Abstract
Cancer metastasis is the leading cause of mortality in patients with solid tumors. The majority of these deaths are associated with metastatic disease that occurs after a period of clinical remission, anywhere from months to decades following removal of the primary mass. This dormancy is prominent in cancers of the breast and prostate among others, leaving the survivors uncertain about their longer-term prognosis. The most daunting aspect of this dormancy and re-emergence is that the micrometastases in particular, and even large lethal outgrowths are often show resistance to agents to which they have not been exposed. This suggests that in addition to specific mutations that target single agents, there also exist adaptive mechanisms that provide this pan-resistance. Potential molecular underpinnings of which are the topic of this review.
1. Introduction
Metastatic cancers remain largely incurable with most therapies being temporizing and/or palliative. The therapeutic treatment used are by in large the same as those for primary tumor. Even in the face of objective response, and when the primary tumor does shrink in response to therapies, the overwhelming majority recur and show broader resistance against therapies [1–3]. With respect to chemotherapy, metastatic lesions are frequently resistant [4–6] and more troublingly is that this treatment can facilitate metastatic tumor growth and drug resistance. Chemotherapy elicits the secretion of various paracrine factors from the stromal cells in the surrounding microenvironment which promote resistance [7–10]. For targeted therapy, a few patients achieve a complete response and others a partial response or stable disease, but the majority continue to progress [11, 12]. Holding some promise, immunotherapies have come to the forefront due to unprecedented durable response rates for select carcinoma types [13–15]. However, the majority of patients do not benefit and of those that do respond (being only one quarter or fewer of treated patients), a substantial portion relapse after a period months to years [16, 17].
Mechanisms underlying resistance to chemotherapies, targeted therapies and immunotherapies in metastatic tumors are often assumed to occur via Darwinian selection of mutants. However, genomic studies have revealed that primary tumors and metastases are closely related from a genetic standpoint [18] and failed to identify unique mutations associated with metastatic propensity [19–21]. This would suggest that disseminated tumor cells (DTCs) do not necessarily acquire unique cell-intrinsic effector functions but instead draw from other resources preexisting in normal cells for pathophysiological function at various stages of development. That is to say, all the cell-intrinsic resources required for dissemination are available in the physiological setting, and tumor cells likely only adjust their expression level and combination to hijack them to their advantage.
Within this review, we will discuss the concept of pan-resistance of DTCs, micrometastases and macrometastases. We discuss that the pan-resistance described above is not merely a unicellular resistance mechanism brought about by clonal expansion of mutant cells and focus on how therapeutic resistance is significantly influenced by the powerful, bidirectional relationship that exists between DTCs and the metastatic microenvironment (MME) [22]. We also propose that pan-resistance pertains to multiple aspects; not merely resistance to multiple drugs but to multiple therapeutic types too.
2. Metastasis associated cancer cell plasticity
2.1. Metastatic cascade
Metastasis is the result of DTCs that initiate new lesions in distant organ sites. Briefly, the cascade involves escape from the primary tumor, intravasation into the circulation, systemic dissemination followed by extravasation and colonization of a distant organ. DTCs may then outgrow immediately into an overt metastasis or establish as a dormant, non-proliferating cell or micrometastatic nodule [23, 24] (Fig 1).
Fig.1.
Metastatic cascade post-extravastion. After extravasation, the MME modulates tumor cell plasticity, dormancy and emergence. Successful colonization by DTCs and entrance into a dormant state involves a cancer-associated mesenchymal to epithelial reverting transition (cMErT) and establishing heterotypic E-cadherin connections with the resident cells of the metastatic organ. After a period of time, dormant cells may be stimulated by inflammatory signals to outgrow and undergo a second cancer-associated epithelial to mesenchymal transition (cEMT). The inflammatory signals are produced following homeostatic disruption (either systemically or locally). They may act to stimulate outgrowth of dormant cells directly or indirectly by activating innate immune or stromal cells in the MME which then produce mitogenic signals. Made with Affinity Designer 1.7.0.
Metastasis can be an early event such that even small tumors < 5mm can establish metastasis long before they become detectable at the primary site [25, 26]. This appears to be the case for some types of solid cancers (e.g. breast, renal cell, lung) [27–29] whereas evidence for others indicates a late escape (e.g. prostate, colorectal and pancreatic) [30–32]. Regardless, it is clear that undetected DTCs are the cause of latent metastatic disease. Throughout the course of the metastatic cascade, the microenvironment around the tumor cells is continually changing and evolving. Subsequently, it is exceedingly important to take into account the dynamic influence it has on protecting metastatic cells from the immune system and therapies whether that be chemo-, target and immunotherapy. The non-genetic factors as well as the stochastic phenotypic switching in DTCs post-extravasation, which results from this relationship, will be a focus of this review.
2.2. Epithelial/mesenchymal cell plasticity
Successful metastatic seeding of distant organs depends on the capability of cancer cells escaping from the primary tumor, surviving during migration and colonization in the ectopic sites. Cell epithelial/mesenchymal plasticity, particularly in carcinomas, confers the ability for cells to change behaviors in terms of movement through tissues and avoidance of contact inhibition of proliferation. The concept of epithelial-mesenchymal transition (EMT) evolved from initial observations that embryonic and adult epithelial cells converted to migratory and invasive fibroblast-like cells when embedded in 3D collagen gels [33]. Since then, EMT has been well studied, given way from “transformation” to “transition”, and “plasticity” more recently, in development, wound healing, fibrosis, and cancer metastasis [34]. Different from EMT in embryogenesis and organ development, which is subtle and controlled and reflects a defined set of transcriptomic and proteomic dichotomies, cancer-associated EMT (cEMT) is aggressive and uncontrolled [35]. Instead of a complete transition to a mesenchymal state as noted during development, carcinoma cells often exhibit a spectrum of epithelial/mesenchymal phenotype. Compared to extremely Epithelial (E) or mesenchymal (M), the hybrid E/M state has been shown to be stable over multiple passages in vitro and “metastable”. Such hybrid E/M state cancer cells are broadly existing in primary and metastases, as well as circulating tumor cells [36–42]. It is even questioned if there are any fully E or M phenotypes in cancer. Therefore, cEMT, and its reverse cancer-associated mesenchymal-epithelial transition (cMET) relfect the relative state shift with respect to the original tumor cells.
In development, the M versus E phenotypic state is characterized by the expression of a set of transcription factors including Twist, Snail1/2 (Slug) and Zeb1, along with the intermediate filament protein vimentin replacing cytokeratins, and the loss of E-cadherin [43–45]. However, in cancer-associated switching, the fidelity of co-expression is lost, rendering definitive phenotypic assignment inconclusive [44, 45]. One consistent marker of the E versus M state, is the cell-cell cohesion homotypic binding receptor E-cadherin [24]. As this is the first receptor to bring cell sheets together and enable the formation of adhesion and then tight junctions that allow for cell polarization, the sine qua non of an “Epithelial” phenotype, its presence or loss from the cell surface can be used to define E and M, respectively [24]. In fact, forced loss of E-cadherin drives a cancer-associated EMT and metastatic behaviors [46], while forced re-expression of this receptor can be viewed as a metastasis suppressor [47–50]. For this discussion, and in recognition of the fluid nature of cancer cell phenotypes, E will be defined as cell surface ligandated E-cadherin, and M by its absence.
