Abstract
Background:
Early detection of ovarian cancer could significantly improve patient outcomes. CA125 is elevated in sera from approximately 60% of patients with early stage I-II disease. Sensitivity might be improved by combining CA125 with other biomarkers. Among potential biomarkers, antigen-autoantibody complexes have received relatively little attention.
Methods:
Luminex-based immunoassays were used to measure HE4, anti-HE4 autoantibody (AAb) and HE4 antigen-autoantibody (Ag-AAb) complexes in sera from patients with early stage (n=73) and late stage (n=49) ovarian cancers at the time of diagnosis and from asymptomatic women with (n=15) or without (n=212) ovarian cancer enrolled in the Normal Risk Ovarian Screening Study (NROSS).
Results:
At 98% specificity for healthy asymptomatic women, 7% of patients with early stage (I-II) ovarian cancer and 4% of patients with late stage (III-IV) disease had elevated levels of HE4 AAb, whereas elevated levels of HE4 Ag-AAb complexes were detected in sera from 38% of early stage and 31% of late stage cases. Complementarity was observed in ROC curves between HE4 Ag-AAb complexes and CA125 levels in early stage ovarian cancer (P<0.001). CA125 detected 63% of cases and a combination of CA125 and HE4 Ag-AAb complexes detected 81%. Complementarity was also observed in ROC curves for an independent validation set with 69 early stage patients (P=0.039). HE4 Ag-AAb complexes were detected in serial preclinical serum samples from women destined to develop ovarian cancer, correlating with CA125, but not providing lead time.
Conclusions:
HE4 Ag-AAb complexes could complement CA125 in detecting a higher fraction of early stage ovarian cancers.
Keywords: ovarian cancer, biomarkers, CA125, HE4, early detection, antigen-autoantibody complexes
Precis:
Early detection of ovarian cancer remains an important unmet medical need. Multiple biomarkers are likely to increase sensitivity, provided that specificity can be maintained. HE4-Ag-AAb complexes compliment CA125 and enhance sensitivity at high specificity, detecting early stage cases missed by CA125. Consequently, HE4-Ag-AAb might be included in the first stage of a two-stage screening strategy.
INTRODUCTION
Over the last three decades, the 5-year survival rate has improved for patients with ovarian cancer, but the cure rate has not. Poor outcomes relate, at least in part, to late diagnosis. More than 75% of patients with ovarian cancer are diagnosed in advanced stage (III-IV) where the overall cure rate is less than 30%1. Less than 25% of ovarian cancers are detected in early stage (I-II) where 70–90% of patients can be cured with conventional surgery and chemotherapy. At present, there is no established method for early detection of ovarian cancer2. Development of improved screening strategies for the detection of early-stage ovarian cancer is a critical unmet need. Results generated from computer simulations predict that improvements in early detection could reduce the mortality of ovarian cancer by as much as 10%−30%3.
To develop a successful screening strategy, blood biomarkers must achieve greater sensitivity and lead time, while maintaining a high specificity. Use of CA125 alone is limited by the fact that only 80% of ovarian cancers express adequate levels of CA125 and elevated levels of CA125 are found in the blood of approximately 60% of patients with early stage (I-II) disease4. The addition of a panel of blood biomarkers to the CA125 assay could enhance sensitivity for detection of early stage ovarian cancer. Despite identification of numerous potential biomarkers for monitoring ovarian cancer5, 6, only CA125 and human epididymis protein 4 (HE4) serum antigens have been approved for clinical use7–9. HE4 is a member of the whey acidic four-disulfide core (WFDC) protein family secreted by epithelial cells10. Compared to its expression in normal tissues including ovary, the WFDC2 (HE4) gene is overexpressed in the majority of ovarian cancers11, 12. While CA125 has slightly greater sensitivity than HE4 for distinguishing women with ovarian cancer from healthy individuals, HE4 has higher sensitivity for distinguishing ovarian cancer from benign pelvic masses13. Neither CA125 nor HE4 is optimally sensitive for detecting early stage disease14, 15. There remains an important unmet clinical need to identify novel biomarkers that would detect patients with early stage disease missed by CA125 and HE4 and detect disease before elevation of these conventional biomarkers.
A major challenge for protein biomarkers is a requirement for low volume, early stage cancer to secrete sufficient amounts of protein to elevate levels in peripheral blood. In many cancers, the aberrant expression of proteins or alterations in their function, structure, or localization can provoke an autoimmune response16. In such cases, small volumes of early stage disease could induce the production of autoantibodies to tumor associated antigens long before the cancer grew large enough to shed enough protein antigen to be detected.
Accumulating evidence suggests that autoantibodies against tumor-associated antigens may be useful diagnostic biomarkers for detecting ovarian cancer at an early stage2, 5, 17. Over the past decades, autoantibodies against numerous cancer antigens have been discovered in several types of cancer, and some autoantibodies exhibit potential to serve as biomarkers for diagnosis and/or early detection of ovarian cancer2. We previously have reported that elevated levels of anti-TP53 autoantibodies can be found in sera from 20% of patients with ovarian cancer and in 16% of patients who had normal levels of CA125. In addition, elevated titers of anti-TP53 autoantibody appeared 8 months before elevation of CA125 and 22 months before diagnosis in patients not identified by CA125 testing, suggesting that anti-TP53 autoantibody is a promising biomarker for early detection of ovarian cancer18. Since anti-TP53 autoantibody detected only 20% of patients with ovarian cancer, additional autoantibodies will be required to provide a panel that will provide earlier detection in >90% of ovarian cancers.
In this study, we have demonstrated that autoantibody against HE4 could be detected in 5 of 73 cases (7%) with early stage ovarian cancer and 2 of 49 cases (4%) with late stage cancer. When, however, sera were treated transiently with glycine buffer at pH 3.0 to dissociate HE4 antigen-autoantibody (Ag-AAb) complexes, free autoantibody could be detected in 28 of 73 cases (38%) with early stage cancer and 15 of 49 cases (31%) with late stage cancer. We have asked whether HE4 Ag-AAb complexes might complement CA125 to detect a larger fraction of early stage ovarian cancers and whether HE4 Ag-AAb complexes might be detected before the rise of CA125.
