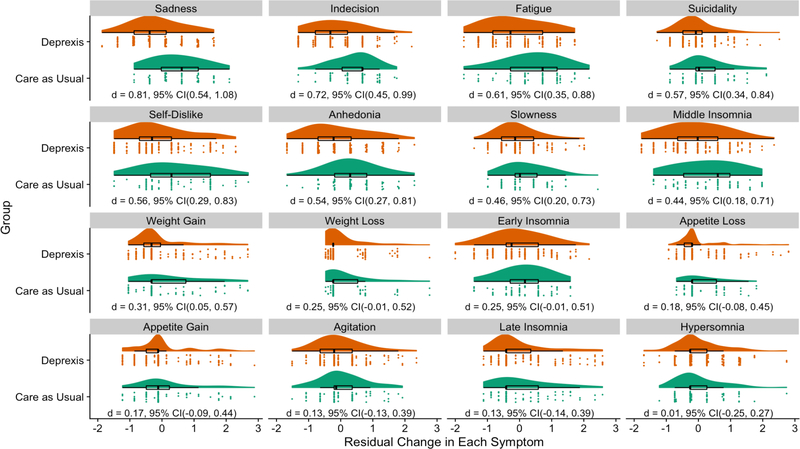
Differential treatment-related symptom change is also observed in treatment effect sizes on individual symptoms. Effect sizes ranged from d = 0.01 (favoring immediate treatment ) for hypersomnia to d = 0.81 for sadness (favoring immediate treatment). See Figure 2 for a visual depiction of treatment effect sizes for all symptoms.
Figure 2.
Raincloud plots of individual depression symptom change in Deprexis and Care As Usual. Symptoms are presented from largest to smallest treatment effects. Raincloud plots allow us to visualize the distribution, boxplot, raw data, and effect size with a confidence interval for each treatment group, an improvement on other visualizations that give less complete information about the data (Allen, Poggiali, Whitaker, Marshall, & Kievit, 2018)
The largest effect sizes were for the symptoms identified by network analyses as being directly targeted by treatment (d = 0.81 for sadness and d = 0.72 for indecision). After using a Holm-Bonferroni correction for multiple testing, treatment significantly improved sadness (p < .001), indecision (p < .001), fatigue (p < .001, d = 0.61), suicidality (p = .002, d = 0.57), self-dislike (p = .001, d = 0.56), anhedonia (p .001, d = 0.54),, slowness (p = .01, d = 0.46), and middle insomnia (p = .01, d = 0.44). We used 10-fold cross-validation repeated 10 times to evaluate the out-of-sample prediction of symptom improvement by treatment condition for each significant effect , and the findings generalized well to out of sample data (maximum variance predicted drop-off from original data to predicted R2 for out-of-sample data = 1.4%, see Supplementary Materials, section 7). Treatment marginally improved slowness (p = .097) and did not significantly improve early insomnia, middle insomnia, late insomnia, hypersomnia, appetite loss, appetite gain, weight loss, weight gain, and agitation (all p values following Holm-Bonferroni correction = 1).
Discussion
Taking a symptom-level approach to treatment efficacy, Deprexis directly decreased two symptoms (sadness and indecision), indirectly decreased six other symptoms (self-dislike, suicidality, anhedonia, fatigue, slowness, and middle insomnia), and did not significantly change half of the symptoms (early insomnia, late insomnia, hypersomnia, appetite loss, appetite gain, weight loss, weight gain, and agitation). Although prior work with this sample found an overall reduction in depression symptoms using a sum score (Beevers et al., 2017), analyses from the present study clearly reveal that treatment had a differential impact on symptoms of depression.
The wide heterogeneity in treatment effects on symptoms, with effects ranging from d = 0.01 (favoring immediate treatment) for hypersomnia to d = 0.81 for sadness (favoring immediate treatment), is especially notable given the previously observed meta-analytic effect size of g = 0.54 for Deprexis on depression sum scores (Twomey et al., 2017). In the present study, six symptoms (sadness, indecision, fatigue, suicidality, self-dislike, and anhedonia) had an effect size of at least d = 0.54. This indicates Deprexis may be more effective for these symptoms than would be expected based on sum score data. On the other hand, this result also implies that Deprexis could be less effective for many other depression symptoms.