3. Early stage metastatic lesions
By this step of colonization, DTCs have escaped from the primary mass, survived the sheer forces and thrombotic events and hematopoietic cells while in the circulation and are now confronted with a foreign and hostile environment for seeding [23, 24, 51]. Within this section, we discuss the how DTCs survive through communications with the MME to successfully colonize at single cells or form micrometastases as well as the molecular and cellular mechanisms that confer pan-resistance at this stage of the cascade.
Presently, it is not possible to determine the presence of dormant DTCs in patients but evidence to indicate that dormant DTCs are resistant to common therapies derives from multiple observations where they have been recovered from bone marrow aspirates of patients several years after systemic therapeutic regimes (both chemotherapy and targeted therapies) [52–55]. While one aspect may be that the dormant cells are often out of the cell cycle, and most chemotherapies and biological agents target dividing cells [56, 57], this is not the complete story, as even growing micrometastases show similar chemoresistance [58]. We also discuss that dormancy involves the convergence of multiple mechanisms; cellular proliferation, angiogenesis and immunological factors, all of which have made the task of finding possibilities for therapeutic targeting and efficacy exceptionally difficult.
3.1. E-cadherin as a mediator of dormancy and chemoresistance
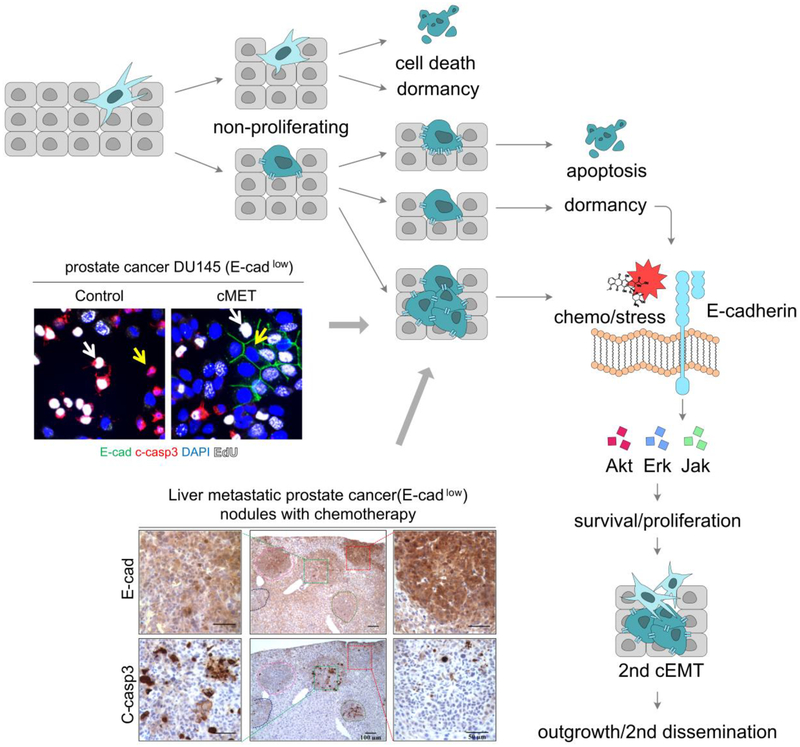
Though under provocative discussions on whether any of these phenotypic transitions are indispensable for metastases formation [34, 44, 45], it is well accepted cEMT enhances the tumor cells capability of migration to escape from the primary sites. It is also becoming acknowledged that a cMET aids survival and colonization in the metastatic sites [23, 24] (Fig 2).
Fig. 2.
E-cadherin mediates micrometastases dormancy and chemoresistance. (A) An overview schematic depicting entry into dormancy and acquisition of chemoresistance. (B) E-cadherin promotes cell survival independent of cell arrest or proliferation. E-cadherin, green; cleaved caspase-3, red; EdU, white; DAPI, blue. White arrow shows proliferating cells; yellow arrow shows nonproliferating cells. (C) E-cadherin protects tumor cells in the MME from chemotherapy. Representative images of DU145 prostate cancer cells and cleaved caspase-3 on sister sections of the liver. Same tumor on the sister sections is lined by same color. (B&C) Adapted from [58]. Made with Affinity Designer 1.7.0.
After an initial cEMT involved in tumor cell breaking away from the primary mass and intravasating into a vascular conduit, the DTC is confronted with a foreign and hostile environment for seeding. Only a diminishing fraction (on the order of 0.01% or less; based on in vivo mouse models [59]) can accomplish seeding and survival. Ectopic seeding requires a cMET with re-expression of E-cadherin. This is based upon the observation that many small metastases express epithelial markers [60, 61]. Chao et. al., examined E-cadherin expression in primary tumors and matched metastases and found that 62% of cases had increased E-cadherin at the metastatic sites compared to the primary tumors [62]. More importantly, not only was E-cadherin presented on the cell membrane in the metastases, whereas it was mainly cytoplasmic in the primary tumors; nearly all of the smallest metastases were epithelial in this manner, with the fraction of presentation decreasing as the nodule became larger, indicating a secondary cEMT being involved in the outgrowth. The cMET is also a partial transition, in that these tumor nodules simultaneously express mesenchymal markers [62]. This is also true in experimental prostate cancer, with E-cadherin expression level is conversely associated with tumor size [58, 62].
This phenotypic switching begs the question of the inductive signals that drive the changes, as E-cadherin is downregulated at multiple levels of control, but is neither mutated nor deleted genetically [24]. In the primary site, cancer-associated fibroblasts [63, 64] and macrophages [65, 66] are considered to express signals that drive cell scattering. Much less is known about the microenvironment at the site of initial intravasation and seeding that allows for cMET and cell survival.
The liver and lung are epithelial organs, in which parenchyma express E-cadherin and can form cell-heterotypic E-cadherin homotypic binding. Tumors cells regain E-cadherin at the cell surface membrane in the lung in patients and in vivo, and increase E-cadherin, be more cuboidal morphology when co-culturing with normal lung epithelial cells in vitro, similar to the situation in liver [47, 67]. Surprisingly, this cMET even occurs in the brain and bone marrow, containing mesenchymal environments that do not present E-cadherin on their resident cells for homotypic ligation and signaling [61, 62].
In prostate cancer, we have shown that E-cadherin can be presented on the membrane in the ectopic site upon abrogation of the EGF receptor (EGFR) activating autocrine loop that initially provides for a mesenchymal phenotype [68]. The blockade of EGFR by hepatocytes, allows for catenin stabilization of E-cadherin on the cell surface, and cell-homotypic and -heterotypic binding with functional gap junctions being formed with the hepatocytes [69]. In breast cancer cells, E-cadherin is downregulated by promoter hypermethylation [47], in addition to EGFR autocrine signaling. Still, the liver microenvironment can revert this suppression, but with breast cancer, this requires limited carcinoma cell proliferation to lose the imprinted methylation.
In both breast and prostate cancers other transcriptional regulators also play a role in further suppressing E-cadherin. Kaiso binds to hypermethylated DNA and prevents transcription, leading to a mesenchymal phenotype [70–72]. However, as Kaiso nuclear localization occurs downstream of E-cadherin abrogation, re-establishing E-cadherin signaling then eliminates this blockade of transcription.
This re-expression of E-cadherin during the earliest stages of metastatic seeding may provide for pan-resistance in two ways. The simplist is that as the carcinoma cells undergo a epithelial reversion, the fraction and velocity of cells actively proliferating is reduced. As most chemotherapeutics and biologics (such as CDK inhibitors and anti-RPTK cascades) target cells in cycle [73–75] this would limit the effectiveness of all these therapies. Even agents that cause cells damage or stress (e.g. metabolic blockade and proteosome inhibitors) would be rendered less efficacious as the quiescent cell have lower metabolic requirements and extended repair times.
