Abstract
Malignant pulmonary granular cell tumor (GCT) is extremely rare and difficult to distinguish from benign GCT. Most GCTs are neural-type and express S-100. However, a small subset of tumors sub-classified as the non-neural type do not express S-100. We report a case of malignant non-neural-type GCT in the lungs.
A 77-year-old woman felt chest discomfort and dyspnea in July 2019. She had never smoked and had no medical history other than hypertension and diabetes mellitus.
She was initially evaluated at a local hospital. Flexible bronchoscopy showed total occlusion of the right main bronchus by a mass-like lesion. Biopsy of the mass lesion revealed chronic inflammation. The patient visited for re-evaluation in September 2019. Rigid bronchoscopy showed worsening of the total obstruction of the right main bronchus by a tumor mass, such that the carina was not visible. Additionally, endobronchial nodules were observed on the medial side of left main bronchus. The tumor masses of both main bronchi were removed by bronchoscopic intervention, but the right main bronchus was not opened. Biopsy revealed malignant GCT, favoring the non-neuronal type (S-100-negative).
We report an extremely rare case of malignant pulmonary GCT negative for S-100 in immunohistochemistry. In this case, surgical resection was not possible because the tumor was diagnosed at a fairly advanced stage and had spread to involve the contralateral main bronchus. The patient chose to be treated at another hospital and was thereafter lost to follow-up.
Keywords: Malignant pulmonary granular cell tumor, S-100-Negative, Non-neural type
Abbreviations: GCT, granular cell tumor; CT, computed tomography; IHC, immunohistochemistry; N:C, nuclear-cytoplasmic; HPF, high-power fields
1. Introduction
Granular cell tumors (GCTs), first described by Abirokossoff in 1926, are common in the head and neck region but can occur at any site [1]. Pulmonary GCT, first reported by Kramer in 1938, is rare [2], with fewer than 80 cases reported through 1995 [3] and only a few more cases reported subsequently. Malignant pulmonary GCT is even more unusual and difficult to distinguish from benign GCT [[4], [5], [6], [7]]. Their proposed histological origins include neural cells, especially Schwann cells [1,8]; thus, most GCTs are considered neural-type and express S-100 protein regardless of the location [9]. However, a small subset of tumors sub-classified as non-neural, do not express S-100 [10,11]. This report describes a case of malignant GCT of the non-neural type occurring in the lungs.
2. Case report
A 77-year-old woman felt chest discomfort and dyspnea in July 2019. The patient had never smoked and had no medical history other than hypertension and diabetes mellitus diagnosed 10 and 5 years prior, respectively. The patient indicated that a chest X-ray performed one year ago was normal. She was admitted to the local hospital and examination for the above symptoms revealed total collapse of her entire right lung. Flexible bronchoscopy revealed total occlusion of the right main bronchus by a mass-like lesion (Fig. 1A). Tissue biopsy by flexible bronchoscopy showed chronic inflammation. The patient then visited Samsung Medical Center for re-evaluation and medical treatment for the endobronchial lesion in September 2019. At that time, the patient constantly complained of dyspnea and intermittent coughing. A chest x-ray showed total atelectasis of her right lung. Since the airway occlusion had persisted for at least two months, the medical staff believed that the probability of reopening of the right lung through bronchoscopic intervention was low. Therefore, the main purpose of rigid bronchoscopy was to establish a treatment plan after an accurate diagnosis with tissue biopsy. The secondary purpose was to remove as much of the obstruction as possible.
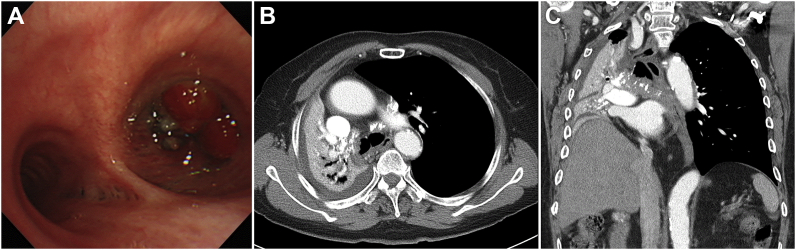
Fig. 1.
(A) Flexible bronchoscopy showing total occlusion of the right main bronchus by a tumor mass. Chest CT showing a calcified tumor mass extending to the right main bronchus and collapse of the right lung. (B) Transverse view (C) Coronal view.
Chest computed tomography (CT) images showed a calcified mass extending to the right main bronchus that had probably originated in the bronchus intermedius and appeared to grow towards the subcarinal area (Fig. 1B and C). Results from the Department of Radiology at Samsung Medical Center indicated that the lesion was more likely to be a central bronchogenic carcinoma than a benign lesion. The other side of the thorax or the upper abdomen did not show any lesions on the CT imaging.
