Abstract
Background:
Poly adenosine diphosphate ribose polymerase inhibitors (PARPi) exhibit promising activity against ovarian cancers, but efficacy can be limited by acquired drug resistance. Here, we explore the role of autophagy in regulating sensitivity of ovarian cancer cells to PARPi.
Methods:
Induction of autophagy was detected by punctate LC3 fluorescence staining, LC3I to LC3II conversion on western blot analysis and electron microscopy. Enhanced growth inhibition and apoptosis were observed using PARPi with hydroxycloroquine (HCQ), chloroquine (CQ), or LYS05 to block hydrolysis of proteins and lipids in autophagosomes or with siRNA against ATG5 or ATG7 to prevent formation of autophagosomes. The preclinical efficacy of the combination of CQ and olaparib was evaluated using a patient-derived xenograft (PDX) and the OVCAR8 human ovarian cancer cell line.
Results:
Four PARP inhibitors (olaparib, niraparib, rucaparib and talazoparib) induced autophagy in a panel of ovarian cancer cells. Inhibition of autophagy with CQ enhanced sensitivity of ovarian cancer cells to PARPi. In vivo, olaparib and CQ produced additive growth inhibition in OVCAR8 xenografts and a PDX. Olaparib inhibited PARP activity leading to increased ROS and accumulation of ɣ-H2AX. Inhibition of autophagy also increased ROS and ɣ-H2AX, enhancing the effect of olaparib on both entities. Treatment with olaparib increased phosphorylation of ATM and PTEN, while decreasing the phosphorylation of AKT and mTOR, and inducing autophagy.
Conclusions:
PARPi-induced autophagy provides an adaptive mechanism of resistance to PARPi in cancer cells with wtBRCA and the combination of PARPi with CQ or other autophagy inhibitors could improve outcomes for ovarian cancer patients.
Keywords: Ovarian cancer, autophagy, resistance, PARP (Poly (ADP) ribose polymerase) inhibitors
Precis:
The data presented here show that PARP inhibitors induce autophagy in 6 BRCA wt ovarian cancer cells that provides an adaptive mechanism of drug resistance.Combination of PARP inhibitors and autophagy inhibitors can overcome resistance and improve survival in different experimental models of ovarian cancer. These findings provide evidence of unique combinations of therapy for improving drug therapeutic efficacy, decrease resistance and extend the use of PARP inhibitors in the clinic.
Introduction
Inhibitors of Poly adenosine diphosphate ribose polymerase (PARP) have shown promising activity against ovarian cancers. 1 While only 15–20% of ovarian cancers contain germ line or somatic mutations of BRCA1 or BRCA2, approximately 30–50% have defects in homologous recombination (HR) DNA repair. Cells with a deficiency in HR repair depend on alternate pathways for DNA repair in order to survive, providing a potential target for DNA-damaging agents. PARP is an enzyme with a key role in detecting DNA single strand breaks and mediating DNA repair through pathways that complement HR.1 The inhibition of PARP in cancer cells with HR deficiency results in the accumulation of DNA double strand breaks and cell death.
The FDA has approved three PARP inhibitors (PARPi) - olaparib, rucaparib and niraparib - for different indications in the management of ovarian cancer. Even though PARPi have shown promising anti-cancer activity, only a fraction of patients respond. Across five independent clinical trials, approximately half of the patients carrying BRCA1/2 mutations fail to respond to PARPi treatment, with an average response rate of 47%, compared to 20% in patients with wild-type BRCA.2 A better understanding of factors which mediate resistance to PARPi, might improve these response rates and benefit patients with ovarian cancer.
While many cancers harbor subpopulations with genetically or epigenetically programmed mechanisms of resistance, increasing interest has centered on the adaptive response of cancer cells to targeted therapy where survival pathways are upregulated only in response to drug treatment. Induction of autophagy has been documented in response to several conventional cytotoxic agents and to targeted therapy.3, 4 Autophagy is a process in which damaged organelles and long-lived proteins can be sequestered and degraded to yield amino acids and fatty acids that can provide energy to cells under nutrient poor conditions or in the presence of stress. During the process of autophagy, subcellular cargo is first sequestered within double membrane vesicles known as autophagosomes. The autophagosomes then fuse with lysosomes, forming structures known as autophagolysosomes that contain proteases and lipases which are activated by decreasing pH within these structures. Proteins and lipids are hydrolyzed to amino and fatty acids which are released into the cytoplasm to be catabolized providing energy.5 Recent studies have found that autophagy can protect cancer cells from chemotherapy or can enhance the response to certain drugs, which can contribute to the efficacy of the anticancer drugs as well as to drug resistance.6, 7 Based on these observations with other drugs and the increasing use of PARPi to treat ovarian cancer, we have explored the role of autophagy on the sensitivity of ovarian cancer cells to PARPi. Here, we document for the first time that PARPi induce autophagy in ovarian cancer cells and that autophagy provides an adaptive mechanism of PARPi resistance that can be overcome confirmed by the functional or phenotypic inhibition of autophagy, providing a new strategy for treatment with PARPi in combination with autophagy inhibitors.
Materials and Methods
Cell lines and cultures.
Human ovarian cancer cell lines UWB1.289, HEY and A2780 were purchased from American Type Culture Collection (Manassas, VA). OVCAR3, OVCAR5, OVCAR8, ES-2, OC316, SKOV3, and IGROV1 were from Dr. Gordon Mills’ laboratory. All the cell lines were validated by genotyping and were mycoplasma-free, as previously described.8 SKOv3 cells were cultured in McCoy’s 5A medium; OVCAR3, OVCAR5, OVCAR8, HEY, OC316, A2780, IGROV1, and ES-2 cells were grown in RPMI1640 medium. BRCA1/2 was sequenced by Stordal et.al and found to be wt in all cell lines.9
Sulforhodamine B (SRB) assay.
Cells were seeded in 96-well plates and incubated overnight. Attached cells were treated and SRB assay was used to measure the growth inhibition as previously described.10
Flow cytometry.
Apoptosis was measured by fluorescence activated cell sorting (FACS) with the FITC Annexin V Apoptosis Detection Kit I (Grand Island, NY, USA) according to the manufacturer’s recommendations.
Clonogenic assays.
200–400 cells/well were seeded in 6-well plates and treated with olaparib (5 μM), CQ (5 μM) or the combination of CQ with olaparib for 14 days. Colonies in each well were stained with Coomassie blue (sigma, B7920) and counted.
Transmission Electron Microscopy (TEM).
HEY and OVCAR8 cells were washed in PBS and fixed with 2.5% glutaraldehyde in 0.1 M Na-cacodylate buffer and further fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer. Specimens were stained with aqueous uranyl acetate and lead citrate before they were observed with a Jeol-100 CX II (JEOL) TEM at 80 kV.
Measurement of Intracellular ROS.
Cells were washed with PBS then stained with H2DCFDA (C6827) (Thermo Fisher, Grand Island, NY) in 1X PBS for 30 min at 37°C in the dark. Samples were centrifuged and re-suspended in PBS then analyzed on the FL-1 channel of a flow cytometer.
Human cell line xenografts.
Experiments using female athymic nu/nu mice were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 00001195) (M. D. Anderson Cancer Center). OVCAR8 cells were implanted intraperitoneally on 6–8 weeks old female nu/nu mice. Mice were randomized into 4 treatment groups: (1) vehicle control (4% DMSO with 30% polyethylene glycol (PEG) 300 and double distilled H2O, (2) olaparib (50mg/kg 5 days/week ip), (3) chloroquine (50mg/kg 5 days/week) and (4) olaparib and chloroquine (5 days/week by ip) for 4 weeks. Tumor weight was measured at the end of the 4 weeks.
Patient-derived tumor xenograft (PDX) models.
Primary patient tumors were collected from a consenting patient with High Grade (IIIC), BRCAwt, ovarian cancer under an approved Mayo Clinic Institutional Review Board (IRB) protocol as previously described.11 Briefly, tumor specimens were minced and mixed with media containing penicillin/streptomycin and Rituximab (10 mg/kg) (Rituxan; Genentech, Inc., San Francisco, CA).12 Minced tissue pieces were injected intraperitoneally into 7–9 weeks old female SCID-bg mice (C.B.−17/IcrHsd-Prkdcscid Lystbg; ENVIGO), in accordance with the Mayo Clinic Institutional Animal Care and Use Committee under an approved protocol (IACUC ID: A37615). PDX models are assigned a patient heterotransplant (PH) number in accordance with the Health Insurance Portability and Accountability Act.