However, this can only be a part of the story, as we have found that E-cadherin expression, and the accompanying cMET, provides for relative protection from multiple chemotherapies independent of proliferation [58, 62]. In experimental models of both breast and prostate cancers, the E micrometastases were protected from induced death while the M phenotype metastases (in both liver and lung) and the primary tumors were effectively treated. This was recapitulated in cell culture and mixed co-culture systems wherein proliferating E-cadherin positive carcinoma cells were not induced to apoptosis whereas the neighboring mesenchymal cells were despite similar proliferation fractions.
This pan resistance results from E-cadherin signaling through canonical survival pathways including PI3-kinase/AKT and MEK/Erk [58, 62]. Superficially, this may create a conundrum as these same pathways along with the p38 Erk pathway, prevent cMET, and their downregulation in the micrometastatic microenvironment is what enables the shift to E with re-expression of E-cadherin [76]. But it appears that the signaling through ligandated E-cadherin is qualitatively and functionally distinct in that it is a low level, barely detectable in non-stressed cells but evident when the cells are challenged by chemotherapies and stressors [58]. The low level and tonicity of the activation of the intermediary kinases is similar to that noted with EGFR signaling when restricted to the cell surface by either tethered ligand or the matricellular proteins tenascin C and laminin V; this signaling enhances survival of stem cells challenged by death signals both in vivo and in vitro, while the pulsatile high level activation by growth factors actually sensitize the cells to death [77–80].
This mode of protection opens new pathways to reverting the chemoresistance of dormancy in that targeting these key kinases would re-sensitize tumors to normal chemotherapy. As MEK inhibitors are in clinical use, particularly for metastatic melanoma, and various inhibitors of the PI3-kinase/AKT pathway have been developed for human use (though limited by systemic toxicity when used at high doses), the use of low doses of these agents would serve to ‘rescue’ classical therapies for increased efficacy against the dormany micrometastases [58].
3.2. Dormancy signals from the MME
Following dissemination, the vascular basement membrane is the first thing DTCs encounter. Consequently, it is not surprising that the common metastatic locations for solid tumors are those with a mostly vascular primary basement membrane (e.g. lung, liver, brain and bone marrow) [81]. This perivascular niche has been shown to confer growth arrest and survival in DTCs [82, 83]. In addition to E-cadherin, various tissue-specific dormancy-inducing signals have also been associated with promoting dormancy (e.g. BMP7 [84], BMP4 [85], TSP-1 [82]) CXCR12/CXCR4 [86]). Mechanistically, many of the dormancy-inducing signals lead to activation of the p38 MAPK pathway and in the absence of mitogenic signals; this promotes an ERKlo/p38hi state in DTCs that in turn drives G0/G1 cell cycle arrest and dormancy [87].
Entry into dormancy is also mediated in some instances by interactions with the extracellular matrix (ECM) proteins. Integrin binding to ECM and stromal cells an also cause cell cycle arrest and confer reduced sensitivity cytotoxic therapies [88]. Notably, although DTCs may up-regulate and alter the repertoire of integrins they express, the ones used to interact with ECM proteins in the MME are typically not mutated and their function remains the same as that for normal cells. Additionally, a collagen I-rich environment suppresses tumor progression via DDR signaling [89, 90]. This provides further credence to the need for a high degree of cellular plasticity exhibited by a DTC rather than genetic mutations being key to surviving the metastatic cascade.
Additional examples have been identified that support the model above wherein active signaling from MME imparts resistance to dormant cells, either in addition to, or predominantly over mitogenic suppression. Resident liver endothelial cells (liver sinusoidal endothelial cells; LSECs) were found to promote survival by inhibiting antitumor immune responses in the early metastatic niche. Interaction of tumor activated-LSECs with lymphocytes decreased their antitumor cytotoxicity and interferon-γ (IFN-γ) secretion [91]. This inhibition of cytotoxic T cells may also further enhance the resistance of dormant cells to immunotherapies, but remains to be determined. DTCs that grow in the bone marrow microenvironment stimulate the host MME to produce survival factors, such as IL-6 and insulin-like growth factors, which protect the tumor cells from cytotoxic drugs [92]. Other recent work has posited that chemotherapy resistant cells predominantly resided in the perivascular niche and the protection from therapy was from the vascular endothelium via VCAM1 and von Willebrand factor binding. These dormant cells could be sensitized to chemotherapy and bone metastases prevented by inhibiting integrin mediated interactions with VCAM1 and von Willebrand factor [93]. These studies combined with our work with E-cadherin, are starting to shed light on the significant role the MME play in shaping resistance in dormant tumor populations.
3.3. Immune escape mechanisms in dormancy
Little is known about whether and how immune cells interact with dormant DTCs. Reports indicate that dormant DTCs may exhibit immune evasive characteristics as we note above [23] rather than directly immune suppressive characteristics that have been posited to exist in later macrometastases [94]. Emerging evidence showed DTCs reduced or lacked tumor antigen presentation via down-regulation of MHC I, making them unrecognizable by the adaptive immune system [95]. Additionally, breast and lung DTCs were reported to broadly down-regulate NK cell-activating ligands, which are important for recognition and eradication by NK cells. The ability appears to be firmly linked with entrance into dormancy [96]. This is also reflected by reduced PD-L1 on E phenotype metastatic tumor cells [23], and while this may appear to prime cells for immune clearance, the concomitant reduction in MHC I renders these cells invisible to the cytotoxic immune cells, and subvert the efficacy of checkpoint inhibitors currently being applied. This suggests that dormant cells may also be inherently resistant to immune checkpoint inhibitors or vaccination therapies that enhance T cell and NK cell mediated killing.
3.4. Immunosuppressive MME protections in dormancy
Immunosuppressive immune cells are involved from the point of DTCs extravagating into the metastatic organ. They begin by creating a permissible pre-metastatic niche that includes metastasis-associated macrophages (MAMs; mainly M2-like), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [97, 98]. They are recruited by soluble factors and extracellular vesicles derived from the primary tumor to help form pre-metastatic niches [94]. All three create a protective microenvironment for the DTCs to arrive in to by suppressing the cytotoxic functions of T and/or NK cells and in doing so [99–101]. Those from the myeloid lineage (MAMs and MDSCs) appear important for promoting the colonization and survival of DTCs [97, 102]. MAMs expressing integrin-α4 aid this process by binding VCAM-1 on DTCs which in turn activates survival signaling [103]. Collectively, these cells serve to protect DTCs from a hostile microenvironment as well as surveillance by immune attack, which can also block the effects of immunotherapy and enable colonization and establishment of metastasis [98, 104, 105].
4. Late stage metastatic lesions
Although mechanisms involved in the early stages metastatic progression are partly understood, those that enable or provoke re-emergence of the dormant tumors remain unclear. Studies point towards the microenvironment as being the one with the license to drive the transition, suggesting the progression to an overt metastatic lesion in dormant cells is irrespective of their underlying genetics. From a progression perspective, we know that reactivation and exit from dormancy in DTCs or micrometastases requires a second cEMT induction, initiation of proliferation, continued survival signaling, and potentially continued evasion of the immune system. The instigators that initiate this sequence of events are beginning to unfold. We and others have shown that it involves disruption of the systemic (macro-environmental level) and local homeostasis (micro-environmental level) [23, 106–109].