MATERIALS AND METHODS
Reagents
Recombinant human HE4 protein with a His-tag was purchased from ACRO Biosystems (Newark, DE). MagPlex microspheres, xMAP antibody coupling kits and reagents for the immunoassay were obtained from Luminex Corp. (Austin, TX). Mouse anti-HE4 monoclonal IgG antibody was purchased from OriGene Technologies (Rockville, MD). Biotin-conjugated mouse anti-His-tag antibody was purchased from R&D Systems (Minneapolis, MN). Biotin-conjugated goat anti-human IgG antibody and anti-mouse IgG antibody were obtained from Jackson ImmunoResearch (West Grove, PA). Streptavidin-R-phycoerythrin (SAPE) was purchased from Life Technologies (Carlsbad, CA). Zeba Spin Desalting columns, 0.5 mL were obtained from Thermo Fisher Scientific (Waltham, MA). The enzyme-linked immunosorbent assays (ELISA) for HE4 antigen by MILLIPLEX MAP Human Circulating Cancer Biomarker Magnetic Bead Panel Kit were purchased from MilliporeSigma (Burlington, MA). Automated immunoassay kits for determining concentration of CA125 antigen were purchased from Roche Diagnostics USA (Indianapolis, IN).
Patient Serum Samples
Sera were drawn from the MD Anderson Gynecologic Cancer Bank and from the Normal Risk Ovarian Cancer Screening Study (NROSS). Ethical approval was obtained for these studies from the appropriate IRB/ethical committees at MD Anderson and collaborating institutions. All participants had provided consent for use of samples in ethically approved secondary studies. The MD Anderson Gynecologic Cancer Bank provided two sets of clinical serum samples. The first sample set (training set) consisted of 73 preoperative sera from patients with stage I-II invasive epithelial ovarian/tubal/peritoneal cancer and 49 preoperative sera from patients with stage III-IV invasive epithelial ovarian/tubal/peritoneal cancer. Sera were obtained from 212 healthy controls from the NROSS study who did not develop ovarian cancer19. The NROSS trial has involved 6,379 postmenopausal women at average risk for developing ovarian cancer who have been followed with annual CA125 measurements and who have been referred for transvaginal ultrasound and gynecologic evaluation if CA125 values increase from each individual’s baseline judged by the Risk of Ovarian Cancer Algorithm (ROCA)20. All healthy controls were followed for at least 7 years to be certain that they did not develop cancer21. This sample set was used to characterize the newly developed immunoassay for detecting and quantitating HE4 autoantibody. The second sample set (validation set) consisted of 69 preoperative sera from patients with stage I-II invasive epithelial ovarian/tubal/peritoneal cancer and from 200 healthy controls who did not develop ovarian cancer from the NROSS study. This sample set was used to verify complementarity between CA125 and HE4 antigen autoantibody complexes.
Within the NROSS trial, 15 ROCA-indicated operations were performed to identify 12 cases of ovarian cancer. Ten of the 12 cancers were invasive and 7 of the 10 invasive cancers were in stage I-II. In the NROSS trial, 3 patients presented with ovarian cancer without an elevation of CA125. One was in stage I and 2 high grade invasive cancers were diagnosed in stage III. From these 15 cases, we analyzed 145 preclinical serial serum samples that predated diagnosis of invasive epithelial ovarian/tubal/peritoneal cancer by up to 11 years. We also evaluated serum specimens from 212 healthy controls who did not develop any type of cancer during follow up on the NROSS trial. This sample set was used to evaluate whether HE4 autoantibody exhibits lead time over CA125 and the ROCA in pre-diagnostic serial patient samples.
Luminex-based HE4 Autoantibody Assays
The MagPlex/xMAP technology (Luminex Corp., Austin, TX) was used to develop an immunoassay for detecting autoantibody against human HE4 antigen. Coupling recombinant HE4 antigen to microspheres, confirmation of coupling efficiency and calibration of the assay were achieved using protocols modified from the xMAP Cookbook, 3rd version (Luminex Corp.). In brief, one million microspheres were coupled with recombinant human HE4 protein using an xMAP antibody coupling kit as specified by the manufacturer. Coupling was accomplished by a carbodiimide reaction linking the primary amino groups on HE4 protein and the carboxyl groups on the microsphere surface. The antigen conjugation procedure was performed according to the manufacturer’s instructions. Biotin-conjugated anti-His tag antibody was used to confirm coupling efficiency. A mouse anti-HE4 monoclonal antibody (0.5 μg/mL) was used to set up a standard curve for quantitating the titer of HE4 autoantibody in human serum samples. A standard operating protocol for performing the immunoassay for clinical serum sample analysis was established during assay development.
For each assay, a suspension of HE4 antigen-microspheres was prepared by diluting the coupled microsphere stocks (one million beads/mL) to a final concentration of 50 beads/μL in phosphate buffered saline (PBS), pH 7.0. Aliquots of microsphere suspension were placed in each well of a 96-well polystyrene microplate. Standard curves for quantitating HE4 autoantibody were plotted from triplicate assays of half-log dilution series of the HE4 autoantibody calibrator with concentrations ranging from 8 ng/mL to 250 ng/mL. Serum samples for assay (2 μL) were diluted with PBS buffer. Diluted serum samples were added to 96-well polystyrene microplates and incubated with HE4-coupled microspheres for 1 hour at room temperature with gentle shaking. To wash the beads, each plate was clipped onto a magnetic plate separator for 1 min and the liquid was discarded by inverting the plate. Following washing with PBS with 0.1% Tween-20 (PBST) buffer, microspheres were incubated with detection antibody: biotinylated goat anti-human IgG for serum samples and biotinylated goat anti-mouse IgG for serial diluted samples of HE4 autoantibody calibrator. The plates were covered to protect them from light for 30 minutes at room temperature with gentle shaking. Plates were washed two times with PBST buffer and microspheres were incubated with fluorescence reporter SAPE. After the final wash, beads were re-suspended in PBS buffer and fluorescence measured on the MAGPIX system (Luminex Corp., Austin, TX) with a minimum of 50 beads read per well. The data were acquired and analyzed by xPONENT software version 4.2 (Luminex Corp., Austin, TX).