PDX passaging and treatment.
An ovarian cancer (OC) model (labeled PH063) from the fifth-generation of passage was chosen based on previous experience and ease of engraftment and established intraperitoneally in female SCID mice. PH063 was revived from cryogenic storage as previously described and reestablished in SCID mice 11. Animals were monitored for engraftment and when tumor size reached 0.5 – 1.0 cm in diameter by transabdominal ultrasound (SonoSite S-Series, SLAx 13–6 MHz linear transducer), mice were randomized to treatment arms (n>9). Olaparib and Chloroquine were given by daily oral gavage in 0.5% methylcellulose. The largest tumor cross-sectional area was measured weekly during 8 weeks. Mice were euthanized individually when moribund or as a cohort after 8 weeks. The primary endpoint was change in tumor area by ultrasound, normalized to the day 1 area of the same tumor and plotted as a ratio vs. time.
Statistics.
Cellular assays were repeated at least twice; the mean and SD were calculated for each assay. Significance of findings was assessed by using 2-tailed Student’s t test or ANOVA. A P Value<0.05 was considered statistically significant. *p < 0.05, **p<0.01, ***p<0.001 and ****P<0.0001. For the OVCAR8 Xenograft model we used One-way ANOVA for comparison of the different groups. Differences were considered to be significant at *p<0.05. GraphPad prism software was used for data analysis and to prepare graphs. PDX data were analyzed via linear mixed effects modeling performed in SAS to assess differences between study groups. Change over time in tumor area from baseline on the natural log scale was compared between groups using a two-parameter growth model framework. The time variable was centered for hypothesis testing. Linear and quadratic terms were included, along with the interaction of each with time. The intercept and linear slope were specified as random effects with unstructured correlation, allowing per-mouse regression lines. Because of occasional differences in measurement intervals, a spatial power correlation structure was used, which assumes any two observations from the same mouse are correlated and that this correlation decreases exponentially with time between the observations. For visualization, model estimates with 95% confidence intervals were plotted for each treatment group. Three degree of freedom pairwise contrasts were performed to assess simultaneous difference in slope and intercept between group trajectories (test of coincident trajectories).
Results
Olaparib and three other PARP inhibitors induce autophagy in ovarian cancer cells.
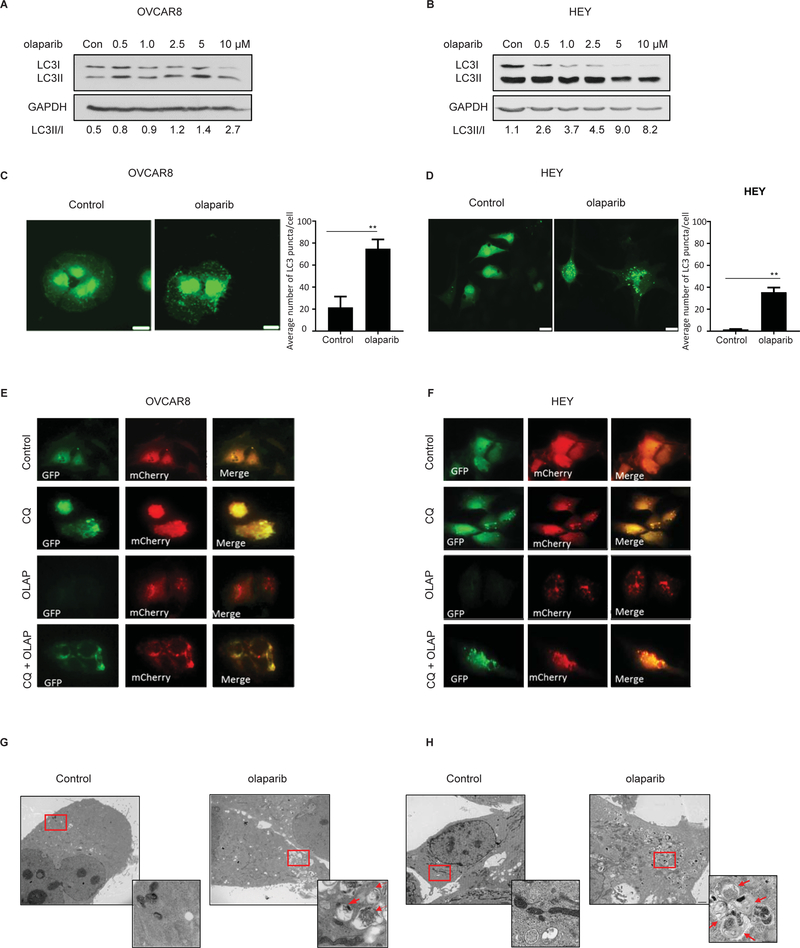
To determine the effect of olaparib on the induction of autophagy, we performed western blot analysis to detect LC3-I to LC3-II conversion. Treatment with olaparib increased the induction of autophagy, evidenced by the conversion of LC3I to LC3II in 9 ovarian cancer cell lines (Figure 1A–B and S1A). Rucaparib and niraparib, as well as talazoparib, increased the LC3-II /LC3-I ratio in OVCAR8, consistent with the induction of autophagy (Figure S1B). Moreover, increased number of LC3 puncta was observed in olaparib-treated cells, documenting the presence of autophagosomes and accumulation of LC3 on autophagic vesicles (Figure 1C –D and S1C).
Figure 1. Olaparib induces autophagy in ovarian cancer cells.
A and B. Cells were treated with olaparib for 72 hours. Western blots were performed to examine the conversion of LC3-I to LC3-II. C and D. Immunofluorescence staining of punctate GFP-LC3 after treatment with olaparib (5 μM). E and F. Fluorescence microscopy of OVCAR8 and HEY cells with or without olaparib (OLAP) (5 μM) or chloroquine (CQ) (12 μM) treatment for 24 hours post infection with GFP-mCherry-LC3B-expressing lentivirus. Yellow puncta indicate the presence of GFP and mCherry signal. Red puncta indicate the fusion of the autophagosome with the lysosome and quenching of GFP. G-H. Measurement of olaparib-induced autophagy with transmission electron microscopy (TEM) in OVCAR8 and HEY cells. Two magnifications of ultrastructure are shown. Red arrows indicate typical membrane autophagosomes. Data represents results from at least 2 experiments.
Using a pH-sensitive mRFP-GFP-LC3 fluorescence probe, we examined autophagic flux. After treatment with olaparib, the number of red punctae increased, consistent with the acidification of autophagolysosomes (Figure 1E–F). In the presence of chloroquine (CQ) which neutralizes the contents of autophagolysosomes there was accumulation of vacuoles that fluoresced both red and green and appeared “yellow” when images were fused. Using transmission electron microscopy (TEM) we detected double membrane vacuolar structures 48 hours after treatment with olaparib in HEY and OVCAR8 cells. Typical autophagosomes containing multiple lamellae and digested material were identified (Figure 1G–H). Substantially fewer and smaller autophagic vesicles were observed in control cells.
Inhibition of autophagy by multiple methods sensitizes ovarian cancer cells to olaparib and other PARP inhibitors.