Resistance in this stage of the metastatic cascade is conferred via the multicellular nature of the MME (e.g. cell-cell and cell-ECM interactions as well as soluble factors and EVs-mediated signaling) and is discussed below (Fig 3). Cellular and epigenetic resistance mechanisms are also barriers but have been discussed extensively elsewhere [4, 6, 11, 13–15, 110].
Fig.3.
Mechanisms of resistance conferred by the MME. (A) Chemotherapeutic resistance observed in dormant cells may be conferred by the MME through E-cadherin re-expression, interactions with endothelial cells (ECs), tumor cells integrin binding to extracellular matrix (ECM), and signals, such as IL-6 and IGFs from bone marrow, contribute chemoresistance. Immune recognition and efficacy of immunotherapy is limited through downregulation of immune target molecules (e.g. PD-L1 and MHC I) and possibly some immunosuppressive effects of ECs, macrophages, myeloid derived suppressor cells (MDSCs) and regulatory T cells (Tregs) which suppress the cytotoxic activity or induce apoptosis of anti-tumor T cells and NK cells. (B) Chemotherapeutic resistance observed in outgrowing metastases may be conferred through creation of dense ECM with survival signals from matricellular molecules, tenascin C in particular, and factors produced by activated stromal cells or innate immune cells. Despite upregulated expression of MHC I and PD-L1 on the tumor cells, efficacy of immunotherapy is inhibited through immunosuppressive effects of macrophages, MDSCs and regulatory T cells. Macrophages produced numerous immunosuppressive molecules, while MDCs produce inflammatory mediators which lead to apoptosis of T and NK cells. MDSCs and Tregs express immune checkpoint proteins PD-L1 and CTLA-4, respectively, which suppress effector T cells. Insulin like growth factos (IGFs); Von Willebrand factor (vWf); Indoleamine 2,3-dioxygenase (IDO); cancer –associated fibroblasts (CAFs). Made with Affinity Designer 1.7.0.
4.1. Mesenchymal transitions in the MME contain elements of protection from death
A cMErT is required for DTCs landing and seeding in the ectopic organ, whereas a secondary cEMT has been found concomitant with metastatic outgrowth in both mouse model and patients. This is based on the observations that E-cadherin expression levels reversely correlate with tumor size [58, 62]. These growing mesenchymal cells are active in cycle and metabolism, which should be responsive to therapies as the primary lesion. However, this is not the case, and so does even the immediate lethal outgrowth (such as triple negative breast cancer). Rather than exposure-related selection or mutation, signals awakening dormant cells or proliferation promoters from the MME are likely the key player.
Simultaneously with the re-active MME secreting signals that drive the secondary cEMT, these cells also produce matrix elements and signals to provide for survival [23]. Chief among these is the ECM matricellular protein tenascin C, that tonically signals via the EGFR in a cell survival mode [111]. This is in addition to soluble signals, which are noted below in “MME and initiation of proliferation programs”.
4.2. Angiogenic switch
In select situations, the exit from dormancy into a proliferative nodule may occur through vascular sprouting and triggering the angiogenic-switch [57, 82, 83]. The relative importance of this event is open to question, as many metastatic tumors coopt existing vasculature, and thus do not require angiogenic expansion [112]. This may explain why angiogenic inhibition has had limited success in the treatment of most metastatic disease, though it is clear that some tumor types are more susceptible to such therapies [113, 114].
Despite the question as to universality of this mechanism, there is evidence that such sprouting may drive emergence [82]. In such a situation, the endothelial cells would not only promote growth by supplying oxygen and nutrients, they also secrete angiocrine factors, which comprise an array of growth factors, trophogens, chemokines, adhesions molecules [115]. In particular, sprouting endothelial cells break dormancy and promote proliferation via secretion of TGF- β1 and periostin [82]. At this stage of metastasis, extracellular vesicles derived from endothelial cells are thought to confer an increased resistance to multiple chemotherapies [116]. Furthermore, as the nodule transitions from a macrometastasis to an overt metastasis the vascular becomes increasing disorganized and abnormal leading to high interstitial fluid pressure. Together these two aspects confer a physiological pharmacokinetic resistance mechanism as they limit the ability of drugs to penetrate.
4.3. MME and initiation of proliferation programs
Changes in the MME that disrupt the stable dormant state are likely the main instigators of emergence as well as resistance. It is becoming increasing evident that the homeostasis of the body as a whole and not simply the local MME is involved in regulating dormancy. In particular the inflammation appear to be key initiator of emergent growth. We, and others, have shown that a systemic and chronic inflammatory are linked to re-emergence from dormancy [106–109, 117, 118]. Examples of homeostatic disruption leading to metastatic progression include surgery [118], gut perturbations [106], alcohol [119] and obesity [120].
Recent studies by others and ourselves have identified the resident non-parenchymal cells of a metastatic organ, particularly endothelial cells and stromal cells, as being activated by the disrupted homeostasis and in turn promoting metastasis [82, 106, 117, 121–123]. These studies demonstrate that stressed endothelial cells [123] or stromal elements (e.g. hepatic stellate cells and CAFs) [117, 124] in the metastatic host organ respond by producing growth factors (such as EGF receptor ligands) and, cytokines and chemokines (e.g. IL-8, MCP-1, IL-6) can awaken the dormant cells. Such a re-entry into the cell cycle would be expected to re-sensitize the metastatic cells if the main mode of chemo-resistance was quiescence, but as this is not the case, concomitant changes in MME also need to provide for tumor cell resistance/survival.
In addition to soluble signals, activated stromal cells, particularly cancer associated fibroblasts (CAFs) and hepatic stellate cells, have been shown to secrete high levels of ECM proteins, which create a dense fibrotic MME. They promote outgrowth by producing ECM rich in collagen, fibronectin and laminin in and around the metastasis [125, 126]. Subsequent binding to these ECM components confers resistance to cytotoxic drugs by inhibiting drug-induced apoptosis [127] and increases resistance to targeted antibody therapies [128]. Resistance to therapies also occurs due to the increasing ECM stiffness, leading to pronounced desmoplasia that creates high interstitial pressure and limits drug penetration [8].
4.4. Immunosuppressive MME in late metastases
The immunotherapy resistance noted in late stage metastasis is posited to occur due to the creation of an overall suppressive MME. Evidence is emerging that points towards the innate immune system as being the main immune protagonist in late stage MME [129, 130]. Research has predominantly focused the activity of macrophages at the primary site, but data is beginning to emerge on the specific role of macrophages in the MME (recently extensively reviewed [131]). Consistently, innate immune cells are widely observed to varying degrees almost all metastatic tumors and can represent up to half of the cells in a tumor nodule [132], while the presence of T cells is variable [133–135]. In some tumors there is a robust infiltrations of T cells while in others they are almost absent or spatially restricted to the tumor margin [133–135], that would limit functionality in clearing the tumor.
With respect to emergence from dormancy, the systemic inflammation described above has also been shown to recruit macrophages, which subsequently trigger the outgrowth of previously dormant tumor cells [118]. Our investigations indicate this is likely induced by the M2-like macrophages and is imparted via induction of a second cEMT [122]. Others found MAMs could be recruited to the MME by DTC-derived endothelin-1 in the lung wherein they secreted trophic and immunosuppressive factors (e.g. MCP-1 and IL-6) that were essential for the transition of micrometastases to over metastases [136]. Resident macrophages in the liver also directly stimulate proliferation through secretion of HGF, TNF-α, IL1β and IL-10 [137, 138]. Notably, the secretion of IL-6 by macrophages also confers chemoresistance [139]. MAMs also indirectly promote sustain metastatic outgrowth by activating stromal cells that then secrete ECM proteins (e.g. periostin), resulting in a fibrotic MME [140] and through inhibition of tumoricidal immune response [141]. Although the role of MAMs in promoting and sustaining metastatic growth is gaining ground, their role in mediating therapeutic resistance at metastatic sites remains unclear, and represents an opportunity for further study and potential therapeutic approach.