Dissociation of Antigen-antibody Complexes in Serum
A procedure for dissociating antigen-antibody complexes in serum has been applied to measure the level of HE4 autoantibody associated with immune complexes in the HE4 autoantibody immunoassay. Serum samples were pretreated with 0.1 M glycine-HCl buffer, pH 3.0 for 30 minutes at room temperature with gentle shaking to allow dissociation of all antigen-bound antibodies. Autoantibodies were purified and brought back to neutral pH by centrifugation on Zeba spin desalting columns (Invitrogen) according to the manufacturer’s instructions. Pretreated samples were immediately analyzed in the anti-HE4 autoantibody immunoassay.
CA125 Antigen Immunoassays
Levels of CA125 antigen in serum samples were measured with Roche’s CA125 immunoassay kit with Cobas e411 automated analyzer (Roche Diagnostics USA, Indianapolis, IN) according to the manufacturer’s instructions. CA125 positivity was determined using a single cut-off value (35 U/mL).
HE4 Antigen Immunoassays
Levels of HE4 antigen in serum samples were measured with a MILLIPLEX MAP Kit – Cancer Biomarker Panel (MilliporeSigma, Burlington, MA) according to the manufacturer’s instructions. Results were measured using the Luminex MAGPIX system (Luminex Corp., Austin, TX) with a minimum of 50 beads read per well. The data were acquired and analyzed using xPONENT software version 4.2 (Luminex Corp., Austin, TX).
Statistical Analysis
Analysis was performed separately for early and late stage data sets. Summary statistics were used to characterize biomarkers for cases and controls. Pairwise Spearman’s correlation coefficients were calculated for all biomarkers. Biomarkers were dichotomized based on cut offs that achieved 98% specificity. Logistic regression models were utilized to assess the prognostic values for biomarkers and to combine biomarkers for calculating AUC values. Multivariable logistic regression models were developed to determine whether adding HE4 AAb or HE4 Ag-AAb complexes to CA125 improved prediction. AUC, sensitivity at FPR of 0.02, and partial AUC (pAUC) were calculated for each model. Bootstrapping with 1000 bootstrap samples was used to compute 95% confidence intervals for the AUC and pAUC, as well as P values for testing the differences in AUC and pAUC values. One-sided test was conducted when comparing AUC, sensitivity, and pAUC values. In order to assess trends in biomarker levels over time, we developed linear mixed effects models (LMM) with each biomarker by using longitudinal measurements before the diagnosis. A random intercept and a random slope of time (months pre-diagnosis) were included in the LMM. All models were adjusted for stage.
Lead time was defined as the difference between the first time an individual’s marker crossed the threshold (Supporting Table S4) and their diagnosis time. Means, standard deviations and ranges were calculated for lead times. All statistical analyses were performed using Stata/MP v15.0 (College Station, TX).
RESULTS
Elevated HE4 autoantibody, antigen-autoantibody complexes and antigen levels are found in sera from early stage (I/II) ovarian cancer patients.
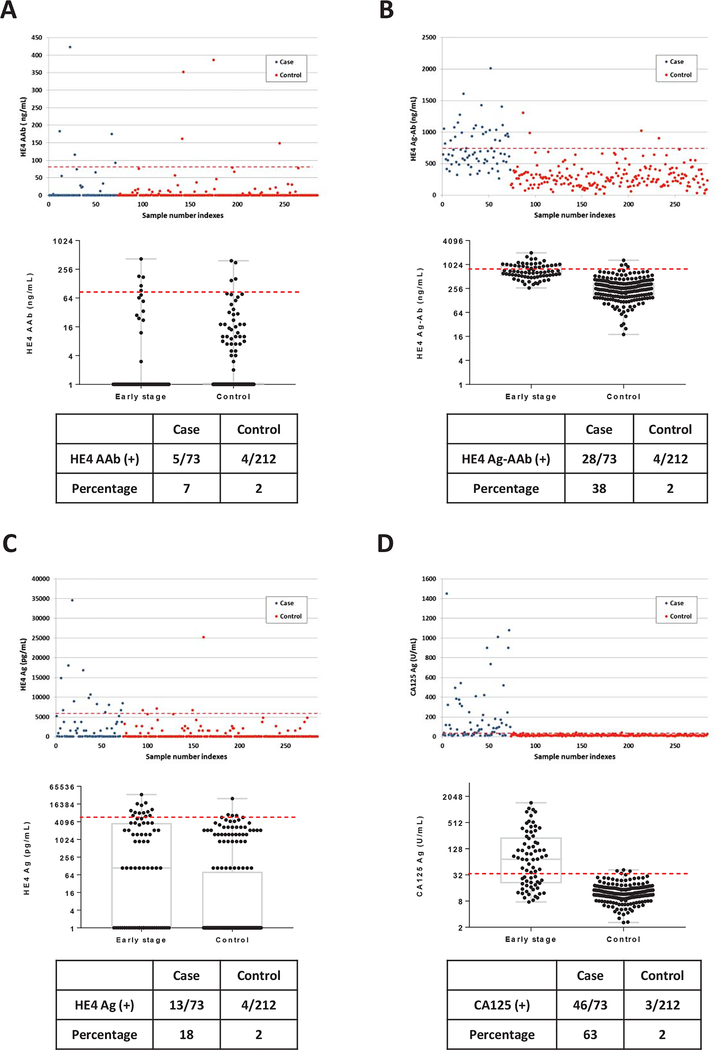
A MagPlex/xMAP-based immunoassay was developed to measure specific autoantibodies against the HE4 antigen in small volumes (2 μL) of human serum. The procedure for immunoassay development, validation and assessment of performance has been outlined in Supporting Fig. S1 and detailed above. We first screened preoperative sera from 73 patients with early stage (I/II) ovarian cancer and 212 healthy women from the NROSS trial (training set). Patient and tumor characteristics are described in Supporting Table S1. At 98% specificity in healthy controls, 5 of 73 sera (7%) from early stage ovarian cancer cases had elevated levels of HE4 autoantibody (Fig. 1A).
