Abstract
Some of the most disabling aspects of mild traumatic brain injury (mTBI) include lingering deficits in executive functioning. It is known that mTBI can damage white matter tracts, but it remains unknown how this structural brain damage translates into cognitive deficits. This experiment utilized theta band phase synchrony to identify the dysfunctional neural operations that contribute to cognitive problems following mTBI. Sub-acute stage (< 2 weeks) mTBI patients (N=52) and healthy matched controls (N=32) completed a control-demanding task with concurrent EEG. Structural MRI was also collected. While there were no performance-specific behavioral differences between groups in the dot probe expectancy task, the degree of theta band phase synchrony immediately following injury predicted the degree of symptom recovery two months later. Although there were no differences in fractional anisotropy (FA) between groups, joint independent components analysis revealed that a smaller network of lower FA-valued voxels contributed to a diminished frontal theta phase synchrony network in the mTBI group. This finding suggests that frontal theta band markers of cognitive control are sensitive to sub-threshold structural aberrations following mTBI.
Keywords: mTBI, DTI, EEG, Theta, Control, Executive, Brain Injury
The ability to effectively exert executive control is critical for work-force participation and meaningful interpersonal relationships (Carney et al. 1999; Kreuter et al. 1998; Sander and Struchen 2011). Brain injuries can complicate executive functions to the detriment of these real-world quality of life measures (McDonald et al. 2002). Executive deficits are amongst the most common and disabling aspects of mTBI (Karr et al. 2014; McDonald et al. 2002), and these might be related to structural brain damage caused by the trauma (Huang et al. 2009; Wallace et al. 2018). Unfortunately the heterogeneity of injuries across individuals complicates attempts to develop imaging biomarkers that could assess damage or assist in predicting recovery trajectories. The current study aimed to test whether EEG-based theta band phase synchrony may act as a functional link between damaged white matter structure and symptomatic phenotype following a mild traumatic brain injury (mTBI).
Phenotype: Executive deficits following mTBI
Post-concussive symptoms include a variety of somatic, cognitive, and emotional complaints (Bigler, 2008; Quinn, Mayer, Master, & Fann, 2018; Rose, Fischer, & Heyer, 2015). A large number of individuals experience varied symptoms after mTBI, with up to 3/4 of individuals expressing at least one persistent symptom 6 months later (van der Naalt et al. 2017), and 1/4 still experiencing significant impairment 1 year later (McMahon et al. 2014). The extent to which these problems reflect preexisting tendencies (e.g. a “miserable minority”) vs. an incomplete recovery is actively debated (McDonald et al. 2002; Quinn et al. 2018; Rohling et al. 2012; Ruff 2005), and complicated by the breadth of cognitive and affective symptoms that may be endorsed following injury.
A growing appreciation of the “domain general” nature of executive control has described how common neural systems contribute to both cognitive and affective processes (Chang et al. 2013; De La Vega et al. 2016; Shackman et al. 2011). Thus, it is possible that a variety of post-concussive symptoms like mood, apathy, and impulsivity may all be linked to aspects of a common deficit in executive functioning (Carroll et al. 2004). Behavioral outcomes of varied frontal processes also appear to be manifest expressions of a smaller number of tightly coordinated neural processes broadly involved in executive control (Niendam et al. 2012). Common neuropsychological tests of executive dysfunction in mTBI tend to load on a single common factor (Busch et al. 2005; Serino et al. 2006), often characterized by globally slowed reaction time (Dimoska-Di Marco et al. 2011; Dockree and Robertson 2011; Frencham et al. 2005). The generality of this finding may not reflect a specific executive deficit following mTBI; rather, it may be a consequence of the inability of standard performance tasks to quantify fine-grained deficits in cognitive control.
In the current experiment, we aimed to leverage a well-characterized task that assesses specific abilities for planning and maintaining rules due to varying stimulus-response demands. The Dot Probe Expectancy (DPX) variant of the common AX-Continuous performance task (AX-CPT) is a major component of the NIH CNTRICS battery (Barch et al. 2009), and is translatable to animal studies (Blackman et al. 2016), bolstering the generalizability of this choice. Similar AX-CPT variants have revealed generalized performance and EEG deficits following mTBI (Larson et al. 2006; Zhao et al. 2018); the current report aims to assess higher cognition in mTIB with a much larger sample and with a novel integration of structural and functional indices proposed to underlie poor cognitive performance.
Structure: White matter damage following mTBI
In the absence of clearly-defined lesions, research-specific scans have been used to derive subtle structural differences based on statistical deviations from non-patients. The most common consequences of acute trauma appear to be expressed in centrally-located, transversal tracts of white matter like the corpus callosum, corona radiata, and cingulum (Aoki and Inokuchi 2016; Eierud et al. 2014; Hulkower et al. 2013; Ling et al. 2012; Roberts et al. 2016; Wallace et al. 2018). Accumulated findings suggest that deficits in cognitive control in mTBI may be due to degraded structural connectivity between neural areas (Wallace et al. 2018), and axonal injury due to shearing and stretching may directly contribute to post-concussive symptoms (Huang et al. 2009). Diffusion tensor imaging (DTI) has been utilized to assess how acute trauma can damage these white matter tracts (Dockree & Robertson, 2011; Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013; Mayer et al., 2010; McDonald et al., 2002; Strauss et al., 2016). Unfortunately, the intuitive appeal of this measure is complicated by the low reliability of findings across samples, the large dimensionality of whole-brain fractional anisotropy (FA) assessments, and the subtle and individualized nature of acute traumas.
Recent literature reviews have described a bidirectional nature of group differences in FA, (where an mTBI group may have lower or higher FA values than matched controls depending on time post-injury) and bidirectional correlations between FA and cognitive behavioral characteristics (Eierud et al. 2014; Hulkower et al. 2013; Kamins et al. 2017; Roberts et al. 2016; Wozniak et al. 2007). Some injury characteristics appear to be important moderators of this variance in FA. More severe forms of TBI are more reliably associated with lower FA values compared to controls (Dodd et al. 2014; Eierud et al. 2014), and negative correlations with performance may be most reliably observed within the first two weeks after injury (Eierud et al. 2014), but beyond that there remains considerable variance in FA findings within mTBI groups. One way to advance beyond these direct observations of structural indices is to link individual FA measures with a known functional process that is tightly associated with cognitive control and is correlated with white matter integrity.
Function: The missing link in structure-to-phenotype assessment
In order to bridge the structure-phenotype gap, we aimed to identify functional deficits that mediate the pathway between structural damage and cognitive outcome. For the current study, we utilized a previously-defined candidate neural mechanism of cognitive control. Frontal theta band (4–8 Hz) EEG activities are proposed to underlie cognitive control in a two-part process: 1) a frontal midline theta power burst that is thought to act as an “alarm bell” indicating the realization of the need for control, and 2) medio-lateral theta band phase synchronous interaction between neural areas that is thought to underlie the implementation of control (Cavanagh and Frank 2014). This model appears robust, with over twenty replications of this phase-synchronous phenomenon occurring after a variety of eliciting circumstances, including causal manipulations and observed deficits in clinical disorders (reviewed in Cavanagh and Cohen 2019). Previous investigations have also described how FA positively correlates with both frontal theta power (Cohen, 2011) and long-distance theta band phase synchrony (Liu et al. 2017). A recent report showed that individuals with a history of mTBI have diminished medio-lateral frontal phase synchrony during reactive control (Smith & Allen, 2019). In sum, a collection of prior findings offers promising evidence that individual differences in medio-lateral phase synchrony should correlate with FA, and that these may be expected to covary due to structural damage following mTBI.
This approach is notably different from other EEG investigations of mTBI. Assessments of resting activities are common, exemplified by the QEEG approach (Hanley et al. 2017; Naunheim et al. 2010; Nuwer et al. 2005; Prichep et al. 2014; Thatcher et al. 1989) or direct assessment of frontal theta at rest (Kaltiainen et al. 2018). While such findings are intriguing, frequency-specific differences at rest may not reflect the same neural phenomenon as task-evoked frequency-specific signatures (e.g. theta-like activity could be due to alpha slowing). Other studies have observed slowing in late cognitive ERP features (Dupuis et al. 2000; Folmer et al. 2011; Larson et al. 2011; Lavoie et al. 2004; Moore et al. 2014; Weinberg 2000; Wilson et al. 2014), yet the majority of these studies tested individuals in the chronic injury state and/or combined patients across the TBI spectrum. There is a considerable lack of studies investigating known EEG features tightly tied to cognitive control in the sub-acute phase of mTBI, when effects are likely to be most prominent.