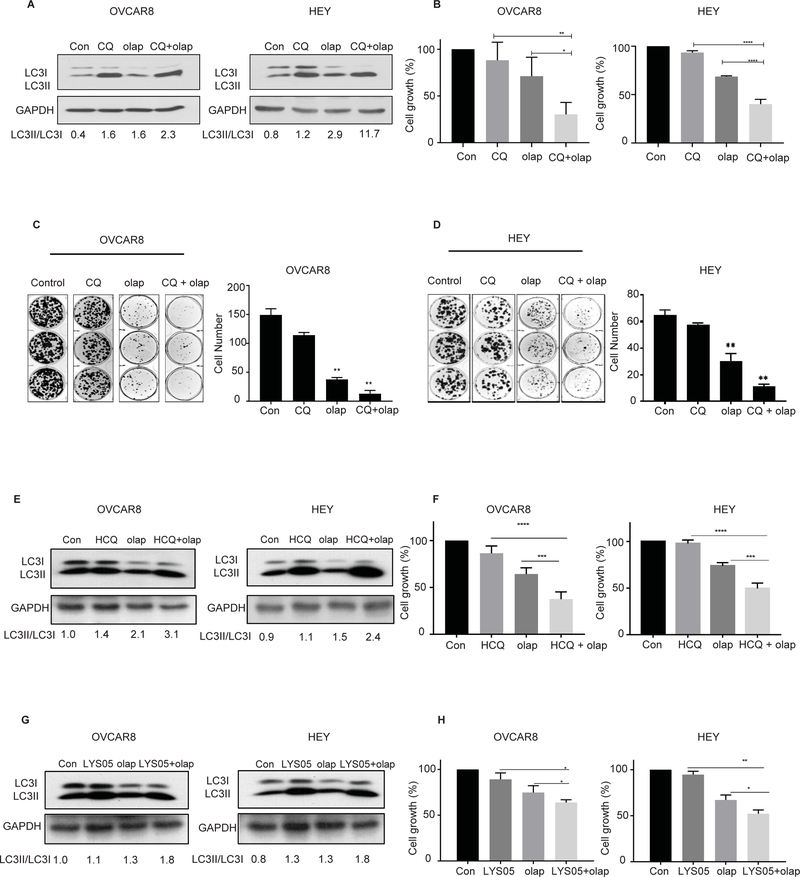
To assess whether inhibition of autophagy could increase the efficacy of olaparib, we combined olaparib with pharmacologic inhibitors of autophagy including CQ, hydroxychloroquine (HCQ) and LYS05. We also used RNAi to knockdown ATG5 and ATG7, proteins required for autophagosome formation, before treatment with olaparib. After olaparib treatment we observed an increase in the LC3-II to LC3-I ratio, that could be further enhanced by chloroquine (Figure 2A, S2A–B). The combination of olaparib with CQ synergistically decreased cell viability compared to either drug alone (*p<0.05, ****p<0.0001) (see Figure 2B, Figure S2C–F) in 6 BRCA Wild-Type ovarian cancer cell lines (OVCAR8, HEY, A2780, OVCAR5, SKOV3 and OC316). Similar results were observed in the BRCA mutant cell line, UWB1.289, (Figure S3A). We also examined the clonogenic survival of HEY and OVCAR8 cells treated with CQ in combination with olaparib. The addition of CQ to olaparib significantly sensitized both ovarian cancer cell lines to treatment with olaparib, further decreasing their clonogenic growth (Figure 2C and D). Inhibition of autophagy also increased the sensitivity of OVCAR8 ovarian cancer cells to rucaparib, niraparib and talazoparib judged by SRB assays (Figure S4A–F). Similar results were obtained with the treatment combination of olaparib and HCQ or LYS05 (Figure 2E –H). HCQ is a derivative of chloroquine that has a more favorable safety profile for administration to humans 13. LYSO5 is a dimeric chloroquine with greater potency for inhibiting autophagy, with no significant toxicity in animal studies and the potential to inhibit autophagy in vivo as a single agent 14. Similar to the experiments performed with CQ, treatment with the combination of olaparib and LYS05 significantly decreased cell viability of OVCAR8 and HEY cells (*P<0.05).
Figure 2. Inhibition of olaparib-induced autophagy decreases cell viability.
Cells were pretreated for 4 hours with 12 μM of chloroquine (CQ), with or without 5 μM of olaparib (olap) or both for 5 days. A. Whole cell lysates were analyzed for conversion of LC3I to LC3II by western blot. B. 4,000 cells/well were plated in 96-well plate, then sequentially treated with olaparib (5 μM) or chloroquine (12 μM) and incubated for 5 days before fixation and staining by sulforhodamine. C and D. OVCAR8 and HEY cells were seeded, in a 6 well plate and cultured with olaparib (5 μM), CQ (10 μM), or olaparib + CQ for 14 days and stained with Coomassie blue. Cells were pretreated for 4 hours with hydroxychloroquine (HCQ) (10 μM) or LYS05 (2 μM) with or without 5 μM of olaparib (olap) or both for 5 days. E and G. Conversion of LC3I to LC3II was analyzed by western blot. F and H. 4,000 cells/well were plated in 96-well plate, then sequentially treated with olaparib (5 μM) or chloroquine (12 μM) and incubated for 5 days before fixation and staining by sulforhodamine. Data represents results from at least 3 experiments.
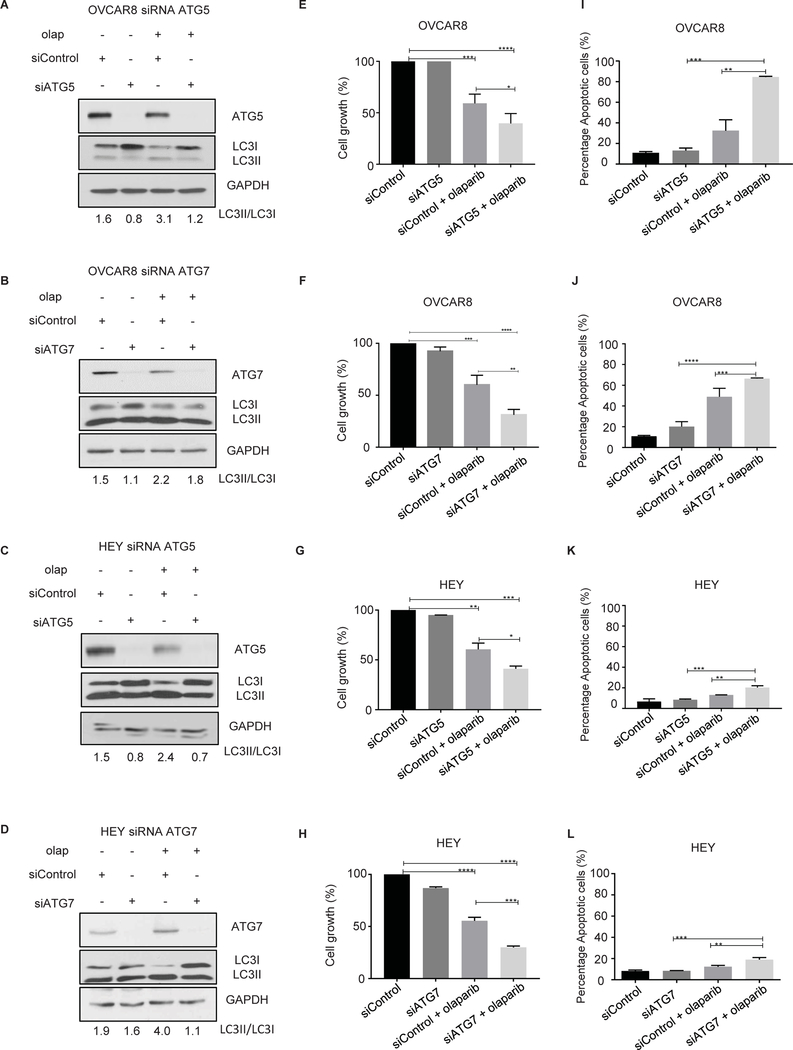
As CQ, HCQ and LYSO5 might have effects on cell function other than the functional inhibition of autophagy, we inhibited induction of autophagy by knockdown of two proteins essential to the process, ATG7 and ATG5. As shown in Figure 3A–D, cells lacking ATG5 or ATG7 have marked decrease in the LC3-II to LC3-I ratio. ATG5 or ATG7 downregulation resulted in enhancement of sensitivity of OC cells to olaparib as shown by a decrease in cell viability and an increase in apoptosis (Figure 3E–L). Collectively, these results suggest that induction of autophagy is a key mechanism by which ovarian cancer cells grow and survive the cytotoxic effects of olaparib.
Figure 3. Inhibition of olaparib-induced autophagy by knocking down ATG5 and ATG7 decreases cell viability and enhances apoptosis.