Nine of the ten Deprexis treatment modules (Psychoeducation, Behavioral Activation, Cognitive Modification, Acceptance and Mindfulness, Problem-solving, Childhood experiences, Interpersonal Skills, Positive Psychology, and Emotion-Focused) primarily target emotions and thoughts rather than vegetative symptoms. Only one module (Relaxation, Physical Exercise, and Lifestyle Modification) primarily target vegetative symptoms. Therefore, it appears that Deprexis may be able to effectively change, directly or indirectly, symptoms that are explicitly targeted by a vast majority of its modules. Future work using larger samples could use baseline characteristics and treatment module usage to directly predict who will be more likely to improve on certain symptoms (e.g., perhaps people who more often utilize the Relaxation, Physical Exercise, and Lifestyle Modification module will be more likely to improve on vegetative symptoms) similar to prior work predicting response using depression sum scores (Pearson, Pisner, Meyer, Shumake, & Beevers, 2018).
Importantly, this pattern of differential treatment effects is obscured when sum scores are used to examine change in depression symptom severity. Other Deprexis trial data should be examined at the symptom-level to determine whether treatment consistently has strong, positive effects for these six symptoms and weaker or null effects for other symptoms. Given the minimal reduction in variance explained in the cross-validated models for the prediction of symptom change by treatment condition, we expect the results to replicate out-of-sample. It would also be very interesting to determine whether a similar pattern of symptom change is observed for other treatment modalities, including more traditional CBT and/or pharmacotherapy (cf. Bekhuis et al., 2018).
One of the directly targeted symptoms, sadness, has been previously identified as a more central symptom of adult depression in cross-sectional network analyses (e.g., Beard et al., 2016; Fried, Epskamp, et al., 2016; Santos, Fried, Asafu-Adjei, & Ruiz, 2017). Network theory predicts that effectively targeting central symptoms within the network will lead to a cascading decrease in other symptoms (Borsboom, 2017). In line with that idea, treatment directly decreased sadness and, in turn, was associated with significant decreases in all symptoms (middle insomnia, self-dislike, suicidality, slowness, anhedonia, and fatigue) connected to change in sadness. However, in the current design, change in sadness and other symptoms were measured using the same two time points. Future symptom-level focused studies could measure symptoms more frequently to make stronger claims about cascading decreases in symptoms.
In addition, the treatment directly changed indecision, a symptom rarely identified as a more central symptom of depression (Bringmann, Lemmens, Huibers, Borsboom, & Tuerlinckx, 2015). Prior work using temporal designs has shown that indecision was more likely to be predicted by other symptoms (e.g., indegree strength) but did not predict change in other symptoms (e.g., outdegree strength) over time (Bringmann et al., 2015). Previous interpretations of temporal networks have emphasized symptoms with high outdegree strength as potentially fruitful targets for intervention (e.g., Rubel, Fisher, Husen, & Lutz, 2018). However, in the current study, change in indecision was associated with significant decreases in three symptoms (slowness, anhedonia, and fatigue) but also non-significant decreases in two other symptoms (early insomnia and agitation). Given this pattern of results, perhaps future investigations should consider whether high indegree symptoms may be higher value intervention targets than previously considered.
To determine whether change in sadness or indecision has a unique cascading effect on other symptoms, a randomized intervention that specifically targets one node or the other, but not both, would need to be developed. This “fat hand” problem, where interventions target multiple potential causal mechanisms rather than a single one, can impede the identification of causal mechanisms (Eberhardt, 2009). Interventions designed to target specific symptoms could be helpful for better understanding how and when changes in certain symptoms lead to changes in other symptoms.
