Natural products have been an unprecedented starting point for drug and agrochemical discovery. They represent the only validated source of chemical diversity capable of delivering a sustainable pipeline for novel drug candidates. As of 1990, 80% of clinically used drugs were natural products or natural product-inspired or semi-synthetic derivatives. From 1994 to 2007, almost half of the new chemical entities approved by FDA were based on natural products [1]. By the end of 2013, natural products or their inspired compounds comprised about 40% of the FDA approved drugs [2]. Streptomyces are by far the most prolific natural product producing organisms and as such this genus has been the research subjects for lead compound discovery for decades. Streptomyces natural products display a diverse array of complex chemical structures including polyketides, peptides, alkaloids and terpenoids. Polyketides (PKSs) produced by Streptomyces contribute many clinically used drugs including immunosuppressants, antiparasitics and antitumor agents [3]. Organic chemists have developed many innovative regio- and stereo-selective strategies to chemically synthesize these structurally complex molecules. However, these total synthetic methods have suffered poor atomic economy, which renders them not economically viable [4]. Microbial fermentation instead is an attractive method in biopharmaceutical industry to produce these polyketide drugs. It is common observation that these PKSs are produced by wild-type Streptomyces strains in a minor amount of materials with the range of submilligram to milligram per litre of liquid culture in the tamed conditions. In the past, untargeted random mutagenesis has been applied with the hope that, after several rounds of mutagenesis, the mutants can generate enhanced titres (preferable grams or kilograms per litres) for the industry to be economically viable. Although having been successfully applied to many PKS producing strains to generate clinically used medicines, this method has been considered time-consuming and extensively laborious, causing difficulty in wider applications.
With advance in sequencing and recombinant DNA technology in the new millennium, we now understand how these chemically complex yet valuable PKSs are produced by Streptomyces. Biosynthetically the PKS pathways are similar to the fatty acid pathway in which chain elongation undergoes via a Claisen-like condensation of acyl-coenzyme A extender units. As with fatty acid synthases, polyketide synthases (PKSs) can be large multifunctional proteins (Type I) or a set of individual monofunctional proteins (type II) or a group of multifunctional enzymes of the chalcone synthase type (type III) [5]. With an ever-expanding toolbox of biosynthetic components, synthetic biology enabling metabolic engineering is an increasingly powerful method to improve PKS titres and generate novel compounds. This can be exemplified by manipulating regulation and signal transduction to stimulate environmental cues, improving precursor and cofactor pools, removing or suppressing competing pathways, improving enzyme conversion rates for bottleneck reactions and overexpressing genes conferring tolerance for the toxicity of produced metabolites [6]. Among these strategies, enriching the pool of acetyl-CoA, a key precursor of polyketides, has been widely used for the titre improvement, by blocking glycolysis or reducing TCA cycle activity to redirect the carbon fluxes, or transcriptionally enhancing the formation of acetyl-CoA precursor etc. [3,7].
Due to the importance in lead compound discovery, the biology of Streptomyces has been well-studies. It is known that natural products are produced by Streptomyces or other microorganisms via secondary metabolisms. Unlike primary metabolites, which are essential for growth in all organisms, secondary metabolites are produced in the stationary phase. During the growth phase in the fermentation, cells utilise external nutrients and grow exponentially. When external nutrients are expanded, cells stop growing and enter stationary phase, in which secondary metabolites start to be produced [7]. However, it has remained poorly understood which factor(s) trigger the metabolic switch from primary metabolisms to secondary metabolisms during the course of fermentation and how cells divert intracellular precursors from primary metabolisms to PKS production in stationary phase during fermentation.
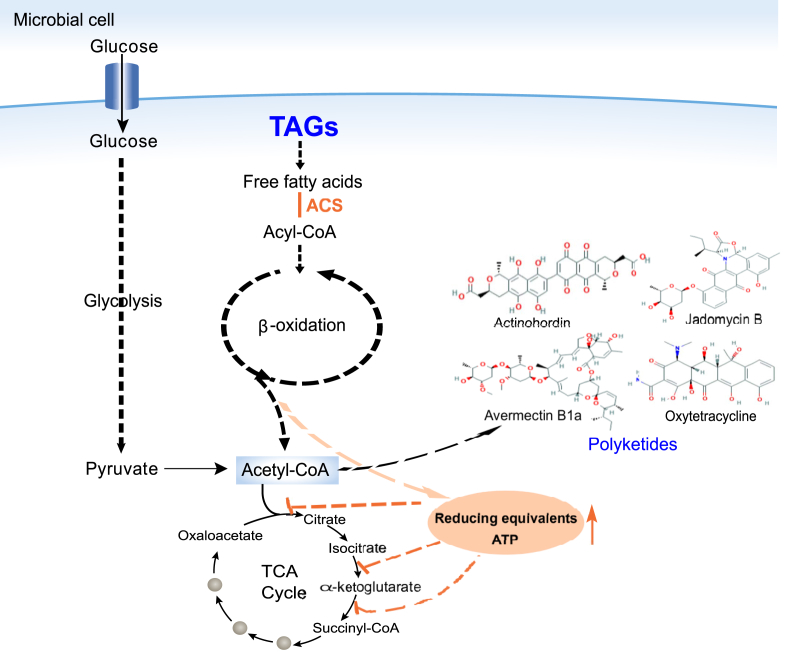