OVCAR8 and HEY cells were transfected with ATG5 or ATG7 siRNA and treated with olaparib (5 μM). A-D. Knockdown efficacy was observed by western blot analysis. E-H. 4,000 cells/well were plated in 96-well plates, then sequentially transfected with ATG5 or ATG7 siRNA. After 24 hours cells were treated with olaparib (5 μM) for 5 days. Cells were fixed and stained by sulforhodamine B. I-L. Transfected cells were treated with olaparib for 96 hours. After incubation with Annexin V-FITC in a buffer containing propidium iodide, cells were analyzed using flow cytometry. Data represents results from at least two experiments.
Inhibition of autophagy with chloroquine sensitizes patient-derived and cell line-derived human ovarian cancer xenografts to olaparib.
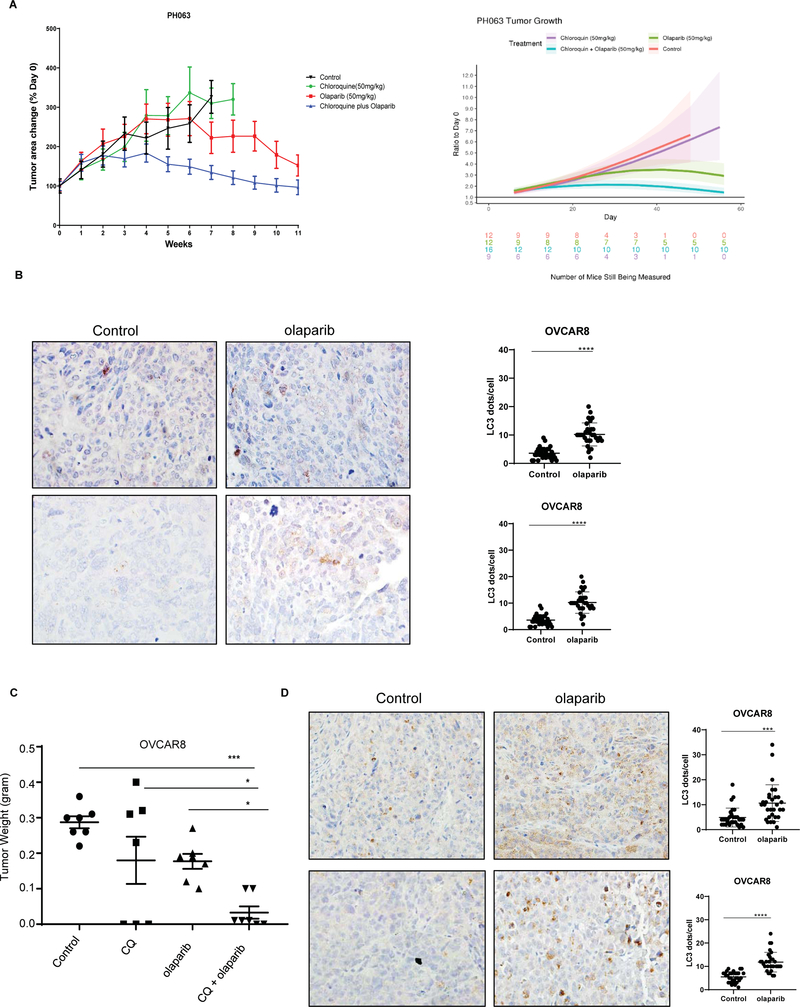
Given the enhanced cytotoxicity observed in cell culture using a combination of olaparib and CQ, we asked whether CQ would augment response to olaparib treatment in an ovarian cancer patient-derived xenograft (PDX) model.15 The preclinical efficacy of the combination of CQ + olaparib was evaluated using the PH-063 OC PDX model. Nu/nu mice were randomized into 4 groups for treatment as follows: 1) control (0.5% methylcellulose), 2) CQ alone, 3) olaparib alone and 4) a combination of CQ and olaparib. The effects of treatment were assessed by weekly transabdominal ultrasound as previously described 11. CQ treatment alone did not inhibit tumor growth relative to controls (p=0.966) but some tumor growth inhibition (TGI) was observed with olaparib (p=0.026). However, the combination of CQ plus olaparib resulted in significant TGI compared to controls and olaparib monotherapy (p<0.001 and p=0.002, respectively) (Figure 4A). No loss in body weight was detected in any of the animals (Data not shown). To confirm these results, we tested the combination of CQ and olaparib in the OVCAR8 human ovarian cancer cell line. Similar results were obtained to those in the PDX model. The tumor size with a combination of CQ and olaparib was significantly smaller compared to controls (***p<0.001), CQ (*p<0.05) or olaparib (*p<0.05) as a single agent (Figure 4C). No loss in body weight was detected during the 4 weeks of treatment compared to control group (Data not shown). Tumor tissues were sectioned for LC3 Immunohistochemistry and western blot analysis. A marked accumulation of punctate LC3 was observed after olaparib administration, consistent with the induction of autophagy (Figure 4B (PDX) and 4D, S8 A–B (OVCAR8).
Figure 4. Chloroquine sensitizes Patient-derived and cell line-derived human ovarian cancer xenografts to olaparib.
A. PH063-PDX model was treated with 50 mg/kg chloroquine (CQ), 50 mg/kg olaparib or a combination of olaparib plus CQ, by daily gavage for 8 weeks (n=12). B and D. LC3 protein was detected by immunohistochemistry using paraffin-embedded sections of tumor excised from PDX mice treated with olaparib or diluent. C. OVCAR8 cells (5× 106) were injected intraperitoneally into nu/nu mice that were treated with 40 mg/kg olaparib, 50mg/kg CQ or both for 4 weeks. After treatment, mice were sacrificed, and tumor weight were measured. The tumor weight in each group is plotted (n=7 in each group).
Taken together, our in vivo data is consistent with in vitro findings, autophagy inhibitors can increase the therapeutic efficacy of PARP inhibitors.
Olaparib blocks PARP activity, stimulates ROS, increases ɣ-H2AX, and induces apoptosis in ovarian cancer cells.
Having established that inhibition of olaparib-induced autophagy enhances the ability of olaparib to decrease ovarian cancer cell viability and inhibit clonogenic growth, we examined mechanism(s) by which olaparib affects ovarian cancer cell growth. Initially, we confirmed the ability of olaparib to block PARP activity in a panel of ovarian cancer cell lines that were treated with increasing doses of olaparib for 24 hours. (Figure S5A and B). Olaparib treatment resulted in a dose-dependent decrease in PARP activity in all ovarian cancer cell lines, leading to accumulation of ɣ-H2AX. (Figure S5C–F, S6A). Furthermore, treatment of ovarian cancer cell lines with olaparib for 96 hours resulted in a dose-dependent decrease in cell proliferation (data not shown). Based on the decreased cell proliferation observed, we evaluated cell cycle distribution and apoptosis after olaparib treatment. FACS analysis of ovarian cancer cells after 96 hours of treatment with olaparib detected a significant increase in the G2/M phase of the cell cycle (Figure S5G – H, S6B). This analysis also demonstrated that olaparib induced apoptosis in ovarian cancer cells (Figures S5I −5J, S6C).