The MDSC innate immune cells also may promote both outgrowth and therapeutic resistance. Evidence suggests they are main orchestrators of immune suppression [142, 143]. Their presence in metastatic lesions correlates with advanced disease stage [144], poor responses to chemotherapy [145, 146] and resistance to immune checkpoint inhibitors [147, 148]. Furthermore, in a murine model, elimination of MDSCs led to cures of experimental metastatic tumors [148]. Induction of metastatic outgrowth was reported to occur via mutual activation loop with metastatic cells involving IL-6 [149]. MDSCs are able generate and enhance suppressive microenvironment via various mechanisms, including expression of PD-L1 [150], inducible nitric oxide synthase (iNOS) [151], arginase-1 [152], indoleamine 2,3-dioxygenase (IDO) [153] and secretion osteopontin [101]. These mechanisms serve to strongly inhibit anti-tumor activities of T and NK cells and stimulate Treg cells; this creates significant obstacles for immune checkpoint blockade therapies.
5.0. Conclusions/perspectives/future
The microenvironment that is part and parcel of all tumor organoids during progression provides not just the support for the disseminated carcinoma cells, but also the protection of these cells from dying in these foreign hostile milieus. Unfortunately, the same strategies that confer survival on a vanishingly small fraction of successful metastatic cells also lead to generalized resistance to therapies. That the disseminated cells must overcome a nutritionally and metabolically challenging microenvironment is linked to chemo- and targeted-therapy resistance, with the avoidance of the intrinsic inflammatory response being phenocopied as immune-therapy escape. The molecular bases of these survival strategies are both multifaceted and changing from the earliest stages of seeding, through dormancy, and persisting in aggressive outgrowth.
The plastic and pleiotropic nature of survival in the face of innate and induced/pharmaceutical death signals presents challenges to effective therapies. This is particularly problematic as most persons harbor multiple metastases (including in diverse organs) that often reside at different stages of dormancy and outgrowth. Thus, targeting one organ or progression stage is likely to be temporizing at best, as the ‘non-targeted’ nodules will persist. Further confounding decisions as to whether and how to treat is the fact that these therapies are not just damaging to non-tumor tissues, but may aggravate those nodules not cleared. This is noted as hyperproliferative disease in the face of immune checkpoint blockade therapy [154], or adaptive cEMT as a consequence of chemotherapy [155], or both [156].
This complexity of therapeutic escape being an integral feature (and not a ‘bug’) of the cancer progression cascade calls for a number fundamental principles in developing curative or persistent treatment regimens. Clinical trials are needed to determine if treatments should target systemic organ changes [106], or try to maintain non-emergent nodule in their dormant state [157]. It is only with a better understanding of the underlying tumor cell and organ biology that we can develop the new diagnostic, theragnostic and interventional approaches that may turn metastatic cancer into a manageable chronic condition rather than a lethal progression.
Acknowledgements
These concepts were informed by studies funded by the VA Merit Award program, and the National Institutes of Health (UH3TR000496, GM69668 and GM63569). The funders had no input over any aspects of this work. The authors thank members of the Wells laboratory and those of Partha Roy (Univ Pittsburgh) and Doug Lauffenburger and Linda Griffith (MIT) for informed suggestions and commentaries. We also thank Jiang Yuhan for generating the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no Conflicts of Interest to declare.
No primary data are presented herein.
REFERENCES:
- 1.Mieog JS, van der Hage JA, and van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. The British journal of surgery 94, 1189–1200 [DOI] [PubMed] [Google Scholar]
- 2.Redden MH, and Fuhrman GM (2013) Neoadjuvant chemotherapy in the treatment of breast cancer. The Surgical clinics of North America 93, 493–499 [DOI] [PubMed] [Google Scholar]
- 3.Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, and Valero V (2016) Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA oncology 2, 508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Angulo AM, Morales-Vasquez F, and Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. Advances in experimental medicine and biology 608, 1–22 [DOI] [PubMed] [Google Scholar]
- 5.Jones SE (2008) Metastatic breast cancer: the treatment challenge. Clinical breast cancer 8, 224–233 [DOI] [PubMed] [Google Scholar]
- 6.Lohiya V, Aragon-Ching JB, and Sonpavde G (2016) Role of Chemotherapy and Mechanisms of Resistance to Chemotherapy in Metastatic Castration-Resistant Prostate Cancer. Clinical Medicine Insights. Oncology 10, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert LA, and Hemann MT (2010) DNA damage-mediated induction of a chemoresistant niche. Cell 143, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, and Golub TR (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, and Nelson PS (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine 18, 1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, and Massague J (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarrin B, Zarifi F, Vaseghi G, and Javanmard SH (2017) Acquired tumor resistance to antiangiogenic therapy: Mechanisms at a glance. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences 22, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arozarena I, and Wellbrock C (2017) Overcoming resistance to BRAF inhibitors. Annals of translational medicine 5, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, and investigators K.-. (2015) Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine 372, 2521–2532 [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, and Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, and CheckMate I (2015) Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine 373, 1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, and Robert C (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390, 1853–1862 [DOI] [PubMed] [Google Scholar]
- 17.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, Gangadhar TC, Hersey P, Dronca R, Joseph RW, Zarour H, Chmielowski B, Lawrence DP, Algazi A, Rizvi NA, Hoffner B, Mateus C, Gergich K, Lindia JA, Giannotti M, Li XN, Ebbinghaus S, Kang SP, and Robert C (2016) Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama 315, 1600–1609 [DOI] [PubMed] [Google Scholar]
- 18.Naxerova K, and Jain RK (2015) Using tumour phylogenetics to identify the roots of metastasis in humans. Nature reviews. Clinical oncology 12, 258–272 [DOI] [PubMed] [Google Scholar]
- 19.Garraway LA, and Lander ES (2013) Lessons from the cancer genome. Cell 153, 17–37 [DOI] [PubMed] [Google Scholar]
- 20.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., and Kinzler KW (2013) Cancer genome landscapes. Science 339, 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozic I, Antal T, Ohtsuki H, Carter H, Kim D, Chen S, Karchin R, Kinzler KW, Vogelstein B, and Nowak MA (2010) Accumulation of driver and passenger mutations during tumor progression. Proceedings of the National Academy of Sciences of the United States of America 107, 18545–18550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correia AL, and Bissell MJ (2012) The tumor microenvironment is a dominant force in multidrug resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy 15, 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells A, Clark A, Bradshaw A, Ma B, and Edington H (2018) The great escape: How metastases of melanoma, and other carcinomas, avoid elimination. Experimental biology and medicine 243, 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells A, Yates C, and Shepard CR (2008) E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clinical & experimental metastasis 25, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, Farias EF, Condeelis J, Klein CA, and Aguirre-Ghiso JA (2016) Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540, 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Guzvic M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, and Klein CA (2016) Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park SH, McKenna A, Chevalier A, Rosenberg M, Barker FG 2nd, Gill CM, Van Hummelen P, Thorner AR, Johnson BE, Hoang MP, Choueiri TK, Signoretti S, Sougnez C, Rabin MS, Lin NU, Winer EP, Stemmer-Rachamimov A, Meyerson M, Garraway L, Gabriel S, Lander ES, Beroukhim R, Batchelor TT, Baselga J, Louis DN, Getz G, and Hahn WC (2015) Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer discovery 5, 1164–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissig H, Richter J, Desper R, Meier V, Schraml P, Schaffer AA, Sauter G, Mihatsch MJ, and Moch H (1999) Evaluation of the clonal relationship between primary and metastatic renal cell carcinoma by comparative genomic hybridization. The American journal of pathology 155, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuukasjarvi T, Karhu R, Tanner M, Kahkonen M, Schaffer A, Nupponen N, Pennanen S, Kallioniemi A, Kallioniemi OP, and Isola J (1997) Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer research 57, 1597–1604 [PubMed] [Google Scholar]