Identifying which interventions directly target which symptoms could be clinically useful. There has been a long-standing debate over whether all psychological treatments are equally effective in addressing mental health problems (Huibers & Cuijpers, 2015; Marcus, O’Connell, Norris, & Sawaqdeh, 2014; Wampold et al., 2017). However, it may be the case that treatments have a similar impact on depression sum scores, but the pattern of symptom change may differ across treatment modalities. For instance, a recent review found that many psychological treatments for depression were similarly efficacious (Cuijpers et al., 2008), but symptom network change could be quite variable across these treatments. Additionally, different treatments may impact the same symptoms via different causal processes (Jones, Heeren, & McNally, 2017), and including these processes in addition to symptoms in future networks may help further elucidate these differing causal processes (e.g., Kraft et al., 2019).
Further, a symptom-level approach could allow for optimally combining treatments by identifying treatments that target different symptoms within the network. For example, the symptoms not targeted by Deprexis in this study were primarily related to sleep and appetite (early insomnia, late insomnia, hypersomnia, appetite loss, appetite gain, weight loss, weight gain, and agitation). Combining Deprexis with an internet intervention that targets sleep (e.g., Carney et al., 2017) could result in greater symptom change across the network than combining Deprexis with an intervention that leads to change in a similar set of symptoms. Investigating a variety of interventions at the symptom-level using methods similar to this study could promote more optimal treatment combinations.
There are limitations to the current analysis that can be addressed by future studies. Depression symptoms were self-reported, and while the overlap between clinician reported depression and self-reported depression is high (Rush et al., 2003), it is possible that symptom-level dynamics may differ across informants, though previous investigations have yielded minimal differences (e.g., Moshier et al., 2018). Future studies utilizing multi-informant reports could therefore measure each symptom using more than one questionnaire item, which could increase the reliability and validity of symptom-level measurement (Flake, Pek, & Hehman, 2017). Further, as non-DSM and DSM defined symptoms of depression appear to be equally central to depression when estimated in the same network (Fried, Epskamp, et al., 2016), future analyses could examine the effects of treatment on non-DSM symptoms. However, the current analysis does provide evidence of which DSM defined depression symptoms may be specifically affected by Deprexis. The current analyses also do not include post-treatment follow-up, limiting our ability to draw conclusions about long-term effects.
In conclusion, this study provides a framework for examining treatment efficacy at the symptom-level. Understanding the effects (or lack thereof) of interventions on specific symptoms could facilitate a variety of theoretical and clinical advances. For example, symptom-level knowledge could help us better understand how treatments work and more effectively prescribe combined treatments that target non-redundant symptoms. Ultimately, taking a symptom-level approach to depression treatment could allow us to address a heterogeneous syndrome with appropriately heterogeneous treatments rather than one-size-fits-all programs.
Supplementary Material
Acknowledgements
This study was supported by Gaia AG, the developer and owner of Deprexis, by providing access to the intervention at no cost to the researchers or participants, as well as providing technical support to the research participants as needed. All research data was collected and analyzed independently by researchers affiliated with the University of Texas at Austin. Financial support for this study was also provided to CGB by an NIH award (R21MH110758), an Independent Investigator award from the Brain and Behavior Foundation, and an unrestricted grant from the KCL Foundation, a 501(c)(3) not-for-profit philanthropic foundation that supports mental health programs in Austin, TX. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank James Hoffman for his help with study coordination.
The data that support the findings of this study are openly available in the Open Science Framework at http://doi.org/10.17605/OSF.IO/SFBZU.
Footnotes
Declaration of Interests
None of the authors are employed by Gaia AG, have received remuneration for participating in this project, or have any other conflicts of interest to declare.