The patient was hospitalized and underwent rigid bronchoscopy, which showed total obstruction of the right main bronchus by a mass. The mass had worsened such that the carina was not readily visible (Fig. 2A). Additionally, small round black endobronchial nodules were observed on the medial side of the left main bronchus (Fig. 2B). The mass in the right main bronchus was removed using a rigid tube and snare but the right main bronchus was not opened (Fig. 2C). The endobronchial nodules on the left main bronchus were removed by biopsy forceps. After rigid bronchoscopy, the right main bronchus did not open and chest X-ray showed persisting total collapse of the patient's right lung.
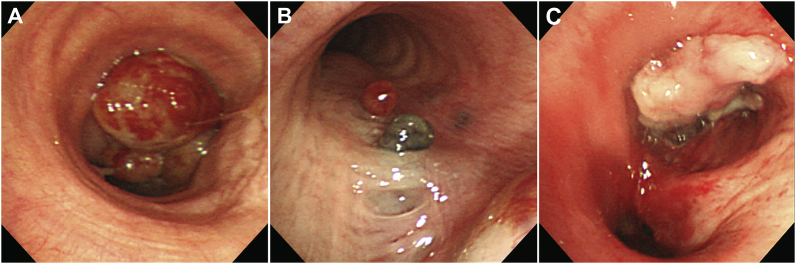
Fig. 2.
(A) Rigid bronchoscopy showing total obstruction of the right main bronchus by the worsening mass such that the carina is not easily observed. (B) Small round black endobronchial nodules are visible on the medial side of the left main bronchus. (C) The mass in the right main bronchus was removed using a rigid tube and snare but the right main bronchus was not opened.
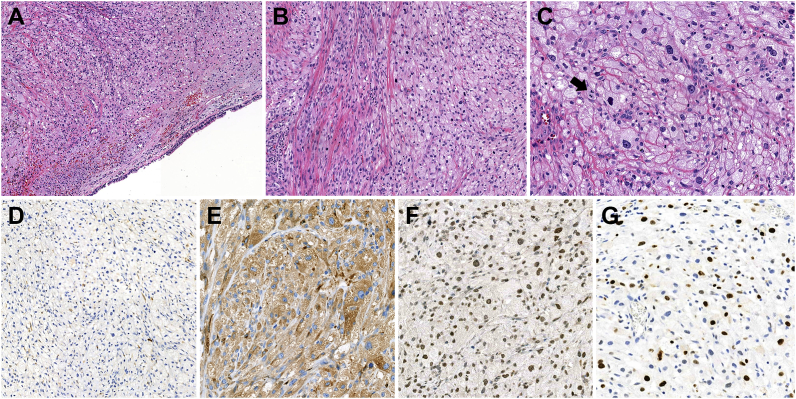
The mass specimen comprised several fragmented clusters of tumor cells arranged in solid sheets or cords separated by thin collagenous bands (Fig. 3A). Spindling of the tumor cells was observed in multifocal areas of the tumor (Fig. 3B). The tumor cells were round to polygonal in shape and had abundant eosinophilic and granular cytoplasm, pleomorphic nuclei with vesicular chromatin, and occasional prominent nucleoli (Fig. 3C). The nuclear-cytoplasmic (N:C) ratio was increased compared to that of conventional GCT. Increased mitotic activity was identified (up to 3 per 10 high-power fields [HPF]) (Fig. 3C). The histological findings were consistent with malignant GCT. Immunohistochemistry (IHC) revealed that the tumor cells were negative for S-100 (Fig. 3D) but diffusely positive for CD68 and TFE-3 (Fig. 3E and F), suggesting non-neural malignant GCT. The Ki-67 labeling index was up to 30% (Fig. 3G).
Fig. 3.
Histologic findings and immunohistochemical staining results from the malignant granular cell tumor of the bronchus. (A) Tumor cells proliferating in the submucosa of the bronchus, forming solid sheets or ribbon-like arrangements separated by thin fibrous tissue. (B) Frequent tumor cell spindling in multifocal areas of the tumor. (C) Tumor cells possessing abundant eosinophilic and coarse granular cytoplasm with distinct cell borders. Severe nuclear pleomorphism with vesicular nuclei, prominent nucleoli, increased N:C ratio, and brisk mitotic activity (arrow) are observed. (D–F) Immunostaining indicating that the tumor cells were negative for S-100 but had strong cytoplasmic and nuclear reactivity for CD 68 and TFE-3, respectively. (G) Increased Ki-67 labeling index (up to 30%) in tumor cells.