The current study
Here we aimed to assess the interaction between structure (FA), function (theta phase synchrony), and phenotype (self-report) in participants who recently experienced an mTBI, as well as matched controls with no history of brain injury. Cognitive function was assessed with a demanding cognitive control task while EEG was recorded. To reduce the dimensionality of these combined measures, we used joint independent components analysis (jICA) of co-varying FA and phase synchrony to identify the structural network that was best captured by the theta band activity of interest.
Materials and Method
Experimental Design
The University of New Mexico Health Sciences Center (UNMHSC) Human Research Protections Office approved the study and all participants provided written informed consent. All participants were aged 18–55 years, were fluent in English, had no premorbid major medical or psychiatric conditions, no history of substance abuse, and were not currently taking medications that interfere with cognitive functioning with the exception of selective serotonin reuptake inhibitors (SSRIs). Three participants from the control group and four from the mTBI group were on SSRIs. None of the sub-acute or control participants had a previous head injury.
Sub-acute mTBI patients were recruited from the Departments of Neurosurgery and Emergency Medicine from UNMHSC within two weeks following their injury. Injury history was assessed with a modified version of the Rivermead semi-structured interview (King, Crawford, Wenden, Moss, & Wade, 1995; Mayer et al., 2018). Participants were enrolled into the sub-acute mTBI group if they met American Congress of Rehabilitation Medicine criteria, assessed by an endorsement of a loss of consciousness following injury of 30 minutes or less as well as a Glasgow Coma Scale of 13–15 if available. Control participants included sex- and age-matched individuals from the Albuquerque, New Mexico community.
Sub-acute mTBI and control participants were invited to three assessment sessions, where symptoms, behavioral performance, and EEG were assessed. Session 1 was from 3–14 days post-injury and was the only session with MRI. Session 2 was ~2 months (1.5 to 3) and Session 3 was ~4 months (3 to 5) following Session 1. Participants were paid $20, $25, or $30 per hour for participation in each respective session. One sub-acute mTBI participant was removed from Session 3 as they experienced a head injury between their second and third sessions. Supplemental Figures 1–3 show the flow diagram of recruitment, participant exclusion, and data availability for each measure and all combined analyses.
Questionnaires and Neuropsychological Assessments
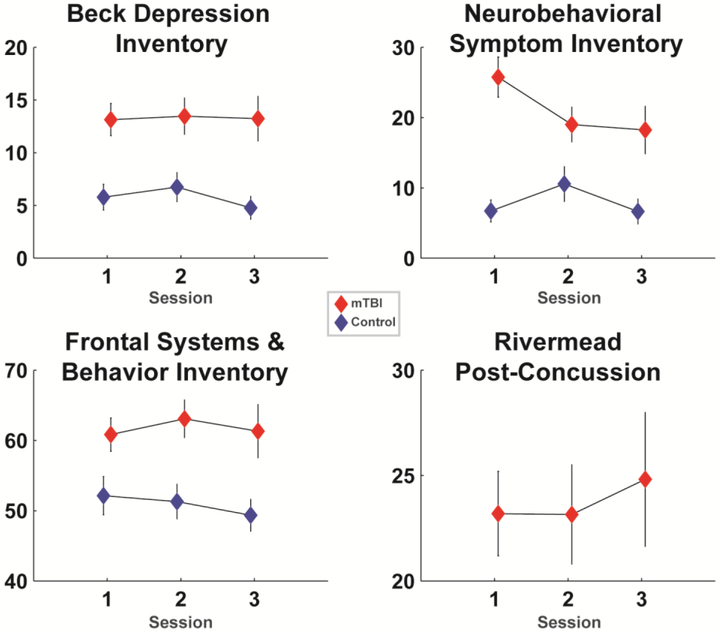
All participants completed demographic and neuropsychological assessments in their first session. All participants scored 45 or above on the Test of Memory Malingering (TOMM). Other assessments included the Test of Premorbid Functioning (TOPF), the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) Digit Span and Coding subtests, and the Hopkins Verbal Learning Test-Revised (HVLT-R). These values are shown in Table 1, which also details the number of participants that completed each assessment session. While the control group had relatively stable enrollment, the sub-acute mTBI group suffered from attrition, with about 79%of participants returning for session 2 and about 58% returning for session 3. Table 2 details the injury characteristics of the sub-acute group. Symptom-related questionnaires were administered in each session, including the Neurobehavioral Symptom Inventory (NSI), the Beck Depression Inventory-Second Edition (BDI), the Frontal Systems Behavior scale (FrSBe), and the Rivermead; these values are shown in Figure 1.
Table 1. Sample size, demographics, and neuropsychological test scores for all groups.
Neuropsychological data were only collected at the first session. All data are mean +/− SD except for session counts. n/a = not applicable. Asterisks indicate statistically significant differences from controls:*p<.05, **p<.01.
| Control | Sub-Acute mTBI | |
|---|---|---|
| Session 1: N (female) | 32 (16) | 52 (19) |
| Session 2: N (female) | 31 (15) | 41 (15) |
| Session 3: N (female) | 27 (12) | 30 (10) |
| Age | 29.59 (10.60) | 28.96 (10.25) |
| Years of Education | 14.78 (2.70) | 13.53 (2.21)* |
| TOPF | 105.38 (12.65) | 94.51 (14.27)** |
| WAIS Coding | 10.53 (2.37) | 9.23 (2.73) * |
| WAIS Span | 11.03 (2.52) | 9.52 (2.57) * |
| HVLT-R Trials 1–3 | 9.01 (1.50) | 7.77 (1.58)** |
| HVLT-R Delay Recall | 48.66 (11.67) | 37.90 (12.02)** |
Table 2. Injury information for the sub-acute mTBI group.
Data are presented as counts (GCS, LOM), median +/− intra-quartile range (LOC minutes, Time since injury), or percentages. Any LOC described as “less than a minute” was coded as .5 minutes. n/a = not applicable (not gathered or reported). GCS=Glasgow Coma Scale, LOC=loss of consciousness, LOM=loss of memory (yes/no question).
| Sub-Acute mTBI | |
|---|---|
| GCS 13–15 | 13 (2), 14 (1), 15 (38), n/a (11) |
| LOC Minutes | 4.5 (13.25) |
| LOM | Yes (27), No (23), n/a (2) |
| Time Since Injury | 10 (5) days |
| Accident / Fall | 17% |
| Assault | 15% |
| Motor Vehicle | 52% |
| Sport | 15% |
Figure 1. Responses to symptom questionnaires for each group and each session.
The sub-acute mTBI group had higher levels of depression (BDI), physical and mental symptoms (NSI), and executive problems in daily living (FrSBe). Only the NSI physical symptoms had interactive trends over time, where symptoms became more similar between groups. The Rivermead was only assessed in the sub-acute mTBI group. Data are mean +/− SEM.
Among the questionnaires assessed in this study, the FrSBe is the only scale related to the executive functions putatively reflected by frontal theta processes; the specificity of the BDI to depression and high reliance of the NSI to somatic distress are divergent from the executive constructs measured here1. The FrSBe score is scaled by the age, sex, and years of education of the participant (Grace and Malloy 2001), making it well-suited for zero-order correlation with brain variables within this diverse sample. Based on our prior investigation of EEG activities to auditory orienting from a subset of this cohort (Cavanagh et al., in press), we were particularly interested in the degree of an individual’s symptomatic recovery, defined as the change over time in FrSBe score (session 2 minus session 1; lower scores thus mean reduced symptoms over time).
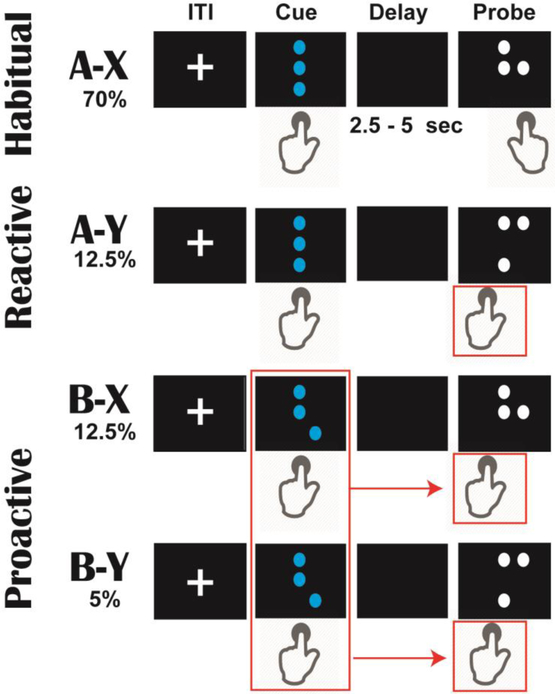
Dot Pattern Expectancy Task
The Dot Pattern Expectancy (DPX) variant of the AX-Continuous Performance Task (Barch et al. 2009; MacDonald et al. 2005) was programmed in Matlab using Psychtoolbox (Brainard 1997; Kleiner et al. 2007; Pelli 1997), see Figure 2. In this two-alternative-forced choice task, cues and probes were depicted as combinations of dots (akin to a braille pattern). These patterns are described with single letter terms: a cue stimulus (A or B) is presented before a probe stimulus (X or Y). Each cue-probe pair had a different probability and different response requirement. Participants were instructed to respond to all cues with a left trigger button on a joystick; responses to the probe stimuli varied depending on the cue-probe pairing.