References
- Allen M, Poggiali D, Whitaker K, Marshall TR, & Kievit R. (2018). Raincloud plots: a multi-platform tool for robust data visualization. 10.7287/peerj.preprints.27137v1 [DOI] [PMC free article] [PubMed]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Retrieved from https://books.google.com/books/about/Diagnostic_and_Statistical_Manual_of_Men.html?hl=&id=-JivBAAAQBAJ
- Andersson G, & Titov N. (2014). Advantages and limitations of Internet-based interventions for common mental disorders. World Psychiatry: Official Journal of the World Psychiatric Association , 13(1), 4–11. 10.1002/wps.20083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Basu A, Cuijpers P, Craske MG, McEvoy P, English CL, & Newby JM (2018). Computer therapy for the anxiety and depression disorders is effective, acceptable and practical health care: An updated meta-analysis. Journal of Anxiety Disorders, 55, 70–78. 10.1016/j.janxdis.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Beard C, Millner AJ, Forgeard MJC, Fried EI, Hsu KJ, Treadway MT, … Björgvinsson T. (2016). Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychological Medicine, 46(16), 3359–3369. 10.1017/s0033291716002300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Pearson R, Hoffman JS, Foulser AA, Shumake J, & Meyer B. (2017). Effectiveness of an internet intervention (Deprexis) for depression in a united states adult sample: A parallel-group pragmatic randomized controlled trial. Journal of Consulting and Clinical Psychology, 85(4), 367–380. 10.1037/ccp0000171 [DOI] [PubMed] [Google Scholar]
- Bekhuis E, Schoevers R, de Boer M, Peen J, Dekker J, Van H, & Boschloo L. (2018). Symptom-Specific Effects of Psychotherapy versus Combined Therapy in the Treatment of Mild to Moderate Depression: A Network Approach. Psychotherapy and Psychosomatics, 87(2), 121–123. 10.1159/000486793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken TF, Van Der Zweerde T, Van Straten A, Van Someren EJW, Borsboom D, & Lancee J. (2019). Introducing Network Intervention Analysis to Investigate Sequential, Symptom-Specific Treatment Effects: A Demonstration in Co-Occurring Insomnia and Depression. Psychotherapy and Psychosomatics, 88(1), 52–54. 10.1159/000495045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D. (2017). A network theory of mental disorders. World Psychiatry: Official Journal of the World Psychiatric Association, 16(1), 5–13. 10.1002/wps.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AOJ (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. 10.1146/annurev-clinpsy-050212-185608 [DOI] [PubMed] [Google Scholar]
- Borsboom D, Fried EI, Epskamp S, Waldorp LJ, van Borkulo CD, van der Maas HLJ, & Cramer AOJ (2017). False alarm? A comprehensive reanalysis of “Evidence that psychopathology symptom networks have limited replicability” by Forbes, Wright, Markon, and Krueger (2017). Journal of Abnormal Psychology, 126(7), 989–999. 10.1037/abn0000306 [DOI] [PubMed] [Google Scholar]
- Bower P, & Gilbody S. (2005). Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. The British Journal of Psychiatry: The Journal of Mental Science, 186, 11–17. 10.1192/bjp.186.1.11 [DOI] [PubMed] [Google Scholar]
- Bringmann LF, Lemmens LHJM, Huibers MJH, Borsboom D, & Tuerlinckx F. (2015). Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychological Medicine, 45(4), 747–757. 10.1017/S0033291714001809 [DOI] [PubMed] [Google Scholar]
- Carlbring P, Andersson G, Cuijpers P, Riper H, & Hedman-Lagerlöf E. (2018). Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cognitive Behaviour Therapy, 47(1), 1–18. 10.1080/16506073.2017.1401115 [DOI] [PubMed] [Google Scholar]
- Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD, & Shapiro CM (2017). Cognitive Behavioral Insomnia Therapy for Those With Insomnia and Depression: A Randomized Controlled Clinical Trial. Sleep, 40(4). 10.1093/sleep/zsx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Andersson G, & van Oppen P. (2008). Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology, 76(6), 909–922. 10.1037/a0013075 [DOI] [PubMed] [Google Scholar]