The malignant lesions in this patient extended to the right main bronchus, carina, and left main bronchus. Therefore, the medical staff planned chemotherapy or radiotherapy for further treatment rather than surgical resection. However, the patient desired easy access for undergoing additional treatment, so she was transferred to a local hospital and thereafter lost to follow-up.
3. Discussion
Approximately 6–10% of GCTs occur in the lungs, 90% of which are endobronchial lesions [1,12]. Most are benign, with very few malignant pulmonary GCTs having been reported. To date, four cases of malignant pulmonary GCT have been reported [[4], [5], [6], [7]]. One case suggested that malignant pulmonary GCTs coexist with small cell lung cancer; however, the histological evidence for malignant GCTs was unclear [4].
Regardless of benign and malignant status, GCT is a very rare tumor. Furthermore, the scarcity of cases and related studies make the diagnosis of malignant GCT difficult. In 1998, Fanburg-Smith et al. reported a large series of GCTs and proposed histologic criteria for the diagnosis of malignant GCT [13] in which GCTs were categorized as malignant, atypical, or benign according to the following six histologic features: 1) nuclear pleomorphism, 2) tumor cell spindling, 3) vesicular nuclei with large nucleoli, 4) increased N:C ratio, 5) necrosis, and 6) increased mitotic rate (>2 mitoses/10 HPF). Tumors with more than three of these features were classified as malignant GCT. Tumors with only one or two features were classified as atypical, while tumors with none of these features or with only focal nuclear pleomorphism were considered benign [13]. Despite the worldwide use of the Fanburg-Smith classification, other criteria for the diagnosis of malignancy have also been proposed. Nasser et al. re-classified GCTs according to the presence of necrosis and/or mitotic activity (>2 mitoses/10 HPF). Tumors with at least one of these features were defined as GCTs with uncertain malignant potential. Additionally, the presence of metastasis was the only factor required for the diagnosis of malignant cases [14]. In the present case, the GCT tissue showed histological evidence of nuclear pleomorphism, tumor cell spindling, and vesicular nuclei with large nucleoli. Furthermore, the tumor cells also had an increased N:C ratio and increased mitotic rate (>2 mitoses/10 HPF). Thus, the tumor met five of the six criteria in the Fanburg-Smith classification and one of two in the Nasser classification. Clinically, the lesion had started in the right side bronchus and spread to the left main bronchus. Fanburg-Smith et al. also reported a Ki-67 index <5% in benign GCTs, 5–10% in atypical tumors, and 10–50% in malignant GCTs [13]. Kapur et al. observed similar correlations and suggested that the Ki-67 index might be useful in distinguishing malignant or atypical GCT from benign GCT [15]. In the present case, the Ki-67 index was over 30%, corresponding to the range of 10–50%, providing an additional clue for diagnosing malignant GCT. By combining these findings, the patient could be definitively diagnosed with malignant pulmonary GCT.
All four previously reported cases of malignant pulmonary GCT were S-100-positive according to IHC [[4], [5], [6], [7]]. However, the present case was S-100-negative on IHC. GCTs negative for S-100 on IHC, known as “non-neural GCT”, were first described in 1991 as “primitive polypoid granular cell tumors” [16]. Chen et al. reported a non-neural GCT of the bronchus in a 9-year-old female patient and suggested that their case was the first example of a non-neural GCT in the lungs [11]. However, that tumor was not malignant. Another report of a case with S-100-negative GCT with malignant potential occurring on the scalp suggested that the absence of S-100 expression in malignant GCTs might be due to altered differentiation processes in the malignant tumors [17].
Benign GCTs are slow-growing and have a good prognosis; moreover, over half of GCT patients are asymptomatic at diagnosis [18]. As described above, malignant pulmonary GCT cases are extremely rare, and thus, their prognosis is unknown. Two of the three cases described above had pleural or lung metastasis and only one case presented a single solitary lesion for which surgical resection was performed [[5], [6], [7]]. Among GCTs occurring in locations other than the pulmonary system, compared to slow-growing benign GCTs, the prognosis of malignant GCTs seems to be poor because of their rapid progression and metastasis [13]. In the present case, upon patient presentation to the hospital with symptoms, a lesion completely blocking the right main bronchus was found and the degree of invasion had progressed rapidly when the patient reported for reevaluation of the lesion by bronchoscopy just two months later.