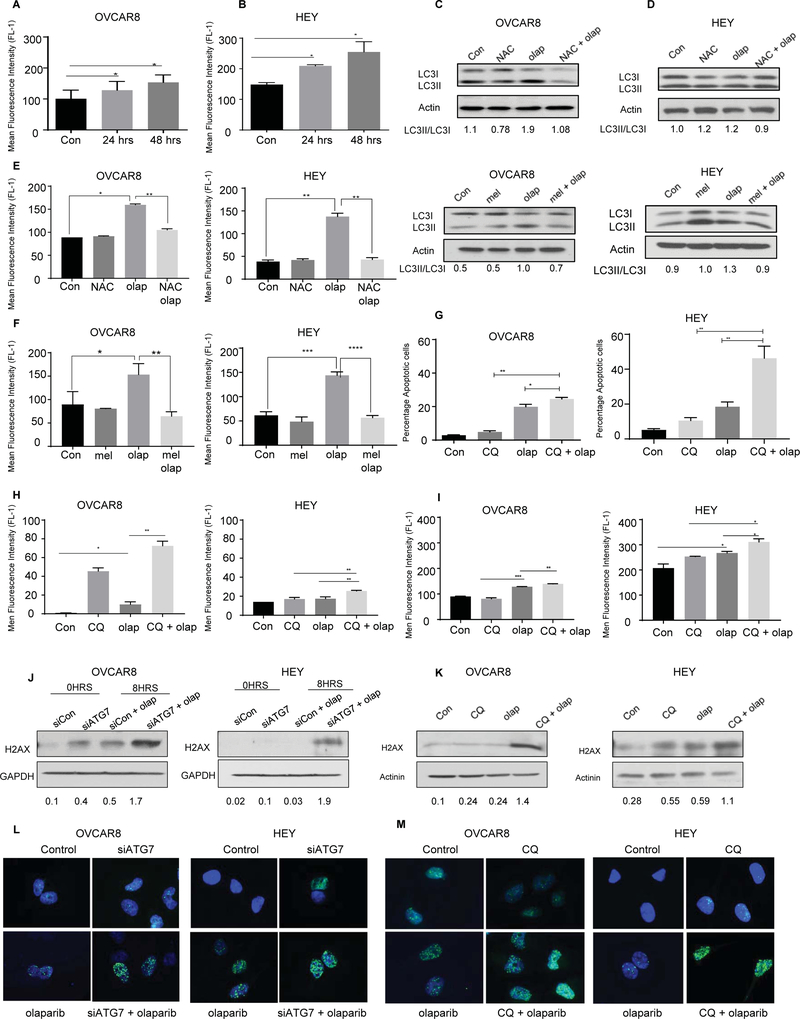
We also evaluated levels of reactive oxygen species (ROS) in HEY and OVCAR8 ovarian cancer cells after treatment with olaparib for 24 and 48 hours. The number of CM-H2DCFDA-positive cells after treatment with olaparib was compared with diluent treated control cells (Figure 5A and 5B). The percentage of CM-H2DCFDA-positive cells increased after treatment with olaparib for 24 or 48 hours (*p<0.05). Based on the fact that olaparib induces autophagy and that ROS are known to induce autophagy 16, we then tested whether olaparib’s effect on autophagy was mediated by ROS production. OVCAR8 and HEY cells were treated with olaparib and N-acetylcysteine (NAC), an antioxidant that replenishes intracellular glutathione (GSH) to prevent formation of oxidative stress.17 NAC treatment blocked LC3 conversion and ROS generation as confirmed by CM-H2DCFDA fluorescence intensity by flow cytometry and reduced LC3I/LC3II conversion (Figure 5C and D). Our results were confirmed by using melatonin which has also been shown to be an effective antioxidant and ROS scavenger by stimulating GSH synthesis 18 (Figure 5E and F). Thus, olaparib induces autophagy, in part, through ROS generation.
Figure 5. Olaparib Induces formation of ROS.
A and B. OVCAR 8 and HEY ovarian cancer cells were treated with olaparib (5 uM) for 24 and 48 hours and assayed for ROS production. The attached cells were stained with 5 mM H2DCFDA, collected and analyzed by flow cytometry. C and D. Western blot for conversion of LC3-I to LC3II after treatment with NAC (10 mM), or melatonin (MEL) (10 μM) or in combination with olaparib (5 μM). E and F. Flow cytometry analysis of ROS after treatment with NAC or melatonin in combination with olaparib. G and H. Cells were treated with olaparib alone, CQ alone, or CQ combined with olaparib for 96 hours. After incubation with Annexin V-FITC in a buffer containing propidium iodide or H2DCFDA, cells were analyzed using flow cytometry for Apoptosis or ROS. I. Evaluation of apoptosis after Knockdown of ATG7. Western blot (J-K) and fluorescence staining (L-M) were performed to evaluate expression of ɣ-H2AX after ATG5/7 knockdown or CQ treatment. Data represents results from at least three experiments.
Inhibition of autophagy with chloroquine enhances olaparib-induced apoptosis and enhances olaparib-induced formation of ROS and DNA damage.
Given the enhanced inhibition of cell viability judged by SRB and clonogenic growth observed with combined treatment, we asked whether chloroquine might enhance the apoptosis produced by olaparib. Different ovarian cancer cell lines were treated with CQ and olaparib individually and in combination before measuring Annexin V-propidium iodide (PI) staining by flow cytometry to determine the percentage of apoptotic cells over 96 hrs. Combined treatment increased apoptosis (*P< 0.05, OVCAR8 and **p<0.01, HEY) in the BRCA WT OVCAR8 and HEY cell lines (Figure 5G) and the BRCA mutant cell line, UWB1.289 (Figure S3 B). To determine whether enhanced apoptosis related to enhanced levels of ROS, OVCAR8 and HEY ovarian cancer cells were treated with CQ or siATG7, individually and in combination with olaparib, and ROS levels were measured by staining with CM-H2DCFDA measuring fluorescence with flow cytometry. When cells were treated with olaparib, ROS levels were even higher compared to either treatment alone (*P<0.05, **P < 0.001) (Figure 5H–I).
ROS from mitochondria can induce DNA damage, but it is also well documented that DNA damage plays an important role in the formation of ROS.19 We evaluated the expression of ɣ-H2AX, a marker of DNA double strand breaks (DSBs), by western blot and immunofluorescence. ATG7 siRNA and CQ pretreatment increased ɣ-H2AX expression in both OVCAR8 and HEY cells. Even higher expression of ɣ-H2AX was observed with combined treatment when compared to treatment with each drug individually. (Figure 5J–M). These results suggest that decrease of cell viability produced by olaparib when autophagy was inhibited may be, at least in part, associated with increased intracellular ROS levels.
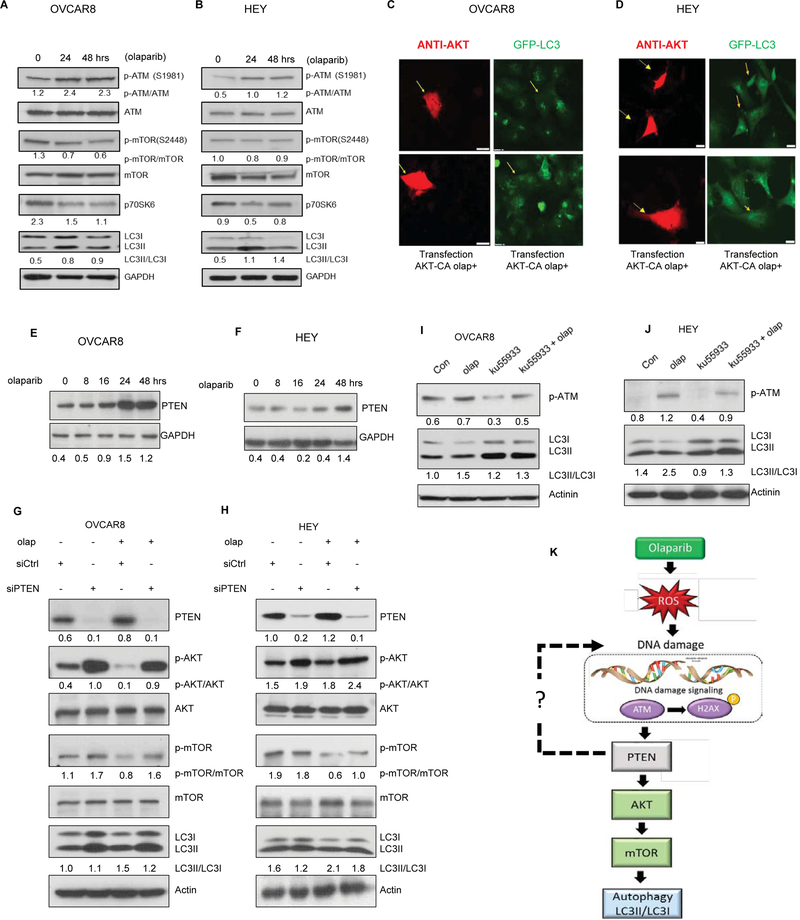
PTEN/AKT-mTOR signaling Pathways are involved in olaparib induced- protective autophagy in ovarian cancer cells.