Figure 2.
The Dot Probe Expectancy Task (DPX). By convention, cues and probes are referred to as A/B and X/Y, respectively. Cues and probes were presented as unique dot combinations, separated by an unpredictable delay. Each stimulus required a specific type of response. On 70% of trials, the standard (A-X) sequence fostered habitual responding of left then right button pushes. Rare (A-Y) trials require participants to break this response with a left button push to the probe; this is suggested to induce a reactive version of cognitive control to overcome the habit. On 17.5% of trials, the B cue can be used to proactively control probe responses, since a left button push is always required for probes after a B cue. In the current study, we were interested in the ability of sub-acute mTBI participants to proactively plan and maintain a controlled response plan following these B cues.
In the most common (70% of trials) A-X sequence, X probes required a right trigger response, creating a habitual left-right response pattern to cue and probe. In the A-Y sequence (12.5% of trials), participants were required to break this expectancy by responding with a left button press to the Y probe. This A-Y probe is thus designed to elicit reactive control. In the B-X (12.5% of trials) and B-Y (5% of trials) sequence, participants were also required to respond with a left button press to the X or Y probe. The B cue is specifically designed to elicit proactive control, since participants can use the forewarning from the B cue to change their response habit to either X or Y probe.
Following convention, there were 5 unique B cues and 5 unique Y cues. Cues and probes remained on the screen until a button press was made. The cue-probe delay was selected from a random distribution of 2500 to 5000 ms. Trials were separated by an inter-trial interval (ITI) selected from a uniform distribution of 750–1000 ms. Participants first received intensive instructions with practice on each rule, and then they completed 25 full practice trials. Participants then completed 5 blocks with 50 trials per block. Including instructions, the task took about 36 minutes to complete.
Only correct trials were used for assessment of response time (RT). Session data were excluded if participants failed to achieve >25% accuracy on bX probe trials. Our main interest was performance on the bX probes as an index of proactive control following B cues. Although the difference between bX and aY probe performance is commonly assessed in this task as a measure of the balance between proactive and reactive control (i.e. the behavioral shift index), we were not interested in the relative tradeoff between these measures, only the ability to apply and maintain proactive control. Analysis of the behavioral shift index is provided in Supplemental Table 1.
EEG Recording and Preprocessing
EEG was recorded continuously from sintered Ag/AgCl electrodes across .1 to 100 Hz with a sampling rate of 500 Hz, an online CPz reference, and a ground at AFz on a 64 channel Brain Vision system. The vertical electrooculogram (VEOG) was recorded from bipolar auxiliary inputs. Data were epoched around the cue onset (−2000 to 7000 ms), from which the associated cue-locked responses were isolated. Activity at the reference electrode CPz was re-created via re-referencing. Very ventral temporal sites were then removed, as they tend to be unreliable, leaving 60 electrodes. Bad channels and bad epochs were identified using a conjunction of the FASTER algorithm (Nolan et al. 2010) and pop_rejchan from EEGlab (Delorme and Makeig 2004) and were subsequently interpolated and rejected respectively. Data were then re-referenced to an average reference. Eye blinks were removed following independent components analysis in EEGlab (Delorme and Makeig 2004).
All EEG analyses were limited to correct performance trials. In order to equate trial counts for comparable signal to noise between conditions, a random selection of A cues were retained, matching the number of B cues remaining following the pre-processing step described above. All analyses were performed on data with a minimum of 20 epochs. Cue-locked event-related potentials averaged over group and sessions are included in Supplemental Figure 4. A Laplacian transform of the data was used to minimize volume conduction prior to time-frequency decomposition, which is a necessary step for connectivity analyses (Cohen, 2014). Time-Frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets defined as a Gaussian-windowed complex sine wave: ei2πtfe−t^2/(2xσ^2), where t is time, f is frequency (which increased from 1–50Hz in 50 logarithmically spaced steps) and the width (or ‘cycles’) of each frequency band were set to increase from 3/(2πf) to 10/(2πf) as frequency increased. Then, the time series was recovered by computing the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in estimates of instantaneous power (the magnitude of the analytic signal) and phase angle (the arctangent of the analytic signal).
Each epoch was then cut in length (−500 to +1000 ms). Averaged power was normalized by conversion to a decibel (dB) scale (10*log10[power(t)/power(baseline)]) from a common cross-condition averaged baseline of −300 to −200 ms, allowing a direct comparison of effects across frequency bands. Inter-site phase clustering (ISPC) over trials was quantified as the length of the average of unit-length vectors that were distributed according to the difference in phase angles between FCz and separate lateral frontal electrodes (F5 and F6). These electrode combinations are the most commonly used pairs for assessing medio-lateral interactions (Cavanagh, Cohen, & Allen, 2009; Smith & Allen, 2019). Based on a recent description of the hemisphericity of this phenomenon we formally compared left and right sided synchrony, although we have previous justification to suggest that proactive conflict might engage a left-hemisphere dominant network (Cavanagh and Cohen 2019), see Supplemental Figure 5.
The full time-frequency spectrum of EEG data was analyzed using t-tests on the B vs. A contrast for both FCz power and medio-lateral ISPC (cluster corrected based on 500 condition shuffles). Since this B vs. A contrast was orthogonal to all group and session hypotheses, time-frequency ROIs (tf-ROIs) were selected from the dominant patterns in this contrast. FCz power was quantified as the average of 300 to 600 ms at 4.5 Hz and FCz-F5 and FCz-F6 ISPC was quantified as the average of 400 to 700 ms at 4.5 Hz.
MRI Recording and Preprocessing
MRI images were obtained with a Siemens 3T Trio TIM scanner and a 32-channel coil. Diffusion weighted images (TR=4000ms, TE=108 ms, 70 slices, flip angle = 84°; voxel size 2 × 2 × 2 mm) were acquired using a twice-refocused spin echo sequence. Four scans were acquired, with 44, 47, 42 or 40 diffusion gradients with a maximum b-value of 2400 s/mm2. The b=0 experiment was repeated eight times. DTI analyses were performed using FSL software (Smith et al., 2004). Data were corrected for bias and distortion (FSL TOPUP), motion and eddy currents (FSL EDDY), and visually checked for quality. FA was then calculated (FSL DTIFIT) and the data were spatially normalized (FSL FNIRT) to the Johns Hopkins University (JHU) white matter atlas (Mori and van Zijl 2007).
Subject-specific abnormalities were calculated in the same manner as prior studies (Mayer et al., 2012). First, data were masked by voxels that exceeded a mask of >0.25 from the average of all subject’s FA values. Subject-specific abnormalities were computed as the statistical distributions of FA values that were greater or less than expected by chance based on the z-score of the control group (16 pixel cluster minimum, p<0.05 chance level adjusted to the distribution corrected z-scores (DisCo-Z: Mayer et al., 2017)). As in prior research, the number and size of both positive and negative clusters were square root transformed to approximate normality and contrasted between groups (Mayer et al., 2012).
Joint Independent Component Analysis (jICA)
jICA uses blind source separation to resolve a single mixing matrix that identifies the maximal covariance between two measures (Calhoun, 2006; Calhoun, Liu, & Adali, 2009; Moosmann, Eichele, Nordby, Hugdahl, & Calhoun, 2008); here we use spatial areas in MRI images and temporal patterns in EEG data. Since MRI data were only acquired at the first session, jICA fusion only included the first session of EEG data. The JHU-masked FA and the full time-course of medio-lateral ISPC (−500 to 1000 ms for FCz-F5 and FCz-F6) of each participant were submitted to jICA. Both groups were included in the jICA decomposition, with a fixed factor to distinguish the groups. The default number of eight components were estimated and individual mixing coefficients for each component were compared between groups using a t-test in the jICA software to determine the maximally discriminant combination of spatial and temporal features. These individual mixing coefficients are also known as loading factors, and they indicate how much each participant expresses or contributes to each latent component (Stephen et al. 2013; Sui et al. 2010). A group difference between these loading factors indicates that one group has a greater net contribution to that component (for example there may be a higher correlation between the specific ISPC time series and FA voxels identified in that component in group A than in group B). Our a priori hypotheses of the medio-lateral ISPC constrained our interpretation of jICA components to the approximate range of 400 to 700 ms where the ISPC was maximal.