Featured in the December issue (vol. 38, pp. 76–83) of Nature Biotechnology in 2019, a Chinese team led by Professor Lixin Zhang from East China University of Science and Technology published a research article, revealing a breakthrough observation of a process that channels carbon flux from triacylglycerols (TAGs), accumulated lipids during the growth phase, into PKS biosynthesis via enzymatic β-oxidation [8]. They applied multi-omics to confirm that the accumulated TAG pool is the intracellular carbon source for PKS production during the stationary phase by comparing the metabolomic and transcriptomic data of wild-type and high-yielding PKS Streptomyces strains. Through metabolic flux analyses and a series of assays, the team uncovered the mystery: more degradation of TAGs in the high-yielding strain via multi-cycle β-oxidation increased cellular acetyl-CoA, as well as reducing equivalents and ATP. The high level of the latter two metabolites further inhibit TCA cycles, which in turn weakens the carbon flux from acetyl-CoA to TCA cycle but increases the precursor supply towards polyketide biosynthesis (Fig. 1). To make good use of this finding, the team designed a new strategy named dynamic degradation of TAG (ddTAG) to rationally control the mobilization of TAGs towards PKS production during stationary phase of fermentation by overexpressing a fatty acyl-CoA synthetase (ACS) encoding the gene sco6196 under a cumate-inducible promoter. The ACS can activate more fatty acids via thioesterification with coenzyme A (CoA) and therefore enhance the β-oxidation process. This ddTAG module enabled significantly improved titres of four different polyketide lead compounds, namely actinorhodin, jadomycin B, oxytetracycline and avermectin B1a from four different Streptomyces producing strains in the shake flask fermentation conditions, respectively, suggesting the wider application of this biotechnology. Finally, the team applied this ddTAG strategy in the scale-up industrial bioreactor to persuade the engineered strain A56-DT to produce 50% higher titres of avermectin B1a to 9.31 g l−1, which is the highest titre ever reported. Interestingly, a recent report by Alper et al. demonstrated a similar β-oxidation up-regulation strategy on intracellular lipid pool in the oleaginous yeast Yarrowia liplytica that enables increased production of a variety of fungal polyketide metabolites, naringenin, resveratrol and bisdemethoxycurcumin in the mutated variants [9]. This observation further demonstrates that rational control of β-oxidation strategy on lipid mobilization during stationary phase can be widely applied to enhance microbial polyketide titres for pharmaceutical production.
Fig. 1.
β-Oxidation up-regulation strategy on intracellular triacylglycerols (TAGs) employed in Ref. [8]. Intracellular TAGs which accumulate in primary metabolism are mobilized to produce polyketides via β-oxidation during stationary phase. Overexpression of the fatty acyl-CoA synthetase (ACS) allows overproduction of acyl-CoA to enter β-oxidation cycle, and therefore increasing the cellular level of acetyl-CoA, the key precursor of polyketides, as well as reducing equivalents and ATP. The high level of the latter two metabolites further inhibit TCA cycles, which in turn weakens the carbon flux from acetyl-CoA to TCA cycle but increases the precursor supply towards polyketide biosynthesis.
Acknowledgement
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC) award (BB/P00380X/1) to H.D.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Harvey A.L. Natural products in drug discovery[J] Drug Discov Today. 2008;13(19–20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Katz L., Baltz R.H. Natural product discovery: past, present, and future[J] J Ind Microbiol Biotechnol. 2016;43(2–3):155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 3.Hwang K.S., Kim H.U., Charusanti P. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol Adv. 2014;32(2):255–268. doi: 10.1016/j.biotechadv.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Trost B.M., Frederiksen M.U., Rudd M.T. Ruthenium‐catalyzed reactions—a treasure trove of atom‐economic transformations. Angew Chem Int Ed. 2005;44(41):6630–6666. doi: 10.1002/anie.200500136. [DOI] [PubMed] [Google Scholar]
- 5.Hertweck C. The biosynthetic logic of polyketide diversity. Angew Chem Int Ed. 2009;48(26):4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- 6.Bilyk O., Luzhetskyy A. Metabolic engineering of natural product biosynthesis in actinobacteria. Curr Opin Biotechnol. 2016;42:98–107. doi: 10.1016/j.copbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Liu G., Chater K.F., Chandra G. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol Mol Biol Rev. 2013;77(1):112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Li S., Li Z. Harnessing the intracellular triacylglycerols for titer improvement of polyketides in streptomyces. Nat Biotechnol. 2020;38(1):76–83. doi: 10.1038/s41587-019-0335-4. [DOI] [PubMed] [Google Scholar]
- 9.Palmer C.M., Miller K.K., Nguyen A. Engineering 4-coumaroyl-CoA derived polyketide production in Yarrowia lipolytica through a β-oxidation mediated strategy. Metab Eng. 2020;57:174–181. doi: 10.1016/j.ymben.2019.11.006. [DOI] [PubMed] [Google Scholar]