To identify potential mechanisms underlying induction of autophagy by olaparib, a high-throughput antibody array was used to evaluate the phosphorylation status of different kinases. We treated OVCAR8 ovarian cancer cells for 48 hours and evaluated the cellular protein content using a Human Phospho-kinase Antibody Array (R and D) Systems. Numerous kinases exhibited changes in phosphorylation status in response to olaparib treatment (Figure S7). Several key modulators of autophagy were included. We observed changes in the phosphorylation of both S473 AKT and S4228 mTOR after olaparib treatment. Furthermore, olaparib also reduces proline-rich AKT substrate of 40kDa (PRAS40), a substrate of AKT.20 As shown in Figure 6A–B, western blot analysis confirmed these observations.
Figure 6. ATM/PTEN/AKT-mTOR signaling Pathways are involved in olaparib-induced autophagy in ovarian cancer cells.
A-B. Western blot analysis for the expression of ATM, mTOR, p70S6K, and LC3. OVCAR8 and HEY ovarian cancer cell lines were treated with olaparib (5 μM) for up to 48hours. C-D. Cells were cotransfected with AKT-CA plasmid and treated with olaparib for 24 hours. Cells were stained with anti-AKT and LC3 and examined with fluorescence microscopy. Arrowheads indicate cells transfected. A-B. Western blot analysis of PTEN after treatment of OVCAR 8 and HEY ovarian cancer cells with olaparib (5 μM) for different intervals (0–48 hours). E-H. Western blotting result of autophagy regulatory proteins including PTEN, AKT and mTOR, post PTEN siRNA in OVCAR8 and HEY cells. I-J. OVCAR8 and HEY cells were treated with the ATM inhibitor, ku55933 (10 μM) and/or olaparib during 48 hours; cell lysates were collected and analyzed by western blot analysis for p-ATM and LC3 expression. K. Schematic demonstration of effects on the ATM/PTEN/AKT/mTOR signaling pathway. Data represents results from at least two experiments.
To further elucidate whether the induction of autophagy by olaparib depends on AKT inhibition, we transfected HEY and OVCAR8 cells with a constitutively active AKT (AKT-CA) plasmid which contains two-point mutations, S473D and T308D. Olaparib increased LC3 punctae as determined by immunofluorescence staining, which could be inhibited in cells transfected with CA-AKT (Figure. 6C–D), suggesting that olaparib-induced autophagy is at least partially dependent on inhibiting the PI3K pathway.
An upstream regulator of AKT is the tumor suppressor gene PTEN (phosphatase and tensin homologue. The best-known biological role of PTEN resides in the negative regulation of PI3K class I/AKT/mTOR pathway.21 Therefore, we investigated the expression of PTEN in OVCAR8 and HEY cells after treatment with olaparib. Immunoblot analysis shows that expression of PTEN was upregulated by olaparib in a time-dependent manner (Figure 6E–F). Inhibition of PTEN reversed olaparib induced increased LC3II (Figure 6G–H). We also treated OVCAR8 and HEY cells with the ATM inhibitor KU-55933 and evaluated its effect on olaparib-induced autophagy. As shown in Figure 6I and 6J, inhibition of ATM decreases conversion of LC3. These results suggest that PTEN activation by DNA damage induces protective autophagy in association with the inhibition of the p-AKT /p-mTOR pathway, in response to olaparib.
Discussion
Despite the promising anti-cancer effects of PARPi in OC patients with germline and somatic mutations in BRCA1/2, at most only half of patients respond to treatment 2. Acquired resistance to PARP inhibitors is common and our knowledge of the molecular mechanisms of resistance in ovarian cancer are poorly understood. Autophagy is a cellular response process to stressful conditions, not limited only to nutrient deprivation, hypoxia or starvation but also to chemotherapy and radiation. Recent studies have found that autophagy can protect cancer cells from chemotherapy or can enhance the response to certain drugs, which can contribute to the efficacy of the anticancer drugs as well as to drug resistance.6 Based on these observations with other drugs and the increasing use of PARPi to treat ovarian cancer, we have explored the role of autophagy on the sensitivity of ovarian cancer cells to PARPi. Previous studies by Arun et. al. has shown that olaparib induces autophagy/mitophagy in BRCA mutant breast cancer cells, that is involve in cell death mechanism.22 In this paper, we report for the first time that PARPi-induced autophagy is a potentially important source of adaptive resistance to this class of drugs in ovarian cancer and have elucidated the mechanism by which autophagy is induced. Importantly, these effects were observed in BRCA wt cells, suggesting that this approach is more generally applicable.
Remarkably, all 4 of the PARPi evaluated could induce autophagy. Overall, functional inhibition of autophagy with chloroquine enhanced olaparib’s activity in cell culture in 6 ovarian cancer cell lines. Moreover, a combination of olaparib and chloroquine proved more effective than either single agent in a xenograft model of the OVCAR8 ovarian cancer cell line and in a patient derived xenograft. Importantly, activation of autophagy was detected within the therapeutic plasma concentration (Cmax) (5 μM) of olaparib. Cmax for patients following dosing at 400 mg bid, is estimated at steady state to range from 1.18 to 14.2 μg/mL (2.7 μM-32.64 μM).23
We demonstrated that combination of CQ, HCQ or LYS05 with olaparib triggered increased cell death, reflecting sensitization of OC cells to olaparib and cell-growth inhibition. A major concern is that the high concentration of CQ and its analogue HCQ that is required to block autophagy in cell culture may be difficult to achieve and maintain in the patients. However, the PDX experiments herein indicate that biologically meaningful concentrations of CQ are achievable in vivo. In addition, the cytotoxic effects of CQ on cancer cells death may extend beyond autophagy, as recent studies have implicated cell cycle arrest, upregulation of TP53 and induction of apoptosis. 24–26
This conclusion is supported not only by the pharmacological experiments using CQ and HCQ, but also by using LYS05 and the siRNA inhibition of ATG5 and ATG7. Quinacrine dimers have recently been developed that have 1000-fold greater potency than HCQ 13 and could facilitate clinical trials of PARP inhibitors with inhibitors of autophagy.
Treatment with either CQ or siRNA against ATG7 in OVCAR8 and HEY -treated cells there is an increase in ROS and ɣ-H2AX as compared to control and each drug alone. Consequently, the cause of apoptosis induction in the combination treatment might be related to the increase in DNA damage and accumulation of ROS. In accordance with these results Qu et al. showed that CQ increases the sensitivity to cisplatin in cholangiocarcinoma cells by increasing ROS.27 Also, Ganguli et al. found that inhibition of autophagy by CQ potentiates the anti-cancer properties of artemisinin by promoting ROS dependent apoptosis.28
Mechanistically, our report has revealed several key molecules and pathways important for olaparib-induced autophagy. Olaparib inhibited PARP activity. Ɣ-H2AX increased after treatment with olaparib, consistent with the induction of DNA damage. Phosphorylation of ATM was increased by olaparib, which was inhibited by the ATM inhibitor, KU-55933, suggesting that DNA damage response was mediated by ATM pathway. Olaparib also increased formation of ROS, increased cell cycle arrest and induced apoptosis. Olaparib-induced ROS played an important role in olaparib-induced autophagy as suggested by the decreased in LC3 conversion after treatment with NAC or melatonin. Conversely, additive accumulation of ROS and ɣ-H2AX could enhance olaparib-induced apoptosis.
The PI3K-AKT-mTOR signaling pathway is an important negative regulator of autophagy.29 Here, olaparib inactivated the AKT/mTOR pathway and upregulated LC3II expression, suggesting that olaparib induced autophagy mediated by AKT/mTOR pathway. In addition, we found that olaparib increased PTEN expression. Furthermore, silencing PTEN reversed olaparib-induced autophagy, suggesting that PTEN was involved in olaparib-induced cytoprotective autophagy. These results were consistent with a study in human colon cancer cells (HT-29) in which expression of PTEN increases autophagy via the PI3K signaling pathway.30 In addition, inhibiting ATM decreased olaparib-induced autophagy. Collectively, these results showed that ATM and PTEN are essential for the subsequent induction of autophagy.