Statistical Analysis
The analysis of change over time utilized mixed linear models (MLMs) to account for individual change within each group in the context of missing data (e.g. attrition). MLMs used restricted maximum likelihood estimation with fixed effects for group (binary), session (continuous), and the group*session interaction. Session was modeled as a repeated factor with autoregressive covariance. Only results with a main or interactive effect of group are discussed. Since the Rivermead was only assessed in the sub-acute mTBI group, a one-way ANOVA was used to examine change over sessions. Reliabilities were assessed using intraclass correlation coefficients (ICCs) within a two-way mixed effects model defined by absolute agreement (Koo and Li 2016). These analyses were performed separately for each group. For simplicity, the output of all MLM and ICC tests are included in Table 3.
Table 3. Statistical values and reliability estimates.
Under the reliability columns, X indicates an average negative covariance between items, making ICC impossible to compute. The effective reliability of these measures is very poor.
| Main Effect of Group | Group * Session Interaction | CTL alpha | mTBI alpha | |
|---|---|---|---|---|
| FrSBe | F(1,82.58)=10.15, p=.002 | F(2,129.27)=0.47, p=.63 | .90 | .79 |
| BDI | F(1,79.38)=13.06, p=.001 | F(2,124.98)=0.26, p=78 | .87 | .89 |
| NSI | F(1,78.25)=15.21, p=.0002 | F(2,123.00)=4.91, p=.009 | .90 | .87 |
| DPX: RT | F(1,73.42)=6.61, p=.01 | F(2,107.02)=1.76, p=18 | .69 | .86 |
| DPX: Acc | F(1,76.57)=3.24, p=.08 | F(2,110.31)=1.13, p=33 | .74 | .95 |
| DPX: bX-aX RT | F(1,74.93)=3.35, p=.07 | F(2,114.71)=1.62, p=.20 | .55 | .76 |
| DPX: bX-aX Acc | F(1,72.85)=0.25, p=.62 | F(2,113.03)=1.40, p=25 | .71 | .29 |
| FCz Power | F(1,75.53)=.19, p=67 | F(2,105.29)=.92, p=.40 | .68 | .85 |
| Bilateral Synch | F(1,115.67)=2.31, p=.12 | F(2,254.75)=1.85, p=.16 | .13 | .02 |
| FCz-F5 Synch | F(1,79.77)=3.81, p=.05 | F(2,126.46)=3.71, p=.03 | .13 | X |
| FCz-F6 Synch | F(1,88.36)=0.25, p=62 | F(2,132.81)=0.20, p=82 | X | X |
Results
Questionnaires
All questionnaire scales significantly differed between groups, yet only the NSI had a significant interaction between group and session where the groups became more similar over time (Table 3 and Figure 1). All questionnaire measures had good reliabilities (.79 to .90). The Rivermead had no main effect of session within the sub-acute mTBI group (t=.41, p=.69; Cronbach’s alpha = .92). In total, the sub-acute mTBI group had greater symptoms of depression, somatic and cognitive complaints, and executive problems in daily living, with very little change over time.
Task Performance
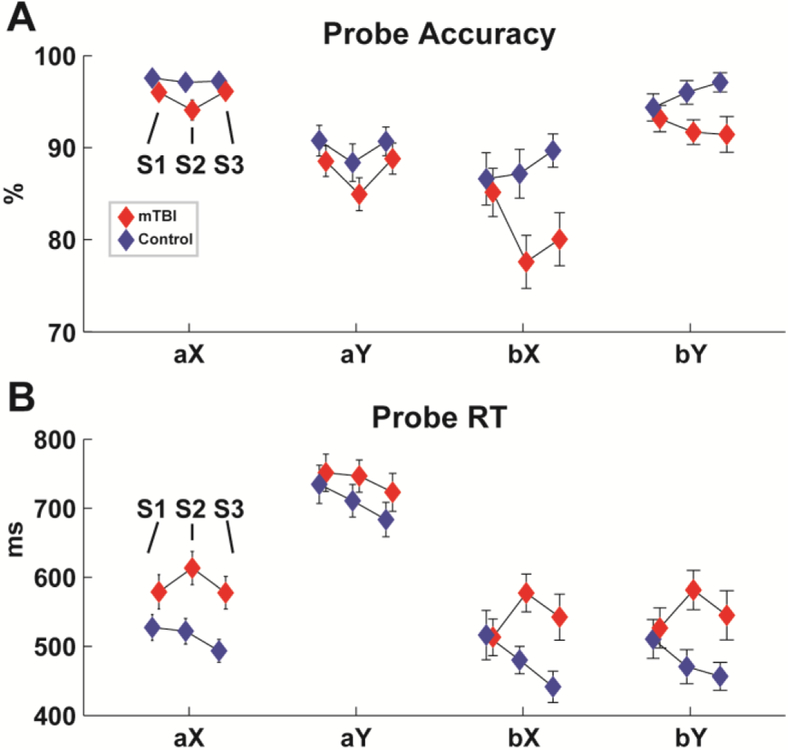
Figure 3 shows the performance on the probe trials of the DPX task. Sub-acute mTBI participants were significantly slower and non-significantly less accurate than controls across all conditions, but there were no effects of mTBI on performance specific to the control demands in the bX condition (i.e. bX minus aX; Table 3). Reliabilities for RT and accuracy spanned a wide range across measures and groups (.29 to .95). This range is in line with findings from a recent review of the psychometric properties of this task that suggested that RT has higher reliability than accuracy since there is more intrinsic variability, and that patients may show more variability than controls over sessions, which can artificially increase reliability estimates (Cooper et al. 2017).
Figure 3. Performance on the DPX task.
Each triplet of icons represents the mean and +/− SD of session 1, 2 and 3 (S1:S3). A) Accuracy was not significantly different between groups. B) The sub-acute mTBI group had significantly slower RTs.
Identifying the Functional EEG Network for Cognitive Control
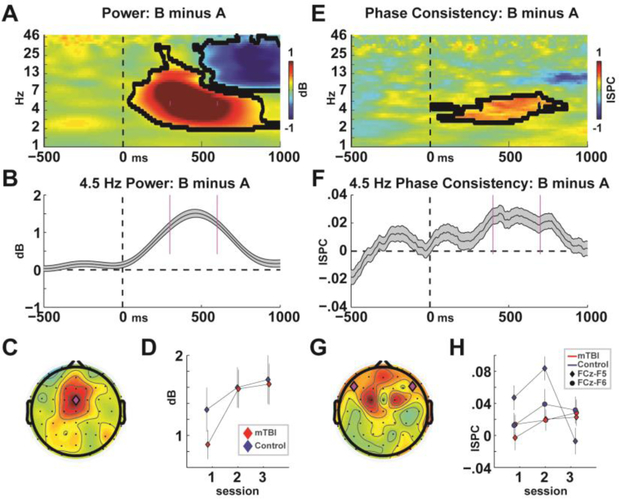
Figure 4 shows the time-frequency data from the theta-band measures. As expected, the difference in power between B and A conditions was dominant in the theta band over the frontal midline at FCz (Figure 4A–C). However there were no significant main or interaction effects of group (Figure 4D and Table 3), nor was there a significant difference between groups at the first session alone (t(66)=1.24, p=.22). Bilateral ISPC was similarly dominant at 4.5 Hz within a slightly later time window, but these did not differ between groups either, nor were there any interactions with laterality (Figure 4E–H and Table 3).
Figure 4. EEG data collapsed across all groups and conditions.
A-C) Time-frequency plot of the B-A difference highlighting how frontal midline theta band power is associated with the need for control. D) There were no differences between groups in theta power. E-F) Average of medio-lateral (left and right) phase consistency highlighting the candidate mechanism for the communication of control. H) There were no differences between groups in bilateral theta phase consistency, but when the left-specific activities were investigated on their own they were significantly different between groups.