- Eberhardt F. (2009). Introduction to the Epistemology of Causation. Philosophy Compass, 4(6), 913–925. 10.1111/j.1747-9991.2009.00243.x [DOI] [Google Scholar]
- Epskamp S, Borsboom D, & Fried EI (2018). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50(1), 195–212. 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake JK, Pek J, & Hehman E. (2017). Construct Validation in Social and Personality Research. Social Psychological and Personality Science, 8(4), 370–378. 10.1177/1948550617693063 [DOI] [Google Scholar]
- Fried EI (2017). The 52 symptoms of major depression: Lack of content overlap among seven common depression scales. Journal of Affective Disorders, 208, 191–197. 10.1016/j.jad2016.10.019 [DOI] [PubMed] [Google Scholar]
- Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, & Borsboom D. (2016). What are “good” depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. Journal of Affective Disorders, 189, 314–320. 10.1016/j.jad.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Fried EI, & Haslbeck J. (2018). Using network analysis to examine links between individual depression symptoms, inflammatory markers, and covariates. 10.31234/osf.io/84ske [DOI] [PubMed]
- Fried EI, & Nesse RM (2015a). Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. Journal of Affective Disorders, 172, 96–102. 10.1016/j.jad.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2015b). Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Medicine, 13(1). 10.1186/s12916-015-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, van Borkulo CD, Epskamp S, Schoevers RA, Tuerlinckx F, & Borsboom D. (2016). Measuring depression over time … Or not? Lack of unidimensionality and longitudinal measurement invariance in four common rating scales of depression. Psychological Assessment, 28(11), 1354–1367. 10.1037/pas0000275 [DOI] [PubMed] [Google Scholar]
- Haslbeck JMB, & Fried EI (2017). How predictable are symptoms in psychopathological networks? A reanalysis of 18 published datasets. Psychological Medicine, 47(16), 2767–2776. 10.1017/S0033291717001258 [DOI] [PubMed] [Google Scholar]
- Huibers MJH, & Cuijpers P. (2015). Common (Nonspecific) Factors in Psychotherapy. In The Encyclopedia of Clinical Psychology (pp. 1–6). 10.1002/9781118625392.wbecp272 [DOI] [Google Scholar]
- Jones P. (2018). networktools: Tools for Identifying Important Nodes in Networks. R package version 1.2. 0. Retrieved Form https://CRAN.R-Project.Org/package=Networktools Retrieved from https://CRAN.R-project.org/package=networktools [Google Scholar]
- Jones PJ, Heeren A, & McNally RJ (2017). [Review of Commentary: A network theory of mental disorders]. Frontiers in psychology, 8, 1305 10.3389/fpsyg.2017.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karyotaki E, Riper H, Twisk J, Hoogendoorn A, Kleiboer A, Mira A, … Cuijpers P. (2017). Efficacy of Self-guided Internet-Based Cognitive Behavioral Therapy in the Treatment of Depressive Symptoms: A Meta-analysis of Individual Participant Data. JAMA Psychiatry , 74(4), 351–359. 10.1001/jamapsychiatry.2017.0044 [DOI] [PubMed] [Google Scholar]
- Kraft B, Jonassen R, Heeren A, Harmer C, Stiles T, & Landrø NI (2019). Attention Bias Modification in Remitted Depression Is Associated With Increased Interest and Leads to Reduced Adverse Impact of Anxiety Symptoms and Negative Cognition. Clinical Psychological Science, 2167702618822480. 10.1177/2167702618822480 [DOI] [Google Scholar]
- Marcus DK, O’Connell D, Norris AL, & Sawaqdeh A. (2014). Is the Dodo bird endangered in the 21st century? A meta-analysis of treatment comparison studies. Clinical Psychology Review, 34(7), 519–530. 10.1016/j.cpr.2014.08.001 [DOI] [PubMed] [Google Scholar]
- McNally RJ, Robinaugh DJ, Wu GWY, Wang L, Deserno MK, & Borsboom D. (2015). Mental Disorders as Causal Systems: A Network Approach to Posttraumatic Stress Disorder. Clinical Psychological Science, 3(6), 836–849. 10.1177/2167702614553230 [DOI] [Google Scholar]
- MDRC. (2016, July 12). Statistical Power in Evaluations That Investigate Effects on Multiple Outcomes. Retrieved August 8, 2019, from MDRC website: https://www.mdrc.org/publication/statistical-power-evaluations-investigate-effects-multiple-outcomes