Figure 1. Levels of HE4 autoantibody, HE4 antigen-autoantibody complexes, HE4 antigen and CA125 antigen in sera from patients with early stage (I/II) ovarian cancer and from healthy controls.
(A) HE4 autoantibody; (B) HE4 antigen-autoantibody complexes; (C) HE4 antigen and (D) CA125 antigen. In the upper panels, each dot represents the average of duplicate serum samples from a single case (red) or control (blue). The middle panels contain a box plot where each box represents the maximum, upper quartile, median, lower quartile and minimum values in case and control groups, respectively. The red dashed lines in each plot represent the cut-off value (at 98% specificity) for each biomarker. The lower panel contains a table displaying the sensitivity for cases and controls near 98% specificity.
Since soluble HE4 protein is secreted from cancer cells and levels are elevated in blood from a majority of ovarian cancer patients10, anti-HE4 autoantibodies may form circulating immune complexes with secreted HE4 antigen. The anti-HE4 autoantibody assay can only detect free HE4 autoantibody and not antigen-autoantibody complexes. To measure autoantibody in HE4 antigen-autoantibody complexes, serum samples were pre-treated with acid glycine buffer (pH 3.0) to dissociate all complexes, before measuring free anti-HE4 autoantibodies. Remarkably, at 98% specificity for healthy individuals, 28 of 73 sera (38%) from early stage ovarian cancer cases had elevated levels of HE4 antigen-autoantibody (Ag-AAb) complexes (Fig. 1B). Thus, most of the anti-HE4 autoantibody in sera from early stage cancer patients was complexed with shed HE4 antigen.
HE4 antigen is a well-known biomarker for monitoring ovarian cancer and for distinguishing malignant from benign pelvic masses. At 98% specificity in sera from healthy women, HE4 antigen levels were elevated in 13 of 73 patients (18%) with early stage ovarian cancer (Fig. 1C). By contrast, using either a CA125 clinical cut-off value of 35 U/mL or a cut-off that included 98% of healthy controls in the present study, CA125 levels were elevated in 46 of 73 patients (63%) with early stage cancer (Fig. 1D). These results suggest that HE4 Ag-AAb complexes had greater sensitivity than HE4 autoantibody or HE4 antigen and were second only to CA125 for detecting early stage disease.
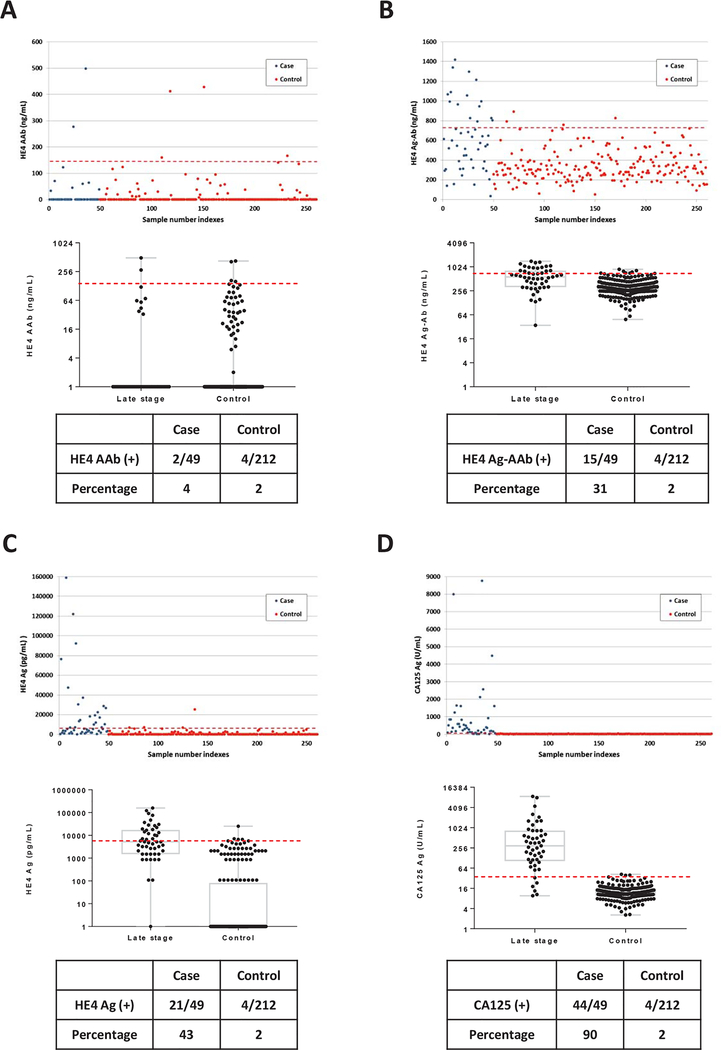
Elevated HE4 autoantibody, antigen-autoantibody complexes and antigen levels are found in sera from late stage (III/IV) ovarian cancer patients.
We next assayed preoperative sera from 49 patients with late stage (III/IV) ovarian cancer. Patient characteristics are described in Supporting Table S1. At 98% specificity for healthy women, 2 of 49 sera (4%) from late stage cancer cases had elevated levels of anti-HE4 autoantibody (Fig. 2A), similar to the 7% observed in early stage ovarian cancer patients. After acid buffer pretreatment, 15 of 49 sera (31%) from cancer cases were detected as HE4 Ag-AAb complexes at 98% specificity (Fig. 2B). Thus HE4 Ag-AAb complexes were found inn 38% of early stage ovarian cancer and 31% of late stage disease. HE4 antigen levels were, however, elevated in 21 of 49 patients with late stage cancer (43%) at 98% specificity (Fig. 2C). When compared to sera from early stage cancer cases (Fig. 1C), serum levels of HE4 antigen were increased in late stage cancer cases. CA125 antigen levels were elevated in 44 of 49 patients (90%) with late stage disease (Fig. 2D). These results suggest that HE4 Ag-AAb complexes levels may decline as cancers grow in volume and HE4 antigen levels rise.