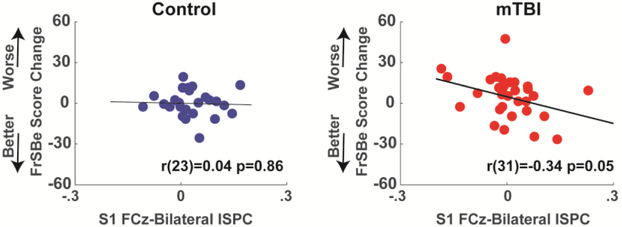
When a posteriori split by hemisphere, left-side ISPC was significantly different between groups with an interaction between group and session, and there was no effect for right-sided ISPC (Table 3). This group*time interaction effect in left-sided ISPC was largely due to a diminished value in the third time point in the control group; this pattern is explained in the discussion (see Supplemental Figure 6). Although power had good reliability, there was little to no reliability in measures of ISPC (Table 3). Indeed, some measures had an average negatively signed covariance, making ICC impossible to compute. This lack of temporal reliability of ISPC is likely related to the low temporal reliability in task performance. Figure 5 shows that bilateral session 1 ISPC was able to predict future recovery in FrSBe scores within the mTBI group (r(31)=−.34, p=.05, R2=.12) whereas there was no meaningful relationship in the control group (r(23)=−.04, p=.86, R2=0). This pattern was most robust on the left side, but right sided ISPC had the same general trend (Supplemental Figure 7).
Figure 5. Function-phenotype relationships.
Session 1 theta band phase synchrony (ISPC) predicts recovery from executive deficits (FrSBe) in the mTBI group between the first and second sessions.
No FA Differences between Groups
Supplemental Figure 8 shows all outputs from conventional FA analyses. There were no group differences in an FDR-corrected whole-brain analysis, and the distribution of JHU region-specific t-tests was indistinguishable from chance. Subject-specific abnormalities were quantified for each individual within each group. There were no differences in the number or mass of positive or negative clusters between groups. In sum, conventional FA analyses failed to distinguish mTBI from control groups.
Linking Structure and Function with jICA
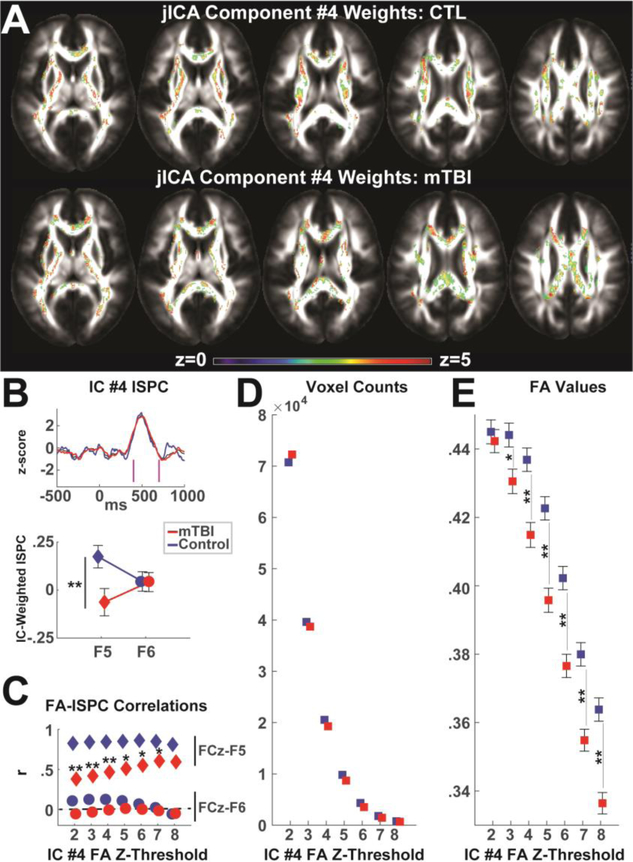
Joint ICA was used to merge structural (FA) and functional (ISPC) data for the first session data in both groups. Only one component (IC #4) captured the a priori temporal window of phase synchrony between 400 to 700 ms, and only this component significantly differentiated groups (Figure 6A–B, p=.025). See Supplemental Figure 9 for all ISPC component time courses. To examine the inter-relation of these relevant voxels and time points, the component weights from IC #4 were used to mask the FA and EEG for each participant. IC#4-weighted ISPC was averaged above a z-score threshold of 2.5, which captured much of the a priori time window. Given the lack of a spatial ROI, we investigated FA weights over a range of z-score thresholds. Within each z-score threshold bin, values of FA, voxel count, and the correlation between FA and ISPC were computed.
Figure 6. Joint ICA component #4 significantly differentiated groups.
A) Z-scores of IC #4 weighted FA for each group. These demonstrate grand average contribution of each FA voxel to the structure-function network identified in IC #4. B) IC #4 weighted ISPC peaked within the a priori temporal region of 400 to 700 ms (magenta lines) and primarily captured left-sided ISPC, which differed between groups. Plots are mean +/− SEM. C) Across varying levels of threshold for the FA weights, FA values correlated with left-sided ISPC but not right-sided ISPC. The difference in correlation between mTBI and control groups was significant for nearly all levels of z-threshold. D) The number of voxels naturally diminished as the discrimination threshold increased, as did the relative number of voxels in the mTBI compared to the control group. E) As the discrimination threshold increased, FA values in IC #4 became increasingly smaller in the mTBI compared to the control group. Plots are mean +/− SEM. ** p<.01, *p<.05
Figure 6B shows how IC #4-weighted ISPC had a significant laterality by group interaction (F(1,59)=4.02, p=.05). Follow-up t-tests indicated that only the left lateralized activity was different between groups (t(59)=2.42, p=.02) whereas right lateral activity was not (t(59)=.04, p=.97). Although the groups differed on pre-morbid IQ, there was no influence of TOPF on this interaction (laterality * group * TOPF: F(2,57)=2.30, p=.11) nor on left-lateralized activity (group * TOPF: F(1,56)=.98, p=.32), nor did TOPF correlate with ISPC or FrSBe change within either group (Supplemental Figure 10).
Figure 6C shows how both groups had positive correlations between IC#4-weighted FA and left-specific IC#4-weighted ISPC regardless of threshold, but no relationship with right-lateralized activity. In the simplest explanatory terms, this correlation depicts how the jICA process identified structural and functional data points that co-vary with each other but not with other data points. The difference in the correlation (z tests of r-to-z transformed coefficients) between FA and left-specific ISPC between mTBI and control groups was significant for nearly all levels of z-threshold, with the mTBI group having smaller correlations than the control group. Figure 6D shows how the number of voxels naturally diminished as the FA discrimination threshold increased, but the relative number of voxels in the mTBI decreased more than the control group. Figure 6E shows that as the discrimination threshold increased, FA values became increasingly smaller in the mTBI group compared to the control group; these effects were statistically significant at all contrasts of z>3. In sum, the structure-function relationship in the mTBI group was characterized by relatively smaller left-specific ISPC, fewer voxels involved in this network, lower FA in these voxels, and a diminished relationship between FA and ISPC.
Discussion
This investigation revealed that when different imaging indices are combined, a deficient structure-function relationship affected by mTBI becomes particularly expressed. Given the a priori interpretability of the theta band functional network, the spatial, temporal, and frequency characteristics in the EEG were able to guide the independent component selection to highlight the FA network of interest. Notably, the only EEG component that met the a priori time window (see Figure 4E–H) was the one that statistically distinguished groups. The mTBI group was characterized by a smaller left-specific ISPC, fewer voxels, lower FA in those voxels, and a diminished relationship between FA and ISPC, suggesting an impoverished structure-function relationship for exerting cognitive control. In sum, this data fusion technique highlighted sub-threshold structural features (Fig 6A, 6D, & 6E) that were presumably altered by mTBI that would otherwise have been unobservable.
There was a notable lack of self-reported symptomatic recovery across the sub-acute mTBI group. Only the NSI, which is dominated by somatic complaints, decreased over time. Other measures of somatic complaints (Rivermead), depression (BDI), and executive function (FrSBe), remained consistently high over the four months following injury. However, within this static group-level trend there was notable individual variation. Motivated by our recent study of P3a and P3b amplitudes on FrSBe change in a sub-set of this cohort (Cavanagh et al. in press), theta band phase synchrony predicted individual trajectories of recovery from FrSBe symptoms over time (Figure 5). A recent review by Karr et al. (2014) suggested that cognitive functions tended to recover within 90 days post-injury, yet they remain one of the vulnerable symptomatic domains following multiple TBIs. This suggests that the ISPC findings reported here may be particularly important for tracking normative recovery as well as quantifying deficits in more chronic cases.