- 30.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, and Iacobuzio-Donahue CA (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, Lucas JM, Brown LG, Dumpit RF, DeSarkar N, Higano C, Yu EY, Coleman R, Schultz N, Fang M, Lange PH, Shendure J, Vessella RL, and Nelson PS (2016) Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nature medicine 22, 369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, Kinzler KW, Vogelstein B, Willis J, and Markowitz SD (2008) Comparative lesion sequencing provides insights into tumor evolution. Proceedings of the National Academy of Sciences of the United States of America 105, 4283–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenburg G, and Hay ED (1982) Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. The Journal of cell biology 95, 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jolly MK, Ware KE, Gilja S, Somarelli JA, and Levine H (2017) EMT and MET: necessary or permissive for metastasis? Molecular oncology 11, 755–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JM, Dedhar S, Kalluri R, and Thompson EW (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of cell biology 172, 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss R, Li ZY, Liu Y, Beyer I, Persson J, Sova P, Moller T, Pesonen S, Hemminki A, Hamerlik P, Drescher C, Urban N, Bartek J, and Lieber A (2011) Analysis of epithelial and mesenchymal markers in ovarian cancer reveals phenotypic heterogeneity and plasticity. PloS one 6, e16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schliekelman MJ, Taguchi A, Zhu J, Dai X, Rodriguez J, Celiktas M, Zhang Q, Chin A, Wong CH, Wang H, McFerrin L, Selamat SA, Yang C, Kroh EM, Garg KS, Behrens C, Gazdar AF, Laird-Offringa IA, Tewari M, Wistuba II, Thiery JP, and Hanash SM (2015) Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. Cancer research 75, 1789–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson VB, David JM, Puig I, Patil PU, de Herreros AG, Thomas GV, and Rajasekaran AK (2014) Wilms’ tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma. PloS one 9, e102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, and Farace F (2011) Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. British journal of cancer 105, 1338–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang D, and Du X (2008) Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World journal of gastroenterology 14, 1823–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosse-Wilde A, Fouquier d’Herouel A, McIntosh E, Ertaylan G, Skupin A, Kuestner RE, del Sol A, Walters KA, and Huang S (2015) Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PloS one 10, e0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, Hopt UT, Keck T, Stickeler E, Passlick B, Schilling O, Reiss CP, Vashist Y, Brabletz T, Berger J, Lotz J, Olesch J, Werner M, and Wellner UF (2014) Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. The Journal of pathology 234, 410–422 [DOI] [PubMed] [Google Scholar]
- 43.Kang Y, and Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279 [DOI] [PubMed] [Google Scholar]
- 44.Tarin D, Thompson EW, and Newgreen DF (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer research 65, 5996–6000; discussion 6000–5991 [DOI] [PubMed] [Google Scholar]
- 45.Thompson EW, Newgreen DF, and Tarin D (2005) Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer research 65, 5991–5995; discussion 5995 [DOI] [PubMed] [Google Scholar]
- 46.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, and Weinberg RA (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer research 68, 3645–3654 [DOI] [PubMed] [Google Scholar]
- 47.Chao YL, Shepard CR, and Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Molecular cancer 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, and Herlyn M (2000) E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. The American journal of pathology 156, 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacGrogan D, and Bookstein R (1997) Tumour suppressor genes in prostate cancer. Seminars in cancer biology 8, 11–19 [DOI] [PubMed] [Google Scholar]
- 50.Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA, Dopazo J, and Martin AG (2013) Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PloS one 8, e77281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells A, Grahovac J, Wheeler S, Ma B, and Lauffenburger D (2013) Targeting tumor cell motility as a strategy against invasion and metastasis. Trends in pharmacological sciences 34, 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, and Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. The New England journal of medicine 353, 793–802 [DOI] [PubMed] [Google Scholar]
- 53.Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, Sommer H, and Pantel K (2000) Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 18, 80–86 [DOI] [PubMed] [Google Scholar]
- 54.Krawczyk N, Banys M, Neubauer H, Solomayer EF, Gall C, Hahn M, Becker S, Bachmann R, Wallwiener D, and Fehm T (2009) HER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial HER2 status on primary tumor. Anticancer research 29, 4019–4024 [PubMed] [Google Scholar]
- 55.Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Juckstock J, Borgen E, Rack B, Braun S, Sommer H, Solomayer E, Pantel K, Nesland J, Friese K, and Naume B (2011) Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clinical cancer research : an official journal of the American Association for Cancer Research 17, 2967–2976 [DOI] [PubMed] [Google Scholar]
- 56.Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, Hoffman RM, and Tarin D (2003) Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clinical cancer research : an official journal of the American Association for Cancer Research 9, 3808–3814 [PubMed] [Google Scholar]
- 57.Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, and Almog N (2006) A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. Journal of the National Cancer Institute 98, 316–325 [DOI] [PubMed] [Google Scholar]
- 58.Ma B, Wheeler SE, Clark AM, Whaley DL, Yang M, and Wells A (2016) Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 64, 1725–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. Journal of the National Cancer Institute 45, 773–782 [PubMed] [Google Scholar]
- 60.Ikeguchi M, Makino M, and Kaibara N (2001) Clinical significance of E-cadherin-catenin complex expression in metastatic foci of colorectal carcinoma. Journal of surgical oncology 77, 201–207 [DOI] [PubMed] [Google Scholar]
- 61.Kowalski PJ, Rubin MA, and Kleer CG (2003) E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast cancer research : BCR 5, R217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao Y, Wu Q, Acquafondata M, Dhir R, and Wells A (2012) Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer microenvironment : official journal of the International Cancer Microenvironment Society 5, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, Huang D, Zhao J, Yang L, Liao D, Su F, Li M, Liu Q, and Song E (2018) CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 172, 841–856 e816 [DOI] [PubMed] [Google Scholar]
- 64.Yang X, Hao J, Mao Y, Jin ZQ, Cao R, Zhu CH, Liu XH, Liu C, Ding XL, Wang XD, Chen D, and Wu XZ (2016) bFGF Promotes Migration and Induces Cancer-Associated Fibroblast Differentiation of Mouse Bone Mesenchymal Stem Cells to Promote Tumor Growth. Stem cells and development 25, 1629–1639 [DOI] [PubMed] [Google Scholar]
- 65.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, and Condeelis JS (2005) Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer research 65, 5278–5283 [DOI] [PubMed] [Google Scholar]
- 66.Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J, Condeelis J, Merad M, and Aguirre-Ghiso JA (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nature communications 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furukawa M, Wheeler S, Clark AM, and Wells A (2015) Lung epithelial cells induce both phenotype alteration and senescence in breast cancer cells. PloS one 10, e0118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner T, Chen P, Goodly LJ, and Wells A (1996) EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clinical & experimental metastasis 14, 409–418 [DOI] [PubMed] [Google Scholar]
- 69.Yates C, Shepard CR, Papworth G, Dash A, Beer Stolz D, Tannenbaum S, Griffith L, and Wells A (2007) Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Advances in cancer research 97, 225–246 [DOI] [PubMed] [Google Scholar]