Figure 2. Levels of HE4 autoantibody, HE4 antigen autoantibody complexes, HE4 antigen and CA125 antigen in sera from patients with late stage (III/IV) ovarian cancer and from healthy controls.
(A) HE4 autoantibody; (B) HE4 antigen-autoantibody complexes; (C) HE4 antigen and (D) CA125 antigen. In the upper panels, each dot represents the average of duplicate serum samples from a single case (red) or control (blue). The middle panels contain a box plot where each box represents the maximum, upper quartile, median, lower quartile and minimum values in case and control groups, respectively. The red dashed lines in each plot represent the cut-off value (at 98% specificity) for each biomarker. The lower panel contains a table displaying the sensitivity for cases and controls near 98% specificity.
HE4 antigen-autoantibody complexes complement CA125 for detecting ovarian cancer.
While complementarity has been observed between serum HE4 and CA125 antigen levels in previous studies8, 9, 15, 22, 23, the addition of HE4 to CA125 did not detect additional ovarian cancer patients either in early or late stage disease (Table 1). Complementarity was observed between HE4 antigen and HE4 Ag-AAb complexes. In early stage disease, HE4 antigen detected 18%, HE4 Ag-AAb complexes detected 38% and the combination detected 51% of cases. Similarly, in late stage disease, HE4 antigen detected 43%, HE4 Ag-AAb complexes detected 31% and the combination detected 67% of cases. The most impressive impact of HE4 Ag-AAb complexes was seen in improving detection by CA125 in early stage (I-II) disease, where CA125 identified 63%, HE4 Ag-AAb complexes identified 38%, CA125 63% and the combination identified 81% of cases. Complementarity was observed when only stage I cases were considered where CA125 detected 52% and the combination 73%. Thus HE4 Ag-AAB complexes enhanced detection of ovarian cancer by 18–21% in both groups.
Table 1.
Complementarity of serum CA125, HE4 antigen and HE4 antigen-autoantibody complexes in early and late stage ovarian cancer
| BIOMARKERS | ||||
|---|---|---|---|---|
| STAGE | CA125 | CA125+HE4 Ag | ||
| Positive | % | Positive | % | |
| Early | 46/73 | 63 | 46/73 | 63 |
| Late | 43/49 | 88 | 43/49 | 88 |
| CA125 | CA125+HE4 Ag-AAb | |||
| Positive | % | Positive | % | |
| Early | 46/73 | 63 | 59/73 | 81 |
| Late | 44/49 | 90 | 46/49 | 94 |
| HE4 | HE4 Ag+HE4 Ag-AAb | |||
| Positive | % | Positive | % | |
| Early | 13/73 | 18 | 37/73 | 51 |
| Late | 21/49 | 43 | 33/49 | 67 |
| HE4 | HE4 Ag-AAb+CA125 | |||
| Positive | % | Positive | % | |
| Early | 28/73 | 38 | 59/73 | 81 |
| Late | 15/49 | 31 | 46/49 | 94 |
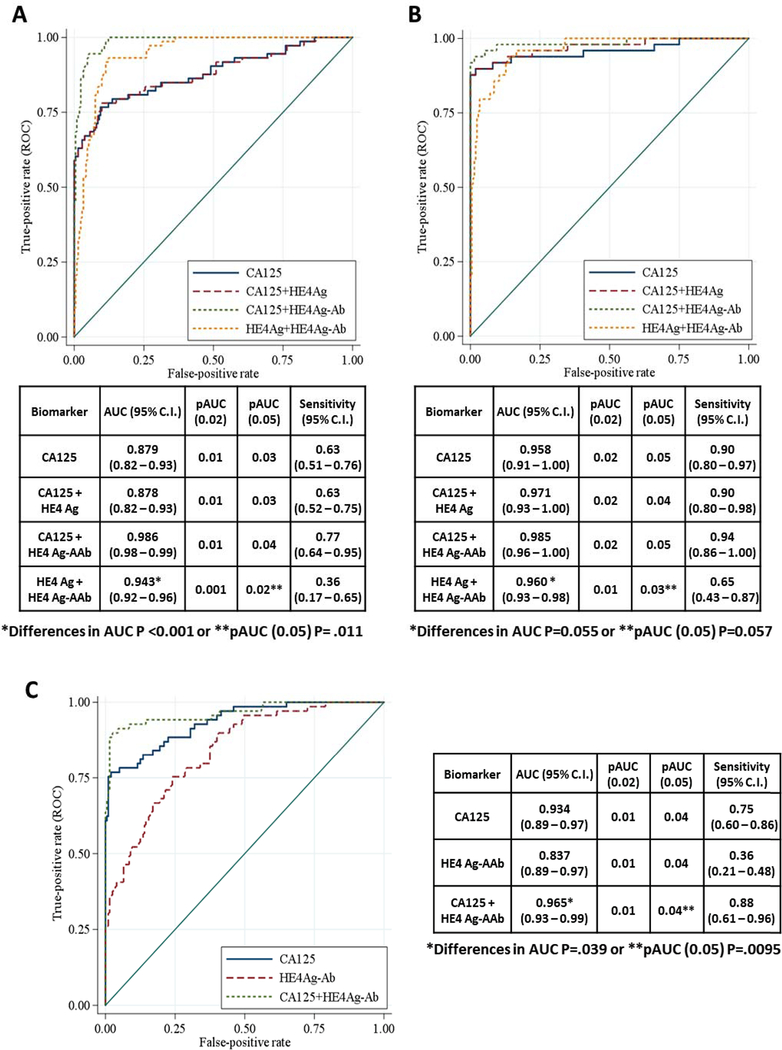
The AUC for the ROC curve for CA125 plus HE4 Ag-AAb complexes in stage I-II disease was significantly greater than either individual component (Fig. 4, P<0.001). The partial AUC for the ROC curve at 95% specificity was also significantly improved over CA125 alone (Fig. 4, P=0.011). In late stage disease, where CA125 was elevated in most cases, the addition of HE4 Ag-AAb complexes to CA125 increased detection from 90% to 94%. The AUCs for the ROC curves for CA125 plus HE4 Ag-AAb complexes and CA125 alone neared statistical significance (Fig. 4, P=0.055) as did the differences in partial AUC at 95% specificity (P=0.057).