Interestingly, the only group differences in EEG measures described here were specific to the phase-synchronous network activities and not to the frontal midline power burst. Prior reports of clinical groups with compromised frontal functions have shown that power and phase synchrony can vary independently, where they can both be larger (anxiety: Cavanagh et al. 2017), they can both be smaller (schizophrenia: Ryman et al. 2018), or a power diminishment can occur in the absence of a phase synchrony difference (Parkinson’s disease: Singh et al. 2018). Here, the finding of relatively maintained power yet diminished (left-specific) phase synchrony over time is commensurate with the hypothesis that frontal midline power and medio-lateral phase dynamics represent two different features of control: namely the initial alarm bell and the following implementation of control (Cavanagh and Frank 2014). It was hypothesized that compromised white matter following mTBI would specifically affect this latter aspect of the ability to communicate the need for control across brain regions.
The lack of whole-brain FDR corrected differences, individual JHU ROI analyses, and single subject abnormalities in FA clusters may be expected as a natural consequence of subtle deficits and profound inter-subject variability. These null effects were not surprising given the lack of a consensus in the literature (Dodd et al. 2014; Eierud et al. 2014). Here we propose that by leveraging a known EEG process, jICA can provide a data-driven solution that more effectively isolates white matter involved in a specific cognitive process. This idea motivates a discussion of the limitations of the current study prior to a summary of the main conclusions.
Limitations
The first limitation of these findings is the problematic reliability of behavioral performance and EEG measures on the dot probe task. Extant findings in sub-acute mTBI participants have tended to show generalized slowing in the absence of specific performance deficits, and this same broad pattern was observed here (Figure 3). However, contrary to the aims of the study, we were unable to determine if there were more fine-grained control-specific deficits in mTBI, possibly due to the unreliability of task performance over time. Although frontal midline theta power was reliable over time (as in prior studies: Cooper et al. 2019), it is likely that phase-based measures differed depending on how control was implemented. Given the unreliability of task measures, it is likely that individuals tend to alter their proactive control, response caution, alertness, or other factors for subtle but meaningful changes in task performance. As recently reviewed by D’Souza et al. (2019), there is limited evidence of reliability across cognitive assessment tools for TBI populations, suggesting that this issue is pervasive across current measurement techniques.
A second limitation is that the analysis of a left-specific FCz-F5 ISPC effect (Figure 4) was not motivated by a higher-level interaction between group, time, and laterality. However, it was a priori motivated by a recent literature review of the hemisphericity of ISPC effects, which also suggested a need for formal statistical comparison between hemispheres (Cavanagh and Cohen 2019). This a posteriori effect was included in the present report since it helps explain the findings from the jICA, which revealed a formal statistical differentiation between hemispheres. The statistical interaction of left-hemisphere phase synchrony between the groups over time points was largely dependent on the strongly diminished value in the control group at the third time point. Exploratory analyses revealed that the F5 electrode did not capture the spatial peak of phase synchrony in session 3 (see Supplemental Figure 6). Despite this spatial variance, we thought it was most appropriate to keep the commonly used a priori spatial locations for assessing these effects; to change these based on the data would invite circular analysis and compromise generalizability. In addition, dimension reduction methods like PCA failed to reveal consistent and interpretable effects across all groups and time points, although supplemental analyses revealed that there were no differences at equidistant posterior electrode sites, demonstrating frontal specificity (Supplemental Figure 11). This spatial unreliability within the frontal cortex may be related to the individual behavioral and phase synchrony unreliability across time points, which may be in turn affected by variation in strategic performance.
Finally, a summary limitation of the current study is that although the sample sizes were good for a sub-acute mTBI sample and comparison group, they were underpowered for the three time points and the complexity of interactions between multiple measures. Despite careful recruitment strategies, groups still differed on pre-morbid IQ (TOPF score, Table 1). TOPF did not relate to ISPC or FrSBe score, nor did it interact with group differences following jICA, yet this issue still complicates generalizability. Future studies of data fusion may aim for a single session assessment with a range of simpler tasks that have demonstrated reliabilities, as well as a control group characterized by acute orthopedic (non-brain) injuries. Despite these limitations, the ultimate conclusion from the current investigation is that EEG measures can boost the inferential value of MRI assessments of mTBI.
Conclusions
Our primary aim in this investigation was to leverage a candidate functional mechanism of cognitive control to link structural damage to distressed behavioral phenotype following mTBI. EEG is uniquely sensitive to canonical neural operations that underlie emergent psychological constructs like cognitive control (Cavanagh 2019; Fries 2009; Siegel et al. 2012). This makes it particularly well-suited for identification of aberrant neural mechanisms that underlie complicated disease states (Insel et al. 2010; Montague et al. 2012). In this study, the control processes reflected by frontal theta band phase synchrony were able to distinguish groups, predict future recovery, and identify a diminishment in structural areas formally linked to the functional process. Together, these provide compelling evidence of the usefulness of combined imaging measures when assessing mTBI.
Supplementary Material
Acknowledgements:
The authors thank Jacqueline Hope Story-Remer, Violet Fratzke, Davin Quinn, Rick Campbell, Ron Yeo, and Jacki Janowich for help with this project and Vince Calhoun for helpful discussions of jICA. This research was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM109089.
Funding: This study was funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM109089.
Footnotes
Although the NSI is sometimes reported with a three factor structure (Caplan et al. 2010), the somatic, cognitive, and affective dimensions were highly correlated here (r values from .78 to .84). Given that the somatic dimension has the most items (11 out of 22 items) and there are somatic features in the other dimensions (headache, fatigue), in this sample these sub-scales appear to reflect common variance in a somatic-dominant dimension.
Data Accessibility: Data and code for this experiment are available on the PRED+CT website: www.predictsite.com; Accession #d010.
Disclosure Statement: None of the authors have potential conflicts of interest to be disclosed.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Aoki Y, & Inokuchi R (2016). A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neuroscience and Biobehavioral Reviews, 66, 119–126. doi: 10.1016/j.neubiorev.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Barch DM, Berman MG, Engle R, Jones JH, Jonides J, Macdonald A, et al. (2009). CNTRICS final task selection: working memory. Schizophrenia bulletin, 35(1), 136–52. doi: 10.1093/schbul/sbn153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2008). Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. Journal of the International Neuropsychological Society : JINS, 14(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Crowe DA, DeNicola AL, Sakellaridi S, MacDonald AW, & Chafee MV (2016). Monkey Prefrontal Neurons Reflect Logical Operations for Cognitive Control in a Variant of the AX Continuous Performance Task (AX-CPT). The Journal of neuroscience : the official journal of the Society for Neuroscience, 36(14), 4067–79. doi: 10.1523/JNEUROSCI.3578-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. doi: 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Busch RM, McBride A, Curtiss G, & Vanderploeg RD (2005). The components of executive functioning in traumatic brain injury. Journal of clinical and experimental neuropsychology, 27(8), 1022–32. doi: 10.1080/13803390490919263 [DOI] [PubMed] [Google Scholar]
- Calhoun V (2006). A Feature-Based Approach To Combine Multimodal Brain Imaging Data. Proceedings 14th Scientific Meeting, International Society for Magnetic Resonance in Medicine, 832. doi: 10.1109/CISS.2014.6814108 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, & Adali T (2009). A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage, 45(1 Suppl), 163–172. doi: 10.1016/j.neuroimage.2008.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LJ, Ivins B, Poole JH, Vanderploeg RD, Jaffee MS, & Schwab K (2010). The structure of postconcussive symptoms in 3 us military samples. Journal of Head Trauma Rehabilitation, 25(6), 447–458. doi: 10.1097/HTR.0b013e3181d5bdbd [DOI] [Google Scholar]