These results support further investigation into the combination of CQ or other autophagy inhibitors with PARPi as a possible novel strategy to improve PARPi therapeutic effect and the outcome of OC patients. To date, most of the clinical studies have been undertaken with hydroxychloroquine rather than chloroquine due to its predictable toxicity profile. The PK and PD for clinically tolerable doses of hydroxychloroquine are at the margin of those required to inhibit autophagy functionally. Despite these limitations, encouraging phase II results have been reported. 31 Drugs that target Atg4 and Vps34 required for the development of autophagic vesicles are also being developed.32 As these become available, trials can be developed combining anti-autophagic drugs and PARPi. To date, most of these drugs have not been myelotoxic, so that combination therapy should be feasible.
Supplementary Material
Figure S1. Olaparib induces autophagy in ovarian cancer cells. Cell lysate was collected after 72 hours treatment with olaparib and western blot was performed to examine the conversion of LC3-I to LC3-II. GAPDH was used as a loading control. (B) OVCAR8 cells were treated with rucaparib, niraparib and talazoparib (0–10uM) for 72 hrs and conversion of LC3-I to LC3-II was analyzed by western blot. (C) Immunofluorescence staining of LC3 of cells treated with olaparib. Data represents results from at least two experiments.
Figure S2. Inhibition of autophagy increases the sensitivity of ovarian cancer cells to Olaparib. A-B. Cells were pretreated for 4 hours with 12μM of chloroquine (CQ), 5μM of olaparib or both for 5 days. Whole cell lysates were analyzed for conversion LC3I to LC3II by Western blot. GAPDH was used as a loading control. C –F. Cells were plated in 96-well plate, then sequentially treated with olaparib, chloroquine or combination of both for 5 days. SRB assay was performed to evaluate cell viability. (n=3). *P<0.05, **p < 0.01, ***P<0.001, Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S3. Inhibition of olaparib-induced autophagy decreases cell viability and enhances apoptosis of UWB1.289 (BRCA1 mutant) cells. A. Cells were pretreated for 4 hours with 15 μM chloroquine (CQ), with or without 0.3 μM of olaparib (olap) or both and incubated for 5 days before fixation and staining by sulforhodamine. B. After incubation with Annexin V-FITC in a buffer containing propidium iodide, cells were analyzed for apoptosis using flow cytometry. Data represents results from at least two experiments.
Figure S4. Inhibition of autophagy increases the sensitivity of ovarian cancer cells to other PARPi. A-C. OVCAR8 cells were pretreated for 4 hours with 12μM of chloroquine (CQ), 5μM of rucaparib, niraparib or talazaoparib. Whole cell lysates were analyzed for conversion of LC3I to LC3II by Western blot. GAPDH was used as a loading control. (D -F) Cells were plated in 96-well plate, then sequentially treated with rucaparib, niraparib or talazoparib with or without chloroquine for 5 days. SRB assay was performed to evaluate cell viability. (n=3). *P<0.05, **p < 0.01, ***P<0.001. Results were obtained using one-way ANOVA. Data represents results from at least three experiments.
Figure S5. Olaparib inhibits PARP activity, induces apoptosis, cell cycle arrest and DNA damage in ovarian cancer cells. A-B. Effect of olaparib on PARP activity. OVAR8 and HEY cells were treated with different concentrations of olaparib (0.05–2.5μM) for 24 hours and subjected to a modified PARP activity assay. C-D. Cells were treated with olaparib for 24 hrs and Immunofluorescence staining was performed for H2AX (green) and counterstaining with DAPI (blue). E-F.Cells were treated with different concentrations of olaparib and western analysis was performed to detect ɣ-H2AX as a marker for DNA damage. G-J. Cells were labeled with PI/annexin V-FITC and DAPI and analyzed for apoptosis and cell cycle by flow cytometry. (n=3). *p< 0.05, **p<0.01, ***p<0.001, ****P<0.0001. Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S6. Olaparib induces apoptosis, cell cycle arrest and DNA damage in ovarian cancer cells. A. Cells were treated with olaparib for 24 hrs and Immunofluorescence staining was performed for H2AX (green) and counterstaining with DAPI (blue). B-C. Cells were treated with different concentrations of olaparib and labeled with PI/annexin V-FITC and DAPI and analyzed for apoptosis and cell cycle by flow cytometry. *p< 0.05, **p<0.01, ***p<0.001, ****P<0.0001. (n=3). Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S7. A high-throughput profile reveals numerous phosphorylated kinases during adenovirus infection. The Human Phospho-kinase Antibody Array (R&D Systems) was employed to examine the phosphorylation status of a variety of kinases in OVCAR8 cells following olaparib treatment. Cells were treated with 5uM of olaparib 48hrs and whole-cell protein lysates were analyzed by the array. The spots indicate phospho-protein levels and controls in duplicate. Please see table 1 for list of kinases. Data represents results from one experiment.
Figure S8. Olaparib induces autophagy in OVCAR8 xenografts. Mice with OVCAR8 xenografts were treated with 40mg/kg of olaparib and 50mg/kg of chloroquine. After 4 weeks, tissue was collected and frozen for LC3 analysis. Each tissue was lysed, and total protein extracted. Whole cell lysates were analyzed for conversion of LC3I to LC3II by Western blot. GAPDH was used as a loading control. Data represents from at least two experiments.
Figure S9. A combination of olaparib and chloroquine inetracted synergistcally in inhibiting growth of ovarian cancer cells. A-B. OVCAR8 and HEY ovarian cancer cells were treated with different concentrations of olaparib and chloroquine using a 1:1.5 ratio. The combination of olaparib with chloroquine demonstrated a synergistic effect judged by a combination index in OVCAR8 and HEY cells. Data represents results from at least two experiments.
Acknowledgments
Funding Support
This work was supported by grants from the Cancer Prevention and Research Institute of Texas RP110595-P1, NCI R01 CA 135354, NIH/NCI Diversity Supplement #R01CA135354, Texas, the MD Anderson SPOREs in Ovarian Cancer NCI P50 CA 83639 and CA 217685, the Mayo Clinic SPORE in Ovarian Cancer CA136393, the Shared Resources of the MD Anderson CCSG grant NCI P30 CA16672, The National Foundation for Cancer Research, and philanthropic support from generous donations from Stuart and Gaye-Lynn Zarrow, the Mossy Foundation and the Roberson Endowment.
Footnotes
Conflict of Interest Disclosure
Dr. Ravi K. Amaravadi is founder of Pinpoint Therapeutics and inventor on 3 patents related to Lys05.