Figure 4. ROC curves for CA125, HE4 antigen, HE4 antigen-autoantibody complexes and a combination of the three biomarkers.
(A) Early stage ovarian cancer and (B) Late stage ovarian cancer. A combination of HE4 antigen-autoantibody complexes and CA125 had a significantly greater AUC (0.986) than did CA125 alone (0.879) in early stage disease (P<0.001) and slightly greater AUC (0.985) than did CA125 alone (0.958) in late stage disease (P=0.055). (C) Early stage ovarian cancer from validation sample set. A combination of HE4 antigen-autoantibody complexes and CA125 had a greater AUC (0.965) than did CA125 alone (0.934) in late stage disease P =0.039).
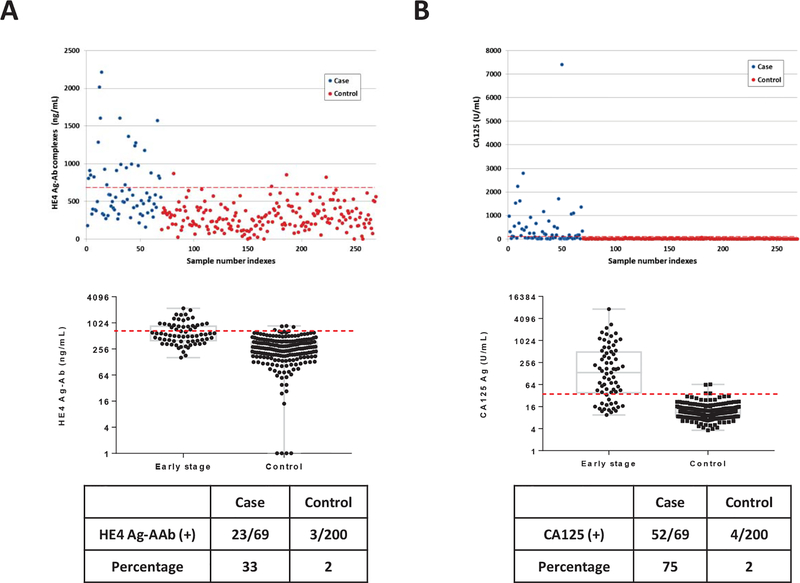
To validate our previous findings, we measured HE4 Ag-AAb complexes and CA125 in a new sample set that included sera from 69 patients with early stage (I/II) ovarian cancer and 200 healthy women from the NROSS trial (validation set). Patient characteristics are described in Supporting Table S2. At 98% specificity for healthy women, 23 of 69 sera from cancer cases (33%) had elevated levels of HE4 Ag-AAb complexes (Fig. 3A). CA125 antigen levels were elevated in 52 of 69 patients (75%) with early stage cancer (Fig. 3B). The combination detected 61 of 69 patients (88%) and improved the ROC curve (Fig. 4C, P=0.039). The partial AUC at 95% specificity was also enhanced significantly by the combination (P=0.0095). Thus, complementarity was observed between HE4 Ag-AAb complexes and CA125 in this validation set. These data suggest that HE4 Ag-AAb complexes might serve as part of a panel to improve detection of early stage ovarian cancer.
Figure 3. Levels of HE4 antigen autoantibody complexes and CA125 antigen in sera from patients with early stage (I/II) ovarian cancer and from healthy controls.
(A) HE4 antigen-autoantibody complexes; and (B) CA125 antigen. In the upper panels, each dot represents the average of duplicate serum samples from a single case (red) or control (blue). The middle panels contain a box plot where each box represents the maximum, upper quartile, median, lower quartile and minimum values in case and control groups, respectively. The red dashed lines in each plot represent the cut-off value (at 98% specificity) for each biomarker. The lower panel contains a table displaying the sensitivity for cases and controls near 98% specificity.
Both HE4 autoantibody and HE4 antigen-autoantibody complex can be elevated with CA125 in preclinical samples of ovarian cancer patients.
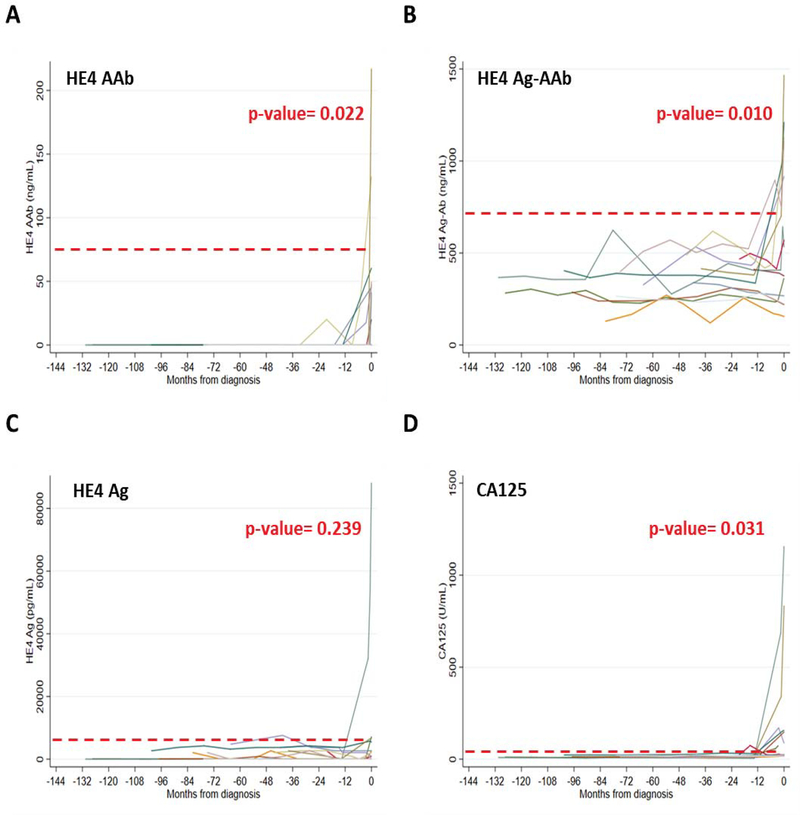
Availability of sera from the MDACC-NROSS trial permitted detection of HE4 autoantibody and HE4 Ag-AAb complexes in preclinical sera from 15 women who subsequently developed invasive epithelial ovarian cancer. Twelve of the 15 had been detected by rising CA125 analyzed by the ROCA algorithm and 3 had not been detected with CA125 and were diagnosed by conventional methods. Patient characteristics are described in Supporting Table S3. HE4 AAb, HE4 Ag-AAb and CA125 all showed increasing marker levels pre diagnosis of ovarian cancer (Fig. 5, A and B). This provides evidence with an independent data set that increasing trends over time of HE4 Ag-AAb complexes can be associated with early stage disease. Elevation of HE4 Ag-AAb complexes levels did not, however, precede elevation of CA125.