- Carney N, Chesnut RM, Maynard H, Mann NC, Patterson P, & Helfand M (1999). Effect of cognitive rehabilitation on outcomes for persons with traumatic brain injury: A systematic review. Journal of Head Trauma Rehabilitation, 14(3), 277–307. [DOI] [PubMed] [Google Scholar]
- Carroll L, Cassidy JD, Peloso P, Borg J, von Holst H, Holm L, et al. (2004). Prognosis for mild traumatic brain injury: results of the who collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36, 84–105. doi: 10.1080/16501960410023859 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, & Cohen MX (2019). Frontal theta as a model specimen for cortical theta In Gable PA, Miller MW, & Bernat E (Eds.), Handbook of EEG Frequency. New York, NY: Oxford University Press. [Google Scholar]
- Cavanagh JF, Wilson JK, Rieger RE, Gill D, Broadway JM, Story-Remer JH, et al. (in press). ERPs predict symptomatic distress and recovery in sub-acute mild traumatic brain injury. Neuropsychologia [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF (2019). Electrophysiology as a theoretical and methodological hub for the neural sciences, (November 2018), 1–13. doi: 10.1111/psyp.13314 [DOI] [PMC free article] [PubMed]
- Cavanagh JF, Cohen MX, & Allen JJB (2009). Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29(1), 98–105. doi: 10.1523/JNEUROSCI.4137-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 1–8. doi: 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Meyer A, & Hajcak G (2017). Error-Specific Cognitive Control Alterations in Generalized Anxiety Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(5). doi: 10.1016/j.bpsc.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, & Sanfey AG (2013). Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cerebral Cortex, 23(3), 739–749. doi: 10.1093/cercor/bhs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, Michael X (2011). Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage, 55(3), 1373–83. doi: 10.1016/j.neuroimage.2010.12.072 [DOI] [PubMed] [Google Scholar]
- Cohen, Mike X (2014). Analyzing Neural Time Series Data: Theory and Practice. Cambridge, MA: MIT Press. [Google Scholar]
- Cooper PS, Karayanidis F, McKewen M, McLellan-Hall S, Wong ASW, Skippen P, & Cavanagh JF (2019). Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. NeuroImage, 189(November 2018), 130–140. doi: 10.1016/j.neuroimage.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SR, Gonthier C, Barch DM, & Braver TS (2017). The role of psychometrics in individual differences research in cognition: A case study of the AX-CPT. Frontiers in Psychology, 8(SEP), 1–16. doi: 10.3389/fpsyg.2017.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Vega A, Chang LJ, Banich MT, Wager TD, & Yarkoni T (2016). Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. doi: 10.1523/JNEUROSCI.4402-15.2016 [DOI] [PMC free article] [PubMed]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods, 134(1), 9–21. doi: 10.1016/j.jneumeth.2003.10.009S0165027003003479[pii] [DOI] [PubMed] [Google Scholar]
- Dimoska-Di Marco A, McDonald S, Kelly M, Tate R, & Johnstone S (2011). A meta-analysis of response inhibition and Stroop interference control deficits in adults with traumatic brain injury (TBI). Journal of clinical and experimental neuropsychology, 33(4), 471–85. doi: 10.1080/13803395.2010.533158 [DOI] [PubMed] [Google Scholar]
- Dockree PM, & Robertson IH (2011). Electrophysiological markers of cognitive deficits in traumatic brain injury: a review. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 82(1), 53–60. doi: 10.1016/j.ijpsycho.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Dodd AB, Epstein K, Ling JM, & Mayer AR (2014). Diffusion Tensor Imaging Findings in Semi-Acute Mild Traumatic Brain Injury. Journal of Neurotrauma, 31(14), 1235–1248. doi: 10.1089/neu.2014.3337 [DOI] [PubMed] [Google Scholar]
- Dupuis F, Johnston KM, Lavoie M, Lepore F, & Lassonde M (2000). Concussions in athletes produce brain dysfunction as revealed by event-related potentials. Clinical Neuroscience and Neuropathology, 11(18), 4087–4092. doi: 10.1097/00001756-200012180-00035 [DOI] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, & Laconte SM (2014). Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage: Clinical, 4, 283–294. doi: 10.1016/j.nicl.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, Billings CJ, Diedesch-Rouse AC, Gallun FJ, & Lew HL (2011). Electrophysiological assessments of cognition and sensory processing in TBI: Applications for diagnosis, prognosis and rehabilitation. International Journal of Psychophysiology, 82(1), 4–15. doi: 10.1016/j.ijpsycho.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Frencham K.a R., Fox AM, & Maybery MT (2005). Neuropsychological studies of mild traumatic brain injury: a meta-analytic review of research since 1995. Journal of clinical and experimental neuropsychology, 27(3), 334–51. doi: 10.1080/13803390490520328 [DOI] [PubMed] [Google Scholar]
- Fries P (2009). Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual review of neuroscience, 32, 209–24. doi: 10.1146/annurev.neuro.051508.135603 [DOI] [PubMed] [Google Scholar]
- Grace J, & Malloy PF (2001). Frontal systems behavior scale. Professional manual. [DOI] [PubMed]
- Hanley D, Prichep LS, Badjatia N, Bazarian J, Chiacchierini R, Curley KC, et al. (2017). A brain electrical activity (EEG) based biomarker of functional impairment in traumatic head injury: a multisite validation trial. Journal of Neurotrauma, 47, neu.2017.5004. doi: 10.1089/neu.2017.5004 [DOI] [PubMed] [Google Scholar]
- Huang M-X, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, et al. (2009). Integrated Imaging Approach with MEG and DTI to Detect Mild Traumatic Brain Injury in Military and Civilian Patients. Journal of Neurotrauma, 26(8), 1213–1226. doi: 10.1089/neu.2008.0672 [DOI] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, & Lipton ML (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. American Journal of Neuroradiology, 34(11), 2064–2074. doi: 10.3174/ajnr.A3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751. doi:167/7/748[pii]10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Kaltiainen H, Helle L, Liljeström M, Renvall H, & Forss N (2018). Theta-Band Oscillations as an Indicator of Mild Traumatic Brain Injury. Brain Topography, 31(6), 1037–1046. doi: 10.1007/s10548-018-0667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins J, Bigler E, Covassin T, Henry L, Kemp S, Leddy JJ, et al. (2017). What is the physiological time to recovery after concussion? A systematic review. British Journal of Sports Medicine, 51(12), 935–940. doi: 10.1136/bjsports-2016-097464 [DOI] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, & Garcia-barrera MA (2014). The Neuropsychological Outcomes of Concussion : A Systematic Review of Meta-Analyses on the Cognitive Sequelae of Mild Traumatic Brain Injury, 28(3), 321–336. doi: 10.1037/neu0000037 [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, & Wade DT (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. Journal of neurology, 242(9), 587–92. http://www.ncbi.nlm.nih.gov/pubmed/8551320. Accessed 20 December 2018 [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard DH, & Pelli D (2007). What’s new in Psychtoolbox-3? Perception 36 ECVP Abstract Supplement. [Google Scholar]
- Koo TK, & Li MY (2016). A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine, 15(2), 155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter M, Sullivan M, Dahllöf a G., & Siösteen a. (1998). Partner relationships, functioning, mood and global quality of life in persons with spinal cord injury and traumatic brain injury. Spinal cord, 36(4), 252–61. http://www.ncbi.nlm.nih.gov/pubmed/9589525 [DOI] [PubMed] [Google Scholar]
- Larson MJ, Farrer TJ, & Clayson PE (2011). Cognitive control in mild traumatic brain injury: conflict monitoring and conflict adaptation. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 82(1), 69–78. doi: 10.1016/j.ijpsycho.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM, Demery J. a, & Stigge-Kaufman D. a. (2006). Cognitive control impairments in traumatic brain injury. Journal of clinical and experimental neuropsychology, 28(6), 968–86. doi: 10.1080/13803390600646860 [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Johnston KM, Leclerc S, Lassonde M, & Dupuis F (2004). Visual P300 Effects Beyond Symptoms in Concussed College Athletes. Journal of Clinical and Experimental Neuropsychology (Neuropsychology, Development and Cognition: Section A), 26(1), 55–73. doi: 10.1076/jcen.26.1.55.23936 [DOI] [PubMed] [Google Scholar]
- Ling JM, Peña A, Yeo RA, Merideth FL, Klimaj S, Gasparovic C, & Mayer AR (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: A longitudinal perspective. Brain, 135(Pt 4), 1281–1292. doi: 10.1093/brain/aws073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yu Y, Gao S, Sun J, Yang X, Liu P, & Qin W (2017). Structural Integrity in the Genu of Corpus Callosum Predicts Conflict-induced Functional Connectivity Between Medial Frontal Cortex and Right Posterior Parietal Cortex. Neuroscience, 366(October), 162–171. doi: 10.1016/j.neuroscience.2017.10.017 [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Goghari VM, Hicks BM, Flory JD, Carter CS, & Manuck SB (2005). A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology, 19(6), 814–821. doi: 10.1037/0894-4105.19.6.814 [DOI] [PubMed] [Google Scholar]