4. Conclusion
We report an extremely rare case of malignant pulmonary GCT that was S-100-negative on IHC. To our knowledge, ours is the first report of GCT with both malignancy and of the non-neural type in the lungs. In this case, surgical resection was not possible because the tumor was diagnosed at a fairly advanced stage and had spread to involve the contralateral main bronchus.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Meyer M.A., Becker J.M., Quinones W. Endobronchial granular cell tumor: a case report. J. Radiol. Case Rep. 2010;4:29–35. doi: 10.3941/jrcr.v4i8.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H.J., An S., Kim H.R. Primary bronchial granular cell tumor in an adult male. Korean J Thorac Cardiovasc Surg. 2014;47:193–196. doi: 10.5090/kjtcs.2014.47.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deavers M., Guinee D., Koss M.N., Travis W.D. Granular cell tumors of the lung. Clinicopathologic study of 20 cases. Am. J. Surg. Pathol. 1995;19:627–635. doi: 10.1097/00000478-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Lauro S., Trasatti L., Bria E., Gelibter A., Larosa G., Vecchione A. Malignant bronchial Abrikossoff's tumor and small cell lung cancer: a case report and review. Anticancer Res. 2001;21:563–565. [PubMed] [Google Scholar]
- 5.Jiang M., Anderson T., Nwogu C., Tan D. Pulmonary malignant granular cell tumor. World J. Surg. Oncol. 2003;1:22. doi: 10.1186/1477-7819-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S., Zhao Q., Wei S., Wu Y., Liu J., Shi T. Next generation sequencing uncovers potential genetic driver mutations of malignant pulmonary granular cell tumor. J. Thorac. Oncol. 2015;10:e106–109. doi: 10.1097/JTO.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 7.Davis R., Deak K., Glass C.H. Pulmonary granular cell tumors: a study of 4 cases including a malignant phenotype. Am. J. Surg. Pathol. 2019;43:1397–1402. doi: 10.1097/PAS.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 8.van der Maten J., Blaauwgeers J.L., Sutedja T.G., Kwa H.B., Postmus P.E., Wagenaar S.S. Granular cell tumors of the tracheobronchial tree. J. Thorac. Cardiovasc. Surg. 2003;126:740–743. doi: 10.1016/s0022-5223(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 9.Machado I., Cruz J., Lavernia J., Llombart-Bosch A. Solitary, multiple, benign, atypical, or malignant: the "Granular Cell Tumor" puzzle. Virchows Arch. 2016;468:527–538. doi: 10.1007/s00428-015-1877-6. [DOI] [PubMed] [Google Scholar]
- 10.Rawal Y.B., Dodson T.B. S-100 negative granular cell tumor (So-called primitive polypoid non-neural granular cell tumor) of the oral cavity. Head Neck Pathol. 2017;11:404–412. doi: 10.1007/s12105-016-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S.Y., Sadanand A., Dillon P.A., He M., Dehner L.P., Leonard D.S. Non-neural (S-100 negative) bronchial granular cell tumor causing acute respiratory failure. Fetal Pediatr. Pathol. 2019:1–5. doi: 10.1080/15513815.2019.1636431. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ghamdi A.M., Flint J.D., Muller N.L., Stewart K.C. Hilar pulmonary granular cell tumor: a case report and review of the literature. Ann. Diagn. Pathol. 2000;4:245–251. doi: 10.1053/adpa.2000.8128. [DOI] [PubMed] [Google Scholar]
- 13.Fanburg-Smith J.C., Meis-Kindblom J.M., Fante R., Kindblom L.G. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am. J. Surg. Pathol. 1998;22:779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Nasser H., Ahmed Y., Szpunar S.M., Kowalski P.J. Malignant granular cell tumor: a look into the diagnostic criteria. Pathol. Res. Pract. 2011;207:164–168. doi: 10.1016/j.prp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Kapur P., Rakheja D., Balani J.P., Roy L.C., Amirkhan R.H., Hoang M.P. Phosphorylated histone H3, Ki-67, p21, fatty acid synthase, and cleaved caspase-3 expression in benign and atypical granular cell tumors. Arch. Pathol. Lab Med. 2007;131:57–64. doi: 10.5858/2007-131-57-PHHKPF. [DOI] [PubMed] [Google Scholar]
- 16.LeBoit P.E., Barr R.J., Burall S., Metcalf J.S., Yen T.S., Wick M.R. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent nonneural origin. Am. J. Surg. Pathol. 1991;15:48–58. doi: 10.1097/00000478-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Schoedel K.E., Bastacky S., Silverman A. An S100 negative granular cell tumor with malignant potential: report of a case. J. Am. Acad. Dermatol. 1998;39:894–898. doi: 10.1016/s0190-9622(98)70375-5. [DOI] [PubMed] [Google Scholar]
- 18.Guarnieri T., Cardinale L., Macchia G., Cortese G., Veltri A. Multiphasic multidetector computed tomography study of a rare tracheal tumor: granular cell tumor. Case Rep Pulmonol. 2014;2014:807430. doi: 10.1155/2014/807430. [DOI] [PMC free article] [PubMed] [Google Scholar]