Figure 5. Longitudinal analysis of HE4 autoantibody, antigen-autoantibody complexes, HE4 antigen and CA125 antigen values in pre-diagnostic serial serum samples from women destine to develop ovarian cancer on the NROSS trial.
Fifteen individuals screened on the NROSS trial developed ovarian cancer. Twelve were detected by rising CA125 and the ROCA, whereas three were not. Multiple pre-clinical serum samples from each participant were analyzed for: (A) HE4 autoantibody; (B) HE4 antigen-autoantibody complexes; (C) HE4 antigen; and (D) CA125 antigen. Red dash line represents 98% specificity for each biomarker. Significant increases prior to diagnosis were observed with HE4 autoantibody (P=0.022), HE4 antigen-autoantibody complexes (P=0.010) and CA125 antigen (P=0.031), but not with HE4 antigen (P=0.239).
DISCUSSION
Having developed a novel Luminex-based immunoassay for HE4 autoantibodies and HE4 Ag-AAb complexes, we have found that HE4 Ag-AAb complexes provide a promising biomarker for early detection of ovarian cancer. HE4 Ag-AAb complexes enhanced the ability of CA125 to detect early stage (I-II) ovarian cancer in up to 81–88% of patients (Table1). ROC analysis also indicated that a combination of HE4 Ag-AAb complexes and CA125 significantly improved the AUC when compared with CA125 alone (Fig. 4). While other investigators have detected HE4 autoantibodies in infertile women and in women with ovarian cancer24, the potential importance of Ag-AAb complexes in detection of ovarian cancer has not been previously appreciated.
An effective strategy for early detection of ovarian cancer remains an unmet need. Given the prevalence of ovarian cancer in the general postmenopausal population at average risk (1:2500), a screening strategy must not only be sufficiently sensitive to detect early stage or small volume disease in 75% of asymptomatic women, but also achieve a specificity of 99.6% to provide a positive predictive value of 10% (i.e., 10 operations for each case of ovarian cancer detected)25. Neither single values of blood biomarkers such as CA125 and HE4 nor imaging modalities including transvaginal sonography (TVS) have sufficient sensitivity and specificity for a cost-effective screening when used alone2. Recent studies suggest, however, that two stage strategies where rising biomarkers trigger imaging can achieve adequate specificity. In both the NROSS study conducted in the United States19 and the United Kingdom Collaborative Study of Ovarian Cancer Screening (UKCTOCS)26, rising CA125 analyzed with the risk of ovarian cancer algorithm triggered TVS in 2–3% of participants, 99.6% specificity was achieved and only 3–4 operations were required to diagnose each ovarian cancer. Moreover, the two-stage multi-modal approach in the UKCTOCS produced a 20% decrease in mortality among incident cases that developed after 7 years of screening27. While there are wide confidence limits about this estimate of reduced mortality that will narrow with time, requiring reanalysis next year, the two-stage strategy appears promising, provided that sensitivity of the biomarkers and of imaging can be improved.
CA125 has limitations as a first step in a two-stage strategy. Only 80% of ovarian cancers express CA1251. Despite evaluation of more than 110 biomarkers, only 2 protein biomarkers have been identified - HE4 and CA72–4 - that detect 16% of early stage cases missed by CA12528, 31. Consequently, the ability of HE4 Ag-AAb complexes to improve detection of early stage disease by approximately 20% is a significant advance. As cited above18, anti-TP53 autoantibodies can also detect approximately 20% of all ovarian cancers and 16% of cases missed by CA125 in the UKCTOCS study. Anti-TP53 autoantibodies were the first biomarkers to detect ovarian cancer 8 months prior to CA125. In the present study, we did not observe comparable lead time with HE4 Ag-AAb complexes. These complexes could, however, become part of a small panel of biomarkers that improve the sensitivity of CA125 alone to detect early stage ovarian cancer in a two-stage approach.
Little free anti-HE4 autoantibody was detected either in early (7%) or late stage disease (4%) (Fig. 1A and 2A). Between early and late stage disease we did observe a change in the ratio of cases with elevated HE4 Ag-AAb complexes to cases with elevated antigen. In early stage disease, 38% of cases had elevated HE4 Ag-AAb complexes and 18% elevated HE4 antigen levels, whereas in late stage disease 31% had elevated Ag-AAb complexes and 43% elevated antigen levels. As HE4 is actively secreted by ovarian cancer cells, this could reflect growth in the volume of the source of the HE4 antigen without an increase in autoantibody production. Conversely decreased antibody production could be caused by the immunosuppression induced by the progressively growing ovarian cancer. Similar observations have been made in two previous studies2, 3 with immune complexes that include MUC1 and MUC16 (CA125). Gourevitch et al. identified anti-MUC1 antibody in serum samples from patients with breast and ovarian cancers and those antibodies formed immune complexes with MUC129. They also demonstrated an inverse correlation between MUC1 immune complexes and MUC1 antigen. Cramer et al.30 found a similarly inverse correlation between CA125 immune complexes and free CA125 antigen.