- 70.Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, Christgen M, Lehmann U, Daniel J, van Diest PJ, and Derksen PW (2012) Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer. PloS one 7, e37864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Liu W, Black S, Turner O, Daniel JM, Dean-Colomb W, He QP, Davis M, and Yates C (2016) Kaiso, a transcriptional repressor, promotes cell migration and invasion of prostate cancer cells through regulation of miR-31 expression. Oncotarget 7, 5677–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones J, Wang H, Zhou J, Hardy S, Turner T, Austin D, He Q, Wells A, Grizzle WE, and Yates C (2012) Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. The American journal of pathology 181, 1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vijayaraghavan S, Moulder S, Keyomarsi K, and Layman RM (2018) Inhibiting CDK in Cancer Therapy: Current Evidence and Future Directions. Targeted oncology 13, 21–38 [DOI] [PubMed] [Google Scholar]
- 74.Pernas S, Tolaney SM, Winer EP, and Goel S (2018) CDK4/6 inhibition in breast cancer: current practice and future directions. Therapeutic advances in medical oncology 10, 1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segaliny AI, Tellez-Gabriel M, Heymann MF, and Heymann D (2015) Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. Journal of bone oncology 4, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma B, and Wells A (2014) The mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase (ERK) are involved in hepatocyte-mediated phenotypic switching in prostate cancer cells. The Journal of biological chemistry 289, 11153–11161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodrigues M, Griffith LG, and Wells A (2010) Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem cell research & therapy 1, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodrigues M, Blair H, Stockdale L, Griffith L, and Wells A (2013) Surface tethered epidermal growth factor protects proliferating and differentiating multipotential stromal cells from FasL-induced apoptosis. Stem cells 31, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodrigues M, Yates CC, Nuschke A, Griffith L, and Wells A (2013) The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue engineering. Part A 19, 1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nuschke A, Rodrigues M, Rivera J, Yates C, Whaley D, Stolz D, Griffith L, and Wells A (2016) Epidermal Growth Factor Tethered to beta-Tricalcium Phosphate Bone Scaffolds via a High-Affinity Binding Peptide Enhances Survival of Human Mesenchymal Stem Cells/Multipotent Stromal Cells in an Immune-Competent Parafascial Implantation Assay in Mice. Stem cells translational medicine 5, 1580–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chambers AF, Groom AC, and MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nature reviews. Cancer 2, 563–572 [DOI] [PubMed] [Google Scholar]
- 82.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, and Bissell MJ (2013) The perivascular niche regulates breast tumour dormancy. Nature cell biology 15, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, and Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine 16, 116–122 [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, and Watabe K (2011) Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. The Journal of experimental medicine 208, 2641–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, and Giancotti FG (2012) The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L, Comatas K, Magnani J, Kim Lyerly H, Cheng Q, McCall CM, and Sipkins DA (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Science translational medicine 8, 340ra373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, and Aguirre-Ghiso JA (2011) ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clinical cancer research : an official journal of the American Association for Cancer Research 17, 5850–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, Saporta S, Boulware D, Moscinski L, Alsina M, and Dalton WS (2003) Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer research 63, 7900–7906 [PubMed] [Google Scholar]
- 89.Badiola I, Olaso E, Crende O, Friedman SL, and Vidal-Vanaclocha F (2012) Discoidin domain receptor 2 deficiency predisposes hepatic tissue to colon carcinoma metastasis. Gut 61, 1465–1472 [DOI] [PubMed] [Google Scholar]
- 90.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, and Weiss SJ (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 91.Arteta B, Lasuen N, Lopategi A, Sveinbjornsson B, Smedsrod B, and Vidal-Vanaclocha F (2010) Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology 51, 2172–2182 [DOI] [PubMed] [Google Scholar]
- 92.Dalton WS (1999) The tumor microenvironment as a determinant of drug response and resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy 2, 285–288 [DOI] [PubMed] [Google Scholar]
- 93.Carlson P, Dasgupta A, Grzelak CA, Kim J, Barrett A, Coleman IM, Shor RE, Goddard ET, Dai J, Schweitzer EM, Lim AR, Crist SB, Cheresh DA, Nelson PS, Hansen KC, and Ghajar CM (2019) Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nature cell biology 21, 238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McAllister SS, and Weinberg RA (2014) The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature cell biology 16, 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pantel K, Schlimok G, Kutter D, Schaller G, Genz T, Wiebecke B, Backmann R, Funke I, and Riethmuller G (1991) Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer research 51, 4712–4715 [PubMed] [Google Scholar]
- 96.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, and Massague J (2016) Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 165, 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Condamine T, Ramachandran I, Youn JI, and Gabrilovich DI (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annual review of medicine 66, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero I, Garrido F, and Garcia-Lora AM (2014) Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer research 74, 6750–6757 [DOI] [PubMed] [Google Scholar]
- 99.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, and Moller A (2012) Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer research 72, 3906–3911 [DOI] [PubMed] [Google Scholar]
- 100.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, and Biragyn A (2009) Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer research 69, 5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sangaletti S, Tripodo C, Sandri S, Torselli I, Vitali C, Ratti C, Botti L, Burocchi A, Porcasi R, Tomirotti A, Colombo MP, and Chiodoni C (2014) Osteopontin shapes immunosuppression in the metastatic niche. Cancer research 74, 4706–4719 [DOI] [PubMed] [Google Scholar]
- 102.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, and Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PloS one 4, e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Q, Zhang XH, and Massague J (2011) Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer cell 20, 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kitamura T, Qian BZ, and Pollard JW (2015) Immune cell promotion of metastasis. Nature reviews. Immunology 15, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, and Morton JP (2016) CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer cell 29, 832–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clark AM, Kumar MP, Wheeler SE, Young CL, Venkataramanan R, Stolz DB, Griffith LG, Lauffenburger DA, and Wells A (2018) A Model of Dormant-Emergent Metastatic Breast Cancer Progression Enabling Exploration of Biomarker Signatures. Molecular & cellular proteomics : MCP 17, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Cock JM, Shibue T, Dongre A, Keckesova Z, Reinhardt F, and Weinberg RA (2016) Inflammation Triggers Zeb1-Dependent Escape from Tumor Latency. Cancer research 76, 6778–6784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grzelak CA, and Ghajar CM (2017) Metastasis ‘systems’ biology: how are macro-environmental signals transmitted into microenvironmental cues for disseminated tumor cells? Current opinion in cell biology 48, 79–86 [DOI] [PubMed] [Google Scholar]
- 109.Clark AM, Wheeler SE, Young CL, Stockdale L, Shepard Neiman J, Zhao W, Stolz DB, Venkataramanan R, Lauffenburger D, Griffith L, and Wells A (2016) A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab on a chip 17, 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharma P, Hu-Lieskovan S, Wargo JA, and Ribas A (2017) Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168, 707–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shao H, Kirkwood JM, and Wells A (2015) Tenascin-C Signaling in melanoma. Cell adhesion & migration 9, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, and Reynolds AR (2019) Vessel co-option in cancer. Nature reviews. Clinical oncology [DOI] [PubMed] [Google Scholar]
- 113.Pignata S, S CC, Du Bois A, Harter P, and Heitz F (2017) Treatment of recurrent ovarian cancer. Annals of oncology : official journal of the European Society for Medical Oncology 28, viii51–viii56 [DOI] [PubMed] [Google Scholar]