- Meyer B, Berger T, Caspar F, Beevers CG, Andersson G, & Weiss M. (2009). Effectiveness of a novel integrative online treatment for depression (Deprexis): randomized controlled trial. Journal of Medical Internet Research, 11(2), e15. 10.2196/jmir.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshier SJ, Bovin MJ, Gay NG, Wisco BE, Mitchell KS, Lee DJ, … Marx BP (2018). Examination of posttraumatic stress disorder symptom networks using clinician-rated and patient-rated data. Journal of Abnormal Psychology, 127(6), 541–547. 10.1037/abn0000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey MC, Stein AT, Pearson R, & Beevers CG Supplemental materials for preprint: Network analyses reveal which symptoms improve (or not) following an Internet intervention (Deprexis) for depression. 10.17605/OSF.IO/SFBZU [DOI] [PMC free article] [PubMed]
- Pearson R, Pisner D, Meyer B, Shumake J, & Beevers CG (2018). A machine learning ensemble to predict treatment outcomes following an Internet intervention for depression. Psychological Medicine, 1–12. 10.1017/S003329171800315X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar P, Wainwright MJ, & Lafferty JD (2010). High-dimensional Ising model selection using ℓ 1 -regularized logistic regression. Annals of Statistics, 38(3), 1287–1319. 10.1214/09-aos691 [DOI] [Google Scholar]
- R Core Team. (2019). R: A Language and Environment for Statistical Computing. Retrieved from https://www.R-project.org/
- Rubel JA, Fisher AJ, Husen K, & Lutz W. (2018). Translating Person-Specific Network Models into Personalized Treatments: Development and Demonstration of the Dynamic Assessment Treatment Algorithm for Individual Networks (DATA-IN). Psychotherapy and Psychosomatics, 87(4), 249–251. 10.1159/000487769 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, … Fava M. (2006). An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biological Psychiatry, 59(6), 493–501. 10.1016/j.biopsych.2005.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, John Rush A, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, … Keller MB (2003). The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, … Keller MB (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Santos H Jr, Fried EI, Asafu-Adjei J, & Ruiz RJ (2017). Network Structure of Perinatal Depressive Symptoms in Latinas: Relationship to Stress and Reproductive Biomarkers. Research in Nursing & Health, 40(3), 218–228. 10.1002/nur.21784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. (2011). Regression shrinkage and selection via the lasso: a retrospective. Journal of the Royal Statistical Society. Series B, Statistical Methodology, 73(3), 273–282. 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- Twomey C, O’Reilly G, & Meyer B. (2017). Effectiveness of an individually-tailored computerised CBT programme (Deprexis) for depression: A meta-analysis. Psychiatry Research, 256, 371–377. 10.1016/j.psychres.2017.06.081 [DOI] [PubMed] [Google Scholar]
- Wampold BE, Flückiger C, Del Re AC, Yulish NE, Frost ND, Pace BT, … Hilsenroth MJ (2017). In pursuit of truth: A critical examination of meta-analyses of cognitive behavior therapy. Psychotherapy Research: Journal of the Society for Psychotherapy Research, 27(1), 14–32. 10.1080/10503307.2016.1249433 [DOI] [PubMed] [Google Scholar]
- Yarkoni T, & Westfall J. (2017). Choosing Prediction Over Explanation in Psychology: Lessons From Machine Learning. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 12(6), 1100–1122. 10.1177/1745691617693393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Ellison W, Young D, Chelminski I, & Dalrymple K. (2015). How many different ways do patients meet the diagnostic criteria for major depressive disorder? Comprehensive Psychiatry, 56, 29–34. 10.1016/j.comppsych.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, & Mattia JI (2001). A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Archives of General Psychiatry, 58(8), 787–794. 10.1001/archpsyc.58.8.787 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.