In conclusion, multiple biomarkers will be required to detect cases of early stage ovarian cancer that would be missed by CA125 using the current ROCA algorithm. Previous studies have found that the HE4 and CA72.4 protein antigens and ant-TP53 autoantibodies can detect a fraction of cases with normal levels of CA125. Here we report that HE4 Ag-AAb complexes can complement CA125 in detecting early stage ovarian cancer, providing another potential member of a panel of biomarkers to be included in a new multi-marker ROCA algorithm.
Supplementary Material
FUNDING SUPPORT
This work was supported by funds from the Early Detection Research Network (5 U01 CA200462–02) and the MD Anderson Ovarian SPOREs (P50 CA83639 and P50CA217685), and the M.D. Anderson NCI Cancer Center Support Grant CA016672, National Cancer Institute, Department of Health and Human Services; the Cancer Prevention Research Institute of Texas (RP160145), Golfer’s Against Cancer, the Mossy Foundation, the Roberson Endowment, National Foundation for Cancer Research; UT MD Anderson Women’s Moon Shot; and generous donations from Stuart and Gaye Lynn Zarrow.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Bast receives royalties from Fujirebio Diagnostics Inc. for the discovery of CA125.
Disclosure of Conflict of Interest: *Royalties from Fujirubio Diagnostics, Inc. for the discovery of CA125
REFERENCES
- 1.Bast RC Jr., Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang WL, Lu Z, Bast RC, Jr. The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagn. 2017;17: 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havrilesky LJ, Sanders GD, Kulasingam S, et al. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity: implications for potential mortality reduction. Cancer. 2011;117: 545–553. [DOI] [PubMed] [Google Scholar]
- 4.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276: 27371–27375. [DOI] [PubMed] [Google Scholar]
- 5.Shi JX, Qin JJ, Ye H, Wang P, Wang KJ, Zhang JY. Tumor associated antigens or anti-TAA autoantibodies as biomarkers in the diagnosis of ovarian cancer: a systematic review with meta-analysis. Expert Rev Mol Diagn. 2015;15: 829–852. [DOI] [PubMed] [Google Scholar]
- 6.El Bairi K, Kandhro AH, Gouri A, et al. Emerging diagnostic, prognostic and therapeutic biomarkers for ovarian cancer. Cell Oncol (Dordr). 2017;40: 105–118. [DOI] [PubMed] [Google Scholar]
- 7.Wei SU, Li H, Zhang B. The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer. Biomed Rep. 2016;5: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gorp T, Cadron I, Despierre E, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamed EO, Ahmed H, Sedeek OB, Mohammed AM, Abd-Alla AA, Abdel Ghaffar HM. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol. 2013;8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63: 3695–3700. [PubMed] [Google Scholar]
- 11.Ono K, Tanaka T, Tsunoda T, et al. Identification by cDNA microarray of genes involved in ovarian carcinogenesis. Cancer Res. 2000;60: 5007–5011. [PubMed] [Google Scholar]
- 12.Welsh JB, Zarrinkar PP, Sapinoso LM, et al. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2001;98: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108: 402–408. [DOI] [PubMed] [Google Scholar]
- 14.Badgwell D, Bast RC, Jr. Early detection of ovarian cancer. Dis Markers. 2007;23: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaenker P, Gray ES, Ziman MR. Autoantibody Production in Cancer-The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun Rev. 2016;15: 477–483. [DOI] [PubMed] [Google Scholar]
- 17.Fortner RT, Damms-Machado A, Kaaks R. Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol Oncol. 2017;147: 465–480. [DOI] [PubMed] [Google Scholar]
- 18.Yang WL, Gentry-Maharaj A, Simmons A, et al. Elevation of TP53 Autoantibody Before CA125 in Preclinical Invasive Epithelial Ovarian Cancer. Clin Cancer Res. 2017;23: 5912–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu KH, Skates S, Hernandez MA, et al. A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013;119: 3454–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skates SJ. Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer. 2012;22 Suppl 1: S24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon U, Gentry-Maharaj A, Ryan A, et al. Recruitment to multicentre trials--lessons from UKCTOCS: descriptive study. BMJ. 2008;337: a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao T, Hu W. CA125 and HE4: Measurement Tools for Ovarian Cancer. Gynecol Obstet Invest. 2016;81: 430–435. [DOI] [PubMed] [Google Scholar]
- 23.Ghasemi N, Ghobadzadeh S, Zahraei M, et al. HE4 combined with CA125: favorable screening tool for ovarian cancer. Med Oncol. 2014;31: 808. [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom I, Swisher E, Hellstrom KE, Yip YY, Agnew K, Luborsky JL. Anti-HE4 antibodies in infertile women and women with ovarian cancer. Gynecol Oncol. 2013;130: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bast RC Jr., Urban N, Shridhar V, et al. Early detection of ovarian cancer: promise and reality. Cancer Treat Res. 2002;107: 61–97. [DOI] [PubMed] [Google Scholar]
- 26.Menon U, Ryan A, Kalsi J, et al. Risk Algorithm Using Serial Biomarker Measurements Doubles the Number of Screen-Detected Cancers Compared With a Single-Threshold Rule in the United Kingdom Collaborative Trial of Ovarian Cancer Screening. J Clin Oncol. 2015;33: 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terry KL, Schock H, Fortner RT, et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin Cancer Res. 2016;22: 4664–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gourevitch MM, von Mensdorff-Pouilly S, Litvinov SV, et al. Polymorphic epithelial mucin (MUC-1)-containing circulating immune complexes in carcinoma patients. Br J Cancer. 1995;72: 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer DW, O’Rourke DJ, Vitonis AF, et al. CA125 immune complexes in ovarian cancer patients with low CA125 concentrations. Clin Chem. 2010;56: 1889–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons A, Fourkala EO, Gentry-Maharaj A, Ryan A, Baggerly KA, Zheng H, Lu KH, Jacobs A, Skates SJ, Menon U*, Bast RC Jr*. Validation of longitudinal performance of a multi-marker panel for the early detection of ovarian cancer. Cancer Prevention Res, In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.