- Mayer a R., Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D., et al. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology, 74(8), 643–50. doi: 10.1212/WNL.0b013e3181d0ccdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Dodd AB, Ling JM, Wertz CJ, Shaff NA, Bedrick EJ, & Viamonte C (2017). An evaluation of Z-transform algorithms for identifying subject-specific abnormalities in neuroimaging data. doi: 10.1007/s11682-017-9702-2 [DOI] [PMC free article] [PubMed]
- Mayer AR, Hanlon FM, Claus ED, Dodd AB, Miller B, Mickey J, et al. (2018). An Examination of Behavioral and Neuronal Effects of Comorbid Traumatic Brain Injury and Alcohol Use. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(3), 294–302. doi: 10.1016/j.bpsc.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Yang Z, Pena A, Yeo R. a, & Klimaj S (2012). Diffusion abnormalities in pediatric mild traumatic brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(50), 17961–9. doi: 10.1523/JNEUROSCI.3379-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Flashman L. a, & Saykin AJ (2002). Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabilitation, 17(4), 333–44. http://www.ncbi.nlm.nih.gov/pubmed/12547981 [PubMed] [Google Scholar]
- McMahon PJ, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, et al. (2014). Symptomatology and Functional Outcome in Mild Traumatic Brain Injury: Results from the Prospective TRACK-TBI Study. Journal of Neurotrauma, 31(1), 26–33. doi: 10.1089/neu.2013.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, & Dayan P (2012). Computational psychiatry. Trends in cognitive sciences, 16(1), 72–80. doi: 10.1016/j.tics.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Hillman CH, & Broglio SP (2014). The persistent influence of concussive injuries on cognitive control and neuroelectric function. Journal of Athletic Training, 49(1), 24–35. doi: 10.4085/1062-6050-49.1.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Eichele T, Nordby H, Hugdahl K, & Calhoun VD (2008). Joint independent component analysis for simultaneous EEG-fMRI: Principle and simulation. International Journal of Psychophysiology, 67(3), 212–221. doi: 10.1016/j.ijpsycho.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, & van Zijl P (2007). Human White Matter Atlas. American Journal of Psychiatry, 164(7), 1005–1005. doi: 10.1176/ajp.2007.164.7.1005 [DOI] [PubMed] [Google Scholar]
- Naunheim RS, Treaster M, English J, Casner T, & Chabot R (2010). Use of brain electrical activity to quantify traumatic brain injury in the emergency department. Brain Injury, 24(11), 1324–1329. doi: 10.3109/02699052.2010.506862 [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective and Behavioral Neuroscience, 12(2), 241–268. doi: 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H, Whelan R, & Reilly RB (2010). FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. Journal of neuroscience methods, 192(1), 152–62. doi: 10.1016/j.jneumeth.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Hovda D. a, Schrader LM, & Vespa PM (2005). Routine and quantitative EEG in mild traumatic brain injury. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 116(9), 2001–25. doi: 10.1016/j.clinph.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. doi: 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Prichep LS, Ghosh Dastidar S, Jacquin A, Koppes W, Miller J, Radman T, et al. (2014). Classification algorithms for the identification of structural injury in TBI using brain electrical activity. Computers in Biology and Medicine, 53, 125–133. doi: 10.1016/j.compbiomed.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Quinn DK, Mayer AR, Master CL, & Fann JR (2018). Prolonged postconcussive symptoms. American Journal of Psychiatry, 175(2), 103–111. doi: 10.1176/appi.ajp.2017.17020235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RM, Mathias JL, & Rose SE (2016). Relationship Between Diffusion Tensor Imaging (DTI) Findings and Cognition Following Pediatric TBI: A Meta-Analytic Review. Developmental Neuropsychology, 41(3), 176–200. doi: 10.1080/87565641.2016.1186167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohling ML, Larrabee GJ, Millis SR, Rohling ML, Larrabee GJ, & Millis SR (2012). The “Miserable Minority” Following Mild Traumatic Brain Injury : Who Are They and do Meta-Analyses Hide Them ?, 4046. doi: 10.1080/13854046.2011.647085 [DOI] [PubMed] [Google Scholar]
- Rose SC, Fischer AN, & Heyer GL (2015). How long is too long? the lack of consensus regarding the post-concussion syndrome diagnosis. Brain Injury, 29(7–8), 798–803. doi: 10.3109/02699052.2015.1004756 [DOI] [PubMed] [Google Scholar]
- Ruff R (2005). Two Decades of Advances in Understanding of Mild Traumatic Brain Injury, 20(1), 5–18. [DOI] [PubMed] [Google Scholar]
- Ryman SG, Cavanagh JF, Wertz CJ, Shaff NA, Dodd AB, Stevens B, et al. (2018). Impaired Midline Theta Power and Connectivity During Proactive Cognitive Control in Schizophrenia. Biological psychiatry, 84(9), 675–683. doi: 10.1016/j.biopsych.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander AM, & Struchen M. a. (2011). Interpersonal relationships and traumatic brain injury. The Journal of head trauma rehabilitation, 26(1), 1–3. doi: 10.1097/HTR.0b013e3182068588 [DOI] [PubMed] [Google Scholar]
- Serino A, Ciaramelli E, Di Santantonio A, Malagù S, Servadei F, & Làdavas E (2006). Central executive system impairment in traumatic brain injury. Brain injury : [BI], 20(1), 23–32. doi: 10.1080/02699050500309627 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter H. a, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews. Neuroscience, 12(3), 154–67. doi: 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, & Engel AK (2012). Spectral fingerprints of large-scale neuronal interactions. Nature reviews. Neuroscience, 13(2), 121–34. doi: 10.1038/nrn3137 [DOI] [PubMed] [Google Scholar]
- Singh A, Pirio Richardson S, Narayanan N, & Cavanagh JF (2018). Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia, 117, 113–122. doi: 10.1016/J.NEUROPSYCHOLOGIA.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, & Allen JJB (2019). Theta-Band Functional Connectivity and Single-Trial Cognitive Control in Sports-Related Concussion : Demonstration of Proof-of- Concept for a Potential Biomarker of Concussion, 1–10. doi: 10.1017/S135561771800108X [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 Suppl 1, S208–19. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Souza AD, Mollayeva S, Pacheco N, & Javed F (2019). Measuring Change Over Time : A Systematic Review of Evaluative Measures of Cognitive Functioning in Traumatic Brain Injury, 10(May). doi: 10.3389/fneur.2019.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Coffman BA, Jung RE, Bustillo JR, Aine CJ, & Calhoun VD (2013). Using joint ICA to link function and structure using MEG and DTI in schizophrenia. NeuroImage, 83, 418–430. doi: 10.1016/j.neuroimage.2013.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SB, Kim N, Branch CA, Kahn ME, Kim M, Lipton RB, et al. (2016). Bidirectional changes in anisotropy are associated with outcomes in mild traumatic brain injury. American Journal of Neuroradiology, 37(11), 1983–1991. doi: 10.3174/ajnr.A4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Adali T, Pearlson G, Yang H, Sponheim SR, White T, & Calhoun Vince D., V. D. (2010). A CCA+ICA based model for multi-task brain imaging data fusion and its application to schizophrenia. NeuroImage, 51(1), 123–134. doi: 10.1016/j.neuroimage.2010.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Gerson I, & Geisler FH (1989). EEG discriminant analyses of mild head trauma. Electroencephalography and Clinical Neurophysiology, 73(2), 94–106. doi: 10.1016/0013-4694(89)90188-0 [DOI] [PubMed] [Google Scholar]
- van der Naalt J, Timmerman ME, de Koning ME, van der Horn HJ, Scheenen ME, Jacobs B, et al. (2017). Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. The Lancet Neurology, 16(7), 532–540. doi: 10.1016/S1474-4422(17)30117-5 [DOI] [PubMed] [Google Scholar]
- Wallace EJ, Mathias JL, & Ward L (2018). The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: A meta-analysis. Neuroscience and Biobehavioral Reviews, 92(March), 93–103. doi: 10.1016/j.neubiorev.2018.05.023 [DOI] [PubMed] [Google Scholar]
- Weinberg MGH (2000). Electrophysiological indices of persistent post-concussion symptoms. Brain Injury, 14(9), 815–832. doi: 10.1080/026990500421921 [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Harkrider AW, & King KA (2014). The effects of visual distracter complexity on auditory evoked p3b in contact sports athletes. Developmental Neuropsychology, 39(2), 113–130. doi: 10.1080/87565641.2013.870177 [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, et al. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology, 22(5), 555–568. doi: 10.1016/j.acn.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wu R, Wang S, Qi H, Qian Y, & Wang S (2018). Behavioral and neurophysiological abnormalities during cued continuous performance tasks in patients with mild traumatic brain injury, (November 2017), 1–11. doi: 10.1002/brb3.966 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.