References
- 1.Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. British Journal of Cancer. 2011;105: 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrail DJ, Lin CC-J, Garnett J, et al. Improved prediction of PARP inhibitor response and identification of synergizing agents through use of a novel gene expression signature generation algorithm. npj Systems Biology and Applications. 2017;3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang ZNJ, Chee CE, Huang SB, Sinicrope FA. The Role of Autophagy in Cancer: Therapeutic Implications. Molecular Cancer Therapeutics. 2011;10: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulda S Autophagy in Cancer Therapy. Frontiers in Oncology. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. Journal of Pathology. 2010;221: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskelinen EL. The dual role of autophagy in cancer. Current Opinion in Pharmacology. 2011;11: 294–300. [DOI] [PubMed] [Google Scholar]
- 7.Santiago-O’Farrill JM, Kleinerman ES, Hollomon MG, et al. Phosphorylated heat shock protein 27 as a potential biomarker to predict the role of chemotherapy-induced autophagy in osteosarcoma response to therapy. Oncotarget. 2018;9: 1602–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou JH, Alfredi A, Zhang S, et al. A novel compound ARN-3236 inhibits SIK2 and sensitizes ovarian cancer to paclitaxel. Cancer Research. 2016;76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stordal B, Timms K, Farrelly A, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Molecular Oncology. 2013;7: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols. 2006;1: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 11.Weroha SJ, Becker MA, Enderica-Gonzalez S, et al. Tumorgrafts as In Vivo Surrogates for Women with Ovarian Cancer. Clinical Cancer Research. 2014;20: 1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler KA, Hou XN, Becker MA, et al. Prevention of Human Lymphoproliferative Tumor Formation in Ovarian Cancer Patient-Derived Xenografts. Neoplasia. 2017;19: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebecca VW, Nicastri MC, McLaughlin N, et al. A Unified Approach to Targeting the Lysosome’s Degradative and Growth Signaling Roles. Cancer Discovery. 2017;7: 1266–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaravadi RK, Winkler JD. Lys05 A new lysosomal autophagy inhibitor. Autophagy. 2012;8: 1383–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo M, Amant F, Biankin AV, et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discovery. 2014;4: 998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad MB, Chen YQ, Gibson SB. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxidants & Redox Signaling. 2009;11: 777–790. [DOI] [PubMed] [Google Scholar]
- 17.Xu JA, Wu YH, Lu GA, et al. Importance of ROS-mediated autophagy in determining apoptotic cell death induced by physapubescin B. Redox Biology. 2017;12: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koksal M, Kurcer Z, Erdogan D, et al. Effect of melatonin and n-acetylcysteine on hepatic injury in rat induced by methanol intoxication: a comparative study. European Review for Medical and Pharmacological Sciences. 2012;16: 437–444. [PubMed] [Google Scholar]
- 19.Kang MA, So EY, Simons AL, Spitz DR, Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death & Disease. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinonen H, Nieminen A, Saarela M, et al. Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. Bmc Genomics. 2008;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu YN, Sun W, Lu JJ, et al. PTEN Activation by DNA Damage Induces Protective Autophagy in Response to Cucurbitacin B in Hepatocellular Carcinoma Cells. Oxidative Medicine and Cellular Longevity. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arun B, Akar U, Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B. The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. International Journal of Oncology. 2015;47: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astrazeneca. OLAPARIB MONOTHERAPY AS MAINTENANCE TREATMENT OF PATIENTS WITH PLATINUM-SENSITIVE RELAPSED GERMLINE BRCA MUTATED (gBRCAm) OVARIAN CANCER 2014. [Google Scholar]
- 24.Hu T, Li P, Luo ZG, et al. Chloroquine inhibits hepatocellular carcinoma cell growth in vitro and in vivo. Oncology Reports. 2016;35: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakhter AJ, Sahu RP, Sun Y, et al. Chloroquine Promotes Apoptosis in Melanoma Cells by Inhibiting BH3 Domain-Mediated PUMA Degradation. Journal of Investigative Dermatology. 2013;133: 2247–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EL, Wustenberg R, Rubsam A, et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncology. 2010;12: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu XZ, Sheng JY, Shen LY, et al. Autophagy inhibitor chloroquine increases sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by mitochondrial ROS. Plos One. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganguli A, Choudhury D, Datta S, Bhattacharya S, Chakrabarti G. Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie. 2014;107: 338–349. [DOI] [PubMed] [Google Scholar]
- 29.Liang C Negative regulation of autophagy. Cell Death and Differentiation. 2010;17: 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arico S, Petiot A, Bauvy C, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. Journal of Biological Chemistry. 2001;276: 35243–35246. [DOI] [PubMed] [Google Scholar]
- 31.Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting Autophagy in Cancer. Cancer. 2018;124: 3307–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronan B, Flamand O, Vescovi L, et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nature Chemical Biology. 2014;10: 1013-+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Olaparib induces autophagy in ovarian cancer cells. Cell lysate was collected after 72 hours treatment with olaparib and western blot was performed to examine the conversion of LC3-I to LC3-II. GAPDH was used as a loading control. (B) OVCAR8 cells were treated with rucaparib, niraparib and talazoparib (0–10uM) for 72 hrs and conversion of LC3-I to LC3-II was analyzed by western blot. (C) Immunofluorescence staining of LC3 of cells treated with olaparib. Data represents results from at least two experiments.
Figure S2. Inhibition of autophagy increases the sensitivity of ovarian cancer cells to Olaparib. A-B. Cells were pretreated for 4 hours with 12μM of chloroquine (CQ), 5μM of olaparib or both for 5 days. Whole cell lysates were analyzed for conversion LC3I to LC3II by Western blot. GAPDH was used as a loading control. C –F. Cells were plated in 96-well plate, then sequentially treated with olaparib, chloroquine or combination of both for 5 days. SRB assay was performed to evaluate cell viability. (n=3). *P<0.05, **p < 0.01, ***P<0.001, Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S3. Inhibition of olaparib-induced autophagy decreases cell viability and enhances apoptosis of UWB1.289 (BRCA1 mutant) cells. A. Cells were pretreated for 4 hours with 15 μM chloroquine (CQ), with or without 0.3 μM of olaparib (olap) or both and incubated for 5 days before fixation and staining by sulforhodamine. B. After incubation with Annexin V-FITC in a buffer containing propidium iodide, cells were analyzed for apoptosis using flow cytometry. Data represents results from at least two experiments.
Figure S4. Inhibition of autophagy increases the sensitivity of ovarian cancer cells to other PARPi. A-C. OVCAR8 cells were pretreated for 4 hours with 12μM of chloroquine (CQ), 5μM of rucaparib, niraparib or talazaoparib. Whole cell lysates were analyzed for conversion of LC3I to LC3II by Western blot. GAPDH was used as a loading control. (D -F) Cells were plated in 96-well plate, then sequentially treated with rucaparib, niraparib or talazoparib with or without chloroquine for 5 days. SRB assay was performed to evaluate cell viability. (n=3). *P<0.05, **p < 0.01, ***P<0.001. Results were obtained using one-way ANOVA. Data represents results from at least three experiments.
Figure S5. Olaparib inhibits PARP activity, induces apoptosis, cell cycle arrest and DNA damage in ovarian cancer cells. A-B. Effect of olaparib on PARP activity. OVAR8 and HEY cells were treated with different concentrations of olaparib (0.05–2.5μM) for 24 hours and subjected to a modified PARP activity assay. C-D. Cells were treated with olaparib for 24 hrs and Immunofluorescence staining was performed for H2AX (green) and counterstaining with DAPI (blue). E-F.Cells were treated with different concentrations of olaparib and western analysis was performed to detect ɣ-H2AX as a marker for DNA damage. G-J. Cells were labeled with PI/annexin V-FITC and DAPI and analyzed for apoptosis and cell cycle by flow cytometry. (n=3). *p< 0.05, **p<0.01, ***p<0.001, ****P<0.0001. Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S6. Olaparib induces apoptosis, cell cycle arrest and DNA damage in ovarian cancer cells. A. Cells were treated with olaparib for 24 hrs and Immunofluorescence staining was performed for H2AX (green) and counterstaining with DAPI (blue). B-C. Cells were treated with different concentrations of olaparib and labeled with PI/annexin V-FITC and DAPI and analyzed for apoptosis and cell cycle by flow cytometry. *p< 0.05, **p<0.01, ***p<0.001, ****P<0.0001. (n=3). Results were obtained using one-way ANOVA. Data represents results from at least two experiments.
Figure S7. A high-throughput profile reveals numerous phosphorylated kinases during adenovirus infection. The Human Phospho-kinase Antibody Array (R&D Systems) was employed to examine the phosphorylation status of a variety of kinases in OVCAR8 cells following olaparib treatment. Cells were treated with 5uM of olaparib 48hrs and whole-cell protein lysates were analyzed by the array. The spots indicate phospho-protein levels and controls in duplicate. Please see table 1 for list of kinases. Data represents results from one experiment.
Figure S8. Olaparib induces autophagy in OVCAR8 xenografts. Mice with OVCAR8 xenografts were treated with 40mg/kg of olaparib and 50mg/kg of chloroquine. After 4 weeks, tissue was collected and frozen for LC3 analysis. Each tissue was lysed, and total protein extracted. Whole cell lysates were analyzed for conversion of LC3I to LC3II by Western blot. GAPDH was used as a loading control. Data represents from at least two experiments.
Figure S9. A combination of olaparib and chloroquine inetracted synergistcally in inhibiting growth of ovarian cancer cells. A-B. OVCAR8 and HEY ovarian cancer cells were treated with different concentrations of olaparib and chloroquine using a 1:1.5 ratio. The combination of olaparib with chloroquine demonstrated a synergistic effect judged by a combination index in OVCAR8 and HEY cells. Data represents results from at least two experiments.