- 114.Zhang W, Shen Z, Luo H, Hu X, Zheng L, and Zhu X (2017) The Benefits and Side Effects of Bevacizumab for the Treatment of Recurrent Ovarian Cancer. Current drug targets 18, 1125–1131 [DOI] [PubMed] [Google Scholar]
- 115.Butler JM, Kobayashi H, and Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature reviews. Cancer 10, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pasquier J, Thawadi HA, Ghiabi P, Abu-Kaoud N, Maleki M, Guerrouahen BS, Vidal F, Courderc B, Ferron G, Martinez A, Al Sulaiti H, Gupta R, Rafii S, and Rafii A (2014) Microparticles mediated cross-talk between tumoral and endothelial cells promote the constitution of a pro-metastatic vascular niche through Arf6 up regulation. Cancer microenvironment : official journal of the International Cancer Microenvironment Society 7, 41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khazali AS, Clark AM, and Wells A (2018) Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. British journal of cancer 118, 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh HL, Dougan SK, and Weinberg RA (2018) The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Science translational medicine 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maeda M, Nagawa H, Maeda T, Koike H, and Kasai H (1998) Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer 83, 1483–1488 [DOI] [PubMed] [Google Scholar]
- 120.Osman MA, and Hennessy BT (2015) Obesity Correlation With Metastases Development and Response to First-Line Metastatic Chemotherapy in Breast Cancer. Clinical Medicine Insights. Oncology 9, 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu X, and Kang Y (2007) Organotropism of breast cancer metastasis. Journal of mammary gland biology and neoplasia 12, 153–162 [DOI] [PubMed] [Google Scholar]
- 122.Yang M, Ma B, Shao H, Clark AM, and Wells A (2016) Macrophage phenotypic subtypes diametrically regulate epithelial-mesenchymal plasticity in breast cancer cells. BMC cancer 16, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor DP, Clark A, Wheeler S, and Wells A (2014) Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast cancer research and treatment 144, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, Huang T, Chan MW, Marini FC, Rosol TJ, Bonafe M, and Hall BM (2008) Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer research 68, 9087–9095 [DOI] [PubMed] [Google Scholar]
- 125.Burnier JV, Wang N, Michel RP, Hassanain M, Li S, Lu Y, Metrakos P, Antecka E, Burnier MN, Ponton A, Gallinger S, and Brodt P (2011) Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene 30, 3766–3783 [DOI] [PubMed] [Google Scholar]
- 126.Eveno C, Hainaud P, Rampanou A, Bonnin P, Bakhouche S, Dupuy E, Contreres JO, and Pocard M (2015) Proof of prometastatic niche induction by hepatic stellate cells. The Journal of surgical research 194, 496–504 [DOI] [PubMed] [Google Scholar]
- 127.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, and Haslett C (1999) Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature medicine 5, 662–668 [DOI] [PubMed] [Google Scholar]
- 128.Hanker AB, Estrada MV, Bianchini G, Moore PD, Zhao J, Cheng F, Koch JP, Gianni L, Tyson DR, Sanchez V, Rexer BN, Sanders ME, Zhao Z, Stricker TP, and Arteaga CL (2017) Extracellular Matrix/Integrin Signaling Promotes Resistance to Combined Inhibition of HER2 and PI3K in HER2(+) Breast Cancer. Cancer research 77, 3280–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beatty GL (2016) Overcoming Therapeutic Resistance by Targeting Cancer Inflammation. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting 35, e168–173 [DOI] [PubMed] [Google Scholar]
- 130.Nielsen SR, and Schmid MC (2017) Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators of inflammation 2017, 9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doak GR, Schwertfeger KL, and Wood DK (2018) Distant Relations: Macrophage Functions in the Metastatic Niche. Trends in cancer 4, 445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qian BZ, and Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, and Kalluri R (2017) Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nature communications 8, 15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li X, Gruosso T, Zuo D, Omeroglu A, Meterissian S, Guiot MC, Salazar A, Park M, and Levine H (2019) Infiltration of CD8(+) T cells into tumor cell clusters in triple-negative breast cancer. Proceedings of the National Academy of Sciences of the United States of America 116, 3678–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stanton SE, and Disis ML (2016) Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for immunotherapy of cancer 4, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Said N, Smith S, Sanchez-Carbayo M, and Theodorescu D (2011) Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. The Journal of clinical investigation 121, 132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gangopadhyay A, Lazure DA, and Thomas P (1998) Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clinical & experimental metastasis 16, 703–712 [DOI] [PubMed] [Google Scholar]
- 138.Thomas P, Hayashi H, Zimmer R, and Forse RA (2004) Regulation of cytokine production in carcinoembryonic antigen stimulated Kupffer cells by beta-2 adrenergic receptors: implications for hepatic metastasis. Cancer letters 209, 251–257 [DOI] [PubMed] [Google Scholar]
- 139.Yin Y, Yao S, Hu Y, Feng Y, Li M, Bian Z, Zhang J, Qin Y, Qi X, Zhou L, Fei B, Zou J, Hua D, and Huang Z (2017) The Immune-microenvironment Confers Chemoresistance of Colorectal Cancer through Macrophage-Derived IL6. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 7375–7387 [DOI] [PubMed] [Google Scholar]
- 140.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, and Schmid MC (2016) Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nature cell biology 18, 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, and De Palma M (2011) Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer cell 19, 512–526 [DOI] [PubMed] [Google Scholar]
- 142.Baniyash M (2016) Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer immunology, immunotherapy : CII 65, 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parker KH, Beury DW, and Ostrand-Rosenberg S (2015) Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Advances in cancer research 128, 95–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, and Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII 58, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang A, Zhang B, Wang B, Zhang F, Fan KX, and Guo YJ (2013) Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer immunology, immunotherapy : CII 62, 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, and Ghiringhelli F (2016) Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer research 76, 5241–5252 [DOI] [PubMed] [Google Scholar]
- 147.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, and Speiser DE (2014) Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer immunology, immunotherapy : CII 63, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr., Papadopoulos N, Kinzler KW, Vogelstein B, and Zhou S (2014) Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proceedings of the National Academy of Sciences of the United States of America 111, 11774–11779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, and Lee DS (2013) A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast cancer research : BCR 15, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, and Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. The Journal of experimental medicine 211, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, and Gabrilovich DI (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of immunology 182, 5693–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodriguez PC, and Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunological reviews 222, 180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, and Ren X (2013) Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. Journal of immunology 190, 3783–3797 [DOI] [PubMed] [Google Scholar]
- 154.Fuentes-Antras J, Provencio M, and Diaz-Rubio E (2018) Hyperprogression as a distinct outcome after immunotherapy. Cancer treatment reviews 70, 16–21 [DOI] [PubMed] [Google Scholar]
- 155.Hwang M, Gordonov S, Wells A, Gertler F, Lauffenburger DA, and Yaffe MB (2019) The checkpoint kinase-regulated phosphoproteome drives a cytoskeleton-associated adaptive response to DNA damaging chemotherapy. in revision [Google Scholar]
- 156.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, Leroy L, Duchemann B, Lefebvre C, Veillon R, Westeel V, Koscielny S, Champiat S, Ferte C, Planchard D, Remon J, Boucher ME, Gazzah A, Adam J, Bria E, Tortora G, Soria JC, Besse B, and Caramella C (2018) Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA oncology 4, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beckwitt CH, Brufsky A, Oltvai ZN, and Wells A (2018) Statin drugs to reduce breast cancer recurrence and mortality. Breast cancer research : BCR 20, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]